Abstract
The surface properties of carbon based electrodes are critically important for the detection of biomolecules and can modulate electrostatic interactions, adsorption and electrocatalysis. Carbon nanotube (CNT) modified electrodes have previously been shown to have increased oxidative sensitivity and reduced overpotential for catecholamine neurotransmitters, but the effect of surface functionalities on these properties has not been characterized. In this study, we modified carbon-fiber microelectrodes (CFMEs) with three differently functionalized single-wall carbon nanotubes and measured their response to serotonin, dopamine, and ascorbic acid using fast-scan cyclic voltammetry. Both carboxylic acid functionalized and amide functionalized CNTs increased the oxidative current of CFMEs by approximately 2–6 fold for the cationic neurotransmitters serotonin and dopamine, but octadecylamine functionalized CNTs resulted in no significant signal change. Similarly, electron transfer was faster for both amide and carboxylic acid functionalized CNT modified electrodes but slower for octadecylamine CNT modified electrodes. Oxidation of ascorbic acid was only increased with carboxylic acid functionalized CNTs although all CNT-modified electrodes showed a trend towards increased reversibility for ascorbic acid. Carboxylic acid-CNT modified disk electrodes were then tested for detection of serotonin in the ventral nerve cord of a Drosophila melanogaster larva, and the increase in sensitivity was maintained in biological tissue. The functional groups of CNTs therefore modulate the electrochemical properties, and the increase in sensitivity from CNT modification facilitates measurements in biological samples.
Introduction
Carbon electrodes have been used for biological analysis because they can rapidly and sensitively monitor specific biomolecules1. The surface properties of carbon electrodes are critically important for detection of biomolecules, particularly for molecules that absorb to the electrode. For example, functional groups such as oxides can promote adsorption of positively charged neurotransmitters, increasing the electrode sensitivity 2–4. In addition, nanostructuring the electrode surface provides more surface area and active, edge plane-like graphite sites for rapid electron transfer 5–8. Recently, there has been a surge of research to incorporate carbon nanotubes into electrochemical sensors because of their unique structural and electronic properties. Electrodes modified with CNTs have been shown to exhibit enhanced electrochemical properties compared to traditional carbon electrodes, such as increased sensitivity, reduced overpotential, and reduced electrode fouling 9,10. However, nanotube properties, such as length, diameter, chirality and purity are highly variable. The CNT fabrication procedure can control some of these parameters but post-fabrication procedures are also often used, especially to increase purity. A common purification procedure is to treat CNTs with strong oxidizing acids, which helps to remove metal catalysts and carbon impurities, but also shortens CNTs, increases sidewall defects, and adds oxygen containing functional groups 11,12. Therefore, most CNT-based sensors use oxygen functionalized nanotubes, although the implications of this are not always addressed. Similar to traditional carbon electrodes, the electrochemical properties of a CNT-based electrode are expected to correlate with CNT functionalization but distinct comparisons have not been made of how functionalization affects electrochemical sensing.
The use of CNT-modified electrodes as biological sensors has been evaluated and reviewed on many occasions 10,13–17. In particular electrochemically-active neurotransmitters, such as dopamine (DA), have been common analytes for sensor development studies. Britto et al. first demonstrated the benefits of CNT based electrodes by demonstrating that a CNT-paste electrode had improved sensitivity for dopamine and near ideal electron transfer kinetics 18. However, few CNT electrodes are practical for direct use in biological systems. CNT-modified glassy carbon electrodes and CNT-paste electrodes are typically larger than 1 mm in diameter and therefore, are not amenable to implantation in biological tissue, especially brain tissue. In addition, the electrochemical techniques employed, such as differential pulse voltammetry or traditional cyclic voltammetry, are too slow to measure rapid changes in neurotransmitters. An ideal CNT-based electrode for measurements in biological systems would be small enough to limit tissue damage, sensitive enough to detect biologically-relevant concentrations, rapid enough to measure fast changes, and simple and inexpensive enough to fabricate reproducibly in large batches 19. A number of strategies might incorporate some of these ideal properties. Fabrication using evaporative immobilization or polymeric entrapment of CNTs is fast and simple. CNT based microelectrodes use small amounts of CNTs and are more amenable to in vivo implantation. When microelectrodes are used in conjunction with rapid electrochemical techniques, such as fast-scan cyclic voltammetry (FSCV), rapid concentration changes can be tracked 20,21. Microelectrodes can also be fabricated in batches and therefore the reproducibility between electrodes evaluated.
In this study, we compared the effect of different chemical functionalities on the electrochemical properties of single-walled carbon nanotube (SWCNT) modified carbon fiber microelectrodes (CFMEs). Commercially-available, functionalized SWCNTs were dip coated onto CFMEs as a simple, quick and inexpensive fabrication procedure. We found that functionalization affected the sensitivity and electron transfer kinetics for dopamine, serotonin (5-HT) and ascorbic acid (AA). The enhanced sensitivity was useful for detection of serotonin in the Drosophila melanogaster ventral nerve cord. The modification technique is simple, cost-effective, and could be easily adopted by anyone using electrochemical detection of biomolecules.
EXPERIMENTAL
Solutions
Dopamine hydrochloride, serotonin hydrochloride (5-hydroxytryptamine hydrochloride,) and ascorbic acid were purchased from Sigma-Aldrich (St. Louis, MO). The Tris-buffer ingredients (15 mM Tris(hydroxymethyl)aminomethane, 3.25 mM KCl, 140 mM NaCl, 1.2 mM CaCl2, 1.25 mM NaH2PO4, 1.2 mM MgCl2 and 2.0 mM Na2SO4 with the pH adjusted to 7.4) were purchased from Fisher Scientific (Suwanee, GA). Stock solutions of 10 mM dopamine, 5 mM 5-HT, and 100 mM AA were made in 0.1 M HClO4 and were diluted daily with the Tris buffer to concentrations of 10 μM DA, 10 μM 5-HT, and 200 μM AA. All aqueous solutions were made with deionized water (Milli-Q Biocel, Millipore, Billerica, MA).
Electrochemistry
Carbon-fiber microelectrodes were fabricated using a previously published technique 20. Briefly, T-650 carbon fibers (7 um diameter, Cytec, West Patterson, NJ) were vacuum-aspirated into a glass capillary and pulled on a Narishige (Tokyo, Japan) vertical electrode puller. The fiber was trimmed at the glass seal, and sealed with Epon Resin 828 (Danbury, CT) with 14% (w/w) m-phenylenediamine (Fisher) hardener heated to 80° C. Electrodes were dipped for 30 s in the epoxy and cured at room temperature overnight, and then were heated to 100° C for 2 h and to 150° C overnight. Electrodes were polished at a 30° angle on a fine diamond abrasive plate (Sutter Instruments model BV-10, Novato, CA) and soaked for at least 10 min in isopropanol before use. 1 M potassium chloride was used as a backfill solution to provide an electrical connection between the fiber and the wire to the headstage.
