Abstract
Objective
Repetitive transcranial magnetic stimulation (rTMS) applied to the dorsolateral prefrontal cortex (DLPFC) has yielded promising results as a treatment for posttraumatic stress disorder (PTSD). However, to date, no quantitative review of its clinical utility has been published.
Method:
We searched for randomized and sham-controlled trials from 1995 to March 2013 using MEDLINE, Embase, PsycINFO, CENTRAL, and SCOPUS. We then performed an exploratory random effects meta-analysis.
Results:
Studies on rTMS applied to the right DLPFC included 64 adults with PTSD. The pooled Hedges g effect size for pre and post changes in clinician-rated and self-reported PTSD symptoms were, respectively, 1.65 (P < 0.001) and 1.91 (P < 0.001), indicating significant and large-sized differences in outcome favouring active rTMS. Also, there were significant pre and post decreases with active rTMS in overall anxiety (Hedges g = 1.24; P = 0.02) and depressive (Hedges g = 0.85; P < 0.001) symptoms. Dropout rates at study end did not differ between active and sham rTMS groups. Regarding rTMS applied to the left DLPFC, there is only one study published to date (using a high frequency protocol), and its results showed that active rTMS seems to be superior overall to sham rTMS.
Conclusions:
Our exploratory meta-analysis shows that active rTMS applied to the DLPFC seems to be effective and acceptable for treating PTSD. However, the small number of subjects included in the analyses limits the generalizability of these findings. Future studies should include larger samples and deliver optimized stimulation parameters.
Keywords: posttraumatic stress disorder, repetitive transcranial magnetic stimulation, meta-analysis, randomized controlled trials, efficacy, acceptability
Abstract
Objectif :
La stimulation magnétique transcrânienne répétitive (SMTr) appliquée au cortex dorsolatéral préfrontal (CDLPF) a produit des résultats prometteurs comme traitement du trouble de stress post-traumatique (TSPT). Cependant, jusqu’ici, aucune revue quantitative de son utilité clinique n’a été publiée.
Méthode :
Nous avons cherché des essais randomisés et contrôlés par simulation de 1995 à mars 2013 à l’aide de MEDLINE, Embase, PsycINFO, CENTRAL, et SCOPUS. Nous avons ensuite procédé à une méta-analyse exploratoire des effets aléatoires.
Résultats :
Les études sur la SMTr appliquée au CDLPF droit comportaient 64 adultes souffrant du TSPT. La taille d’effet calculée selon le g de Hedges pour les changements avant et après des symptômes de TSPT cotés par les cliniciens et auto-déclarés était, respectivement, 1,65 (P < 0,001) et 1,91 (P < 0,001), indiquant des différences significatives et de forte taille des résultats favorisant la SMTr active. De même, il y avait des diminutions significatives avant et après la SMTr active dans les symptômes d’anxiété généralisée (g de Hedges = 1,24; P = 0,02) et dépressifs (g de Hedges = 0,85; P < 0,001). Les taux d’abandon au terme de l’étude ne différaient pas entre les groupes de SMTr active et de SMTr simulée. En ce qui concerne la SMTr appliquée au CDLPF gauche, il n’y a qu’une étude publiée à ce jour (utilisant un protocole de haute fréquence), et les résultats indiquent que la SMTr active semble être généralement supérieure à la SMTr simulée.
Conclusions :
Notre méta-analyse exploratoire indique que la SMTr active appliquée au CDLPF semble être efficace et acceptable pour traiter le TSPT. Toutefois, le nombre réduit de sujets inclus dans les analyses limite d’une certaine manière la généralisabilité de ces résultats. En résumé, les futures études devraient inclure des échantillons plus grands et produire des paramètres de stimulation optimisés.
A severe and often chronic psychiatric illness with a lifetime prevalence in community samples of up to 8%,1,2 PTSD occurs in susceptible people who have been exposed to extremely traumatic events that involved actual or threatened death or serious injury, or a threat to the physical integrity of self or others.3 PTSD is characterized by pervasive hyperarousal, intrusive thoughts, exaggerated startle response, flashbacks, nightmares, sleep disturbances, emotional numbness, and persistent avoidance of trauma-associated stimuli,4 and is associated with significant personal distress and disability, as well as with high social and economic costs.5,6
Current first-line treatment strategies for PTSD include ADs (for example, selective serotonin reuptake inhibitors, and serotonin–norepinephrine reuptake inhibitors) and psychotherapy (for example, prolonged exposure or trauma-focused cognitive-behavioral therapy).7,8 However, a considerable proportion of patients with PTSD remain significantly ill despite undergoing such relatively diverse therapeutic options, and are thereby left with lasting negative repercussions on their global functioning and well-being.9 Therefore, novel treatments for PTSD are of considerable interest, one of which is rTMS.10
rTMS is a noninvasive neuromodulation technique that is able to modulate cortical and subcortical function with the use of rapidly changing electromagnetic fields generated by a coil of wire placed over the scalp.11 Depending on the parameters of stimulation, rTMS can either decrease or increase cortical excitability in relatively focal areas, with frequencies of 1 Hz or less (LF-rTMS) usually being inhibitory and higher frequencies (≥5 Hz; HF-rTMS) usually being excitatory.12 To date, rTMS has been shown, for example, to be an effective treatment for MDD and schizophrenia.13,14
Clinical Implications
A considerable proportion of patients with PTSD remain significantly ill despite receiving several therapeutic interventions.
rTMS applied to the DLPFC is a promising therapeutic approach for patients with resistant PTSD.
