Summary
Across the nervous system, neurons form highly stereotypic patterns of synaptic connections that are designed to serve specific functions. Mature wiring patterns are often attained upon the refinement of early, less precise connectivity. Much work has led to the prevailing view that many developing circuits are sculpted by activity-dependent competition amongst converging afferents, which results in the elimination of unwanted synapses and the maintenance and strengthening of desired connections. Studies of the vertebrate retina, however, have recently revealed that activity can play a role in shaping developing circuits without engaging competition amongst converging inputs that differ in their activity levels. Such neurotransmission-mediated processes can produce stereotypic wiring patterns by promoting selective synapse formation rather than elimination. We discuss how the influence of transmission may also be limited by circuit design, and further highlight the importance of transmission beyond development in maintaining wiring specificity and synaptic organization of neural circuits.
Introduction
The nervous system comprises a rich diversity of circuit designs, yet the structural and functional organizations unique to each circuit are reliably preserved across animals within a species. Not surprisingly, such consistency in circuit arrangements has focused the attention on elucidating the developmental mechanisms that give rise to the immense variety of stereotyped connectivity patterns. Studies across different regions of the nervous system and across species have collectively led to the textbook view that molecular guidance cues limit the matching of pre- and postsynaptic cells, and activity-driven competitive processes refine the initial patterns of connectivity (Fox and Wong, 2005; Margeta and Shen, 2010; Sanes and Yamagata, 2009; Shen and Scheiffele, 2010; Wong and Ghosh, 2002). Here, we will add to and deviate from this established view by highlighting recent studies in the vertebrate retina that have revealed both conventional and unconventional roles for neurotransmission in circuit assembly. We will also discuss how the orderly wiring patterns of the retina (Hoon et al., 2014) facilitate exploration of the roles of neurotransmission in circuit maintenance during normal development and in disease conditions, which would be helpful for designing strategies aimed at retinal circuit repair.
Building basic circuit architectures during development
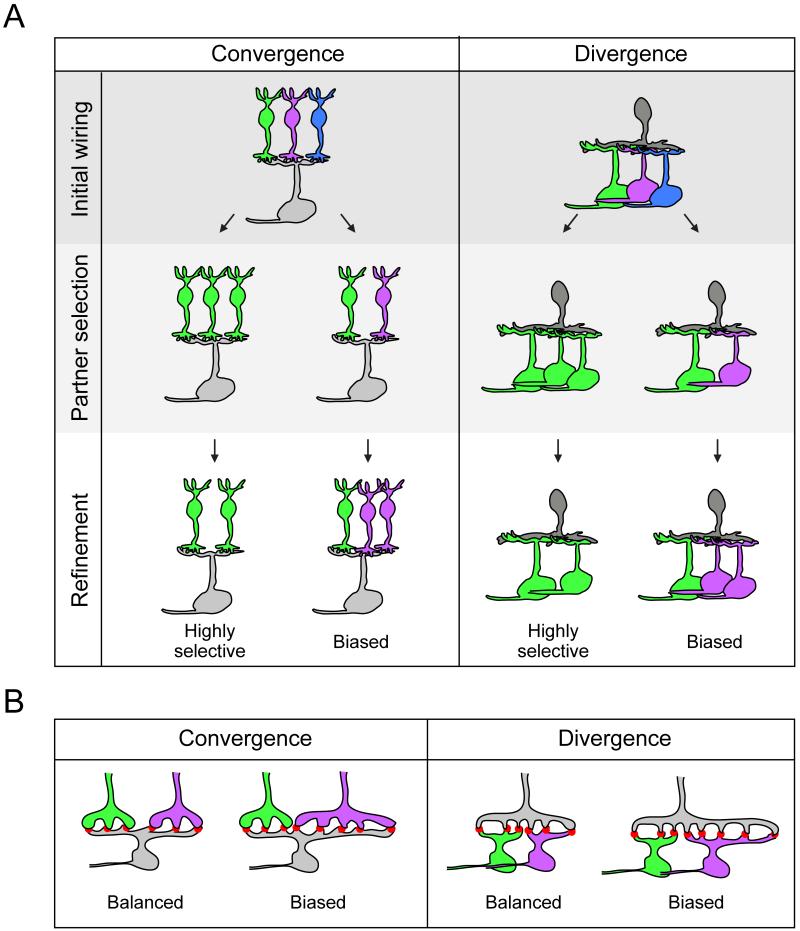
Before discussing the role of neurotransmission in sculpting circuits, it is instructive to present some common circuit designs, and outline the developmental steps that could lead to these basic configurations. Fundamentally, each circuit is made up of a combination of converging and diverging synaptic connections (Figure 1). In many circuits, the postsynaptic cell is contacted by many more presynaptic cell types during development than at maturity. The process of synapse elimination removes erroneous connections. Elimination can result in some postsynaptic cells becoming highly selective, e.g. innervated by only one functional input type in the adult. For example, in the visual system, excitatory neurons in the dorsal lateral geniculate nucleus (dLGN) are initially contacted by retinal ganglion cell axons from both eyes, but are monocularly innervated at maturity (Campbell and Shatz, 1992; Jaubert-Miazza et al., 2005; Shatz and Kirkwood, 1984; Ziburkus and Guido, 2006). Although not always (Seabrook et al., 2013), a further step in circuit refinement can occur even after the input partner type is selected. This is evident for projection neurons in the dLGN in which the number of connected presynaptic cells decreases after eye-specific inputs have been established (Chen and Regehr, 2000; Jaubert-Miazza et al., 2005; Ziburkus and Guido, 2006). In some cases, the pruning process can be dramatic, as seen for cerebellar Purkinje cells (Crepel et al., 1981; Hashimoto et al., 2009b; Mariani and Changeux, 1981), which eliminate all but one climbing fiber from an initial convergence of around half a dozen afferents.
Figure 1. Developmental sequences producing stereotypic neuronal connectivity patterns.
A. Postsynaptic cells receive a diversity of afferent inputs (convergence), and often, presynaptic cells contact multiple postsynaptic targets (divergence). Adult patterns of synaptic convergence and divergence may be derived from initially imprecise sets of connections that undergo refinement upon maturation. A, B. Biased connectivity can arise from differences in the number of connected presynaptic cells (A, shown here for contacts with two purple cells compared to contact with a single green cell), from differences in the number of synapses (red puncta) made by each axon type (B), or from differences in synaptic strength (not shown). Additionally, different input types could utilize disparate receptor types to achieve differential connectivity.
More commonly observed, however, are circuits in which the postsynaptic cell remains connected to more than one type of excitatory presynaptic cell. This is apparent for the AON type ganglion cell in the mouse retina that maintains glutamatergic input from at least two types of cone bipolar cells, but eliminates early contacts with rod bipolar cells (Morgan et al., 2011). Connections with different afferent types may also be stereotypic whereby the postsynaptic cell makes a specific number of connections with each input type. For instance, Type 6 cone bipolar cells provide the majority of the synapses onto the AON type retinal ganglion cell whereas Type 7 cone bipolar cells form the minority (Schwartz et al., 2012). Such biased wiring could be attained after a period of increasing connections with all partner types followed subsequently by selective pruning of connections with one type. Alternatively, the postsynaptic cell could specifically increase synaptogenesis with the major input type. These processes, however, need not be mutually exclusive. How circuits establish stereotypic wiring patterns is not yet well understood but recent studies have uncovered some developmental mechanisms that can generate these stereotypic patterns.
In contrast to studies on synaptic convergence, there are few studies that have followed how synaptic divergence in the circuit is established. It is clear that some long-range axonal projections transiently contact multiple targets during early development, but later retract innervation from some areas. For example, neurons in the occipital cortex initially send axon collaterals into the pyramidal tract which are later removed (Stanfield et al., 1982). Likewise, early in development, axons of olfactory receptor neurons project to more than one glomerulus on each side of the olfactory bulb, but restrict their terminals to a single glomerulus at maturity (Zou et al., 2004). However, even after an individual afferent has matched with the appropriate postsynaptic partner type, there can be further synaptic pruning. Notably, climbing fibers contact many more Purkinje cells early on compared to maturity (Sugihara, 2006). Moreover, although a single axon may eliminate inappropriate partners, it can end up contacting more than one partner type. This has been found to occur for mouse rod bipolar cells that initially contact AON ganglion cells (Morgan et al., 2011), but primarily maintain synapses with two amacrine cell types at maturity (Raviola and Dacheux, 1987; Schubert et al., 2013; Tsukamoto et al., 2001). Finally, after settling on the postsynaptic partner types, biased connectivity with one postsynaptic partner type might occur although clear examples of this process have not yet been found.
The scenarios schematized in Figure 1 suggest that circuit refinement is a key step towards establishing the final wiring diagrams in the nervous system. Classic developmental studies that have focused on the neuromuscular junction (Sanes and Lichtman, 1999) and on sensory systems (Katz and Shatz, 1996; LeVay and Gilbert, 1976; LeVay et al., 1980) have led to the accepted view that such refinement involves synapse elimination, which is driven largely by activity-mediated competition producing ‘winners’ that take over contact area from ‘losers’. We will next briefly review how neurotransmission fuels such competition, and highlight emerging concepts of how transmission can regulate connectivity without necessarily engaging competitive processes.
New roles for neurotransmission in shaping wiring patterns
Circuit alterations by activity imbalances without competition
In the primate and cat visual systems, axonal projections terminating in layer 4 of the visual cortex are arranged into alternating, eye-specific columns of equal width (Hubel and Wiesel, 1972; Shatz et al., 1977; Wiesel et al., 1974). Monocular deprivation leads to a reduction in the width of the deprived eye column and an expansion of the non-deprived eye projections (Hubel and Wiesel, 1977; LeVay and Gilbert, 1976; Shatz and Stryker, 1978). However, binocular deprivation does not affect the widths of the ocular dominance columns (LeVay et al., 1980). These seminal findings demonstrate that an imbalance in afferent activity causes an unequal distribution of axonal coverage between the more active and less active afferents. Since these early findings, numerous examples of circuits in other systems and species support the belief that imbalances in transmission promote connectivity with one input over the other (Ben Fredj et al., 2010; Buffelli et al., 2003; Carrillo et al., 2013; Hashimoto et al., 2009a; Hua et al., 2005; Yasuda et al., 2011; Yu et al., 2004). Notably, in the rodent olfactory system, the axonal projection patterns of olfactory receptor neurons are not altered by genetically driving expression of the light chain of tetanus toxin (TeNT) in all axons (Yu et al., 2004). This toxin cleaves the vesicle associated membrane protein 2 (VAMP2) thereby diminishing exocytosis (Schiavo et al., 1992). Expression of TeNT in one subtype of olfactory sensory neuron, however, results in a failure of the silenced axons to maintain their characteristic convergence onto a single glomerulus (Yu et al., 2004). Strikingly, in zebrafish tectum, application of tetrodotoxin (TTX) to block all retinal transmission does not affect retinal axonal arbor size (Hua et al., 2005). In contrast, individual retinal ganglion cell axons expressing the inward rectifier potassium channel Kir2.1 that suppresses neuronal excitability (Hua et al., 2005) or TeNT (Ben Fredj et al., 2010) alter the size of their axonal arbor compared to wildtype. Together, these examples emphasize that relative, rather than absolute, levels of transmission drive different outcomes for active and inactive inputs competing for synaptic territory. It is important to note, however, that temporal correlations in spiking amongst neighboring afferents, rather than their relative activity level, is also an important determinant of axonal arbor size and synaptic connectivity (Blankenship and Feller, 2010; Dhande et al., 2011; Feller, 2009; Munz et al., 2014; Ruthazer et al., 2003; Sernagor and Mehta, 2001).
