Abstract
The exact origin of the neointimal cells in arteriovenous fistulae (AVFs) has not been conclusively established. This study attempts to elucidate the anatomical source of neointimal cells in AVFs. Experimental fistulas were created in Lewis wild type (WT) and transgenic rats that constitutively expressed the Green Fluorescent Protein (GFP) in all tissues. AVFs were created by anastomosing the left renal vein to the abdominal aorta after unilateral nephrectomy. Three potential sources (bone marrow, feeding artery and the fistula vein itself) were examined. The contribution of bone marrow (BM) derived cells to the AVF neointima was examined in lethally irradiated WT rats that had been rescued with GFP BM cells. Neointimal cells in these chimeric rats were mostly GFP negative indicating the non-BM origin of those cells. Then, the contribution of arterial cells to the AVF neointima was assessed in a fistula made with a GFP aorta that had been implanted orthotopically into a WT rat. In this model, most of the neointimal cells were also GFP negative demonstrating that AVF neointimal cells are not derived from the feeding artery cells. Finally to study local resident cells contribution to the formation of neointimal lesions, a composite fistula was created by interposing a GFP vein between the renal vein and the aorta in a WT recipient rat. GFP neointimal cells were only found in the transplanted vein. This study suggests that neointimal cells originate from the local resident cells of the AVF.
Keywords: arteriovenous fistula, neointimal cell, vein, origin, vascular access
The arteriovenous fistula (AVF) is the preferred type of vascular access for hemodialysis patients (1). However, this type of access is frequently complicated by non-maturation (20–50%) after creation and subsequent dysfunction of mature AVFs (2,3). These complications are mainly attributed to vascular stenosis due to the development of neointimal hyperplasia (NIH). This histological lesion is responsible for a variety of clinical predicaments including prolonged access bleeding after dialysis needle removal, suboptimal urea clearance as well as dialysis adequacy (low Kt/v), high recirculation, and abnormal dialysis machine pressures (2, 3). If uncorrected, the stenosis could lead to thrombosis resulting in the loss of lifeline of the patient (2, 3).
For the most part, NIH in an AVF is formed by myofibroblasts and to lesser extent, by contractile smooth muscle cells (4). The traditional view on the pathogenesis of NIH emphasizes that the lesion is comprised of proliferative smooth muscle cells that migrate and accumulate into the intima (5). However, recent publications in the field of arterial remodeling suggest that adventitial (6) and bone marrow (BM) derived cells can also contribute to neointimal formation (7).
Whether neointimal cells in the AVF originate from BM-progenitors, the feeding artery, or the local venous wall itself remain elusive. We used a novel method to create AVFs in rats and applied high resolution immunofluorescence microscopy to study the anatomical source of neointimal cells in the AVF. Our systematic analysis of the bone marrow, feeding artery and the fistula vein itself for the origin of cells that make up NIH lesion forms the basis of this study.
METHODS
Rats
The heterozygous Lewis transgenic rats from the Rat Resource and Research Center (Columbia, MO) were bred at our facilities. Heterozygous Lewis transgenic rats constitutively express the Green Fluorescent Protein (GFP) in all tissue cells (including vascular wall cells and the neointimal cells) (8). Littermate wild type (WT) animals were used as recipients. All animal procedures were revised and approved by the University of Miami Institutional Animal Care & Use Committee, protocols 08-065 and 10-009.
Bone marrow transplantation
Chimeric rats were generated as shown in Figure 1A (9). Wild type animals were lethally irradiated with a single dose of 1025 cGy (Cs-137 source, Nordion, Ontario) before receiving 8 × 107 GFP BM cells via the jugular vein. One month later, the number of GFP cells in peripheral blood of recipient rats was assessed using fluorescence-activated cell sorting analysis (BD LSR System I, BD Biosciences, San Jose, CA).
Figure 1. Minimal contribution of bone marrow (BM) derived cells to the arteriovenous fistula (AVF) neointima.
