Abstract
DNA methylation is an epigenetic mechanism that can inhibit gene transcription. The aim of this study was to assess changes induced by an obesogenic diet in the methylation profile of genes involved in adipose tissue triacylglycerol metabolism, and to determine whether this methylation pattern can be altered by resveratrol and pterostilbene. Rats were divided into four groups. The control group was fed a commercial standard diet, and the other three groups were fed a commercial high-fat, high-sucrose diet (6 weeks): the high-fat, high-sucrose group, the resveratrol-treated group (RSV; 30 mg/kg/day), and the pterostilbene-treated group (PT; 30 mg/kg/day). Gene expression was measured by RT-PCR and gene methylation by pyrosequencing. The obesogenic diet induced a significant increase in adipose tissue weight. Resveratrol and pterostilbene partially prevented this effect. Methylation pattern of ppnla2 and pparg genes was similar among the experimental groups. In fasn, significant hypomethylation in −90-bp position and significant hypermethylation in −62-bp position were induced by obesogenic feeding. Only pterostilbene reversed the changes induced by the obesogenic diet in fasn methylation pattern. By contrast, the addition of resveratrol to the diet did not induce changes. Both phenolic compounds averted fasn up-regulation. These results demonstrate that the up-regulation of fasn gene induced by an obesogenic feeding, based on a high-fat, high-sucrose diet, is related to hypomethylation of this gene in position −90 bp. Under our experimental conditions, both molecules prevent fasn up-regulation, but this change in gene expression seems to be mediated by changes in methylation status only in the case of pterostilbene.
Electronic supplementary material
The online version of this article (doi:10.1007/s12263-014-0411-9) contains supplementary material, which is available to authorized users.
Keywords: Obesogenic diet, Resveratrol, Pterostilbene, Methylation, Fatty acid synthase
Introduction
Epigenetic processes are those that act to regulate heritable changes in gene activity through remodelling of chromatin, but are not accompanied by changes in the DNA coding sequence. Major epigenetic mechanisms include DNA methylation, nucleosome remodelling, and histone modifications. DNA methylation involves the addition of a methyl group to the fifth position of cytosine in the context of CpG dinucleotides, which are underrepresented in DNA. Clusters of CpGs, called CpG islands, are often found in association with genes, most often in the promoters and first exons (Jones and Takai 2001; Takai et al. 2001). Methylation of these CpG islands prevents gene transcription through different mechanisms in mammals. It can directly repress transcription by blocking the binding of transcriptional activators to cognate DNA sequences. Moreover, methyl-CpG-binding proteins (MBPs) directly recognize methylated DNA and recruit corepressors to silence transcription and to modify surrounding chromatin (Klose and Bird 2006). Epigenetic mechanisms such as DNA methylation have an implication in gene expression regulation, and they have become an interesting topic in the onset, development, and therapy of several diseases. Although epigenetic changes take place mainly during embryogenesis (Morgan et al. 2005), gestation, and lactation (Martínez et al. 2012), it has been demonstrated that some environmental changes could also induce variations in DNA methylation profile during adult life (Paternain et al. 2012).
Diet is one of the most determining modifiable environmental factors that affect epigenome (Park and Lee 2013). It has been reported that a wide range of dietary factors, which includes nutrients (folic acid, vitamin B12, choline, methionine, fatty acids) and some bioactive food components (polyphenols) (Campión et al. 2010; Supic et al. 2013), can modify gene methylation. This methylation pattern can also be modified by the percentage of macronutrients in the diet (cafeteria diets rich in fat, diets rich in sucrose) (Milagro et al. 2009; Lomba et al. 2010a, b). With regard to polyphenols, several studies have shown that these molecules can modify gene methylation patterns, mainly in cancer prevention (Link et al. 2010; Henning et al. 2013; Saha et al. 2013), but very scarce data concerning adipocyte and adipose tissue genes have been reported (Boqué et al. 2013; Alberdi et al. 2013).
In the previous studies from our laboratory, we observed significant reductions in body fat in rats fed an obesogenic diet and treated with resveratrol (Macarulla et al. 2009; Arias et al. 2011; Miranda et al. 2013; Gómez-Zorita et al. 2013) or pterostilbene, a dimethyl ether derivative of resveratrol (manuscript submitted). Interest in pterostilbene is based on the fact that the substitution of a hydroxy with a methoxy group in polyphenols increases the transport into cells and increases the metabolic stability of the molecule (Wen and Walle 2006). Thus, the low bioavailability showed by resveratrol is increased in the case of pterostilbene (Kapetanovic et al. 2011).
In this context, the aim of the present work was to assess changes induced by an obesogenic diet in the methylation of genes involved in white adipose tissue triacylglycerol metabolism, and to determine whether this methylation pattern can be altered by resveratrol and pterostilbene.
