Abstract
Objectives
An ecofriendly alternative to chemical pesticides is bio-pesticides, which are derived from natural sources. The interest in bio-pesticides is based on the disadvantages associated with chemical pesticides.
Methods
We conducted acute toxicity assessments of camphor, a major component of bio-pesticides, by using Daphnia magna (D. magna) as well as assessed the morphological abnormalities that occurred in Danio rerio (D. rerio) embryos.
Results
The median effective concentration of camphor on D. magna after 48 hours was 395.0 μM, and the median lethal concentration on D. rerio embryos after 96 hours was 838.6 μM. The no observed effect concentration and predicted no effect concentration of camphor on D. magna, which was more sensitive than D. rerio, were calculated as 55.2 μM and 3.95 μM, respectively. Morphological abnormalities in D. rerio embryos exposed to camphor increased over time. Coagulation, delayed hatching, yolk sac edema, pericardial edema, and pigmentation of embryos mainly appeared between 24 and 48 hours. Further, symptoms of scoliosis and head edema occurred after 72 hours. In addition, bent tails, ocular defects and collapsed symptoms of fertilized embryonic tissue were observed after 96 hours.
Conclusions
The camphor toxicity results suggest that continuous observations on the ecosystem are necessary to monitor toxicity in areas where biological pesticides containing camphor are sprayed.
Keywords: Acute toxicity, Bio-pesticides, Camphor, Danio rerio embryo, Daphnia magna, Morphological abnormalities
Introduction
The overuse of chemical pesticides for improving agricultural productivity has created numerous problems, including the destruction of natural ecosystems and pesticide-tolerant diseases and insect pests. In addition, there has been increased interest in improving the quality of life by using eco-friendly agricultural products. Recently, in Organization for Economic Cooperation and Development (OECD) countries, including South Korea, the use of chemical pesticides and fertilizers has decreased and eco-friendly agricultural businesses have been increasingly promoted, while interest in bio-pesticides is increasing. As a result, bio-pesticides using eco-friendly, plant-derived ingredients showing broad insecticidal effects with minimal damage to the environment have been increasingly developed. Further, ecofriendly pesticides with plant extracts are used as insecticides, insect repellents, and anti-feedants; they target specific diseases or insect pests and cause minimal harm to birds, insects, and mammals, such as humans, and thus, are useful as insect repellants. In addition, even if used in an open field, they decompose quickly, thereby minimizing the risk of environmental pollution or residual toxicity [1]. Plant-derived insecticidal substances were first commercialized about 160 years ago. Currently, more than 150 related products are produced and sold in 39 countries, and the manufacture of such products is expected to increase [2]. The research institute involved in the present joint research, Environment and Future Ltd., has developed and been selling a bio-pesticide (SSAGRI; Nature & Future, Gokseong, Korea) with camphor extracted from camphor trees as the major ingredient. Eco-friendly bio-pesticides supposedly differ in their degree of influence according to the chemical structure of the extract and toxicity expression mechanism and in their toxicity and susceptibility according to the type of pesticide and the type of organism that is influenced [3,4]. In the “Rural Development Administration’s Bio-pesticide Registration Standard and Assessment Method,” the regulatory authorities for the registration and management of pesticides demand that additional toxicity data for aquatic organisms be included, along with Daphnia magna (D. magna) toxicity assessment results, for pesticides that are considered the most hazardous, to reduce the uncertainty of predictions of ecosystem effects because of differences in susceptibility based on the type of pesticide and organism.
Thus, in the present research, the toxicities to D. magna and Danio rerio (D. rerio) were assessed. D. rerio is relatively easy to rear, affordable to maintain, and produces 200-300 embryos in a single external fertilization, and therefore, are appropriate for toxicity assessment [5]. In addition, the assessment period was set to 96 hours, allowing quick derivation of toxicity results. Moreover, they are commonly used in medical fields as the DNA concordance rate with humans is nearly 90%.
