Abstract
Anthocyanins (AC) are water-soluble natural pigments found in various parts of higher plants. Despite their limited oral bioavailability and very low post-absorption plasma concentrations, the dietary consumption of these pigments has been proposed to be associated with a significant protection against several human pathological conditions, including cardiovascular diseases. Many studies highlighted that some health benefits of AC localize in particular at endothelium level, contributing to vascular homeostasis and also to the control of angiogenesis, inflammation, and platelet aggregation. This review reports and comments on the large existing literature addressing the molecular mechanisms that, beyond the antioxidant properties, may have a significant role in the effects of AC and AC-rich foods on vessel endothelium. Among these, AC have been reported to prevent peroxynitrite-mediated endothelial dysfunction in endothelial cells (ECs), thanks to their capability to modulate the expression and activity of several enzymes involved in NO metabolism. Furthermore, evidence indicates that AC can prevent the expression of adhesion molecules and the adhesion of monocytes to ECs challenged by pro-inflammatory agents. Overall, the activity of AC could be associated with the ability to elicit cell adaptive responses involving the transcription factor Nrf2 by affecting the “nucleophilic tone” of the organism. This review confirms the importance of specific nutritional molecules for human health and suggests new avenues for nutrition-based interventions to reduce the risk of cardiovascular disease in the population.
Keywords: Anthocyanins, Endothelial dysfunction, Nrf2, Inflammation, Nitric oxide
Introduction
Anthocyanins (AC) represent a group of water-soluble natural pigments present in different parts (mainly flowers and fruits, but also leaves, stems, and roots) of higher-order plants. AC are intensely colored pigments conferring blue, purple, red, and orange colors to many edible fruits and vegetables.
The interest of these plant pigments, in addition to the technological relevance due to the sensorial characteristics, is also rising for their potentially beneficial properties for human health. The potential effects of AC in health and disease have attracted and are attracting not only medical research but also the stakeholders of food industry, not only as natural alternatives to synthetic dyes but also for the formulation of novel functional foods or food-derived extracts of food or nutraceuticals.
Up to the beginning of this century, the main feature of AC that attracted scientists involved in disease risk was in the ability to act as “free radical scavengers.” According to this ability, shared in general by all molecules having a phenolic structure characterized by B ring hydroxyl groups and a conjugated double bond system, the wide spectrum of biological effects of AC was attributed, by earlier studies, to an antioxidant activity (Prior and Wu 2006). However, more recently, several reports became available, indicating the involvement of other mechanisms of action beyond such activity, and it seems clear that in vivo AC biological effects cannot be explained solely on the basis of their antioxidant characteristics. The most accredited mechanism indicates that AC are able to modulate crucial signaling pathways and gene regulation (Nöthlings et al. 2008; de Pascual-Teresa et al. 2010; Cimino et al. 2013) in particular thanks to a positive interaction with Nrf2-mediated signaling and gene expression.
In fact, even back to 1939, the Nobel laureate Linus Pauling was the first one to propose that the intensity of the color exhibited by these pigments is caused by the resonant structure of the flavylium ion (Wrolstad et al. 2005). This structure confers to AC-specific chemical properties. In particular, the presence of a positive charge at physiological pH renders AC different from other polyphenols and is associated with strong reactivity with electron-rich substrates. In its quinoidal form, this structure has been convincingly proposed to be an important mechanism contributing to the maintenance of a high cellular nucleophilic tone by the molecules formerly pooled within the broad family of “dietary antioxidants” as it results in the expression of endogenous antioxidant enzymes by a “para-hormetic” effect (Forman et al. 2014a). This feature therefore makes AC good candidates to play a beneficial role in several human pathological conditions (Domitrovic 2011; Huang et al. 2013).
Irrespective of the exact mechanism of action, many studies revealed that a significant part of the effects of AC localize at the level of the vessel endothelium. Vessel endothelium, although composed by a single layer of cells lining the vascular systems, actively contributes in several ways, along with other functions, to vascular homeostasis.
This review deals with some of the molecular mechanisms underlying the effects of AC and AC-rich foods on vascular system, mainly focusing on their possible protective role against the endothelial dysfunction that characterizes several vascular pathological conditions. In this review, we anticipate that, on the basis of the available literature, AC can be considered substances not only “Generally Recognized as Safe—GRAS” but, adopting a recently proposed novel concept and acronym, “Generally Regarded as Beneficial—GRAB” (Forman et al. 2014b). According to this concept, nutritional policies should specifically address the importance of this family of compounds and encourage the consumption of AC-rich foods as an expedient strategy to reduce the risk of vascular dysfunction and disease.
This review aims to provide a critical view of the most relevant available literature addressing the biological effects of AC, with a particular concern to potential weaknesses of previous studies. We have therefore considered the majority of the publications having the term “anthocyanin” or the chemical name of specific molecules belonging to this family (or food items known to be rich sources of these molecules) within the title or the keywords or abstract. Additional sources were added to corroborate our comments to the cited papers. In this case, several different searching criteria were utilized. The search engines selected to retrieve published studies was either PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) or Scopus (www.scopus.com).
Anthocyanin chemistry and dietary sources in western diets
AC are mainly present in nature in the form of heterosides where a hydroxyl group attached to the first carbon is substituted by an alcoholic, a phenolic, or a specific sugar moiety. Structurally, AC are derivatives of 2-phenylbenzopyrylium (flavylium cation) and consist of an aglycone (anthocyanidin), sugar(s), and, in many cases, acyl group(s) (Andersen and Jordheim 2006).
Depending on the numerous existing structural characteristics (number and position of hydroxyl and methoxyl groups as substituents, the nature and the number of bonded sugars to their structure, the aliphatic, or aromatic carboxylates bonded to the sugar in the molecule and the position of these bonds), the literature reports more than 500 different AC. Among them, the six anthocyanidins most commonly found in fruits and vegetables are pelargonidin, cyanidin, delphinidin, petunidin, peonidin, and malvidin (Pg, Cy, Dp, Pt, Pn, Mv) (Harborne 1993; de Pascual-Teresa et al. 2000). Their distribution in plants usually consumed in the western diet is roughly as follows: Cy 50 %, Dp 12 %, Pg 12 %, Pn 12 %, Pt 7 %, and Mv 7 %. 3-monoglycosides, 3-diglycosides, 3,5-diglycosides, and 3-diglycoside-5-monoglycosides are the more known glycosidic variations among these pigments, glucose being the most common conjugated sugar (Pati et al. 2009). The frequency of 3-glucoside derivatives is 2.5-fold than that of 3,5-diglucosides, and the most common AC in the western diet is Cy-3-O-glucoside (C3G) (Kong et al. 2003).
As mentioned above, the major food sources of AC in western dietary profile are red fruits (berries and red grapes), red wine, cereals and purple corn, and some vegetables, such as red cabbage (de Pascual-Teresa and Sanchez-Ballesta 2008). On the basis of the available reports, it is interesting to note that the content of AC often varies within the same food items of a factor of 20 or more. These differences are linked to several determinants, including seasonal and/or local environmental characteristics as well as to specific features of the cultivar. These significant within-item differences render difficult the precise assessment of AC consumption in individuals. On the other hand, the possibility to improve AC consumption by introducing food plants selected for a high AC synthesis is an open strategy to increase the dietary consumption of these pigments.
The mean dietary AC intake obviously depends on the specific dietary profile characteristic of the population under study and has been estimated to be between 3 and 215 mg/day (Wu et al. 2006; Frankel et al. 1995; Kuhnau 1976; Chun et al. 2007). These numbers are significantly higher than those reported for other dietary flavonoids such as genistein and quercetin (estimated range 20–25 mg/day) (Hertog et al. 1993). However, methodological differences in the estimation, as well as geographical, seasonal, social, and cultural diversity of the examined populations, may also contribute to explain the wide reported range of AC consumption (de Pascual-Teresa and Sanchez-Ballesta 2008; Clifford 2000; Manach et al. 2005). For example, the European Prospective Investigation into Cancer and Nutrition (EPIC) study, involving totally 36,037 subjects (ranged between 35 and 74 years old) in ten European countries, has recently estimated the dietary intake and food sources of AC and their derivatives, together with lifestyle factors (sex, age, body mass index, smoking status, educational level, and physical activity) (Zamora-Ros et al. 2011). Interestingly, a clear “south to north” gradient of intake was observed. Cy and Mv have been found to be the most consumed AC, with significant geographical- and gender-related differences. AC intake associated with the consumption of specific food items frequently considered “healthy food” has been found to be higher in non-obese older females, non-smokers (former or never smokers), those having higher education level and those doing moderate or active physical activity. In this study, no specific further “confounders” (such as most frequent medical surveys) were identified. In general, as expected, the most important food sources of AC were fruits, wine, fruit-based non-alcoholic beverages, and some vegetables. Giving for granted the beneficial effects of AC in human health, the available database of AC consumption let us anticipate that a significant space exists to implement nutritional policies targeted to increase the dietary consumption of AC-rich foods, in particular among specific groups of population characterized by “at risk” lifestyles.
Anthocyanin bioavailability and bioefficacy: from the chemical structure to the influence of the food matrix
Obviously, bioactive compounds derived from plant sources need to be bioavailable in order to exert any effect. Therefore, a complete knowledge of their pharmacokinetic is important to understand the real impact of the daily intake on health protection and improvement. This obvious statement has unfortunately been somehow frequently ignored by several investigators who have suggested mechanisms underlying the protective effects of plant-derived molecules obtained on the solely base of in vitro experimental designs. This weakness has, in some cases, brought about significant bias, narrowed the understanding of data, and sometime possibly compromised the robustness of the conclusion of further experimental and supplementation studies.
Similarly to other phenolics, AC are hydrolyzed in the intestine and rapidly absorbed from the gut, entering into epithelial cells by passive diffusion or carrier-mediated permeation (Crozier et al. 2009). In enterocytes, they are further metabolized and eliminated in urine and bile, with an overall rate depending on both the aglycone structure and the sugar moiety (Ichiyanagi et al. 2006). However, available data suggest that AC are poorly bioavailable (Pojer et al. 2013). In fact, the proportion of total AC (native AC and metabolites) absorbed and excreted in urine is very low as compared to the ingested dose (McGhie and Walton 2007). Several factors may be responsible for this apparent low bioavailability, including the fact that a significant number of metabolites can be present at concentration below the analytical detection limits, and that carbinol and chalcone forms of AC, present in blood and urine at neutral pH values, may escape detection or be chemically bound to other components (Mazza 2007).
AC reach maximum concentration in the circulatory system within 3 h in humans. This fast rate is mainly due to the gastric absorption (Passamonti et al. 2003). Subsequently, AC are rapidly removed from plasma by liver metabolism (Milbury et al. 2002, 2010a , b) and, overall, the reported bioavailability is <2 % (Ichiyanagi et al. 2006; Stoner et al. 2005). Data from experimental animals suggest that intact AC may be efficiently taken up into the tissues of GI tract, but not transported into the circulation, and that gut microflora is able to degrade ingested AC in the large intestine (McGhie and Walton 2007). Glycosylated and acylated AC are supposed to be lesser bioavailable; however, AC can be hydrolyzed by glycosidases of GI tract generating the corresponding aglycones. In this form, AC have an increased bioavailability and higher biological potential effects at the expense of their stability.
The specific features of AC aglycones, as well as the specificity of glycosylation, affect plasma AC disappearance half-time (t1/2). In fact, at least in the rat, AC carrying the same sugar moiety have the following plasma t1/2: Dp > Cy > Pt = Pn > Mv. Similarly, considering AC carrying the same aglycone, the t1/2 is decreasing (except for Mv) in the order: galactoside > glucoside > arabinoside (Ichiyanagi et al. 2006).
It has been demonstrated that in humans, absorption, gastrointestinal transit, and plasma elimination times depend on anthocyanin structure. In a study dealing with the adminstration of purple carrots containing five different 3-O-glycosides of Cy, to 12 healthy volunteers (Novotny et al. 2012), the efficiency of absorption of acylated compounds was lower than that observed for non-acylated AC. The same study reports that acylated AC have a shorter half-life for gastrointestinal absorption than non-acylated AC, while the fractional elimination rate of non-acylated compounds was slower than that of acylated AC.
In adult men, a single oral administration of 721 mg of a mixture of Cy-3-glycosides leads to a cumulative serum concentration of both parent AC and their metabolites equal to about 377 nmol/L h, with a peak concentration of 96 nmol/L at 2.8 h (Kay et al. 2005). The total urinary excretion of ingested AC over 24 h was 1,071 µg.