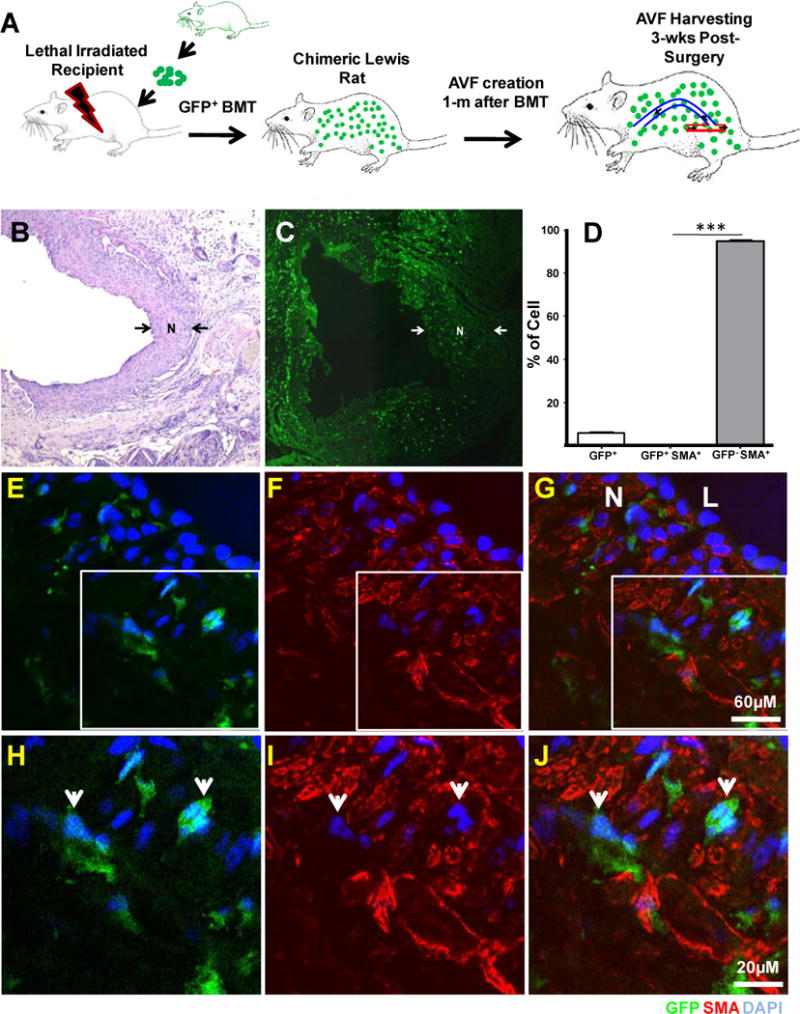
A. Schematic representation of the methodology to generate chimeric rats. Lethal irradiated animals were rescued with a single injection of GFP BM cells from transgenic rats. AVFs were created in chimeras one month after and harvested three weeks post surgery.
B. Representative picture of Hematoxylin and Eosin (H & E) stained fistula created in the chimeric rat. Neointima (N) appears between arrows.
C. Confocal image of a cross-section from fistula placed in a chimeric rat (x100). BM-derived cells (GFP+ cells, green) were found sparse throughout the fistula wall. Neointima (N) is noticed again between arrows
D. Histogram showing the number of GFP and GFP SMA positive and negative cells in the neointima of AV fistulas placed in chimeric rats. Bars represent the mean ± SEM, n=6. ***p<0.01.