In the present research, the acute toxicity of camphor, which is one of the major ingredients of bio-pesticides, was assessed as the median lethal concentration (LC50) by using the median effective concentration (EC50) for killing and immobilization of water fleas and juvenile D. rerio and its embryos. Additionally, simple lethality offers a limit to assessing the effect of toxicity by determining the degree of toxicity. To assess the influence of the major ingredients of pesticides on the morphologies of different organisms, a fish embryo toxicity test [5] was conducted in addition to the existing toxicity assessment method. For measuring the toxicity in the most sensitive species, the no observed effect concentration (NOEC) and predicted no effect concentration (PNEC) [6] were derived from the acute toxicity assessment results for the two study species, which would provide the stable concentration range for the ecosystem. The results will allow the ecosystem toxicity effect of the plant extracts to be assessed ahead of the development of eco-friendly pesticides with plant-derived extracts. Further, this would be helpful for setting a usage standard within the stable range.
Materials and Methods
Structure and Physiochemical Characteristics of Camphor
Camphor (Sigma-Aldrich Co., Louis, MO, USA; GC level), extracted from Cinnamomum camphora, has a molecular weight of 152.23, octanol-water partition coefficient of 2.38, and a low water solubility but high solubility in ethanol. The structure is shown in Figure 1. The concentration of camphor reagent used in the present research is ≥95.0%.
Figure 1.
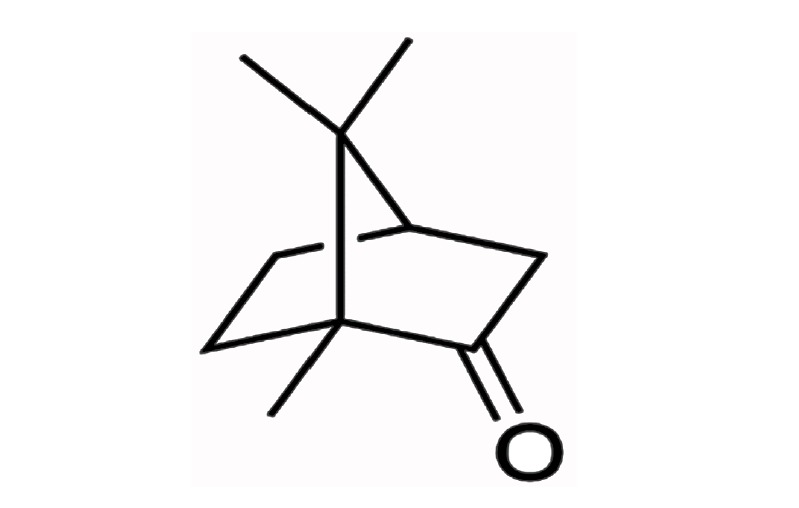
Chemical structure of camphor.
Rearing Conditions of Target Species for Toxicity Assessment
D. magna
D. magna was distributed from a chemical toxicity research institute and was cultured in accordance with OECD test guideline 202 [7]. The rearing condition was set to pH 7.5±0.2, temperature of 20±1°C and photoperiod of 16:8-hour light/dark (L/D). For food, 7-10×107 cells/L of Chlorella vulgaris was provided every day. Rearing water was changed 3 times a week, and the number of female parents was set to 10/L.
D. rerio
The D. rerio used in acute fish toxicity assessment was distributed from Kyungpook National University. Rearing conditions were set to a temperature of 28±1°C and photoperiod of 16:8- hour L/D; rearing water was dissolved with 0.065 g/L of sodium bicarbonate (Daejung Chemicals & Metals Co., Ltd., Siheung, Korea; extra pure level), and the pH was set to pH 7.5±0.2 with 12% sodium phosphate monobasic anhydrous (Daejung Chemicals & Metals) solution, while the conductivity was set to 0.39-0.43 mS/cm with 0.16 g/L of sea salt (Aquarium Systems, France). As for food, small tropical fish feed (Guppy BOb; Jeil Feed Co., Ltd., Daejeon, Korea) was provided 3 times a day, and Ocean Star International Brine Shrimp (Snowville, UT, USA) was provided once a week.
Toxicity Assessment Method
Preparation of toxicity substance
Camphor was dissolved using ethanol (Junsei Chemical Co., Tokyo, Japan; extra pure level) as a volatile essential oil of the plant. Then, 3.125 g of camphor was taken and dissolved in the ethanol and standardized to a total of 50 mL to prepare a 0.395 M of stock solution. In order to turn this crude liquid into a concentration for the actual experiment, the liquid was diluted with rearing water before using it.