Our exploratory meta-analysis has shown that active rTMS applied to the DLPFC seems to be effective and acceptable for treating PTSD.
Limitations
The generalizability of our findings is somewhat limited by the small number of subjects included in the analyses, the heterogeneity of the stimulation protocols, and the lack of medium- to long-term effectiveness data on rTMS for PTSD.
Although the pathophysiology of PTSD remains unclear, growing evidence from functional neuroimaging studies suggest that it is consistently associated with hypoactivation of the prefrontal cortex (including medial and dorsolateral regions) and hyperactivation of deeper brain structures, such as the amygdala.15–17 The DLPFC, in particular, is involved in many complex cognitive and behavioural functions that are relevant to PTSD18,19 and that have been shown to be reliably modulated by rTMS (for example, executive functioning,20,21 working memory,22,23 verbal memory,24 supervisory attentional control,25,26 and reasoning and decision making27,28). As a result, numerous recent open-label trials and RCTs have been carried out and have shown that rTMS targeting the DLPFC is potentially useful for treating PTSD.29–34 However, to date, no attempt has been made to integrate the findings from these studies to produce a more accurate estimation of the efficacy and acceptability of rTMS applied to the right and the left DLPFC for PTSD. Therefore, we conducted the present systematic review and exploratory meta-analysis to examine this issue.
Methodology of the Literature Review
Search Strategy
We identified articles for inclusion in this meta-analysis by searching MEDLINE, Embase, PsycINFO, CENTRAL, and SCOPUS from January 1, 1995, to March 12, 2013, and by screening the bibliography of all included studies. The search syntaxes included a combination of the following key words: “transcranial magnetic,” “rTMS,” “magnetic stimulation,” “stress*,” “PTSD,” and “trauma*.” Detailed information regarding the search procedures (including syntaxes, parameters, and results) are shown in the online eAppendix.
Study Selection
Candidate studies (judged by their title and abstract) had to satisfy the following criteria35: study validity—random allocation, double-blinded, sham-controlled (that is, coil angled on the scalp or use of a sham coil), parallel design, of 5 subjects or more with PTSD randomized per study arm; sample characteristics—subjects aged 18 to 75 years with a diagnosis of primary PTSD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,36 or the International Classification of Diseases, Tenth Revision37 criteria; treatment characteristics—LF- (≤1 Hz) or HF- (≥5 Hz) rTMS applied over the left or the right DLPFC for 5 or more sessions; and, publication-related—articles written in English. Studies were excluded if their protocol involved rTMS started concomitantly with new psychotropics (for example, ADs or APs).
Data Extraction
Data were recorded in a structured fashion as follows: sample characteristics—mean age, sex, and illness severity; rTMS-related—stimulation frequency and intensity (including the total number of stimuli delivered), number of treatment sessions, and type of sham; primary outcome measure—score changes (pre- and post-rTMS) on validated clinician-reported measures (for example, the Treatment-Outcome PTSD Scale38); secondary outcome measure—score changes (pre- and post-rTMS) on validated self-reported measures (for example, the PTSD Checklist39); tertiary outcome measures—score changes (pre- and post-rTMS) on validated anxiety (for example, the Hamilton Anxiety Rating Scale40 and the State-Trait Anxiety Inventory41), and depression (for example, the Hamilton Depression Rating Scale42 and the Beck Depression Inventory43) measures; and, acceptability of treatment—overall dropout rates for active and sham rTMS groups at study end.
Study Quality
We used the well-known Jadad Scale to assess the quality of the included RCTs.44 Criteria evaluated by this scale include randomization, blinding, and report of participant withdrawal, and possible scores range from 0 to 5. A priori, studies with a Jadad score of 3 or more were deemed higher quality and those with a score of 0 to 2 were classified as lower quality.45
Data Synthesis and Analyses
Analyses were performed using Comprehensive Meta-Analyses version 2.0 (Biostat, Englewood, NJ) and IBM SPSS version 20 (IBM Corporation, Chicago, IL).
We used a random-effects model as we assumed that the true ESs had likely varied between the included RCTs.46 If provided, intention-to-treat data, using a method, such as LOCF, were preferred over data from completers.47 Score changes (pre- and post-rTMS) were investigated with pooled Hedges g ESs. As we could not retrieve the correlations between pre- and post-rTMS measures from the individual RCTs, we followed the recommendation by Rosenthal48 and assumed a conservative estimation (r = 0.7). Also, as recommended by the Cochrane Collaboration,35 in studies comparing 3 treatment conditions (that is, 2 active, compared with 1 sham), we split the control group into 2 equal parts (so that the total number of control subjects added up to its original size) and compared each half with the 2 active conditions. For the study by Boggio et al,31 we had to estimate the means and standard deviations by, respectively, reviewing the published graphs and imputting average values from other RCTs included in this meta-analysis.49 Acceptability of treatment was assessed with odds ratios for differential dropout rates between active and sham rTMS groups.50,51 Also, to rule out the presence of baseline between-group differences in illness severity, we computed pooled Hedges g ESs for subjects’ PTSD, depression, and anxiety scores. Finally, we carried out an exploratory analysis by subgrouping the included studies into LF- and HF-rTMS protocols.
Heterogeneity was assessed using the Q statistic and I2 index.52 Values of P < 0.1 for the former and (or) more than 35% for the latter were deemed as indicative of significant between-study heterogeneity.50 We further used funnel plots, the fail-safe number (that is, the number of missing studies that would make a specific result statistically nonsignificant at P > 0.05),53 and Egger’s Regression Intercept54 to test for the presence of publication bias.50,52
Pooled statistical analyses were not conducted when 2 studies or less were available for a particular brain target (that is, left or right DLPFC) owing to the impossibility of producing funnel plots and estimating publication bias.55 Instead, in this context we reported individual study results in a descriptive manner only.
