Abstract
Background
Given their high rates of uncontrolled blood pressure, urban African Americans comprise a particularly vulnerable subgroup of persons with hypertension. Substantial evidence has demonstrated the important role of family and community support in improving patients’ management of a variety of chronic illnesses. However, studies of multilevel interventions designed specifically to improve urban African American patients’ blood pressure self-management by simultaneously leveraging patient, family, and community strengths are lacking.
Methods/Design
We report the protocol of the Achieving Blood Pressure Control Together (ACT) study, a randomized controlled trial designed to study the effectiveness of interventions that engage patient, family, and community-level resources to facilitate urban African American hypertensive patients’ improved hypertension self-management and subsequent hypertension control. African American patients with uncontrolled hypertension receiving health care in an urban primary care clinic will be randomly assigned to receive 1) an educational intervention led by a community health worker alone, 2) the community health worker intervention plus a patient and family communication activation intervention, or 3) the community health worker intervention plus a problem-solving intervention. All participants enrolled in the study will receive and be trained to use a digital home blood pressure machine. The primary outcome of the randomized controlled trial will be patients’ blood pressure control at 12 months.
Discussion
Results from the ACT study will provide needed evidence on the effectiveness of comprehensive multi-level interventions to improve urban African American patients’ hypertension control.
Keywords: hypertension, self-management, community health worker
1. Introduction
Despite the availability of several efficacious pharmacologic [1] and non-pharmacologic [2] approaches to high blood pressure management, blood pressure control in the United States is inadequate, with fewer than 55% of persons undergoing treatment having adequate blood pressure control [3]. African American adults represent a particularly vulnerable subgroup of persons with hypertension, as they are more likely to have hypertension than Whites and equally as likely to be aware of and treated for hypertension but less likely to achieve blood pressure control while receiving treatment [3, 4]. African Americans are also more likely to suffer end organ damage as a result of hypertension, including up to four-fold greater rates of kidney failure [5]. Mechanisms for lower rates of treatment adherence for hypertension control among African Americans have not been completely elucidated, but may be due to differential rates of health insurance coverage, less access to health care and resources needed to care for hypertension, and attitudinal differences [6, 7].
Hypertension self-management behaviors including blood pressure self-monitoring, lifestyle changes (e.g., eating and exercise habits), adherence to medications, and shared medical decision-making (i.e. patients playing an active role in decisions about hypertension care with physicians) represent a cornerstone of recommended care for hypertension and have been associated with significant improvements in hypertension control among treated patients [1, 8–12]. Evidence suggests, however, that patients perform self-management behaviors variably (with as few as 50% persistently adhering to prescribed therapies) and that interventions designed to improve self-management behaviors may have variable effectiveness [13]. Further, hypertensive African Americans demonstrate lower adherence to self-management behaviors than their White counterparts [14–16] and may possess an inaccurate or incomplete understanding of hypertension, the process and goals of hypertension care, and how to perform hypertension self-management [17–19]. Moreover, recent research indicates that some African Americans may lack problem-solving skills to overcome barriers or problems they confront when performing hypertension self-management [20–23].
Evidence suggests interventions likely to improve hypertension self-management behaviors among African Americans are those targeted at multiple levels, including the patients themselves as well as patients’ immediate and extended social networks [16, 24, 25–27]. Additionally, a prior study employing community health workers (CHWs) to provide support and encouragement for patients’ self-management behaviors (e.g., teaching family members how to assist patients’ appointment keeping) found that this strategy facilitated significant improvements in blood pressure control [28, 29]. Culturally sensitive patient-centered interventions that specifically address barriers to hypertension self-management among African Americans and harness strengths of African American patients as well as their immediate and extended social networks to improve self-management behaviors are needed.
We hypothesize that interventions designed to support urban African American patients’ hypertension self-management by simultaneously leveraging patient, family, and community-level strengths will improve patients’ hypertension control.
2. Materials and Methods
2.1 Study Overview
The Achieving Blood Pressure Control Together (ACT) study is a single center randomized controlled trial sponsored by the National Heart, Lung, and Blood Institute of the National Institutes of Health. Utilizing the principles of community-based participatory research and implementation science, we developed three culturally sensitive educational and behavioral interventions that leverage patient, family, and community resources to support patients’ engagement in hypertension self-management behaviors. We engaged community stakeholders throughout all phases of the study design. While developing the grant proposal, we met with community members with hypertension as well as with experience providing hypertension education to East Baltimore community members. During these meetings, we obtained community members’ input regarding the cultural relevance of the intervention and potential usefulness of the proposed interventions for urban African Americans with uncontrolled hypertension in East Baltimore, Maryland. After the grant was funded, we engaged individual community stakeholders and members of our advisory board on a continuing basis through bi-weekly research meetings and quarterly advisory board meetings. We also conducted focus groups of African American hypertensive patients receiving care at the clinical practice site where the trial will be conducted and patients’ family members to elucidate intervention features to address personal, clinical, and community barriers to hypertension control. We also performed directed interviews with the clinical practice medical director, the practice administrator, clinic physicians, nurses, and staff, and representatives from the clinic’s predominant health care payer to gain their perspectives on components of the intervention that might lead to long term sustainability [30, 31].
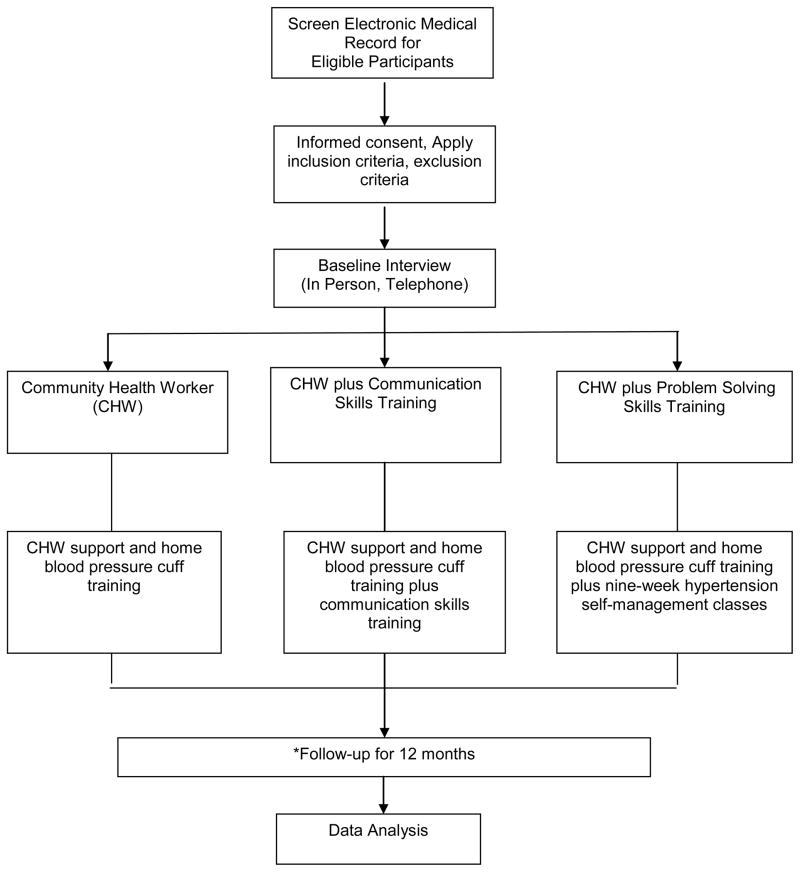
The primary aim of the study is to test the effectiveness of these three behavioral self-management interventions to facilitate urban African American hypertensive patients’ self-management behaviors and ultimate hypertension control (Figure 1). The Johns Hopkins School of Medicine Institutional Review Board has approved all study procedures [Protocol #NA-00078591]. The study is registered with clinicaltrials.gov [NCT01902719].
