Abstract
Background
Release for full activity and return to sport after anterior cruciate ligament reconstruction (ACLR) is often dictated by time from surgery and subjective opinion by the medical team. Temporal guidelines for return to sport may not accurately identify impaired strength and neuromuscular control, which are associated with increased risk for second injury (contralateral and/or ipsilateral limb) after ACLR in athletes.
Hypotheses
Athletes undergoing ACLR and returning to sport would demonstrate functional deficits that would not be associated with time from surgery.
Study Design
Controlled laboratory study.
Methods
Thirty-three male (n = 10) and female (n = 23) athletes with unilateral ACLR, who were cleared by a physician to return to their sport after surgery and rehabilitation, performed the single-legged vertical hop test for 10 seconds on a portable force plate. Matched teammates of each patient were recruited to serve as sex-, sport-, and age-matched controls (CTRL; n = 67). Maximum vertical ground-reaction force (VGRF) was measured during each single-limb landing. Single-limb symmetry index (LSI) was calculated as the ratio of the involved divided by uninvolved limb, expressed as a percentage.
Results
The single-limb vertical jump height LSI was reduced in the ACLR group, 89% (95% confidence interval [CI], 83%–95%), compared with the matched CTRL group, 101% (95% CI, 96%–105%; P<.01). The LSI for VGRF normalized to potential energy achieved during flight of the hop was increased in ACLR at 112% (95% CI, 106%–117%) relative to the CTRL group at 102% (95% CI, 98%–106%; P<.01). Linear regression analysis indicated that time from surgery was not associated with limb symmetry deficits in the ACLR group (P >.05; R2 = .002–.01).
Conclusion
Deficits in unilateral force development (vertical jump height) and absorption (normalized VGRF) persist in an athlete’s single-limb performance after ACLR and full return to sports. These symmetry deficits appear to be independent of time after reconstruction.
Clinical Relevance
On the basis of these results, clinicians should consider assessment of single-limb power performance in the decision-making process for return-to-sport release. Persistent side-to-side asymmetries may increase the risk of contralateral and/or ipsilateral injury.
Keywords: ACL reinjury, anterior cruciate ligament injury risk factors, targeted neuromuscular training, knee injury prevention, clinical assessment tools, sports reentry, knee rehabilitation, lower extremity biomechanics
The “release for full activity” or the determination of “return to sport” (RTS) is a potentially sensitive landmark for the athlete who has a strong desire to return to immediate high-level sports participation after anterior cruciate ligament reconstruction (ACLR). This is in part due to accelerated rehabilitation, decreased activity restrictions, or a combination of both from the treating clinicians. These factors, combined with the athlete’s increased confidence in his or her ability gained from improved function and a concomitant decrease in pain during and after sports-related activities, can strongly influence RTS decision making. The determination of accelerated RTS may be heightened with pressure from coaches, parents, and/or teammates to meet specific sport timelines. In addition, during the advanced phases of rehabilitation, there is often a gap between the athlete’s perceived versus actual sports readiness, as subjective scores do not always correlate with quantified function and strength scores in athletes with ACL injuries and reconstructions.23,24,31 Return to sport The American Journal of after ACLR unfortunately involves an elevated risk of second ACL injury.13,33 Specifically, incidence of graft failure or injury to the contralateral knee may exceed 20% in young athletes who return to competitive activities.29,32 However, athletes who demonstrate limb symmetry during high-level sports activities before sports reintegration after ACL reconstruction may significantly reduce their potential for future ACL injury.16,28,29,34
A recent systematic review was employed to ascertain the factors used to determine return to unrestricted sports activities after ACLR.2 Of the 264 studies that met the inclusion for this investigation, 60% indicated the amount of time postoperatively as at least a part of the patient’s criteria for return to sports activities, and one-third of the investigations reported time from surgery as the only determinant for RTS decision making. Forty of the 264 investigations employed postoperative time combined with subjective criteria for RTS decision making. Unfortunately, only 13% of the studies evaluated indicated objective criteria as a required marker for RTS.2 Although the literature provides strong support for the use of functional performance to guide decision making, release to full activity and RTS are often influenced by temporal guidelines based on time from surgery and medical team opinion.2,17 These factors can be strongly influenced by the athlete’s confidence level or desire to reintegrate back into sport. Without the use of objective measures that identify potential deficits, it may be difficult for clinicians to justify sport restriction and the associated limitations or address any lasting impairments related to the initial ACL injury or reconstruction. Residual strength and proprioceptive deficits may limit an athlete’s ability to reacquire sports skills and may also subsequently increase his or her risk of reinjury.22,27–29 Specific progressive guidelines, based on objective measures, can provide a goal-oriented rehabilitation process that may be an appealing approach for athletes.5,17,20,21
Isolated time-driven guidelines in the late phases of training before RTS, particularly in cases when athletes progress to unrestricted activity, are counterintuitive as this is the time frame when athletes begin to expose the lower extremity to forces and motions that can highly load the knee and reconstructed graft.4,9,16,19,25 Although strength recovery is highly variable in athletes between 3 and 11 months after ACLR,35 significant deficits often remain in muscle strength, motor coordination, and proprioception that are independent of surgical method at 1-year follow-up.39 Often, athletes released for unrestricted activity may be prepared to begin more functional training to better prepare for sport reintegration. However, they may demonstrate deficits that limit their potential for safe integration into full competitive sports.28,29,39,40 Residual biomechanical and neuromuscular deficits can increase reinjury risk during early sports reintegration.7,16,26,28
Regardless of the graft type chosen, there is a broad range (12 weeks to 12 months) of temporal criteria used to determine safe return to unrestricted activity.2 Late-phase rehabilitation and RTS training that is organized to meet predetermined objective guidelines (functional performance, strength, flexibility, postural stability, and fear of reinjury measures), combined with time-driven guidelines based on healing and maturation of tissue, may help to systematically transition the athlete with a reconstructed ACL through RTS training in a safe and efficacious manner.2,17,20,21,29 Efforts to employ combined functional criteria landmarks, as opposed to isolated temporal-driven landmarks, may help athletes to develop bilateral symmetry and a dynamically functional lower extremity that is prepared to safely respond to the high joint forces and torques generated during sports.2,17 The purpose of this study was to evaluate the association of time from surgery with performance on high-level coordinative power measures in athletes following ACLR and RTS activities. We hypothesized that athletes after ACLR and RTS would demonstrate measurable involved limb performance and force absorption deficits during a single-legged vertical power test that would be independent of time from surgery.