Results
Literature Search
We retrieved 98 references (after discarding duplicates) from MEDLINE, PsycINFO, Embase, CENTRAL, and SCOPUS. Among these, 3 met the eligibility criteria,31–33 comprising 4 comparisons between active and sham rTMS applied to the right DLPFC and a single comparison between active and sham rTMS applied to the left DLPFC.
Study Quality and Strategy for Missing Data
The 3 included RCTs had a score of 3 or more in the Jadad Scale and were thus considered to have a high overall methodological quality. Regarding the strategies used by the RCTs to deal with missing data, 1 employed the LOCF approach,31 1 performed completers-only analyses,32 and 1 reported no patient dropout at study end.33
Repetitive Transcranial Magnetic Stimulation Over the Right Dorsolateral Prefrontal Cortex
Included Randomized Controlled Trials: Main Characteristics
Three RCTs on rTMS applied to the right DLPFC were included in this meta-analysis, comprising 64 subjects with PTSD, of whom 38 were randomized to active rTMS (mean age 44.3 years [SD 6.5]; 68.4% males), and 26 were randomized to sham rTMS (mean age 48.8 years [SD 7.9]; 61.5% males). All RCTs administered 10 rTMS sessions in total, and the mean number of magnetic pulses delivered was 6250 (SD 6652).1 The main characteristics of the included RCTs are described in Table 1. The funnel plots and the additional forest plots are shown in the online eAppendix.
Table 1.
Included RCTs on rTMS over the DLPFC for PTSD: main characteristics
| Study | Active rTMS
|
Sham rTMS
|
rTMS parameters | Type of trauma | Missing data approach | Study quality44 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Age, years (SD) | Female/male, n | n | Age, years (SD) | Female/male, n | Strategy | Brain target | Frequency, Hz / Sessions | rMT, % / total pulses | ||||
| Cohen et al32 | 8 | 40.8 (9.9) | 1/7 | 6 | 42.8 (14.8) | 2/4 | 90° angulation | Right DLPFC | 1/10 | 80/1000 | Combat (n = 4), motor vehicle accident (n = 11), sexual abuse (n = 2), assault (n = 2), work accident (n = 4), or death of a relative (n = 1). Time since the trauma = 5.4 years (SD 7.0). |
Completers-only analyses | 4 |
| 10 | 41.8 (11.4) | 4/6 | 6 | 42.8 (14.8) | 2/4 | 90° angulation | 10/10 | 80/4000 | |||||
| Boggio et al31 | 10 | 40.7 (13.6) | 6/4 | 10 | 45.9 (11.4) | 7/3 | Sham coil | Right DLPFC | 20/10 | 80/16 000 | Sexual abuse (n = 3), assault (n = 4), death or severe disease of a relative (n = 11), or kidnapping or death threats (n = 2). Time since the trauma = 3.9 years (SD 4.3). |
LOCF | 4 |
| 10 | 47.1 (12.1) | 7/3 | 10 | 45.9 (11.4) | 7/3 | Sham coil | Left DLPFC | 20/10 | 80/16 000 | Sexual abuse (n = 3), assault (n = 4), death or severe disease of a relative (n = 10), or kidnapping or death threats (n = 3). Time since the trauma = 3.8 years (SD 4.3). |
LOCF | 4 | |
| Watts et al33 | 10 | 54.0 (12.3) | 1/9 | 10 | 57.8 (11.8) | 1/9 | Sham coil | Right DLPFC | 1/10 | 90/4000 | Combat (n = 8), sexual abuse (n = 1), assault (n = 1), or multiple (n = 10). Time since the trauma = 39.7 years (13.9). |
No patient drop out | 3 |
DLPFC = dorsolateral prefrontal cortex; LOCF = last observation carried forward; PTSD = posttraumatic stress disorder; RCT = randomized controlled trial; rMT = resting motor threshold; rTMS = repetitive transcranial magnetic stimulation
Clinician-Reported Posttraumatic Stress Disorder Symptoms
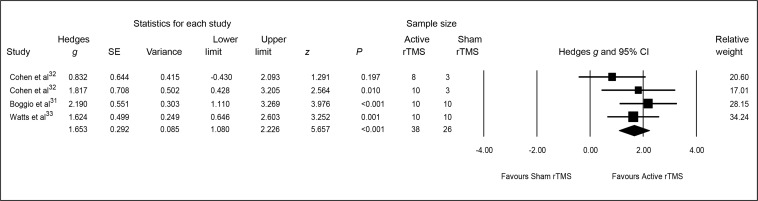
The pooled Hedges g ES for changes from baseline to end point on clinician-reported PTSD symptoms was 1.65 (95% CI 1.08 to 2.23; z = 5.66, P < 0.001), indicating a significant and large-sized difference in outcome favouring active rTMS (Figure 1).
Figure 1.
Meta-analysis of active, compared with sham, rTMS for PTSD: clinician-reported PTSD symptoms
PTSD = posttraumatic stress disorder; rTMS = repetitive transcranial magnetic stimulation; SE = standard error
Heterogeneity between RCTs did not exceed that expected by chance (Q = 2.63, df = 3, P = 0.45; I2 = 0), implying that the variance among the ESs was not greater than expected by sampling error. Finally, the associated funnel plot was reasonably symmetrical, the fail-safe N was 28 and Egger’s regression intercept was −1.77 (t = 0.43, df = 2, 2-tailed P = 0.71), all suggesting a low risk of publication bias.
