Abstract
The neurotrophin receptor p75NTR is utilized by a variety of pathogens to gain entry into the central nervous system (CNS). We tested if this entry portal might be exploited using a phage display library to isolate internalizing antibodies that target the CNS in vivo. By applying a phage library that expressed human single chain variable fragment (scFv) antibodies on their surface to a transected sciatic nerve, we showed that (1) phage conjugated to anti-p75NTR antibody or phage scFv library pre-panned against p75NTR are internalized by neurons expressing p75NTR; (2) subsequent retrograde axonal transport separates internalized phage from the applied phage; and, (3) internalized phage can be recovered from a proximal ligature made on a nerve. This approach resulted in 13-fold increase in the number of phage isolated from the injured nerve compared with the starting population, and isolation of 18 unique internalizing p75NTR antibodies that were transported from the peripheral nerve into the spinal cord, through the blood-brain barrier. In addition, antibodies recognizing other potentially internalized antigens were identified through in vivo selection using a fully diverse library. Because p75NTR expression is upregulated in motor neurons in response to injury and in disease, the p75NTR antibodies may have substantial potential for cell-targeted drug/gene delivery. In addition, this novel selection method provides the potential to generate panels of antibodies that could be used to identify further internalization targets, which could aid drug delivery across the blood-brain barrier.
Keywords: phage display, internalizing antibodies, retrograde transport, blood-brain barrier, scFv, p75NTR
Introduction
The p75 neurotrophin receptor (p75NTR)1 is arguably the most interesting member of the numerous receptors known to regulate neurotrophin signaling. It is promiscuous (i.e., it binds to all neurotrophins), associates with a variety of neurotrophin and other receptors, and therefore is implicated in eliciting or modulating a diverse range of biological actions including neuronal survival, differentiation and cell death.2,3 It is constitutively expressed at a high level in some neurons (e.g., in basal forebrain); however, it is also a marker of injured or degenerating motor neurons being upregulated following an axotomising injury4 or in diseases such as amyotrophic lateral sclerosis.5 In addition to binding neurotrophins, p75NTR interacts with an impressive array of proteins, including lectins (e.g., wheat germ agglutinin), and pathogens (e.g., neurotoxic forms of prion proteins, β-amyloid, rabies viral capsid glycoprotein, tetanus toxin and cholera toxin in association with gangliosides).6,7 This suggests that the trafficking resulting from p75NTR endocytosis allows pathogens access to intracellular pathways important for the control of significant cellular responses.
Such an entry route has been utilized in research by coupling fluorescein to antibodies against p75NTR, resulting in a ‘Trojan Horse’ reagent that labels p75NTR-expressing neurons intracellularly,8 or by coupling toxins to eliminate specific neuronal populations.9 Previously, we used this approach to construct an immunogene complex consisting of an anti-p75NTR antibody MC19210 coupled to a plasmid DNA to achieve functional gene delivery to motor neurons11 and basal forebrain neurons,12 resulting in selective transduction of these neuronal populations expressing the receptor.
While MC192 has proven useful for targeted delivery in the rat as it activates receptor internalization,8 not all antibodies that bind receptors are internalized effectively,13 reflected by the limited availability of other anti-p75NTR antibodies with this functionality. Screening for such antibodies generated from conventional hybridoma technology is slow, laborious and expensive. Phage display is a proven alternative to effectively isolate peptides and antibodies against purified target antigens.14-17 Despite some successes, isolation of targeted internalizing antibodies using phage display remains challenging due to the limitations in the selection approach with cell lines in vitro, necessitating further validations in vivo.13,18-22 Given that a variety of pathogens utilize p75NTR to gain entry to the central nervous system (CNS), we wondered if a phage display library could be applied in vivo to isolate internalizing antibodies useful for targeted therapeutics, and whether the characteristics of the antibodies would reveal insight into the requirement for internalization.
The sciatic nerve is a potential portal of entry into the CNS that bridges the peripheral nerves to the cell bodies within the spinal cord through the blood-brain barrier. It is surgically accessible, and can be ligated to cause an injury to upregulate p75NTR and to trap retrogradely transported receptor.23 The bundle of axons that constitute the sciatic nerve allowed a high concentration of phage to be applied to a large number of neurons with relatively little diffusion in an in vivo environment. Furthermore, the property of retrograde transport physically isolated internalized phage, allowing recovery of accumulated phage from a ligation made on the sciatic nerve proximal to the site of application, away from contaminating input population. The ability to recover functional phage particles from the sciatic nerve using this procedure was an interesting observation given the potential for proteolytic cleavage of the coat proteins of the bacteriophage particles that could affect the ability of the phage to infect E. coli. By using a phage display library of human single chain variable fragment (scFv) antibodies, we isolated in vivo-selected ligands that targeted a specific population of nerves that were transported efficiently into the spinal cord. In addition, by pre-panning the phage library against human p75NTR in vitro, we selectively isolated functional anti-p75NTR scFv phage that may be especially useful for facilitating proof of concept demonstration and transition to clinical research (e.g., MC192 does not recognize human p75NTR). The number of phage transported and recovered post-enrichment of the population for p75NTR binding was increased by over 13-fold compared with an unselected population, demonstrating the efficiency of the novel selection regime. By comparing the sequences of pre-panned anti-p75NTR scFv library to a fully diverse scFv that were isolated in vivo, we found that, although phage displaying antibodies to the p75NTR neurotrophin receptor constituted the greatest number of antibody clones, numerous other potential targets were identified.
