Abstract
In order to follow optic neuritis patients and evaluate the effectiveness of their treatment, a handy, accurate and quantifiable tool is required to assess changes in myelination at the central nervous system (CNS). However, standard measurements, including routine visual tests and MRI scans, are not sensitive enough for this purpose. We present two visual tests addressing dynamic monocular and binocular functions which may closely associate with the extent of myelination along visual pathways. These include Object From Motion (OFM) extraction and Time-constrained stereo protocols. In the OFM test, an array of dots compose an object, by moving the dots within the image rightward while moving the dots outside the image leftward or vice versa. The dot pattern generates a camouflaged object that cannot be detected when the dots are stationary or moving as a whole. Importantly, object recognition is critically dependent on motion perception. In the Time-constrained Stereo protocol, spatially disparate images are presented for a limited length of time, challenging binocular 3-dimensional integration in time. Both tests are appropriate for clinical usage and provide a simple, yet powerful, way to identify and quantify processes of demyelination and remyelination along visual pathways. These protocols may be efficient to diagnose and follow optic neuritis and multiple sclerosis patients.
In the diagnostic process, these protocols may reveal visual deficits that cannot be identified via current standard visual measurements. Moreover, these protocols sensitively identify the basis of the currently unexplained continued visual complaints of patients following recovery of visual acuity. In the longitudinal follow up course, the protocols can be used as a sensitive marker of demyelinating and remyelinating processes along time. These protocols may therefore be used to evaluate the efficacy of current and evolving therapeutic strategies, targeting myelination of the CNS.
Keywords: Medicine, Issue 86, Optic neuritis, visual impairment, dynamic visual functions, motion perception, stereopsis, demyelination, remyelination
Introduction
Optic neuritis as a model for tracking tissue degeneration and repair
Multiple sclerosis (MS) is a chronic inflammatory neurodegenerative disease of the central nervous system (CNS) and is the leading cause of nontraumatic neurological disability in young adults in developed countries. Demyelination is considered the most characteristic histopathological feature of MS. Recent studies, however, revealed that MS is also a neurodegenerative disease with early neuroaxonal damage1-3.
Optic neuritis (ON), inflammation of the optic nerve, is the presenting symptom in 20% of MS patients and at least 50% of those suffering from MS experience at least one episode of ON during their lifetime4. Unlike other locations of MS lesions that do not always correlate to the clinical manifestations, demyelinating episode of the optic nerve typically results in distinctive manifestation of acute visual loss. Given its comorbidity with MS and its prominent clinical features, ON offers a unique opportunity for tracking tissue degeneration and repair and their consequences in a single MS lesion.
The need for improved methods for tracking tissue degeneration and repair in vivo
Pathologic studies in MS implicate demyelination as a principal cause of axonal transection and subsequent axonal degeneration. Remyelination may prevent demyelinated axons from degenerating; however, effective remyelination may be limited as a result of repeated attacks. Therefore, current and evolving neuroprotective and regenerative therapeutic strategies in MS are aimed to prevent new attacks and promote remyelination processes in the CNS5.
In order to follow up optic neuritis patients and evaluate the efficacy of their treatment, a fine tool to quantify changes in myelination at the CNS is required. However, the standard measurements, including routine visual tests and MRI scans, are not sensitive enough for this purpose. Routine visual tests (i.e. visual acuity, contrast sensitivity, visual fields, and color perception) may reveal cases of reduced input projection along the visual pathways but are insensitive to identify delayed projection rates, which is the role of the demyelinated fibers6,7. T2 hyperintense lesions, which are the hallmark of the disease, result from residual mixture of edema, inflammation, demyelination, axonal loss and gliosis and thus cannot differentiate between demyelination and other brain pathologies. Furthermore, standard MRI is designed to reveal qualitative tissue contrast. While these are adequate for identifying the location of unusual tissue, they are insufficient to quantitatively assess tissue properties.
Dynamic visual tests may be used as markers of demyelination and remyelination
We argue that dynamic visual functions are more appropriate than static functions to identify and quantify changes in projection latencies along the visual pathways. While accomplishment of both static and dynamic visual functions requires sufficient amount of visual input projection, only dynamic visual functions depend on projection rates. Optic nerve demyelination may thus affect dynamic rather than static visual functions, implicating the need for rapid transmission of visual input in order to perceive motion.