E–J. Double immunofluorescence confocal microscopy of BM-derived cells in the AVF neointima (GFP+, green). Microphotographies from the GFP (E and H) and SMA (F and I) channels were overlaid on Figures G and J. Top images were taken at x400. Boxed areas were magnified below (x630). Representative GFP+ SMA− cells are pointed with arrows. Nuclei were DAPI counter stained (blue). N: neointima; L: Lumen
Surgical Procedures
Only male (2–4 month-old) rats were included in the study. All surgeries were performed under isoflurane anesthesia (Webster Veterinary, Ocala, FL). The aorto-caval fistula was created as follow (10). The abdominal cavity was accessed through a midline incision from the xiphoid process to the symphysis pubis. Retractors were placed to keep exposure. The abdominal contents were reflected to the right to expose the inferior vena cave, aorta and the left kidney. The intestines were wrapped in saline- soaked gauze to keep them moist and warm. The Gerota’s Fascia of the left kidney was incised using an electrocautery and the renal artery and the ureter were both ligated with 8.0 prolene suture (Ethicon, San Lorenzo, PR). The renal vein was occluded with a 2 mm microvascular clamp and the left kidney was amputated at the distal renal vein. The infrarenal aorta was dissected from the inferior vena cava and a curve vascular clamp was placed in the free aorta to maintain proximal and distal vascular control.
Approximately 1 mm arteriotomy was created with microvascular scissors and the lumen of the aorta and renal vein were flushed with saline and heparin. The anastomosis of the distal end of renal vein to the aorta was performed using 10.0 monofilament suture (Ethicon). Following completion of the anastomosis the venous and arterial clamps were removed and bleeding assessed. In case of leakage, interrupted #10 monofilament stitch was used when needed. The abdomen was irrigated with sterile normal saline and all abdominal content placed in their normal anatomical position. Closure of abdominal cavity was performed in layers using interrupted suture pattern with 4.0 absorbable sutures. In this model blood circulates from the aorta through the renal vein (AVF) and to the inferior vena cava.