Results
Neurons internalize phage chemically conjugated to p75NTR antibody
We hypothesized that if a neuron could internalize bacteriophage, then retrograde axonal transport would separate internalized phage from applied phage in vivo. A monoclonal antibody MC192 raised against p75NTR is internalized and retrogradely transported in rat neurons.24,25 We chemically conjugated MC192 to a control bacteriophage M13KO7 (Fig. 1A) to test if neurons will internalize and retrogradely transport phage displaying an internalizing antibody. The rat sciatic nerve was ligated with a suture 1 week prior to phage application to upregulate the expression of p75NTR in motor neurons4 (Fig. 1B). The ligature was then cut from the nerve and replaced with gel foam containing the MC192-phage complex and a second ligature was tied 2 cm proximal to block retrograde axonal transport. Internalization and retrograde axonal transport processes continue for at least 24 h after axotomy.26 Accumulation of MC192-phage distal to the ligation was shown by immunohistochemistry 18 h after axotomy (Fig. 1C), while application of M13KO7 phage alone resulted in no visible accumulation (data not shown).
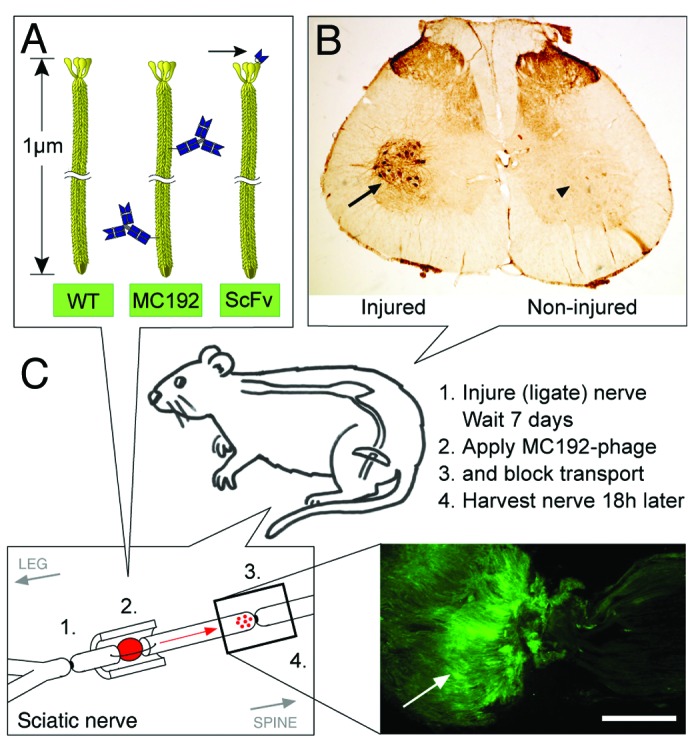
Figure 1. Targeting bacteriophage to an internalizing receptor p75NTR. (A) Phage used in study. WT, M13KO7 phage used as a wild-type control; MC192, M13KO7 phage with chemically conjugated MC192; scFv, M13 phage with genetically encoded scFv on pIII coat protein from a phage library. Note difference in mode of display of antibodies; arrow indicates scFv. (B) Injury upregulates motor neuron expression of p75NTR. Left sciatic nerve ligation 7 d earlier stimulates p75NTR expression in motor neurons in lumbar spinal cord, detected using MC192 as a primary antibody (arrows); p75NTR expression was undetected on contralateral side. (C) Retrograde accumulation of internalized MC192-phage in pre-injured rat sciatic nerve. MC192-phage was applied between cut ends of sciatic nerve, ligation injured 7 d earlier to upregulate p75NTR expression. Eighteen hours later MC192-phage (arrow) was detected accumulating distal to an upstream ligation using anti-phage antibodies (arrowhead). Phage was not detected following application of WT (result not shown). Scale bar, 1 mm.
In vivo selection of internalizing anti-p75NTR scFv phage from a phage library
Having demonstrated that neurons can be used to separate phage displaying internalizing antibodies from non-modified phage, we then used this method to generate a population of internalizing scFv to p75NTR. First, a phage library enriched for display of antibodies to the p75NTR was generated by one or more rounds of in vitro panning against recombinant human p75NTR coated onto plastic tubes. Phage selected by one round of in vitro selection against p75NTR or control phage (M13KO7) were applied to sciatic nerves that had been pre-injured 1 week earlier to upregulate p75NTR or to control nerves without upregulated p75NTR expression. Eighteen hours later, 5 mm of the nerve tissue distal to the ligature was removed to extract and count phage by dilution titering and for additional rounds of in vivo selection (Fig. 2A). Thirteen-fold more phage were recovered from the pre-injured nerve tissue than from control nerve (Fig. 2B, injured). As a control, application of anti-p75NTR phage to non-injured sciatic nerve resulted in background levels of recovery similar to control phage (Fig. 2B, non-injured). The subpopulation of internalized anti-p75NTR phage isolated from one round of in vivo screening was amplified and reapplied to the pre-injured sciatic nerve as before, this time without the blocking ligature to determine if phage is transported to the spinal cord. Immunohistochemistry of a spinal cord section from the lumbar region 24 h later showed clearly that phage were localized in the cell bodies of p75NTR expressing motor neurons (Fig. 2C). Control phage applied to the pre-injured sciatic nerve on the contralateral side showed no visible accumulation of phage, supporting the data from phage titer. Together these experiments demonstrate that internalizing anti-p75NTR scFv can target delivery of other molecules (phage) to nerves expressing high levels of p75NTR due to prior injury and provides a novel route to the selection of retrogradely-transported phage antibodies.