We have developed two behavioral tasks to assess monocular and binocular visual functions which may closely associate with projection latencies along the visual pathways. These include Object From Motion (OFM) extraction and Time-constrained stereo protocols.
In the OFM test, an array of dots compose an object, by moving the dots within the image rightward while moving the dots outside the image leftward or vice versa. The dot pattern generates a camouflaged object that cannot be detected when the dots are stationary or moving as a whole. Importantly, object recognition is dependent on motion perception. Using the OFM protocol, we have demonstrated a sustained deficit in the affected eyes of ON patients, evident even 12 months following the optic neuritis attack, while standard visual functions had recovered8. Furthermore, impaired performance was associated with delayed conductions (delayed P100, reflecting demyelination) and improvement in motion perception was correlated with shortening of conduction rates (reflecting remyelination; linear least squares regression with calculation of the correlation coefficient F=27.3; p=0.0005; r=-0.87)9.
The currently presented OFM protocol was updated in order to fit the test for clinical usage, including test shortening, adjusting the test software to result in an automatic output file, and to result in a motion sensitivity score.
To assess the effect of projection latencies on binocular vision, the Time-constrained Stereo protocol was developed. In this protocol, spatially disparate images are presented for a limited length of time, challenging binocular integration in time. This test was designed to test the hypothesis that due to demyelination at the affected nerve, information from the two eyes will reach the cortex at different time points impairing binocular integration in time. Testing a group of recovered ON patients (1-2.5 years following the attack), we have shown that while most patients had intact performance levels in a standard static stereo task; performance on the time-constrained stereo task was impaired in most cases10.
The OFM and the time-constrained stereo protocols provide a simple, yet powerful, way to identify and quantify processes of demyelination and remyelination along the visual pathways. These protocols may be efficient to diagnose and follow up ON and MS patients in a cost effective manner using an easy to use computer based protocol.
Protocol
The protocol follows the Hadassah Hebrew University Ethics Committee guidelines for studies in human subjects. To avoid the effect of myopia or refractive errors on test results, the protocols should be performed while patients wear their eyeglasses (corrected vision).
Object From Motion (OFM) protocol:
1. Test Initiation and Instructing Subjects
Seat the subject 50 cm in front of the computer screen.
Open the OFM software.
Instruct the subject that he will be presented with motion defined objects. Instruct him to respond as correct and as fast as possible by pressing the "A" keyboard and then verbally naming the perceived object.
Following response, a screen indicating "press the space bar" will appear. Instruct the subject to press the space bar when ready to identify the next stimulus.
Explain to the subject that stimuli may appear at very hard to-perceive velocities or at some easier to perceive ones.
2. Learning Phase
Enter "learning OFM" at the command line. Subject will now be presented with 4 example stimuli. This phase is conducted when subject's both eyes are open.
3. Testing Phase
In general, each OFM test includes 20 stimuli. All are first presented at the lowest velocity of 4 pixels/sec. Those not recognized will be then presented at the next velocity of 5.5 pixels/sec. Those not recognized, will be then presented at the next velocity of 7.5 pixels/sec and so on, going through 10 pixels/sec, 13.5 pixels/sec, 18 pixels/sec, and till the fastest velocity of 24.5 pixels/sec. Velocities were defined based on the exponent y=3*e0.3. If five consecutive stimuli in a certain velocity were not recognized, the next stimuli will be presented at the next faster velocity to avoid frustration in the patients. This will result in shortening test length which generally is longer as recognition is worse (necessitating the passage through larger number of velocities per stimulus).
Cover subject's one eye with an eye patch. Every eye patch may be adequate as long as it supplies full coverage.
Enter "OFM objects" at the command line.
Choose one stimuli set (software includes 4 stimuli sets. Each set includes 20 different objects. Selection may be random. However, make sure that you apply different stimuli sets for each of the tested eyes; Apply different stimuli sets for subsequent learning time points in case of longitudinal assessment).
A prompt asking you to enter subject's name, tested eye and testing date will appear. Complete required information.
- Carefully monitor the subject and respond to the subject while she/he completes the task as follows:
- When a stimulus appears the subject must press the "A" button on the keyboard and name the identity of the presented stimulus.
- Press the left mouse button for a correct answer or the right mouse button for a wrong answer.
- Subject presses the "space bar" on the keyboard for initiation of the next stimulus.