Imbalances in transmission of converging afferents also lead to differences in connectivity of some retinal circuits (Figure 2). But, an important distinction is that changes in synapse density can occur without apparent synaptic competition. Connections in the inner retina are organized into two major functionally distinct layers (Figure 2A), the ON layer comprising synapses of retinal cells that depolarize to light increments, and the OFF layer that composes of synapses of cells that hyperpolarize to such increments (Nelson et al., 1978; Wassle, 2004). ON-OFF bistratified retinal ganglion cells receive input from both ON and OFF bipolar cells. Selective suppression of neurotransmission from ON bipolar cells (grm6-TeNT) causes a reduction in their number of synapses with the ON arbor of the ON-OFF retinal ganglion cell, with no change in connectivity to the OFF arbor (Kerschensteiner et al., 2009 and Figure 2B). Thus, ON and OFF bipolar cells do not compete for synapses in an activity-dependent manner, but transmission does regulate their connectivity. In fact, the number of synapses formed between bipolar cells and retinal ganglion cells is regulated by transmission autonomously on a cell-by-cell basis (Okawa et al., 2014). In transgenic lines in which TeNT is expressed in a sparse population of ON bipolar cells, synaptic connectivity with the ganglion cell is only reduced for the TeNT-expressing axon (Figure 2B). Neighboring active axons of the same type that co-innervate the dendrite of the postsynaptic ganglion cell do not increase their synapse number or density (Okawa et al., 2014). Thus, in this circuit, even though a local imbalance of transmission from neighboring axons results in a differential synaptic distribution amongst the converging axons, there is no ‘takeover’ by the more active axons.
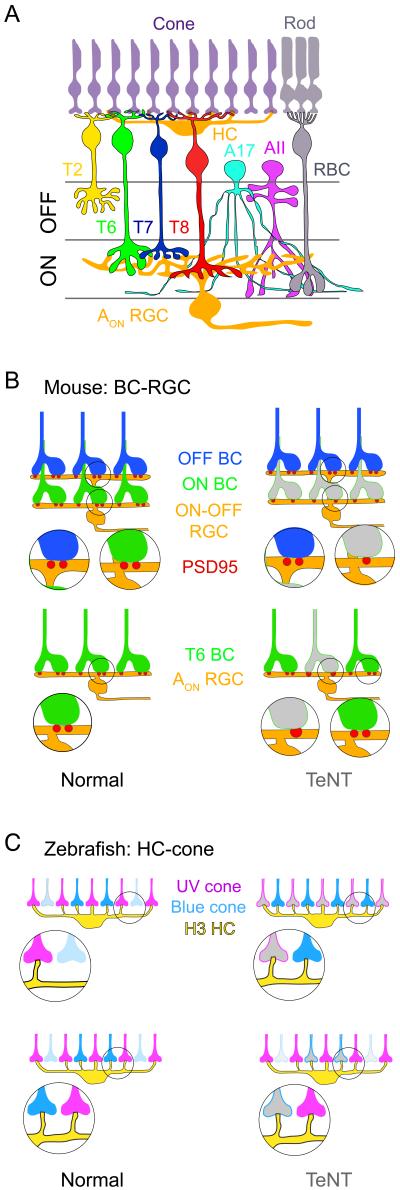
Figure 2. Retinal connections undergo activity-dependent alterations without competition.
A. Basic organization of the vertebrate retina, illustrated here for the mouse. Cone: cone photoreceptor; Rod: rod photoreceptor; HC: horizontal cell; T2, 6, 7, and 8: Cone bipolar cell types; RBC: rod bipolar cell; A17: A17 amacrine cell; AII: AII amacrine cell; AON RGC: AON retinal ganglion cell; ON, OFF: ON and OFF sublaminae of the inner plexiform layer. B. (Top) Suppressing glutamatergic transmission from all ON bipolar cells by expression of tetanus toxin light chain (TeNT; gray) reduces the number of excitatory synaptic sites (marked by PSD95) between ON but not OFF bipolar cells contacting a bistratified ON-OFF retinal ganglion cell (Kerschensteiner et al., 2009). (Bottom) Synaptic number and size of T6 ON cone bipolar cells are regulated by transmission on a cell-by-cell basis (Okawa et al., 2014). C. Zebrafish H3 HCs contact more UV cones than blue cones. (Top) Blocking UV cone transmission (TeNT; gray) results in increased connectivity with blue cones, but no change in connectivity with UV cones. (Bottom) Blocking blue cone transmission has no effect on connectivity with either cone type (Yoshimatsu et al., 2014).
Competitive mechanisms also do not operate in another retinal circuit although perturbing transmission during development clearly alters its wiring pattern (Figure 2C). In the outer retina of larval zebrafish, the H3 type of horizontal cell is contacted by cone photoreceptors with peak sensitivity to ultraviolet light (UV cones), as well as cones that are sensitive to blue light (blue cones) (Li et al., 2009; Yoshimatsu et al., 2014). H3 cells form a stereotypic 5:1 UV to blue cone connectivity; this biased connectivity with UV cones comes about by preferential synaptogenesis with UV cones as the H3 cell matures (Yoshimatsu et al., 2014). This is not because there are more UV cones compared to blue cones in the field because the ratio of the numbers of UV to blue cones across the retina remains relatively constant (~ 1:1) throughout the period of synaptogenesis. A role for neurotransmission was evident when UV cones were genetically silenced or when UV light responses were abolished by knockdown of UV opsin (Yoshimatsu et al., 2014). Perturbing UV cone transmission results in an increased number of blue cones contacted by the H3 cell (Figure 2C), suggesting that transmission from one set of afferents controls connectivity with the other afferent type (Yoshimatsu et al., 2014). There was no evidence, however, for an activity-dependent competitive process because the perturbed UV cones did not lose their connections, and in fact retained a normal number of contacts with the H3 cell (Figure 2C). Also, silencing the blue cones did not trigger an increase in UV cone connections (Figure 2C), nor a loss of blue cone connections (Yoshimatsu et al., 2014). Likewise no ‘punishment’ signal is apparent in mixed co-cultures of wildtype and NMDA receptor knockout (GluN1-negative) hippocampal neurons. In these cultures, synapse density increased on the dendrites of wildtype cells but there was no change in synapse density on GluN1-negative cells (She and Craig, 2011). Thus, these recent studies suggest that transmission can play a key role in establishing stereotypic patterns of converging inputs without evoking competitive mechanisms.
Activity can shape cell type-specific connectivity despite balanced transmission
As mentioned earlier, uniformly perturbing transmission across afferents does not appear to lead to differential changes in their connectivity (Ben Fredj et al., 2010; Hua et al., 2005; LeVay et al., 1980; Yasuda et al., 2011; Yu et al., 2004, but see Huberman et al., 2006; Stryker and Harris, 1986). A recent study in the retina, however, challenges the generality of this outcome. Type 6 but not Type 7 bipolar cells show a decrease in connectivity with the AON ganglion cell when both bipolar cell types express TeNT (Morgan et al., 2011 and Figure 3A, B). Conversely, when transmission from all bipolar cells increases after photoreceptor degeneration in the Crx (cone-rod homeobox gene) deficient mutant, only the Type 6 bipolar cells increase their connectivity with the AON ganglion cell (Soto et al., 2012). Thus, the number of synapses Type 6 bipolar cells make with the AON ganglion cell appears proportional to the bipolar cell transmission level, and this relationship is cell-autonomous (Figure 3B). Moreover, the bidirectional regulation of synapse number by transmission appears to be input type specific, because it does not occur for the Type 7 bipolar cell - AON ganglion cell connections (Figure 3B).
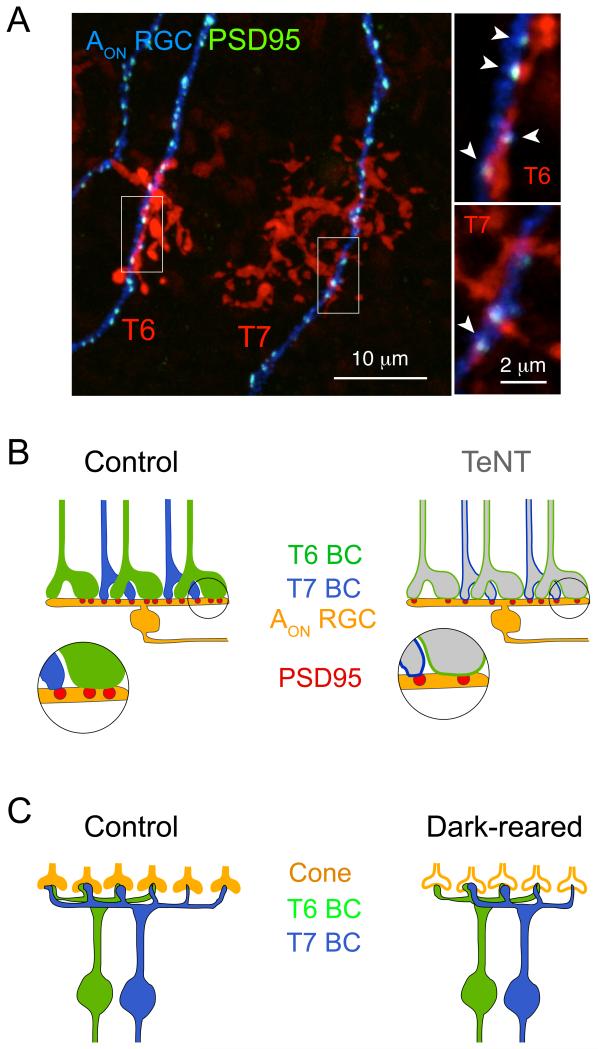
Figure 3. Uniform alteration of transmission from all inputs can lead to differential connectivity.