Acute toxicity assessment of D. magna
The D. magna used in the test were young, healthy individuals aged less than 24 hours. Camphor was prepared in concentrations beakers of 49.4, 98.8, 197.5, 395.0, and 790.0 μM; 10 D. magna were put into each concentration beakers. In addition, during the experimental period, oxygen and food were not provided, and death and immobilization between 24 and 48 hours were observed. Every experiment was repeated three times, and SigmaPlot 12.0 (Systat Software Inc., San Jose, CA, USA) was used for statistical evaluations.
Egg production of D. rerio
During spawning, male and female D. rerio are easily distinguishable, because male D. rerio have an orange body with a silver belt whereas female D. rerio have a red body and swollen abdomen.
The night before the spawning, male and female D. rerio in a 2:1 ratio were transferred to the darkened mating cage. During the copulation, spawning, and fertilization, light was emitted and eggs were produced within 30 minutes. D. rerio tends to eat their eggs; hence, and the adults were separated from the eggs [8]. The collected eggs were washed with methylene blue solution and egg water. The fertilized and unfertilized eggs were separated using an optical microscope (Eclipse E200; Nikon, Tokyo, Japan), and only the fertilized eggs were used for further observations.
Acute toxicity assessment and embryo toxicity assessment of D. rerio
Camphor was prepared in 395, 790, 1,580, 3,160, and 6,320 μM concentrations by diluting with the D. rerio rearing water. Then, 2.5 mL of camphor was put in each concentration in 24- well plates, and three sets of experiments were repeated 10 times. During the test, the temperature was set to 28±1°C, pH was set to 7.5±0.2, conductivity was set to 0.4 mS/cm, and photoperiod was set to 16/8-hour L/D. Individuals that died or showed morphological abnormalities were observed with a microscope and numbered within 0-96 hours, for every 24 hours, and statistical analyses were carried out through SigmaPlot. Embryo toxicity was assessed with 30 replicates per concentration, and the incidents of every abnormality were cumulatively recorded and expressed as a percentage. As there were cases where multiple incidents occurred for a single embryo, the total amount could be over 100%.
Estimation of No Observed Effect Concentration and Predicted No Effect Concentration
If only limited toxicity data were obtained from the toxicity assessment of chemical substances in the OECD, a constant assessment factor was used in each extrapolation stage to predict the PNEC of the ecosystem. The acute toxicity assessment results for the two species of subject organisms were adjusted to a constant assessment factor of 100 to derive the PNEC and NOEC [6,9].
Results
Acute Toxicity Assessment of D. magna
For D. magna, there was no death or immobilization at a concentration below 49.4 μM after 48 hours, but immobilization began at concentrations over 98.8 μM. The survival rate at 48 hour at the highest concentration of 790.0 μM (Figure 2A) was 33.3%. In the material safety data sheet of ethanol used as a solvent, the toxicity effect is described as that could be neglected at concentrations below 0.63%. The 790.0 μM camphor concentration in the present research contains 0.188% ethanol, and thus, is within the stable range as a solvent. In the experimental results, the mortality of D. magna at this ethanol concentration was 10%, which corresponds to the standard OECD guideline 202 [7] of limiting the effect to the control group to 10%, and thus, the toxicity effect of the ethanol can be ignored. As to the acute toxicity assessment result of D. magna for camphor, the EC50 value was 395.0 μM after 48 hours of exposure (Figure 2A).
Figure 2.
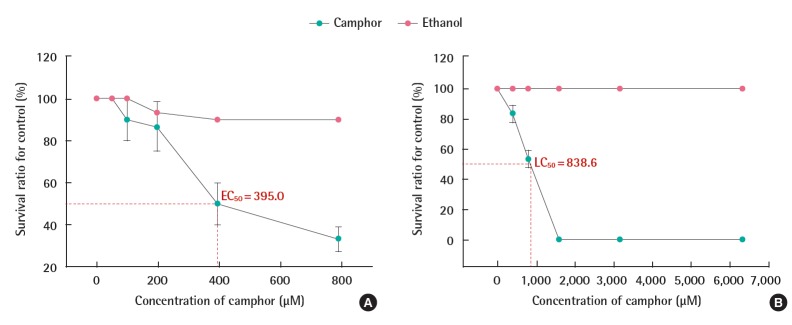
Acute toxicity of camphor to D. magna (A) and D. rerio embryos (B) at 96 hr (n=30). EC50, median effective concentration; LC50, median lethal concentration.