- This procedure (steps 3.5.1-3.1.5.3) continues until all 20 stimuli in the set are either recognized or presented at the fastest velocity.
Repeat the whole procedure (from step 3.1) for the subject's second eye using a different set of stimuli.
Time-constrained Stereo Protocol:
1. Test Initiation and Instructing Subjects
Seat the subject 50 cm in front of the computer screen.
Open the Stereo software.
Instruct the subject that he will be presented with 3 dimensional (3D) shapes and will have to name the perceived shape as correct and fast as possible. Shapes will be one of the following: a circle, a square, a triangle, or a star.
Explain to the subject that stimuli may appear at very hard to perceive conditions or at some easier to perceive ones.
Instruct the subject to wear the 3D glasses.
Turn off the room lightening.
2. Learning Phase
Enter "learning Stereo" at the command line. Subject will be now presented with 4 repetitions of each shape, presented at the longest stimuli duration (500 msec) and at the easiest disparity (840 sec of arc) conditions. Following the 3D presentation condition, a 2D presentation will follow for each shape. In the latter, a line marking shapes contours will be added, to make sure subject perceived the dimensions of the presented shape. Subjects, who did not succeed at this easy condition, will not be tested at the next phase.
3. Testing Phase: Stereopsis Perception as a Function of Binocular Disparity
The 4 shapes will be presented for 500 msec at 4 different disparity conditions: 120, 300, 540, and 840 sec of arc.
Enter "Stereo Disparity" at the command line.
A prompt asking you to enter subject's name and testing date will appear. Complete required information.
Stimulus appears
Subject names the presented shape. Responses are coded by the examiner at key buttons 1-4 (i.e. press the 1, 2, 3, or 4 key buttons for subject's verbal responses of "a circle", "a square", "a triangle", or "a star", respectively). Due to lighting conditions and the fact that the subject wears 3D glasses, he cannot code the responses by himself).
Subject presses any key to continue to the next stimulus. The order of stimuli presentation is random.
4. Testing Phase
Stereopsis perception as a function of stimulus presentation time. The 4 shapes will be presented for disparities of 540 and 840 sec of arc at either 40, 60, or 100 msec durations.
Enter "Stereo Duration" at the command line.
Repeat steps 3.2-3.5.
Representative Results
OFM protocol
The protocol results in a text file, automatically summarizing subject's responses. Outcome can be analyzed in two ways:
Total score: Each stimulus presented is assigned with a stimulus weight, and the sum of weights of all identified stimuli is set as the subject's response score. The weight of a particular stimulus is set based on the speed of the stimulus, with higher weights to lower speeds. Unidentified stimuli are assigned with a zero weight. Generally, let N be the different velocities v1, v2, ..., vN employed (where v1 is the lowest speed, v2 is the next to lowest velocity and so on); w1, w2,...,wN are the assigned weights (where w1 > w2 > ... > wN. w0 is the weight associated with an unidentified or undetected stimulus); and k1 to kN are the number of stimuli identified in each velocity. Then, the response score will be set to w1k1 + w2k2 +...+ wNk N + w0k0, where k0 is the number of unidentified or undetected stimuli and k0 + k1+ k2+...+ kN = K. The response score can therefore be from zero (no stimulus identified) to w1K (all stimuli identified at the lowest speed, hence received the highest score). The current protocol is composed of 20 stimuli presented at 6 different velocities and thus response may range from 0-120. The highest score of 6 will be assigned to stimuli identified at the lowest velocity and the lowest score of 1 will be assigned to stimuli identified at the highest velocity. For example, suppose that no stimulus is identified at speeds v1 and v2, 1 stimulus is identified at each of speeds v3 and v4, 7 stimuli are identified at speed v5, 9 stimuli are identified at speed v6, and 2 stimuli are not identified at all. The response score in this numerical example is, therefore, 0*6 + 0*5 + 1*4 + 1*3 + 7*2 + 9*1 + 2*0 = 30, where the term at the right hand side corresponds to the two unidentified stimuli.
Motion sensitivity score: Will be defined as the slowest dot speed in which a patient had reached 80% correct responses, identifying this dot speed as the patient's threshold to obtain intact motion perception.