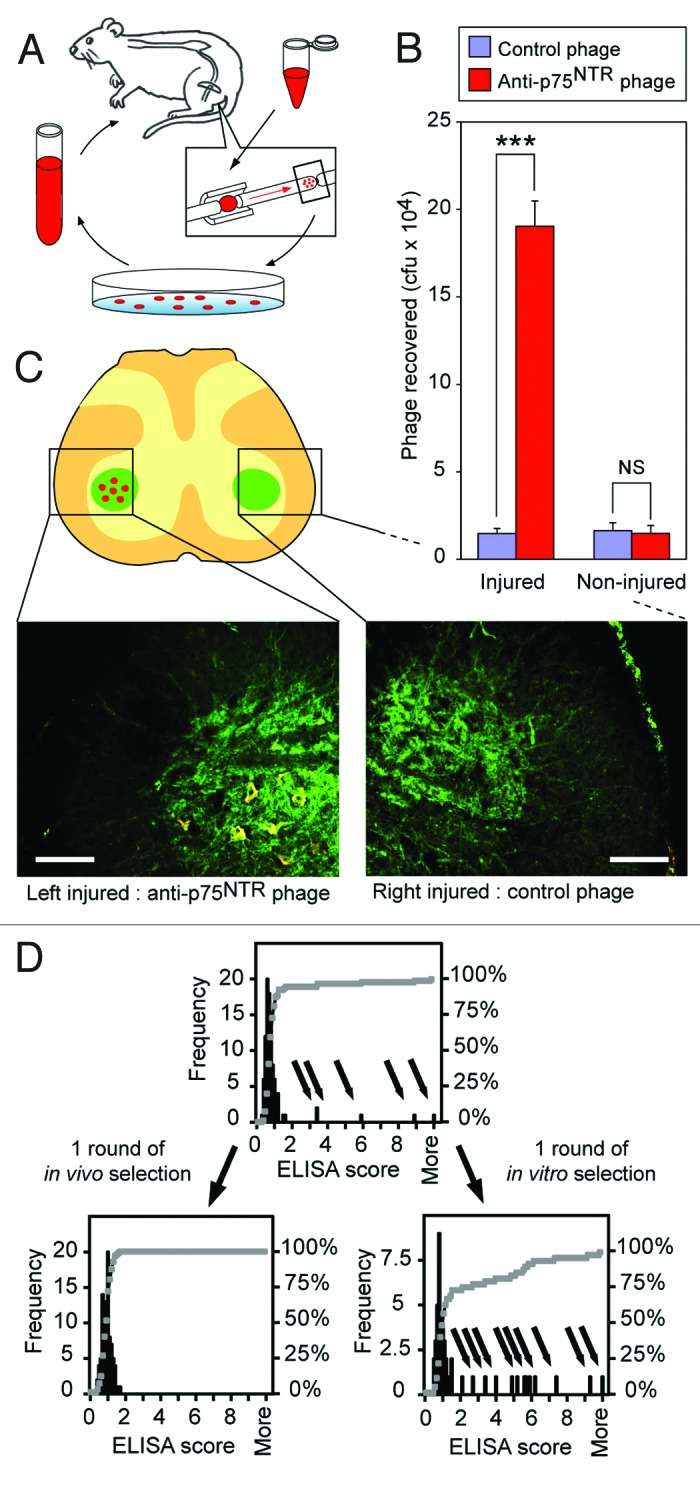
Figure 2. Isolating p75NTR antibodies by neuronal retrograde transport in vivo. (A) The in vivo selection procedure. Phage library enriched for binding to p75NTR from one round of in vitro panning was applied to the rat sciatic nerve in vivo at 5 x 1010 cfu. Phage was recovered 18 h later and used to infect E.coli for titer. The phage was then amplified for further rounds of in vivo selections (optional) or for sequence analysis. (B) Nerve pre-injury increases recovery of anti-p75NTR phage. Phage recovered from nerve ligation 18 h after applying either WT phage (blue columns) or phage enriched for anti-p75NTR by one round of in vitro panning (red columns) to either uninjured sciatic nerve or sciatic nerve injured 7 d earlier by ligation. Phage recovery increased 13-fold by nerve pre-injury: ***p < 0.001 Student’s t-test; NS, not significant; n = 3; cfu, colony forming units. (C) In vivo-selected anti-p75NTR phage are transported to the cell bodies in the spinal cord. Anti-p75NTR phage isolated from first round in vivo screening was reapplied to the injured sciatic nerve without blocking ligation. Twenty-four hours later phage co-localized on the cell bodies of p75NTR expressing neurons. Control phage applied to the contralateral side also with induced injury showed no visible accumulation. p75NTR expression is detected in green, phage as red, co-localization is visualized as yellow. Scale bar 150µm. (D) Anti-p75NTR scFv with high ELISA scores are not internalized in vivo. ELISA scores of a sample of 96 phage clones enriched for anti-p75NTR by one round of in vitro panning, and after either a further in vitro panning round or one round of in vivo phage selection. Note the absence of clones with high ELISA scores (arrows) after a single round of in vivo phage selection.
ELISA scores of internalizing anti-p75NTR scFv phage
We determined the relative binding of all in vitro and in vivo isolated anti-p75NTR phage to recombinant human p75NTR by ELISA. Tumor necrosis factor-related apoptosis-inducing ligand receptor 2 (TRAIL-R2), which belongs to the same tumor necrosis factor receptor superfamily as p75NTR, was used as a control antigen to determine the level of nonspecific binding and to derive an ELISA score relative to background. The first round in vitro selection against p75NTR resulted in a population of anti-p75NTR scFv phage, some of which exhibited high ELISA scores against p75NTR (Fig. 2D, arrows). While a further round of in vitro panning increased the number of these high score p75NTR antibodies in the population, no high score p75NTR antibodies were recovered from the nerve after in vivo selection (note absence of arrows, Figure 2D). The average internalizing anti-p75NTR scFv phage has an ELISA score of 0.987 ± 0.264 (SD) (range 0.400–1.795, n = 96; input population range: 0.326–14.33, n = 96). The loss of high score anti-p75NTR scFv phage following a single round of in vivo selection suggests that p75NTR antibodies with a high ELISA score, in general, do not stimulate internalization and retrograde transport. Potential explanations for this observation include the possibility that different epitopes or conformations of p75NTR are being recognized and some are preferentially internalized over others. Therefore, the failure of high ELISA binders to internalize may simply originate from the epitope or the in vitro confirmation recognized. Similarly, low affinity antibodies may result from failure to recognize rat p75NTR, or given the diversity of the library after only one round of in vitro panning, some of the antibodies may be binding internalizing targets other than p75NTR. It is however also possible that antibodies with faster on and off rates may be preferentially internalized, correlating with lower ELISA scores.