Two additional experimental AVFs were created to study the contribution of arterial versus venous cells to the neointimal formation. The first of those models investigated the contribution of arterial cells to the neointima. In here, the descending thoracic aorta that included adventitia and most of surrounding connective tissue from a GFP or a WT rat were transplanted to the abdominal aorta of a WT or a GFP recipient (9). After the arterial transplant was established, the AVF was created as described above using the graft as the feeding artery. A schematic representation of these fistulae is depicted in Figure 2A and B, and representative microphotography is shown in Figure I of supplemental data. The second model was created to evaluate the venous origin of neointimal cells in the rat AVF. For this, a 2mm fragment of vena cava from a GFP donor rat was interposed using end-to-end anastomosis between the aorta and renal vein of a WT recipient rat (Figure 3A and Figure III of supplemental data). No immunosuppressive or anticoagulant treatment was used postoperatively.
Figure 2. Arterial cells do not contribute to the neointima in the venous limb of the fistula.
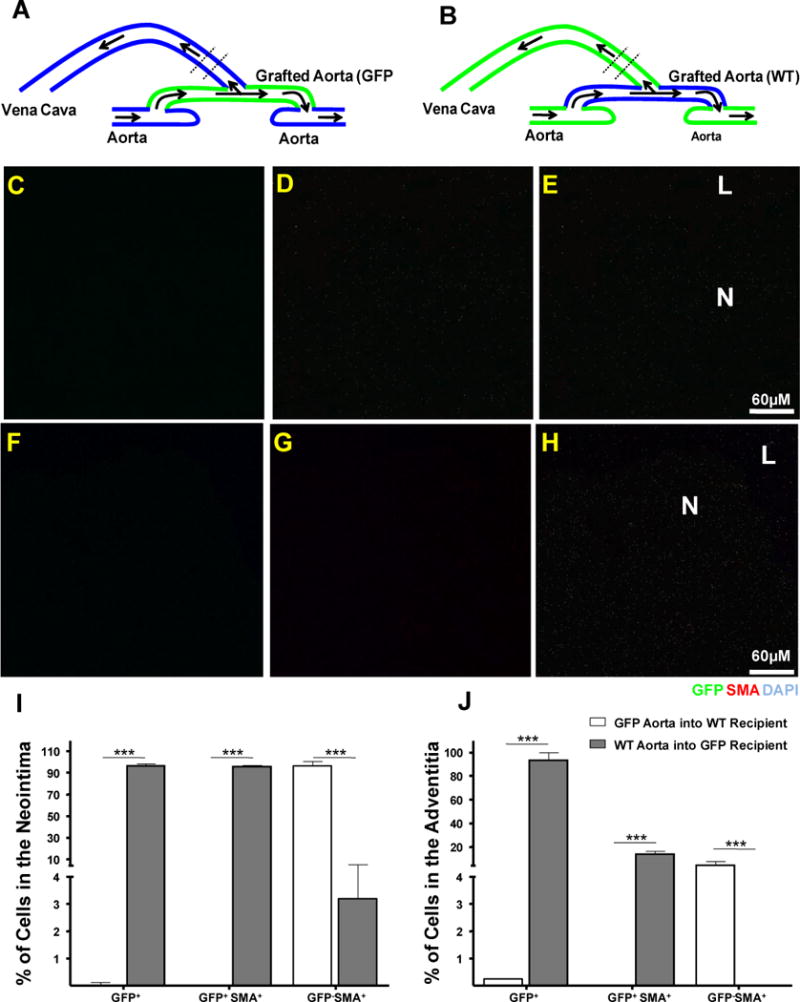
A and B Diagrammatic representations of AVF constructed in WT or GFP rats using previously transplanted GFP or WT arterial limbs, respectively. The area used for cross sections appears between discontinuous lines.
C–E Detection of GFP+ (C, green) SMA+ (D, red) cells in the neointima of a representative WT AV constructed using a GFP grafted aorta.
F–H Detection of GFP+ (F, green) SMA+ (G, red) cells in the neointima of a representative GFP AV fistula constructed using a WT grafted aorta. Nuclei were counter stained with DAPI (blue). Panels in each set are, from left to right, GFP (C and F), SMA (D and G) and GFP/SMA/DAPI merged pictures (E and H). Arrows represent the area where sections were taken.
I and J Histograms showing the number of GFP and GFP SMA positive and negative cells in the neointima and adventitia of above AVFs. Bars represent the mean ± SEM, n=3. *** p<0.01.
N: Neointima; L: Lumen.
Figure 3. Neointimal cells in AVF are originated from cells in the vein.
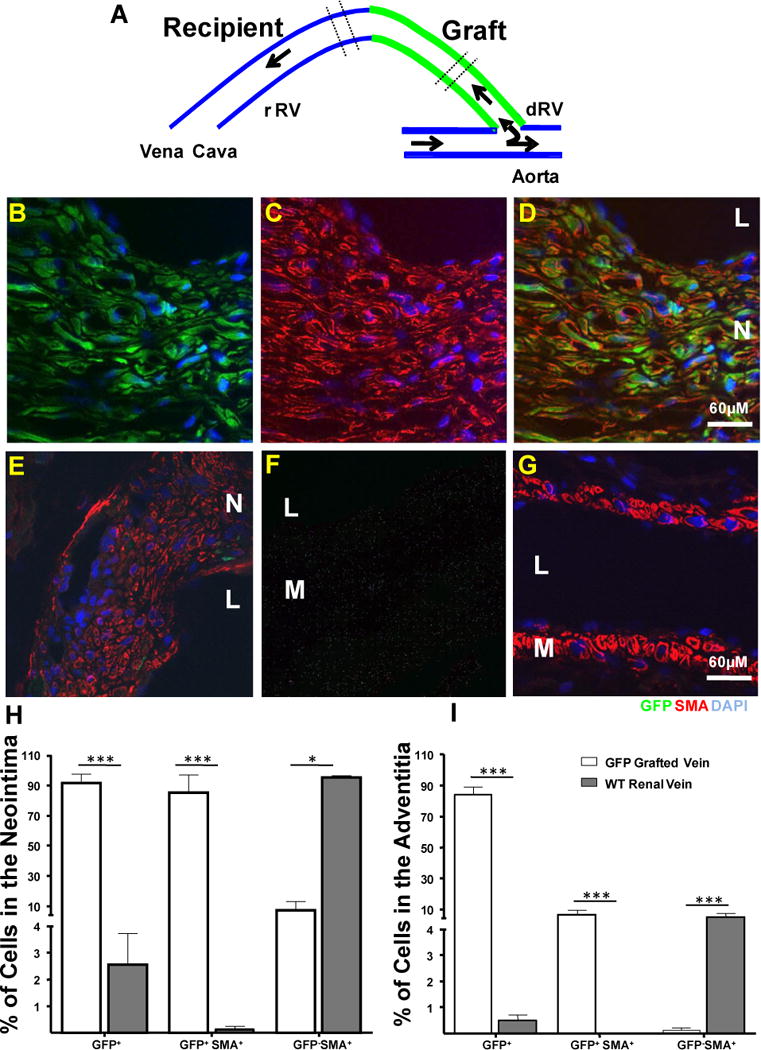
A. Diagrammatic representation of the composite AVF created by interposing a GFP vena cava between the recipient renal vein and the aorta of a WT recipient. The area used for cross sections appears between discontinuous lines.
B–D. Representative pictures of neointimal cells in the grafted vein. Most of those cells stained positively for GFP and SMA which revealed that those cells were originated from local cells in the graft. Microphotographies from the GFP (green, B) and SMA (red, C) channels were overlaid on Figure D.
E. Representative section from the recipient renal vein. This section was taken from an area close to the vein-to-vein anastomotic point. Most of the neointimal cells were SMA+ (red) GFP−(green). Only rare GFP+ cells from the graft were found infiltrated in this part of the neointima.