METHODS
Participant Characteristics
Informed written consent was obtained from all participants (legal guardian if younger than 18 years) and approved by the institutional review board. A total of 33 athletes after ACLR agreed to participate in this study. There were 10 male patients and 23 female patients. Ages ranged from 15.4 to 19.4 years (average, 17.4 years). The participants’ height ranged from 161.5 to 180.1 cm, and mass ranged from 56.2 to 93 kg. They were a mean of 9.7 months from surgery (range, 8.2–11.3 months). Every participant had returned to his or her respective sport (basketball, volleyball, football, soccer, softball, track and field) before testing. Each participant brought 1 to 3 teammates to serve as sex-, sport-, and age-matched controls (CTRL; n = 67). A questionnaire was used to determine history of knee injury and was corroborated with a personal interview with the investigator. Concomitant injuries (eg, meniscal, other ligamentous, chondral) and specifics regarding surgical procedure (eg, graft type) were documented from the interview but were not further analyzed in this study.
Anterior-posterior tibiofemoral translation was quantified using the CompuKT knee arthrometer (Medmetric Corp, San Diego, California) to measure total anteriorposterior displacement of the tibia relative to the secured femur. During the measurement, each leg was placed on the adjustable thigh support with the knee stabilized at 20° to 35° of knee flexion. The arthrometer was secured to the shank such that the patellar sensor pad was rested on the patella with the knee joint line reference mark on the CompuKT aligned with the participant’s joint line. The ankle and foot were stabilized to limit leg rotation. The tester provided posterior and anterior (±134 N) pressure on an axis perpendicular to the tibia. Total displacement (in millimeters) was plotted on the computer and recorded.
Data Collection
The investigators determined leg dominance by asking participants which leg they would use to kick a ball as far as possible.10,11 For the vertical single-limb hop (VSH) test, each athlete was instructed to jump as high as he or she could on one leg, land under control, recover his or her balance, and repeat vertical hopping for 10 seconds. The within-session intraclass reliability of single-leg force production and force attenuation measures using the described testing methods have demonstrated high reliability (≥0.97).14 A counterbalanced testing order was used to eliminate any potential order or learning effect, and each athlete was given 3 practice trials before data acquisition. One successful test trial was recorded for the matched nondominant/injured and dominant/noninjured lower extremity limbs. The test trial was repeated if the athlete placed the contralateral foot onto the force plate and/or landed outside the dimensions of the force plate. If a task was not performed according to instructions or data were unable to be recorded, the participant immediately stopped and rested. The participants in both groups were athletes involved in running and cutting sports with previous experience performing activities similar to the demands of the tasks used for testing. Accordingly, proper test performance was most often obtained after only 1 practice trial by each participant. After each successful test, participants were given a minimum of 2 minutes of rest and were encouraged to wait until they achieved full recovery before testing the opposite limb (Figure 1). During the VSH test, vertical ground-reaction force was measured using a portable force plate (Accupower; AMTI, Watertown, Massachusetts) with dimensions of 76 × 102 × 12 cm (length × width × height). The measurements were sampled at 400 Hz as described by previous investigators.14
Figure 1.
Participant performing vertical single-legged hop test.