Acute Toxic Assessment of D. rerio Embryos
For D. rerio embryos, the survival rate rapidly decreased within the range of 395–1,580 μM after 96 hours, and above 1,580 μM, the lethality was 100%. To exclude the toxicity of the solvent, the effect of the ethanol in each concentration was assessed as well. According to the study of Duan et al. [10], where ethanol up to 0.1% is present in a solvent, there was no toxicity effect on the fertilized eggs of D. rerio. However, according to the present research, although there was a concentration of 1.5% ethanol for 6,320 μM of camphor, 15 times higher than 0.1%, the fertilized eggs of D. rerio had 100% survival. Thus, the effect of ethanol solvent toxicity on the D. rerio embryos can be excluded. The LC50 of camphor at 96 hours for the D. rerio embryo was 838.6 μM (Figure 2B).
Effects of Elapsed Time on Fertilized Eggs of D. rerio
Twenty-four hours after fertilization
D. rerio embryos were absorbed inside the egg membrane at 24 hours after the fertilization after the egg membrane was destroyed due to camphor exposure, and congelation (C) occurred at every concentration. Once the embryo of the fertilized egg was congealed, development did not proceed further and the embryo died. The morphological abnormalities of D. rerio within 24 hours after fertilization are shown in Figure 3 and Table 1.
Figure 3.
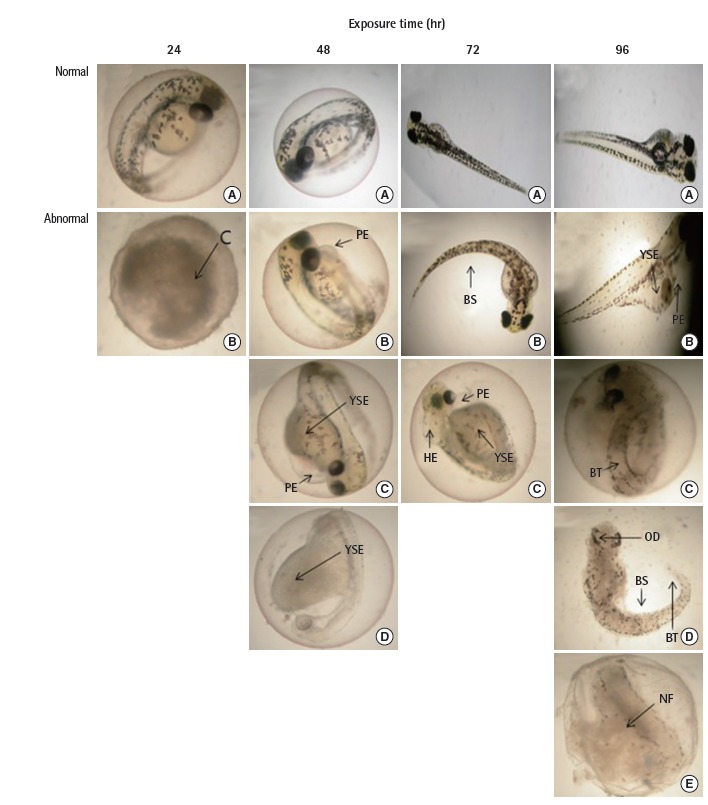
Image summary of the morphological abnormalities of D. rerio embryos caused by camphor. C, congelation; PE, pericardiac edema; BS, bent spine; YSE, yolk sac edema; HE, head edema; BT, bent tail; OD, ocular defect; NF, collapse symptoms of fertilized embryo tissue.
Table 1.