Self-Reported Posttraumatic Stress Disorder Symptoms
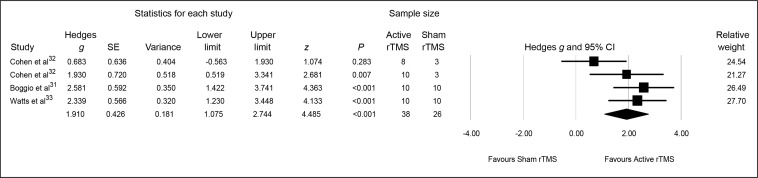
The pooled Hedges g ES for changes from baseline to end point on self-reported PTSD symptoms was 1.91 (95% CI 1.07 to 2.74; z = 4.48, P < 0.001), indicating a significant and large-sized difference in outcome favouring active rTMS (Figure 2).
Figure 2.
Meta-analysis of active, compared with sham, rTMS for PTSD: patient-reported PTSD symptoms
PTSD = posttraumatic stress disorder; rTMS = repetitive transcranial magnetic stimulation; SE = standard error
Heterogeneity between RCTs was significant (Q = 5.58, df = 3, P = 0.13, I2 = 46.21%). Visual inspection of the forest plot suggested that this was caused by the Cohen et al32 LF-rTMS protocol, and its removal from the analysis resulted in nonsignificant heterogeneity (Q = 0.49, df = 2, P = 0.78, I2 = 0%), although the clinical results remained unaltered in terms of a statistically significant between-group difference (Hedges g = 2.33, 95% CI 1.63 to 3.02; z = 6.54, P < 0.001). Finally, the associated funnel plot was reasonably symmetrical, the fail-safe N was 36, and Egger’s regression intercept was −5.46 (t = 0.63, df = 2, 2-tailed P = 0.59), all suggesting a low risk of publication bias.
Overall Anxiety Symptoms
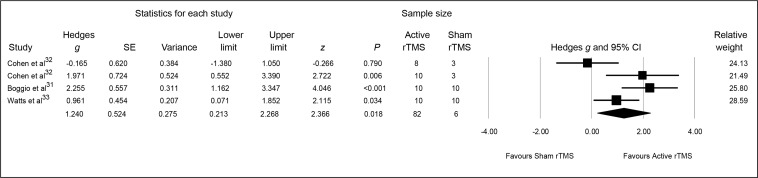
The pooled Hedges g ES for changes from baseline to end point on anxiety symptoms was 1.24 (95% CI 0.21 to 2.27; z = 2.37, P = 0.02), indicating a significant and large-sized difference in outcome favouring active rTMS (Figure 3).
Figure 3.
Meta-analysis of active, compared with sham, rTMS for PTSD: overall anxiety symptoms
PTSD = posttraumatic stress disorder; rTMS = repetitive transcranial magnetic stimulation; SE = standard error
Heterogeneity between RCTs was significant (Q = 9.83, df = 3, P = 0.02, I2 = 69.49%). Visual inspection of the forest plot suggested that this heterogeneity resulted from RCTs clearly subdivided into 2 groups: 1 reporting relatively high pre and post changes in anxiety symptoms (that is, the HF-rTMS protocols of Cohen et al32 and Boggio et al31), and the other reporting relatively low pre and post changes in anxiety symptoms (that is, the LF-rTMS protocols of Cohen et al32 and Watts et al33). Finally, the associated funnel plot was reasonably symmetrical, the fail-safe N was 16, and Egger’s regression intercept was 1.24 (t = 0.19, df = 2, 2-tailed P = 0.87), all suggesting a low risk of publication bias.
Depressive Symptoms
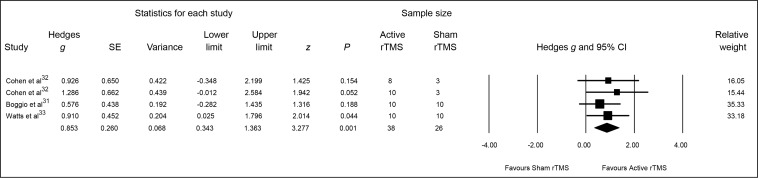
The pooled Hedges g ES for changes from baseline to end point on depressive symptoms was 0.85 (95% CI 0.34 to 1.36; z = 3.28, P = 0.001), indicating a significant and large-sized difference in outcome favouring active rTMS (Figure 4).
Figure 4.
Meta-analysis of active, compared with sham, rTMS for PTSD: depressive symptoms
PTSD = posttraumatic stress disorder; rTMS = repetitive transcranial magnetic stimulation; SE = standard error
Heterogeneity between RCTs was not statistically significant (Q = 0.85, df = 3, P = 0.84, I2 = 0%). Finally, the associated funnel plot was reasonably symmetrical, the fail-safe N was 8, and Egger’s regression intercept was 1.84 (t = 1.6, df = 2, 2-tailed P = 0.25), all suggesting a low risk of publication bias.
Acceptability of Repetitive Transcranial Magnetic Stimulation Treatment
Overall, no differences on dropout rates at study end were observed between active and sham rTMS groups (10.52% [4/38], compared with 21.87% [7/32], respectively; OR 0.42; z = −1.21, P = 0.23).
Heterogeneity between RCTs was not statistically significant (Q = 1.26, df = 3, P = 0.74, I2 = 0%). Finally, the associated funnel plot was reasonably symmetrical, and Egger’s regression intercept was −0.52 (t = 0.26, df = 2, 2-tailed P = 0.82), all suggesting a low risk of publication bias.