Data Management
Raw vertical ground-reaction force data were filtered with a generalized cross-validation spline using a 50-Hz cutoff frequency. Peak vertical ground-reaction force (VGRF) during the landing phase (first 250 ms) was calculated for each jump. Vertical jump height (= ½g(t/2)2, where g = 9.81 m/s2 and t = time in seconds in the air) and potential energy were also calculated from the flight phase before each landing. Potential energy was operationally defined as energy derived from the product of mass of the participant (in kilograms), gravitational acceleration of the Earth (9.81 m/s2), and single-legged jump height (in meters).36 Normalized VGRF was expressed as maximum VGRF divided by potential energy, and normalized force-loading rate was expressed as normalized VGRF (in Newton/joules [N/J]) divided by the time to peak landing force(s). Although no clear gold standard for normalization of maximum vertical ground-reaction force currently exists, previous investigations assessing maximum vertical ground-reaction force of participants during landings have generally used subject mass in normalization procedures.6,8,15,18,30,41 Because of the potential for limb-to-limb differences in vertical jump height and variability of jump height between repetitions, the current investigation selected potential energy as the preferred normalization factor for limb symmetry calculations. For each measurement, a limb symmetry index (LSI) was calculated as the ratio of the involved or nondominant limb divided by the uninvolved or dominant limb for ACLR and CTRL patients, respectively. The LSI is a percentage, with 100% being complete symmetry between the 2 limbs.
Statistics
A mixed-model repeated-measure analysis of variance (side × group) was used to determine significant interactions of involved limb deficits. A 1-way between-groups multivariate analysis of variance was employed to investigate differences between groups (ACLR vs CTRL) in LSI measures for vertical jump height, landing VGRF, and normalized VGRF. Effect size was calculated for each measurement in addition to P values with a priori α established at .05. Linear regression analyses were used to evaluate the association of time from surgery to identified performance deficits during the VSH test.
RESULTS
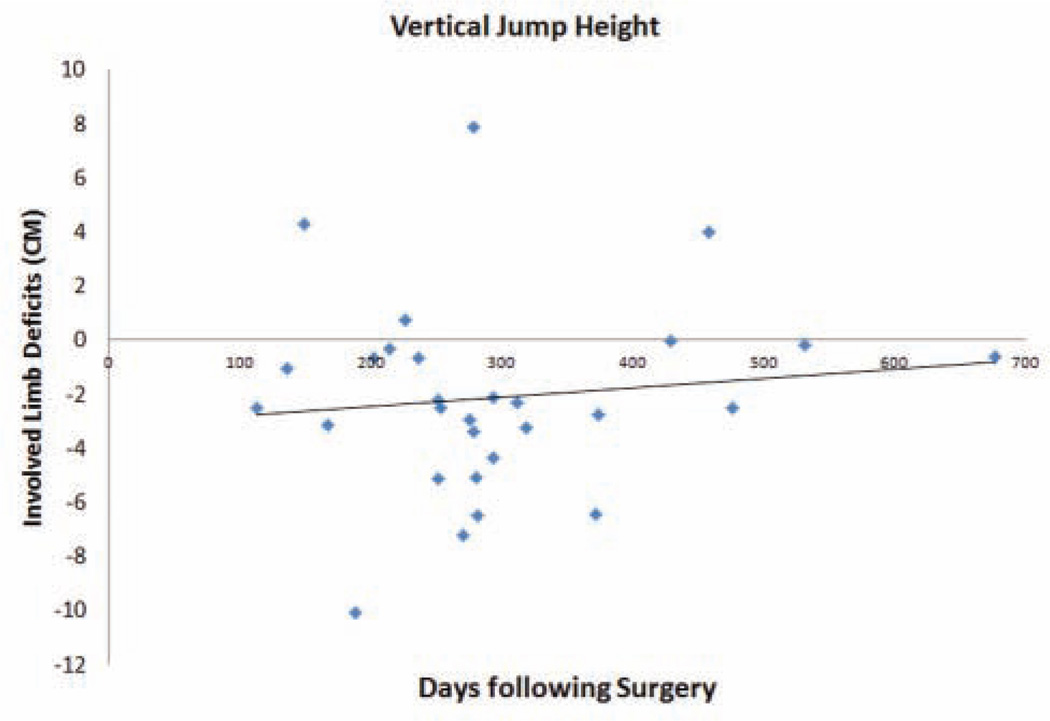
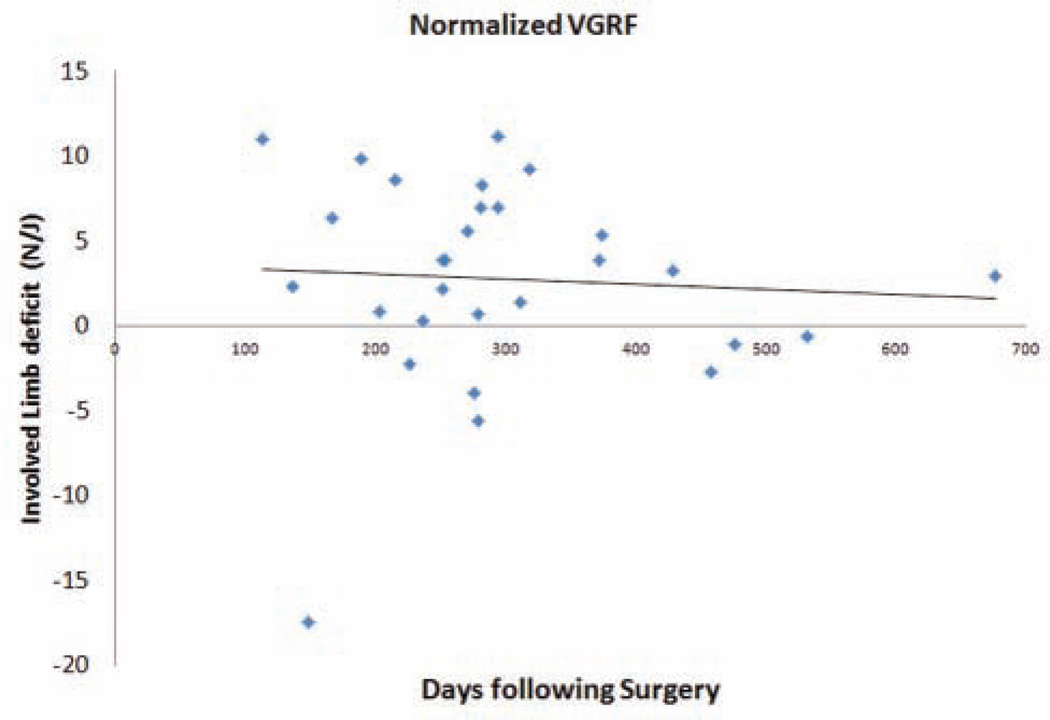