Sequence analysis of internalized anti-p75NTR scFv phage
We then sequenced the entire scFv coding region of a total of 576 individual clones (typically 250 amino acids per scFv) taken from the in vitro and in vivo phage selections. Individual clones were identified by the amino acid sequence of heavy (VH) and light (VL) variable domains. Clones that were frequently occurring (that is with identical sequences that have appeared more than once in the random sample) were assigned an ID letter (Table 1). Eighteen frequently occurring clones were identified through in vivo selection of anti-p75NTR phage populations isolated from pre-injured nerve. The relative proportion of scFv clones isolated from the nerve provides one measure of the relative internalization efficiency of these clones and may be reported as a rank score: scFv with a higher internalization score stimulate internalization more efficiently than lower scores. Given that the input population is not uniform, an alternative measure of internalization efficiency can be calculated from the increase in the number of identical clones during one round of in vivo phage selection. We called this alternative measure of internalization efficiency the expansion score. The ELISA score was used to test for a correlation with the expansion score (Table 1). No correlation was observed between ELISA score and expansion score (Spearman rank correlation coefficient, r = -0.098; Student two tailed t test: t = 0.01; p < 0.01; Figure 3A). A similar lack of correlation (r = 0.089) was observed when the internalization rank score data was used. Interestingly, with increasing rounds of in vitro panning against recombinant p75NTR, internalizing antibodies recovered from in vivo selections were progressively lost (Fig. 3B), and instead the third panning round was dominated by antibodies with high ELISA scores that were not represented from in vivo selections (Fig. 3C).
Table 1. In vivo selection of 1st round in vitro anti-p75NTR population.
| Sequence ID | ELISA Score | Percentage Occurrence | % occurrence Input library | Internalization Score | Expansion Score |
|---|---|---|---|---|---|
| H | 1.38 | 1.04 | 0.69 | 1 | 1.50 |
| F | 1.18 | 3.65 | 0.69 | 5 | 5.25 |
| L | 1.08 | 3.65 | 3.47 | 5 | 1.05 |
| D | 1.02 | 1.56 | 0.69 | 2 | 2.25 |
| K | 0.93 | 1.56 | 0.69 | 2 | 2.25 |
| B | 0.92 | 5.21 | 5.56 | 6 | 0.94 |
| M | 0.90 | 1.56 | 0.69 | 2 | 2.25 |
| E | 0.89 | 1.56 | 0.69 | 2 | 2.25 |
| A | 0.87 | 3.13 | 1.39 | 4 | 2.25 |
| N | 0.86 | 2.08 | 0.69 | 3 | 3.00 |
| I | 0.85 | 1.04 | 0.69 | 1 | 1.50 |
| G | 0.84 | 1.04 | 0.69 | 1 | 1.50 |
| Q | 0.84 | 1.56 | 0.69 | 2 | 2.25 |
| J | 0.81 | 2.08 | 1.39 | 3 | 1.50 |
| C | 0.77 | 1.04 | 0.69 | 1 | 1.50 |
| P | 0.75 | 1.04 | 0.69 | 1 | 1.50 |
| R | 0.71 | 1.56 | 0.69 | 2 | 2.25 |
| O | 0.62 | 2.08 | 0.69 | 3 | 3.00 |
In order to maximise the number of anti-p75NTR phage in the screen without compromising diversity, a naïve parental phage library was first panned against recombinant human p75NTR by one round of in vitro affinity panning. The resulting population was then screened in vivo by neuronal retrograde transport. The table below shows the characteristics of 18 unique anti-p75NTR antibody clones isolated from the in vivo procedure.
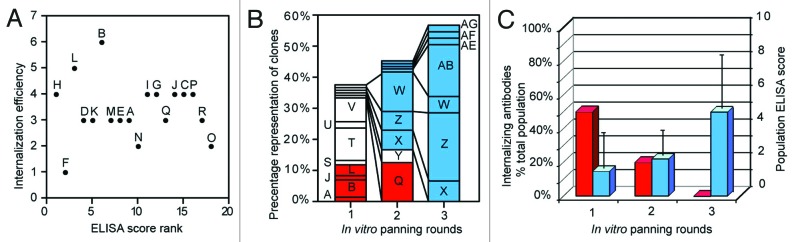
Figure 3. Ability to stimulate internalization does not correlate with ELISA score. (A) Internalization efficiency does not correlate with ELISA score. A scatter plot of internalization efficiency vs. ELISA score rank (listed in the order from higher to lower ELISA scores) for a total of 18 frequently occurring internalized anti-p75NTR clones showed no significant correlation. (B) In vitro panning increased the relative proportion of frequently occurring phage clones displaying anti-p75NTR antibodies with ELISA scores above 1.5 (blue columns), whereas internalized clones with lower ELISA scores were lost through selections (red columns). (C) Internalizing anti-p75NTR antibodies are lost with increasing rounds of in vitro panning. The proportion of the phage population that internalized (red columns) decreased as the mean ELISA scores of the phage population increased with repeated rounds of in vitro panning (blue columns).
De novo selection using parental phage antibody library
Finally, we tested whether it was possible to isolate internalizing antibodies from a fully diverse parental phage library that has not been pre-panned against p75NTR. We predicted that this method would isolate antibodies that bound to potentially all internalizing antigens, and that at least some antibodies would be targeted against p75NTR. Interestingly, a number of frequently occurring clones were isolated with matching sequences to those isolated from in vitro panning against p75NTR (Table 2). Among the clones isolated in vivo, BLAST search based on the sequence similarity of the variable domains to non-redundant databases (nrdb) uncovered high sequence similarities to the heavy or light chains of antibodies raised against transforming growth factor β 1/2 (99%), CD55 (97%) and tenascin C (95%) which are known to be expressed on neurons. The potential of these molecules as carriers of cargo for CNS delivery merits further investigation.