Baseline Illness Severity
Subjects receiving active rTMS (compared with those receiving sham rTMS) had significantly higher baseline scores on clinician-reported PTSD symptoms (Hedges g = 0.74, z = 2.86, P = 0.004), self-reported PTSD symptoms (Hedges g = 0.75, z = 2.87, P = 0.004), and depressive symptoms (Hedges g = 0.59, z = 2.33, P = 0.02). However, they did not differ in their baseline overall anxiety symptoms (Hedges g = 0.34, z = 1.34, P = 0.18).
Subgroup Analyses: High Frequency, Compared With Low Frequency, Repetitive Transcranial Magnetic Stimulation Protocols
Because of the small number of included RCTs we did not perform between-group statistical analyses. HF-rTMS protocols resulted in significant and large-sized improvements in clinician-reported PTSD symptoms (Hedges g = 2.05, z = 4.71, P < 0.001), self-reported PTSD symptoms (Hedges g = 2.32, z = 5.07, P < 0.001), overall anxiety symptoms (Hedges g = 2.15, z = 4.6, P < 0.001), and depressive symptoms (Hedges g = 0.79, z = 2.17, P = 0.03). In contrast, LF-rTMS protocols yielded statistically significant improvements in clinician-reported PTSD symptoms (Hedges g = 1.33, z = 3.36, P = 0.001), self-reported PTSD symptoms (Hedges g = 2.34, z = 4.13, P < 0.001), and depressive symptoms (Hedges g = 0.91, z = 2.47, P = 0.001), although there was no significant change in overall anxiety symptoms (Hedges g = 0.55, z = 1.38, P = 0.17).
Repetitive Transcranial Magnetic Stimulation Over the Left Dorsolateral Prefrontal Cortex
Only the RCT by Boggio et al31 has reported efficacy data on rTMS applied to the left DLPFC. Briefly, they have shown that an HF protocol administered over 10 sessions was associated with a Hedges g ES for pre and post changes in clinician-rated and self-reported PTSD symptoms of 0.97 (95% CI 0.08 to 1.86; P = 0.03) and 1.29 (95% CI 0.36 to 2.22; P = 0.007), respectively. Further, they have reported a significant decrease in clinician-rated overall anxiety symptoms (Hedges g = 1.01; 95% CI 0.12 to 1.91, P = 0.03), but only a statistical trend toward a decrease in clinician-rated depressive symptoms (Hedges g = 0.86; 95% CI –0.02 to 1.75, P = 0.05).
Discussion
The main objective of our meta-analysis was to assess the efficacy and the acceptability of rTMS applied to the right and to the left DLPFC for treating PTSD. Briefly, we have shown that active rTMS applied to the right DLPFC significantly reduced clinician-rated and patient-reported core PTSD symptoms, as well as overall anxiety and depressive symptoms following 10 daily sessions. Importantly, we found that patients who received active rTMS over the right DLPFC were significantly more ill at baseline, and it is possible that active treatment might have been even more effective than sham rTMS if both groups were comparable in terms of baseline psychopathology. Regarding rTMS applied over the left DLPFC, the only RCT published to date has shown promising results for an HF protocol although additional studies are needed to allow for pooled analyses. Finally, rTMS over the DLPFC seemed to be an acceptable treatment for PTSD as indexed by differential dropout rates at study end.
It is of interest that both LF- and HF-rTMS applied to the right DLPFC exerted a positive clinical effect on PTSD and its associated symptoms; this is counterintuitive, at least neurophysiologically, as LF- and HF-rTMS are believed to have opposite effects on cortical excitability (that is, usually inhibitory and excitatory effects, respectively).56,57 At present, there is no clear explanation for this paradoxical observation, although recent studies have shown, for example, that LF-rTMS at relatively low intensities (for example, ≤90% of the resting motor threshold) sometimes fails to induce a measurable change on motor excitability, and that its effects are associated with a large inter-individual variability (perhaps related to the baseline excitability level of the targeted cortical area58–60), with some subjects even showing facilitatory effects.61 Nevertheless, our exploratory subgroup analyses further indicated that HF-rTMS protocols (compared with LF-rTMS protocols) may be associated with slightly greater clinical improvements in core PTSD symptoms (as assessed by clinician-rated measures), as well as in overall anxiety symptoms. This finding, although clearly preliminary, could be explained, at least in part, by the excitatory effects of HF-rTMS on the underlying cortical tissue that may have counteracted the hypofrontality usually observed in patients with PTSD and thus enhanced the modulation of fear-related emotional responses and encoding or consolidation of traumatic memories by indirectly inhibiting the amygdala.15,16,62–64 Additionally, HF-rTMS might have inhibited contralateral brain structures involved in memory retrieval networks.31 In summary, the results of our meta-analysis only partially support the conventional model of the neurocircuitry underlying PTSD (involving relative hypoactivity of frontal regions and hyperactivity of subcortical regions, such as the amygdala65), as we have shown that LF-rTMS (an inhibitory intervention) is also potentially effective for PTSD and might produce its therapeutic effects by inhibiting a possibly lateralized right-sided hyperactivity of the DLPFC.31 However, one has to be extremely careful when attempting to explain the underlying neurobiology of PTSD and (or) the putative mechanisms of action of rTMS based on the results from these small RCTs.