Figure 1. Overview of Study Design and Randomized Controlled Trial.
*Follow-up assessments at 4 months and 12 months.
2.2 Conceptual Framework
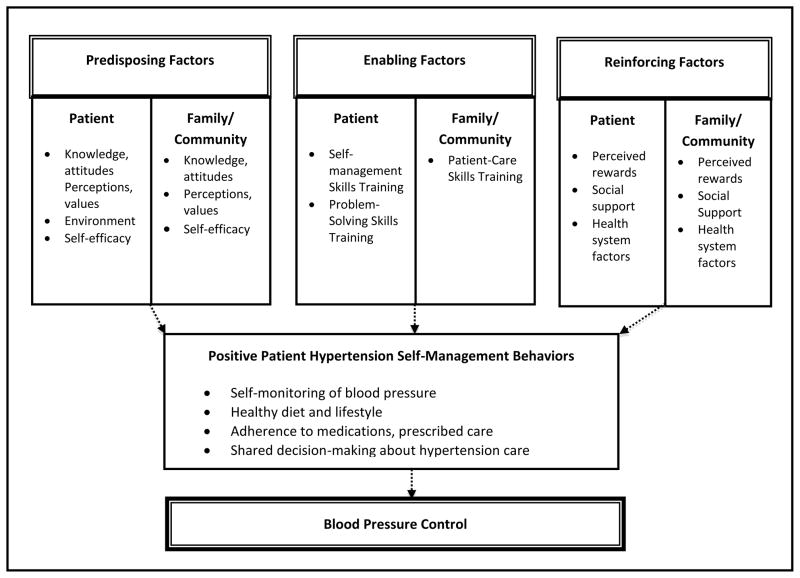
The study’s conceptual framework incorporates key aspects of Social Cognitive Behavioral Theory and the PRECEDE-PROCEED framework, in which individual, social, and environmental factors influence health behaviors and health outcomes [32,33] (Figure 2). In the framework, patients’ hypertension self-management behaviors (i.e. self-monitoring of blood pressure, adherence to healthy diet and lifestyle, adherence to medications and other prescribed care, and their involvement in shared decision-making) act as key mediators of their blood pressure control and are determined by patient, family, and community-level factors. Incorporating the concept of “Reciprocal Determinism”- in which mechanisms for patient behavior change are viewed as dependent on both intrapersonal (patient) and interpersonal (family and community) constructs that continually influence each other- the framework depicts patient-level and family/community-level factors as simultaneous and interdependent determinants of patients’ hypertension self-management behaviors [32].
Figure 2.
Study Conceptual Framework
Determinants of patient hypertension self-management behaviors may be categorized into predisposing, enabling, and reinforcing factors, which mediate patients’ successful enactment of hypertension self-care behaviors [34]. Within the context of this study, Predisposing Factors are antecedents to behavior and provide the rationale for patients’ motivation toward hypertension self-management behaviors, and include: (1) patients’ knowledge and attitudes regarding self-management behaviors, patients’ perceptions of the value of hypertension self-management, and their self-efficacy (i.e. confidence in performing and overcoming barriers) with regard to performing self-management; and (2) family and community knowledge and attitudes regarding self-management behaviors, family perceptions of the value of patients’ self-management behaviors, and family and community capacity and resources to support patients’ hypertension self-management behaviors. Enabling Factors are antecedents to behavior that allow a motivation to be realized directly or indirectly through an environmental factor, and include: (1) the provision of skills training to enhance patients’ performance of hypertension self-management behaviors; and (2) the provision of skills training to enhance family members’ ability to support patients’ successful enactment of hypertension self-management behaviors. Reinforcing Factors are factors that follow a behavior and provide continuing reward or incentive for the persistence or repetition of the behavior, and include: (1) patients’ perceived rewards for continuing hypertension self-management behaviors, social support from others for continuing hypertension self-management behaviors, and vicarious reinforcement of self-management behaviors through interactions with the health care system, and (2) family members’ perceived rewards for their continuing role in supporting patient self-management behaviors through social interactions with patients and through their role in supporting patients’ self-management through interactions with the health care system.
2.3 Study Design
Study Population
A total of 336 patient participants will be recruited for this study. Potentially eligible patient participants will include English-speaking individuals aged 18 years or older, who are currently receiving primary care at a Johns Hopkins Community Physicians urban clinic located in Baltimore, MD. Potentially eligible participants must also self-identify as African American and have a diagnosis of uncontrolled hypertension. We will define uncontrolled hypertension as two measures of systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg (irrespective of patients’ use of antihypertensive medications) obtained at the clinic over a 6 month period prior to screening and recruitment. We will exclude persons from participating if they are pregnant. We will measure potential participants’ cognitive function, but we will not exclude potential participants with low cognitive function.
Primary care physicians from the clinical site primary care practice who care for patients enrolled in the study will also be recruited for the study.