Materials and methods
Animals, diets, and experimental design
The experimental procedure used in the present study followed the guidelines of the Animal Usage of the University of Basque Country (CUEID CEBA/30/2010). Six-week-old male Wistar rats (Harlan Ibérica, Barcelona, Spain) were individually housed in polycarbonate metabolic cages (Techniplast Gazzada, Guguggiate, Italy). Animals were housed in a temperature-controlled facility (22 ± 2 °C) and maintained under a 12:12-h light–dark cycle per day. After a 6-day adaptation period, the animals were randomly divided into four groups (n = 8 per group) and fed the experimental diets for 6 weeks. Experimental diets were supplied by Harlan Ibérica (Barcelona, Spain). One group (control group) was fed a commercial standard diet (TD.06416), which provided 3.7 kcal/g and 10 % of calories as fat. The other three groups, high-fat, high-sucrose group (HFS), resveratrol-treated group (RSV), and pterostilbene-treated group (PT), were fed a commercial high-fat, high-sucrose diet (obesogenic diet) (TD.06415), which provided 4.6 kcal/g and 45 % of kcal as fat. In RSV and PT groups, resveratrol or pterostilbene was added to the fresh diet daily, as previously described by Macarulla et al. (2009) in amounts that ensured a dose of 30 mg/kg body weight/day.
Body weight and food intake were measured daily. At the end of the experimental period, rats were killed after an overnight fast under anesthesia (chloral hydrate) by cardiac exsanguination. Adipose tissues from epididymal, perirenal, mesenteric, and subcutaneous regions were dissected and weighed and then immediately frozen. All samples were stored at −80 °C until analysis.
Epigenetic study
Selection of relevant genes
For the epigenetic study, it was decided to include genes that satisfied the following two criteria: (a) genes involved in triacylglycerol metabolism, such as the lipoprotein lipase (lpl), fatty acid synthase (fasn), acetyl-CoA carboxylase (acaca), hormone-sensitive lipase (lipe), adipose tissue triglyceride lipase (pnpla2), sterol-regulatory-element-binding transcription factor 1 (srebf1), or peroxisome proliferator-activated receptor γ (pparg), and (b) genes with at least one CpG island in the gene promoter or first exon. The CpG Island Searcher Program (http://cpgislands.usc.edu) was used to identify which genes had CpG islands. Based on the fulfillment of these two criteria, only fasn, pnpla2, and pparg genes were selected for methylation analysis.
DNA samples
About 150 mg of perirenal adipose tissue samples was used for DNA extraction. Isolation of DNA was performed using QIAamp DNA Investigator Kit (Qiagen, Valencia, USA; Cat. No. 56504) following the manufacturer’s instructions.
Pyrosequencing
For the pyrosequencing analysis, 1 µg of genomic DNA was bisulfite-converted using the Ez DNA Methylation Gold-Kit (Zymo Research; D5005; Irvine, CA, USA) according to the manufacturer’s protocol. Primers for fasn, pnpla2, and pparg were designed using the Assay Design Software (Qiagen, Valencia, USA) and synthesized by Fisher Scientific (Pittsburgh, PA). For each gene, four, three, and two fragments were analyzed, respectively. PCR amplifications were performed using Qiagen HotStar Taq Plus DNA polymerase kit reagents (Qiagen, Valencia, USA), 7.5 μM biotinylated primer, 15 μM non-biotinylated primer, and 2 μl of bisulfite-treated DNA (60 ng). PCR conditions were as follows: 5 min at 95 °C for enzyme activation followed by 40 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 58 °C and extension for 30 s at 72 °C, with a final extension of 2 min at 72 °C. PCR primer sequences and sequencing primer sequences are given in Table 1. The quality and quantity of the PCR product was confirmed by agarose gel (1 %) electrophoresis before the cleanup and pyrosequencing analysis. Pyrosequencing was carried out using the PyroMark Gold Q96 Reagents (Qiagen, Valencia, USA) on a PyroMark MD pyrosequencer (Qiagen, Valencia, USA), and the methylation level was calculated using the Pyro Q CpG software (Qiagen, Valencia, USA).
Table 1.
PCR and pyrosequencing primer sequences
| Genes | Analyzed zones and primer sequences | |
|---|---|---|
| fasn | ||
| −90 to −62 | Fwd Rev Seq |
5′-GTGTGGAAGTTAGATGATAATT-3′ 5′-BIO-CTTAAAACTCTAATCTTATAACCACCTTAC-3′ 5′-GTTAGATGATAATTTTTAAGTGGG-3′ |
| +754 to +812 | Fwd Rev Seq |
5′-GGTTTGGGATTGGAAAGAGATT-3′ 5′-BIO-ACATATCTAAACTATATCCTTTTTACACCA-3′ 5′-GGATTGGAAAGAGATTGA-3′ |
| +951 to +997 | Fwd Rev Seq |
5′-GAATTTTGGAAAATAAATATAGTGAGTGT-3′ 5′-BIO-ATAACCCTTAATCCCCAACACT-3′ 5′-ATTTTGGAAAATAAATATAGTGAG-3′ |
| pnpla2 | ||
| −205 to −162 | Fwd Rev Seq |
5′-GTAGGGTGTGGTGGAGAT-3′ 5′-BIO-TTCTACCCCTCCTACTACACT-3′ 5′-GGGTGTGGTGGAGAT-3′ |
| +150 to +172 | Fwd Rev Seq |
5′-BIO-GGTGTTTAGATTTGTATAAAATTTG-3′ 5′-CACCTACTAAACAAAACCATCT-3′ 5′-TCTCAAACTTTAACTTCTCAT-3′ |
| +349 to +391 | Fwd Rev Seq |
5′-GATGTTTTTAAGGGAGATTAAGT-3′ 5′-BIO-AACCACTCCAATATAATAAACC-3′ 5′-GATTAAGTGGAATATTT-3′ |
| pparg | ||
| −431 to −388 | Fwd Rev Seq |
5′-GGTTAGGAGGGTTATAGTGGAGTT-3′ 5′-BIO-AACTATCACCAAATCCACACAAT-3′ 5′-GGTTTTTTTAGAAGGTGTT-3′ |
| −214 to −190 | Fwd Rev Seq |
5′-BIO-GGTGATAGTTTAAGTAATTTGGT-3′ 5′-ATAACCCCATTTTCCTCA-3′ 5′-CATTTTCCTCACACCTA-3 |
Fwd forward, Rev reverse, Seq sequence, BIO biotinylated, fasn fatty acid synthase, pnpla2 adipose tissue triglyceride lipase, pparg peroxisome proliferator-activated receptor γ
Functional analysis
ALGGEN-PROMO (http://alggen.lsi.upc.es) bioinformatic program was used to identify potential transcription factors at those CpG sites that showed significant differences in the methylation level among groups.