FSCV was performed using a ChemClamp (Dagan, Minneapolis, MN, n=0.01 headstage), PCI 6711 and 6052 computer interface cards (National Instruments, Austin, TX) and home built break-out box. The triangular waveform applied was from −0.4 V to 1.0 V at 400 V/s vs a Ag/AgCl reference electrode, at a frequency of 10 Hz. For serotonin measurements, another waveform was also used, from 0.2 V to 1.0V to −0.1V and back to 0.2V at 1000 V/s22. Data collection was computer controlled by the TarHeel CV software program23.
Electrodes were tested using a flow-injection system, as previously described in detail24. Analyte injections lasting 5 s were made and current vs time traces were obtained by integrating the current in a 100 mV window centered at the oxidation peak for each cyclic voltammogram (CV). All CVs were collected about 3 s after analyte was introduced. Background-subtracted CVs were calculated by subtracting the average of ten background scans taken before the compound was injected from the average of five CVs when the compound was present.
Carbon nanotube electrode modification
Carboxylic-acid functionalized (COOH-CNT), amide-functionalized (CONH2-CNT) and octadecylamine-functionalized SWCNTs (ODA-CNT) were purchased from Sigma Aldrich, and were used without further chemical purification or modification. One mg/mL of the functionalized-CNT was suspended in N,N-dimethylmethanamide by sonicating for 60 min using a tissue sonicator at a power setting of 30% (model 150V/T; Biologics, Inc., Manassas, VA).
All electrodes were first tested to measure the voltammetric response to each analyte. Then, salts were removed by soaking for 5 min each in water and isopropanol, and air drying for 10 min. Next, the tip of each electrode was dipped into a CNT suspension for 60 s and allowed to evaporatively dry for at least 10 min. The electrode was tested again. Each electrode served as its own control, as the response before CNT modification was compared to the response of the same electrode after CNT modification. The total time for CNT modification, dip coating and drying, was only 11 min and electrodes could be fabricated in batches.
Data from electrodes that had a S/N ratio less than 10 were rejected from the study as too noisy. The rejection rate for each type of CNT varied; approximately 15% of COOH-CNT and ODA-CNT modified electrodes were rejected, while approximately 35% of CONH2-CNTs modified electrodes were rejected. All of the non-functionalized-CNT modified electrodes were rejected, prohibiting a direct comparison between functionalized and non-functionalized CNTs.
Drosophila ventral nerve cord experiments
Homozygous 3-day-old larvae expressing a Tph-GAL4;UAS-ChR2 genotype were fed all-trans retinal for two days 25,26. The central nervous system (CNS) of a wandering, 3rd instar (5-day-old) larvae was dissected and incubated as described previously 27. The optic lobes were removed to yield an isolated ventral nerve cord (VNC), which was adhered to the bottom of a Petri dish containing buffer. In a dark room, an electrode was implanted into the VNC 4–6 segments away from the cut edge. The VNC was allowed to equilibrate for 5 min after electrode insertion. During data collection, 30 s of baseline electrochemistry data was collected followed by 10 s of blue-light illumination from a 10 W halogen lamp with a fluorescein emission filter (450 – 490 nm). A post-data collection band block filter was used (OriginLab OriginPro 7.5) to remove line noise between 59.5 Hz and 60.5 Hz.
Scanning electron microscopy
Scanning electron microscope images were taken on a JEOL JSM-6700F microscope (Tokyo, Japan) using an accelerating voltage of 10 kV and a working distance between 5 mm and 8.2 mm, in secondary electron imaging mode. Samples were sputter-coated before imaging using a precision etching coating system with gold/palladium or carbon (PECS, 682, Gatan Inc, Pleasanton, CA).
Statistics
GraphPad Prism 4.0 was used for all statistics (GraphPad Software, San Diego, CA). All averaged values are given as the mean ± SEM (standard error of the mean) for n number of electrodes, unless otherwise noted. Normalized signals were calculated for each electrode by dividing the post-CNT modification signal by the pretest signal. Significance was determined by paired t-tests and significance was defined as p ≤ 0.05.
RESULTS
The goal of this study was to compare the effects of nanotube functionalization on the electrochemical detection of neurochemicals. Carbon-fiber microelectrodes were modified with CNTs using a simple procedure that was adapted from the evaporative drying of a droplet on an electrode 28. Because it is difficult to retain a droplet on the microelectrode surface long enough to allow it to air dry, electrodes were dipped in suspensions of nanotubes and then dried 20. We chose to use commercially-available nanotubes because they are easy to acquire, do not require further chemical modification, and could easily be adopted by the electrochemical community. FSCV was used because it provides the sub-second time resolution required for monitoring fast concentration changes of neurotransmitters.
Surface morphology of CNT-modification of CFMEs
Electrodes were dip coated with carbon nanotubes and the background currents recorded. The background current is directly proportional to the electroactive surface area of the electrode; therefore, increasing the surface area by adding CNTs was also expected to increase the background current 29. Fig. 1 compares background currents before (dashed-line) and after modification (solid-line) with three different functionalized carbon nanotubes: amide (CONH2-CNT), carboxylic-acid (COOH-CNT), and octadecylamine (ODA-CNT). All CNT-modified electrodes displayed a similar increase in the background current; about a factor of 3 for the CONH2-CNT and COOH-CNT modified electrodes, and about 2.5 for ODA-CNT modified electrodes. Therefore the increase in surface area after coating was likely similar.
Figure 1.
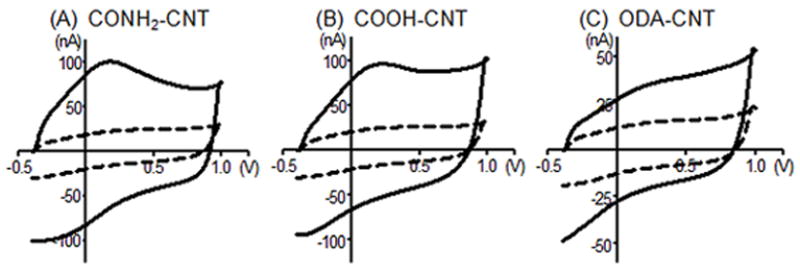
Example fast-scan cyclic voltammograms showing background currents for bare electrodes (dashed line), and functionalized CNT-modified electrodes (solid line) using: (A), amide-, (B) carboxylic acid-, and (C) octadecylamine-functionalized CNTs. Each panel compares data before and after modification for the same electrode. Electrode was scanned from −0.4 to 1.0V and back at 400 V/s at 10 Hz.