Study Interventions
Community Health Worker (CHW) Intervention
The CHW intervention was designed to enhance patients’ hypertension self-management behaviors by (1) providing patients with home blood pressure monitors and training in how to operate them; (2) teaching patients about lifestyle behaviors that improve hypertension management; (3) acting as a liaison between patients and their primary care clinic (e.g., assisting with transportation to a medical visit or helping patients overcome other personal or environmental barriers to care); and (4) engaging in longitudinal behavioral support for patients with telephone calls and home visits throughout the study, including contacts on an ad-hoc basis as initiated by the participants (Table 1). CHWs will also interact with study participants (via telephone, at the clinic site, or participants’ residence or community) during pre-specified study follow-up episodes and on an ad-hoc basis. Community health workers receive standardized training to include “With Every Heartbeat Is Life: A Community Health Worker’s Manual for African Americans,” a culturally sensitive cardiovascular disease training manual created especially for African American communities by the National Heart, Lung, and Blood Institute (NHLBI) [35].
Table 1.
Objectives for Community Health Worker Intervention
| Objective | Description |
|---|---|
| Provide Training and Reinforcement on the Use of Home Blood Pressure Cuff |
|
| Serve as Liaison to Clinical and Non-clinical Services |
|
| Provide Encouragement and Support |
|
Do My Part – Patient and Family Communication Skills Training
The Do My PART intervention is designed to enhance active patient engagement in the medical visit dialogue and encourage shared decision-making in regard to treatment and self-management. Because many chronic disease patients, particularly those most vulnerable in terms of poor health, low literacy and advanced age, are routinely accompanied to their medical visits by a family member or friend (termed ‘companions’ from here on), the Do My PART training was designed so that it could be adapted for use with accompanied or unaccompanied study patients [36]. The intervention is administered by a trained interventionist in a private area adjacent to the clinic waiting area immediately prior to the patient’s first study clinic visit and takes approximately 30 minutes for an accompanied patient and 20 minutes for an unaccompanied patient. Both patients and companions, when present, are instructed by the interventionist in four skill sets represented in the mnemonic “PART”: (P) prepare for their visits; (A) act during their visits; (R) review key recommendations; and (T) take recommendations home (Table 2). Companions, when present, will review skills to assist patients by facilitating their understanding of physician explanations and instructions, facilitate physician understanding of patient concerns and preferences, and facilitate active patient involvement in the medical dialogue and shared decision-making in regard to treatment and self-management behaviors. Delivery of the Do My PART intervention will be audiotaped for later analysis to assess treatment fidelity.
Table 2.
DO MY PART communication skills training
| Skill Area | Patient Objectives | Companion Objectives (when present) |
|---|---|---|
| P – Prepare for your Visit | While waiting to see the doctor, think about any problems, symptoms or questions that have come up since your last visit. Consider what you want to get out of today’s visit. | While waiting to see the doctor, discuss any problems, symptoms or questions that have come up since the patient’s last medical visit. Consider together what you both want to get out of today’s visit. |
| A – Act During the Visit | Talk with your doctor about anything that may be worrying you. | Assist the patient in assuring all concerns are addressed by clarifying or expanding medical history and introducing relevant medical topics when warranted. |
| Let your doctor know if you don’t understand something. | Assist the patient in understanding the doctor’s explanations by repeating terms in plain language when warranted. | |
| Ask questions. | Assist the patient by asking questions or remind the patient of questions identified during the visit preparation when warranted. | |
| Indicate treatment preferences. | Assist the patient by asking directly for their opinion and treatment preferences. | |
| Clarify instructions by using teach back and summarization. | Assist the patient by clarify instructions by using teach back and summarization, when warranted | |
| Identify barriers to self-management and brainstorm ways to address these challenges. | Assist the patient by identifying barriers to self-management and brainstorm ways to address these, when warranted. | |
| R – Review Your Treatment Plan | Write down instructions and new information about your treatment plan given during the visit. This will help you remember what to do when you get home | Assist the patient by taking notes during the visit especially noting new information about the treatment plan. |
| T – Take it Home | Right after your visit, make a “To- Do” list to take home. | Assist the patient after the visit, by working together to make a “To-Do” list to take home. |
Problem-Solving Training Intervention
The Problem-Solving intervention was adapted from a previously developed, tested, and validated self-management intervention for urban African Americans patients with diabetes mellitus [20, 37]. In our prior work, we studied problem solving intervention materials among patients with diabetes with low and average literacy as well as persons with mild to moderate visual and cognitive impairments and found them to be suitable for use among these populations [38, 39].
The intervention trains patients to improve their hypertension self-management by employing skills to overcome their self-identified barriers to self-management behaviors. Participants will learn about behavioral goals for monitoring their blood pressure and making effective diet and lifestyle modifications for blood pressure control. Participants will perform several learning exercises designed to equip them with skills to manage the self-identified barriers they encounter in adhering to hypertension self-care. The intervention will take place over nine consecutive weeks in two-hour group-based sessions led by a trained interventionist (Table 3). We will audiotape each of the group-based sessions for analysis of treatment adherence. Interventionists with a background and previous training in health education, psychology, or social work will receive 25 hours of training specific to the intervention and problem-solving therapy [40, 41].
Table 3.
Objectives of Problem-Solving Sessions
| Session Number | Session Title | Description |
|---|---|---|
| 1 | Overview of Hypertension |
|
| 2 | Overview of Problem Solving |
|
| 3 | “Taking control of stress and emotions” |
|
| 4 | “What makes a problem a problem” |
|
| 5 | “Know thyself: Set goals that fit your life” |
|
| 6 | “Different ways to reach your health goals: knowing your options” |
|
| 7 | “That sounds good, but does it work for me?” |
|
| 8 | “Take action and know the signs” |
|
| 9 | “Putting it all together” |
|
All written materials provided to participants for each intervention are tailored to meet a fourth to sixth grade reading level to accommodate participants with low health literacy.
Participant Recruitment
Patient Participants
Patient participants will be recruited from the clinic practice between September 2013 and September 2014. Using clinical records obtained from the electronic medical record (EMR), we will identify potentially eligible patients seen in the primary care clinic during the previous 6 months. Our preliminary exploration of clinic records indicated a total of 3,074 hypertensive patients in the clinic, of whom 2,934 (95%) were African American. Approximately 41% of these African American patients had uncontrolled hypertension (N=1,212), providing an adequate sample from which we will recruit potential patient participants. Throughout the study, we will prospectively screen the EMR every two months to identify potentially eligible patients receiving care at the clinic using the previously mentioned criteria. We have obtained a HIPAA waiver to screen the EMR for potentially eligible participants. We will mail all potentially eligible participants a letter from “our partner” primary care physician group advising them that they are eligible to participate in the study along with a letter from the study principal investigator with a brief summary of the study eligibility criteria, objectives, what they would be asked to do as a study participant, and information on how to contact the study recruiters by telephone. Letters include a self-addressed stamped envelope, a standard refusal form allowing potential participants to decline participation, and a study brochure. We provide potentially eligible participants ten days to refuse to participate either by mail or phone. After the ten day waiting period, trained recruiters attempt to contact all potential participants by telephone to assess their willingness to participate in the study and complete eligibility screening and a baseline telephone questionnaire using a standardized telephone script and oral consent process. During pre-testing, we deemed the informed consent process for the entire study too lengthy and complex to be performed adequately over the telephone. Potential participants therefore provide consent in a two-phase procedure in which consent is first obtained with an oral consent process using a standardized telephone script to complete the baseline telephone questionnaire. During this oral consent, a general overview of all study procedures is reviewed. After completion of the baseline telephone questionnaire, consented participants are then invited to attend a clinic enrollment visit and a home visit to occur within 14–21 days of telephone recruitment. At the time of the clinic enrollment visit, participants are asked to provide written consent for the clinic enrollment procedures, the study interventions, and all study follow-up assessments.