RNA extraction for expression analysis
Total RNA was isolated from the perirenal adipose tissue (100 mg) using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. In a following step, DNase treatment (Applied Biosystems, Foster City, CA, USA) was carried out. The quantity of the purified RNA was determined using a NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, DE, USA). After checking the suitable integrity (RIN > 7) of RNA (2100 Bioanalyzer, Agilent Technologies, Palo Alto, CA, USA), 1.5 μg of total RNA was reverse-transcribed into complementary DNA (Applied Biosystems Inc., Foster City, CA, USA) according to the manufacturer’s instructions.
Quantitative PCR
A 4.75-μl aliquot of each diluted complementary DNA sample was used for PCR amplification in a 12.5-μl reaction volume. The complementary DNA samples were amplified on an iCycler-MyiQ real-time PCR detection system (Bio-Rad, Hercules, CA, USA) in the presence of SYBR® Green master mix (Applied Biosystems, Foster City, CA, USA) and a 300-nM concentration of each of the sense and antisense primers. Real-time PCR condition as well as fasn and β-actin primers was previously reported (Alberdi et al. 2011). PCR-specific primers were synthesized commercially (Integrated DNA Technologies, Leuven, Belgium): mRNA levels in all samples were normalized to the values of β-actin, and the results are expressed as fold changes of the threshold cycle (Ct) value relative to the controls using the 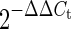 method (Livak and Schmittgen 2001). Three technical replicates of each PCR were done for each sample, and the specificity of a quantitative PCR assay was confirmed by dissociation curve.
method (Livak and Schmittgen 2001). Three technical replicates of each PCR were done for each sample, and the specificity of a quantitative PCR assay was confirmed by dissociation curve.
Nuclear DNA methyltransferase (DNMT) activity assay
Nuclear protein was extracted from perirenal adipose tissue (300 mg) using RIPA buffer (100 μl). About 15 μg nuclear proteins of each sample was used to measure DNMT activity by the EpiSeeker DNMT Activity Quantification Kit (ABCAM, Cambridge, UK; Cat. ab113467) following the manufacturer’s protocol with minor modifications. Briefly, first incubation time was increased to 3 h. Incubation conditions were 37 °C with constant orbital shaking. The results were calculated as optical density/h/mg according to the manufacturer’s instructions.
Statistical analysis
Results are presented as mean ± SE of the means. Statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Analysis of variance (ANOVA) was used to determine the presence or absence of significant differences (P < 0.05) in the analytical variables among the four groups of animals with different diets. Mixed linear model was used including “diet” as fixed effect and “animal” as random effect. The Tukey’s test was used as a posthoc test for multiple comparison analyses among the four groups of animals. Correlation analysis was performed using Pearson’s correlation coefficient to determine the relationships between fasn methylation in different positions and fasn expression (expressed as ΔCt). Statistical significance was set up at P < 0.05.
Results
Body weight and adipose tissue weights
High-fat, high-sucrose feeding resulted in increased body weight as compared to control diet-fed animals. When obesogenic diet supplemented with resveratrol or pterostilbene was fed to the rats, significantly lower body mass was observed in the resveratrol- and pterostilbene-offered groups compared to the HFS group. No differences in energy intake among the three HFS-fed animals were found, and all these groups showed higher caloric intakes than their standard diet-fed counterparts (Table 2).
Table 2.