Scanning electron microscope images were collected to examine the surface coverage of CNTs. Fig. 2 shows representative CNT-modified disk electrode surfaces. Although there is variability in the CNT layer between individual electrodes, the general surface coverage and morphology of the CNT layer is similar for electrodes modified with different functionalized CNTs. Occasionally, large agglomerations were observed on an electrode surface. CNTs are known to self-assemble into agglomerations due to van der Waals forces; thus, separating and suspending individual CNTs can be difficult 30. We observed a qualitative correlation that electrodes with large CNT agglomerations were highly noisy (S/N <10). The large surface area of an agglomeration likely leads to a large capacitive current; however, the noise increases more than the signal because only a small portion of the surface area is accessible to the analyte 31,32. SEM images of non-functionalized CNT modified electrodes showed impurities and a large number of agglomerations (Supplemental Fig. 1). This explains why non-functionalized CNT electrodes displayed such high noise, and could not be compared to functionalized CNTs. A monolayer of CNTs is likely a more ideal coating and improved CNT suspension techniques would lead to more consistent fabrication.
Figure 2.
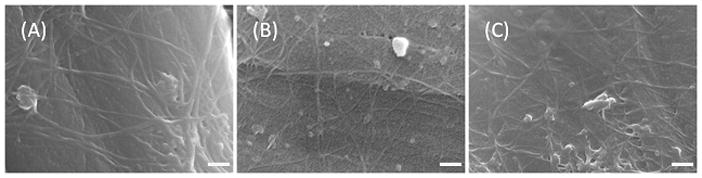
Scanning electron microscopy images of (A) a CONH2-CNT, (B) a COOH-CNT, and (C) an ODA-CNT -modified carbon fiber disk microelectrode display relatively similar layers of nanotubes on the carbon-fiber surface, implying that differences between the three different functionalized CNTs do not largely affect the nanotube layer. The white scale bar represents 100 nm.
Effect of CNT functionalization on neurochemical detection
Fig. 3 shows example background-subtracted cyclic voltammograms from individual electrodes before and after nanotube modification for the detection of three common neurochemicals: 10 μM serotonin (5-HT), 10 μM dopamine (DA), and 200 μM ascorbic acid (AA). 5-HT and DA are cationic, monoamine neurotransmitters at physiological pH, with differing adsorption properties due to differences in chemical structure 22,33,34. Ascorbic acid is an anionic analyte that is often an interferent for catecholamine detection 35,36. The CONH2-CNT modified electrode displays about a 3-fold increase in oxidation current for both 5-HT and DA but a decrease in sensitivity towards AA (Figs. 3A–C). The COOH-CNT modified electrode shows over a 2-fold increase in oxidation current for 5-HT and DA as well as AA (Figs. 3D–F). In contrast, the ODA-CNT modified electrode exhibits little difference in signal after CNT treatment (Figs. 3G–I). Increases in reduction peaks were generally observed only when the oxidation peak increased. One exception was for ODA-CNT modified electrodes, where the reduction peak increased for AA (Fig. 3I inset) but the oxidation peak did not. Because the surface coverage was similar for all the types of nanotubes, the results indicate that functionalization, and not strictly surface area, is the predominant contributor to the change in sensitivity.
Figure 3.
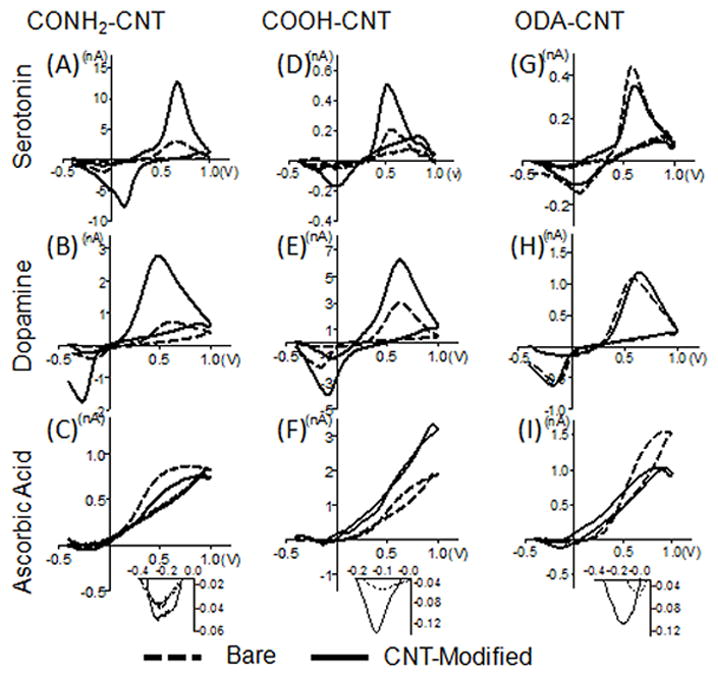
Representative cyclic voltammograms from electrodes before (dashed lines) and after modification with carbon nanotubes (solid line). Vertical columns compare types of functionalized nanotubes: amide-CNTs (panels A–C), carboxylic acid-CNTs (panels D–F) and octadecylamine-CNTs (panels G–I). Horizontal rows compare different compounds 10 μM serotonin (panels A, D, G), 10 μM dopamine (panels B, E, H), and 200 μM ascorbic acid (panels C, F, I). The insets for ascorbic acid show the reduction peaks.
To compare the variance among electrodes, Fig. 4 shows averaged data for each nanotube functionalization. Because carbon-fiber microelectrodes can vary in sensitivity, each electrode was used as its own control. To calculate statistical significance, peak currents for each analyte were compared before and after CNT coatings using paired t-tests. A normalized current increase was also calculated by dividing the current after CNT modification by the current before modification. The average normalized signal for 5-HT oxidation at CONH2-CNT electrodes was 46, signifying a significant, 46-fold increase in signal after CNT modification (p = 0.0052). For COOH-CNT modified electrodes, the average normalized current increase was a significant 5.4-fold increase (p = 0.0036), but was not as large as for CONH2-CNT. Oxidation current did not significantly increase for ODA-CNT electrodes (p = 0.7877). Trends were similar for dopamine oxidation peaks with the significant increases in current being detected with CONH2-CNT (13-fold increase, p = 0.0079) and COOH-CNT modified electrodes (4.6-fold increase, p = 0.003), but not for ODA-CNTs (p = 0.5668). The oxidation signal for AA displayed a different trend than the other analytes. Modification of an electrode with CONH2-CNTs or ODA-CNTs resulted in no significant changes in current (p = 0.6481, 0.7713 respectively) while COOH-CNTs increased the sensitivity toward AA by 4.5 times (p = 0.0184).