Table 2. In vivo selection of a fully diverse parental library.
| Sequence ID | Percentage Occurrence | ELISA Score | Previously Identified? |
|---|---|---|---|
| B | 6.25 | 0.81 | Y |
| K | 4.17 | 0.87 | Y |
| N | 4.17 | 0.65 | Y |
| V | 3.13 | 0.85 | Y |
| AI | 2.08 | 1.24 | ? |
| F | 2.08 | 0.79 | Y |
| AN | 2.08 | 0.71 | ? |
| AO | 2.08 | 0.78 | ? |
| AP | 2.08 | 0.79 | ? |
| AR | 2.08 | 0.88 | ? |
A naïve fully diverse phage library was applied in vivo to characterize the antibodies isolated. Interestingly five antibody clones were already identified previously through in vivo selection of anti-p75NTR antibodies.
Discussion
In the present study we examined whether the common entry route of pathogens can be exploited to screen a phage antibody library that target the CNS. By administering phage conjugated to a p75NTR antibody or phage antibody display library pre-panned against recombinant p75NTR to the sciatic nerve, we showed that neurons expressing the receptor internalize and transport bacteriophage to the spinal cord, through the blood-brain barrier. By harvesting retrogradely transported phage from a proximal ligature, we were able to isolate 18 unique scFv antibody clones that have demonstrated ability to transport cargo via p75NTR. Furthermore, application of a fully diverse phage library to the sciatic nerve yielded not only anti-p75NTR clones, but identified other potential receptor targets. This approach demonstrated the feasibility of a novel in vivo selection method to generate panels of antibodies with substantial potential in mediating delivery of therapeutic gene or drug cargo into the CNS.
Comparison to other phage display approaches to isolate internalizing ligands
Phage display technology conventionally involves in vitro affinity-based selection against purified target antigens.14-17 Panning phage library against cell lines may yield internalizing ligands. Despite some successes, isolation of a small number of internalized phage from the vast number of non-specific phage bound to the cell surface remains a major challenge in devising a routine selection procedure.13,18-22 In addition, ligands isolated in vitro require further screening to test their effectiveness in vivo. Pasqualini, Arap and colleagues pioneered a method that involves injecting a phage library into the tail vein of mice and isolating phage that have homed into the vasculature of particular organs.27 The advantage of this method is that blood circulation eliminates non-specific phage from the target areas, allowing isolation of ligands that bind endothelial cells in a biological environment. Their work has been extended to isolate phage that are targeted to specific types of cancers, which suggests that they have much clinical promise.18 The ligands isolated from this procedure are generally confined to the blood vessels; however, harvesting tissues 24 h after injection may favor selection of phage that are internalized or transcytosed by the endothelial cells.28 There is a unique advantage in isolating phage-displayed ligands in an in vivo setting because it ensures that the antigen is displayed in its original cellular context within their unique environment. Notably, phage library panning against some immobilized antigens do not yield clones that bind in cellular context.29 Furthermore, conventional panning methods against purified antigens or biopanning against cell lines for systematic protein expression and interaction analysis may overlook potential targets because some markers may be exposed in a restricted way in vivo that will make it a good target irrespective of their expression level or affinity.16 Boulis and colleagues have panned phage peptide library against trisialoganglioside (GT1b) and eluted with the tetanus toxin C fragment to isolate a peptide with tetanus toxin C-like binding characteristic. The resulting phage peptide was shown to be selectively internalized by differentiated PC12 cells, and retrogradely transported when injected to the sciatic nerve.30 Because GT1b is also known to be associated with the p75NTR,2 it is possible that this peptide was transported in a similar manner to our anti-p75NTR phage antibody. Frenkel and Solomon have demonstrated that nasally applied phage could be subsequently detected within the brain.31 Similarly, nasal administration of phage display library has yielded brain-targeted phage peptides.32 In our approach, we used intracellular axonal transport of the sciatic nerve to separate internalized phage, allowing physical separation of the useful clones from the library.
It is widely assumed that high affinity and high specificity are equivalent and that antibodies must exhibit both a high rate of attachment and a slow dissociation rate to be therapeutically useful. In our study, we used conventional in vitro panning to isolate and amplify p75NTR scFv antibodies from the phage library to maximize the chances of phage recovery in the subsequent selection in vivo. Phage isolated and amplified from the first round panning was used because we reasoned that this population would have the greatest diversity of anti-p75NTR phage. This population also included antibodies to p75NTR that were isolated in vivo with low ELISA scores. It is interesting to note that nerve growth factor (NGF), an endogenous ligand for the p75NTR, which stimulates internalization and retrograde axonal transport,25 exhibits both a fast association and a fast dissociation rate constant.33 As ELISA only provides a limited measure of antibody interaction to their target, the next step is to further characterize on and off-rates of individual antibody clones using techniques such as Biacore, and to perform competition studies (e.g., with NGF) to map binding epitopes. Is it possible that low affinity (high off-rate) antibodies reminiscent of the characteristics of NGF are recovered from our in vivo selection? It has been proposed that p75NTR might act as a surface reservoir for excess neurotrophins that are encountered in an in vivo environment,34,35 and then passed onto high affinity Trk receptors that are located on the same or other neurons nearby.36 In the physiological context with upregulated p75NTR, high affinity ligands with slow off-rates may simply bind tightly to the large number of p75NTR expressed on the Schwann cells near the lesion site, internalizing into glia and depleting the availability to neurons.37 Thus we speculate that low affinity antibodies are more likely to be internalized and transported by neurons binding to p75NTR along with other endogenous neurotrophins and co-receptors. Interestingly, a recent study using monoclonal antibodies against transferrin receptors as therapeutic carriers to the brain demonstrated that lower affinity antibodies were more efficient at crossing the blood-brain barrier.38 The authors found that although high affinity antibodies bound tightly to the receptors expressed on endothelial cells lining the blood vessels, they failed to detach from the target during transcytosis and hence were not delivered into the brain.