A. Maximum intensity projection of a confocal image stack showing synaptic contacts (fluorescently tagged PSD95) between Type 6 (T6) and Type 7 (T7) bipolar cells with an AON retinal ganglion cell (RGC) in the mouse retina. Bipolar cells shown here are labeled in the grm6-tdTomato transgenic line (Kerschensteiner et al., 2009). Insets show higher magnification of the bipolar cell contacts. Arrows point to synaptic appositions defined by overlap of PSD95 and tdTomato signal in three-dimension. B. Suppressing transmission from all retinal ON bipolar cells differentially reduces the number of synaptic sites (PSD95) between T6, but not T7, bipolar cells (BC) with the AON RGC (Morgan et al., 2011). C. Sensory deprivation alters the number of cone photoreceptors contacted by T7 but not T6 BCs compared at 3 weeks after birth (Dunn et al., 2013).
Altering transmission across the entire population of afferents can also lead to disparate changes in connectivity with distinct postsynaptic cell types. In the outer retina, cone photoreceptors contact every type of cone bipolar cell. In dark-reared animals, the number of cone photoreceptors synapsing onto Type 6 bipolar cells is unaffected throughout development, whereas Type 7 and 8 bipolar cells initially connect with fewer cones than normal, although they eventually settle on control numbers (Dunn et al., 2013 and Figure 3C). Thus, loss of sensory stimulation across cone photoreceptors does not uniformly affect connectivity of all their postsynaptic bipolar cell types. These observations raise the additional point that disruptive effects of altering transmission may be corrected over time. The mechanisms that create disparate effects on connectivity even when afferents are similarly active or inactive remain elusive. It is clear that different outcomes on connectivity can arise when different methods are used to manipulate neuronal transmission (Bleckert and Wong, 2011). Indeed, when excitability of retinal ganglion cells is suppressed by expression of Kir2.1, their axons become smaller, whereas their arbors become larger than normal when they express TeNT, which decreases exocytosis (Ben Fredj et al., 2010; Hua et al., 2005). Similarly, reducing GABA synthesis from basket interneurons upon deletion of GAD1, one of two glutamic acid decarboxylase isoforms, results in a smaller axon arbor and fewer smaller synapses on the cortical pyramidal cell soma (Chattopadhyaya et al., 2007). In contrast, complete blockade of GABAergic transmission, by deleting both GAD isoforms or the vesicular inhibitory amino acid transporter, leads to an overgrowth of the axon arbor and an increased density of smaller synaptic contacts on the pyramidal cell soma (Wu et al., 2012). But, for the photoreceptor circuits discussed above, all presynaptic cells underwent the same perturbation i.e. sensory deprivation. Thus, at least in the retina, there exist separate activity-regulated mechanisms that control the connectivity of disparate postsynaptic cell populations that share a common set of inputs.
Cellular and network design constraints on activity-dependent competition
The diverse actions of neurotransmission beg the question of why activity-dependent mechanisms are sometimes engaged and other times not. One possibility is that there are constraints within the circuit design that limits interactions amongst the converging inputs. Work in the ‘plasticity’ field has elegantly demonstrated that inputs that are close-by can exhibit long-term heterosynaptic plasticity (Engert and Bonhoeffer, 1997; Tao et al., 2001). Competitive mechanisms based on neurotransmission may thus occur during development only when the afferents innervating the postsynaptic cell are intermingled. Indeed, many of the key examples of activity-dependent competition involve circuits for which competing axons initially overlap highly, but become segregated later (Brown et al., 1976; Cline and Constantine-Paton, 1989; Colman and Lichtman, 1993; Godement et al., 1984; Huberman et al., 2008; Letinsky and Morrison-Graham, 1980; LeVay et al., 1978; Linden et al., 1981; Redfern, 1970; Shatz, 1983). Afferents that are normally spatially separated can also show activity-dependent competition when forced to co-innervate a target. An elegant example comes from the ‘three-eyed’ frog experiment in which alternating eye-specific stripes emerge in the optic tectum where retinal axons of the third, implanted eye take over territory normally occupied by the contralateral eye (Reh and Constantine-Paton, 1985). This induced segregation is mediated by an activity-dependent competitive process because the eye-specific stripes fail to form in the presence of TTX (Reh and Constantine-Paton, 1985).
Competition between different input types may be limited in circuits where distinct afferents innervate separate dendritic compartments of the cell as seen for Nucleus Laminaris neurons, cerebellar Purkinje cells and hippocampal CA3 pyramidal neurons (Figure 4A). For example in the chick auditory brainstem, ipsilateral and contralateral afferents from the Nucleus Magnocellularis contact the dorsal and ventral arbors of the Nucleus Laminaris neurons respectively (Parks and Rubel, 1975 and Figure 4A). Accordingly, for these neurons, perturbing transmission from one set of afferents leads to connectivity changes only in their contacted dendritic arbor (Benes et al., 1977; Sorensen and Rubel, 2006; Wang and Rubel, 2012). Some activity-dependent competitive interactions occur between parallel fibers and climbing fibers that overlap marginally (Figure 4A) on the dendritic arbor of cerebellar Purkinje cells (Hashimoto et al., 2001; Ichikawa et al., 2002; Miyazaki et al., 2004; Uemura et al., 2007; Watanabe and Kano, 2011). Climbing fiber elimination proceeds in two phases, an early phase when these fibers undergo elimination before innervation by parallel fibers, and a late phase when further pruning of perisomatic climbing fibers is regulated by transmission between parallel fibers and the Purkinje cell (reviewed in Watanabe and Kano, 2011). This late phase of elimination is dependent on synaptic transmission between parallel fibers and Purkinje cells because impairing transmission in glutamate receptor mutants leads to retention of many weaker climbing fiber inputs (Aiba et al., 1994; Hashimoto et al., 2001; Ichikawa et al., 2002).
Figure 4. Potential architectural constraints on activity-dependent competition.
A. Schematic illustrating the segregated distributions of distinct afferents on the dendritic arbors of four types of neurons: (1) The dorsal and ventral arbors of chick Nucleus Laminaris (NL) neurons are innervated separately by ipsi- and contralateral projections from Nucleus Magnocellularis (NM) neurons; (2) Climbing fibers (CF) and parallel fibers (PF) contact the proximal and distal parts of the purkinje cell (PC) dendritic arbor; (3) Distinct excitatory afferents (PP = Perforant path, MF = mossy fiber, SC = Schaffer collateral) contact different parts of the dendritic arbor of hippocampal CA3 pyramidal neurons; and (4) An ON-OFF retinal ganglion cell (RGC) receives inputs from ON and OFF bipolar cells on dendrites stratifying in separate sublaminae. B. The axonal terminals of each bipolar cell type (blue) tile such that there is minimal overlap of their territories (magenta polygon). C. Comparison of the axon terminals of mouse Type 6 (T6) bipolar cells in control, TeNT and diphtheria toxin (DTA)-expressing retinas. TeNT: suppressed transmission in the axon; DTA: neighboring bipolar cells ablated. Shown are z-projections of the digital masks of individual axon terminals. Scale bar applies to all. D. Within the axonal territory of inactive (TeNT) T6 bipolar cells, there is no increase in the number of synapses (estimated by PSD95 expression) between the postsynaptic RGC and its other bipolar afferents (presumed T7 here). However, T7 bipolar cells gain many more synapses with the RGC when T6 bipolar cells are ablated (DTA). Note that PSD95 puncta apposed to TeNT-expressing T6 bipolar cells are larger compared to those apposed to non-TeNT bipolar cells. For C and D see Okawa et al., 2014.
Spatial constraints on connectivity are particularly evident in many circuits of the retina. As mentioned earlier, the laminar organization of ON and OFF connections may limit competition between ON and OFF bipolar cell inputs because even when convergent, these input types form onto distinct arbors of the ganglion cell (Figure 4A). This may be why blocking ON transmission does not affect OFF connectivity of the bistratified ganglion cells (Kerschensteiner et al., 2009 and Figure 2B). A second spatial constraint involves ‘tiling’ (Figure 4B). Tiling precludes overlap between axonal terminals of the same type of bipolar cells, and may explain why the Type 6 bipolar cells do not exhibit homosynaptic (same input type) activity-dependent competition. Instead, releasing bipolar cells from the tiling constraint upon ablation of a subset of bipolar cells causes the remaining axons to expand (Figure 4C) and increase synaptic contact with the ganglion cell (Okawa et al., 2014). Thus these axons are not intrinsically limited in their ability to acquire more synaptic territory. Tiling may allow each type of bipolar cell to cover the entire visual field uniformly with little gaps or redundancy in the sampling of the image. Such a constraint may be advantageous because activity-dependent competitive mechanisms could cause unpredictable variability in neighboring bipolar cell dendritic and axonal territories and thus coverage of visual space.
Two other observations in retina, however, suggest that even when different input types highly overlap, they do not necessarily exhibit transmission-mediated heterosynaptic competition. The axonal terminals of Types 6 and 7 bipolar cells overlap greatly, and their synapses are intermingled along the dendrites of the AON ganglion cell. Within the axonal territory of a TeNT-expressing bipolar cell, the density of synapses attributed to other bipolar cells does not change (Figure 4D). This suggests that despite the close proximity of the two input types, they do not compete in an activity-mediated manner. However, ablation of large numbers of Type 6 bipolar cells results in an increase in synaptic density made by Type 7 axons on the AON ganglion cell (Figure 4D). Physical loss of Type 6 axons may enable Type 7 axons to more readily overlap with the dendrites of the ganglion cell and form more synapses (Okawa et al., 2014). A second example comes from the zebrafish retina where UV and blue cones contact the same stretch of dendrite of the H3 horizontal cell; although the stereotypic wiring of this circuit is activity-regulated, there is no competition between the cone types (Yoshimatsu et al., 2014).
Why do some converging inputs compete and others not? One possibility is that synaptic resources in both the axons and the postsynaptic target neurons are limited. If presynaptic neurons have a limited amount of resource for maintaining synaptic efficacy, then the distribution of these resources can determine which contact ‘wins’. Observations in the neuromuscular junction (NMJ) support this notion because less divergent motor neurons which can potentially invest more of their synaptic resources at each junction emerge as ‘winners’ in maintaining contacts with the muscle fibers (Kasthuri and Lichtman, 2003). For the postsynaptic neuron, limited resources could restrict synaptic strengthening to one or a few inputs. Cerebellar Purkinje cells preferentially strengthen one climbing fiber contact over the others during the first postnatal week. When calcium channels on the Purkinje cells are mutated, however, multiple climbing fiber contacts become co-strengthened (Hashimoto et al., 2011). The net excitatory postsynaptic currents (EPSCs) generated by the Purkinje cell in the mutant are surprisingly similar to those of wildtype cells, supporting the hypothesis that a limited postsynaptic resource is being equally distributed amongst many contacts (Watanabe and Kano, 2011). In the retina, however, synaptic resources do not seem to be a limiting factor because Type 6 bipolar cells can increase their synapse density with the ganglion cell above their normal density (Soto et al., 2012), and without apparent loss of synaptic contact by other converging axons. H3 horizontal cells can similarly increase connectivity with blue cones without requiring elimination of contacts with silenced UV cones (Yoshimatsu et al., 2014). Together, these observations suggest that if a ‘resource limit’ applies across circuits, then the retinal circuits discussed here must not tap all the resources available during normal development because synapses can be added by one afferent type without a complementary loss in other afferents.