The obtained score should be compared to the normally sighted data, comparing patients' results as relative to the standard range. This is illustrated in Figure 1. The current OFM protocol was tested on a group of 16 first ever unilateral ON patients aged 20-50 (32.1±2.5 mean± SEM); studied 1.5-19 months following an optic neuritis attack (6.5±1.47 months on average). At testing, 12 of the patients were CIS and the other 4 RRMS with the first attack of ON. MRI was normal in 10 patients. All patients were hospitalized in the Neurology or Ophthalmology Departments in Hadassah medical center and were followed up in our Neurology outpatient clinic. ON was defined on clinical grounds by a specialized Neuro ophthalmologist, who had also ruled out the coexistence of other ophthalmological diseases. Perimetry was obtained in all patients but 3. Eyes with corrected visual acuity lower than 20/30 and/or central scotoma were excluded from data analysis. Thus, presented results include 12 affected and 15 fellow eyes. In addition, 29 normally sighted eyes were tested.
As demonstrated in Figure 1, affected eyes of ON patients obtain significantly lower results when compared to normally sighted eyes (p=0.0001, Two-tailed T Test), and to patients' fellow eyes (p=0.006). Significant impairment of the affected eyes is found whether analyzing the results as total scores (as in Figure 1) or as motion sensitivity scores (p=0.0004 and p=0.02 in comparison to eyes of controls and to fellow eyes). Impaired scores on the OFM protocol are found also in cases of recovered visual acuity (i.e. visual acuity ≥ 20/2511; p=0.01 in comparison to controls), suggesting that this measurement is more sensitive to identify patients' persisting visual complains. As seen in Figure 1, the scores of all but one of the affected eyes are placed outside the normal range (defined as ±2.5 SD from controls' mean).
Time-constrained stereo protocol
Stereo perception is tested twice. Each subject obtains two scores, defining his stereo perception as a function of binocular disparity and as a function of stimulus presentation time. Results are given as text files and analyzed as percent correct scores.
The current Stereo protocol was tested on a group of 17 first ever unilateral optic neuritis patients, aged 20-58 years (36.4±2.6 mean± SEM); 12-26 months following an ON attack (15.8±1.34 months on average). All patients were hospitalized in the Neurology or Ophthalmology Departments in Hadassah and were followed up in our Neurology outpatient clinic. All eyes had corrected visual acuity ≥20/25. Perimetry was assessed in all patients; none had central scotoma in either eye. A neuro-ophthalmologist tested the patients to exclude the coexistence of other ophthalmological diseases.
At testing, 10 of the patients were CIS and the other 7 RRMS with first attack of ON. MRI was normal in 7 patients.
Twenty six and seventeen matched healthy controls were tested in the stereo by disparity and stereo by duration protocols, respectively.
Stereo perception as a function of binocular disparity (independent of stimulus duration)
Subjects' scores are summarized for the different disparity conditions: 120, 300, 540, and 840 sec of arc. As expected, performance level improves as binocular disparity increases (Figure 2A). This is true for both ON patients and healthy control subjects. Improved stereo perception as a function of increased disparity cues resemble standard stereo tests and demonstrate the effectiveness of our protocol to assess stereopsis. As seen in Figure 2A, the effects of binocular disparity cues on stereopsis perception resemble in ON patients and control subjects.
Stereo perception as a function of stimulus duration (independent of disparity degree)
Subjects' scores are summarized for the different stimuli durations: 40, 60, and 100 msec. All presented at the highest disparity of 840 sec of arc. For control subjects, reducing stimuli presentation times did not change performance levels; performance was similar for all presentation conditions (92.1%, 90.8%, and 91.4% correct in the 40, 60, and 100 msec stimulus durations, respectively, Figure 2B). In ON patients, on the other hand, performance level was dependent on stimuli duration length. Stereopsis was significantly impaired in the 40 msec compared with the 60 msec stimuli duration conditions (61.7% and 72.2% correct respectively, p=0.04; paired two-tailed T-Test). Performance in the 60 msec condition was indistinguishable from the 100 msec condition (71.2% correct, Figure 2B).
Thus, 40 msec is a sufficient time for healthy eyes to synchronize binocular information, resulting in intact stereopsis perception. This timing is insufficient in cases of ON. This may be explained by the fact that monocular demyelination generates a delay between the information projected via the two eyes, challenging binocular integration in time for brief stimuli.