Use of p75NTR antibody as a therapeutics carrier into the CNS
Early radiolabeling studies have revealed two types of in vivo transport processes for NGF; one with high affinity low capacity and another of lower affinity and higher capacity.39 These two saturable processes likely reflect binding sites for TrkA/p75NTR complex and p75NTR. Studies with PC12 cells indicate that MC192-mediated p75NTR internalization proceeds three times slower than NGF-mediated TrkA/p75NTR complex internalization,8 and interestingly p75NTR associated complex is trafficked to a cellular compartment that escapes lysosomal acidification and proteolytic degradation.8,40 Such a trafficking mechanism could explain why p75NTR is favored by the pathogens to gain entry into the CNS and why it may be an effective target for therapeutic antibodies.
The immunotoxin 192-saporin, formed by conjugating the monoclonal antibody MC192 to saporin,41 has been widely used to precisely target and eliminate p75NTR expressing neurons such as the basal forebrain cholinergic neurons in animal models of neurodegeneration. While this suggests that MC192 may also have potential for delivering therapeutics to such cells, MC192 recognizes only rat p75NTR. To isolate molecules with therapeutic potential, internalizing anti-p75NTR clones that cross-react with both human and rat p75NTR were selected by in vitro panning against human p75NTR followed by in vivo phage selection using the rat. Demonstration of a method for isolating antibodies that stimulate receptor-mediated internalization in vivo enables development of novel products for efficient delivery of drug or gene payloads directly into specific target cells. Use of p75NTR antibodies to target delivery of bacteriophage into neurons demonstrates that neurons are capable of internalizing and retrogradely transporting payloads of substantial molecular size and complexity. While full immunoglobulins are divalent, the scFv fragments displayed on the phage libraries are typically monovalent, suggesting that binding of a single scFv mediates internalization.13 When a ligature is not placed on the sciatic nerve, retrograde axonal transport could deliver a DNA/protein complex to the motor neuron cell body within the spinal cord, behind the protective blood-brain barrier.
We used p75NTR as an example to demonstrate a method of isolating fully human antibodies that can deliver molecules into target neurons in vivo. Our approach also allows isolation of ligands against potentially any internalizing receptors and antigens expressed on neurons, and validates a novel selection method to enrich for such molecules. In addition, our study demonstrates the feasibility of delivering therapeutic molecules intraneuronally through the blood-brain barrier into the CNS.
Materials and Methods
Animals
Six-week-old female Sprague-Dawley rats were used. All animal experiments were performed according to the animal experiment ethical directions approved by the Institutional Animal Welfare Committee [approval number 437/99(b)].
Chemical conjugation of MC192 to M13KO7 phage
MC192 was prepared from a hybridoma cell line using the method of Chandler.10 M13KO7 helper phage was obtained from New England Biolabs. Sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (sulfo-SMCC) (Pierce) was initially reacted with primary amine groups on the antibody. M13KO7 was incubated with 2-iminothiolane (2-IT) (Pierce) to generate free sulfhydryl groups. The activated M13KO7 were then mixed with SMCC conjugated antibody at 1:10 ratio to form a thioester bond. Iodoacetamide (Sigma) was then added to block free reactive sulfhydryl groups and the complex precipitated twice in PEG/NaCl to remove free antibodies.
Immunohistochemistry
For the sciatic nerve, M13KO7 and MC192-phage was detected with biotinylated anti-fd phage antibody (Sigma) and streptavidin-Alexafluor488 (Molecular Probes) on 50 µm longitudinal cryostat cut sections of the sciatic nerve with sutures removed. p75NTR expression was detected on 50 µm free floating sections of rat lumbar spinal cord by DAB staining method using MC192 as a detection antibody and HRP labeled donkey anti-mouse as a secondary reagent. Phage and p75NTR two color expression was detected on 50 µm rat lumbar spinal cord sections using MC192 as a detection antibody and donkey anti-mouse-Alexafluor 488 as a secondary reagent. Phage was detected using anti-fd phage antibody and anti-rabbit Cy3 as a secondary reagent (Sigma).
In vitro panning and selection
Phage library containing 1.3 × 1010 individual recombinants was kindly provided by Cambridge Antibody Technology. Recombinant human p75NTR was obtained from R&D Systems. In vitro panning and selection against p75NTR was performed using immunotubes (Nunc: maxisorp) coated with p75NTR (20 µg/ml at 4°C). Tubes were then incubated with 3% skim milk in phosphate-buffered saline (PBS) for 1 h before removing and adding the phage particles. Phage library was incubated with 3% skim milk in PBS before selection. For the first round, 1 × 1012 titerd units of library phage in a total volume of 1 ml were used per immunotube and incubated for 2 h at room temperature. Tubes were washed three times with PBS and remaining phage used to infect E. coli for screening.
Sciatic nerve ligation injury
Under halothane anesthesia, the left common sciatic nerve was exposed at the level of middle thigh by blunt dissection through the biceps femoris. A 5–7 mm of the nerve was freed from adhering tissue near tibial branch bifurcation, and one tight ligature was made using a 6-0 silk suture, then the wound was closed.
Application of bacteriophage in vivo
Seven days after nerve ligation, under halothane anesthesia, the left common sciatic nerve was exposed at upper thigh level by blunt dissection and one tight ligature was made using a 6-0 silk suture. The wound in the mid-thigh was reopened to locate the first ligation, and an 8 mm length of a silicone silastic cuff (2.6 mm ID, 4.9 mm OD) with a cut along the tube at 2 mm width was then fitted on the sciatic nerve at site of the first ligation. The old ligation was excised and 5–10 µl of phage soaked in 1 mm3 of collagen gel matrix was placed between the cut end stumps within the silicone silastic cuff. The nerve ends were sutured together with the collagen gel matrix in place using a 10-0 nylon suture.