Figure 4.
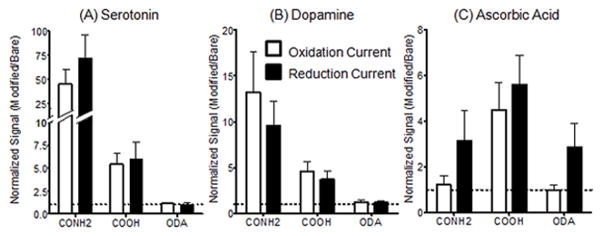
Averaged data comparing normalized peak currents. Signals after CNT modification are normalized by dividing by the current before modification for each electrode. These ratios were then averaged. Error bars are standard error of the mean. The dotted line at 1.0 marks no change in signal after electrode modification. Normalized values for (A) 10 μM serotonin, (B) 10 μM dopamine, and (C) 200 μM ascorbic acid show that the sensitivity to different analytes is dependent on surface functional groups. See Table 1 for sample group sizes.
For the reduction peaks, the normalized signal for 5-HT and DA followed a similar trend as the oxidation peaks, increasing with CONH2-CNT (p = 0.004 and 0.0023, respectively) and COOH-CNTs (p =0.0036 and 0.0026, respectively). However, reduction peaks for 5-HT, DA, and AA did not significantly increase with ODA-CNT modification (p = 0.5684, 0.7500 and p = 0.2584, respectively). The reduction current was significantly increased by CONH2-CNT or COOH-CNT modifications (p = 0.0431 and p = 0.0075, respectively) This suggests that although the oxidation signal is unaffected by CONH2-CNTs, an electrochemical enhancement of the reduction of AA still occurs. While the reduction peak observed after CNT modification is still small (see Fig. 3 insets), the reduction peak at the bare electrode is almost completely absent, which accounts for the larger increases. AA reversibility has previously been found to improve at carbon electrode surfaces modified with oxygen containing functional groups 37,38. The observed increase in reduction peak currents suggests that all of the CNT types also have some properties to increase AA reversibility.
Some of the example data in Fig. 3 is widely different than the average data in Fig. 4. The differences are explained with Fig. 5, which shows scatter plots of normalized 10 μM serotonin signals. Each electrode is depicted as a point and horizontal lines mark the median as well as the quartiles. The normalized signals for the ODA-CNT modified electrodes are clustered around 1, indicating that the signal does not increase. For CONH2-CNT modified electrodes approximately 20 % of the electrodes showed an increase in signal that was over an order of magnitude above the rest, referred to hereafter as “high-current electrodes”. However, 80 % of the electrodes had normalized signals below 30, with most electrodes in the 1.5 to 13 range. If the electrodes with a normalized signal above 30 were excluded, the average normalized signal would be about 3.4 for both 5-HT and DA, similar to COOH-CNT electrodes. The high-current electrodes were not observed with COOH-CNT modified electrodes, where the maximum normalized signal detected was 21.
Figure 5.
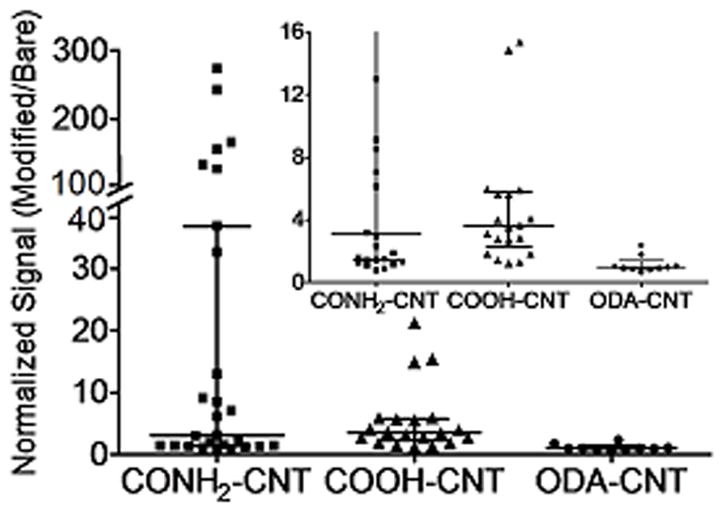
Scatter plots of normalized oxidation signal for 10 μM serotonin comparing the effects of carbon nanotube-modified electrodes with different functional groups: CONH2-CNT, COOH-CNT and ODA-CNT. On the scatter-whisker plots, the short horizontal lines outline the quartiles, long horizontal lines denote the median and points represent individual data points. The inset is magnified to better display lower signals.
Table 1 compares the means, medians, ranges, and standard deviations for all CNT-modifications for each analyte. In general, ODA-CNTs did not alter the oxidation signal of any of the analytes tested. The difference between mean and median of data is largest for CONH2-CNTs, due to the high current electrodes. CONH2-CNTs were the most sensitive toward 5-HT and DA, while COOH-CNTs increased the signal for all of the analytes similarly, about a 2 to 6-fold signal increase. Both COOH-CNTs and CONH2-CNTs had a linear response from 0.1 to 150 μM for dopamine and 0.1 to 20 μM for serotonin (Supplemental Fig. 2). The approximate limit of detection for CONH2-CNT electrodes was 90 nM for 5HT and 130 nM for DA and for COOH-CNT modified electrodes was 70 nM for 5HT and 180 nM for DA.
TABLE 1.