F and G. Sections from the recipient aorta and the vena cava showed the absence of tissue autofluorescence and neointima in those vessels. Vascular smooth muscle cells were stained with an antibody against SMA (red). No green autofluorescence was detected. Nuclei were counter stained with DAPI (blue). M: Media; N: Neointima; L: Lumen.
H and I Histograms showing the number of GFP and GFP SMA positive and negative cells in the neointima and adventitia of the composite fistula. Bars represent the mean ± SEM, n=3. ***p<0.01; * p<0.05.
Tissue harvesting
Three weeks after surgery the fragments of descending aorta, AVF, and superior vena cava were harvested and fixed in 1% paraformaldehyde / 0.1 glutaraldehyde / 20% sucrose for 2h. Specimens were immersed in 20% sucrose for an additional 24h before embedding in OCT for cryosectioning. These conditions ensure low tissue autofluorescence while preserving GFP structure and avoiding protein leaking out of cells (11).
Immunofluorescence confocal microscopy
Ten μM cross-sections were rehydrated in PBS for 20 minutes and non specific binding sites were blocked with 10% goat serum (Chemicon International, Temecula, CA) in PBS for 1h at room temperature. Primary monoclonal antibodies, anti-smooth muscle cell actin (SMA, Dako, Carpinteria, CA) and anti-CD45 (BD Biosciences) were diluted with the blocking solution and applied onto sections overnight at 4°C. After washing twice with PBS for 3 min, tissue sections were incubated with Alexa Fluor 546 goat anti-mouse (Invitrogen, Carlsbad, CA) for 90 min at room temperature. Sections were mounted in Vectashield w/DAPI (Vector Laboratories, Burlingame, CA) and examined with a confocal scanning laser microscope Zeiss LSM 510 META (Carl Zeiss MicroImaging, Inc., Thornwood, NY) in an inverted configuration. Data was captured and analyzed with Zeiss LSM 510 Meta and Image Browser software (Carl Zeiss). Images were acquired with the use of sequential capture mode to avoid potential fluorescence bleed-through between channels. All images were captured with a plain-neofluor 40x/1.3 Oil DIC objective lens. Up to 12 optical slices of 1 μm in depth each were recorded for every sample. The three grayscale images were electronically merged to produce a pseudocolored image: blue for nuclei, red for SMA, and green for GFP. The signal-to-noise ratio was always adjusted with sections from transgenic (positive) and WT (negative) rats.