Jeon et al. (2012) analyzed the pharmacokinetics of C3G in 12 subjects, after a 2-week multiple dosing of black bean (Phaseolus vulgaris, Cheongjakong-3-ho) seed coat extract, confirming that a significant amount of C3G is absorbed in humans following the ingestion of this extract. The accumulation after a 2-week multiple dosing was also excluded to occur. Similarly, the administration of 20 g of a chokeberry extract to healthy volunteers led to average levels of AC and their metabolites equal to 592 nmol/L (range 197–986) within 2 h in the serum and 12 mmol/L (range 11–13) in the urine 24 h after administration (Kay et al. 2004). In another study, AC plasma concentrations in 15 participants with coronary artery disease reached a maximum within 1.5 h after the administration of 480 mL cranberry juice containing about 95 mg AC (Milbury et al. 2010a). Plasma concentrations of the specific AC ranged between 0.6 and 4.6 nmol/L. The pattern of AC glucosides detected in plasma mainly reflected the relative concentration assessed in the juice. Finally, Ohnishi et al. (2006) recovered 5 % of AC contained in the administered cranberry juice in the urine of humans. Other studies reported a recovery between 1.8 and 2 % of strawberry AC in human urine (Felgines et al. 2003; Carkeet et al. 2008).
However, important discrepancies regarding metabolism of AC are present in the available literature. For instance, Miyazawa et al. (1999) reported that, both in rats and humans, Cy glycosides are absorbed as they are, while Kay et al. (2005) found that Cy-3-glycosides, once rapidly absorbed are extensively metabolized into glucuronidated and methylated derivatives following a moderate-to-high oral dose in humans. In fact, the administration of an oral 721 mg dose of Cy-3-glycosides from chokeberry extract leads to a cumulative serum concentration (expressed as the area under the concentration curve over the time) of 376.65 ± 16.20 nmol h/L, of total AC (parent compounds and metabolites) within a window of 0–7 h. The maximum concentration was reached within 2.8 h, and the parent AC represented only 32 % of the total AC detected. A similar picture was observed in urine samples, where only 32.5 % of the AC excreted (total 24 h) were in the same form of parent compounds. Furthermore, pharmacokinetic reports indicate that parent glycosides and glucuronide conjugates are mainly present in the bloodstream up to 5 h from administration. At a later stage, methylation increases (6–24 h), suggesting that metabolism can affect the bioactivity of AC over time (Mazza and Kay 2008).
However, plasma AC levels seems to be sufficiently high to exert their biological activity, especially for specific targets like vessel endothelium cells, where intracellular signaling pathways and gene-regulatory activities have been reported to be significantly modulated by AC (either parent compounds or their derivatives).
Interestingly, Czank et al. (2013) have recently demonstrated that AC are more bioavailable than previously supposed, and that their metabolites are still present in the circulation at ≤48 h from ingestion. These authors investigated the absorption, distribution, metabolism, and excretion (ADME) of a (13)C5-labeled anthocyanin in eight male subjects after the administration of 500 mg isotopically labeled C3G. Different biological samples (blood, breath, urine, and feces) were collected over 48 h, and (13)C and (13)C-labeled metabolite concentrations were measured by isotope-ratio mass spectrometry and liquid chromatography-tandem mass spectrometry. The relative bioavailability was 12.4 ± 1.4 %. Maximum rates of (13)C elimination were achieved 30 min after ingestion, whereas (13)C-labeled metabolites peaked (maximum serum concentration: 5.97 ± 2.14 μmol/L) at 10.2 ± 4.1 h. The half-life for (13)C-labeled metabolites, identified as degradation products, phenolic, hippuric, phenylacetic, and phenylpropenoic acids, ranged between 12.4 ± 4.2 and 51.6 ± 22.6 h.
Finally, individual variation in AC bioavailability is possibly associated with inter-individual differences of xenobiotic metabolism in GI tract, liver, and other tissues. These differences are very likely to be caused by the expression of single nucleotide polymorphisms (SNPs) such as the one reported in specific phase II drug metabolizing enzymes genes, like catechol-O-methyltransferase (Miller et al. 2011), glutathione S-transferases (Lampe 2007), and UDP glucuronosyl transferase (Iwuchukwu et al. 2009), but also to variation of intestinal microflora population (microbiome) (Del Rio et al. 2010).
Anthocyanins effects on endothelium
The vessel endothelium: from function to dysfunction
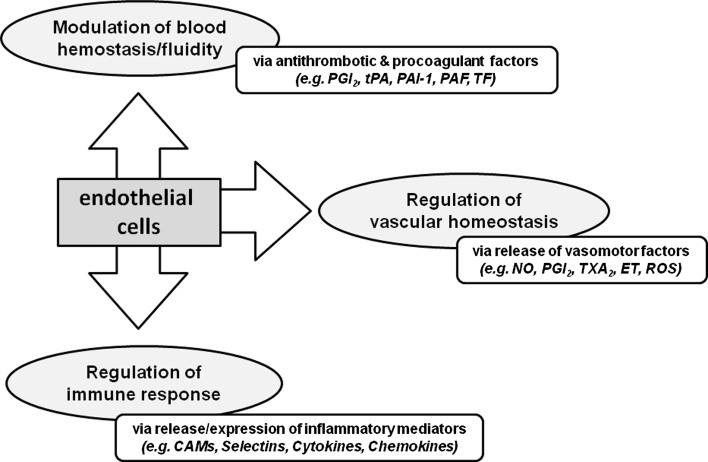
The endothelium is a single layer of cells constituting the inner surface of all blood vessels and acting as an active interface between the blood vessel wall and blood stream. In each organ, the endothelial floor controls the flow of nutrients and of the different biologically active molecules, playing a critical role as a barrier and as a primary sensor of physical and chemical changes occurring in the bloodstream. The endothelium also controls the passage of fluids into tissues, modulates cellular trafficking and coagulation, and contributes to the regulation of blood pressure (Fig. 2) (Endemann and Schiffrin 2004). Endothelial cells (EC) serve as the gateway for leukocyte entry into tissues in response to inflammatory stimuli by a transmigration process called extravasation. They have also a key role in the regulation of several aspects of immune responses. Finally, EC contributes to the control of mitogenesis and angiogenesis (Zania et al. 2008).
Fig. 2.
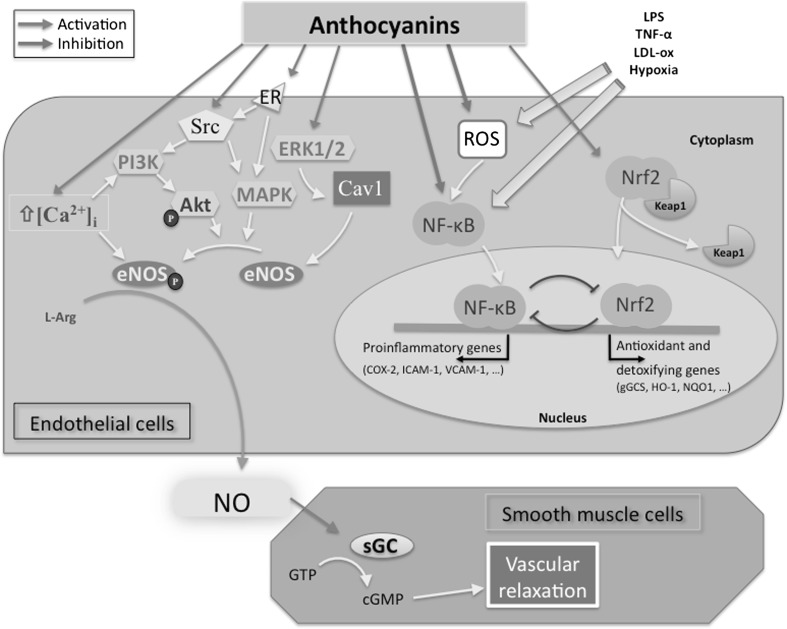
Effects of AC in vascular endothelium. Anthocyanins are potent inducers of the endothelial formation of NO involving different intracellular signaling pathways. Furthermore, AC are able to modulate pro-inflammatory pathway by inhibiting ROS and the redox-sensitive transcription factor NF-κB. Indirect mechanisms involved in ROS scavenging ability of AC could be also linked to acute activation of antioxidant and detoxifying enzymes modulated by Nrf2 transcription factor. ROS reactive oxygen species, PI3K phosphatidylinositol 3-kinase, MAPK mitogen-activated protein kinases, ERK1/2 extracellular regulated kinase 1 and 2, Akt protein kinase B, LDL-ox oxidized low density lipoprotein, TNF-a tumor necrosis factor a, eNOS endothelial NO synthase, NO nitric oxide, ER estrogen receptor, Cav-1 caveolin-1, sGC soluble guanylyl cyclase, GTP guanosine-5′-triphosphate, cGMP cyclic guanosine monophosphate, NQO1 NAD(P)H:quinone oxidoreductase-1, HO-1 heme oxygenase-1, KEAP1 kelch-like-ECH-associated protein 1, Nrf2 nuclear factor erythroid-2 (NF-E2)-related factor 2
The blood flow is regulated by the secretion and absorption of vasoactive substances expressed by the endothelium acting in a paracrine manner to constrict or dilate specific vascular beds in response to stimuli. In fact, ECs can release vasodilators, such as nitric oxide (NO) as well as vasoconstrictor molecules, including endothelin-1 (ET-1) (Schiffrin 2001; Verma and Anderson 2002). Discontinuation of endothelial function is an early indicator of the development of vascular disease and an important area for further research and identification of new potential therapeutic targets. In fact, endothelial dysfunction, the transition from a healthy endothelium to a generalized damaged phenotype, characterized by pro-coagulative, pro-inflammatory, and pro-vasoconstrictive features (van den Oever et al. 2010; Flammer and Luscher 2010), is an early event in many diseases including atherosclerosis (AS), hypertension, diabetes, sepsis, chronic kidney disease, and hyperlipidemia (Virdis et al. 2011; Versari et al. 2009; Kerekes et al. 2008; Peters et al. 2003; Landray et al. 2004). In particular, AS, the most prevalent vascular disease in developed countries, is a multifactorial disease with several predisposing factors, such as smoking, diabetes, hyperlipidemia, hypertension, mechanical stress, and inflammation.
Despite their very low concentrations in plasma, AC incorporation into plasmatic membrane and cytosol of vascular ECs was demonstrated with the capacity to exert significant protective effects against oxidative damage at different cellular level (Youdim et al. 2000). Ziberna et al. (2012) assessed the uptake of physiological concentrations of C3G by human vascular ECs and investigated the involvement of the membrane transporter bilitranslocase, as a key player underlying molecular mechanism for membrane transport. Bilitranslocase is a plasma membrane organic anion carrier, expressed in human and rat aortic primary ECs, involved in the transport of different dietary flavonoids. C3G, similarly to the other AC, MvG, has been demonstrated to be transported via bilitranslocase inside ECs in human and rat aortic primary endothelial cells as well as Ea.hy 926 cells (Maestro et al. 2010). These results suggest that, despite their lower oral bioavailability, dietary AC may exert their biological activity at intracellular level in the endothelium. Interestingly Cy-3-O-rutinoside and Pg-3-O-glucoside may be taken up, in vitro, by two brain EC lines from mouse (b.END5) and rat (RBE4). This evidence suggests that these molecules can cross the blood–brain barrier and contributes to explain, at least in part, their neuroprotective effects (Youdim et al. 2003). Even though it seems quite evident that AC (and in general all polyphenols) do not exert any specific antioxidant activity inside the brain, more recent indications suggest that the possible brain effects of these molecules are also indirect and due to the activation of a hormetic dose–response and to effects on peripheral systems of the body, which in turn affect CNS functions (Schaffer and Halliwell 2012). This concept reinforces the importance of the effects of AC at the level of endothelium and, in general, independently of the tissue or organ considered, the evidence that even though the observed concentrations of plasma AC are not sufficient to exert a bona fide antioxidant activity, both in the extracellular and in intracellular spaces, they may be able to affect signal transduction and/or gene expression (Fig. 1).
Fig. 1.