Although clearly preliminary, our findings are encouraging when viewed in the context of more established treatments for PTSD. For example, a recent large meta-analysis has shown that ADs given for 4 to 12 weeks (compared with placebo) were associated with a Hedges g ES of 0.23 (95% CI 0.15 to 0.31; n = 4112) for reducing clinician-reported PTSD symptoms.7 Further, prolonged exposure, a specific therapy that includes multiple sessions of imaginal and in vivo exposure, has been associated with a Hedges g ES of 1.08 (95% CI 0.69 to 1.46; n = 675) for improving core PTSD symptoms.66 Also, a meta-analysis reported that the atypical APs risperidone and olanzapine, when used as add-ons or monotherapy (compared with placebo), were associated with a small-to-medium ES for reducing clinician-reported PTSD symptoms (that is, standardized mean difference = 0.45, 95% CI 0.14 to 0.75; n = 192).67
Nevertheless, as the optimum rTMS protocol for PTSD has yet to be determined, future studies should investigate the use of more intense treatment protocols (for example, higher numbers of total magnetic pulses and [or] percentage of the resting motor threshold), identify more clinically relevant neuromodulation protocols (for example, preconditioning paradigms or priming and different waveforms),68,69 as well as apply baseline electrophysiological and neuroimaging evaluations to better predict which patients will benefit from treatment.70,71 Also, further research should assess whether rTMS can potentiate the efficacy of a concomitantly administered psychotherapy.10 Finally, novel developments in the field of neuromodulation, such as the H coil,72 might also enhance the clinical utility of rTMS for PTSD by allowing the direct stimulation of relatively deeper brain structures, while theta burst stimulation might produce more consistent and enduring positive after-effects.73
Limitations
First, we only retrieved 3 RCTs with relatively small samples. Nevertheless, pooled analyses for rTMS applied to the right DLPFC already indicated significantly better clinical results for active, compared with sham, rTMS. Second, the quality of the available sham rTMS conditions is still unresolved,12 and the use of coil tilting and (or) first-generation sham coils may not have been optimal.74 In addition, we could not assess the integrity of blinding in the included RCTs owing to the absence of information in this regard. However, we have recently shown that a similar percentage of subjects with MDD receiving active, compared with sham, rTMS were able to correctly guess their treatment allocation at study end (that is, 52%, compared with 59%, respectively; risk difference = –0.04, z = −0.51, P = 0.61).75 Third, the strategy most commonly used for locating the right DLPFC (that is, the 5 cm method) has been recently criticized for its inaccuracy12 and future studies may benefit from the use of neuronavigation.76 Fourth, we could not estimate the stability of the medium- to long-term effects of rTMS for PTSD or its cost-effectiveness, and this is especially relevant considering the labour-intensive and time-consuming nature of this treatment.77 Nevertheless, preliminary evidence suggests that the positive clinical effects of rTMS for PTSD are sustained for up to 3 months following the study end.31,33 Fifth, the subgroup analyses should be seen as exploratory in nature and thus far from conclusive. Sixth, the reliability of the analyses of heterogeneity and publication bias might have been relatively low owing to the small number of included studies.78 Seventh, the extensive use of imputation and the lack of an analysis of rTMS-related side effects are additional limitations of our study. Eight, our decision to pool the results of LF- and HF-rTMS protocols applied to the right DLPFC is also a potential limitation of our study, particularly considering their likely distinct neurophysiological basis. Finally, meta-analyses have often been criticized for the potential of publication bias and for the inclusion of poor-quality trials.50 However, in our study these concerns were addressed by the comprehensive and systematic review of the literature and by the use of stringent inclusion criteria.
Conclusion
Our exploratory meta-analysis suggests that rTMS applied to the DLPFC is a promising new treatment for PTSD, even though its evidence base is still limited to a few clinical studies. Clearly, future larger-scale RCTs should first and foremost investigate the putative differential efficacy of HF-, compared with LF-, rTMS protocols, as well as of left, compared with right DLPFC coil placements. Additionally, they should explore whether patients with distinct subtypes of PTSD preferentially respond to rTMS, whether its beneficial effects are maintained over time, and whether the improvement in PTSD symptoms observed after rTMS treatment is mediated, for example, by changes in related psychopathological dimensions, such as depressive symptoms. Finally, considering its overall safety record74,79 and potential efficacy, we believe that rTMS could be currently offered, on a strictly humanitarian and compassionate basis, to patients with PTSD who remain significantly ill despite having received several conventional treatments for this condition (for example, pharmacotherapy and psychotherapy).
Acknowledgments
Dr Berlim has received a researcher-initiated grant from Brainsway Inc to study the neurobiology of deep transcranial magnetic stimulation (H1 coil) in MDD. Dr Van den Eynde reports no potential conflicts of interest. Our study received no external funding.