Body weight, energy intake, and adipose tissue weights of rats fed control diet or high-fat, high-sucrose diets supplemented or not with resveratrol or pterostilbene for 6 weeks
| Control | HFS | RSV | PT | ANOVA | |
|---|---|---|---|---|---|
| Final weight (g) | 290 ± 4a | 403 ± 7b | 363 ± 6c | 373 ± 9c | P < 0.001 |
| Energy intake (kcal/day) | 65.5 ± 2.3a | 73.0 ± 2.1b | 69.6 ± 2.6b | 72.9 ± 3.1b | P < 0.05 |
| Adipose tissue weights (g) | |||||
| Epididymal | 4.0 ± 0.7a | 15.3 ± 0.7b | 13.0 ± 0.7b,c | 10.0 ± 0.8c | P < 0.001 |
| Perirenal | 4.7 ± 0.7a | 16.9 ± 0.7b | 11.9 ± 0.8c | 10.9 ± 0.8c | P < 0.001 |
| Mesenteric | 1.4 ± 0.6a | 6.5 ± 0.6b | 4.4 ± 0.6c | 4.1 ± 0.6c | P < 0.001 |
| Subcutaneous | 5.2 ± 1.1a | 20.0 ± 1.5b | 13.1 ± 1.9c | 10.9 ± 1.1c | P < 0.001 |
| E + PR + M | 10.4 ± 0.3a | 37.2 ± 2.0b | 30.8 ± 1.2c | 25.8 ± 2.1d | P < 0.001 |
| PR + M | 6.5 ± 0.3a | 22.4 ± 1.0b | 17.7 ± 0.8c | 15.7 ± 1.6c | P < 0.001 |
| ∑ Adipose tissues | 17.3 ± 0.3a | 56.1 ± 2.9b | 42.7 ± 1.9c | 35.6 ± 4.7c | P < 0.001 |
Values are mean ± SEM (n = 8). Differences among groups have been determined by ANOVA and Tukey’s post hoc analysis. Values in the same row with different superscripts are significantly different
E epididymal, PR perirenal, M mesenteric, HFS high fat, high sucrose, RSV resveratrol, PT pterostilbene
The obesogenic diet induced a significant increase in adipose tissue weight in all the anatomical locations analyzed. When resveratrol or pterostilbene was included in the diet, an important reduction in all the adipose depots, except for epididymal adipose tissue in animals fed RSV-supplemented diet, was observed. These molecules prevented the increase in fat accumulation induced by the obesogenic diet but only partially because adipose tissue weights in RSV and PT groups were higher than those in control rats. When comparing resveratrol- and pterostilbene-treated rats, statistical differences were only detected in the sum of epididymal + perirenal + mesenteric adipose depots. PT group presented an additional 16 % reduction in the visceral fat (Table 2).
DNA methylation
Taken as a whole, the methylation pattern of ppnla2 and pparg genes was not significantly different among the four experimental groups (data not shown). With regard to fasn, no relevant differences in total methylation status were found among the groups (79–80 % methylation for all the groups) in the perirenal adipose tissue. However, some variations were observed in specific DNA positions. All the changes observed in HFS, RSV, and PT groups when compared with the control group, expressed as percentage, are summarized in Table 3. Only those higher than 5 % were considered since this is the sensitivity threshold of the pyrosequencing assay.
Table 3.
Changes in the methylation status of the measured positions in fasn gene, expressed as percentage
| Groups | CpG site (position) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| −90 | −62 | +754 | +791 | +812 | +951 | +956 | +962 | +997 | |
| HFS | −11 | +6 | −1 | 0 | −1 | −3 | −7 | +1 | −2 |
| RSV | −10 | +6 | −2 | −2 | −3 | −1 | −2 | +2 | +2 |
| PT | −1 | +3 | +2 | +2 | +1 | −4 | −3 | 0 | −2 |
Data for each CpG site represent the mean percentage of methylation change compared to the control group
HFS high fat high sucrose, RSV resveratrol, PT pterostilbene
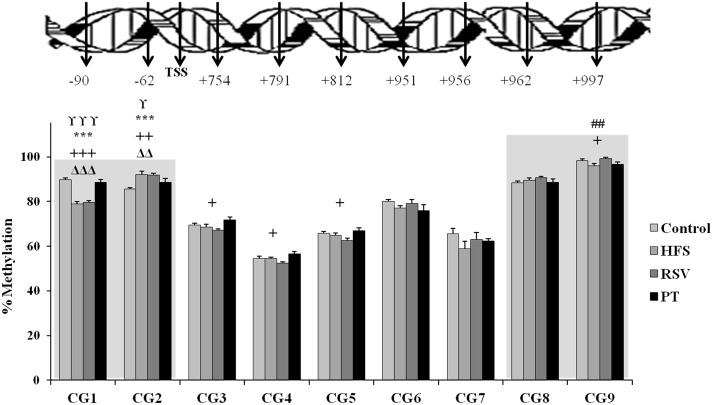
As far as obesogenic feeding is concerned, when rats from HFS group were compared to the control group, significant hypomethylation in −90-bp position (P < 0.001) and significant hypermethylation in −62-bp position (P < 0.01) were observed (Fig. 1; Table 3). The addition of resveratrol and pterostilbene to the obesogenic diet led to different methylation patterns of fasn. In the case of pterostilbene, according to changes in positions −90 bp and −62 bp, it can be stated that this molecule reversed the changes induced by the obesogenic diet (Fig. 1; Table 3). By contrast, when HFS group was compared to the RSV group, no significant changes in the methylation status were observed (Fig. 1; Table 3).
Fig. 1.
Fasn methylation pattern in selected CpG sites in perirenal adipose tissue of rats fed a control diet or high-fat, high-sucrose diets supplemented or not (HFS) with resveratrol (RSV) or pterostilbene (PT) for 6 weeks. Values are presented as mean ± SE of the means. Symbols in bars show statistical differences between groups as follows: control versus HFS: ***P < 0.001; control versus RSV: *P < 0.05; ***P < 0.001; HFS versus RSV: ##P < 0.01; HFS versus PT; ΔΔP < 0.01; ΔΔΔP < 0.001; PT versus RSV; +P < 0.05; ++P < 0.01; +++P < 0.001. TSS transcriptional start site
In the four experimental groups, methylation status was lower in positions after the TSS (+754 bp to +956 bp) (average methylation 66 %) than in the rest of the promoter region (average percentage 90.5 %) (Fig. 1). This fits with a canonical pattern of methylation (Davies et al. 2012).