New perspectives on activity-driven synapse formation and elimination
In many circuits, synapse elimination is evoked by an activity-dependent process. But, recent observations in the mouse retina suggest that elimination can occur irrespective of transmission. Synaptic contacts between developing rod bipolar cells and the AON ganglion cell disappear by maturity regardless of whether transmission in these bipolar cells is suppressed (Morgan et al., 2011) or enhanced (Soto et al., 2012). These contacts are assumed to be synaptic owing to the presence of the postsynaptic scaffold protein, PSD95, at the axon-dendrite appositions, but future studies are needed to ascertain whether there is transient functional contact between these cell types.
More surprising is the finding that the rate of synapse formation is regulated by activity. Time-lapse imaging of hippocampal cell cultures shows that fluorescently tagged PSD95 clusters appear more slowly in cultures whose overall activity is reduced by chronic application of an NMDA receptor antagonist, compared to untreated cultures. Moreover, under this condition, the rate of PSD95 disappearance remains unaffected (Okabe et al., 1999). Recent time-lapse imaging of PSD95 on the dendrites of retinal ganglion cells corroborates these findings in culture. The rate of excitatory synaptogenesis on AON ganglion cells is reduced when bipolar cell transmission is suppressed in grm6-TeNT retinas (Kerschensteiner et al., 2009), whereas it is increased in Crx mutants with enhanced bipolar cell neurotransmitter release (Soto et al., 2012). Moreover, perturbing transmission from ON bipolar cells does not alter the rate of synapse elimination from ganglion cell dendrites (Kerschensteiner et al., 2009).
How does transmitter release influence the rate of synaptogenesis? It has long been proposed that transmitter release promotes synaptogenesis by encouraging the motility of pre- and postsynaptic structures, especially dendritic filopodia (Andreae and Burrone, 2014; Kwon and Sabatini, 2011; Smith and Jahr, 1992; Ziv and Smith, 1996). Filopodia are protrusive and highly motile structures and are thought to facilitate contact between axons and dendrites. The activity-regulated synaptogenesis between the Type 6 bipolar cells and the AON ganglion cell casts another angle on how transmission can influence synaptogenesis. Individual axonal boutons of Type 6 bipolar cells contacting AON ganglion cell dendrites contain more than one ribbon release site, each separately apposed to a PSD95 cluster (Morgan et al., 2011; Okawa et al., 2014). Unlike addition of synapses at separate contact sites such as at spines, new synaptic sites are added over time at stable pre- and postsynaptic appositions between the Type 6 bipolar cell and the ganglion cell. Boutons progressively form with maturation and 1 to 4 separate release sites develop, each with a ribbon opposite a PSD95 cluster, resembling a small version of the Calyx of Held. TeNT-expressing Type 6 bipolar cells are less able to develop ‘multisynaptic’ boutons (Morgan et al., 2011), implicating the involvement of an activity-dependent component for this step of synaptogenesis. Transmitter release thus affects synaptogenesis not only by influencing the motility of dendritic filopodia, but also by controlling the addition of new synaptic sites at stable appositions.
Finally, it is important to realize that whether transmission regulates synapse formation, elimination or both may depend on the developmental stage. For example, binocular deprivation prior to the critical period leads to a decrease in spine formation but no change in spine elimination on the apical dendrites of Layer 5 pyramidal neurons in the visual cortex (Majewska and Sur, 2003). In the mouse retina as well, early transmission between the ON bipolar cells and AON ganglion cell affects the rate of synapse formation and not elimination (Kerschensteiner et al., 2009). Interestingly, however, visual deprivation during the peak of the critical period can lead to increases in both spine formation and elimination on the dendrites of Layer 5 pyramidal neurons (Majewska and Sur, 2003). The complexity by which transmission influences synapse number over time emphasizes the need to perform time-lapse imaging in order to tease apart the separate effects on synapse addition and elimination at each stage.
Activity-mediated modifications at retinal synapses
Neurons have the remarkable capacity to alter their synapses when neurotransmission is increased or decreased, both during development and at maturity. These alterations include changes to connectivity (e.g. number of synapses) as well as modifications at the level of individual synapses (Aizenman and Cline, 2007; Li and Cline, 2010; Zhao et al., 2013). Hippocampal, neocortical and spinal neurons in culture exhibit ‘synaptic scaling’ where globally blocking activity leads to an increase in the amplitudes of miniature EPSCs and ionotropic glutamate receptor expression at excitatory synapses. The converse occurs when network excitability is increased (reviewed in Davis, 2006; Turrigiano, 2007, 2011, 2012). Synaptic scaling evoking postsynaptic changes has also been found in vivo, where for example, denervation causes an enhancement of muscle excitability due to an increase in acetylcholine receptors at the NMJ (Berg and Hall, 1975; Sharpless, 1975). Presynaptic changes, such as in the probability of transmitter release, have also been documented at the fly NMJ when postsynaptic receptors are blocked (Frank et al., 2006; Frank et al., 2009; Kauwe and Isacoff, 2013), or when postsynaptic cells are chronically hyperpolarized (Paradis et al., 2001). Similarly, blocking activity leads to increased transmitter release in mammalian neuronal cell culture systems (Murthy et al., 2001).
Like these other circuits, suppressing transmission from retinal ON bipolar cells also leads to both pre- and postsynaptic alterations. PSD95 clusters apposed to silenced bipolar cell contacts are enlarged (Okawa et al., 2014 and Figure 2B), and multiple presynaptic ribbons are commonly found apposed to these abnormal postsynaptic sites (Kerschensteiner et al., 2009). In transgenic lines where individual or small groups of ON bipolar cells express TeNT, PSD95 clusters opposite the silenced but not the active axon terminals are enlarged (Figure 2B), suggesting that the effect is local, and that there is no compensatory decrease in PSD95 cluster size at active synapses (Okawa et al., 2014). This increase in PSD95 size at the silenced bipolar cell synapses differs from observations in hippocampal cultures where a single neuron is made to express TeNT. PSD95 clusters at the TeNT synapses are normal, but the expression of the AMPA receptor subunit GluA1 is decreased possibly because these receptors are found to be more motile at inactive synapses (Ehlers et al., 2007). However, GluA1 puncta size does not change when vesicular release is blocked in all neurons in the culture (Harms et al., 2005). In contrast, expressing TeNT in virtually all ON bipolar cells produces the same phenotype as when transmission from only a few bipolar cells is suppressed (Kerschensteiner et al., 2009; Okawa et al., 2014). Thus, the bipolar-ganglion cell synapse is modified by neurotransmission on an axon-by-axon basis i.e. without requiring differences in synaptic drive amongst neighboring converging axons, and without adjustments in active synapses to rebalance the net input onto the ganglion cell.
Sensory drive has also been found to influence glutamate receptor accumulation in the visual system. A short period of visual deprivation in the developing rat results in an increased amplitude of EPSCs at recurrent connections of Layer 4 star pyramidal cells in the visual cortex (Maffei et al., 2004 and Figure 5A). Such deprivation also causes an increase in AMPA (GluA)-receptor mediated miniature EPSCs in Layers 2/3 of the adult mouse visual cortex (Goel and Lee, 2007). In contrast, dark-rearing results in a decrease in metabotropic glutamate receptor 6 (mGluR6) at the dendritic tips of mouse ON cone bipolar cells (Figure 5B), thus affecting the visual system at its very first synapse (Dunn et al., 2013). mGluR6 is solely responsible for mediating light responses in ON bipolar cells. One potential explanation for the disparate effects of visual deprivation is that ionotropic and metabotropic glutamate receptors may be regulated in distinct ways. This may not be surprising given that at the photoreceptor synapse, light suppresses glutamate release onto bipolar cells i.e. this is a sign-inverting synapse, whereas ionotropic glutamate receptors are found at sign-preserving synapses. Downregulation of mGluR6 at ON bipolar dendrites is in response to the continuous release of glutamate from photoreceptors in the dark, similar to the reduction in ionotropic glutamate receptors observed when network excitation is increased (Lissin et al., 1998). However, physiological recordings are necessary to ascertain whether the loss in mGluR6 is a consequence of increased or decreased glutamate release from cones because as yet we do not know whether photoreceptors of dark-reared animals are capable of homeostatic adjustments. Importantly, mGluR6 expression in rod bipolar cells is unaffected by dark-rearing (Figure 5B), suggesting that regulation of glutamate receptors in the visual system by sensory drive may not only be receptor type specific, but is also cell or pathway specific.
Figure 5. Neurotransmission regulates synapse formation and maintenance.
A. Monosynaptic inputs from neighboring star pyramidal cells in Layer 4 of the visual cortex increase in amplitude (Amp) in monocularly deprived rats (Maffei et al., 2004) B. Sensory deprivation selectively reduces mGluR6 expression on dendritic tips of cone bipolar cells (CBC) but not rod bipolar cells (RBC) (Dunn et al., 2013). C. Star pyramidal neurons in the visual cortex receive GABAergic input from fast spiking (FS) and regular-spiking nonpyramidal (RSNP) interneurons. These two inhibitory synapses are differentially modulated by visual deprivation. Response amplitude of RSNP synapses increases whereas synapses made by FS neurons show reduced amplitude. D. RBC axon terminals and A17 amacrine cell neurites form reciprocal synapses. RBCs release glutamate (glu) that drives release of GABA from the A17, which inhibits further release of glutamate from the RBC terminal. In GAD1 knockout (KO) mouse retina, GABA release is impaired (gray) resulting in a selective decrease of α1 subunit-containing GABAA receptors (GABAARα1) at RBC terminals. α3 subunit-containing GABAA receptors (GABAARα3) and GABAC receptors (GABACR) are unaffected. AMPA receptor (GluA) expression on A17 processes assessed by glutamate evoked responses remains unperturbed in the GAD1 KO retina (Schubert et al., 2013).