Endothelial cells functions. Endothelium plays a critical role in maintaining vascular homeostasis. ECs function as a barrier regulating the flow of nutrient substances and different biologically active molecules. The endothelium is essential in controlling the passage of fluid into tissue, in modulating cellular trafficking and coagulation, and in regulating blood pressure. The endothelium also plays a key role in the regulation of immune responses, and the endothelial cell layer serves as the gateway for the entry of leukocytes into tissue in response to inflammatory stimuli. CAMs cell adhesion molecules, NO nitric oxide, PGI2 prostacyclin, TXA2 thromboxane A2, ET endothelin, ROS reactive oxygen species, tPA tissue plasminogen activator, PAI-1 plasminogen activator inhibitor 1, PAF platelet-activating factor, TF tissue factor
Anthocyanin consumption, endothelial functions and CVD risk
Numerous epidemiological reports suggest that the consumption of fruits containing high concentrations of AC, such as pomegranate, purple grapes, and berries, are associated with the reduction of specific CVD risk factors, particularly with respect to hypertension, platelet aggregation, and endothelial-dependent vasodilatation. Furthermore, the incorporation of AC-rich plant foods into the diet is more “biologically effective” than consuming a single AC extract/supplement, possibly due to the synergy with other bioactive compounds contained in them (Titta et al. 2010). Kay et al. (2012) have recently carried out a systematic review of randomised controlled trials based on the administration of flavonoid-rich food products to evaluate the relative impact of flavonoid composition, dose, and structure on vascular function. The effects of six flavonoid subgroups on flow-mediated dilation (an index of endothelial function; FMD) and blood pressure were assessed. Meta-analyses of combined flavonoid subclasses showed significant improvements in FMD and systolic/diastolic blood pressure. Similar benefits were observed for the flavan-3-ol, catechol flavonoids (catechins, quercetin, Cy, etc.), procyanidins, epicatechin, and catechin subgroups. Dose–response relationships were nonlinear for FMD, with greater associations observed when a polynomial regression analyses was applied. Similarly, Cassidy et al. (2013) examined the relationship between AC and other flavonoids and myocardial infarction (MI) in a group of 93,600 women (25–42 years old, healthy at baseline and followed for 18 years) from the Nurses’ Health Study II. Flavonoid intake, divided into subclasses, was calculated from validated food-frequency questionnaires collected every 4 years, using an updated and extended database provided by the US Department of Agriculture. An inverse association between higher intakes of AC and risk of MI was observed, and the addition of intermediate conditions, including history of hypertension, did not significantly attenuate the relationship. The combined intake of 2 AC-rich foods, blueberries and strawberries, had a better effect in decreasing the risk of MI in comparison with the consumption of >3 servings a week and with lower intakes. Interestingly, the intake of other flavonoid subclasses was not significantly associated with MI risk, apparently putting AC in a very special position among the numerous bioactive phenolic molecules.
Indeed, the majority of the studies available in the literature largely differ by design and outcomes. For instance, 12 weeks supplementation with AC isolated from berries (320 mg/day) improved endothelium-dependent vasodilation (as shown measuring artery FMD, circulating HDL cholesterol concentrations, soluble VCAM-1 and LDL cholesterol concentrations) in 150 hypercholesterolemic patients (Zhu et al. 2011). More recently, the same authors (Zhu et al. 2013) observed a significant decrease in the serum levels of high-sensitivity C-reactive protein and soluble VCAM-1 and of plasma IL-1β in 50 hypercholesterolemic subjects consuming a purified AC mixture (320 mg/day) for 24 weeks. Dohadwala et al. (2011) carried out a chronic crossover study, administering a double-strength cranberry juice (54 % juice, 835 mg total polyphenols, and 94 mg AC) or a matched placebo beverage for 4 weeks to 59 subjects with coronary heart disease. In these experimental conditions, the carotid femoral pulse wave velocity, a clinically relevant marker of arterial stiffness, was reduced by cranberry juice consumption. Similarly, Aviram et al. (2004) investigated the effects of consuming pomegranate juice (PJ, an important source of AC and bioactive tannins) for 1–3 years by ten patients with carotid artery stenosis (CAS) on the progression of carotid lesions and blood pressure. PJ consumption resulted in a significant reduction (up to 30 %) of common carotid intima-media thickness (IMT) and of systolic blood pressure (by about 12 %). After 1 year of PJ consumption, an increase in serum total antioxidant status was also observed, whereas serum LDL basal oxidative state and susceptibility to copper ion-induced oxidation and serum levels of antibodies against oxidized LDL were all significantly reduced.
On the contrary, a more recent study reports that 6 weeks of regular consumption of a wild blueberries drink containing flavonols, phenolic acids, and AC, at the daily dose of 25 g freeze-dried powder (providing 375 mg of AC), resulted in reduced levels of oxidized DNA bases with no effect on endothelial function markers in 18 male volunteers with risk factors for cardiovascular disease (Riso et al. 2013).
Overall, the evidence supporting conclusions about the efficacy of AC in modulating endothelial functions still appear limited and somehow inconsistent, partly due to the heterogeneity in the design of studies, the lack of controls, the relatively short intervention periods, and the low power in several studies (Chong et al. 2010; Hooper et al. 2008; van Dam et al. 2013). It would be advisable that an agreement will be reached among researchers actively involved in this topic, in order to set the parameters for shared “standardized” experimental designs, allowing a solid comparison between different studies.
Pharmacological effects of AC on endothelium: molecular basis
NO-related mechanisms
NO is a heterodiatomic free radical product generated through oxidation of l-arginine to l-citrulline playing a key role in vasodilation (Stamler et al. 1992). Its generation may be catalyzed by two different Ca2+/calmodulin-dependent NO synthases, the constitutively active endothelial NO synthase (eNOS) and neuronal NO synthase, and by the Ca-insensitive inducible NO synthase (iNOS) (Vallance and Leiper 2002).
A balanced release of NO is involved in various important physiological functions including relaxation of blood vessels and platelet aggregation inhibition (Lowenstein et al. 1994). While the constitutive enzyme eNOS synthesizes low amounts of NO regulating physiological homeostasis and cell signaling, the inducible form iNOS, induced by cytokines like interferon gamma, interleukin 1α, or TNF-α, produces large quantities of NO (up to μM concentration in the microenvironment of activated macrophages) (Domitrovic 2011). High concentrations of NO can then result in cytotoxicity, as it is a relatively reactive molecule potentially able to react with superoxide (O−·2) generating peroxynitrite (ONOO−) (Guzik et al. 2002), a highly reactive species which can directly react with various biological targets and components of the cell including lipids, thiols, amino acid residues, DNA bases, and low molecular weight antioxidants (O’Donnell et al. 1999).
Several studies are available indicating that the consumption of dietary AC is associated with an enhanced production of the vasodilator factor NO. This is corroborated by results obtained by supplementation studies demonstrating that the intake of foods rich in AC leads to a significant improvement of endothelium-dependent vasodilation. Zhu et al. (2011), in a long-term intervention trial on hypercholesterolemic individuals, found that a long-term supplementation of AC isolated from berries (320 mg/day for 12 weeks) improved FMD of the brachial artery (an index of endothelial function). Similarly, a rise in plasma concentrations of cGMP (an index of NO activity and, therefore, an indirect indicator of endothelium-dependent vasodilation) was observed. On the other hand, in a chronic randomized crossover human study, Dohadwala et al. (2011) did not show any chronic effect of cranberry juice on the primary endpoint of the brachial artery FMD, but observed a highly significant effect on central aortic stiffness, increasingly recognized as being a significant measure of vascular function relevant for cardiovascular disease. A recent study conducted by Thandapilly et al. (2012) demonstrated that the treatment with whole grape powder (rich in AC, as well as in resveratrol, catechins, flavonoids, and several other flavonoids) was associated with a spectrum of vascular and cardiac benefits in spontaneously hypertensive rats, with a remarkable decrease of blood pressure, increased arterial relaxation and vascular compliance, and attenuated cardiac hypertrophy. In Sprangue-Dawley rats, wild blueberries incorporated into the diet (8 % w/w) improved vascular tone and the artery responsiveness to factors increasing vessel contractility only if maintained for more than 4 weeks (Del Bo’ et al. 2012). Therefore, the length of dietary supplementation appears as a critical component for wild blueberries bioactive components to exert their beneficial effects. This particular effect was explained by the authors according to the documented age-related changes in postsynaptic α1-adrenoreceptor mechanisms in rat aorta and might involve the interaction between agonist membrane receptors and blueberry components.
Some in vitro studies provided indications suggesting that at high concentrations, AC may have a role in protecting against alterations of the cellular redox status mediated by NO, directly acting as peroxynitrite scavenger. Serraino et al. (2003), for example, demonstrated that blackberry juice and its main component C3G, are capable of preventing peroxynitrite-mediated endothelial dysfunction in human umbilical vein endothelial cells (HUVECs) and vascular insufficiency in rat thoracic aorta rings.
Similarly, malvidin-3-O-glucoside (M3G) has been reported to efficiently protect bovine arterial endothelial cells (BAECs) from peroxynitrite induced apoptotic death. It is important to note that oxidative stress was assessed by a non-specific test (dichlorodihydrofluorescein diacetate assay), known to be reactive toward a broad range of oxidizing species that are likely to be increased during intracellular oxidant stress, rather than specifically toward peroxynitrite. In the same study, carbonyl groups were significantly reduced in the presence of M3G treatment, indicating that also a protein oxidative damage associated with an altered cellular redox status was countered (Paixão et al. 2012b). Furthermore, M3G administration inhibited mitochondrial apoptotic signaling pathways caused by peroxynitrite, counteracting the depolarization of mitochondrial membrane, the activation of caspase-3 and caspase-9, and the increase of the expression of the pro-apoptotic protein Bax. In a different study utilizing a similar experimental model, the same authors found that M3G administration was associated with the upregulation of eNOS mRNA, leading to a significant enhancement of eNOS expression and NO synthesis. Pro-inflammatory mediators (iNOS and COX-2 expression, and IL-6 synthesis) were also significantly reduced thanks to the inhibition of the NF-kB pathway (Paixão et al. 2012a). As mentioned, an evident limit of these studies is in the very high concentrations considered (12.5–25 μM) that are far from any achievable level.
It is interesting to remark that the observed protective effects of AC are not necessarily due to their direct antioxidant (reductant) activity, as the regulation of enzymes involved in NO activity is clearly involved. Accordingly, Xu et al. (2004a, b) reported that C3G can act as an eNOS natural activator in BAEC. In fact C3G increased production of NO by phosphorylation of the protein kinase C (also known as Akt) and of extracellular signal-regulated kinase 1/2 (ERK1/2) and enhanced eNOS activity by promoting its phosphorylation at Ser1179 and dephosphorylation at Ser116 (Fig. 2). Phosphorylation of Akt (Ser473) and ERK1/2 paralleled that of eNOS (Ser1179), and then increased its activity, as evidenced by the regulated association between eNOS and soluble guanylyl cyclase (sGC), the increase in cGMP production, and the induced phosphorylation at Ser239 of the vasodilator-stimulated phosphoprotein (VASP) after treatment with C3G. In contrast to the studies mentioned above, these experiments were designed considering C3G concentrations (0.5 μM) close to the levels potentially achievable in vivo, corroborating the indication that the observed effects of C3G on NO activity were not an experimental artifact, therefore supporting the potential importance of this molecule in human health and disease.
Lazzè et al. (2006) showed that Dp, to a greater extent than Cy, decreases ET-1 production and induces eNOS in HUVECs. Interestingly, also protocatechuic acid, an AC metabolite, exhibits a mild inhibitory effect on NO production and TNF-α secretion in lipopolysaccharide (LPS)- treated macrophages (Hidalgo et al. 2012). Bell and Gochenaur (2006) also noted that AC-rich chokeberry and bilberry extracts produced a dose-dependent relaxation of coronary artery rings from mature female pigs, with chokeberry extracts exhibiting the highest power. Also in these cases, some concerns about the effective AC concentrations considered (up to 100 μM and 0.5–5 mM, respectively) can be raised, partially reducing the possibility of inferring significant similar effects in vivo.
Interestingly, C3G has been reported to have opposite effects on the different NOS isoforms. Even though also in this case high concentration was considered (about 20–200 μM), Pergola et al. (2006) could demonstrate that C3G exerts an inhibitory activity on iNOS. In their study, an AC-rich fraction from blackberry extract and purified C3G reduced iNOS protein levels at the transcriptional level in LPS-treated J774 macrophages through the inhibition NF-κB activation (Fig. 2). This differential effect might have a high concern considering the different roles exerted by the two isoforms in different pathophysiological contexts. In fact, while NO generation by eNOS has an important role in maintaining cardiovascular homeostasis, on the contrary, a chronic induction of NO production by iNOS, occurring for instance in heart failure and ischemia–reperfusion, can have detrimental effects on the circulatory function (Calderone 2003). Similarly to C3G, other polyphenolic compounds have been reported to exert opposite effects on NO production in different experimental models (Chan et al. 2000). Once corroborated by more solid studies on experimental models and confirmed in vivo, the differential effect on NOS isoforms may be pivotal for the generation of preventive/therapeutic strategies based on the establishment of a “good” balance between iNOS and eNOS in various pathophysiological systems.