Comparison of normalized signals from functionalized CNT modification for 10 μM serotonin, 10 μM dopamine and 200 μM ascorbic acid detection
| Oxidation | Serotonin | Dopamine | Ascorbic Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| CNT | CONH2 | COOH | ODA | CONH2 | COOH | ODA | CONH2 | COOH | ODA |
| Mean | 46 | 5.4 | 1.1 | 13 | 4.6 | 1.3 | 1.2 | 4.5 | 1.0 |
| Median | 3.2 | 3.6 | 0.93 | 2.0 | 2.3 | 1.0 | 0.91 | 2.4 | 0.80 |
| Range | 0.75 – 270 | 1.2 – 21 | 0.64 – 2.3 | 0.38 – 99 | 0.83 – 22 | 0.72 – 4.4 | 0.43 – 5.6 | 1.0 – 16 | 0.51 – 2.3 |
| Standard Deviation | 80 | 5.4 | 0.52 | 27 | 5.5 | 0.92 | 1.36 | 5.07 | 0.59 |
| Sample Size | 27 | 20 | 10 | 39 | 26 | 15 | 13 | 18 | 8 |
| Reduction | Serotonin | Dopamine | Ascorbic Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| CNT | CONH2 | COOH | ODA | CONH2 | COOH | ODA | CONH2 | COOH | ODA |
| Mean | 72 | 6.2 | 1.1 | 8.3 | 3.7 | 1.2 | 3.2 | 5.6 | 2.9 |
| Median | 3.2 | 3.0 | 0.91 | 1.9 | 1.9 | 1.1 | 1.6 | 4.52 | 1.8 |
| Range | 0.75 – 370 | 0.99 – 31 | 0.56 – 1.8 | 0.28 – 69 | 0.57 – 17 | 0.75 – 3.3 | 0.82 – 15 | 0.85 – 46 | 0.81 – 9.6 |
| Standard Deviation | 79 | 8.0 | 0.44 | 15 | 4.7 | 0.63 | 4.7 | 5.3 | 2.9 |
| Sample Size | 27 | 20 | 10 | 39 | 26 | 15 | 13 | 18 | 8 |
Electron Transport Kinetics
The catalytic effect of CNTs on electron transport kinetics has been described for various analytes, including catecholamines 39–42. Electron transfer kinetics can be compared using the difference between peak oxidation and reduction potentials (ΔEp), with a decrease in ΔEp denoting faster electron transport kinetics. Table 2 compares the average ΔEp for bare and CNT-modified electrodes for serotonin, dopamine, and ascorbic acid. For 5-HT, ΔEp decreased significantly after modification with either CONH2-CNTs or COOH-CNTs (about 89 mV and 70 mV, respectively). The decrease in ΔEp for DA after CNT modification was smaller than for 5-HT using both CONH2-CNT and COOH-CNT (about 31 mV and 54 mV, respectively). The trends at CONH2-CNT and COOH-CNT modified electrodes were similar for AA but the variance was higher because the reduction peak potential was difficult to determine on the small peak; thus, the results were not statistically significant. In contrast, ODA-CNT modified electrodes displayed significant increases in ΔEp for both 5-HT and DA (44 mV and 43 mV, respectively). These results indicate that not all CNTs cause a catalytic effect on electron transfer kinetics and that functional groups modulate the electron transfer rate.
TABLE 2.
Effect of CNT functionalization on electron transport kinetics
| Serotonin | Dopamine | Ascorbic Acid | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| CNT | CONH2 | COOH | ODA | CONH2 | COOH | ODA | CONH2 | COOH | ODA |
|
|
|||||||||
| Bare Mean ΔEp | 0.52 ± 0.05 | 0.55 ± 0.08 | 0.50 ± 0.04 | 0.74 ± 0.08 | 0.77 ± 0.08 | 0.74 ± 0.05 | 0.97 ± 0.14 | 1.02 ± 0.15 | 0.88 ± 0.05 |
| CNT Mean ΔEp | 0.43 ± 0.17 | 0.48 ± 0.14 | 0.54 ± 0.05 | 0.70 ± 0.12 | 0.72 ± 0.09 | 0.78 ± 0.05 | 0.93 ± 0.17 | 0.97 ± 0.10 | 0.85 ± 0.14 |
| P-value | 0.0098 | 0.008 | 0.0141 | 0.0463 | 0.0015 | 0.0001 | 0.3735 | 0.3149 | 0.6596 |
| Significance | ** | ** | * | * | ** | *** | N.S. | N.S. | N.S. |
| Sample Size | 27 | 19 | 10 | 39 | 26 | 15 | 13 | 18 | 8 |
p-values determined using paired t-tests.
95% confidence,
99% confidence,
99.9% confidence,
N.S. not significant
The shape of the current vs time plots for a flow injection experiment provides insight into changes in mass transport or adsorption to the electrode. An ideal response for diffusion controlled detection would be square but adsorbed analytes, such as dopamine, deviate from that response and are rounded 43. Fig. 6 illustrates normalized, average current vs time response traces before (dashed line) and after modification (solid line) with CONH2-CNT, COOH-CNT, or ODA-CNT for 10 μM 5-HT (Fig. 6A, B, C, respectively) and 10 μM DA (Fig. 6D, E, F). Traces are normalized to account for differences in peak heights so variations in shape can be clearly observed. CONH2-CNT modification had the largest effect on time response, slowing the response for DA and 5-HT at both the introduction and removal of the analyte. Interestingly, the time response for COOH-CNT modification is about the same as the bare electrode. Therefore, COOH-CNTs increase sensitivity and electron transport kinetics without slowing the time response. The ODA-CNT modified electrodes display a slightly slower time response to dopamine but not serotonin. This change in response is likely due to inhibited mass transport of the analyte to the electrode surface caused by the long-chain alkyl groups.
Figure 6.
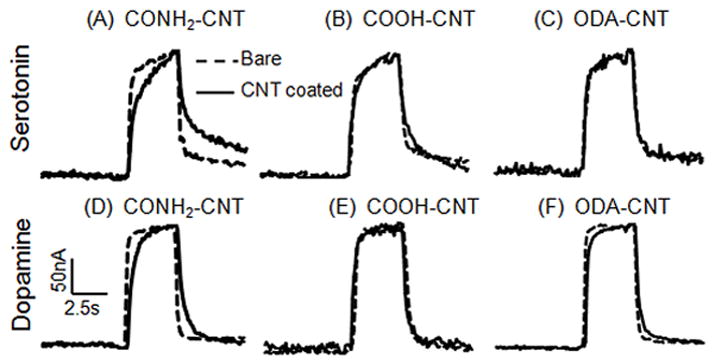
Normalized current vs time plots comparing the electrode time response to a bolus of 10μM serotonin (A–C), and 10μM dopamine (D–F). These responses were averaged from 10 electrodes each, but error bars are not shown for clarity. Bare electrodes are dashed lines and electrodes after CNT-modification are solid lines. Plots of COOH-CNT (A, D), CONH2-CNT (B, E), and ODA-CNT (C, F) responses are compared.