In the ACLR cohort, each participant had returned to his or her respective sport before testing with a mean of 9.7 months from surgery (95% confidence interval [CI], 8.2–11.3 months) and at the time of testing reported functional stability without giving-way episodes. For the ACLR group, the mean side-to-side difference in anterior knee laxity was 3.7 ± 2.4 mm measured at 134 N and 2.6 ± 2.0 mm measured at 89 N of force. Importantly, linear regression analysis indicated that time from surgery was not a significant determinant of absolute limb asymmetry differences (P >.05; R2 = .002–.01). Figures 2 and 3 present the lack of association of deficits in the ACLR group’s involved limb to generate force (vertical jump height; Figure 2) and absorb forces (normalized VGRF; Figure 3) relative to the time from surgical reconstruction.
Figure 2.
Association of limb-to-limb (uninvolved – involved) deficits for vertical jump height with time from surgery. CM, centimeter.
Figure 3.
Association of limb-to-limb (uninvolved – involved) deficits for normalized vertical ground-reaction force (VGRF) during single-legged landing with time from surgery.
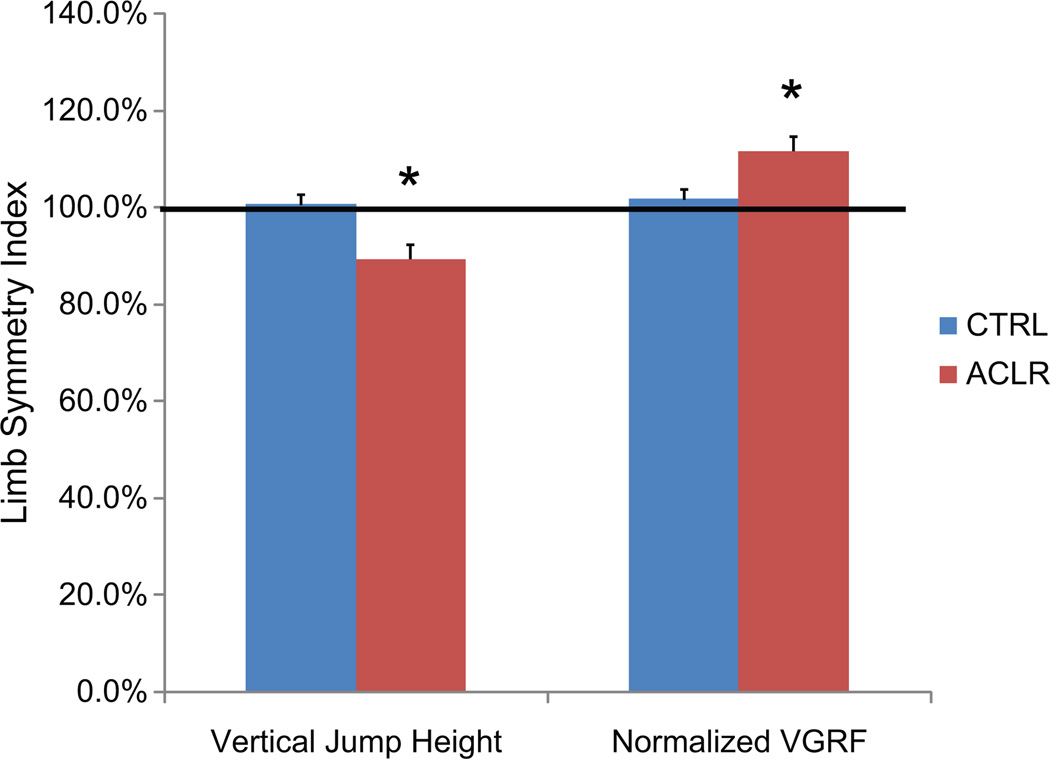
Group comparisons for the VSH test results demonstrated significant interactions for deficits in force generation (vertical jump) and normalized force absorption in the involved limb of the ACLR group (P<.05). This was demonstrated by a significant difference in LSI compared with CTRL for all measurements. The single-limb vertical jump height LSI was lower in the ACLR group (89%; 95% CI, 83%–95%) in comparison with the CTRL group (101%; 95% CI, 96%–105%; P<.01, large effect size). Peak vertical ground-reaction force LSI during landing in the ACLR group was 95% (95% CI, 90%–100%), which was marginally reduced compared with the CTRL group (102%; 95% CI, 98%–106%; P < .05 and a moderate effect). Interestingly, the LSI for VGRF when normalized to potential energy was also significantly different between groups, but with the ACLR group demonstrating increased relative load in the involved limb with an LSI of 112% (95% CI, 106%–117%) compared with CTRL, which had 102% (95% CI, 98%–106%) and a small to moderate effect size for group differences. Figure 4 provides a pictorial representation of the reduced ability of the participants in the ACLR group’s involved limb to generate force (vertical jump height) in combination with a reduced ability to absorb forces (normalized VGRF) relative to the uninvolved limb.