Gene expression of fatty acid synthase
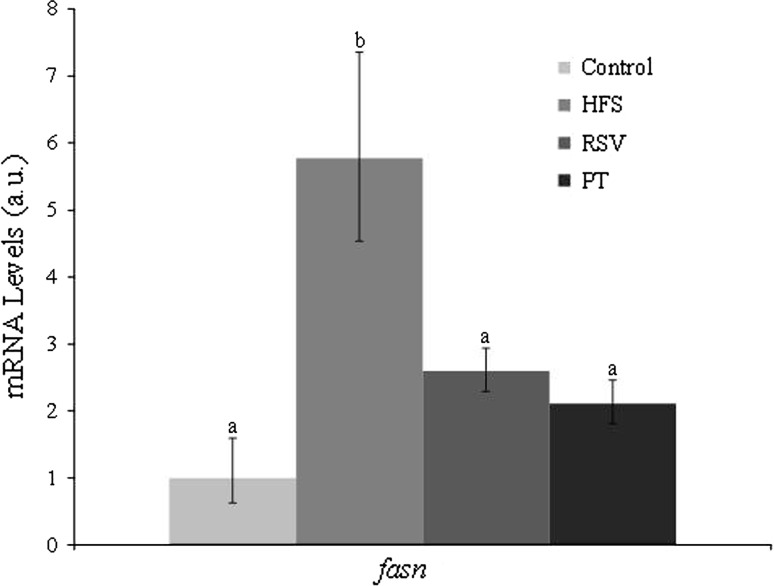
Fasn expression was significantly increased (fivefold) in rats fed the obesogenic diet (HFS group) as compared to the control rats. Resveratrol and pterostilbene prevented this effect. Thus, expression values in these two groups were significantly lower than that of the HFS group, but similar to that of control rats (Fig. 2).
Fig. 2.
Gene expression of fatty acid synthase in perirenal adipose tissue of rats fed a control diet or high-fat, high-sucrose diets supplemented or not (HFS) with resveratrol (RSV) or pterostilbene (PT) for 6 weeks. Values are presented as mean ± SE of the means. a,b Bars not sharing common letter are significantly different (P < 0.05). Fasn fatty acid synthase, HFS high-fat, high-sucrose
Correlation analysis between methylation and expression in fasn gene
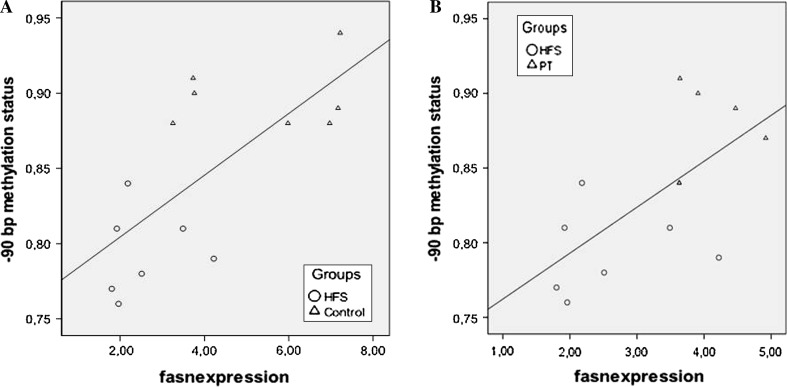
Pearson’s correlation coefficients were calculated when significant differences in fasn methylation status were found between groups (Fig. 1). Significant correlations were only found in −90-bp position when control and HFS groups (P = 0.01; Pearson’s coefficient: 0.708), as well as HFS and PT groups (P = 0.05; Pearson’s coefficient: 0.648) were considered (Fig. 3).
Fig. 3.
Pearson’s correlations between percentage of DNA methylation and gene expression for fasn in rats from control and HFS groups (a) and in rats from HFS and PT groups (b) were compared. Statistical significance was set up at P < 0.05. HFS high-fat, high-sucrose, PT pterostilbene
Nuclear DNA methyltransferase activity
Nuclear DNA methyltransferase (DNMT) activity showed a decrease in HFS group when compared with the control group, which showed a P = 0.061. No differences were found among the other three experimental groups (Fig. 4).
Fig. 4.
Nuclear DNA methyltransferase activity in perirenal adipose tissue of rats fed a control diet or high-fat, high-sucrose diets supplemented or not (HFS) with resveratrol (RSV) or pterostilbene (PT) for 6 weeks. Values are presented as mean ± SE of the means. OD optical density
Discussion
As expected, feeding a diet rich in fat and sucrose led to increased energy intake and, consequently to increased fat accumulation, as observed when rats from HFS group were compared with rats fed the control diet. The addition of resveratrol or pterostilbene in the HSF diet at a dose of 30 mg/kg/day prevented this effect, but only partially because rats in groups RSV and PT did not reach the values showed by control animals in body weight and adipose tissue weights. In general terms, no important differences were observed between both phenolic compounds.