CNT-modified Electrodes in Biological Tissue
The goal of our experiments was to develop a simple, relatively quick method to increase the sensitivity of carbon microelectrodes for use in vivo. To examine the biological usefulness of these microelectrodes, COOH-CNT modified electrodes were used to measure endogenous serotonin changes in the ventral nerve cord (VNC) of the fruit fly, Drosophila melanogaster. The flies were genetically modified to express Channelrhodopsin-2, a blue light sensitive cation channel, in only serotonergic neurons 25. Serotonin release was stimulated by exposing the nerve cord to blue light for 10 s. Both CONH2-CNT and COOH-CNT modified electrodes could be used to measure serotonin in a Drosophila VNC, but COOH-CNTs were chosen for further study because of the faster time response and lower noise.
For measurements of serotonin in biological tissue, a modified applied waveform is normally used to reduce fouling by the reactive oxidation byproducts 22. With this waveform, a positive holding potential is used (+0.2 V) and the potential is ramped up to 1.0 V, down to −0.1 V and back to 0.2 V at 1000 V/s. Fig. 7A shows that using this serotonin waveform, the serotonin signal was larger after the COOH-CNT modification. The inset shows this increase in sensitivity was on average about 3-fold. Fig. 7B shows the CVs for 5-HT in two separate VNCs using a bare electrode (black dotted curve) or a COOH-CNT electrode (solid curve). Separate samples are used because multiple electrodes cannot be inserted into the same sample. Both electrodes were pre-calibrated, and the measured concentration of 5-HT was calculated for each VNC; about 650 nM was detected at the COOH-CNT modified electrode and about 870 nM was detected at the bare CFME. The COOH-CNT electrodes detected about 2.5-fold more current per micromolar serotonin than bare electrodes (Fig. 7B inset). Therefore, the increases in sensitivity observed during the in vitro calibration experiments were also observed in the biological tissue. The electrodes were stable enough to use for several hours in tissue.
Figure 7.
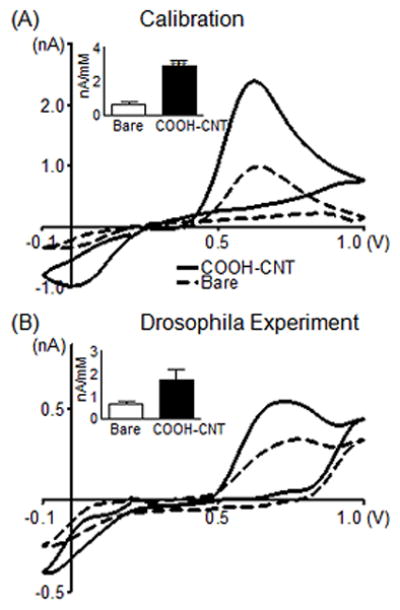
Measurements of endogenous serotonin in Drosophila. (A) Effect of COOH-CNT modification on the detection of 10 μM serotonin using the “serotonin waveform.” Electrode was scanned from 0.2 to 1.0 to −0.1 and back to 0.2 V at 1000 V/s at 10 Hz. The CV compares an electrode before (dashed) and after (solid) CNT modification during in vitro calibration. The inset shows average sensitivity for the oxidation peak (n=5). (B) Detection of serotonin in a larval Drosophila melanogaster ventral nerve cord. Different nerve cords are used for the bare (dashed) and COOH-CNT (solid) electrodes. Converting current to concentration using calibration data shows the COOH-CNT detected 650 nM serotonin with a peak oxidation current of 0.54 nA, while the bare electrode detected 870 nM serotonin with a peak oxidation current of 0.33 nA. The inset shows the average sensitivity for bare electrodes and COOH-CNT modified electrodes in Drosophila (n=5).
DISCUSSION
Variability among electrodes
The “high current electrodes” observed with CONH2-CNTs modification can be explained by differences in the thickness of the CNT layer. Electrodes with large CNT agglomerations displayed large signals but also much higher noise that resulted in a reduced S/N ratio. Therefore, the “high-current electrodes” were not ideal for use in biological tissue. CONH2-CNTs were generally more difficult to suspend and CNT agglomerations were more common than other functionalized CNTs. Agglomerations also likely caused more CONH2-CNT modified electrodes to be rejected for having a S/N less than 10 (35 %) compared to 15 % for COOH-CNT and ODA-CNT. Similarly, agglomerations and impurities caused all the electrodes that were modified with non-functionalized, pristine CNTs to be rejected because of low S/N ratios. Therefore, direct comparison between functionalized and non-functionalized CNTs was not possible with identical fabrication methods.
Most CNT electrode studies fabricate one, or a small number of electrodes and do not address reproducibility of CNT-modified electrode fabrication, likely because it is a challenging issue. In our study, variability among electrodes was high, even when the high-current electrodes were excluded. The variability was lower among COOH-CNT modified electrodes, which might make them the best candidates for routine use. Alternatively, CONH2-CNT electrodes with high S/N ratios could be chosen, as electrodes are normally tested before biological use and noisy electrodes could be excluded without adding extra procedural steps. Reproducibility will be a key area for future research. Because a thin and uniform layer of CNTs appears to be optimal, strategies to achieve this, such as depositing CNTs into controllable polymer layers or using charge assisted assembly of nanotubes, might lead to more order.
Effect of functional groups on electrochemical detection
Oxygen-containing functional groups on carbon electrodes affect electrode properties such as analyte adsorption, electron transfer kinetics, and electrocatalysis, particularly for cationic analytes such as dopamine 1,5. Similarly, functional groups on carbon nanotubes are also expected to affect electrode properties. The faster electron kinetics at CNT-modified electrodes are generally attributed to the intrinsic properties of the nanotubes but the typical CNT purification procedure in strong acids produces COOH functional groups, so the functional group could be playing a role in the electrode properties. By comparing differently functionalized nanotubes, we determined that functional groups on CNTs modulate sensitivity, electron transfer, and electrocatalysis, although the effects are dependent on analyte properties.