Other mechanisms might be involved in the vascular effects of AC. Chalopin et al. (2010) reported that Dp, in particular, activates molecular pathways (such as Src, ERK1/2, and eNOS) in Ea.hy926 ECs, through direct interaction with estrogen receptor-α (ERα), which leads to NO endothelial production and consequent vasorelaxation (see Fig. 2 for a simplified description of pathways involved). In addition, they carried out a docking study of Dp on ER-α. The expected binding mode of the ligand binding domain on ER-α was similar to that observed in the X-ray structure of the ER-α with 17β-estradiol (E2). Also in this case, a very high concentration of Dp (33 μM) was utilized, partially compromising the possibility to robust conclusions about the presence of this effect in vivo.
In ECs, the activation of eNOS in response to circulating hormones, local autacoids, and substances released from platelets, coagulation cascade, and autonomic nervous system is mainly dependent on an increase in cytosolic-free calcium concentration ([Ca2+]i) (Domitrovic 2011; Mombouli and Vanhoutte 1999). Dp, at a concentration of 10 µg/mL (about 30 μM), induced a significant increase in [Ca2+]i that led to the formation of NO in BAECs (Martin et al. 2002). However, it is important to note that the amplitude of Dp-induced calcium signal, less than 200 nM, is relatively low with respect to the one induced by physiological agonists such as bradykinin (Schini-Kerth et al. 2010). Therefore, even if an increase in [Ca2+]i is an important route that leads to eNOS activation in ECs, it is likely that other additional mechanisms contribute to the stimulatory effect of AC on eNOS activity (Fig. 2).
Additionally, Martin et al. (2003) showed that Dp inhibits apoptotic response in ECs exposed to actinomycin D, a DNA transcription suppressor, by increasing eNOS expression via the MEK1/2 inhibitor-sensitive pathway. The effect of Dp also involves NO and guanylyl cyclase-dependent pathway and is associated with the ability in maintaining endothelial [Ca2+]i level within a physiological range, and with the reduction of mitochondrial release of cytochrome c. It is likely that NO, via cGMP (resulting from guanylyl cyclase hydrolysis of guanosine triphosphate), plays a significant part in this process. The authors have therefore hypothesized that the arrest of mitochondrial release of cytochrome c is the mechanism whereby NO can mediate the antiapoptotic effect of Dp. Another mechanism by which the NO-cGMP pathway inhibits apoptosis in ECs is the negative feedback on [Ca2+]i homeostasis (Perrier et al. 2009), since increase of [Ca2+]i is one of the fundamental signals that lead to cellular apoptosis (Martin et al. 2003).
NF-κB and other signal transduction pathways
A chronic pro-inflammatory condition is considered a typical feature in vascular endothelial dysfunction triggered by the activation of transcription factors such as NF-KB, functionally dependent on the cellular redox state. Thus, several pro-inflammatory agents, such as oxidized low density lipoprotein (ox-LDL), free radicals/ROS, and TNF-α, are able to act as triggering agents in AS (Libby 2007).
A robust amount of positive evidence supporting the protective effect of AC against vascular endothelial dysfunction has been achieved in vivo using experimental animal models, and in particular in apolipoprotein E-deficient (apoE−/−) mice. The lack of a functional gene ApoE makes these mice incapable of producing a key glycoprotein, apoE, essential for lipids transport and metabolism. (apoE−/−) mice are healthy when born, but with a markedly altered plasma lipid profile in comparison with wild-type mice, and quickly develop severe “human-like” atherosclerotic lesions, regardless of the diet (Kolovou et al. 2008).
Wang et al. (2012a) reported that in 8-week-old male apoE (−/−) mice fed with a high-fat, cholesterol-rich diet, the supplementation with C3G (2 g/kg diet) for 8 weeks prevented or reversed hypercholesterolemia-induced endothelial dysfunction by inhibiting accumulation of cholesterol and 7-oxysterol in the aorta, with a subsequent reduction in superoxide production, thus preserving eNOS activity and NO bioavailability.
According to the evidence that accelerated AS in diabetes mellitus is primarily due to limited availability and function of endothelial progenitor cells (EPC), Zhang et al. (2013) investigated the protective effects of a very high dietary supplementation of C3G (0.2 % wt:wt for 6 weeks) on EPC function and endothelial repair in streptozotocin-induced diabetic apoE (−/−) mice, underscoring the potential role of C3G in prevention and treatment of diabetic vascular complications. In fact, the endothelium-dependent relaxation response to acetylcholine in aortas of C3G-fed mice was 51 % higher than that of controls and similar to that observed in non-diabetic apoE (−/−) mice. The ability of in vitro adhesion to fibronectin, migration, and tube formation was significantly affected in diabetic EPCs and was significantly saved in response to C3G. At the molecular level, a higher phosphorylation of AMPK Thr172 and eNOS Ser1177 was observed in EPCs obtained from C3G-treated diabetic mice in comparison with non-diabetic mice.
Furthermore, 2 weeks of supplementation with an AC-rich extracts of blueberry (0.02 % wt/wt in diet) mitigated the development of atherosclerotic lesions in apo E (−/−) mice, and this appeared to be mediated by the overexpression of genes involved in bile acid synthesis and cholesterol absorption in the liver and by a down-regulation of pro-inflammatory gene expression (Mauray et al. 2010). The in vivo mechanisms of action of bilberry extract have been studied using a transcriptomic approach, allowing the detection of a modulated expression of 1,261 genes in the aorta (Mauray et al. 2012). These sets of genes are involved in various cellular processes, such as oxidative stress, inflammation, transendothelial migration, and angiogenesis. All these processes are associated with AS development/protection. Some of the most significantly down-regulated genes included genes coding for AOX1, CYP2E1, or TXNIP involved in response to oxidative stress, JAM-A coding for adhesion molecules, or VEGFR2 involved in angiogenesis regulation. Others were upregulated genes, such as CRB3, CLDN14, or CDH4, potentially associated with an increase of cell–cell adhesion and a decrease in paracellular permeability.
However, it is unclear whether the protective effect shown in these studies is due to the parent AC contained in the extracts studied or to metabolites having a specific biochemical activity. For example, the administration of protocatechuic acid (one of the major AC metabolites) in apoE-deficient mice reduces aortic VCAM-1 and ICAM-1 expression, NF-κB activity, and the levels of plasma-soluble VCAM-1 and ICAM-1, thus delaying the development of AS (Fig. 2) (Wang et al. 2010).
Ox-LDL has a pivotal role in atherogenesis. It is widely used in vitro to implement experimental models for the pathogenesis of AS, frequently carried out by means of cultured ECs from different sources (human, bovine), also aimed to the investigation of the protective effect of AC against endothelial dysfunction. In fact, ox-LDL induces inflammation and apoptosis in ECs, inhibits their proliferation, and stimulates the expression of adhesion molecules. Its atherogenic mechanism within ECs and monocytes is hypothesized to include the induction of the transcription of genes relevant for atherogenesis, such as NF-κB, adhesion molecules, and NOS. Moreover, ox-LDL activates lectin-like oxidized low density lipoprotein receptor (LOX)-1, a specific receptor that helps ox-LDL uptake in ECs and therefore improves monocytes adhesion (Pirillo et al. 2013).
A number of in vitro studies in cultured ECs demonstrate that AC can effectively protect vessel ECs against damage effects induced by ox-LDL. In ECs exposed to ox-LDL, the pre-treatment with 0.1 µg/mL of a phenolic fraction from Lonicera caerulea L. (blue honeysuckle) having an AC content of 18.5 % had significant protective effects against cell damage detected by means of some non-specific cell injury markers such as lactate dehydrogenase leakage and thiobarbituric acid reactive substances formation (Palíková et al. 2009). Even though it provides some indication about the ability of AC metabolites to counter the detrimental effect of ox-LDL, this study appears outdated due to the limitation of the methodology chosen by the authors to identify cell damage and dysfunction.
Few purified molecules have also been tested within this context. Chen et al. (2010, 2011) reported that the pre-incubation with 100 µM Dp had significant protective effects against injury induced by ox-LDL in HUVECs. In this report, even though “biased” by an experimental design providing a concentration not achievable in vivo, Dp was able to prevent ox-LDL-induced cell viability loss and apoptosis, to reduce intracellular ROS overproduction, to restore the activity of endogenous antioxidants, and to increase NO levels. These effects are overall considered “protective” within the pathogenesis of AS and were mediated by the upregulation of the expression of Bax and by the down-regulation of Bcl-2 protein, pivotal players in apoptosis-related signaling pathways, that can either promote cell survival or cell death (Ola et al. 2011).
Similar effects have been demonstrated in vitro for delphinidin-3-O-glucoside (D3G) from dark-skin berries, in porcine aortic endothelial cells (PAECs) exposed to ox-LDL (Xie et al. 2012). In fact, co-treatment with 100 μM D3G countered the detrimental effects of ox-LDL in cultured vascular ECs, including the increase in intracellular superoxide (measured by lucigenin assay), NADPH oxidase (NOX2 and NOX4, some of the main sources of ROS in vascular ECs through the catabolism of NADPH), and caspase-3 protein levels. Even though the very high D3G concentration utilized is objectionable, this study seems to demonstrate that cell viability, the activities of mitochondrial enzymes and of Bcl-2 were positively affected, suggesting a potential role of D3G in AS prevention and treatment.
Oxidation products of cholesterol are present in human atherosclerotic plaques and show significant atherogenic properties. 7-Oxysterols are the major cytotoxic components found in ox-LDL, 7-ketocholesterol (7-KC), and 7α-hydroxycholesterol having the most detrimental effects on vessel endothelium (Poli et al. 2013). In particular, 7-KC is believed to cause foam cell transformation in macrophages and toxicity to vascular endothelial and smooth muscle cells. 7-KC is generated in lipoprotein deposits by a free radical involving mechanism, also known as Fenton reaction, requiring transition metal catalysis, usually by iron or copper. One of the major consequences of 7-KC generation and accumulation is a pro-inflammatory response, mainly acting through three kinase signaling pathways, AKT-PKCζ-NFκB, p38MAPK, and ERK. 7-KC inflammatory pathways have been described in different cell types, responding to 7-KC with the subsequent activation of NF-κB.
A number of studies addressing the protective effect of AC against oxysterol-induced damage in ECs are available. Pretreatment with “Aronox” (1–50 µg/mL), an AC-rich extract from Aronia melanocarpa E., has been reported to protect HUVECs against damage induced by 7β-hydroxycholesterol resulting in cytochrome c release, caspase-3 activation, and down-regulation of anti-apoptotic Bcl-2 (Zapolska-Downar et al. 2008). Similarly, a very high, non-physiologically achievable dose of Dp (about 33 μM) has been reported to inhibit apoptosis elicited by 7β-hydroxycholesterol in BAECs. The antiapoptotic effect of Dp was associated with an increase of endothelial NOS expression mediated by a MAP kinase (Martin et al. 2003).
Wang et al. (2012b) suggested a further mechanism by which AC protect from oxidative damage induced by oxysterol on ECs. They showed that the administration of C3G (ranging from 0.5 to 50 µM) to human aortic ECs (HAECs) abrogates the increase of ROS and inhibits apoptosis induced by 7-KC. These effects are accompanied by the preservation of NO bioavailability thanks to the maintenance of high eNOS activity. The authors attributed this effect to the ability of C3G to upregulate the expression of ATP-binding cassette subfamily G member 1 (ABCG1) and subfamily A member 1 (ABCA1), and thus to promote oxysterols efflux.
The adhesion of monocytes to the vascular endothelium and their subsequent trans-endothelial migration are recognized as crucial early events in atherogenesis. Cell adhesion molecules (CAMs) mediate different phases of migration of leukocytes from the bloodstream to the inflammatory foci and play a pivotal role in pathologic inflammation such as in AS. Even though the expression of both VCAM-1 and ICAM-1 is upregulated in atherosclerotic lesions, VCAM-1 has been reported to play an important role in the initiation of AS opening the avenue to the development of new therapeutic agents having specific suppressive effects on CAMs. With regard to differential mechanisms regulating the expression of VCAM-1 and ICAM-1, it has been reported that functional transcription factor binding motifs for NF-κB, interferon regulatory transcription factor-1 (IRF-1), activator protein-1 (AP-1), and the transcription factor genes binding to the DNA sequence GATA (GATAs) exist in VCAM-1 gene promoter region (Iademarco et al. 1992; Neish et al. 1995; Lechleitner et al. 1998; Papi and Johnston 1999). ICAM-1 promoter has also NF-κB, AP-1, and specificity protein-1 (SP-1)- binding sites (Stade et al. 1990).