Statistical analysis
The number of GFP positive versus total number of cells (frequency) in the neointima and adventitia of each AVF is presented in Table I (Supplemental Data). At least 10 independent sections were inspected for each specimen. Data were expressed as means ± SEM. Two-group comparison was performed using Student’s t-test for independent samples. Statistics were calculated with GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
RESULTS
The contribution of BM circulating progenitor cells to AVF neointimal formation
The contribution of BM-derived cells to the fistula neointima was studied in chimeric rats that specifically expressed GFP in the BM and BM-derived cells (Figure 1A). In most of those rats the 96.40 ± 7.51 % of blood peripheral cells were GFP+ at the time of surgery (9). Only six out of eight chimeric rats (75%) that underwent surgery for AVF creation reached the end of the experiment (3 wks). Neointimal hyperplasia was detected in all fistulae (Figure 1B).
A significant number of GFP cells (BM-derived) were found infiltrated into the AVF vascular wall at three weeks post surgery. GFP cells were less abundant in the neointima than in the adventitia (5.65 ± 2.03% vs. 40.12 ± 5.40 %, n=6, Figure 1C). The frequency of GFP cells (BM-derived) in the neointima of the AV fistula wall is shown in Figure 1D and in Table I of supplemental data. The infiltrated GFP cells either in neointima or adventitia were negative for the neointimal cell marker SMA (Figure 1E–J). These findings indicated that neointimal cells in the venous limb of the fistula do not come from BM.
The contribution of arterial cells to AVF neointimal formation
As mentioned above, a fistula was created using a GFP aortic graft transplanted to a WT recipient to assess the contribution of arterial versus recipient cells to the neointimal formation (Figure 2A). In this model, double positive cells for GFP and SMA in the neointima would indicate mobilization of pathogenic cells from the aorta towards the venous limb of the fistula. No GFP+ cells were found in those fistulas near the anastomosis point which indicated minimal to no contribution of arterial cells to the neointima (0.045 ± 0.09 %, n=4, Figures 2 C–E). The feeding artery showed no signs of inflammation. The cells (smooth muscle and endothelial) in the graft were appeared normal and, as expected, expressed GFP and SMA. This fact ruled out the possibility of anti-GFP immune reactivity that could potentially compromise the graft survival (Figure II of supplemental data). We also confirmed the results by creating an AV fistula with a WT aorta that had been placed in a GFP recipient (Figure 2B). As expected, most of the neointimal cells in the venous side of this fistula were GFP+ SMA+ (95.74 ± 1.48 %, n=3, Figure 2F–H). The frequency of GFP+ cells in the neointima and adventitia of these fistulas is shown on Figure I and J.
The venous origin of neointimal cells in the rat AVF
Finally, we aimed at studying the resident cells of the vein contribution to the AVF neointimal formation. For this purpose, a GFP-WT composite fistula was created (Figure 3A and Figure III of supplemental data) as mentioned above. If neointimal cells are originating from pre-existing cells in the vein, the neointima in the graft has to be largely populated by GFP+ SMA+ cells. The results revealed that neointimal cells in the graft were mostly GFP+ SMA+ (84.94 ± 11.76 %, n=3, Figure 3B–D) and GFP− SMA+ (95.19 ± 1.09 %, n=3,) in the recipient vein (Figure 3E). Figures I and J depict the frequency of GFP cells in the neointima and adventitia of composite AVFs. Rare GFP cells from the graft were found infiltrated in the recipient neointima (Figure 3E). No neointima formation was observed in the aorta and vena cava of those animals (Figure F and G). These results confirmed that neointimal cells in AVFs are mostly derived from pre-existing cells in the vein.