As explained in the “Materials and methods” section, in the present study, we were interested in the methylation of lpl, fasn, acaca, lipe, pnpla2, srebf1, and pparg because these genes play important roles in the control of triacylglycerol metabolism in adipose tissue. Moreover, these genes have been shown to be targets for resveratrol (Rivera et al. 2009; Szkudelska and Szkudelski 2010; Baile et al. 2011; Kim et al. 2011; Alberdi et al. 2011; Gómez-Zorita et al. 2012; Lasa et al. 2012). Nevertheless, when CpG-rich areas were investigated, we observed that only fasn, pnpla2, and pparg genes showed these areas near to the gene promoter. Consequently, only these three genes were studied.
The pattern of methylation showed by pnpla2 and pparg in control rats was not altered in rats from HFS, RSV, and PT groups, suggesting that under the present dietary conditions, this epigenetic mechanism was not important in the regulation of these genes. By contrast, several changes were observed in fasn. Thus, the following discussions will focus on this gene.
Feeding a high-fat, high-sucrose diet led to a significant increase in fasn expression. It is well documented that while high-fat diets decrease the expression of this gene (Duran-Montgé et al. 2009; Jiang et al. 2009), diets rich either in simple carbohydrates (sucrose) or carbohydrates with high glycemic index induce an increase (Kim and Freake 1996; Kabir et al. 1998; Morris et al. 2003). It seems that in the present study, the effect of high-sucrose content was greater than that of high-fat content, and the final effect was an up-regulation of fasn. These results agree with those reported by Yang et al. (2012) when using this type of diet. Taking into account that this enzyme catalyzes the synthesis of long-chain fatty acids from acetyl-CoA and malonyl-CoA, and thus, it is one of the rate-limiting enzyme in de novo lipogenesis, it can be proposed that the increase in body fat induced by the high-fat, high-sucrose diet was due, at least in part, to increase in fatty acid synthesis.
Obesogenic diet induced significant DNA methylation changes with respect to the controls. We observed a mild but significant methylation increase in −62-bp position, while a decrease in −90 bp, being only this one significantly correlated with the overexpression of fasn. It has been previously reported that small methylation changes can be associated with gene expression variability that exert significant effects on phenotype (Irizarry et al. 2009), and even if we think that not all the expression change observed for fasn should be attributed to this methylation change at position −90 bp, we believe that it truly contributed to the down-regulation of fasn. Interestingly, nuclear DNMT activity showed a similar pattern of response to DNA methylation level at −90 bp, this meaning that it could be a mechanism, among others, which justifies the observed effects of high-fat, high-sucrose feeding and pterostilbene on fasn methylation.
To better understand the mechanism by which DNA methylation level in the −90-bp position of fasn could be related to the decrease in gene expression, we searched for consensus response elements around this −90-bp position; this −90-bp position of fasn promoter is a binding site for Sp1, an ubiquitous transcription factor that acts as a glucose sensor (Vaulont et al. 2000). It has been demonstrated that Sp1 is crucial for fasn gene promoter activity in adipocytes (Rolland et al. 1996). Consequently, the influence of the observed methylation status changes on the regulation of fasn transcription mediated by Sp1 cannot be discarded.
Hypomethylation of fasn induced by a high-fat, high-sucrose feeding was previously reported by Uriarte et al. (2013). When comparing this study with the present one, two important issues should be underlined. On the one hand, Uriarte et al. observed hypomethylation in fasn after 20 weeks of feeding a high-fat, high-sucrose diet. In the present study, this effect was observed after 6 weeks of the same dietary treatment, meaning that this epigenetic mechanism does not need very long periods to take place. On the other hand, the method used by Uriarte et al. to measure DNA methylation (MALDI-TOF mass spectrophotometry) was different from that used in the present study. The fact of finding similar results by using two methods with differences in specificity, sensibility, and accuracy reinforces the hypomethylating action of this dietary pattern on fasn.
Resveratrol and pterostilbene treatments led to significant decreases in fasn expression. mRNA levels found in RSV and PT groups were not different from those found in the control group, meaning that these molecules totally prevented the alteration induced by the obesogenic diet. This effect was involved, at least in part, in the obesity prevention action showed by these phenolic compounds. As far as resveratrol effects are concerned, the methylation pattern in the investigated regions of fasn gene remained unchanged in RSV group when compared with the HSF group, suggesting that gene expression changes were not likely to be associated with modifications in DNA methylation. By contrast, pterostilbene reversed the changes induced by the obesogenic diet in positions −90 and −62 bp, and the percentage of methylation in these regions was similar in PT and control groups. Pearson’s correlations revealed that only hypermethylation in −90-bp position showed significant correlation with fasn expression. These results, together with those obtained when the effects of obesogenic diet were analyzed, reinforce the relevance of methylation in −90-bp position in the control of fasn expression by the diet.
Taken as a whole, the results obtained in the present study demonstrate that the up-regulation of fasn gene induced by an obesogenic feeding based on a high-fat, high-sucrose diet is related to hypomethylation of this gene in position −90 bp. Under our experimental conditions, both resveratrol and pterostilbene prevent fasn up-regulation, but this change in gene expression seems to be mediated by changes in methylation status only in the case of pterostilbene.