There is robust evidence indicating that AC may inhibit expression of CAMs and monocytes adhesion to ECs challenged by pro-inflammatory agents. TNF-α is widely used as a pro-inflammatory agent in ECs as it is capable of inducing endothelial dysfunction, promoting formation of intracellular ROS and NF-κB activation. Kim et al. (2006) demonstrated that high concentrations of AC isolated from black soybean seed coat (in a range between 10 and 100 μg/mL) inhibit TNF-α-induced increase of VCAM-1, ICAM-1, and cyclooxygenase-2 levels in BAECs, by affecting NF-κB-dependent gene expression. In the same study, a single dose of AC (25–100 mg AC from black soybean coat per kg b.w.) protected the hearts of rats subjected to 30 min occlusion of myocardial left descending coronary artery, followed by 24 h reperfusion. Overall, these results were confirmed by Nizamutdinova et al. (2009), who reported that treatment with 50–100 μg/mL AC from black soybean seed coat inhibited ROS accumulation (measured with dichlorofluorescein diacetate dye assay) and VEGF production in ECs challenged with TNF-α, reducing, in a dose-dependent manner, the adhesion of inflammatory monocytes to ECs. Under these experimental conditions, AC also blocked NF-κB translocation into the nucleus. In a different study conducted on HAECs exposed to TNF-α, 100 μg/mL of a Cy-rich purple sweet potato leaf extract (PSPLE) significantly inhibited monocyte adhesion to ECs and attenuated the expression of VCAM-1, IL-8, and CD40. The authors hypothesized that this effect was due to the modulation of NF-κB and MAPK signaling (Chao et al. 2013).
We have demonstrated that C3G at 20–40 μM counters CAMs overexpression and adhesion of leukocytes to the endothelium induced by TNF-α in HUVECs. In the same study, C3G also decreased the activation of the transcription factor NF-κB as well as the intracellular levels of H2O2 and lipid peroxidation by-products, triggered by this pro-inflammatory cytokine (Speciale et al. 2010) (see also Fig. 2). Similarly, Chen et al. (2011) studied the effect of Dp on ox-LDL-induced adhesion of monocytes to cultured ECs. The authors showed that pretreatment with 50–200 μM Dp decreased in a dose-dependent manner ox-LDL-induced upregulation of ICAM-1 and P-selectin expression resulting in an inhibition of monocytes adhesion and transmigration. Moreover, Dp treatment mitigated ox-LDL-induced ROS production and p38MAPK protein expression. The transcriptional activity of NF-κB was also inhibited subsequently to a decrease of IκB-α degradation, leading to a reduced expression of mRNA and protein for NADPH oxidase subunit.
Most recently, Zhu et al. (2013) reported that D3G and C3G act synergistically in inhibiting LPS-induced VCAM-1 (see Fig. 2) secretion in PAECs. The authors suggest that dietary supplementation with plant-based foods rich in different AC compounds is likely to be more beneficial than consuming a single AC supplement. Also vitisin A (a class of pigments formed through chemical interaction of the original AC with pyruvic acid during wine aging) have been reported to have an inhibiting effect on TNF-α-induced monocyte chemoattractant protein expression in primary human ECs, but to a much lower extent in comparison with the original AC (García-Alonso et al. 2004).
AC have been also reported to be involved in some of the molecular events underlying the development of diabetic nephropathy (DN), a major complication of diabetes and the leading cause of end-stage renal disease. In early DN, both renal damage and accumulation of macrophages take place in the pathological environment of glomerular vessels adjacent to the kidney mesangial cells expressing pro-inflammatory mediators. Kang et al. (2012) performed an interesting study to show that AC-rich purple corn extract (PCA) can be a potential protective agent for treatment of diabetes-associated kidney glomerulosclerosis, both in vitro and in vivo. When human ECs and THP-1 monocytes were grown in media of human renal mesangial cells (HRMCs) exposed to 33 mM glucose, the administration of PCA was associated with a decreased expression of endothelial VCAM-1, E-selectin, and monocyte integrins-β1 and -β2 due to the blockade of mesangial Tyk2 pathway. In the in vivo study conducted on leptin receptor deficient db/db mice (a rodent model for obesity and type 2 diabetes), the administration of 10 mg/kg/day PCA for 8 weeks mitigated CXCR2 induction and activation of Tyk2 and STAT1/3. In the kidneys of PCA supplemented mice, a reduced infiltration and accumulation of macrophages was observed, associated with a significant modulation of mesangial IL-8-Tyk-STAT signaling pathway.
The interaction with other cell signaling pathways may also be involved in the ability of AC to protect ECs against the deleterious effect of TNF-α. In a study conducted on BAECs, Xu et al. (2007) have shown that supplementation with 50 μM Cy significantly reduced the number of apoptotic cells, the levels of cleaved caspase-3 and poly(ADP-ribose)polymerase (PARP), all events triggered by the exposure to TNF-α. Inhibitors of Akt, ERK-1/2, and Src kinases and transfection with a dominant negative Akt cDNA blocked the inhibitory effect of Cy on cleaved caspase-3. The treatment with Cy was also associated with a significant increase of eNOS and thioredoxin (Trx) expression. This effect was inhibited by siRNA transfection of cGMP-dependent protein kinase (PKG) and by PKG inhibitor KT5823 demonstrating the involvement of this kinase in Cy-induced effects. Finally, the treatment with Cy countered TNF-α-induced decrease of Trx S-nitrosylation, restored caspase-3 S-nitrosylation, and reduced the increase in expression and acetylation of p53 tumor suppressor gene. Overall, these results indicate that Cy inhibits apoptosis induced by TNF-α, acting through multiple pathways.
According to the concept of AS as a chronic inflammatory disease, many studies suggested that the immune mediator CD40 and its counterpart CD40 ligand (CD40L), members of the TNF and TNF-receptor (TNFR) family, are important factors in the pathogenesis of AS (Tousoulis et al. 2010). Even though the role of the CD40-CD40L pair has been considered to be limited to T and B lymphocyte interactions, it is has been more recently found also expressed by a variety of non-immune cells, such as vascular ECs, where it exerts a broad range of functions. The binding of CD40 to CD40L induces the production of a number of inflammatory cytokines and chemokines, such as interleukins, monocyte chemoattractant protein-1 (MCP-1), and adhesion molecules, able to activate atherogenesis (Pamukcu et al. 2011). Along with these, CD40–CD40L interactions are hypothesized to play a major role in plaque rupture through the upregulation of MMPs expression (DeGraba 2004).
It is known that TNF-receptor-associated factor 2 (TRAF-2) plays a critical role in CD40-NF-κB pathway, and its overexpression increases CD40-mediated NF-κB activation, and that TRAF-2 is almost completely recruited to lipid rafts after stimulation by CD40 ligand (Tewari and Dixit 1996). Xia et al. (2007) found that 1–100 μM C3G and pelargonidin-3-O-glucoside (Pn3G) prevents CD40-induced pro-inflammatory state, as measured by the production of IL-6, IL-8, and monocyte chemoattractant protein-1, by inhibition of CD40-induced NF-κB activation in HUVECs. The exposure of cells to AC interrupted not only TRAF-2 recruitment to lipid rafts, but also induced a reduction of lipid rafts cholesterol content, without affecting the interaction between CD40 ligand and CD40 receptor. Thus, it can be speculated that AC counter CD40-induced pro-inflammatory signaling by preventing TRAF-2 translocation to lipid rafts via the regulation of cholesterol distribution.
Finally, some authors reported the presence of indirect mechanisms involved in intracellular modulation of redox status by AC, which could be linked to acute activation of antioxidant and detoxifying enzymes, such as heme oxygenase-1 (HO-1) (Lazzè et al. 2006; Sorrenti et al. 2007) and, as reported earlier, eNOS (Xu et al. 2004b). HO-1 overexpression, in turn, increases the production of bilirubin, an endogenous antioxidant, which, at physiological concentrations, has been proposed to protect ECs against hydrogen peroxide-mediated injury and to reduce cardiovascular events in humans (Minetti et al. 1998; Huang et al. 2012). C3G, both at high (μM range) and low (nM range) concentrations, has been observed to be able to significantly induce HO-1 protein expression in ECs (Sorrenti et al. 2007).
According to these observations, studies from our laboratory demonstrated that AC are able to induce a cellular adaptive response (Cimino et al. 2013). Genetic analysis has revealed that the coordinated induction of cytoprotective genes is regulated through a cis-regulatory DNA sequence in the promoter or enhancer region named electrophiles responsive element (EpRE), also frequently referred as antioxidant responsive element (ARE), located within the regulatory region of a number of target genes, including glutathione S-transferases (GST1-4, GSTMI-6, and MGST2-3), NAD(P)H: quinone oxidoreductase 1 (NQO1), HO-1, glutamate cysteine ligase, and γ-glutamylcysteine synthetase heavy and light subunits. The binding to EpRE sequences mediate transcriptional activation of genes in cells exposed to different kinds of stress and therefore plays a pivotal role in cellular defense (Chen and Kong 2004).
Numerous studies provided evidence that the activation of a redox-sensitive gene-regulatory network mediated by the NF-E2-related factor-2 (Nrf2) is intimately involved in inducing EpRE-driven response to oxidative stress and xenobiotics (Fig. 2). Nrf2 is a member of bZIP transcription factors. In basal conditions, in the absence of any kind of specific cellular stress, Nrf2 is sequestered in the cytoplasm after binding to an actin-bound protein, Keap1, that promotes Nrf2 degradation by the ubiquitin proteasome pathway (Fig. 2) (Forman et al. 2014a; Wakabayashi et al. 2004). In the presence of electrophiles, an alkylated form of Keap1 is formed by Michael addition, which evades from Nrf2 allowing its accumulation within the nucleus, and the transactivation of EpRE-regulated target genes. Since adaptive and pharmacologically induced expression of Nrf2/EpRE-regulated cytoprotective proteins may contribute to the atheroprotective and anti-inflammatory phenotype in ECs, dietary phytochemicals, able to act as nucleophilic modulators of signal transduction pathways, might represent a potential therapeutic strategy to protect vascular system against various stressors and to prevent several pathological conditions (Speciale et al. 2011a, b). According to this mechanism, in HUVECs challenged with TNF-α, C3G is able to counteract TNF-α induced alterations, including activation of NF-κB, increased gene expression of adhesion molecules, leukocyte adhesion to endothelium, and intracellular accumulation of H2O2 and lipid peroxidation byproducts (Speciale et al. 2010). Furthermore, pretreatment of TNF-α exposed HUVECs with C3G activates Nrf2/EpRE pathway and consequently improves cellular antioxidant systems (Speciale et al. 2013) through the involvement of specific MAPKs (ERK1/2). Under these conditions, the inactivation of ERK1/2 activity by the inhibitor PD98059 abolishes the increase of nuclear accumulation of Nrf2 induced by C3G. Interestingly, the mechanism involved in the protective effect of C3G could be associated mainly with a capability to elicit cell adaptive responses, since C3G was able to induce Nrf2 nuclear accumulation not only following TNF-α exposure but also without any kind of stimulus. Also this effect seems to be mediated by the activation of specific kinases ERK1/2 and is abolished by the specific MAP kinase inhibitor PD98059. As consequence of Nrf2 activation, HO-1 and NQO-1 expression are upregulated by C3G treatment. It is interesting to note that this effect is present in cells both exposed and not exposed to TNF-α, and it is abolished by treatment with PD98059. Finally, in C3G treated cells challenged with TNF-α, the inhibition of ERK1/2 kinases by PD98059 not only abolishes the increase of Nrf2 nuclear accumulation and the overexpression of HO-1 and NQO-1, but also increases NF-κB p65 nuclear translocation, confirming the cross talk between NF-κB and Nrf2 (Bellezza et al. 2010).
Similarly, AC metabolites are able to protect HUVECs against damage induced by moderate hyperoxia (O2 32 %). In fact, the cytotoxic effect of mild hyperoxia came along with a significant decrease in Nrf2 nuclear accumulation, as well as in the expression of Nrf2-regulated antioxidant and cytoprotective genes. In this experimental model, AC metabolites have been reported to be able to activate the Nrf2 pathway not only under hyperoxic but also in normoxic conditions, suggesting the presence of an adaptive, protective effect of AC in mild hyperoxia (Cimino et al. 2013). The same protective mechanism has been demonstrated for C3G in ECs exposed to hypoxic condition by modulating intracellular oxidative stress induced by low-oxygen tension (Anwar et al. 2014).
However, the effect of AC appears to be different from that of their glycosides. In HUVECs, aglycon anthocyanidin forms, such as Cy and Dp, have been observed to display a major action compared to Cy in inducing a significant dose-dependent inhibitory effect on both protein and mRNA levels of ET-1 (Lazzè et al. 2006). Cy and Dp both increased the protein level of eNOS, but Dp showed the major effect raising eNOS protein in a dose-dependent manner. In the same study HO-1 protein induction by Cy was apparent only at very high concentrations (100 µM).