Modification of carbon-fiber electrodes with CNTs is expected to change the conductivity of the surface44,45, the availability of edge plane active sites for adsorption and the density of electronic states available for electron transfer 1. The addition of defect sites on CNTs decreases conductivity by disrupting the sp2 hybridization 46. Adding octadecylamine, a long chain alkane with low electron conductivity, to the defect sites would be expected to further reduce conductivity. However, COOH and CONH2 groups would be expected to have higher conductivity than ODA-CNTs because both have electron rich oxygen atoms with open pi orbital sites to allow electron conduction. The size of the functional groups could also affect analyte interactions with the electrode surface, as octadecylamine is a long chain that would prevent close approach by the analyte and therefore increase the electron tunneling distance. Functional groups may affect intermolecular interactions of analytes with the electrode including dipole-dipole interactions, induced dipoles, hydrophobic effects, and electrostatic interactions 1. Both amide and carboxyl groups are polar, facilitating strong dipole-dipole interactions with the analytes. Also, the charge of the COOH groups would allow direct electrostatic interactions with positively charged analytes such as dopamine and serotonin, although negatively charged analytes like ascorbic acid would be electrostatically repelled. Intermolecular interactions between the analytes and ODA-CNTs were expected to be weaker.
The data largely followed the predictions made from intermolecular interactions. Little increase in signal was observed for any of the analytes with ODA-CNT modified electrodes. COOH-CNT and CONH2-CNT modified electrodes had large increases in sensitivity for dopamine and serotonin (Table 1) demonstrating polar functional groups enhanced the electrochemistry of cations. This increase could be due to the addition of adsorption sites or a modulation of the adsorption/desorption coefficients due to changes in interactions between the analyte and the electrode. The time response curves in Fig. 6 help distinguish between these effects. CONH2-CNT electrodes had slower time response traces after CNT modification for serotonin and dopamine but COOH-CNT modified electrodes did not. Increasing the adsorption rate constant is expected to slow the electrode response 47. Thus, the larger signal increases and slow response of CONH2-CNT modified electrodes may be due to stronger adsorption caused by interactions between the indolamine or catecholamine and the amide functional group 5,48,49. The increase in signal for COOH-CNT modified electrodes is likely due to more adsorption sites and an increased number of functional groups, rather than a change in the adsorption constant since the time response does not change. The COOH-CNT modified electrodes provide higher sensitivity without compromising temporal resolution.
Ascorbic acid oxidation currents are controlled primarily by diffusion to the electrode, not by adsorption 36. At CONH2-CNT modified electrodes, the signal for AA does not increase and AA may be repelled by the electron rich amide functional groups. Similarly, no increase in signal was observed for AA at ODA-CNT electrodes. At COOH-CNT electrodes, AA has an equivalent signal increase to both dopamine and serotonin, which was not expected based on electrostatic interactions. However, AA electrochemistry is known to depend on surface chemistry. Previous studies with glassy carbon electrodes have shown that while AA did not adsorb to the electrode, per se, masking oxide groups interfered with the electrochemistry, showing that AA did interact with them 50. Likewise, other studies have shown that surface oxide groups can lead to enhanced AA reversibility 38,51. We observed larger reduction peaks and therefore electrocatalysis for AA at all CNT modified electrodes, but especially for COOH-CNTs.
Functional groups affected electron transfer (Table 2) as the polar CONH2-CNT and COOH-CNT modified electrodes had significantly faster electron transfer for both serotonin and dopamine, as evident from a decrease in ΔEp. In contrast, ODA-CNT modified electrodes significantly increased ΔEp for serotonin and dopamine, suggesting that the ODA group impeded electron transfer. These data follow the predictions made based on conductivity and electron tunneling distance. Thus, electron transfer is governed not only by the electronic properties of the nanotube, but also by the functional group.
Practical Use and Conclusions
Ideal electrodes for neurotransmitter experiments would be easily and reproducibly fabricated, small enough to cause minimal tissue damage, and able to track fast changes in neurochemicals. CNT-modified microelectrodes meet many of these criteria, as fabrication using commercial nanotubes is easy and FSCV allows rapid measurements of neurotransmitter changes. Improvements in reproducibility are still necessary, but the inexpensive nature of fabricating the electrodes allows a small number of electrodes to be discarded with limited loss in productivity. The electrodes are small enough to be implanted in the VNC of a Drosophila larva, which is only 50 μm wide. Previous experiments measuring endogenous neurotransmitters in Drosophila25–27 have used cylindrical electrodes because they have higher surface areas and higher sensitivity. The increase in sensitivity imparted by COOH-CNT modification makes disk electrodes, which are easier to localize in specific areas, more feasible for use in these studies. The sensitivity increases are maintained after tissue implantation, so CNT modification is a valid strategy for detecting small changes in neurotransmitters. These electrodes would also be amenable to detection of neurotransmitters in other animal models as well, such as in brain slices or in vivo. COOH-CNT electrodes offer fast time response and better reproducibility while CONH2-CNT electrodes could be used when sensitivity to cations over anions is desired or when larger increases in sensitivity are needed. Being able to tune the electrochemical properties by implementing different functionalized CNTs is advantageous, as the functionalized CNTs can be chosen according to which analytes or interferents are expected to be present in the tissue. Thus, CNT-modified electrodes are promising for real applications as neurotransmitter sensors.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Science Foundation (Grant CHE 0645587522) and the National Institutes of Health (Grant R01 MH085159) to B.J.V.