DISCUSSION
The development of neointimal hyperplasia is the most common cause of arteriovenous fistulae dysfunction (2–4). In this context, understanding the cellular and molecular mechanisms underlying NIH becomes a critical component for the application of therapeutic interventions to halt the process. Such interventions might be an approach to ameliorate stenosis and prolong access patency.
The current study investigated the anatomical source for NIH. In the first part of this study, we investigated the contribution of BM-derived cells to the AVF neointimal formation. In line with previous studies (12,13), our results demonstrated that BM-derived cells (progenitor cells) do not directly contribute to the NIH formation in AVFs. These results are also consistent with previous investigators who demonstrated that in coronary and peripheral circulation that neointimal cells in the arterial lesions (post-injury model) do not come from BM (9,14,15). However, BM-derived cells infiltrating the adventitia of the vascular wall may still have a crucial role on vascular remodeling as they can actively secrete growth factors and extracellular matrix components (16). Further research is needed to clarify whether BM-derived cells are essential for pathogenic remodeling of the fistula wall.
In the second part of this study, we challenged the idea of having migrating cells from the feeding artery to contribute to the neointimal formation in the fistula (venous side). The hypothesis is supported by previous literature emphasizing that arterial adventitial progenitor cells can differentiate into SMCs and contribute to the neointimal formation in vein grafts (17). Nonetheless, we could not find clues for the participation of arterial cells in the formation of the AVF neointimal hyperplasia. Finally, we aimed at providing direct evidence to prove that neointimal cells originated from pre-existing cells in the vein. It was achieved by the mean of a composite AVF formed by both, WT and GFP veins. As expected, GFP neointimal cells were only found in the GFP segment of the fistula. Interestingly, only a small number of cells from the graft (GFP+) were found within the recipient’s neointima documenting the restricted mobility of neointimal cells in the fistula.
The important question now is which cells within the fistula wall migrate to the sub-endothelial space to cause NIH formation and the development of subsequent stenosis? There are three obvious possibilities: endothelial cells, smooth muscle cells and adventitial fibroblasts. Because of their extraordinary plasticity, the smooth muscle cells have been traditionally considered to be the major precursor of neointimal cells (5). Conversely, the role of the adventitia in vascular remodeling has become more evident. The resident cells in the adventitia can undergo substantial structural and functional changes in response to stress/injury and other factors. Adventitial fibroblasts can migrate through the vascular wall and form the neointima (6). The possibility of having endothelial-to-mesenchymal transition in the AV fistula cannot be excluded. It is well accepted now that under certain stimulus endothelial cells can acquire the myofibroblast phenotype and perhaps participate in the neointimal formation (18). Indeed more research is needed to further clarify the contribution of these cell types to the AVF neointimal formation.
Limitations
The major limitation of the current study is that rats included in this study were not end stage renal disease models. Perhaps the use of rat models with end stage renal disease would have provided closer results to what is observed in hemodialysis patients. However, outcomes from the current proposal will generate baseline data on the mechanism underlying neointimal formation in AVFs and confer further support to dispensing more complex studies. The neointimal development in this rat aorto-caval fistula model was limited to the renal vein and no significant remodeling of the feeding artery (aorta) was found at the end of the experiment (4 weeks post surgery). The lack of significant arterial abnormalities may be due the fact that duration of the study was restricted to a 4-week period. Other limitations of the study are the anatomical location of the experimental fistula (abdominal cavity) versus human superficial AVF and the differences in hemodynamic forces between humans and rodents which may modify the degree of intimal hyperplasia (19).
CONCLUSION
The current study demonstrates that: 1) neointimal cells do not come from BM, 2) arterial cells do not contribute to the neointimal formation in AVF, and 3) neointimal cells originate from pre-existing cells in the vein.