Abbreviations
- AD
antidepressant
- AP
antipsychotic
- DLPFC
dorsolateral prefrontal cortex
- ES
effect size
- HF
high frequency
- LF
low frequency
- LOCF
last observation carried forward
- MDD
major depressive disorder
- PTSD
posttraumatic stress disorder
- RCT
randomized controlled trial
- rTMS
repetitive transcranial magnetic stimulation
References
- 1.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.Bisson JI. Post-traumatic stress disorder. BMJ. 2007;334:789–793. doi: 10.1136/bmj.39162.538553.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein DJ, Seedat S, Iversen A, et al. Post-traumatic stress disorder: medicine and politics. Lancet. 2007;369:139–144. doi: 10.1016/S0140-6736(07)60075-0. [DOI] [PubMed] [Google Scholar]
- 5.Brunello N, Davidson JR, Deahl M, et al. Posttraumatic stress disorder: diagnosis and epidemiology, comorbidity and social consequences, biology and treatment. Neuropsychobiology. 2001;43:150–162. doi: 10.1159/000054884. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Davidson JR. Trauma: prevalence, impairment, service use, and cost. J Clin Psychiatry. 1997;58(Suppl 9):5–11. [PubMed] [Google Scholar]
- 7.Ipser JC, Stein DJ. Evidence-based pharmacotherapy of post-traumatic stress disorder (PTSD) Int J Neuropsychopharmacol. 2012;15:825–840. doi: 10.1017/S1461145711001209. [DOI] [PubMed] [Google Scholar]
- 8.Ponniah K, Hollon SD. Empirically supported psychological treatments for adult acute stress disorder and posttraumatic stress disorder: a review. Depress Anxiety. 2009;26:1086–1109. doi: 10.1002/da.20635. [DOI] [PubMed] [Google Scholar]
- 9.Van Etten ML, Taylor S. Comparative efficacy of treatment for posttraumatic stress disorder: a meta-analysis. Clin Psychol Psychother. 1998;5:126–144. [Google Scholar]
- 10.Novakovic V, Sher L, Lapidus KA, et al. Brain stimulation in posttraumatic stress disorder. Eur J Psychotraumatol. 2011;2 doi: 10.3402/ejpt.v2i0.5609. Epub 2011 Oct 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. 2011;168:356–364. doi: 10.1176/appi.ajp.2010.10060864. [DOI] [PubMed] [Google Scholar]
- 12.Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37:102–116. doi: 10.1038/npp.2011.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berlim MT, Van den Eynde F, Perdomo ST, et al. Response, remission and dropout rates following high frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–239. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- 14.Slotema CW, Aleman A, Daskalakis ZJ, et al. Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: update and effects after one month. Schizophr Res. 2012;142:40–45. doi: 10.1016/j.schres.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Patel R, Spreng RN, Shin LM, et al. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore SA. Cognitive abnormalities in posttraumatic stress disorder. Curr Opin Psychiatry. 2009;22:19–24. doi: 10.1097/YCO.0b013e328314e3bb. [DOI] [PubMed] [Google Scholar]
- 19.McNally RJ. Cognitive abnormalities in post-traumatic stress disorder. Trends Cogn Sci. 2006;10:271–277. doi: 10.1016/j.tics.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Vanderhasselt MA, De Raedt R, Baeken C, et al. The influence of rTMS over the right dorsolateral prefrontal cortex on intentional set switching. Exp Brain Res. 2006;172:561–565. doi: 10.1007/s00221-006-0540-5. [DOI] [PubMed] [Google Scholar]
- 21.Wagner M, Rihs TA, Mosimann UP, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex affects divided attention immediately after cessation of stimulation. J Psychiatr Res. 2006;40:315–321. doi: 10.1016/j.jpsychires.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Osaka N, Otsuka Y, Hirose N, et al. Transcranial magnetic stimulation (TMS) applied to left dorsolateral prefrontal cortex disrupts verbal working memory performance in humans. Neurosci Lett. 2007;418:232–235. doi: 10.1016/j.neulet.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 23.Sandrini M, Rossini PM, Miniussi C. Lateralized contribution of prefrontal cortex in controlling task-irrelevant information during verbal and spatial working memory tasks: rTMS evidence. Neuropsychologia. 2008;46:2056–2063. doi: 10.1016/j.neuropsychologia.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Turriziani P, Smirni D, Oliveri M, et al. The role of the prefrontal cortex in familiarity and recollection processes during verbal and non-verbal recognition memory: an rTMS study. Neuroimage. 2010;52:348–357. doi: 10.1016/j.neuroimage.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Hwang JH, Kim SH, Park CS, et al. Acute high-frequency rTMS of the left dorsolateral prefrontal cortex and attentional control in healthy young men. Brain Res. 2010;1329:152–158. doi: 10.1016/j.brainres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Vanderhasselt MA, De Raedt R, Leyman L, et al. Acute effects of repetitive transcranial magnetic stimulation on attentional control are related to antidepressant outcomes. J Psychiatry Neurosci. 2009;34:119–126. [PMC free article] [PubMed] [Google Scholar]
- 27.van’t Wout M, Kahn RS, Sanfey AG, et al. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005;16:1849–1852. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- 28.Philiastides MG, Auksztulewicz R, Heekeren HR, et al. Causal role of dorsolateral prefrontal cortex in human perceptual decision making. Curr Biol. 2011;21:980–983. doi: 10.1016/j.cub.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 29.McCann UD, Kimbrell TA, Morgan CM, et al. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gen Psychiatry. 1998;55:276–279. doi: 10.1001/archpsyc.55.3.276. [DOI] [PubMed] [Google Scholar]
- 30.Grisaru N, Amir M, Cohen H, et al. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry. 1998;44:52–55. doi: 10.1016/s0006-3223(98)00016-x. [DOI] [PubMed] [Google Scholar]
- 31.Boggio PS, Rocha M, Oliveira MO, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. 2010;71:992–999. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen H, Kaplan Z, Kotler M, et al. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161:515–524. doi: 10.1176/appi.ajp.161.3.515. [DOI] [PubMed] [Google Scholar]