Electronic supplementary material
Acknowledgments
This study was supported by grants from Government of the Basque Country (PTERORES, S-E 12N24 and IT-512-13), Ministerio de Economía y Competitividad (AGL2011-27406-ALI), Instituto de Salud Carlos III (CIBERobn), and University of the Basque Country (UPV/EHU) (ELDUNANOTEK UFI11/32). Ana Gracia is a recipient of a doctoral fellowship from the Ministerio de Economía y Competitividad (UPV/EHU).
References
- Alberdi G, Rodríguez VM, Miranda J, Macarulla MT, Arias N, Andrés-Lacueva C, Portillo MP. Changes in white adipose tissue metabolism induced by resveratrol in rats. Nutr Metab. 2011;8(1):29. doi: 10.1186/1743-7075-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberdi G, Rodríguez VM, Miranda J, Macarulla MT, Churruca I, Portillo MP. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013;141(2):1530–1535. doi: 10.1016/j.foodchem.2013.03.085. [DOI] [PubMed] [Google Scholar]
- Arias N, Macarulla MT, Aguirre L, Martínez-Castaño MG, Gómez-Zorita S, Miranda J, Martínez JA, Portillo MP. The combination of resveratrol and conjugated linoleic acid is not useful in preventing obesity. J Physiol Biochem. 2011;67(3):471–477. doi: 10.1007/s13105-011-0086-2. [DOI] [PubMed] [Google Scholar]
- Baile CA, Yang JY, Rayalam S, Hartzell DL, Lai CY, Andersen C, Della-Fera MA. Effect of resveratrol on fat mobilization. Ann N Y Acad Sci. 2011;1215:40–47. doi: 10.1111/j.1749-6632.2010.05845.x. [DOI] [PubMed] [Google Scholar]
- Boqué N, de la Iglesia R, de la Garza AL, Milagro FI, Olivares M, Bañuelos O, Soria AC, Rodríguez-Sánchez S, Martínez JA, Campión J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res. 2013;57(8):1473–1478. doi: 10.1002/mnfr.201200686. [DOI] [PubMed] [Google Scholar]
- Campión J, Milagro F, Martínez JA. Epigenetics and obesity. Prog Mol Biol Transl Sci. 2010;94:291–347. doi: 10.1016/B978-0-12-375003-7.00011-X. [DOI] [PubMed] [Google Scholar]
- Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, Coarfa C, Harris RA, Milosavljevic A, Troakes C, Al-Sarraj S, Dobson R, Schalkwyk LC, Mill J. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012;13(6):R43. doi: 10.1186/gb-2012-13-6-r43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Montgé P, Theil PK, Lauridsen C, Esteve-Garcia E. Dietary fat source affects metabolism of fatty acids in pigs as evaluated by altered expression of lipogenic genes in liver and adipose tissues. Animal. 2009;3(4):535–542. doi: 10.1017/S1751731108003686. [DOI] [PubMed] [Google Scholar]
- Gómez-Zorita S, Fernández-Quintela A, Macarulla MT, Aguirre L, Hijona E, Bujanda L, Milagro F, Martínez JA, Portillo MP. Resveratrol attenuates steatosis in obese Zucker rats by decreasing fatty acid availability and reducing oxidative stress. Br J Nutr. 2012;107(2):202–210. doi: 10.1017/S0007114511002753. [DOI] [PubMed] [Google Scholar]
- Gómez-Zorita S, Fernández-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition. 2013;29(11–12):1374–1380. doi: 10.1016/j.nut.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Henning SM, Wang P, Carpenter CL, Heber D. Epigenetic effects of green tea polyphenols in cancer. Epigenomics. 2013;5(6):729–741. doi: 10.2217/epi.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Wu H, Feinberg AP. A species-generalized probabilistic model-based definition of CpG islands. Mamm Genome. 2009;20(9–10):674–680. doi: 10.1007/s00335-009-9222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Wang Q, Yu Y, Zhao F, Huang P, Zeng R, Qi RZ, Li W, Liu Y. Leptin contributes to the adaptive responses of mice to high-fat diet intake through suppressing the lipogenic pathway. PLoS ONE. 2009;4(9):e6884. doi: 10.1371/journal.pone.0006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293(5532):1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Kabir M, Rizkalla SW, Quignard-Boulangé A, Guerre-Millo M, Boillot J, Ardouin B, Luo J, Slama G. A high glycemic index starch diet affects lipid storage-related enzymes in normal and to a lesser extent in diabetic rats. J Nutr. 1998;128(11):1878–1883. doi: 10.1093/jn/128.11.1878. [DOI] [PubMed] [Google Scholar]