Differential effects of neurotransmission have been found not only for different cell types but also at different synapses on the same postsynaptic cell (Bartley et al., 2008; Kim and Alger, 2010; Maffei et al., 2004). For instance, in visually deprived rats, inputs of basket interneurons onto Layer 4 star pyramidal neurons decrease in amplitude, but connections with regular-spiking nonpyramidal (RSNP) interneurons increase in amplitude (Maffei et al., 2004 and Figure 5C). Thus, the strength of inhibitory connections onto an individual postsynaptic cell can be altered separately when network activity is perturbed (Figure 5C). A recent study in the retina further demonstrates that transmission can also act differentially on distinct types of GABA receptors even at the same presynaptic contact (Schubert et al., 2013). A17 amacrine cells form large GABAergic presynaptic boutons that are apposed to the rod bipolar cell axonal terminal to form a highly local reciprocal microcircuit. Glutamate is released onto the A17 bouton, and GABAergic feedback from each bouton occurs at distinct clusters of GABAA and GABAC receptors on the rod bipolar cell axon (Figure 5D). Furthermore, both α1 and α3 subunits of the GABAA receptor are found on the rod bipolar axons. The development of GABAA and GABAC responses proceeds normally in mice in which GAD1 is knocked out specifically in the retina. But, over time, α1-containing receptor clusters are reduced, resulting in a decrease in the amplitude of GABAA-evoked currents in the bipolar cell (Schubert et al., 2013). However, α3GABAA and GABAC receptors are maintained. Thus, GABAergic transmission differentially influences maintenance of axonal GABA receptors in a receptor-type specific manner. Interestingly, increasing network activity in hippocampal cultures by chronic blockade of inhibition leads to decreased surface expression of GluA1 but not GluN1 (Lissin et al., 1998). Thus, transmission can differentially regulate distinct types of GABAergic or ionotropic glutamate receptors at individual synapses.
Modifications at synapses when transmission is altered could help rebalance excitation and inhibition in the circuit. Generally, both excitation and inhibition scale together in a coordinated manner (Hartman et al., 2006; Kilman et al., 2002; Rutherford et al., 1997; Turrigiano, 2011; van Zundert et al., 2010). But, inhibition and excitation at the rod bipolar cell-A17 reciprocal synapse (Figure 5D) appear to undergo independent regulation. In the GAD1 KO, there is an initial increase in glutamate release from rod bipolar cells onto the A17 amacrine cell but later this release returns to normal levels (Schubert et al., 2013). As discussed earlier, GABA receptors on rod bipolar cells are either downregulated or unchanged, instead of increased when GABA release is diminished. When TeNT-expressing rod bipolar cells cannot release glutamate, there is no change to the glutamate current recorded in A17 cells by puffing glutamate at their boutons. This suggests that GluA receptors on the A17 cells are at normal levels (Schubert et al., 2013), although it is not confirmed that they are clustered appropriately in the mutant. Also, GABA receptor responses from the bipolar cell axon appear normal in the TeNT-expressing axons. Therefore, despite the close proximity of the excitatory and inhibitory sites, separated only by a micrometer or so, the synaptic components of the A17-rod bipolar connection are not adjusted coordinately in response to activity perturbations.
Taken together, it is evident that like other CNS neurons, some but not all retinal synapses can demonstrate scaling of their synapses when activity is altered. Pre- and postsynaptic modifications of transmission can act in a cell-autonomous manner such as in bipolar cell axons, without evoking changes across all converging inputs. The local action of transmission on bipolar cell synapses (Okawa et al., 2014) supports the increasing evidence for homeostatic adjustments occurring locally (Beique et al., 2011; Branco et al., 2008; Hou et al., 2008; Kauwe and Isacoff, 2013). In the retina, local synaptic modifications also do not necessarily require differences in the transmission levels of neighboring inputs. Moreover, excitatory and inhibitory components at synaptic connections may not always be regulated together to reset the balance, as seen for the A17-rod bipolar synapse. How excitatory and inhibitory connections of other retinal neurons are regulated remains to be elucidated.
Overall, retinal synapses share similarities with other CNS synapses with regard to how transmission influences their development, maturation and maintenance. But, retinal circuits appear to recruit unconventional and cell type-specific roles for transmission in order to attain specific connectivity patterns. Notably, the maintenance of GABA and glutamate receptor types at retinal synapses is differentially regulated by activity. We will discuss next how transmission perturbations that occur in retinal disease models can also invoke cellular and synaptic remodeling of the circuit.
Circuit reorganization in models of retinal disease characterized by transmission defects
Circuits are often disrupted in disease due to neurons being disconnected from partners that undergo cell death. However, there are mutations that cause disease that are associated with impaired neurotransmission without immediate neuronal loss. For example, a spontaneous mutation of the Cacna1f gene encoding the α1F subunit of voltage dependent calcium channel CaV1.4 (nob2 mice, a model of congenital stationary nightblindness (Chang et al., 2006)), or the α1F knockout (CaV1.4(α1F) KO), perturbs calcium-dependent transmission from photoreceptors (Mansergh et al., 2005; Specht et al., 2009). Transmission from photoreceptors is also abnormal when the cyclic nucleotide gated phototransduction channels A3 and B1 (CNGA3 and CNGB1), found at cone and rod photoreceptors respectively, are absent in a mouse model of retinitis pigmentosa (Michalakis et al., 2013). Loss of these CNG channels could alter the membrane potential of photoreceptors and thus affect neurotransmitter release from their terminals (Busskamp et al., 2010). Several studies based on these mouse mutants using synaptic markers as well as electron microscopy reveal some startling changes in connectivity in the outer retina and in the organization of the photoreceptor synapse (Figure 6).
Figure 6. Structural and connectivity defects in transmission mutant models of retinal disease.
A. Mice lacking phototransduction channels from both rods and cones (blue: CNGA3/B1) are a model of retinitis pigmentosa. Neurotransmission is also perturbed in mice with mutations in the voltage-gated calcium channel CaV1.4 (red) located at photoreceptor axon terminals, amongst which nob2 mice are a model for congenital stationary night blindness. Rod: rod photoreceptor, Cone: cone photoreceptor, HC: horizontal cell, RBC: rod bipolar cell, CBC: cone bipolar cell. B. Rod terminals retract whereas RBC dendrites and HC processes sprout into the outer nuclear layer, in both CNGA3/B1 double knockout (dKO) mice and CaV1.4 mutants (shown by the stripes of blue and red). In CaV1.4 mutants, retraction of cone terminals is rarely observed. But the cones often form extra branches (shown in red). CBCs in general appear to be less affected in all transmission mutants. C. Defects at the rod photoreceptor synapse found in different transmission mutants. nob4 mice lack metabotropic glutamate receptor expression (mGluR6). See text for references.
A common finding in Cacna1f mutants and CNGA3/CNGB1 double knockout (dKO) mice is the sprouting of postsynaptic processes and the retraction of rod axon terminals into the outer nuclear layer (ONL) (Bayley and Morgans, 2007; Chang et al., 2006; Mansergh et al., 2005; Michalakis et al., 2013; Raven et al., 2008; Regus-Leidig et al., 2014; Zabouri and Haverkamp, 2013). In CaV1.4(α1F) KO mice, cone photoreceptor axons also show abnormal branching, including elaboration of several primary processes from the cell body. Sprouting occurs post eye-opening, after a period of normal development. Thus, proper transmission from photoreceptors is necessary to maintain the laminated organization of the outer synaptic layer (Figure 6A, B). However, dark-rearing does not induce sprouting, indicating that light-driven activity may not be critical for restraining processes of the bipolar and horizontal cells to the outer plexiform layer (OPL) (Dunn et al., 2013; Raven et al., 2008). Another finding common to transmission mutants is that cone bipolar cells examined thus far are less susceptible to dendritic sprouting. In the Cacna1f mutant, the dendrites of OFF bipolar cells expressing the neurokinin receptor 3 remain stratified in the OPL (Chang et al., 2006). Whether other OFF cone bipolar cells and ON cone bipolar cells are resilient to dendritic sprouting has yet to be investigated for the mutants in detail.
Why do the pre- and postsynaptic processes in the outer retina elaborate in such an abnormal manner? Although the cellular mechanisms are not yet well understood, a parsimonious explanation is that photoreceptor terminals and their connected postsynaptic partners extend together to maintain synaptic contact. Examination of fixed tissue across ages suggests that horizontal cells are the first to sprout processes into the ONL, followed by rod bipolar cells and the retraction of rod axons (Bayley and Morgans, 2007; Michalakis et al., 2013). Rod bipolar cell dendrites are sometimes found to fasciculate with the horizontal cell sprouts, implying that horizontal cell sprouting could pave the way for the extension of bipolar cell terminals into the ONL (Michalakis et al., 2013). However, rod bipolar cell dendrites do not always sprout when horizontal cell processes elaborate outside the OPL, as found in a knockout of the synaptic adhesion molecule, netrin-G ligand 2 (NGL-2) (Soto et al., 2013) and in the retina lacking the transmembrane protein Semaphorin6A or its receptor PlexinA4 (Matsuoka et al., 2012). Thus, axonal sprouting in horizontal cells and rod bipolar cells may be regulated separately.
The neuritic sprouts of horizontal cells and rod bipolar cells (Figure 6B) seem to form ectopic synapses with photoreceptors, as assessed by immunostaining for pre- and postsynaptic markers, and by ultrastructural analysis (Bayley and Morgans, 2007; Michalakis et al., 2013; Regus-Leidig et al., 2014; Zabouri and Haverkamp, 2013). For example in the Cacna1f mutant, horizontal cell neuritic sprouts seem to form synapses ectopically with sprouting cone photoreceptor terminals (Zabouri and Haverkamp, 2013), an observation that supports the ‘move together to maintain contact’ hypothesis. It is however not yet certain that all the ectopically located synapses in the transmission deficient mutants contact appropriate synaptic partners. Despite evidence of horizontal and rod bipolar neuritic sprouts being apposed to vesicle associated proteins such as VGluT1 (Regus-Leidig et al., 2014), it is not as yet known whether transmission occurs at these sites. What is certain, however, is that an optimum level of calcium influx is needed in the photoreceptor terminal to prevent sprouting because altering the calcium current without physical disruption of the channel leads to abnormal elaboration of rod bipolar cell and horizontal cell neurites (Haeseleer et al., 2004; Knoflach et al., 2013; Liu et al., 2013; Regus-Leidig et al., 2014). Perhaps of greater significance is to determine whether or not such ectopic synapses develop as a direct consequence of perturbed transmission. Observations from mouse mutants in which postsynaptic components are altered do not support this notion. Dendrites in the OPL remain normally stratified in mGluR6 KO mice (Tagawa et al., 1999), as in other mice with perturbations in postsynaptic components in ON bipolar cells such as nyctalopin (nob1) (Ball et al., 2003), TRPM1 channels (Koike et al., 2010; Morgans et al., 2009), and the G-protein associated with the mGluR6 signaling cascade, GαO (Dhingra et al., 2000). A clearer role for transmission is, however, evident when the connections of rod and cone bipolar cells are compared in mice lacking CNGA3 or rhodopsin (photopigment in rods), or both (Haverkamp et al., 2006). In CNGA3 KO mice, cone bipolar cells, while still synapsing with cones, elaborate dendrites to contact nearby rods. Conversely, in the rhodopsin mutant (Haverkamp et al., 2006), rod bipolar cells wire with cones. Miswiring does not occur for rod or cone bipolar cells in CNGA3/rhodopsin dKO mice, suggesting that the bipolar cells may be searching to form new partnerships with ‘active’ presynaptic cells (Claes et al., 2004; Haverkamp et al., 2006). These observations in mice differ from those of zebrafish where expression of TeNT in both UV and blue cones does not direct H3 horizontal cell dendrites towards active rod photoreceptors, or towards red or green cones (Yoshimatsu et al., 2014).