The presence of an ortho-dihydroxyphenyl structure on the B ring seems to be required for most of the AC pharmacological activities, including their inhibitory effects on endothelial dysfunction. Yi et al. (2012) have recently investigated the relationship between AC chemical structure and their endothelial protective properties. In the EA.hy926 cell line (frequently considered a good model for HUVECs), the exposure to ox-LDL-induced decrease of cell viability, generation of O−·2 and other ROS, p38MAPK activation, NF-κB nuclear translocation, and transcriptional activity, and the expression of mRNA of genes, such as ICAM-1, VCAM-1, E-selectin, MMP-1, MMP-2, and MMP-9, were inhibited by pretreatment with Dp and Cy. The number of hydroxyl groups in total, a 3′,4′-ortho-dihydroxyl group on the B ring, and 3-hydroxyl group on the C ring of flavonoids, were important structure characteristics for the in vitro inhibitory effects. Thus, Dp exerts more significant endothelium-protective effects compared to Cy. These results are consistent with those previously reported by the same authors who compared the inhibitory effect of 21 AC against ox-LDL-induced endothelial damage and their endothelial protective properties, as measured by cell viability, radical scavenging activity, production of malondialdehyde as breakdown product of lipid peroxidation, and NO release (Yi et al. 2010). However, in vivo, the activity observed after the consumption of these compounds could be also due to their metabolites since C3G and D3G have been reported to be rapidly methylated by catechol-O-methyl transferase (COMT) into Pn3G and Pt3G (Vanzo et al. 2011).
Conclusions
Prevention and management of vascular diseases are one of the major public health challenges worldwide. These pathologies are associated with high risk of cardiovascular complications such as uncontrolled high blood pressure, coronary heart disease (which leads to heart attack) and stroke, congestive heart failure, heart rhythm irregularities, kidney failure resulting in shortened life expectancy and higher morbidity.
A proper nutrition, assuring an optimal intake of bioactive food constituents, may be at the base of new therapeutic approaches for cardiovascular disease prevention and treatment and contribute to a “healthy cardiovascular” population (Huang et al. 2013). The dietary consumption of AC has been frequently associated with health-promoting benefits, and therefore, components of this class of phytopigments have been proposed to be active part of “functional foods” such as red, blue, and purple berries are the most important ingredient in the formulation of “nutraceuticals.”
Early reports suggested that the biological activities of AC were solely related to their antioxidant power, but animal and human bioavailability studies indicated that the concentrations in tissues and biofluids are well below those required for direct antioxidant action. Many studies are now available indicating the existence of other multiple and complex activities that cannot be explained on the basis of the antioxidant characteristic and point out that the “playground” of AC is in the modulation of critical signaling pathways and genes regulation.
Interestingly, the consumption of AC has not been associated with adverse health effects. The risk of toxicity and undesirable side effects from food supply, in spite of a high dietary consumption of phenolic compounds in certain countries, is relatively low, largely due to their overall low absorption (Martin 2010; He and Giusti 2010).
This paper summarized the cardiovascular health-promoting effects of AC and highlighted the current knowledge about the molecular mechanisms involved in such effects, focusing in particular on the ability to modulate gene expression and cell signaling pathways at the level of vessel endothelium.
Overall, AC bioavailability in plasma has been reported to be less than 2 % of the ingested amount, suggesting that they either pass through the gastrointestinal tract or are metabolized in the gut or by the liver. However, a lot of papers provide evidence that dietary AC, despite their limited oral bioavailability and very low post-absorption plasma concentrations, may provide protection against vessel endothelium damage in several pathological conditions. Probably low plasma concentrations of AC could be sufficient to justify their biological activity when their targets are the vessel endothelium cells, which are proven able to incorporate AC into the membrane and cytosol, and then to exert significant protective effects against oxidative and inflammatory stressors. However, it is evident that this field of knowledge requires further development. In fact there is insufficient evidence to draw conclusions about AC efficacy, since available data are frequently inconsistent. This inconsistency is in large part due to the heterogeneity of the experimental design, to a frequent lack of controls, and overall to a low power of several studies. Furthermore, a confusing factor is represented by the fact that dietary sources of AC, such as berries, are rich in a wide range of phenolic compounds, and AC can provide health-promoting effects with synergistic actions in combination with other compounds, due to interaction between substances. On the other hand, AC are present in food matrix in different structures and this heterogeneity leads to different pharmacological outcomes. We have remarked several time throughout this review that many in vitro studies, addressing the understanding of AC mechanisms of action, have considered concentrations between 10 and 100 μM which are too high to be achieved in vivo within the target site in physiological conditions. Quite obviously, the direct transfer of these data to “real life” condition is very difficult to be implemented. Finally, the literature suggests that the metabolites may actually be responsible for much of the beneficial properties on ECs (Cimino et al. 2013). Further studies are needed in order to establish the real implications of AC metabolites and the specific mechanisms through which they can contribute to the observed AC health-promoting properties. We hope that our review can contribute to a background to reach an agreement to build consensus statements describing the set of prerequisites needed to uniform future protocols and studies, in terms of concentration utilized, ways of administration, and methodology adopted to assess the outcomes.
Conflict of interest
Antonio Speciale, Francesco Cimino, Antonella Saija, Raffaella Canali, and Fabio Virgili declare that they have no conflict of interest. This article does not contain any studies with human or animal subjects performed by the any of the authors.
Abbreviations
- AC
Anthocyanins
- AS
Atherosclerosis
- C3G
Cyanidin-3-O-glucoside
- CVD
Cardiovascular disease
- Cy
Cyanidin
- D3G
Delphinidin-3-O-glucoside
- Dp
Delphinidin
- ERK
Extracellular signal-regulated kinases
- FMF
Flow-mediated dilation
- HO-1
Heme oxygenase-1
- ICAM-1
Intercellular adhesion molecule-1
- M3G
Malvidin-3-O-glucoside
- MAPKs
Mitogen-activated protein kinases
- MI
Myocardial infarction
- Mv
Malvidin
- NF-κB
Nuclear factor-kappaB
- NQO-1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
NF-E2-related factor-2
- Pg
Pelargonidin
- Pn
Peonidin
- Pt
Petunidin
- ROS
Reactive oxygen species
- TNF-α
Tumor necrosis factor-α
- VCAM-1
Vascular cell adhesion molecule-1
References
- Andersen ØM, Jordheim M. The anthocyanins. In: Andersen ØM, Markham KR, editors. Flavonoids—chemistry, biochemistry and application. Boca Raton: Taylor & Francis; 2006. pp. 471–551. [Google Scholar]
- Anwar S, Speciale A, Fratantonio D, Cristani M, Saija A, Virgili F, Cimino F. Cyanidin-3-O-glucoside modulates intracellular redox status and prevents HIF-1 stabilization in endothelial cells in vitro exposed to chronic hypoxia. Toxicol Lett. 2014;226(2):206–213. doi: 10.1016/j.toxlet.2014.01.048. [DOI] [PubMed] [Google Scholar]
- Aviram M, Rosenblat M, Gaitini D, Nitecki S, Hoffman A, Dornfeld L, Volkova N, Presser D, Attias J, Liker H, Hayek T. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin Nutr. 2004;23(3):423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Bell DR, Gochenaur K. Direct vasoactive and vasoprotective properties of anthocyanin-rich extracts. J Appl Physiol. 2006;100(4):1164–1170. doi: 10.1152/japplphysiol.00626.2005. [DOI] [PubMed] [Google Scholar]
- Bellezza I, Mierla AL, Minelli A. Nrf2 and NF-κB and their concerted modulation in cancer pathogenesis and progression. Cancers (Basel) 2010;2(2):483–497. doi: 10.3390/cancers2020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone A. The therapeutic effect of natriuretic peptides in heart failure; differential regulation of endothelial and inducible nitric oxide synthases. Heart Fail Rev. 2003;8(1):55–70. doi: 10.1023/a:1022147005110. [DOI] [PubMed] [Google Scholar]
- Carkeet C, Clevidence BA, Novotny JA. Anthocyanin excretion by humans increases linearly with increasing strawberry dose. J Nutr. 2008;138:897–902. doi: 10.1093/jn/138.5.897. [DOI] [PubMed] [Google Scholar]
- Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127(2):188–196. doi: 10.1161/CIRCULATIONAHA.112.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalopin M, Tesse A, Martínez MC, Rognan D, Arnal JF, Andriantsitohaina R. Estrogen receptor alpha as a key target of red wine polyphenols action on the endothelium. PLoS One. 2010;5(1):e8554. doi: 10.1371/journal.pone.0008554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MM, Mattiacci JA, Hwang HS, Shah A, Fong D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem Pharmacol. 2000;60(10):1539–1548. doi: 10.1016/s0006-2952(00)00471-8. [DOI] [PubMed] [Google Scholar]
- Chao PY, Huang YP, Hsieh WB. Inhibitive effect of purple sweet potato leaf extract and its components on cell adhesion and inflammatory response in human aortic endothelial cells. Cell Adhes Migr. 2013;7(2):237–245. doi: 10.4161/cam.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Kong AN. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Biol Med. 2004;36(12):1505–1516. doi: 10.1016/j.freeradbiomed.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Chen CY, Yi L, Jin X, Mi MT, Zhang T, Ling WH, Yu B. Delphinidin attenuates stress injury induced by oxidized low-density lipoprotein in human umbilical vein endothelial cells. Chem Biol Interact. 2010;183(1):105–112. doi: 10.1016/j.cbi.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Chen CY, Yi L, Jin X, Zhang T, Fu YJ, Zhu JD, Mi MT, Zhang QY, Ling WH, Yu B. Inhibitory effect of delphinidin on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via ROS/p38MAPK/NF-κB pathway. Cell Biochem Biophys. 2011;61(2):337–348. doi: 10.1007/s12013-011-9216-2. [DOI] [PubMed] [Google Scholar]
- Chong MF, Macdonald R, Lovegrove JA. Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr. 2010;104(Suppl 3):S28–S39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- Cimino F, Speciale A, Anwar S, Canali R, Ricciardi E, Virgili F, Trombetta D, Saija A. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8(4):391–399. doi: 10.1007/s12263-012-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford MN. Anthocyanins-nature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1063–1072. [Google Scholar]
- Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26(8):1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am J Clin Nutr. 2013;97(5):995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- de Pascual-Teresa S, Sanchez-Ballesta MT. Anthocyanins: from plant to health. Phytochem Rev. 2008;7:281–299. [Google Scholar]
- de Pascual-Teresa S, Santos-Buelga C, Rivas-Gonzalo JC. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J Agric Food Chem. 2000;48(11):5331–5337. doi: 10.1021/jf000549h. [DOI] [PubMed] [Google Scholar]
- de Pascual-Teresa S, Moreno DA, García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11(4):1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGraba TJ. Immunogenetic susceptibility of atherosclerotic stroke: implications on current and future treatment of vascular inflammation. Stroke. 2004;35(11 Suppl 1):2712–2719. doi: 10.1161/01.STR.0000143788.87054.85. [DOI] [PubMed] [Google Scholar]
- Del Bo’ C, Kristo AS, Kalea AZ, Ciappellano S, Riso P, Porrini M, Klimis-Zacas D. The temporal effect of a wild blueberry (Vaccinium angustifolium)-enriched diet on vasomotor tone in the Sprague-Dawley rat. Nutr Metab Cardiovasc Dis. 2012;22(2):127–132. doi: 10.1016/j.numecd.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr. 2010;104(Suppl 3):S67–S90. doi: 10.1017/S0007114510003958. [DOI] [PubMed] [Google Scholar]