- Kapetanovic IM, Muzzio M, Huang Z, Thompson TN, McCormick DL. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother Pharmacol. 2011;68(3):593–601. doi: 10.1007/s00280-010-1525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Freake HC. High carbohydrate diet and starvation regulate lipogenic mRNA in rats in a tissue-specific manner. J Nutr. 1996;126(3):611–617. doi: 10.1093/jn/126.3.611. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kang MJ, Choi HN, Jeong SM, Lee YM, Kim JI. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr Res Pract. 2011;5(2):107–111. doi: 10.4162/nrp.2011.5.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31(2):89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Lasa A, Schweiger M, Kotzbeck P, Churruca I, Simón E, Zechner R, Portillo MP. Resveratrol regulates lipolysis via adipose triglyceride lipase. J Nutr Biochem. 2012;23(4):379–384. doi: 10.1016/j.jnutbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Link A, Balaguer F, Goel A. Cancer chemoprevention by dietary polyphenols: promising role for epigenetics. Biochem Pharmacol. 2010;80(12):1771–1792. doi: 10.1016/j.bcp.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lomba A, Martínez JA, García-Díaz DF, Paternain L, Marti A, Campión J, Milagro FI. Weight gain induced by an isocaloric pair-fed high fat diet: a nutriepigenetic study on FASN and NDUFB6 gene promoters. Mol Genet Metab. 2010;101(2–3):273–278. doi: 10.1016/j.ymgme.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Lomba A, Milagro FI, García-Díaz DF, Marti A, Campión J, Martínez JA. Obesity induced by a pair-fed high fat sucrose diet: methylation and expression pattern of genes related to energy homeostasis. Lipids Health Dis. 2010;9:60. doi: 10.1186/1476-511X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarulla MT, Alberdi G, Gómez S, Tueros I, Bald C, Rodríguez VM, Martínez JA, Portillo MP. Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J Physiol Biochem. 2009;65(4):369–376. doi: 10.1007/BF03185932. [DOI] [PubMed] [Google Scholar]
- Martínez JA, Cordero P, Campión J, Milagro FI. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc Nutr Soc. 2012;71(2):276–283. doi: 10.1017/S0029665112000055. [DOI] [PubMed] [Google Scholar]
- Milagro FI, Campión J, García-Díaz DF, Goyenechea E, Paternain L, Martínez JA. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 2009;65(1):1–9. doi: 10.1007/BF03165964. [DOI] [PubMed] [Google Scholar]
- Miranda J, Portillo MP, Madrid JA, Arias N, Macarulla MT, Garaulet M. Effects of resveratrol on changes induced by high-fat feeding on clock genes in rats. Br J Nutr. 2013;110(8):1421–1428. doi: 10.1017/S0007114513000755. [DOI] [PubMed] [Google Scholar]
- Morgan HD, Santos F, Green K, Dean W, Reik W (2005) Epigenetic reprogramming in mammals. Hum Mol Genet 14(1):R47–R58. doi:10.1093/hmg/ddi114 [DOI] [PubMed]
- Morris KL, Namey TC, Zemel MB. Effects of dietary carbohydrate on the development of obesity in heterozygous Zucker rats. J Nutr Biochem. 2003;14(1):32–39. doi: 10.1016/S0955-2863(02)00249-8. [DOI] [PubMed] [Google Scholar]
- Park KM, Lee SH. Anti-hyperlipidemic activity of Rhynchosia nulubilis seeds pickled with brown rice vinegar in mice fed a high-fat diet. Nutr Res Pract. 2013;7(6):453–459. doi: 10.4162/nrp.2013.7.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martínez JA, Campión J. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology. 2012;96(3):249–260. doi: 10.1159/000341684. [DOI] [PubMed] [Google Scholar]
- Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77(6):1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Rolland V, Liepvre XL, Jump DB, Lavau M, Dugail I. A GC-rich region containing Sp1 and Sp1-like binding sites is a crucial regulatory motif for fatty acid synthase gene promoter activity in adipocytes. Implication In the overactivity of FAS promoter in obese Zucker rats. J Biol Chem. 1996;271(35):21297–21302. doi: 10.1074/jbc.271.35.21297. [DOI] [PubMed] [Google Scholar]
- Saha K, Hornyak TJ, Eckert RL. Epigenetic cancer prevention mechanisms in skin cancer. AAPS J. 2013;15(4):1064–1071. doi: 10.1208/s12248-013-9513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supic G, Jagodic M, Magic Z. Epigenetics: a new link between nutrition and cancer. Nutr Cancer. 2013;65(6):781–792. doi: 10.1080/01635581.2013.805794. [DOI] [PubMed] [Google Scholar]
- Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635(1–3):1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- Takai D, Gonzales FA, Tsai YC, Thayer MJ, Jones PA. Large scale mapping of methylcytosines in CTCF-binding sites in the human H19 promoter and aberrant hypomethylation in human bladder cancer. Hum Mol Genet. 2001;10(23):2619–2626. doi: 10.1093/hmg/10.23.2619. [DOI] [PubMed] [Google Scholar]
- Uriarte G, Paternain L, Milagro FI, Martínez JA, Campion J. Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J Physiol Biochem. 2013;69(3):601–611. doi: 10.1007/s13105-012-0231-6. [DOI] [PubMed] [Google Scholar]
- Vaulont S, Vasseur-Cognet M, Kahn A. Glucose regulation of gene transcription. J Biol Chem. 2000;275(41):31555–31558. doi: 10.1074/jbc.R000016200. [DOI] [PubMed] [Google Scholar]
- Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos. 2006;34(10):1786–1792. doi: 10.1124/dmd.106.011122. [DOI] [PubMed] [Google Scholar]
- Yang ZH, Miyahara H, Takeo J, Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol Metab Syndr. 2012;4(1):32. doi: 10.1186/1758-5996-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.