Figure 4.
Mean and standard error of the mean for limb symmetry index (in percentages) for the anterior cruciate ligament reconstruction (ACLR) group compared with the control (CTRL) group for vertical jump height and normalized vertical ground-reaction force (VGRF) during single-legged landing.
DISCUSSION
Large-scale epidemiological analyses indicate that 2 years after ACLR, there is only a 3% risk of ipsilateral reinjury and a 3% risk of contralateral ACL injury when new surgery is used as the primary outcome variable.37 At 5 years’ follow-up after ACLR, the contralateral knee (11.8%) has double the risk of injury compared with ACL graft rupture in the ipsilateral knee (5.8%), whereas the relative risk of reinjury is still relatively low over the extended time period in older and less active populations relative to the current cohort.38 Of importance, epidemiological data from highly active cohorts who actually return to sports activities exceed 20% second injury rates in just the first year after RTS.28,32 Based on this large discrepancy for reinjury between young athletic populations and older general populations after ACLR, it is critical to develop specific, objective evidence-based guidelines and population-specific risk analyses to drive rehabilitation and RTS decision making for highly active populations. The purpose of this study was to evaluate the association of time from surgery with high-level functional coordinative power (function that requires multijoint coordination of strength, balance, and proprioception to optimize power output) measures specific to young athletes after ACLR and RTS activities. We hypothesized that athletes after ACLR and RTS would demonstrate measurable involved limb performance and force absorption deficits during a single-legged vertical power test that would be independent of time from surgery.
The current findings revealed that there was significant asymmetry between limbs of the ACLR group, as shown by their combined reductions in force generation (evidenced by vertical jump height) and force absorption (evidenced by normalized landing VGRF). In addition, the reported asymmetry values were significantly different from those of the CTRL group. The current results indicate that up to 11 months after surgery and after release to sport, there are still significant deficits between the reconstructed limb and noninjured limb, as well as significant limb asymmetry compared with noninjured matched controls. Importantly, these deficits showed no association to the time from surgery in young athletes.
Too often, release for full activity and RTS after ACLR is based on time from surgery and subjective clinical impression.2 This time frame may be accelerated by an athlete with a strong desire to reintegrate back into sport. Objective measures that identify deficits during high-level coordinative tasks may aid clinicians in their justification for sport restrictions and the associated limitations even after the athlete has reached or passed a time-driven landmark. In addition, the residual power generation reductions (jump height) in combination with force absorption deficits (normalized GRF) identified in the current study may help direct end-stage rehabilitation to address any specific force generation or force absorption impairments associated with the initial ACL injury or reconstruction. Quantitative guidelines specific to sports-related power can provide a goal-oriented rehabilitation process that may be an appealing approach for athletes as opposed to “waiting” for the time frame they are allowed to return to sport.5,21 Targeted rehabilitation can also alleviate residual strength and proprioceptive deficits that would limit an athlete’s ability to reacquire sports skills and may ultimately reduce his or her risk of reinjury.20,29
The goal of the current study was 2-fold. First, we aimed to determine if functional deficits existed during a demanding motor coordination and power development task in young athletes. If these deficits were observed, we intended to determine if there was an association of objectively measurable deficit in athletes who have undergone ACLR to the time from surgery. We employed the VSH test to quantify potential deficits as prior reports indicated that single-limb hopping tasks were the most sensitive deficit measures in athletes at the time of RTS.22 A prior study employed a preexhaustion protocol to evaluate single-legged hop symmetry values to determine functional deficits in patients after ACLR. Similar to the current results, functional asymmetries were reported in athletes up to 11 months after surgery.1 When comparing healthy and ACL-injured individuals, as well as patients with reconstructed knees, with 5 different hop tests for asymmetry, only 10% of patients had restored hop performance ability 11 months after the initial ACL injury and 6 months after ACL reconstruction.12
Although strength recovery is highly variable in adolescent athletes between 3 and 11 months after ACLR,35 the current results indicate that significant deficits remain present in muscular power and motor coordination, independent of time from surgery in our highly active population. Muscular strength and basic functional performance deficits can show significant improvement during the RTS phase of rehabilitation. However, in higher level functional tasks that require more coordination and neuromuscular control, similar adaptations are not consistently identified during this same period.40 Thus, assessment criteria focused on isolated strength measures that do not include motor coordination and power development may be inadequate to identify persistent deficits that may increase reinjury risk in patients who wish to return to competitive sport.40 Objective assessment of aberrant neuromuscular strategies (asymmetry in measures of functional performance, strength, flexibility, and/or postural stability) that potentially lead to abnormal loading of the lower extremity, especially during high-risk single-limb maneuvers such as those used in the current investigation, could establish a foundation for efficacious RTS rehabilitation programs for young athletes.