- 33.Watts BV, Landon B, Groft A, et al. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012;5:38–43. doi: 10.1016/j.brs.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg PB, Mehndiratta RB, Mehndiratta YP, et al. Repetitive transcranial magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. J Neuropsychiatry Clin Neurosci. 2002;14:270–276. doi: 10.1176/jnp.14.3.270. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. 1st ed. West Sussex (GB): John Wiley & Sons Ltd; 2008. [Google Scholar]
- 36.American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders (DSM-IV) 4th ed. Washington (DC): APA; 1994. [Google Scholar]
- 37.World Health Organization (WHO) The ICD-10 Classification of Mental and Behavioural Disorders: clinical descriptions and diagnostic guidelines. 10th ed. Geneva (CH): WHO; 1992. [Google Scholar]
- 38.Davidson JR, Colket JT. The eight-item treatment-outcome post-traumatic stress disorder scale: a brief measure to assess treatment outcome in post-traumatic stress disorder. Int Clin Psychopharmacol. 1997;12:41–45. doi: 10.1097/00004850-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28:596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 41.Kendall PC, Finch AJ, Jr, Auerbach SM, et al. The State-Trait Anxiety Inventory: a systematic evaluation. J Consult Clin Psychol. 1976;44:406–412. doi: 10.1037//0022-006x.44.3.406. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 44.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 45.Boutron I, Guittet L, Estellat C, et al. Reporting methods of blinding in randomized trials assessing nonpharmacological treatments. PLoS Med. 2007;4:e61. doi: 10.1371/journal.pmed.0040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 47.Fergusson D, Aaron SD, Guyatt G, et al. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325:652–654. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenthal R. Meta-analytic procedures for social research. Newbury Park (CA): Sage Publications; 1993. [Google Scholar]
- 49.Furukawa TA, Barbui C, Cipriani A, et al. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. West Sussex (GB): Wiley & Sons Ltd; 2009. [Google Scholar]
- 51.Grissom RJ, Kim JJ. Effect sizes for research—univariate and multivariate application. New York (NY): Routledge; 2012. [Google Scholar]
- 52.Cooper H, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. New York (NY): Russell Sage Foundation Publications; 2009. [Google Scholar]
- 53.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–641. [Google Scholar]
- 54.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothstein HR, Sutton AJ, Borenstein M. Publication bias in meta-analysis: prevention, assessment and adjustments. 1st ed. London (GB): John Wiley & Sons Ltd; 2005. [Google Scholar]
- 56.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Bestmann S. The physiological basis of transcranial magnetic stimulation. Trends Cogn Sci. 2008;12:81–83. doi: 10.1016/j.tics.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci. 2003;23:10867–10872. doi: 10.1523/JNEUROSCI.23-34-10867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Touge T, Gerschlager W, Brown P, et al. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- 60.Weisz N, Steidle L, Lorenz I. Formerly known as inhibitory: effects of 1-Hz rTMS on auditory cortex are state-dependent. Eur J Neurosci. 2012;36:2077–2087. doi: 10.1111/j.1460-9568.2012.08097.x. [DOI] [PubMed] [Google Scholar]
- 61.Daskalakis ZJ, Moller B, Christensen BK, et al. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- 62.Pare D, Duvarci S. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol. 2012;22:717–723. doi: 10.1016/j.conb.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin LM, Handwerger K. Is posttraumatic stress disorder a stress-induced fear circuitry disorder? J Trauma Stress. 2009;22:409–415. doi: 10.1002/jts.20442. [DOI] [PubMed] [Google Scholar]
- 66.Powers MB, Halpern JM, Ferenschak MP, et al. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Pae CU, Lim HK, Peindl K, et al. The atypical antipsychotics olanzapine and risperidone in the treatment of posttraumatic stress disorder: a meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Int Clin Psychopharmacol. 2008;23:1–8. doi: 10.1097/YIC.0b013e32825ea324. [DOI] [PubMed] [Google Scholar]
- 68.Peterchev AV, Wagner TA, Miranda PC, et al. Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul. 2012;5:435–453. doi: 10.1016/j.brs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) Neuropsychopharmacology. 2010;35:301–316. doi: 10.1038/npp.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matthews PM, Honey GD, Bullmore ET. Applications of fMRI in translational medicine and clinical practice. Nat Rev Neurosci. 2006;7:732–744. doi: 10.1038/nrn1929. [DOI] [PubMed] [Google Scholar]
- 71.Khodayari-Rostamabad A, Reilly JP, Hasey GM, et al. Using pre-treatment electroencephalography data to predict response to transcranial magnetic stimulation therapy for major depression. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6418–6421. doi: 10.1109/IEMBS.2011.6091584. [DOI] [PubMed] [Google Scholar]
- 72.Isserles M, Shalev AY, Roth Y, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder—a pilot study. Brain Stimul. 2013;6(3):377–383. doi: 10.1016/j.brs.2012.07.008. Epub 2012 Aug 18. [DOI] [PubMed] [Google Scholar]
- 73.Huang Y-Z, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 74.Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berlim MT, Broadbent H, Van den Eynde F. Blinding integrity in clinical trials of high frequency repetitive transcranial magnetic stimulation (rTMS) for depression: a meta-analysis. Int J Neuropsychopharmacol. 2013;16(5):1173–1181. doi: 10.1017/S1461145712001691. Epub 2013 Feb 11. [DOI] [PubMed] [Google Scholar]
- 76.Schonfeldt-Lecuona C, Lefaucheur JP, Cardenas-Morales L, et al. The value of neuronavigated rTMS for the treatment of depression. Neurophysiol Clin. 2010;40:37–43. doi: 10.1016/j.neucli.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 77.Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacol Ther. 2012;133:98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 79.Machii K, Cohen D, Ramos-Estebanez C, et al. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117:455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.