Recovery of bacteriophage applied in vivo
Eighteen hours after the application of phage, the rats were killed by anesthetic overdose, the sciatic nerve exposed, and another ligation was made 0.5 cm below the upper thigh ligation. The sciatic nerve was then excised with the two ligations in place to avoid loss of phage from cut end stumps, scrubbed extensively with sterile tissue paper soaked in PBS until the nerve sheath was removed. The sciatic nerve was then transferred, sutures removed and cut into small pieces in lysis buffer (1% Triton X-100, 10 mM Tris, 2 mM EDTA, pH 8, plus 1/100 volume of protease inhibitor cocktail; Sigma). The sample was then vortexed for 1 h at room temperature and microfuged to pellet nerve tissue. The supernatant was collected and 20% volume of CaCl2 was added. The phage in the supernatant was used to infect E.coli for titer/amplification.
ELISA
Individual colonies of E.coli grown on agar plates were picked randomly from each experiment group and amplified in a 96-well plate in a growth media. Antigen plates were prepared by adding 100 µl of 5 µg/ml recombinant human p75NTR/ Fc chimera (R&D Systems) in PBS. To the ELISA plate well, anti-p8-HRP conjugate (Pharmacia) was added and the color reaction monitored after the addition of TMB substrate and H2SO4 at optical density of 450 nm using a microtiter plate reader. Positive clones were detected by comparing the ELISA signal to that of a control well coated with 5 µg/ml recombinant TRAIL R2 receptor/Fc chimera (R&D Systems). ELISA scores were calculated by dividing the reading of p75NTR by TRAIL R2 receptor, i.e., fold increase above control.
Statistical analysis
Rank correlation coefficient was calculated using the formula r = 1-{(6Σd2)/(n(n2-1))} where d = difference between the ranks of paired observations, n = number of paired observations. One ≥ r ≥ -1 where r = 1 indicated a perfect correlation, whereas r = -1 indicated a perfect inverse correlation. r = 0 indicated no correlation.
Sequencing of scFv antibodies
Cells from an individual colony on a 2TYAG agar plate were used as the template for a PCR amplification of the inserted DNA using the primers pUC19reverse and fdtetseq using the Taq-Dye-terminator cycle sequencing system (Applied Biosystems).
Acknowledgments
We thank Cambridge Antibody Technology (now integrated with MedImmune, LLC) for kindly providing the human scFv phage antibody display library. HT was supported by an Australian Post-graduate Award Scholarship.
Disclosure of Potential Conflicts of Interest
MedImmune Ltd. has patents covering the scFv display library. Patent applications have been filed on the procedure.
References
- 1.Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–7. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- 2.Schecterson LC, Bothwell M. Neurotrophin receptors: Old friends with new partners. Dev Neurobiol. 2010;70:332–8. doi: 10.1002/dneu.20767. [DOI] [PubMed] [Google Scholar]
- 3.Underwood CK, Coulson EJ. The p75 neurotrophin receptor. Int J Biochem Cell Biol. 2008;40:1664–8. doi: 10.1016/j.biocel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Ernfors P, Henschen A, Olson L, Persson H. Expression of nerve growth factor receptor mRNA is developmentally regulated and increased after axotomy in rat spinal cord motoneurons. Neuron. 1989;2:1605–13. doi: 10.1016/0896-6273(89)90049-4. [DOI] [PubMed] [Google Scholar]
- 5.Lowry KS, Murray SS, McLean CA, Talman P, Mathers S, Lopes EC, et al. A potential role for the p75 low-affinity neurotrophin receptor in spinal motor neuron degeneration in murine and human amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2001;2:127–34. doi: 10.1080/146608201753275463. [DOI] [PubMed] [Google Scholar]
- 6.Butowt R, von Bartheld CS. Connecting the dots: trafficking of neurotrophins, lectins and diverse pathogens by binding to the neurotrophin receptor p75NTR. Eur J Neurosci. 2003;17:673–80. doi: 10.1046/j.1460-9568.2003.02497.x. [DOI] [PubMed] [Google Scholar]
- 7.Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–6. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- 8.Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M. Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J Neurosci. 2003;23:3209–20. doi: 10.1523/JNEUROSCI.23-08-03209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Book AA, Wiley RG, Schweitzer JB. Specificity of 192 IgG-saporin for NGF receptor-positive cholinergic basal forebrain neurons in the rat. Brain Res. 1992;590:350–5. doi: 10.1016/0006-8993(92)91121-T. [DOI] [PubMed] [Google Scholar]
- 10.Chandler CE, Parsons LM, Hosang M, Shooter EM. A monoclonal antibody modulates the interaction of nerve growth factor with PC12 cells. J Biol Chem. 1984;259:6882–9. [PubMed] [Google Scholar]
- 11.Barati S, Hurtado PR, Zhang SH, Tinsley R, Ferguson IA, Rush RA. GDNF gene delivery via the p75(NTR) receptor rescues injured motor neurons. Exp Neurol. 2006;202:179–88. doi: 10.1016/j.expneurol.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 12.Berhanu DA, Rush RA. Targeted silencing of TrkA expression in rat forebrain neurons via the p75 receptor. Neuroscience. 2008;153:1115–25. doi: 10.1016/j.neuroscience.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Poul MA, Becerril B, Nielsen UB, Morisson P, Marks JD. Selection of tumor-specific internalizing human antibodies from phage libraries. J Mol Biol. 2000;301:1149–61. doi: 10.1006/jmbi.2000.4026. [DOI] [PubMed] [Google Scholar]
- 14.Bratkovic T. Progress in phage display: evolution of the technique and its application. Cell Mol Life Sci. 2010;67:749–67. doi: 10.1007/s00018-009-0192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths AD, Duncan AR. Strategies for selection of antibodies by phage display. Curr Opin Biotechnol. 1998;9:102–8. doi: 10.1016/S0958-1669(98)80092-X. [DOI] [PubMed] [Google Scholar]