The height jumped during the single-legged hop is indicative of the athlete’s ability to generate push-off force. The current investigational sample demonstrated a large reduction in jump height for the involved side. As expected with the reduced height of the jump, the ACLR patients also showed reduced landing forces (raw VGRF). Interestingly, when the landing VGRF were normalized to potential energy achieved with each limb, the ACLR showed increased relative landing force in the involved limb, which may indicate a mechanism in which the deficits after ACLR may further exacerbate the reinjury risk. This combination of deficits evidenced during jump performance and landing coordination may indicate that the ACLR limb is insufficiently prepared to generate and handle forces needed to protect the passive knee structures during sport. These structures, possibly including the newly reconstructed ACL, which is structurally weakened relative to the native tissue, may be unable to withstand the potential for increased relative load, thus putting athletes after ACLR at increased risk for reinjury.3,29 Thus, late-phase rehabilitation and RTS training may be best when organized to meet predetermined objective guidelines, such as symmetry measures of functional performance, strength, flexibility, and postural stability, combined with time-driven guidelines based on healing and maturation of tissue, which may ultimately help provide the best approach to safely transition the athlete through RTS phases.2,17,21
Prior investigations have used high-tech biomechanical laboratories to identify proprioceptive and functional deficits that place patients at risk for initial ACL injury and that are likely persistent after ACL reconstruction, increasing the athlete’s risk for subsequent injury.16,29 The present study took advantage of a portable force plate to establish deficits during a high-level task. The portability of these measures may increase the clinical utility of late-phase rehabilitation guidelines driven to prevent risk of secondary ACL injury. However, future work is needed to establish this linkage between the current measures and sport-specific risk of secondary injury.29 In addition, although there are prior reports of unilateral deficits related to second injury risk in individuals after ACL reconstruction and RTS, they may not be as easily evident during bipedal performance or during modified versions of double-limb performance activities. Isolation of the involved limb with unilateral hopping tasks has been suggested to identify performance deficits in running and cutting sport athletes.22 Although correction of side-to-side asymmetry is achieved with integrated neuromuscular training in healthy populations, future work is needed to determine the potential to modify residual force production and absorption asymmetries in patients after ACLR using similar targeted neuromuscular training programs.
Limitations
There are potential limitations to the current investigation to consider when applying these results in a clinical setting. First, maximum effort could not be verified during VSH. This limitation was partially minimized by continuous encouragement provided to the participants during testing and further minimized by reduction and analysis of force peaks that achieved at least 70% of the maximum VGRF in the 10-second trial. In addition, participants performed VSH in variable athletic footwear. Shoe type could potentially have an effect on neuromuscular control during the landing phase of VSH, which has been designated as a critical period when noncontact knee injuries are considered to occur.10 Although shoe types varied among participants, they all consisted of rubber outsoles manufactured for athletic performance. Finally, this study focuses on young, highly active athletes; practitioners should be conservative if they choose to generalize the current results to patient populations who are not young athletes. However, we included all young athletes from a variety of surgeons using various surgical reconstruction techniques and rehabilitation protocols that aid in the generalizability of the current results to similar populations. In addition, previous reports indicate that muscle strength, motor coordination, and proprioception recovery are often independent of surgical method at 1-year follow-up, which also aid in the generalizability of the current results.39 The authors also acknowledge the potential for type II error due to the small sample size, but given the very low, strongest association between day from surgery and vertical jump height (correlation of 0.01), which would require more than 600 participants to be statistically significant, it is very unlikely that the reported lack of association from time from surgery is affected by β error. In addition, the range of days since surgery is 100 to 700, which may further diminish concerns for errors in the reported lack of association between time from surgery and functional deficits.
CONCLUSION
The results of the current investigation indicate that young athletes assessed after medical release and RTS activity demonstrate measurable functional deficits after ACLR that are independent of time from surgery. This finding is important as the exclusive use of temporal guidelines by clinicians may be an inaccurate scale to measure readiness to safely return to sport when in fact neuromuscular deficits with high-risk maneuvers persist. Our study emphasizes the need to use objective tools that are sensitive to limb-to-limb deficits and to develop rehabilitation protocols that are targeted to eliminate limb asymmetries. The current results indicate that objective methods can be used to quantify the resolution of these deficits. Late-phase rehabilitation and RTS training that is organized to meet predetermined objective guidelines, combined with time-driven guidelines based on healing and maturation of tissue, may help to systematically transition the athlete with a reconstructed ACL through RTS. This approach may help an athlete develop bilateral symmetry and a dynamically functional lower extremity that is prepared to safely respond to the extreme forces generated and absorbed during sports. In addition, an objective late-phase rehabilitation program that is focused on symmetry restoration may reduce the athlete’s risk of reinjury and optimally prepare him or her to meet, and potentially exceed, preinjury performance levels.
