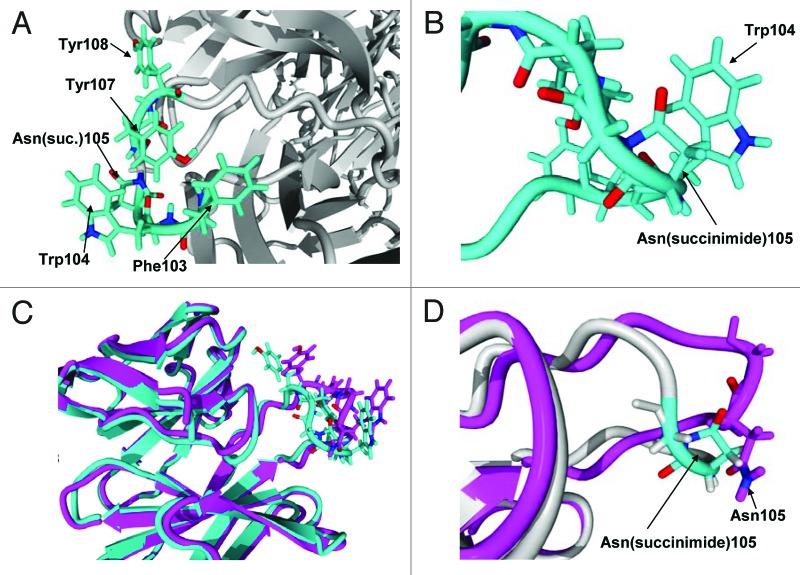
Figure 12. (A) Hydrophobic rich region in CDR3. There are four hydrophobic residues with high solvent accessibility shown in the figure: Phe103, Trp104, Tyr107 and Tyr108. (B) Expanded view of Asn(succinimide)105 and Trp104. The two π bond rich systems have close to a planar relationship as is typical for π stacking interactions. (C) Tertiary alignment of molecular models of Fabs. The energy minimized model of the CDR3 containing an asparagine at position 105 is shown in magenta and the energy minimized region showing the cyclic imide (succinimide105) is shown in teal. Changes to both structures are observed in both the backbone and side chain orientations within CDR3. (D) Comparison of the backbones of the two energy minimized regions of the native structure and the stable cyclic imide.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.