- 16.Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev. 2006;58:1622–54. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 18.Brown KC. Peptidic tumor targeting agents: the road from phage display peptide selections to clinical applications. Curr Pharm Des. 2010;16:1040–54. doi: 10.2174/138161210790963788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fransson J, Borrebaeck CA. Selection and characterization of antibodies from phage display libraries against internalizing membrane antigens. Methods Mol Biol. 2009;480:113–27. doi: 10.1007/978-1-59745-429-2_8. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Cao BB. Screening of specific internalization Fab fragment from human naive phage library by combinational bio-panning. Methods Mol Biol. 2009;525:161–74, xv. doi: 10.1007/978-1-59745-554-1_8. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Y, Marks JD. Identification of target and function specific antibodies for effective drug delivery. Methods Mol Biol. 2009;525:145–60, xv. doi: 10.1007/978-1-59745-554-1_7. [xv.] [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Zou H, Zhang S, Marks JD. Internalizing cancer antibodies from phage libraries selected on tumor cells and yeast-displayed tumor antigens. J Mol Biol. 2010;404:88–99. doi: 10.1016/j.jmb.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson EM, Jr., Taniuchi M, Clark HB, Springer JE, Koh S, Tayrien MW, et al. Demonstration of the retrograde transport of nerve growth factor receptor in the peripheral and central nervous system. J Neurosci. 1987;7:923–9. doi: 10.1523/JNEUROSCI.07-03-00923.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Q, Snider WD, Pinzone JJ, Johnson EM., Jr. Retrograde transport of nerve growth factor (NGF) in motoneurons of developing rats: assessment of potential neurotrophic effects. Neuron. 1988;1:335–43. doi: 10.1016/0896-6273(88)90082-7. [DOI] [PubMed] [Google Scholar]
- 25.Johnson EM, Jr., Taniuchi M, Clark HB, Springer JE, Koh S, Tayrien MW, et al. Demonstration of the retrograde transport of nerve growth factor receptor in the peripheral and central nervous system. J Neurosci. 1987;7:923–9. doi: 10.1523/JNEUROSCI.07-03-00923.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin JW, Price DL, Engel WK, Drachman DB. The pathogenesis of reactive axonal swellings: role of axonal transport. J Neuropathol Exp Neurol. 1977;36:214–27. doi: 10.1097/00005072-197703000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–6. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Zhang Q, Pang Z, Wang Y, Liu Q, Guo L, et al. Identification of peptide sequences that target to the brain using in vivo phage display. Amino Acids. 2012;42:2373–81. doi: 10.1007/s00726-011-0979-y. [DOI] [PubMed] [Google Scholar]
- 29.Huls GA, Heijnen IA, Cuomo ME, Koningsberger JC, Wiegman L, Boel E, et al. A recombinant, fully human monoclonal antibody with antitumor activity constructed from phage-displayed antibody fragments. Nat Biotechnol. 1999;17:276–81. doi: 10.1038/7023. [DOI] [PubMed] [Google Scholar]
- 30.Federici T, Liu JK, Teng Q, Yang J, Boulis NM. A means for targeting therapeutics to peripheral nervous system neurons with axonal damage. Neurosurgery. 2007;60:911–8, discussion 911-8. doi: 10.1227/01.NEU.0000255444.44365.B9. [DOI] [PubMed] [Google Scholar]
- 31.Frenkel D, Solomon B. Filamentous phage as vector-mediated antibody delivery to the brain. Proc Natl Acad Sci U S A. 2002;99:5675–9. doi: 10.1073/pnas.072027199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan XM, Chen YP, Xu WR, Yang WJ, Wen LP. Identification of nose-to-brain homing peptide through phage display. Peptides. 2009;30:343–50. doi: 10.1016/j.peptides.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 33.Eveleth DD, Bradshaw RA. Internalization and cycling of nerve growth factor in PC12 cells: interconversion of type II (fast) and type I (slow) nerve growth factor receptors. Neuron. 1988;1:929–36. doi: 10.1016/0896-6273(88)90150-X. [DOI] [PubMed] [Google Scholar]
- 34.Taniuchi M, Clark HB, Johnson EM., Jr. Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–8. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson EM, Jr., Taniuchi M, DiStefano PS. Expression and possible function of nerve growth factor receptors on Schwann cells. Trends Neurosci. 1988;11:299–304. doi: 10.1016/0166-2236(88)90090-2. [DOI] [PubMed] [Google Scholar]
- 36.Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron. 1994;13:203–15. doi: 10.1016/0896-6273(94)90470-7. [DOI] [PubMed] [Google Scholar]
- 37.Kahle P, Hertel C. Nerve growth factor (NGF) receptor on rat glial cell lines. Evidence for NGF internalization via p75NGFR. J Biol Chem. 1992;267:13917–23. [PubMed] [Google Scholar]
- 38.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 39.Dumas M, Schwab ME, Thoenen H. Retrograde axonal transport of specific macromolecules as a tool for characterizing nerve terminal membranes. J Neurobiol. 1979;10:179–97. doi: 10.1002/neu.480100207. [DOI] [PubMed] [Google Scholar]
- 40.Makkerh JP, Ceni C, Auld DS, Vaillancourt F, Dorval G, Barker PA. p75 neurotrophin receptor reduces ligand-induced Trk receptor ubiquitination and delays Trk receptor internalization and degradation. EMBO Rep. 2005;6:936–41. doi: 10.1038/sj.embor.7400503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Book AA, Wiley RG, Schweitzer JB. Specificity of 192 IgG-saporin for NGF receptor-positive cholinergic basal forebrain neurons in the rat. Brain Res. 1992;590:350–5. doi: 10.1016/0006-8993(92)91121-T. [DOI] [PubMed] [Google Scholar]