Companion Participants
Patient participants randomized to receive the Do My PART intervention will be asked if they have a single companion or multiple companions who regularly accompany them to their doctor visits with their primary care physician at the clinical site and if they think their companion would be interested in participating in the study. If so, the patient is provided with a study brochure to be given to their companion(s) describing the objectives of the study, what companion participants will be required to do if they participate, and contact information. To be eligible, companions must routinely accompany the patient to their medical visits, be at least 18 years of age and be English-speaking. To participate, a companion must be present at the patient’s clinic visit, at which time the staff will establish eligibility, obtain written consent and include them in the Do My PART intervention. When a companion is not identified, the participant is trained on his or her own.
Primary Care Physician Participants
Eight physicians providing primary care at the clinical site for patients enrolled in the ACT study will also be enrolled to assess their communication and shared decision-making about hypertension via audio recordings of all clinic visits of study patients throughout the study as well as a brief post-visit questionnaire administered after each visit. Physician providers must be English-speaking and identify themselves as a care provider for a patient enrolled in the study. We will obtain written consent of clinical site primary care providers.
Screening and randomization
Patient participants must complete both a clinic visit and a home visit to be enrolled in the study. At the time of the clinic visit, trained study staff obtains written consent for enrollment in the study. Using blind and secure allocation by computer, we randomly assign enrolled patient participants in a ratio of 1:1:1 to one of three arms: (1) CHW intervention alone, (2) CHW intervention plus the ACT Do My PART intervention, and (3) CHW intervention plus the nine-week ACT problem-solving intervention.
2.4 Measures
All patient participants enrolled in the ACT study are assessed using a structured telephone interview and an in-person questionnaire prior to randomization. Trained study staff will also administer a structured telephone interview comprising instruments with known properties of reliability and validity at 4 months and twelve 12 months (Table 4). We have pre-tested interviews to assess their length (60 minutes for the baseline telephone interview, 50 minutes for the in-home enrollment interview, and 60 minutes for follow-up interviews) and feasible completion by participants. During administration, all questions and responses are read to participants. If participants indicate they are not able to complete interviews during a single sitting, interviewers will offer alternative dates and times for completing the interviews.
Table 4.
Study Assessments
| Baseline | 4 Months | 12 Months | |
|---|---|---|---|
| Primary Outcomes | |||
| Change in systolic and diastolic blood pressure | C | C | C |
| Mediators of Hypertension Control | |||
| Hypertension self-care (High Blood Pressure Self-Care Profile)44 | T | T | T |
| Medication adherence (Morisky Scale)45 | T | T | T |
| Patient engagement in shared decision-making46 | T | T | T |
| Perceived usefulness of CHW (CHW Evaluation Questionnaire)47 | T | T | T |
| Cultural competence of primary care providers (CAHPS Cultural Competence Item Set)48 | T | ||
| Health literacy practices at the clinic (CAHPS Item Set for Addressing Health Literacy)49 | T | ||
| Patient centered medical home (CAHPS Patient Centered Medical Home)50 | T | T | T |
| Community Health Worker Communication | A* | A* | A* |
| Provider Communication | A* | A* | A* |
| Biomedical Correlates | |||
| Hyperlipidemia51 | L | L | L |
| Glycemic control52 | L | L | L |
| eGFR53 | L | L | L |
| Microalbumin54 | L | L | L |
| Body mass index | C | C | C |
| Co-morbidities (Charlson Co-Morbidity Index)55 | I | ||
| Physical function (PROMIS Physical Function) 56 | I | ||
| Physical participation (PROMIS Ability to Participate in Social Roles and Activities)57 | I | ||
| Tobacco or cigarette use (National Health Interview Survey)58 | I | T | |
| Cigar use (National Health Interview Survey)58 | I | T | |
| Alcohol use (CAGE)59,60 | T | T | |
| Drug use (Single Question Screening Test)61 | T | T | |
| Physical activity (National Health and Nutrition Exam Survey)62 | I | T | T |
| Diet (Block Fruit-Vegetable-Fiber Screener)63 | I | T | T |
| Diet (Block Dietary Fat Screener)64 | I | T | T |
| Core healthy days (Behavioral Risk Factor Surveillance System)65 | T | T | T |
| Awareness/Understanding of progression to CKD | I | T | T |
| Knowledge of kidney problem/CKD (Kidney Knowledge Survey)66 | I | ||
| Psychological Correlates | |||
| Decision self-efficacy (Decision Self Efficacy Scale)67–69 | T | T | T |
| Problem-focused coping (Health Problem Solving Scale)70,71 | T | T | T |
| Patient activation (Patient Activation Measure)72 | T | T | T |
| Depression (PHQ-8)73 | T | T | T |
| Perceived stress (Perceived Stress Scale)74 | T | T | T |
| Resilience (Resilience Scale)75,76 | T | ||
| History of traumatic events (List of Threatening Experiences-Questionnaire)77 | T | ||
| Environmental Correlates | |||
| Food Security (USDA Food Security Questionnaire)78 | I | ||
| Survey of food and appliances in the home | I | ||
| Presence of a medical companion | I | ||
| Current in-home outreach efforts from clinic (NHIS)58 | I | ||
| Residential mobility | I | ||
| Neighborhood cohesion79,80 | I | ||
| Neighborhood health (Neighborhood Health Questionnaire)81 | T | T | |
| Social and Demographic Correlates | |||
| Age, Gender, Ethnicity/Race, Employment, Insurance status, Income, and Wealth | T | ||
| Discrimination (Everyday Discrimination Scale)82–84 | T | T | |
| Social status (MacArthur Scale of Subjective Social Status)85 | I | ||
| Social support (PROMIS Social Functioning Scale)86,87 | I | ||
| Family functioning (Family APGAR Scale)88 | I | ||
| Health literacy (Newest Vital Sign)89–91 | I | ||
| Health numeracy (Subjective Numeracy Scale)92,93 | I | ||
| Cognitive Function (Telephone Interview Cognition Scale-Modified)94,95 | I | ||
| Information Technology Usage (NHIS)96 | I | ||
| Literacy (Wide Range Achievement Test, 4th edition)97 | I | ||
| Inventory of egocentric social network including attributes and perceived health behaviors of network members | I |
Key: C = Clinical Measure; T = Questionnaire Administered via Telephone; L=Laboratory Measure; I = Questionnaire Administered In-person; A=Audiorecordings; CKD = Chronic Kidney Disease; CHW = Community Health Worker; eGFR = Estimated Glomerular Filtration Rate
Audio recordings obtained throughout baseline and follow-up periods (all patient participant-CHW telephone interactions and all patient participant-physician visits audiorecorded)
Primary Outcome
The primary outcome of interest is patient participants’ hypertension control during study follow-up. This outcome is based on guidelines from the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) [9]. The Report from the Panel Members Appointment to the Eighth Joint National Committee (JNC-8) [42] recommendations is being released during the year of our study launch. To account for potential changes in clinical practice which may be reflected by the JNC 8 recommendations, we will perform secondary analyses with BP control defined according to JNC 8 recommendations, which will specify less stringent BP management goals (i.e., SBP <150 and DBP <90 for participants over the age of 60 years and SBP <140 and DBP <90 for participants with diabetes or chronic kidney disease).