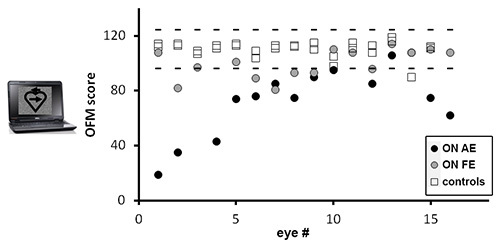 Figure 1. Scores in the OFM protocols for optic neuritis and control eyes. Total scores obtained in patients' affected eyes (AE) – black circles; fellow eyes (FE) – gray circles, and control eyes – open squares. Dotted lines represent normative range, defined as ±2.5 SD from controls' mean.
Figure 1. Scores in the OFM protocols for optic neuritis and control eyes. Total scores obtained in patients' affected eyes (AE) – black circles; fellow eyes (FE) – gray circles, and control eyes – open squares. Dotted lines represent normative range, defined as ±2.5 SD from controls' mean.
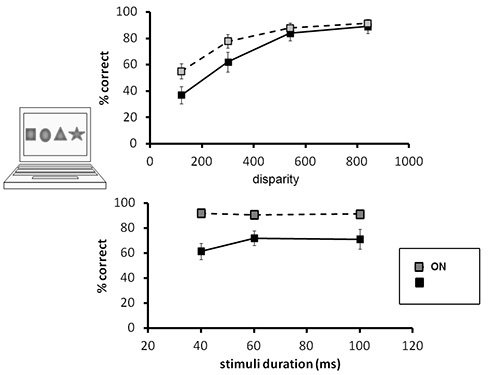 Figure 2. Stereo perception as a function of binocular disparity and as a function of stimuli duration in optic neuritis and control subjects.(A) Stereopsis perception as a function of binocular disparity. As seen, similar trend is found in both ON and controls (gray and black symbols, respectively), demonstrating improved stereopsis perception as a function of increased disparity. (B) Stereopsis perception as a function of stimuli duration. As seen, while controls' perception is independent of stimuli duration, stereo perception in the patients is a function of stimulus presentation length; significantly worse performance is found when stimuli are presented for 40 msec compared with stimuli presented for 60 and 100 msec.
Figure 2. Stereo perception as a function of binocular disparity and as a function of stimuli duration in optic neuritis and control subjects.(A) Stereopsis perception as a function of binocular disparity. As seen, similar trend is found in both ON and controls (gray and black symbols, respectively), demonstrating improved stereopsis perception as a function of increased disparity. (B) Stereopsis perception as a function of stimuli duration. As seen, while controls' perception is independent of stimuli duration, stereo perception in the patients is a function of stimulus presentation length; significantly worse performance is found when stimuli are presented for 40 msec compared with stimuli presented for 60 and 100 msec.
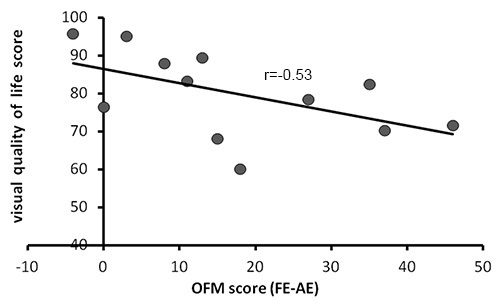 Figure 3. The relationship between OFM scores and visual quality of life in optic neuritis patients. Visual quality of life scores (obtained via the NEI-VFQ-25 and the 10 Item Neuro Ophthalmic Supplement questionnaires) plotted against patients' OFM scores. OFM score in each patient were defined as the delta between his affected and fellow eyes' scores (scores of the fellow eyes minus scores of the affected eyes, to minimize individual differences). As seen, subjective visual quality of life scores correlate with patients' scores on the OFM protocol; impaired quality of life associates with impaired motion perception, while better quality of life associates with intact motion perception.
Figure 3. The relationship between OFM scores and visual quality of life in optic neuritis patients. Visual quality of life scores (obtained via the NEI-VFQ-25 and the 10 Item Neuro Ophthalmic Supplement questionnaires) plotted against patients' OFM scores. OFM score in each patient were defined as the delta between his affected and fellow eyes' scores (scores of the fellow eyes minus scores of the affected eyes, to minimize individual differences). As seen, subjective visual quality of life scores correlate with patients' scores on the OFM protocol; impaired quality of life associates with impaired motion perception, while better quality of life associates with intact motion perception.