Supplementary Material
Acknowledgments
We thank Lucinda Greenaway and Irene Hung for clerical assistance. This work was supported by the National Heart Lung and Blood Institute [1K01HL096413-01] to RIVP.
References
- 1.Fistula First National Vascular Access Improvements Initiative. Available at: http://www.fistulafirst.org/. Accessed January 192007.
- 2.Asif A, Roy-Chaudhury P, Beathard GA. Early arteriovenous fistula failure: a logical proposal for when and how to intervene. Clin J Am Soc Nephrol. 2006;1:332–339. doi: 10.2215/CJN.00850805. [DOI] [PubMed] [Google Scholar]
- 3.Asif A, Lenz O, Merrill D, Cherla G, Cipleu CD, Ellis R, Francois B, Epstein DL, Pennell P. Percutaneous management of perianastomotic stenosis in arteriovenous fistulae: results of a prospective study. Kidney Int. 2006;69:1904–1909. doi: 10.1038/sj.ki.5000358. [DOI] [PubMed] [Google Scholar]
- 4.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24:2786–2791. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 7.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 8.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline Transmission and Tissue-Specific Expression of Transgenes Delivered by Lentiviral Vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Menocal L, St-Pierre M, Wei Y, Khan S, Mateu D, Calfa M, Rahnemai-Azar AA, Striker G, Pham SM, Vazquez-Padron RI. The origin of post-injury neointimal cells in the rat balloon injury model. Cardiovasc Res. 2009;81:46–53. doi: 10.1093/cvr/cvn265. [DOI] [PubMed] [Google Scholar]
- 10.Manning E, Nikolaos S, Velazquez Omaida JG-CP, Asif A, Salman LH, Vazquez-Padron RI. A New Aorto-Caval Fistula Model to Study the Development of Neointimal Hyperplasia. Under Revision in J Vas Surg. doi: 10.1159/000332327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brazelton TR, Blau HM. Optimizing techniques for tracking transplanted stem cells in vivo. Stem Cells. 2005;23:1251–1265. doi: 10.1634/stemcells.2005-0149. [DOI] [PubMed] [Google Scholar]
- 12.Kokubo T, Ishikawa N, Uchida H, Chasnoff SE, Xie X, Mathew S, Hruska KA, Choi ET. CKD accelerates development of neointimal hyperplasia in arteriovenous fistulas. J Am Soc Nephrol. 2009;20:1236–1245. doi: 10.1681/ASN.2007121312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castier Y, Lehoux S, Hu Y, Foteinos G, Tedgui A, Xu Q. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. Kidney Int. 2006;70:315–320. doi: 10.1038/sj.ki.5001569. [DOI] [PubMed] [Google Scholar]
- 14.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time-course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 15.Iwata H, Manabe I, Fujiu K, Yamamoto T, Takeda N, Eguchi K, Furuya A, Kuro-o M, Sata M, Nagai R. Bone Marrow-Derived Cells Contribute to Vascular Inflammation but Do Not Differentiate Into Smooth Muscle Cell Lineages. Circulation. 2010:2048–2057. doi: 10.1161/CIRCULATIONAHA.110.965202. [DOI] [PubMed] [Google Scholar]
- 16.Simper D, Mayr U, Urbich C, Zampetaki A, Prokopi M, Didangelos A, Saje A, Mueller M, Benbow U, Newby AC, Apweiler R, Rahman S, Dimmeler S, Xu Q, Mayr M. Comparative proteomics profiling reveals role of smooth muscle progenitors in extracellular matrix production. Arterioscler Thromb Vasc Biol. 2010;30:1325–1332. doi: 10.1161/ATVBAHA.110.204651. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 19.Greve JM, Les AS, Tang BT, Draney Blomme MT, Wilson NM, Dalman RL, Pelc NJ, Taylor CA. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am J Physiol Heart Circ Physiol. 2006;291:H1700–1708. doi: 10.1152/ajpheart.00274.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