Blood pressure is measured by trained and certified observers during regularly scheduled medical visits at the clinic using an automatic oscillometric monitor (Omron HEM 907-XL). We will use two measures: (1) the average of all three measurements and (2) the average of the last two measurements, obtained at baseline, 4-months, and 12-month follow-up. Blood pressure control is dichotomized as uncontrolled SBP ≥140mmHg or DBP ≥90mmHg; SBP ≥130mmHg or DB P≥80 mmHg for patients with diabetes, chronic kidney disease or coronary heart disease) or controlled (SBP <140mmHg and DBP<90mmHg; SBP <130mmHg and DB P<80 mmHg for patients with diabetes, chronic kidney disease or coronary heart disease).
Mediators of Hypertension Control
We will assess patient participants’ performance of self-management behaviors including their self-monitoring of blood pressure, self-reported treatment compliance (i.e., reduced sodium intake and appointment keeping), and medication adherence throughout the study via self-reported measures and administrative clinical records at baseline, 4 months, and 12 months follow-up.
We will provide all patient participants enrolled in the study with an Omron BP791IT (arm circumference 17.0–42.0 cm) or Life Source UA-789PC (arm circumference 42.1–50 cm) digital home blood pressure cuff and instruct participants to record their blood pressure measurements according to the guidelines set forth by the American Heart Association [43]. Participants will be directed to bring the home blood pressure cuff with them to their clinic visits and study staff will download stored measures into the study database prior to the visit and create a report for patient participants to share with their primary care provider during the clinic visit. Participants will also complete a hypertension self-care instrument, designed specifically for patients with high blood pressure, to assess their current health behaviors and confidence in following treatment recommendations for hypertension [44]. Medication adherence will be assessed via a medication diary and self-reported measures [45].
We will audio record all clinical encounters between enrolled patients and their primary care provider during the study period to assess patient engagement in shared medical decision-making. We will also audio record encounters between CHW and patient participants. Audio recordings will be analyzed using the Roter Interaction Analysis System (RIAS), a measure for assessing provider-patient communication during face-to-face consultation [46]. RIAS will be used to describe and categorize communication behaviors and quantify communication events between patient participants and their primary care provider.
Patient participants will have the opportunity to assess the perceived usefulness of the CHW by answering questions about the benefits and disadvantages of interacting with the CHW, quality of CHW services, communication with the CHW, and overall satisfaction of the role of the CHW throughout the study [47].
Using questions adapted from the Consumer Assessment of Healthcare Providers and Systems (CAHPS) program, we will collect self-reported patient assessment of their primary care provider’s cultural competence [48], communication and health literacy [49] at baseline, and attributes of patient-centered medical home [50] at baseline as well as at 4 and 12 months follow-up.
Biomedical Correlates
Patient participants will have blood and spot urine collected at the clinic during scheduled study visits. When patient participants are not able to have measures obtained in the clinic, we will obtain them during home visits. We will obtain samples at baseline, 4 months, and 12 months. We will assess the presence of hyperlipidemia (e.g., fasting serum total cholesterol, low-density lipoprotein, high-density lipoprotein, and triglycerides) based on previously published guidelines [51], glycemic control (i.e., fasting serum glucose and serum hemoglobin A1c for patient participants previously diagnosed with diabetes --new onset of diabetes will be defined as a fasting random glucose of 126 mg/dL or above for patients without a previous diagnosis, and abnormal glycemic control defined as hemoglobin A1c ≥7% for those with an established diagnosis) [52], chronic kidney disease (CKD) (i.e., calculation of eGFR using the CKD-epidemiology collaboration equation) [53] and will assess the presence of kidney damage by obtaining urine microalbumin levels [54]. Trained study staff will also assess patient participants’ height and weight during clinic study visits at baseline, 4 months, and 12 months of follow-up and use them to calculate participants’ body mass index as their weight in kilograms divided by height in meters squared.
We will also assess other measures, including patient participants’ medical comorbidities [55], self-reported physical function and ability to participate in social roles [56, 57], health behaviors of tobacco or cigarette use [58], alcohol use [59, 60], drug use [61], physical activity [62], diet [63, 64], core health days [65], awareness or understanding of progression to CKD, and knowledge of kidney disease [66].
Psychological Correlates
We will assess patient participants’ decision self-efficacy [67–69], problem-solving skills [70, 71], patient activation [72], depression [73], perceived stress [74], resilience [75, 76], and history of traumatic events [77] via telephone questionnaire at baseline, 4 months and 12 months follow-up.
Environmental Correlates
We will assess patient participants’ food security [78], food and appliances in the home, current in-home outreach efforts from health workers [58], residential mobility, neighborhood cohesion [79, 80], and neighborhood health [81] at baseline and 12 month follow-up.