At the synaptic level, a range of deficits have been found in mice with transmission defects (Figure 6C). The photoreceptor synapse exhibits a triad arrangement whereby horizontal cell and bipolar cell processes invaginate the axon pedicle opposite the anchored synaptic ribbon. In the CNGA3/B1 dKO, this arrangement is preserved even for the ectopically located contacts in the ONL (Michalakis et al., 2013). In the mGluR6 loss of function mutant (nob4), horizontal cell processes invaginate the rod photoreceptor, but bipolar cell dendrites do not (Cao et al., 2009). For photoreceptor terminals located at the OPL or at ectopic locations in the Cacna1f mutant, both horizontal and bipolar cell processes fail to invaginate the photoreceptor pedicle from early stages of development onwards (Raven et al., 2008; Regus-Leidig et al., 2014; Zabouri and Haverkamp, 2013). Moreover, ribbons are abnormally shaped and unanchored in the Cacna1f mutant (Bayley and Morgans, 2007; Raven et al., 2008; Regus-Leidig et al., 2014; Zabouri and Haverkamp, 2013). These diverse effects suggest that the structural arrangement of the retinal photoreceptor triad synapse is not influenced by transmitter release in a simple way.
Comparison of the proteins found at photoreceptor terminals in wildtype and in transmission mutants also underscores the complexity by which synaptic proteins are affected by activity perturbations. In the Cacna1f mutant, active zone proteins including bassoon and Rim2 are downregulated. Interestingly, Veli3, an adaptor protein, is also abnormally low in expression in rods but appears to be maintained in cones. Vesicle associated proteins such as VAMP2 and VGluT1 appear normal (Zabouri and Haverkamp, 2013). Whether or not these molecular changes at photoreceptor terminals are specific to the loss of CaV1.4 remains to be explored by investigation of other transmission mutants. Furthermore, in contrast to the photoreceptor terminal, little is known about the structural and molecular rearrangements at their postsynaptic sites. One intriguing observation thus far is the unusual accumulation of mGluR7 on ON bipolar cell dendrites in the absence of mGluR6 (Tagawa et al., 1999; Tsukamoto et al., 2007). Future comparisons of the expression and localization of pre- and postsynaptic proteins across mutants with impaired transmission are necessary, and should help uncover the cellular and molecular interactions at individual retinal synapses that are regulated by activity. In addition, electrophysiological analysis is needed to directly correlate changes in neurotransmission with disorganizations in the structure and molecular composition of retinal synapses in disease conditions.
Unifying the roles of activity in retinal circuit assembly and maintenance
The recent studies of the developing and diseased retina we discussed portray a diversity of roles for neurotransmission in shaping and maintaining synaptic connectivity. Can we find common ‘rules’ or strategies despite the multifaceted roles of activity that have emerged? These may become apparent if we consider whether or not defects in transmission affect retinal connectivity in ways that are specific to: (i) cell types (ii) synapse types (excitatory versus inhibitory, single versus multiple release sites), and (iii) transmitter receptor types. We explore this complexity by considering the bipolar-ganglion cell connectivity further.
The differential effects of suppressing transmission from ON bipolar cells onto AON ganglion cell raise several questions. The first is: Is the differential loss of synapses between TeNT-expressing Type 6 and 7 bipolar cells with their common target, the AON ganglion cell, a bipolar cell-type specific phenomenon? That is, do Type 6 bipolar cells uniformly adjust their connectivity with all their postsynaptic partners according to their activity? It is known that overall, the number of ribbon release sites is reduced in each Type 6 axon (Kerschensteiner et al., 2009), but it is not yet known whether there is a differential loss with some or all of its postsynaptic partners. Examining the changes in Type 6 connections with other yet to be identified postsynaptic targets would provide an answer to this question. Another possibility is that regardless of the pre- and postsynaptic partner types, only connections with the ‘dominant’ afferent type are readily altered by activity manipulations. If so, then one would expect that Type 7 bipolar cells show the same activity-dependent regulation with ganglion cells for which they provide the majority of synapses. Yet another possibility is that the failure to add synaptic sites by the TeNT-positive bipolar cell axon is specific to a particular synapse arrangement. Perhaps Type 6 bipolar cells make multisynaptic bouton contacts only with AON ganglion cells; examining the TeNT effects at contact sites with other postsynaptic partners would be instructive. Also, like Type 6 bipolar cells, Type 2 bipolar cells can make multisynaptic contacts with the ectopic dendrites of AON ganglion cells in a retina where the majority of ON bipolar cells are ablated (Okawa et al., 2014). Examining synaptic development in mice in which Type 2 bipolar cells express TeNT would be instructive. Furthermore, connections between Type 6 and Type 7 bipolar cells with the AON ganglion cell may be differentially modified by activity because the composition of the glutamate receptors at these sites may differ. In this regard, it might be interesting to ascertain the types of glutamate receptors at Type 6 and Type 7 connections. Finally, we do not yet know how changes in the excitatory drive from Type 6 and 7 bipolar cells affect inhibition onto the ganglion cell dendrites, or feedback inhibition onto their own axon terminals. The inhibitory synapses on cone bipolar terminals may also differentially modify inhibitory drive onto the ganglion cell dendrite. As yet, inhibitory synapses on AON ganglion cells and cone bipolar terminals have not been investigated in grm6-TeNT retinas.
Defining the precise role for transmission and uncovering the common ‘rules’ will require identification of the complete ensemble of converging and diverging inputs within the circuit. Such efforts are currently underway in large-scale connectome reconstructions using serial electron microscopy (Briggman et al., 2011; Helmstaedter et al., 2013; Kim et al., 2014). Information from the connectome is, however, currently based on axon-dendrite appositions, and thus future confirmation that each apposition is a synapse is necessary. In addition, the types of transmitter receptors at each synapse need to be identified. Furthermore, electrophysiological recordings will be essential for elucidating the functional relevance of the ‘wires’, and for determining the kinetic properties of the synapses. It is also important to realize that functional rearrangements can occur during development without apparent changes in physical connectivity (Wei et al., 2011).
Finally, we need to define what is meant by ‘the circuit’ beyond synaptic convergence and divergence, because there is a tremendous amount of ‘cross-talk’ between the classically-defined parallel processing pathways like the rod versus cone, and ON versus OFF pathways of the retina (Werblin, 2010). The many structural and functional ‘connectome’ considerations posit a difficult task ahead for gaining a complete understanding of how disrupting transmission between one set of synaptic partners impacts connectivity in other parts of the circuit. Despite these challenges, the compact circuitry of the retina and the already vast information about its connectivity and function, together offer a highly tractable system for unmasking and unifying the roles of neurotransmission in the development and maintenance of neuronal circuits.
Acknowledgments
Supported by NIH grants (EY10699, 17101, 14358 to R.O.L.W and a Vision Core grant EY01730), a Human Frontier Science Program grant (RGP0035 to L. Lagnado, F. Schmitz and R. O. L. Wong) and a Knights Templar Eye Foundation career starter grant (M.H.). We would like to thank P. Mardoum and C. Gamlin for critical reading of the manuscript and helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Aizenman CD, Cline HT. Enhanced visual activity in vivo forms nascent synapses in the developing retinotectal projection. J Neurophysiol. 2007;97:2949–2957. doi: 10.1152/jn.00452.2006. [DOI] [PubMed] [Google Scholar]
- Andreae LC, Burrone J. The role of neuronal activity and transmitter release on synapse formation. Curr Opin Neurobiol. 2014;27C:47–52. doi: 10.1016/j.conb.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SL, Pardue MT, McCall MA, Gregg RG, Peachey NS. Immunohistochemical analysis of the outer plexiform layer in the nob mouse shows no abnormalities. Vis Neurosci. 2003;20:267–272. doi: 10.1017/s0952523803203059. [DOI] [PubMed] [Google Scholar]
- Bartley AF, Huang ZJ, Huber KM, Gibson JR. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J Neurophysiol. 2008;100:1983–1994. doi: 10.1152/jn.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PR, Morgans CW. Rod bipolar cells and horizontal cells form displaced synaptic contacts with rods in the outer nuclear layer of the nob2 retina. J Comp Neurol. 2007;500:286–298. doi: 10.1002/cne.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proc Natl Acad Sci U S A. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Fredj N, Hammond S, Otsuna H, Chien CB, Burrone J, Meyer MP. Synaptic activity and activity-dependent competition regulates axon arbor maturation, growth arrest, and territory in the retinotectal projection. J Neurosci. 2010;30:10939–10951. doi: 10.1523/JNEUROSCI.1556-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Parks TN, Rubel EW. Rapid dendritic atrophy following deafferentation: an EM morphometric analysis. Brain Res. 1977;122:1–13. doi: 10.1016/0006-8993(77)90658-8. [DOI] [PubMed] [Google Scholar]
- Berg DK, Hall ZW. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975;244:659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship AG, Feller MB. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat Rev Neurosci. 2010;11:18–29. doi: 10.1038/nrn2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Wong RO. Identifying roles for neurotransmission in circuit assembly: insights gained from multiple model systems and experimental approaches. Bioessays. 2011;33:61–72. doi: 10.1002/bies.201000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco T, Staras K, Darcy KJ, Goda Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron. 2008;59:475–485. doi: 10.1016/j.neuron.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Brown MC, Jansen JK, Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976;261:387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffelli M, Burgess RW, Feng G, Lobe CG, Lichtman JW, Sanes JR. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- Busskamp V, Duebel J, Balya D, Fradot M, Viney TJ, Siegert S, Groner AC, Cabuy E, Forster V, Seeliger M, Biel M, Humphries P, Paques M, Mohand-Said S, Trono D, Deisseroth K, Sahel JA, Picaud S, Roska B. Genetic reactivation of cone photoreceptors restores visual responses in retinitis pigmentosa. Science. 2010;329:413–417. doi: 10.1126/science.1190897. [DOI] [PubMed] [Google Scholar]