Effect degree on the abnormal morphological symptoms of D. rerio embryo after exposure to camphor
| Time (hr) | Symptoms (%) |
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C |
YSE |
PE |
P |
BS |
BT |
HE |
OD |
NF |
||||||||||||||||||||||||||||
| 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | 24 | 48 | 72 | 96 | |
| 395 (μM) | 10.0 | 10.0 | 10.0 | 10.0 | - | - | 3.3 | 6.7 | - | 3.3 | 3.3 | 3.3 | - | - | - | - | - | - | 6.7 | 16.7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6.7 |
| 790 (μM) | 20.0 | 20.0 | 20.0 | 20.0 | - | 23.3 | 26.7 | 26.7 | - | 20.0 | 23.3 | 26.7 | - | - | - | - | - | - | 3.3 | 3.3 | - | - | - | - | - | - | - | - | - | - | - | 16.7 | - | - | - | 26.7 |
| 1,580 (μM) | 43.3 | 60.0 | 60.0 | 70.0 | - | 40.0 | 40.0 | 40.0 | - | 40.0 | 40.0 | 40.0 | - | 30.0 | 16.7 | - | - | - | - | 3.3 | - | - | - | 3.3 | - | - | 3.3 | 3.3 | - | - | - | 26.7 | - | - | - | 30.0 |
| 3,160 (μM) | 100. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 6,320 (μM) | 100. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
C, congelation; YSE, yolk sac edema; PE, pericardiac edema; P, pigmentation; BS, bent spine; BT, bent tail; HE, head edema; OD, ocular defect; NF, collapse symptoms of fertilized embryo tissue.
Forty-eight hours after fertilization
At 395 μM at 48 hourrs after fertilization, there was 10.0% C and 3.3% pericardial edema (PE); at 790 μM, there was 20% C, 23.3% yolk sac edema (YSE), and 20% PE; and at 1,580 μM, there was 60% C, 40% YSE, 40% PE, and 30% pigmentation (P) (Figure 3, Table 1).
Seventy-two hours after fertilization
At 395 μM at 72 hours after fertilization, there was 10.0% C, 3.3% YSE, 3.3% PE, and 6.7% bent spine (BS); at 790 μM, there was 20% C, 26.7% YSE, 23.3% PE, and 3.3% BS; and at 1,580 μM, there was 60% C, 40% YSE, 40% PE, 16.7% P, and 3.3% head edema (HE) (Figure 3, Table 1).
Ninety-six hours after fertilization
At 395 μM at 96 hours after fertilization, there was 10.0% C, 6.7% YSE, 3.3% PE, 16.7% BS, and 6.7% abnormalities where the form of the fertilized egg tissue showed symptoms of fertilized embryo tissue collapse (NF). At 790 μM, there was 20% C, 26.7% YSE, 26.7% PE, 3.3% BS, 16.7% ocular defects (OD), and 26.7% NF. At 1,580 μM, there was 70% C, 40% YSE, 40% PE, 3.3% BS, 3.3% bent tail (BT), 3.3% HE, 26.7% OD, and 30% NF (Figure 3, Table 1).
Morphological abnormality changes with concentration and time
Based on the observed abnormality rates within 96 hours after fertilization, morphological abnormalities in D. rerio embryo development increased as camphor concentration increased. Bent spine was found in 6.7% and 3.3% of embryos at 395 and 790 μM, respectively, and the occurrence rate appeared to be two times higher at low concentrations. When D. rerio embryos were exposed to camphor in high concentrations, the spine was formed; thus, C occurs largely within 24 hours, before the generation of BS incidents, eventually causing a high occurrence of BS incidents at low concentration (Figures 3 and 5, Table 1). In other words, when D. rerio embryos are exposed to camphor at high concentrations, they cannot go through a normal development process and show strong toxicity from going through C at an early stage. The major toxicity-related symptoms of people exposed to camphor are neurasthenia, excessive muscle reflex, myotonic myopathy, dyspnea, seizure, and even death in the case of an oral administration to a child [11]. After inferring the effects to D. rerio embryos based on the symptoms, it is deemed that BS occurred as the muscle contracted due to the effect of camphor on the muscle.
Figure 5.
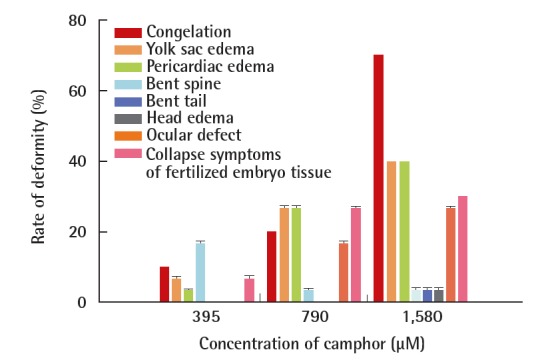
Morphological abnormalities of D. rerio based on different concentrations of camphor at 96 hr.