Social and Demographic Correlates
We will assess patient participants’ sociodemographic characteristics (e.g., self-reported age, gender, ethnicity/race, educational attainment, occupation, income class, wealth, and health insurance status), experiences of discrimination [82–84], social status [85], social support [86, 87], family functioning [88], health literacy [89–91], health numeracy [92–93], cognitive function [94, 95], internet technology use [96], literacy [97], and social networks at baseline.
2.5 Statistics
Sample Size
We hypothesize the CHW plus Do My PART and CHW plus problem-solving interventions will improve hypertension control to a greater extent than the CHW intervention alone. We also hypothesize the CHW plus problem-solving intervention will improve hypertension control to a greater extent than the CHW plus Do My PART intervention. Our sample size estimates are based on data from a previous randomized controlled trial (Triple P study) testing the effect of physician and CHW interventions to improve shared decision-making in hypertension care [98] as well as preliminary data from a pilot study of a self-management and problem-solving intervention implemented for African Americans with diabetes (Project DECIDE) [20]. In Triple P, which did not directly address patients’ performance of self-management skills or problem solving, patients receiving the physician and CHW interventions experienced a 10 to 15% improvement in blood pressure control compared to participants receiving a less intensive intervention [98]. Other interventions consisting of self-monitoring blood pressure and patient education have reported similar findings [99]. In Project DECIDE, participants receiving a problem-solving intervention experienced a 50% improvement in blood pressure control [20]. Our own prior systematic review of patient-centered behavioral interventions for hypertension revealed that patient-centered interventions combining multiple components such as education and behavioral strategies yield improved blood pressure outcomes over singular interventions [100, 101]. We therefore make the conservative estimate that at 12 month follow-up, 50% of participants receiving the CHW intervention will experience blood pressure control. If the Do My PART and problem-solving interventions have additive effects above and beyond the CHW intervention, we will have 98% power to detect a trend in which at least 75% and 80% achieve blood pressure control in these two arms, respectively, while accounting for 20% participant attrition and multiple comparisons of these arms with the CHW arm. We further estimate with 20% attrition over 12 months, we will have 80% power to detect these differences across study arms. We have also estimated that we will be able to detect a 12mmHg difference in SBP between arms given our planned sample size.
Randomization
We will use a variable-block sized non-stratified randomization with an allocation ratio of 1:1:1. A study statistician constructed the randomization before the start of the trial.
Statistical Analysis
We will test the adequacy of randomization by means of comparing baseline characteristics of patient participants randomized to each group, including gender, educational attainment, and income, as well as by utilizing comparative statistics (ANOVA or Kruskal-Wallis test) to detect differences in groups possibly attributable to inadequacies of randomization. Random assignment of patients into their originally assigned group (i.e., CHW intervention, CHW plus Do My PART, or CHW plus problem-solving intervention) will be the main independent variable for intent-to-treat analyses. The general approach for intent to treat analyses is to employ highly flexible core statistical models for the intervention effect on BP measure and BP control outcomes. These core models are valid if data are missing at random (MAR) and will be implemented through mixed effects generalized linear modeling approach to take full advantage of the repeated outcome assessments at randomization, 4 months, and 12 months follow-up visits. We will conduct sensitivity analyses to assess the potential impacts on inferences in scenarios where the missing data patterns deviate from the MAR process. We will also carry out secondary, on treatment analyses and analyses examining potential mediating factors using standard analytic approaches for longitudinal observational studies.
Sustainability
Once the ACT study has concluded, we will assess the intervention’s potential for long-term sustainability by presenting study findings and obtaining qualitative feedback from patients, community members, clinic stakeholders (staff, clinicians, administrators), and payers. We will also assess costs incurred as part of the interventions. If interventions are consistently deemed potentially sustainable, we will work with translation and dissemination researchers, community stakeholders, clinic leaders, and payers to develop strategies for interventions’ long-term implementation. The study clinic currently employs medical staff, CHWs, and behaviorally trained social workers that are adequately trained to carry out the interventions if they are found to be effective. Numerous activities performed during the intervention development phase (including meeting with clinic administrators, health care providers, and staff as well as shadowing clinic staff) have contributed to our development of highly pragmatic training protocols and workflows that minimize intervention impact on daily clinic activities [31]. If effective, we will provide refined training protocols and workflows to clinic staff and clinical administrators so that protocols can be readily implemented in the clinic as they were implemented during the study.
3. Conclusions
Patient-centered approaches to improve hypertension self-management are acknowledged as an effective mechanism through which improved adherence to patient self-management can be achieved [27]. Published systematic reviews of studies testing interventions to improve hypertension self-management adherence, including interventions employing patient education, patient support strategies (i.e. social support from a health care professional or a family member), and encouraging greater patient involvement in self-management, suggest such approaches should be multifaceted, targeting patients’ self-management through several mechanisms simultaneously, including the broader social and environmental context [27, 100–103]. Despite recommendations and prior evidence, studies of multifaceted patient-centered behavioral interventions specifically addressing African Americans’ self-identified barriers or facilitators to carrying out hypertension self-management, as well as barriers or facilitators identified in patients’ immediate (i.e. families/friends) and extended social networks (i.e. communities) are lacking.
We designed ACT study interventions with the objective of designing and rigorously testing the effectiveness of interventions that simultaneously engage patient, family, and community-level strengths to facilitate urban African American hypertensive patients’ self-management behaviors. Interventions have been previously demonstrated to have efficacy, but were designed specifically in ACT to be practical and to be readily implemented in routine clinical practice settings serving urban African Americans. If effective, interventions may add tremendous value to current efforts to improve hypertension care among urban African Americans in at least four ways. First, in addition to benefitting patients’ immediate and long-term health, identifying mechanisms through which urban African American patients with uncontrolled blood pressure can achieve blood pressure control will help clinicians and other health care providers (e.g. health care insurers) seeking adjunctive non-pharmacological approaches to enhance the effectiveness of prescribed therapies among a particularly high-risk group of patients. Second, interventions leveraging patients’ family and community resources to help them overcome barriers to hypertension self-management may be more powerful and more sustainable than other interventions that do not leverage these resources. Third, interventions engaging patients’ families could influence family behaviors and potentially broaden the impact of the intervention. Finally, we designed the ACT study to assess, pragmatically, the effectiveness of interventions in a manner that they would be employed in real-world clinical practice settings. Rigorously evaluated interventions to enhance blood pressure control, implemented as they would be in routine clinical practice, could provide health care providers and policy makers with the confidence to endorse feasibly implemented interventions in in a variety of clinical settings and among other vulnerable populations.