- Campbell G, Shatz CJ. Synapses formed by identified retinogeniculate axons during the segregation of eye input. J Neurosci. 1992;12:1847–1858. doi: 10.1523/JNEUROSCI.12-05-01847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Masuho I, Okawa H, Xie K, Asami J, Kammermeier PJ, Maddox DM, Furukawa T, Inoue T, Sampath AP, Martemyanov KA. Retina-specific GTPase accelerator RGS11/G beta 5S/R9AP is a constitutive heterotrimer selectively targeted to mGluR6 in ON-bipolar neurons. J Neurosci. 2009;29:9301–9313. doi: 10.1523/JNEUROSCI.1367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, Nishiyama N, Nishiyama H. Dendritic translocation establishes the winner in cerebellar climbing fiber synapse elimination. J Neurosci. 2013;33:7641–7653. doi: 10.1523/JNEUROSCI.4561-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Heckenlively JR, Bayley PR, Brecha NC, Davisson MT, Hawes NL, Hirano AA, Hurd RE, Ikeda A, Johnson BA, McCall MA, Morgans CW, Nusinowitz S, Peachey NS, Rice DS, Vessey KA, Gregg RG. The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci. 2006;23:11–24. doi: 10.1017/S095252380623102X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Wu CZ, Knott G, Kuhlman S, Fu Y, Palmiter RD, Huang ZJ. GAD67-mediated GABA synthesis and signaling regulate inhibitory synaptic innervation in the visual cortex. Neuron. 2007;54:889–903. doi: 10.1016/j.neuron.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Claes E, Seeliger M, Michalakis S, Biel M, Humphries P, Haverkamp S. Morphological characterization of the retina of the CNGA3(−/−)Rho(−/−) mutant mouse lacking functional cones and rods. Invest Ophthalmol Vis Sci. 2004;45:2039–2048. doi: 10.1167/iovs.03-0741. [DOI] [PubMed] [Google Scholar]
- Cline HT, Constantine-Paton M. NMDA receptor antagonists disrupt the retinotectal topographic map. Neuron. 1989;3:413–426. doi: 10.1016/0896-6273(89)90201-8. [DOI] [PubMed] [Google Scholar]
- Colman H, Lichtman JW. Interactions between nerve and muscle: synapse elimination at the developing neuromuscular junction. Dev Biol. 1993;156:1–10. doi: 10.1006/dbio.1993.1054. [DOI] [PubMed] [Google Scholar]
- Crepel F, Delhaye-Bouchaud N, Dupont JL. Fate of the multiple innervation of cerebellar Purkinje cells by climbing fibers in immature control, x-irradiated and hypothyroid rats. Brain Res. 1981;227:59–71. doi: 10.1016/0165-3806(81)90094-8. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB, Crair MC. Development of single retinofugal axon arbors in normal and beta2 knockout mice. J Neurosci. 2011;31:3384–3399. doi: 10.1523/JNEUROSCI.4899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra A, Lyubarsky A, Jiang M, Pugh EN, Jr., Birnbaumer L, Sterling P, Vardi N. The light response of ON bipolar neurons requires G[alpha]o. J Neurosci. 2000;20:9053–9058. doi: 10.1523/JNEUROSCI.20-24-09053.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Della Santina L, Parker ED, Wong RO. Sensory experience shapes the development of the visual system’s first synapse. Neuron. 2013;80:1159–1166. doi: 10.1016/j.neuron.2013.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert F, Bonhoeffer T. Synapse specificity of long-term potentiation breaks down at short distances. Nature. 1997;388:279–284. doi: 10.1038/40870. [DOI] [PubMed] [Google Scholar]
- Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K, Wong RO. A comparison of experience-dependent plasticity in the visual and somatosensory systems. Neuron. 2005;48:465–477. doi: 10.1016/j.neuron.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J, Imbert M. Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol. 1984;230:552–575. doi: 10.1002/cne.902300406. [DOI] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Maeda T, Possin DE, Maeda A, Lee A, Rieke F, Palczewski K. Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nat Neurosci. 2004;7:1079–1087. doi: 10.1038/nn1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms KJ, Tovar KR, Craig AM. Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci. 2005;25:6379–6388. doi: 10.1523/JNEUROSCI.0302-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman KN, Pal SK, Burrone J, Murthy VN. Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat Neurosci. 2006;9:642–649. doi: 10.1038/nn1677. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Kitamura K, Watanabe M, Kano M. Translocation of a “winner” climbing fiber to the Purkinje cell dendrite and subsequent elimination of “losers” from the soma in developing cerebellum. Neuron. 2009a;63:106–118. doi: 10.1016/j.neuron.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ichikawa R, Takechi H, Inoue Y, Aiba A, Sakimura K, Mishina M, Hashikawa T, Konnerth A, Watanabe M, Kano M. Roles of glutamate receptor delta 2 subunit (GluRdelta 2) and metabotropic glutamate receptor subtype 1 (mGluR1) in climbing fiber synapse elimination during postnatal cerebellar development. J Neurosci. 2001;21:9701–9712. doi: 10.1523/JNEUROSCI.21-24-09701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Tsujita M, Miyazaki T, Kitamura K, Yamazaki M, Shin HS, Watanabe M, Sakimura K, Kano M. Postsynaptic P/Q-type Ca2+ channel in Purkinje cell mediates synaptic competition and elimination in developing cerebellum. Proc Natl Acad Sci U S A. 2011;108:9987–9992. doi: 10.1073/pnas.1101488108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Yoshida T, Sakimura K, Mishina M, Watanabe M, Kano M. Influence of parallel fiber-Purkinje cell synapse formation on postnatal development of climbing fiber-Purkinje cell synapses in the cerebellum. Neuroscience. 2009b;162:601–611. doi: 10.1016/j.neuroscience.2008.12.037. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Michalakis S, Claes E, Seeliger MW, Humphries P, Biel M, Feigenspan A. Synaptic plasticity in CNGA3(−/−) mice: cone bipolar cells react on the missing cone input and form ectopic synapses with rods. J Neurosci. 2006;26:5248–5255. doi: 10.1523/JNEUROSCI.4483-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, Briggman KL, Turaga SC, Jain V, Seung HS, Denk W. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–174. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- Hoon M, Okawa H, Della Santina L, Wong RO. Functional Architecture of the Retina: Development and Disease. Prog Retin Eye Res. 2014 doi: 10.1016/j.preteyeres.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–1026. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J Comp Neurol. 1972;146:421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Speer CM, Chapman B. Spontaneous retinal activity mediates development of ocular dominance columns and binocular receptive fields in v1. Neuron. 2006;52:247–254. doi: 10.1016/j.neuron.2006.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa R, Miyazaki T, Kano M, Hashikawa T, Tatsumi H, Sakimura K, Mishina M, Inoue Y, Watanabe M. Distal extension of climbing fiber territory and multiple innervation caused by aberrant wiring to adjacent spiny branchlets in cerebellar Purkinje cells lacking glutamate receptor delta 2. J Neurosci. 2002;22:8487–8503. doi: 10.1523/JNEUROSCI.22-19-08487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, Guido W. Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci. 2005;22:661–676. doi: 10.1017/S0952523805225154. [DOI] [PubMed] [Google Scholar]
- Kasthuri N, Lichtman JW. The role of neuronal identity in synaptic competition. Nature. 2003;424:426–430. doi: 10.1038/nature01836. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kauwe G, Isacoff EY. Rapid feedback regulation of synaptic efficacy during high-frequency activity at the Drosophila larval neuromuscular junction. Proc Natl Acad Sci U S A. 2013;110:9142–9147. doi: 10.1073/pnas.1221314110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong RO. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat Neurosci. 2010;13:592–600. doi: 10.1038/nn.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Greene MJ, Zlateski A, Lee K, Richardson M, Turaga SC, Purcaro M, Balkam M, Robinson A, Behabadi BF, Campos M, Denk W, Seung HS. Space-time wiring specificity supports direction selectivity in the retina. Nature. 2014;509:331–336. doi: 10.1038/nature13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach D, Kerov V, Sartori SB, Obermair GJ, Schmuckermair C, Liu X, Sothilingam V, Garrido MG, Baker SA, Glosmann M, Schicker K, Seeliger M, Lee A, Koschak A. Cav1.4 IT mouse as model for vision impairment in human congenital stationary night blindness type 2. Channels (Austin) 2013;7:503–513. doi: 10.4161/chan.26368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Obara T, Uriu Y, Numata T, Sanuki R, Miyata K, Koyasu T, Ueno S, Funabiki K, Tani A, Ueda H, Kondo M, Mori Y, Tachibana M, Furukawa T. TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci U S A. 2010;107:332–337. doi: 10.1073/pnas.0912730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature. 2011;474:100–104. doi: 10.1038/nature09986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky MS, Morrison-Graham K. Structure of developing frog neuromuscular junctions. J Neurocytol. 1980;9:321–342. doi: 10.1007/BF01181540. [DOI] [PubMed] [Google Scholar]
- LeVay S, Gilbert CD. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976;113:1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- LeVay S, Stryker MP, Shatz CJ. Ocular dominance columns and their development in layer IV of the cat’s visual cortex: a quantitative study. J Comp Neurol. 1978;179:223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- LeVay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- Li J, Cline HT. Visual deprivation increases accumulation of dense core vesicles in developing optic tectal synapses in Xenopus laevis. J Comp Neurol. 2010;518:2365–2381. doi: 10.1002/cne.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YN, Matsui JI, Dowling JE. Specificity of the horizontal cell-photoreceptor connections in the zebrafish (Danio rerio) retina. J Comp Neurol. 2009;516:442–453. doi: 10.1002/cne.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DC, Guillery RW, Cucchiaro J. The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol. 1981;203:189–211. doi: 10.1002/cne.902030204. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci U S A. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kerov V, Haeseleer F, Majumder A, Artemyev N, Baker SA, Lee A. Dysregulation of Ca(v)1.4 channels disrupts the maturation of photoreceptor synaptic ribbons in congenital stationary night blindness type 2. Channels (Austin) 2013;7:514–523. doi: 10.4161/chan.26376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Majewska A, Sur M. Motility of dendritic spines in visual cortex in vivo: changes during the critical period and effects of visual deprivation. Proc Natl Acad Sci U S A. 2003;100:16024–16029. doi: 10.1073/pnas.2636949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansergh F, Orton NC, Vessey JP, Lalonde MR, Stell WK, Tremblay F, Barnes S, Rancourt DE, Bech-Hansen NT. Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet. 2005;14:3035–3046. doi: 10.1093/hmg/ddi336. [DOI] [PubMed] [Google Scholar]