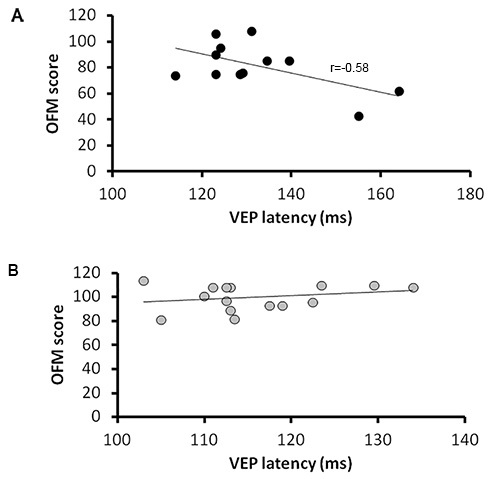 Figure 4. The relationship between OFM scores and VEP latencies in optic neuritis patients. OFM scores plotted against patients VEP latencies (P100) for the affected eyes (A) and fellow eyes (B). As seen, OFM scores are correlated with VEP latencies, indicating projection rates. This is true in the affected but not the fellow eyes of the patients.
Figure 4. The relationship between OFM scores and VEP latencies in optic neuritis patients. OFM scores plotted against patients VEP latencies (P100) for the affected eyes (A) and fellow eyes (B). As seen, OFM scores are correlated with VEP latencies, indicating projection rates. This is true in the affected but not the fellow eyes of the patients.
Discussion
Optic neuritis is a demyelinative disease of the optic nerve, causing acute visual loss. Though considered transient when using standard visual testing1, patients continue to perceive difficulties in performing everyday visual tasks. We argue that dynamic visual tests are adequate to identify and quantify these sustained deficits. This is since dynamic but not static visual functions depend on projection rates, and may be more vulnerable to delayed projection following demyelination.
The OFM protocol
In order to define how dynamic visual function deficit are reflected in patients' daily life, patients were assessed via the vision specific and overall quality of life questionnaires; the 25 Item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ-25)12 and the 10 Item Neuro Ophthalmic Supplement13. These include questions regarding the patient's subjective estimation of his visual abilities as well as questions regarding his frustration feelings, difficulty performing certain tasks, and the need for assistance from others due to visual deficiencies. For each item, the patient is asked to choose the response that best describes his perception at the time of examination. Visual quality of life score ranges between 0-100.
We have found that scores in the OFM protocol explain a notable amount of patients' subjective visual complains, after controlling for visual acuity deficits.
Scores in the OFM protocol (defined as the delta between scores in the affected and fellow eyes, to minimize individual differences) were correlated with visual quality of life scores (r=-0.53, see details in Figure 3). Correlation was close to statistical significance (p=0.077). OFM scores predicted 28% of the variance in the visual quality of life questionnaires, explaining a major part of the patients' subjective visual complains.
In addition to its role in explaining patients' visual complains, the OFM protocol can be used as an easy marker of myelination degree along the visual pathways; deterioration in OFM scores reflecting delayed projection rates will mark demyelination while improved OFM scores reflecting shortening of latencies will mark remyelination. Furthermore, the amount of change in the OFM scores may signal the amount of demyelination or remyelination. Currently, Visual Evoked Potential (VEP) latencies are the gold standard tool to identify and quantify demyelination and remyelination in the visual pathways. As known for many years, prolonged VEP latencies are frequently found following ON, and this prolongation may persist for years6,14.
In order to validate the role of the OFM scores as an index of myelination along the visual pathways, VEP latencies using a pattern reversal VEP were assessed in each patient. As demonstrated in Figure 4, scores in our OFM protocol were significantly correlated with absolute VEP latencies. This was true for the affected eyes (linear least squares regression with calculation of the correlation coefficient F=5.2; p=0.046; r=-0.58) but not for the fellow eyes (F=0.85; p>0.05; r=0.25) of optic neuritis patients. This is in accordance with our previous findings on a different group of ON patients10 which may suggest the specificity of the OFM protocol to reveal a demyelinative attack. The correlation between OFM scores and VEP (P100) latency values indicate that OFM may not only identify, but also quantify the demyelinative damage. The correlation between improvement in the OFM protocol and shortening of VEP latencies along time9 suggest that the OFM protocol may also sensitively quantify remyelination.