Acknowledgments
The authors would like to thank everyone who contributed to the planning phase of this study, patients and their families, members of the Johns Hopkins Center to Eliminate Cardiovascular Health Disparities Community Advisory Board, and clinical practice staff, providers, and administrators.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Patti L. Ephraim, Email: pephraim@jhsph.edu.
Felicia Hill-Briggs, Email: fbriggs3@jhmi.edu.
Debra Roter, Email: droter@jhsph.edu.
Lee Bone, Email: lbone@jhsph.edu.
Jennifer Wolff, Email: jwolff@jhsph.edu.
LaPricia Lewis-Boyer, Email: llewis3@jhmi.edu.
David Levine, Email: dlevine@jhmi.edu.
Hanan Aboumatar, Email: habouma1@jhmi.edu.
Lisa A Cooper, Email: lisa.cooper@jhmi.edu.
Stephanie Fitzpatrick, Email: stephanie_fitzpatrick@rush.edu.
Kimberly Gudzune, Email: kgudzun1@jhu.edu.
Michael Albert, Email: malber11@jhmi.edu.
Dwyan Monroe, Email: dymonroe@gmail.com.
Michelle Simmons, Email: msimmons090349@gmail.com.
Debra Hickman, Email: dhickman@sisterstogetherandreaching.org.
Leon Purnell, Email: lpurnell2003@yahoo.com.
Annette Fisher, Email: Annette.Fisher@heart.org.
Richard Matens, Email: Richard.Matens@co.union.nc.us.
Gary Noronha, Email: Gary_Noronha@URMC.Rochester.edu.
Peter Fagan, Email: pfagan1@jhmi.edu.
Hema Ramamurthi, Email: hramamu1@jhmi.edu.
Jessica Ameling, Email: jamelin1@jhmi.edu.
Jeanne Charlston, Email: jeannec@jhmi.edu.
Tanyka Sam, Email: tssam1@yahoo.com.
Kathryn A. Carson, Email: kcarson@jhmi.edu.
Nae-Yuh Wang, Email: naeyuh@jhmi.edu.
Deidra Crews, Email: dcrews1@jhmi.edu.
Raquel Greer, Email: rfcharle@jhmi.edu.
Valerie Sneed, Email: vlee2@jhmi.edu.
Sarah J. Flynn, Email: sjflynn9@gmail.com.
Nicole DePasquale, Email: ndepasqu@jhsph.edu.
L. Ebony Boulware, Email: ebony.boulware@duke.edu.
References
- 1.Wright JT, Jr, Probstfield JL, Cushman WC, et al. ALLHAT findings revisited in the context of subsequent analyses, other trials, and meta-analyses. Arch Intern Med. 2009 May 11;169(9):832–842. doi: 10.1001/archinternmed.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2006;(4):CD005182. doi: 10.1002/14651858.CD005182.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290(2):199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Giles T, Aranda JM, Jr, Suh DC, et al. Ethnic/racial variations in blood pressure awareness, treatment, and control. J Clin Hypertens (Greenwich) 2007 May;9(5):345–354. doi: 10.1111/j.1524-6175.2007.06432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.System USRD. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008. [Google Scholar]
- 6.Ogedegbe G. Barriers to optimal hypertension control. J Clin Hypertens (Greenwich) 2008 Aug;10(8):644–646. doi: 10.1111/j.1751-7176.2008.08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper LA. A 41-year-old African American man with poorly controlled hypertension: review of patient and physician factors related to hypertension treatment adherence. JAMA. 2009 Mar 25;301(12):1260–1272. doi: 10.1001/jama.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wassertheil-Smoller S, Shumaker S, Ockene J, et al. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI) Arch Intern Med. 2004;164(3):289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 10.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. J Cardiovasc Nurs. 2008 Jul-Aug;23(4):299–323. doi: 10.1097/01.JCN.0000317429.98844.04. [DOI] [PubMed] [Google Scholar]
- 11.Schoenthaler A, Chaplin WF, Allegrante JP, et al. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009 May;75(2):185–191. doi: 10.1016/j.pec.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006 Apr 4;144(7):485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 13.Juncos LI. Patient compliance and angiotensin converting enzyme inhibitors in hypertension. J Cardiovasc Pharmacol. 1990;15 (Suppl 3):S22–25. doi: 10.1097/00005344-199000153-00005. [DOI] [PubMed] [Google Scholar]
- 14.Dickson M, Plauschinat CA. Racial differences in medication compliance and healthcare utilization among hypertensive Medicaid recipients: fixed-dose vs free-combination treatment. Ethn Dis. 2008 Spring;18(2):204–209. [PubMed] [Google Scholar]
- 15.Shaya FT, Du D, Gbarayor CM, Frech-Tamas F, Lau H, Weir MR. Predictors of compliance with antihypertensive therapy in a high-risk medicaid population. J Natl Med Assoc. 2009 Jan;101(1):34–39. doi: 10.1016/s0027-9684(15)30808-7. [DOI] [PubMed] [Google Scholar]
- 16.Trivedi RB, Ayotte B, Edelman D, Bosworth HB. The association of emotional well-being and marital status with treatment adherence among patients with hypertension. J Behav Med. 2008 Dec;31(6):489–497. doi: 10.1007/s10865-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlomann P, Schmitke J. Lay beliefs about hypertension: an interpretive synthesis of the qualitative research. J Am Acad Nurse Pract. 2007 Jul;19(7):358–367. doi: 10.1111/j.1745-7599.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 18.Ayotte BJ, Trivedi R, Bosworth HB. Racial differences in hypertension knowledge: effects of differential item functioning. Ethn Dis. 2009 Winter;19(1):23–27. [PubMed] [Google Scholar]
- 19.Boutin-Foster C, Ogedegbe G, Ravenell JE, Robbins L, Charlson ME. Ascribing meaning to hypertension: a qualitative study among African Americans with uncontrolled hypertension. Ethn Dis. 2007 Winter;17(1):29–34. [PubMed] [Google Scholar]
- 20.Hill-Briggs F, Lazo M, Peyrot M, Doswell A, Chang YT, Hill MN, Levine D, Wang NY, Brancati FL. Effect of problem-solving-based diabetes self-management training on diabetes control in a low income patient sample. J Gen Intern Med. 2011 Sep;26(9):972–8. doi: 10.1007/s11606-011-1689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesley ML. Social problem solving training for African Americans: effects on dietary problem solving skill and DASH diet-related behavior change. Patient Educ Couns. 2007 Jan;65(1):137–146. doi: 10.1016/j.pec.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Whitfield KE, Allaire JC, Wiggins SA. Relationships among health factors and everyday problem solving in african americans. Health Psychol. 2004 Nov;23(6):641–644. doi: 10.1037/0278-6133.23.6.641. [DOI] [PubMed] [Google Scholar]