- Dohadwala MM, Holbrook M, Hamburg NM, Shenouda SM, Chung WB, Titas M, Kluge MA, Wang N, Palmisano J, Milbury PE, Blumberg JB, Vita JA. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am J Clin Nutr. 2011;93(5):934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domitrovic R. The molecular basis for the pharmacological activity of anthocyans. Curr Med Chem. 2011;18(29):4454–4469. doi: 10.2174/092986711797287601. [DOI] [PubMed] [Google Scholar]
- Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- Felgines C, Talavéra S, Gonthier MP, Texier O, Scalbert A, Lamaison JL, Rémésy C. Strawberry anthocyanins are recovered in urine as glucuroand sulfoconjugates in humans. J Nutr. 2003;133:1296–1301. doi: 10.1093/jn/133.5.1296. [DOI] [PubMed] [Google Scholar]
- Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Davies KJ, Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic Biol Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Traber M, Ursini F. Antioxidants: GRABbing new headlines. Free Radic Biol Med. 2014;66:1–2. doi: 10.1016/j.freeradbiomed.2013.10.808. [DOI] [PubMed] [Google Scholar]
- Frankel EN, Waterhouse AL, Teissedre PL. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J Agric Food Chem. 1995;43:890–894. [Google Scholar]
- García-Alonso M, Rimbach G, Rivas-Gonzalo JC, De Pascual-Teresa S. Antioxidant and cellular activities of anthocyanins and their corresponding vitisins A—studies in platelets, monocytes, and human endothelial cells. J Agric Food Chem. 2004;52(11):3378–3384. doi: 10.1021/jf035360v. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension. 2002;39(6):1088–1094. doi: 10.1161/01.hyp.0000018041.48432.b5. [DOI] [PubMed] [Google Scholar]
- Harborne JB. The flavonoids: advances in research since 1986. London: Chapman and Hall; 1993. [Google Scholar]
- He J, Giusti MM. Anthocyanins: natural colorants with health-promoting properties. Annu Rev Food Sci Technol. 2010;1:163–187. doi: 10.1146/annurev.food.080708.100754. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr Cancer. 1993;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- Hidalgo M, Martin-Santamaria S, Recio I, Sanchez-Moreno C, de Pascual-Teresa B, Rimbach G, de Pascual-Teresa S. Potential anti-inflammatory, anti-adhesive, anti/estrogenic, and angiotensin-converting enzyme inhibitory activities of anthocyanins and their gut metabolites. Genes Nutr. 2012;7(2):295–306. doi: 10.1007/s12263-011-0263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper L, Kroon PA, Rimm EB, Cohn JS, Harvey I, Le Cornu KA, Ryder JJ, Hall WL, Cassidy A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- Huang SS, Huang PH, Wu TC, Chen JW, Lin SJ. Association of serum bilirubin with contrast-induced nephropathy and future cardiovascular events in patients undergoing coronary intervention. PLoS One. 2012;7(8):e42594. doi: 10.1371/journal.pone.0042594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WY, Davidge ST, Wu J. Bioactive natural constituents from food sources-potential use in hypertension prevention and treatment. Crit Rev Food Sci Nutr. 2013;53(6):615–630. doi: 10.1080/10408398.2010.550071. [DOI] [PubMed] [Google Scholar]
- Iademarco MF, McQuillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1) J Biol Chem. 1992;267:16323–16329. [PubMed] [Google Scholar]
- Ichiyanagi T, Shida Y, Rahman MM, Hatano Y, Konishi T. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. J Agric Food Chem. 2006;54:6578–6587. doi: 10.1021/jf0602370. [DOI] [PubMed] [Google Scholar]
- Iwuchukwu OF, Ajetunmobi J, Ung D, Nagar S. Characterizing the effects of common UDP glucuronosyltransferase (UGT) 1A6 and UGT1A1 polymorphisms on cis- and trans-resveratrol glucuronidation. Drug Metab Dispos. 2009;37(8):1726–1732. doi: 10.1124/dmd.109.027391. [DOI] [PubMed] [Google Scholar]
- Jeon S, Han S, Lee J, Hong T, Yim DS. The safety and pharmacokinetics of cyanidin-3-glucoside after 2-week administration of black bean seed coat extract in healthy subjects. Korean J Physiol Pharmacol. 2012;16(4):249–253. doi: 10.4196/kjpp.2012.16.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MK, Li J, Kim JL, Gong JH, Kwak SN, Park JH, Lee JY, Lim SS, Kang YH. Purple corn anthocyanins inhibit diabetes-associated glomerular monocyte activation and macrophage infiltration. Am J Physiol Renal Physiol. 2012;303(7):F1060–F1069. doi: 10.1152/ajprenal.00106.2012. [DOI] [PubMed] [Google Scholar]
- Kay CD, Mazza G, Holub BJ, Wang J. Anthocyanin metabolites in human urine and serum. Br J Nutr. 2004;91:933–942. doi: 10.1079/bjn20041126. [DOI] [PubMed] [Google Scholar]
- Kay CD, Mazza G, Holub BJ. Anthocyanins exist in the circulation primarily as metabolites in adult men. J Nutr. 2005;135:2582–2588. doi: 10.1093/jn/135.11.2582. [DOI] [PubMed] [Google Scholar]
- Kay CD, Hooper L, Kroon PA, Rimm EB, Cassidy A. Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res. 2012;56(11):1605–1616. doi: 10.1002/mnfr.201200363. [DOI] [PubMed] [Google Scholar]
- Kerekes G, Szekanecz Z, Dér H, Sándor Z, Lakos G, Muszbek L, Csipö I, Sipka S, Seres I, Paragh G, Kappelmayer J, Szomják E, Veres K, Szegedi G, Shoenfeld Y, Soltész P. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J Rheumatol. 2008;35:398–406. [PubMed] [Google Scholar]
- Kim HJ, Tsoy I, Park JM, Chung JI, Shin SC, Chang KC. Anthocyanins from soybean seed coat inhibit the expression of TNF-alpha-induced genes associated with ischemia/reperfusion in endothelial cell by NF-kappaB-dependent pathway and reduce rat myocardial damages incurred by ischemia and reperfusion in vivo. FEBS Lett. 2006;580(5):1391–1397. doi: 10.1016/j.febslet.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Kolovou G, Anagnostopoulou K, Mikhailidis DP, Cokkinos DV. Apolipoprotein E knockout models. Curr Pharm Des. 2008;14(4):338–351. doi: 10.2174/138161208783497769. [DOI] [PubMed] [Google Scholar]
- Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Kuhnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Dietet. 1976;24:117–191. [PubMed] [Google Scholar]
- Lampe JW. Diet, genetic polymorphisms, detoxification, and health risks. Altern Ther Health Med. 2007;13(2):S108–S111. [PubMed] [Google Scholar]
- Landray MJ, Wheeler DC, Lip GY, Newman DJ, Blann AD, McGlynn FJ, Ball S, Townend JN, Baigent C. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the Chronic Renal Impairment in Birmingham (CRIB) study. Am J Kidney Dis. 2004;43:244–253. doi: 10.1053/j.ajkd.2003.10.037. [DOI] [PubMed] [Google Scholar]
- Lazzè MC, Pizzala R, Perucca P, Cazzalini O, Savio M, Forti L, Vannini V, Bianchi L. Anthocyanidins decrease endothelin-1 production and increase endothelial nitric oxide synthase in human endothelial cells. Mol Nutr Food Res. 2006;50(1):44–51. doi: 10.1002/mnfr.200500134. [DOI] [PubMed] [Google Scholar]
- Lechleitner S, Gille J, Johnson DR, Petzelbauer P. Interferon enhances tumor necrosis factor-induced vascular cell adhesion molecule 1 (CD106) expression in human endothelial cells by an interferon-related factor 1-dependent pathway. J Exp Med. 1998;187:2023–2030. doi: 10.1084/jem.187.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65(12 Pt 2):S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994;120(3):227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Maestro A, Terdoslavich M, Vanzo A, Kuku A, Tramer F, Nicolin V, Micali F, Decorti G, Passamonti S. Expression of bilitranslocase in the vascular endothelium and its function as a flavonoid transporter. Cardiovasc Res. 2010;85(1):175–183. doi: 10.1093/cvr/cvp290. [DOI] [PubMed] [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- Martin K. Polyphenols as dietary supplements: a double-edged sword. Nutr Diet Suppl. 2010;2:1–12. [Google Scholar]
- Martin S, Andriambeloson E, Takeda K, Andriantsitohaina R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br J Pharmacol. 2002;135(6):1579–1587. doi: 10.1038/sj.bjp.0704603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Giannone G, Andriantsitohaina R, Martinez MC. Delphinidin, an active compound of red wine, inhibits endothelial cell apoptosis via nitric oxide pathway and regulation of calcium homeostasis. Br J Pharmacol. 2003;139(6):1095–1102. doi: 10.1038/sj.bjp.0705347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauray A, Felgines C, Morand C, Mazur A, Scalbert A, Milenkovic D. Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr. 2010;5(4):343–353. doi: 10.1007/s12263-010-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauray A, Felgines C, Morand C, Mazur A, Scalbert A, Milenkovic D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr Metab Cardiovasc Dis. 2012;22(1):72–80. doi: 10.1016/j.numecd.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Mazza GJ. Anthocyanins and heart health. Ann Ist Super Sanità. 2007;43(4):369–374. [PubMed] [Google Scholar]
- Mazza G, Kay CD. Bioactivity, absorption, and metabolism of anthocyanins. In: Daayf F, Lattanzio V, editors. Recent advances in polyphenols research. Hoboken: Blackwell; 2008. pp. 228–262. [Google Scholar]
- McGhie TK, Walton MC. The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Cao G, Prior RL, Blumberg J. Bioavailablility of elderberry anthocyanins. Mech Ageing Dev. 2002;123:997–1006. doi: 10.1016/s0047-6374(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Vita JA, Blumberg JB. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr. 2010;140:1099–1104. doi: 10.3945/jn.109.117168. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Vita JA, Blumberg JB. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J Nutr. 2010;140(6):1099–1104. doi: 10.3945/jn.109.117168. [DOI] [PubMed] [Google Scholar]
- Miller RJ, Jackson KG, Dadd T, Mayes AE, Brown AL, Minihane AM. The impact of the catechol-O-methyltransferase genotype on the acute responsiveness of vascular reactivity to a green tea extract. Br J Nutr. 2011;105(8):1138–1144. doi: 10.1017/S0007114510004836. [DOI] [PubMed] [Google Scholar]
- Minetti M, Mallozzi C, Di Stasi AM, Pietraforte D. Bilirubin is an effective antioxidant of peroxynitrite-mediated protein oxidation in human blood plasma. Arch Biochem Biophys. 1998;352(2):165–174. doi: 10.1006/abbi.1998.0584. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Nakagawa K, Kudo M, Muraishi K, Someya K. Direct intestinal absorption of red fruit anthocyanins; cyanidin-3-glucoside and yanidin-3;5-diglucoside; into rats and humans. J Agric Food Chem. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Endothelial dysfunction: from physiology to therapy. J Mol Cell Cardiol. 1999;31(1):61–74. doi: 10.1006/jmcc.1998.0844. [DOI] [PubMed] [Google Scholar]
- Neish AS, Read MA, Thanos D, Pine R, Maniatis T, Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Moll Cell Biol. 1995;15:2558–2569. doi: 10.1128/mcb.15.5.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizamutdinova IT, Kim YM, Chung JI, Shin SC, Jeong YK, Seo HG, Lee JH, Chang KC, Kim HJ. Anthocyanins from black soybean seed coats stimulate wound healing in fibroblasts and keratinocytes and prevent inflammation in endothelial cells. Food Chem Toxicol. 2009;47(11):2806–2812. doi: 10.1016/j.fct.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Nöthlings U, Schulze MB, Weikert C, et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr. 2008;138(4):775–781. doi: 10.1093/jn/138.4.775. [DOI] [PubMed] [Google Scholar]
- Novotny JA, Clevidence BA, Kurilich AC. Anthocyanin kinetics are dependent on anthocyanin structure. Br J Nutr. 2012;107(4):504–509. doi: 10.1017/S000711451100314X. [DOI] [PubMed] [Google Scholar]
- O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12(1):83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- Ohnishi R, Ito H, Kasajima N, Kaneda M, Kariyama R, Kumon H, Hatano T, Yoshida T. Urinary excretion of anthocyanins in humans after cranberry juice ingestion. Biosci Biotechnol Biochem. 2006;70:1681–1687. doi: 10.1271/bbb.60023. [DOI] [PubMed] [Google Scholar]
- Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351(1–2):41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- Paixão J, Dinis TC, Almeida LM. Malvidin-3-glucoside protects endothelial cells up-regulating endothelial NO synthase and inhibiting peroxynitrite-induced NF-kB activation. Chem Biol Interact. 2012;199(3):192–200. doi: 10.1016/j.cbi.2012.08.013. [DOI] [PubMed] [Google Scholar]
- Paixão J, Dinis TC, Almeida LM. Protective role of malvidin-3-glucoside on peroxynitrite-induced damage in endothelial cells by counteracting reactive species formation and apoptotic mitochondrial pathway. Oxid Med Cell Longev. 2012;2012:428538. doi: 10.1155/2012/428538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palíková I, Valentová K, Oborná I, Ulrichová J. Protectivity of blue honeysuckle extract against oxidative human endothelial cells and rat hepatocyte damage. J Agric Food Chem. 2009;57(15):6584–6589. doi: 10.1021/jf9003994. [DOI] [PubMed] [Google Scholar]