Change in the congelation rate
The C rate of fertilized eggs up to 24 hours increased at every concentration except 1,580 μM, and starting at 48 hours, it stayed consistent. In particular, at concentrations of 3,160 and 6,320 μM, strong toxicity was displayed, with 100% C occurring after 24 hours. Also, at every concentration except for 1,580 μM, the level was maintained consistently even as time passed (Figure 4A).
Figure 4.
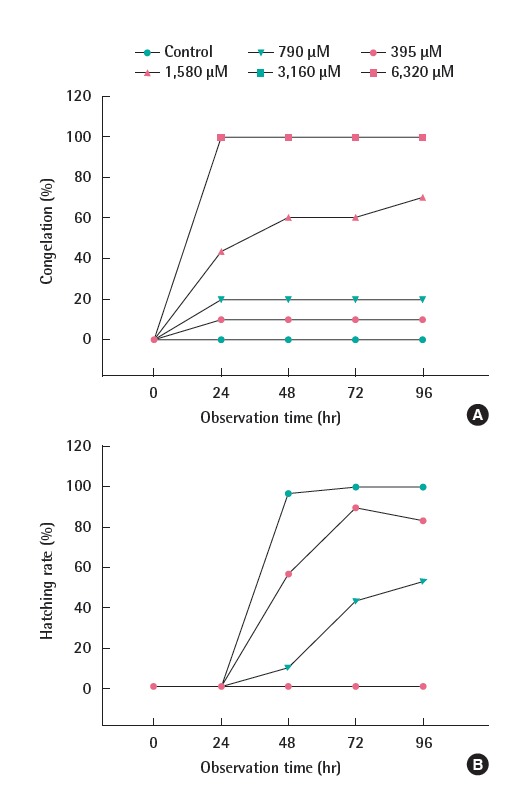
The ratios of congelation (A) and hatching (B) of D. rerio according to the observation time (n=30).
Change in hatchability
At 395 and 790 μM concentration, the hatchability after 96 hours was 83.3 and 53.3, respectively. However, at 1,580 μM concentration, morphological abnormalities such as C, YSE, PE, P, and HE occurred, showing incubation delays until 72 hours, and, at 96 hours, abnormalities such as C and symptoms of fertilized embryo tissue collapse occurred and embryos finally died. At concentrations over 3,160 μM, 100% C occurred by 24 hours, and all subjects died before getting to incubation.
Safe application concentration of bio-pesticide
D. magna, which showed the most susceptive reaction to camphor, had a NOEC of 55.2 μM (7,156 times more diluted water than bio-pesticide), and a PNEC of 3.95 μM (100,000 times more diluted water than bio-pesticide) by applying an uncertainty assessment factor of 100 to the EC50 (395 μM) of D. magna [6]. Thus, the bio-pesticide at a concentration range of 500- 1,000 times would toxicity affect the ecosystem in the region where it is directly applied.
Discussion
From assessing the acute toxicity of camphor with two types of organisms, the 48 hours EC50 for D. magna was found to be 395.0 μM (1,000 times more diluted water than bio-pesticide), and at 96 hours the LC50 for fertilized eggs of D. rerio was 838.6 μM (471 times more diluted water than bio-pesticide).
There were additional incidents of morphological abnormalities in fertilized eggs of D. rerio. The frequency of congelation increased with increasing concentration at 24 hours, and was constant at 48 hours. The reason for congelation has not been confirmed, but as C occurred at a high rate at 24 hours, which is the period of the completion of organs in the embryo, and then stayed constant at every concentration except for 1,580 μM, camphor appears to cause C in early embryo development. Furthermore, hatching delays, YSE, PE, P, etc. were observed in unfertilized eggs where C had not occurred. At 72 hours after fertilization, BS and HE occurred, and at 96 hours, BS, ocular defects, and abnormalities of the form of the fertilized egg tissue going through symptoms of fertilized embryo tissue collapse were observed. At the present stage, the reason for the occurrence of edema has not been revealed, but it is deemed that P occurred because the D. rerio turned opaque due to delay in the generation of melanocytes in the embryo [12].