The advantage of using the OFM protocol to identify and quantify changes in myelination degree along the visual pathways comes from its availability.
In contrast to VEP measurements, applying the OFM protocol does not require a highly expensive device and a trained technician. The protocol results in an automatic output file, which can be evaluated by all clinicians. This makes the OFM accessible inexpensive tools suitable for widely use.
The time-constrained stereo protocol
The time-constrained stereo protocol may be more adequate than standard stereopsis tests to assess binocular visual dysfunctions specific to optic neuritis.
Standard stereopsis tests are designed to reveal impaired binocular vision in cases when one eye transfers insufficient amount of visual input. This may characterize the acute phase of ON as well as other optic neuropathies. These tests, however, will not reveal cases in which both eyes deliver sufficient visual information but at different timing (characterizing chronic optic neuritis). Since visual stimuli are presented for an unlimited time, differences in projection rates are negligible.
While not identified via standard measurements, differences in projection rates among the eyes are expected to significantly harden everyday binocular vision (in which rapid stimuli are common). Thus, the time-constrained stereo protocol is useful to identify binocular visual dysfunction following ON and may explain patients' persisting complains in everyday visual life.
Since our protocols aim to generate standard evaluation of dynamic visual functions, which will result in similar scores independent of external conditions, the protocols are strict and cannot be modified thought running. If following technical problems the test collapses prior to full completion, partial information will be saved. Patient's score in the latter case should be regarded with caution, depending on the amount of information that was already acquired. Alternatively, repeating the test using a different experimental set is possible.
To ensure standard scoring among patients, it is important to be precise on test initiation steps, and especially keeping the required illumination conditions. It is also critical to complete the learning phase step as performance level in the visual tests may be dependent on familiarization with stimuli characteristics. Successful performance in the learning step is a prerequested condition to proceed to the testing phase, indicating that the subject has sufficient motion perception or sufficient binocular vision to be tested in the OFM or stereo protocols. Moreover, to ensure that performance level in the dynamic visual tests reflects the functional status of the optic nerve rather than anterior eye segments, other ophthalmological diseases should be ruled out and protocols should be carried out while patients wear their eyeglasses (controlling out effects of myopia or refractive errors).
One limitation of the OFM protocol results from its finite amount of test stimuli. These 80 objects were selected based on a comprehensive study on optic neuritis and control subjects as the most diagnostic items (out of 240 stimuli tested) between these groups. The 80 items compose 4 different stimuli sets. To avoid inter eye learning, different stimuli set is applied to each tested eye. To avoid learning in time, different stimuli sets are applied between subsequent testing phases. This is adequate for two adjacent testing phases. The 4 currently stimuli sets are controlled for difficulty levels. Inclusion of new stimuli within the testing phase will demand prior evaluation to ensure similar difficulty levels between testing sets.
This paper focused on demyelination as the pathophysiology characterizing optic neuritis. We are familiar with the notion that optic neuritis is more than a demyelination state and that substantial neuroaxonal damage may also occur in the patients, as revealed by recent optical coherence tomography (OCT) studies15-19. However, while the functional consequence of this neuroaxonal damage was previously established20-22, demonstrating reduced visual acuity, contrast acuity and visual quality of life, the functional consequence of demyelination was rarely addressed. Our study was aimed to reveal the functional consequences of demyelination in differentiation from axonal loss and develop clinical tools to quantify these functional changes.
To summarize, we suggest that our novel OFM and stereo protocols may be used in the diagnosis and follow up course of ON patients. In the diagnosis process, these protocols may reveal visual deficits that cannot be identified via standard visual measurements. The protocols are efficient to define a major part of the currently unexplained visual complaints in patients following recovery of visual acuity. In the follow up course, the protocols can be used as a sensitive marker of demyelination and remyelination along time.
These tools may be used to evaluate the efficacy of currently developing neuroprotective and regenerative therapeutic strategies targeting myelination in the central nervous system23.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
This work was supported by the Caesarea Edmond & Benjamin de Rothschild Foundations.