- Margeta MA, Shen K. Molecular mechanisms of synaptic specificity. Mol Cell Neurosci. 2010;43:261–267. doi: 10.1016/j.mcn.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J, Changeux JP. Ontogenesis of olivocerebellar relationships. I. Studies by intracellular recordings of the multiple innervation of Purkinje cells by climbing fibers in the developing rat cerebellum. J Neurosci. 1981;1:696–702. doi: 10.1523/JNEUROSCI.01-07-00696.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka RL, Jiang Z, Samuels IS, Nguyen-Ba-Charvet KT, Sun LO, Peachey NS, Chedotal A, Yau KW, Kolodkin AL. Guidance-cue control of horizontal cell morphology, lamination, and synapse formation in the mammalian outer retina. J Neurosci. 2012;32:6859–6868. doi: 10.1523/JNEUROSCI.0267-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Schaferhoff K, Spiwoks-Becker I, Zabouri N, Koch S, Koch F, Bonin M, Biel M, Haverkamp S. Characterization of neurite outgrowth and ectopic synaptogenesis in response to photoreceptor dysfunction. Cell Mol Life Sci. 2013;70:1831–1847. doi: 10.1007/s00018-012-1230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Hashimoto K, Shin HS, Kano M, Watanabe M. P/Q-type Ca2+ channel alpha1A regulates synaptic competition on developing cerebellar Purkinje cells. J Neurosci. 2004;24:1734–1743. doi: 10.1523/JNEUROSCI.4208-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JL, Soto F, Wong RO, Kerschensteiner D. Development of cell type-specific connectivity patterns of converging excitatory axons in the retina. Neuron. 2011;71:1014–1021. doi: 10.1016/j.neuron.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgans CW, Zhang J, Jeffrey BG, Nelson SM, Burke NS, Duvoisin RM, Brown RL. TRPM1 is required for the depolarizing light response in retinal ON-bipolar cells. Proc Natl Acad Sci U S A. 2009;106:19174–19178. doi: 10.1073/pnas.0908711106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz M, Gobert D, Schohl A, Poquerusse J, Podgorski K, Spratt P, Ruthazer ES. Rapid Hebbian axonal remodeling mediated by visual stimulation. Science. 2014;344:904–909. doi: 10.1126/science.1251593. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Jr., Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Okabe S, Kim HD, Miwa A, Kuriu T, Okado H. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nat Neurosci. 1999;2:804–811. doi: 10.1038/12175. [DOI] [PubMed] [Google Scholar]
- Okawa H, Della Santina L, Schwartz GW, Rieke F, Wong RO. Interplay of cell-autonomous and nonautonomous mechanisms tailors synaptic connectivity of converging axons in vivo. Neuron. 2014;82:125–137. doi: 10.1016/j.neuron.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Parks TN, Rubel EW. Organization and development of brain stem auditory nuclei of the chicken: organization of projections from n. magnocellularis to n. laminaris. J Comp Neurol. 1975;164:435–448. doi: 10.1002/cne.901640404. [DOI] [PubMed] [Google Scholar]
- Raven MA, Orton NC, Nassar H, Williams GA, Stell WK, Jacobs GH, Bech-Hansen NT, Reese BE. Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J Comp Neurol. 2008;506:745–758. doi: 10.1002/cne.21526. [DOI] [PubMed] [Google Scholar]
- Raviola E, Dacheux RF. Excitatory dyad synapse in rabbit retina. Proc Natl Acad Sci U S A. 1987;84:7324–7328. doi: 10.1073/pnas.84.20.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern PA. Neuromuscular transmission in new-born rats. J Physiol. 1970;209:701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regus-Leidig H, Atorf J, Feigenspan A, Kremers J, Maw MA, Brandstatter JH. Photoreceptor degeneration in two mouse models for congenital stationary night blindness type 2. PLoS One. 2014;9:e86769. doi: 10.1371/journal.pone.0086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Constantine-Paton M. Eye-specific segregation requires neural activity in three-eyed Rana pipiens. J Neurosci. 1985;5:1132–1143. doi: 10.1523/JNEUROSCI.05-05-01132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science. 2003;301:66–70. doi: 10.1126/science.1082545. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schubert T, Hoon M, Euler T, Lukasiewicz PD, Wong RO. Developmental regulation and activity-dependent maintenance of GABAergic presynaptic inhibition onto rod bipolar cell axonal terminals. Neuron. 2013;78:124–137. doi: 10.1016/j.neuron.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GW, Okawa H, Dunn FA, Morgan JL, Kerschensteiner D, Wong RO, Rieke F. The spatial structure of a nonlinear receptive field. Nat Neurosci. 2012;15:1572–1580. doi: 10.1038/nn.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, Krahe TE, Govindaiah G, Guido W. Interneurons in the mouse visual thalamus maintain a high degree of retinal convergence throughout postnatal development. Neural Dev. 2013;8:24. doi: 10.1186/1749-8104-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Mehta V. The role of early neural activity in the maturation of turtle retinal function. J Anat. 2001;199:375–383. doi: 10.1046/j.1469-7580.2001.19940375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless SK. Supersensitivity-like phenomena in the central nervous system. Fed Proc. 1975;34:1990–1997. [PubMed] [Google Scholar]
- Shatz CJ. The prenatal development of the cat’s retinogeniculate pathway. J Neurosci. 1983;3:482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Kirkwood PA. Prenatal development of functional connections in the cat’s retinogeniculate pathway. J Neurosci. 1984;4:1378–1397. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Lindstrom S, Wiesel TN. The distribution of afferents representing the right and left eyes in the cat’s visual cortex. Brain Res. 1977;131:103–116. doi: 10.1016/0006-8993(77)90031-2. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat’s visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She K, Craig AM. NMDA receptors mediate synaptic competition in culture. PLoS One. 2011;6:e24423. doi: 10.1371/journal.pone.0024423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Scheiffele P. Genetics and cell biology of building specific synaptic connectivity. Annu Rev Neurosci. 2010;33:473–507. doi: 10.1146/annurev.neuro.051508.135302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Jahr CE. Rapid Induction of Filopodial Sprouting by Application of Glutamate to Hippocampal Neuron book chaper in ‘The Nerve Growth Cone’. Raven Press Ltd; New York: 1992. [Google Scholar]
- Sorensen SA, Rubel EW. The level and integrity of synaptic input regulates dendrite structure. J Neurosci. 2006;26:1539–1550. doi: 10.1523/JNEUROSCI.3807-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Ma X, Cecil JL, Vo BQ, Culican SM, Kerschensteiner D. Spontaneous activity promotes synapse formation in a cell-type-dependent manner in the developing retina. J Neurosci. 2012;32:5426–5439. doi: 10.1523/JNEUROSCI.0194-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Watkins KL, Johnson RE, Schottler F, Kerschensteiner D. NGL-2 regulates pathway-specific neurite growth and lamination, synapse formation, and signal transmission in the retina. J Neurosci. 2013;33:11949–11959. doi: 10.1523/JNEUROSCI.1521-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht D, Wu SB, Turner P, Dearden P, Koentgen F, Wolfrum U, Maw M, Brandstatter JH, tom Dieck S. Effects of presynaptic mutations on a postsynaptic Cacna1s calcium channel colocalized with mGluR6 at mouse photoreceptor ribbon synapses. Invest Ophthalmol Vis Sci. 2009;50:505–515. doi: 10.1167/iovs.08-2758. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, O’Leary DD, Fricks C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature. 1982;298:371–373. doi: 10.1038/298371a0. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I. Organization and remodeling of the olivocerebellar climbing fiber projection. Cerebellum. 2006;5:15–22. doi: 10.1080/14734220500527385. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Sawai H, Ueda Y, Tauchi M, Nakanishi S. Immunohistological studies of metabotropic glutamate receptor subtype 6-deficient mice show no abnormality of retinal cell organization and ganglion cell maturation. J Neurosci. 1999;19:2568–2579. doi: 10.1523/JNEUROSCI.19-07-02568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao HW, Zhang LI, Engert F, Poo M. Emergence of input specificity of ltp during development of retinotectal connections in vivo. Neuron. 2001;31:569–580. doi: 10.1016/s0896-6273(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ishii M, Takao M, Iwatsuki K, Nakanishi S, Fukuda Y. A novel connection between rods and ON cone bipolar cells revealed by ectopic metabotropic glutamate receptor 7 (mGluR7) in mGluR6-deficient mouse retinas. J Neurosci. 2007;27:6261–6267. doi: 10.1523/JNEUROSCI.5646-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–324. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Kakizawa S, Yamasaki M, Sakimura K, Watanabe M, Iino M, Mishina M. Regulation of long-term depression and climbing fiber territory by glutamate receptor delta2 at parallel fiber synapses through its C-terminal domain in cerebellar Purkinje cells. J Neurosci. 2007;27:12096–12108. doi: 10.1523/JNEUROSCI.2680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Zhao JP, Constantine-Paton M. Synaptic drive at developing synapses: transient upregulation of kainate receptors. Dev Neurobiol. 2010;70:737–750. doi: 10.1002/dneu.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rubel EW. In vivo reversible regulation of dendritic patterning by afferent input in bipolar auditory neurons. J Neurosci. 2012;32:11495–11504. doi: 10.1523/JNEUROSCI.1737-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Kano M. Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur J Neurosci. 2011;34:1697–1710. doi: 10.1111/j.1460-9568.2011.07894.x. [DOI] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2011;469:402–406. doi: 10.1038/nature09600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS. Six different roles for crossover inhibition in the retina: correcting the nonlinearities of synaptic transmission. Vis Neurosci. 2010;27:1–8. doi: 10.1017/S0952523810000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, Lam DM. Autoradiographic demonstration of ocular-dominance columns in the monkey striate cortex by means of transneuronal transport. Brain Res. 1974;79:273–279. doi: 10.1016/0006-8993(74)90416-8. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]
- Wu X, Fu Y, Knott G, Lu J, Di Cristo G, Huang ZJ. GABA signaling promotes synapse elimination and axon pruning in developing cortical inhibitory interneurons. J Neurosci. 2012;32:331–343. doi: 10.1523/JNEUROSCI.3189-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda M, Johnson-Venkatesh EM, Zhang H, Parent JM, Sutton MA, Umemori H. Multiple forms of activity-dependent competition refine hippocampal circuits in vivo. Neuron. 2011;70:1128–1142. doi: 10.1016/j.neuron.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimatsu T, Williams PR, D’Orazi FD, Suzuki SC, Fadool JM, Allison WT, Raymond PA, Wong RO. Transmission from the dominant input shapes the stereotypic ratio of photoreceptor inputs onto horizontal cells. Nat Commun. 2014;5:3699. doi: 10.1038/ncomms4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Power J, Barnea G, O’Donnell S, Brown HE, Osborne J, Axel R, Gogos JA. Spontaneous neural activity is required for the establishment and maintenance of the olfactory sensory map. Neuron. 2004;42:553–566. doi: 10.1016/s0896-6273(04)00224-7. [DOI] [PubMed] [Google Scholar]
- Zabouri N, Haverkamp S. Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One. 2013;8:e63853. doi: 10.1371/journal.pone.0063853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JP, Murata Y, Constantine-Paton M. Eye opening and PSD95 are required for long-term potentiation in developing superior colliculus. Proc Natl Acad Sci U S A. 2013;110:707–712. doi: 10.1073/pnas.1215854110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziburkus J, Guido W. Loss of binocular responses and reduced retinal convergence during the period of retinogeniculate axon segregation. J Neurophysiol. 2006;96:2775–2784. doi: 10.1152/jn.01321.2004. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Smith SJ. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron. 1996;17:91–102. doi: 10.1016/s0896-6273(00)80283-4. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, Firestein S. Postnatal refinement of peripheral olfactory projections. Science. 2004;304:1976–1979. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]