D. magna, which showed susceptibility to camphor, had a NOEC of 55.2 μM (7,156 times more diluted water than biopesticide), and a PNEC assessed to be 3.95 μM (100,000 times more diluted water than bio-pesticide). As the results correspond to the EC50 value of camphor, the onsite application concentration, which is a 1,000-fold dilution ratio (395.0 μM), appeared to show local toxicity during onsite application. In the actual environment, however, the concentration appears to exist below the PNEC due to soil infiltration, decomposition by microorganisms, and excellent dilution effects. In addition, since a bio-pesticide is an eco-friendly substance existing in the natural world as a plant extract-derived compound, it is predicted that the actual situation would not necessarily correspond to the experimental results. Observation through more sustainable environment monitoring of bio-pesticide use will be necessary to be certain of the effects on the ecosystem. Some studies of ecotoxicity assessment have used the entire life cycle of the organism through acute, subchronic, and chronic tests, but a previous study reported that the chronic toxicity threshold concentration obtained through susceptibility test results for the early life stage is not majorly different from the toxicity value calculated from the entire life toxicity test result [6]. As such, through the ecotoxicity assessment at the early development stage of D. rerio within 96 hours, the ecosystem toxicity effect could be quickly assessed.
Acknowledgments
We sincerely thank the Ministry of Trade, Industry and Energy who supported the present research, as part of a program sponsoring the leading businesses in the Honam Economic Region (no. R0001955).
Footnotes
The authors have no conflicts of interest with material presented in this paper.
References
- 1.Nam HS. Environmentally-friendly agriculture & biotic pesticide. KIC News. 2011;14(4):12–18. (Korean) [Google Scholar]
- 2.You AS, Choi YW, Jeong MH, Hong SS, Park YK, Jang HS, et al. Acute ecotoxicity evaluation of thyme white, clove bud, cassia, lavender, lemon eucalyptus essential oil of plant extracts. Korean J Pestic Sci. 2011;15(4):350–356. (Korean) [Google Scholar]
- 3.Hashimoto Y, Okubo E, Ito T, Yamaguchi M, Tanaka S. Changes in susceptibility of carp to several pesticides with growth. J Pestic Sci. 1982;7(4):457–461. [Google Scholar]
- 4.Matsunake S. Pesticide design –strategy and tactics. In: Yamamoto I, Fukami J, editors. Pesticide design. Tokyo: Soft Sci Inc.; 1979. pp. 1061–1062. [Google Scholar]
- 5.Organization for Economic Cooperation and Development (OECD) OECD guideline for the testing of chemicals: fish embryo toxicity (FET) test. 2006 [cited 2014 Jun 20]. Available from: http://www.oecd.org/chemicalsafety/testing/36817070.pdf.
- 6.van Leeuwen CJ, Vermeire T. Risk assessment of chemicals: an introduction. Dordrecht: Springer; 2007. pp. 326–340. [Google Scholar]
- 7.Organization for Economic Cooperation and Development (OECD) Test NoTest No. 202: Daphnia sp. acute immobilization test 2004. [cited 2014 Jun 20]. Available from: http://www.oecd-ilibrary.org/docserver/download/9720201e.pdf?expires=1410363690&id=id&accname=guest&checksum=E89C93D5677FBC62C384B06802BFC0AD.
- 8.Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp Biochem Physiol C Toxicol Pharmacol. 2009;149(2):196–209. doi: 10.1016/j.cbpc.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Yim EC, Shin JJ, Park IT, Han HK, Kim SW, Cho H, et al. Acute toxicity assessment of new algicide, thiazolidinedione derivative (TD53) to marine ecosystem. Korean Soc Biotechnol Bioeng J. 2011;26(1):7–12. (Korean) [Google Scholar]
- 10.Duan Z1, Zhu L, Zhu L, Kun Y, Zhu X. Individual and joint toxic effects of pentachlorophenol and bisphenol A on the development of zebrafish (Danio rerio) embryo. Ecotoxicol Environ Saf. 2008;71(3):774–780. doi: 10.1016/j.ecoenv.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 11.Love JN, Sammon M, Smereck J. Are one or two dangerous? Camphor exposure in toddlers. J Emerg Med. 2004;27(1):49–54. doi: 10.1016/j.jemermed.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Choi TY, Kim SM, Sohn KC, Kim CD, Lee JH, Yoon TJ. Zebrafish as an in vivo model for the study of skin cells. Korean J Invest Dermatol. 2007;4(2):37–44. (Korean) [Google Scholar]