References
- Rovaris M, et al. Axonal injury in early multiple sclerosis is irreversible and independent of the short-term disease evolution. Neurology. 65(10):1626–1630. doi: 10.1212/01.wnl.0000184493.06254.a6. [DOI] [PubMed] [Google Scholar]
- Tallantyre EC, et al. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult. Scler. 4(4):406–411. doi: 10.1177/1352458510364992. [DOI] [PubMed] [Google Scholar]
- Oberwahrenbrock T, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult. Scler. 2013;19(14):1887–1895. doi: 10.1177/1352458513489757. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Dalton CM, Miller DH, Plant GT. Management of acute optic neuritis. Lancet. 360(9349):1953–1962. doi: 10.1016/s0140-6736(02)11919-2. [DOI] [PubMed] [Google Scholar]
- Keough MB, Yong VW. Remyelination therapy for multiple sclerosis. Neurotherapeutics. 2013;10(1):44–54. doi: 10.1007/s13311-012-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday AM, McDonald WI, Mushin J. Delayed pattern-evoked responses in optic neuritis in relation to visual acuity. Trans. Ophthalmol. Soc. U.K. 93(0):315–324. [PubMed] [Google Scholar]
- Jones SJ, Brusa A. Neurophysiological evidence for long-term repair of ms lesions: Implications for axon protection. J. Neurol. 206(2):193–198. doi: 10.1016/s0022-510x(02)00428-8. [DOI] [PubMed] [Google Scholar]
- Raz N, et al. Sustained motion perception deficit following optic neuritis: Behavioral and cortical evidence. Neurology. 76(24):2103–2111. doi: 10.1212/WNL.0b013e31821f4602. [DOI] [PubMed] [Google Scholar]
- Raz N, Dotan S, Chokron S, Ben-Hur T, Levin N. Demyelination affects temporal aspects of perception: An optic neuritis study. Ann. Neurol. 2012;71(4):531–538. doi: 10.1002/ana.22692. [DOI] [PubMed] [Google Scholar]
- Raz N, Chokron S, Ben-Hur T, Levin N. Temporal reorganization to overcome monocular demyelination. Neurology. 2003;81(8):702–709. doi: 10.1212/WNL.0b013e3182a1aa3e. [DOI] [PubMed] [Google Scholar]
- Kniestedt C, Stamper RL. Visual acuity and its measurement. Ophthalmol. Clin. North. 16(2):155–170. doi: 10.1016/s0896-1549(03)00013-0. [DOI] [PubMed] [Google Scholar]
- Mangione CM, et al. Development of the 25-item national eye institute visual function questionnaire. Arch. Ophthalmol. 2001;119(7):1050–1058. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- Raphael BA, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the nei-vfq-25. Am. J. Ophthalmol. 142(6):1026–1035. doi: 10.1016/j.ajo.2006.06.060. [DOI] [PubMed] [Google Scholar]
- Halliday AM, McDonald WI, Mushin J. Delayed visual evoked response in optic neuritis. Lancet. 1(7758):982–985. doi: 10.1016/s0140-6736(72)91155-5. [DOI] [PubMed] [Google Scholar]
- Costello F, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann. Neurol. 59(6):963–969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- Albrecht P, et al. Degeneration of retinal layers in multiple sclerosis subtypes quantified by optical coherence tomography. Mult. Scler. 18(10):1422–1429. doi: 10.1177/1352458512439237. [DOI] [PubMed] [Google Scholar]
- Bock M, et al. Time domain and spectral domain optical coherence tomography in multiple sclerosis: A comparative cross-sectional study. Mult. Scler. 16(7):893–896. doi: 10.1177/1352458510365156. [DOI] [PubMed] [Google Scholar]
- Dorr J, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 6(4):18132–18. doi: 10.1371/journal.pone.0018132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult. Scler. 2013;19(4):443–450. doi: 10.1177/1352458512457844. [DOI] [PubMed] [Google Scholar]
- Saidha S, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult. Scler. 2011;17(12):1449–1463. doi: 10.1177/1352458511418630. [DOI] [PubMed] [Google Scholar]
- Costello F, et al. Tracking retinal nerve fiber layer loss after optic neuritis: A prospective study using optical coherence tomography. Mult. Scler. 2008;14(7):893–905. doi: 10.1177/1352458508091367. [DOI] [PubMed] [Google Scholar]
- Walter SD, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119(6):1250–1257. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taveggia C, Feltri ML, Wrabetz L. Signals to promote myelin formation and repair. Nat. Rev. Neurol. 6(5):276–287. doi: 10.1038/nrneurol.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