ACKNOWLEDGMENT
The authors acknowledge the Sports Medicine Biodynamics Team, who worked together to make this large data collection session possible. The authors also thank St Xavier High School, including Wellington Orthopaedics (Richelle Gwin, John Brehm, and Michael Gordon), for their invaluable support to complete this project.
source of funding: The authors acknowledge funding support from NFL Charities. The authors also acknowledge funding support from National Institutes of Health/NIAMS grants R01-AR049735, R01-AR05563, and R01-AR056259.
Footnotes
One or more of the authors has declared the following potential conflict of interest
REFERENCES
- 1.Augustsson J, Thomee R, Karlsson J. Ability of a new hop test to determine functional deficits after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004;12(5):350–356. doi: 10.1007/s00167-004-0518-4. [DOI] [PubMed] [Google Scholar]
- 2.Barber-Westin SD, Noyes FR. Factors used to determine return to unrestricted sports activities after anterior cruciate ligament reconstruction. Arthroscopy. 2011;27(12):1697–1705. doi: 10.1016/j.arthro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31(6):519–525. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 4.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 5.Cascio BM, Culp L, Cosgarea AJ. Return to play after anterior cruciate ligament reconstruction. Clin Sports Med. 2004;23(3):395–408. doi: 10.1016/j.csm.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Coventry E, O’Connor KM, Hart BA, Earl JE, Ebersole KT. The effect of lower extremity fatigue on shock attenuation during single-leg landing. Clin Biomech (Bristol, Avon) 2006;21(10):1090–1097. doi: 10.1016/j.clinbiomech.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Decker MJ, Torry MR, Noonan TJ, Riviere A, Sterett WI. Landing adaptations after ACL reconstruction. Med Sci Sports Exerc. 2002;34(9):1408–1413. doi: 10.1097/00005768-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Decker MJ, Torry MR, Wyland DJ, Sterett WI, Richard Steadman J. Gender differences in lower extremity kinematics, kinetics and energy absorption during landing. Clin Biomech (Bristol, Avon) 2003;18(7):662–669. doi: 10.1016/s0268-0033(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 9.DeMorat G, Weinhold P, Blackburn T, Chudik S, Garrett W. Aggressive quadriceps loading can induce noncontact anterior cruciate ligament injury. Am J Sports Med. 2004;32(2):477–483. doi: 10.1177/0363546503258928. [DOI] [PubMed] [Google Scholar]
- 10.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 11.Ford KR, Myer GD, Smith RL, Vianello RM, Seiwert SL, Hewett TE. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin Biomech (Bristol, Avon) 2006;21(1):33–40. doi: 10.1016/j.clinbiomech.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Gustavsson A, Neeter C, Thomee P, et al. A test battery for evaluating hop performance in patients with an ACL injury and patients who have undergone ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):778–788. doi: 10.1007/s00167-006-0045-6. [DOI] [PubMed] [Google Scholar]
- 13.Harner CD, Paulos LE, Greenwald AE, Rosenberg TD, Cooley VC. Detailed analysis of patients with bilateral anterior cruciate ligament injuries. Am J Sports Med. 1994;22(1):37–43. doi: 10.1177/036354659402200107. [DOI] [PubMed] [Google Scholar]
- 14.Harrison AD, Ford KR, Myer GD, Hewett TE. Sex differences in force attenuation: a clinical assessment of single-leg hop performance on a portable force plate. Br J Sports Med. 2011;45(3):198–202. doi: 10.1136/bjsm.2009.061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hass CJ, Schick EA, Chow JW, Tillman MD, Brunt D, Cauraugh JH. Lower extremity biomechanics differ in prepubescent and postpubescent female athletes during stride jump landings. J Appl Biomech. 2003;19:139–152. [Google Scholar]
- 16.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 17.Kvist J. Rehabilitation following anterior cruciate ligament injury: current recommendations for sports participation. Sports Med. 2004;34(4):269–280. doi: 10.2165/00007256-200434040-00006. [DOI] [PubMed] [Google Scholar]
- 18.Madigan ML, Pidcoe PE. Changes in landing biomechanics during a fatiguing landing activity. J Electromyogr Kinesiol. 2003;13(5):491–498. doi: 10.1016/s1050-6411(03)00037-3. [DOI] [PubMed] [Google Scholar]
- 19.Markolf KL, Burchfield DM, Shapiro MM, Shepard MF, Finerman GA, Slauterbeck JL. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13(6):930–935. doi: 10.1002/jor.1100130618. [DOI] [PubMed] [Google Scholar]
- 20.Myer GD, Paterno MV, Ford KR, Hewett TE. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res. 2008;22(3):987–1014. doi: 10.1519/JSC.0b013e31816a86cd. [DOI] [PubMed] [Google Scholar]