Reference List
- 1.McCreery RL. Chem Rev. 2008;108:2646. doi: 10.1021/cr068076m. [DOI] [PubMed] [Google Scholar]
- 2.Bath BD, Martin HB, Wightman RM, Anderson MR. Langmuir. 2001;17:7032. [Google Scholar]
- 3.Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM. Analyst. 2003;128:1413. doi: 10.1039/b307024g. [DOI] [PubMed] [Google Scholar]
- 4.Duvall SH, McCreery RL. J Am Chem Soc. 2000;122:6759. [Google Scholar]
- 5.Allred CD, McCreery RL. Anal Chem. 1992;64:444. [Google Scholar]
- 6.McCreery RL, Cline KK, Mcdermott CA, Mcdermott MT. Colloids Surf, A. 1994;93:211. [Google Scholar]
- 7.Swain GM, Kuwana T. Anal Chem. 1991;63:517. [Google Scholar]
- 8.Alsmeyer YW, McCreery RL. Langmuir. 1991;7:2370. [Google Scholar]
- 9.Dumitrescu I, Unwin PR, Macpherson JV. Chem Commun. 2009:6886. doi: 10.1039/b909734a. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs CB, Peairs MJ, Venton BJ. Anal Chim Acta. 2010;662:105. doi: 10.1016/j.aca.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Forrest GA, Alexander AJ. J Phys Chem C. 2007;111:10792. [Google Scholar]
- 12.Ismail AF, Goh PS, Tee JC, Sanip SM, Aziz M. Nano. 2008;3:127. [Google Scholar]
- 13.Rivas GA, Rubianes MD, Rodriguez MC, Ferreyra NE, Luque GL, Pedano ML, Miscoria SA, Parrado C. Talanta. 2007;74:291. doi: 10.1016/j.talanta.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Yun YH, Dong ZY, Shanov V, Heineman WR, Halsall HB, Bhattacharya A, Conforti L, Narayan RK, Ball WS, Schulz MJ. Nano Today. 2007;2:30. [Google Scholar]
- 15.Wang J. Electroanalysis. 2005;17:7. [Google Scholar]
- 16.Trojanowicz M. TrAC, Trends Anal Chem. 2006;25:480. [Google Scholar]
- 17.Gooding JJ. Electrochim Acta. 2005;50:3049. [Google Scholar]
- 18.Britto PJ, Santhanam KSV, Ajayan PM. Bioelectrochem Bioenergetics. 1996;41:121. [Google Scholar]
- 19.Phillips PEM, Wightman RM. TrAC, Trends Anal Chem. 2003;22:509. [Google Scholar]
- 20.Swamy BEK, Venton BJ. Analyst. 2007;132:876. doi: 10.1039/b705552h. [DOI] [PubMed] [Google Scholar]
- 21.Hocevar SB, Wang J, Deo RP, Musameh M, Ogorevc B. Electroanalysis. 2005;17:417. [Google Scholar]
- 22.Jackson BP, Dietz SM, Wightman RM. Anal Chem. 1995;67:1115. doi: 10.1021/ac00102a015. [DOI] [PubMed] [Google Scholar]
- 23.Heien MLAV, Johnson MA, Wightman RM. Anal Chem. 2004;76:5697. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- 24.Strand AM, Venton BJ. Anal Chem. 2008;80:3708. doi: 10.1021/ac8001275. [DOI] [PubMed] [Google Scholar]
- 25.Borue X, Cooper S, Hirsh J, Condron B, Venton BJ. J Neurosci Methods. 2009;179:300. doi: 10.1016/j.jneumeth.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borue X, Condron B, Venton BJ. J Neurochemistry. 2010;113:188. doi: 10.1111/j.1471-4159.2010.06588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickrey TL, Condron B, Venton BJ. Anal Chem. 2009;81:9306. doi: 10.1021/ac901638z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo HX, Shi ZJ, Li NQ, Gu ZN, Zhuang QK. Anal Chem. 2001;73:915. doi: 10.1021/ac000967l. [DOI] [PubMed] [Google Scholar]
- 29.Wightman RM, Wipf DO. In: Electroanalytical Chemistry: A Series of Advances. Bard AJ, editor. Vol. 15. Marcel Dekker, Inc; New York City: 1989. p. 267. Voltammetry at Ultramicroelectrodes. [Google Scholar]
- 30.Thess A, Lee R, Nikolaev P, Dai HJ, Petit P, Robert J, Xu CH, Lee YH, Kim SG, Rinzler AG, Colbert DT, Scuseria GE, Tomanek D, Fischer JE, Smalley RE. Science. 1996;273:483. doi: 10.1126/science.273.5274.483. [DOI] [PubMed] [Google Scholar]
- 31.Long JT, Weber SG. Anal Chem. 1988;60:2309. doi: 10.1021/ac00166a002. [DOI] [PubMed] [Google Scholar]
- 32.Weber SG. Anal Chem. 1989;61:295. doi: 10.1021/ac00179a004. [DOI] [PubMed] [Google Scholar]
- 33.Ikarashi Y, Blank CL, Maruyama Y. J Chromatogr. 1993;645:219. doi: 10.1016/0021-9673(93)83381-2. [DOI] [PubMed] [Google Scholar]
- 34.Zhou FM, Liang Y, Salas R, Zhang LF, De Biasi M, Dani JA. Neuron. 2005;46:65. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 35.Wightman RM, Deakin MR, Kovach PM, Kuhr WG, Stutts KJ. J Electrochem Soc. 1984;131:1578. [Google Scholar]
- 36.Baur JE, Kristensen EW, Wightman RM. Anal Chem. 1988;60:2334. doi: 10.1021/ac00172a005. [DOI] [PubMed] [Google Scholar]
- 37.Deakin MR, Kovach PM, Stutts KJ, Wightman RM. Anal Chem. 1986;58:1474. doi: 10.1021/ac00298a046. [DOI] [PubMed] [Google Scholar]
- 38.Stutts KJ, Kovach PM, Kuhr WG, Wightman RM. Anal Chem. 1983;55:1632. [Google Scholar]
- 39.Wang J, Musameh M, Lin Y. J Am Chem Soc. 2003;125:2408. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- 40.Banks CE, Davies TJ, Wildgoose GG, Compton RG. Chem Commun. 2005:829. doi: 10.1039/b413177k. [DOI] [PubMed] [Google Scholar]
- 41.Banks CE, Compton RG. Analyst. 2005;130:1232. doi: 10.1039/b508702c. [DOI] [PubMed] [Google Scholar]
- 42.Gong KP, Chakrabarti S, Dai LM. Angewandte Chemie-International Edition. 2008;47:5446. doi: 10.1002/anie.200801744. [DOI] [PubMed] [Google Scholar]
- 43.Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM. Anal Chem. 2000;72:5994. doi: 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- 44.Sulong AB, Muhamad N, Sahari J, Ramli R, Deros BM, Park J. European Journal of Scientific Research. 2009;29:13. [Google Scholar]
- 45.Kim YJ, Shin TS, Choi HD, Kwon JH, Chung YC, Yoon HG. Carbon. 2005;43:23. [Google Scholar]
- 46.Garcia-Lastra JM, Thygesen KS, Strange M, Rubio A. Phys Rev Lett. 2008;101 doi: 10.1103/PhysRevLett.101.236806. [DOI] [PubMed] [Google Scholar]
- 47.Stamford JA, Hurst PR, Kuhr WG, Wightman RM. J Electroanal Chem. 1989;265:291. [Google Scholar]
- 48.Runnels PL, Joseph JD, Logman MJ, Wightman RM. Anal Chem. 1999;71:2782. doi: 10.1021/ac981279t. [DOI] [PubMed] [Google Scholar]
- 49.Michael AC, Justice JB. Anal Chem. 1987;59:405. doi: 10.1021/ac00130a006. [DOI] [PubMed] [Google Scholar]
- 50.Chen PH, McCreery RL. Anal Chem. 1996;68:3958. [Google Scholar]
- 51.Zhang MN, Liu K, Xiang L, Lin YQ, Su L, Mao LQ. Anal Chem. 2007;79:6559. doi: 10.1021/ac0705871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