- Pamukcu B, Lip GY, Snezhitskiy V, Shantsila E. The CD40-CD40L system in cardiovascular disease. Ann Med. 2011;43(5):331–340. doi: 10.3109/07853890.2010.546362. [DOI] [PubMed] [Google Scholar]
- Papi A, Johnston SL. Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its upregulation by rhinovirus infection via NF-kappaB and GATA transcription factors. J Biol Chem. 1999;274:30041–30051. doi: 10.1074/jbc.274.42.30041. [DOI] [PubMed] [Google Scholar]
- Passamonti S, Vrhovsek U, Vanzo A, Mattivi F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003;544:210–213. doi: 10.1016/s0014-5793(03)00504-0. [DOI] [PubMed] [Google Scholar]
- Pati S, Liberatore MT, Gambacorta G, Antonacci D, La Notte E. Rapid screening for anthocyanins and anthocyanin dimers in crude grape extracts by high performance liquid chromatography coupled with diode array detection and tandem mass spectrometry. J Chromatogr A. 2009;1216(18):3864–3868. doi: 10.1016/j.chroma.2009.02.068. [DOI] [PubMed] [Google Scholar]
- Pergola C, Rossi A, Dugo P, Cuzzocrea S, Sautebin L. Inhibition of nitric oxide biosynthesis by anthocyanin fraction of blackberry extract. Nitric Oxide. 2006;15(1):30–39. doi: 10.1016/j.niox.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Perrier E, Fournet-Bourguignon MP, Royere E, Molez S, et al. Effect of uncoupling endothelial nitric oxide synthase on calcium homeostasis in aged porcine endothelial cells. Cardiovasc Res. 2009;82(1):133–142. doi: 10.1093/cvr/cvp034. [DOI] [PubMed] [Google Scholar]
- Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res. 2003;60:49–57. doi: 10.1016/s0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pojer E, Mattivi F, Johnson D, Stockley CS. The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food Saf. 2013;12(5):483–508. doi: 10.1111/1541-4337.12024. [DOI] [PubMed] [Google Scholar]
- Poli G, Biasi F, Leonarduzzi G. Oxysterols in the pathogenesis of major chronic diseases. Redox Biol. 2013;1(1):125–130. doi: 10.1016/j.redox.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior RL, Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006;40(10):1014–1028. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]
- Riso P, Klimis-Zacas D, Del Bo’ C, Martini D, Campolo J, Vendrame S, Møller P, Loft S, De Maria R, Porrini M. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr. 2013;52(3):949–961. doi: 10.1007/s00394-012-0402-9. [DOI] [PubMed] [Google Scholar]
- Schaffer S, Halliwell B. Do polyphenols enter the brain and does it matter? Some theoretical and practical considerations. Genes Nutr. 2012;7(2):99–109. doi: 10.1007/s12263-011-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL. A critical review of the role of endothelial factors in the pathogenesis of hypertension. J Cardiovasc Pharmacol. 2001;38(Suppl 2):S3–S6. doi: 10.1097/00005344-200111002-00002. [DOI] [PubMed] [Google Scholar]
- Schini-Kerth VB, Auger C, Kim JH, Etienne-Selloum N, Chataigneau T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflugers Arch. 2010;459(6):853–862. doi: 10.1007/s00424-010-0806-4. [DOI] [PubMed] [Google Scholar]
- Serraino I, Dugo L, Dugo P, Mondello L, Mazzon E, Dugo G, Caputi AP, Cuzzocrea S. Protective effects of cyanidin-3-O-glucoside from blackberry extract against peroxynitrite-induced endothelial dysfunction and vascular failure. Life Sci. 2003;73(9):1097–1114. doi: 10.1016/s0024-3205(03)00356-4. [DOI] [PubMed] [Google Scholar]
- Sorrenti V, Mazza F, Campisi A, Di Giacomo C, Acquaviva R, Vanella L, Galvano F. Heme oxygenase induction by cyanidin-3-O-beta-glucoside in cultured human endothelial cells. Mol Nutr Food Res. 2007;51(5):580–586. doi: 10.1002/mnfr.200600204. [DOI] [PubMed] [Google Scholar]
- Speciale A, Canali R, Chirafisi J, Saija A, Virgili F, Cimino F. Cyanidin-3-O-glucoside protection against TNF-α-induced endothelial dysfunction: involvement of nuclear factor-κB signaling. J Agric Food Chem. 2010;58(22):12048–12054. doi: 10.1021/jf1029515. [DOI] [PubMed] [Google Scholar]
- Speciale A, Anwar S, Ricciardi E, Chirafisi J, Saija A, Cimino F. Cellular adaptive response to glutathione depletion modulates endothelial dysfunction triggered by TNF-α. Toxicol Lett. 2011;207(3):291–297. doi: 10.1016/j.toxlet.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Speciale A, Chirafisi J, Saija A, Cimino F. Nutritional antioxidants and adaptive cell responses: an update. Curr Mol Med. 2011;11(9):770–789. doi: 10.2174/156652411798062395. [DOI] [PubMed] [Google Scholar]
- Speciale A, Anwar S, Canali R, Chirafisi J, Saija A, Virgili F, Cimino F. Cyanidin-3-O-glucoside counters the response to TNF-alpha of endothelial cells by activating Nrf2 pathway. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201300102. [DOI] [PubMed] [Google Scholar]
- Stade BG, Messer G, Riethmüller G, Johnson JP. Structural characteristics of the 5’ region of the human ICAM-1 gene. Immunobiology. 1990;182(1):79–87. doi: 10.1016/S0171-2985(11)80585-1. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stoner GD, Sardo C, Apseloff G, Mullet D, Wargo W, Pound V, Singh A, Sanders J, Aziz R, Casto B, Sun X. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries for 7 days. J Clin Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- Tewari M, Dixit VM. Recent advances in tumor necrosis factor and CD40 signaling. Curr Opin Genet Dev. 1996;6(1):39–44. doi: 10.1016/s0959-437x(96)90008-8. [DOI] [PubMed] [Google Scholar]
- Thandapilly SJ, LeMaistre JL, Louis XL, Anderson CM, Netticadan T, Anderson HD. Vascular and cardiac effects of grape powder in the spontaneously hypertensive rat. Am J Hypertens. 2012;25(10):1070–1076. doi: 10.1038/ajh.2012.98. [DOI] [PubMed] [Google Scholar]
- Titta L, Trinei M, Stendardo M, Berniakovich I, Petroni K, Tonelli C, Riso P, Porrini M, Minucci S, Pelicci PG, Rapisarda P, Reforgiato Recupero G, Giorgio M. Blood orange juice inhibits fat accumulation in mice. Int J Obes (Lond) 2010;34(3):578–588. doi: 10.1038/ijo.2009.266. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Androulakis E, Papageorgiou N, Briasoulis A, Siasos G, Antoniades C, Stefanadis C. From atherosclerosis to acute coronary syndromes: the role of soluble CD40 ligand. Trends Cardiovasc Med. 2010;20(5):153–164. doi: 10.1016/j.tcm.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat Rev Drug Discov. 2002;1(12):939–950. doi: 10.1038/nrd960. [DOI] [PubMed] [Google Scholar]
- van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: review of recent findings. Curr Opin Lipidol. 2013;24(1):25–33. doi: 10.1097/MOL.0b013e32835bcdff. [DOI] [PubMed] [Google Scholar]
- van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzo A, Vrhovsek U, Tramer F, Mattivi F, Passamonti S. Exceptionally fast uptake and metabolism of cyanidin 3-glucoside by rat kidneys and liver. J Nat Prod. 2011;74(5):1049–1054. doi: 10.1021/np100948a. [DOI] [PubMed] [Google Scholar]
- Verma S, Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation. 2002;105:546–549. doi: 10.1161/hc0502.104540. [DOI] [PubMed] [Google Scholar]
- Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br J Pharmacol. 2009;157:527–536. doi: 10.1111/j.1476-5381.2009.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virdis A, Ghiadoni L, Taddei S. Effects of antihypertensive treatment on endothelial function. Curr Hypertens Rep. 2011;13:276–281. doi: 10.1007/s11906-011-0207-x. [DOI] [PubMed] [Google Scholar]
- Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wei X, Yan X, Jin T, Ling W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J Agric Food Chem. 2010;58(24):12722–12728. doi: 10.1021/jf103427j. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Wang X, Liu Y, Xia M. Supplementation with cyanidin-3-O-β-glucoside protects against hypercholesterolemia-mediated endothelial dysfunction and attenuates atherosclerosis in apolipoprotein E-deficient mice. J Nutr. 2012;142(6):1033–1037. doi: 10.3945/jn.112.157701. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Wang X, Liu Y, Xia M. Cyanidin-3-O-β-glucoside induces oxysterol efflux from endothelial cells: role of liver X receptor alpha. Atherosclerosis. 2012;223(2):299–305. doi: 10.1016/j.atherosclerosis.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Wrolstad RE, Durst RW, Lee J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol. 2005;16(9):423–428. [Google Scholar]
- Wu XL, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J Agric Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- Xia M, Ling W, Zhu H, Wang Q, Ma J, Hou M, Tang Z, Li L, Ye Q. Anthocyanin prevents CD40-activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arterioscler Thromb Vasc Biol. 2007;27(3):519–524. doi: 10.1161/01.ATV.0000254672.04573.2d. [DOI] [PubMed] [Google Scholar]
- Xie X, Zhao R, Shen GX. Influence of delphinidin-3-glucoside on oxidized low-density lipoprotein-induced oxidative stress and apoptosis in cultured endothelial cells. J Agric Food Chem. 2012;60(7):1850–1856. doi: 10.1021/jf204461z. [DOI] [PubMed] [Google Scholar]
- Xu JW, Ikeda K, Yamori Y. Cyanidin-3-glucoside regulates phosphorylation of endothelial nitric oxide synthase. FEBS Lett. 2004;574(1–3):176–180. doi: 10.1016/j.febslet.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Xu JW, Ikeda K, Yamori Y. Upregulation of endothelial nitric oxide synthase by cyanidin-3-glucoside, a typical anthocyanin pigment. Hypertension. 2004;44(2):217–222. doi: 10.1161/01.HYP.0000135868.38343.c6. [DOI] [PubMed] [Google Scholar]
- Xu JW, Ikeda K, Yamori Y. Inhibitory effect of polyphenol cyanidin on TNF-alpha-induced apoptosis through multiple signaling pathways in endothelial cells. Atherosclerosis. 2007;193(2):299–308. doi: 10.1016/j.atherosclerosis.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Yi L, Chen CY, Jin X, Mi MT, Yu B, Chang H, Ling WH, Zhang T. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett. 2010;584(3):583–590. doi: 10.1016/j.febslet.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Yi L, Chen CY, Jin X, Zhang T, Zhou Y, Zhang QY, Zhu JD, Mi MT. Differential suppression of intracellular reactive oxygen species-mediated signaling pathway in vascular endothelial cells by several subclasses of flavonoids. Biochimie. 2012;94(9):2035–2044. doi: 10.1016/j.biochi.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Martin A, Joseph JA. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic Biol Med. 2000;29(1):51–60. doi: 10.1016/s0891-5849(00)00329-4. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem. 2003;85(1):180–192. doi: 10.1046/j.1471-4159.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- Zamora-Ros R, Knaze V, Luján-Barroso L, et al. Estimation of the intake of anthocyanidins and their food sources in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Br J Nutr. 2011;106(7):1090–1099. doi: 10.1017/S0007114511001437. [DOI] [PubMed] [Google Scholar]
- Zania P, Papaconstantinou M, Flordellis CS, Maragoudakis ME, Tsopanoglou NE. Thrombin mediates mitogenesis and survival of human endothelial cells through distinct mechanisms. Am J Physiol Cell Physiol. 2008;294(5):C1215–C1226. doi: 10.1152/ajpcell.00452.2007. [DOI] [PubMed] [Google Scholar]
- Zapolska-Downar D, Nowicka G, Sygitowicz G, Jarosz M. Anthocyanin-rich Aronox extract from Aronia melanocarpa E protects against 7beta-hydroxycholesterol-induced apoptosis of endothelial cells. Ann Nutr Metab. 2008;53(3–4):283–294. doi: 10.1159/000191290. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Wang Y, Liu Y, Xia M. Supplementation of cyanidin-3-O-β-glucoside promotes endothelial repair and prevents enhanced atherogenesis in diabetic apolipoprotein E-deficient mice. J Nutr. 2013;143(8):1248–1453. doi: 10.3945/jn.113.177451. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Xia M, Yang Y, Liu F, Li Z, Hao Y, Mi M, Jin T, Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin Chem. 2011;57(11):1524–1533. doi: 10.1373/clinchem.2011.167361. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ling W, Guo H, Song F, Ye Q, Zou T, Li D, Zhang Y, Li G, Xiao Y, Liu F, Li Z, Shi Z, Yang Y. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis. 2013;23(9):843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Ziberna L, Tramer F, Moze S, Vrhovsek U, Mattivi F, Passamonti S. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic Biol Med. 2012;52(9):1750–1759. doi: 10.1016/j.freeradbiomed.2012.02.027. [DOI] [PubMed] [Google Scholar]