- 21.Myer GD, Paterno MV, Ford KR, Quatman CE, Hewett TE. Rehabilitation after anterior cruciate ligament reconstruction: criteria based progression through the return to sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- 22.Myer GD, Schmitt LC, Brent JL, et al. Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011;41:377–387. doi: 10.2519/jospt.2011.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neeb TB, Aufdemkampe G, Wagener JH, Mastenbroek L. Assessing anterior cruciate ligament injuries: the association and differential value of questionnaires, clinical tests, and functional tests. J Orthop Sports Phys Ther. 1997;26(6):324–331. doi: 10.2519/jospt.1997.26.6.324. [DOI] [PubMed] [Google Scholar]
- 24.Noyes FR, McGinniss GH. Controversy about treatment of the knee with anterior cruciate laxity. Clin Orthop Relat Res. 1985;198:61–76. [PubMed] [Google Scholar]
- 25.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 26.Orchard J, Seward H, McGivern J, Hood S. Intrinsic and extrinsic risk factors for anterior cruciate ligament injury in Australian footballers. Am J Sports Med. 2001;29(2):196–200. doi: 10.1177/03635465010290021301. [DOI] [PubMed] [Google Scholar]
- 27.Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17(4):258–262. doi: 10.1097/JSM.0b013e31804c77ea. [DOI] [PubMed] [Google Scholar]
- 28.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Hewett TE. Effects of sex on compensatory landing strategies upon return to sport after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2011;41(8):553–559. doi: 10.2519/jospt.2011.3591. [DOI] [PubMed] [Google Scholar]
- 29.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittenger VM, McCaw ST, Thomas DQ. Vertical ground reaction forces of children during one- and two-leg rope jumping. Res Q Exerc Sport. 2002;73(4):445–449. doi: 10.1080/02701367.2002.10609044. [DOI] [PubMed] [Google Scholar]
- 31.Ross MD, Irrgang JJ, Denegar CR, McCloy CM, Unangst ET. The relationship between participation restrictions and selected clinical measures following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2002;10(1):10–19. doi: 10.1007/s001670100238. [DOI] [PubMed] [Google Scholar]
- 32.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37:246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 33.Shelbourne KD, Klootwyk TE, Wilckens JH, De Carlo MS. Ligament stability two to six years after anterior cruciate ligament reconstruction with autogenous patellar tendon graft and participation in accelerated rehabilitation program. Am J Sports Med. 1995;23(5):575–579. doi: 10.1177/036354659502300510. [DOI] [PubMed] [Google Scholar]
- 34.Shelbourne KD, Klotz C. What I have learned about the ACL: utilizing a progressive rehabilitation scheme to achieve total knee symmetry after anterior cruciate ligament reconstruction. J Orthop Sci. 2006;11(3):318–325. doi: 10.1007/s00776-006-1007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells L, Dyke JA, Albaugh J, Ganley T. Adolescent anterior cruciate ligament reconstruction: a retrospective analysis of quadriceps strength recovery and return to full activity after surgery. J Pediatr Orthop. 2009;29(5):486–489. doi: 10.1097/BPO.0b013e3181aa2197. [DOI] [PubMed] [Google Scholar]
- 36.Wikstrom EA, Tillman MD, Kline KJ, Borsa PA. Gender and limb differences in dynamic postural stability during landing. Clin J Sport Med. 2006;16(4):311–315. doi: 10.1097/00042752-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Wright RW, Dunn WR, Amendola A, et al. Risk of tearing the intact anterior cruciate ligament in the contralateral knee and rupturing the anterior cruciate ligament graft during the first 2 years after anterior cruciate ligament reconstruction: a prospective MOON cohort study. Am J Sports Med. 2007;35(7):1131–1134. doi: 10.1177/0363546507301318. [DOI] [PubMed] [Google Scholar]
- 38.Wright RW, Magnussen RA, Dunn WR, Spindler KP. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am. 2011;93(12):1159–1165. doi: 10.2106/JBJS.J.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yosmaoglua HB, Baltaci G, Kaya D, Ozer H, Atay A. Comparison of functional outcomes of two anterior cruciate ligament reconstruction methods with hamstring tendon graft. Acta Orthop Traumatol Turc. 2011;45(4):240–247. doi: 10.3944/AOTT.2011.2402. [DOI] [PubMed] [Google Scholar]
- 40.Yosmaoglu HB, Baltaci G, Kaya D, Ozer H. Tracking ability, motor coordination, and functional determinants after anterior cruciate ligament reconstruction. J Sport Rehabil. 2011;20(2):207–218. doi: 10.1123/jsr.20.2.207. [DOI] [PubMed] [Google Scholar]
- 41.Zheng N, Barrentine SW. Biomechanics and motion analysis applied to sports. Phys Med Rehabil Clin North Am. 2000;11(2):309–322. [PubMed] [Google Scholar]