- 23.Whitfield KE, Wiggins S. The influence of social support and health on everyday problem solving in adult African Americans. Exp Aging Res. 2003 Jan-Mar;29(1):1–13. doi: 10.1080/03610730303703. [DOI] [PubMed] [Google Scholar]
- 24.Ogedegbe G, Harrison M, Robbins L, Mancuso CA, Allegrante JP. Barriers and facilitators of medication adherence in hypertensive African Americans: a qualitative study. Ethn Dis. 2004 Winter;14(1):3–12. [PubMed] [Google Scholar]
- 25.Morenoff JD, House JS, Hansen BB, Williams DR, Kaplan GA, Hunte HE. Understanding social disparities in hypertension prevalence, awareness, treatment, and control: the role of neighborhood context. Soc Sci Med. 2007 Nov;65(9):1853–1866. doi: 10.1016/j.socscimed.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorman BK, Sivaganesan A. The role of social support and integration for understanding socioeconomic disparities in self-rated health and hypertension. Soc Sci Med. 2007 Sep;65(5):958–975. doi: 10.1016/j.socscimed.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004 Apr 12;164(7):722–732. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 28.Levine DM, Bone LR, Hill MN, et al. The effectiveness of a community/academic health center partnership in decreasing the level of blood pressure in an urban African-American population. Ethn Dis. 2003 Summer;13(3):354–361. [PubMed] [Google Scholar]
- 29.Levine DM, Becker DM, Bone LR. Narrowing the gap in health status of minority populations: a community-academic medical center partnership. Am J Prev Med. 1992 Sep-Oct;8(5):319–323. [PubMed] [Google Scholar]
- 30.Flynn SJ, Ameling JM, Hill-Briggs F, Wolff JL, Bone LR, Levine DM, Roter DL, Lewis-Boyer L, Fisher A, Purnell L, Ephraim PL, Barbers J, Fitzpatrick SL, Albert MC, Cooper LA, Fagan PJ, Martin D, Ramamurthi HC, Boulware LE. Facilitators and barriers to hypertension self-management in urban African Americans: Perspectives of patients and family members. Patient Preference and Adherence. 2013;7:741–749. doi: 10.2147/PPA.S46517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ameling JM, Ephraim PL, Bone LR, Levine DM, Roter DL, Wolff JL, Hill-Briggs F, Fitzpatrick SL, Noronha GJ, Fagan PJ, Lewis-Boyer L, Hickman D, Simmons M, Purnell L, Fisher A, Cooper LA, Aboutamar HJ, Albert MC, Flynn SJ, Boulware LE. Adapting hypertension self-management interventions to enhance their sustained effectiveness among urban African Americans. Family and Community Health. 2013 doi: 10.1097/FCH.0000000000000020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Green LW, Levine DM, Deeds SG. Clinical trials of health education for hypertensive outpatients: design and baseline data. Preventive Medicine. 1975;4(4):417–25. doi: 10.1016/0091-7435(75)90030-4. [DOI] [PubMed] [Google Scholar]
- 33.Gielen AC, McDonald EM. In: Using the Precede-Proceed Planning Model to Apply Health Behavior Theories. Glanz K, Rimer BK, Lewis FM, editors. Vol. 3. San Francisco, CA: Jossey-Bass; 2002. pp. 409–436. [Google Scholar]
- 34.Bandura A. Social Foundations of Thought and Actions. Englewood Clifffs, NJ: Prentice Hall; 1896. [Google Scholar]
- 35.National Heart, Lung, and Blood Institute, U.S. Department of Health and Human Services (DHHS) [Accessed February 14, 2014.];With Every Heartbeat is Life: A Community Health Worker’s Manual for African Americans. https://www.nhlbi.nih.gov/health/prof/heart/other/chdblack/aa_manual.htm.
- 36.Wolff JL, Roter DL. Hidden in plain sight: Medical visit companions as a resource for vulnerable older adults. Archives of Internal Medicine. 2008;168(13):1409–1415. doi: 10.1001/archinte.168.13.1409. [DOI] [PubMed] [Google Scholar]
- 37.Hill-Briggs F, Gary TL, Yeh HC, Batts-Turner M, Powe NR, Saudek CD, Brancati FL. Association of social problem solving with glycemic control in a sample of urban African Americans with type 2 diabetes. J Behav Med. 2006 Feb;29(1):69–78. doi: 10.1007/s10865-005-9037-0. [DOI] [PubMed] [Google Scholar]
- 38.Hill-Briggs F, Renosky R, Lazo M, et al. Development and pilot evaluation of literacy-adapted diabetes and CVD education in urban, diabetic African Americans. J Gen Intern Med. 2008;23:1491–4. doi: 10.1007/s11606-008-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill-Briggs F, Lazo M, Renosky R, Ewing C. Usability of a diabetes and cardiovascular disease education module in an African American, diabetic sample with physical, visual, and cognitive impairment. Rehabil Psychol. 2008;53:1–8. [Google Scholar]
- 40.D’Zurilla TJ, Nezu AM. Problem-Solving Therapy: A Positive Approach to Clinical Interventions. 3. Springer Publishing Company; 2006. [Google Scholar]
- 41.Nezu AM, Nezu Maguth C, D’Zurilla TJ. Solving Life’s Problems: A 5-Step Guide to Enhanced Well-Being. Springer Publishing Company; 2006. [Google Scholar]
- 42.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014 Feb 5;311(5):507–20. doi: 10.1001/jama.2013.284427. Erratum in: JAMA. 2014 May 7;311(17):1809. [DOI] [PubMed] [Google Scholar]
- 43.Home blood pressure monitoring. American Heart Association; [Accessed September 23, 2013.]. http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/SymptomsDiagnosisMonitoringofHighBloodPressure/Home-Blood-Pressure-Monitoring_UCM_301874_Article.jsp. [Google Scholar]
- 44.Han HR, Lee H, Commodore-Mensah Y, Kim MT. Development and validation of the Hypertension Self-Care Profile: A practical tool to measure hypertension self-care. Journal of Cardiovascular Nursing. doi: 10.1097/JCN.0b013e3182a3fd46. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morisky DE, Ang A, Krousel-Wood MA, Ward H. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Roter D, Larson S. The Roter Interaction Analysis System (RIAS): utility and flexibility for analysis of medical interactions. Patient Education and Counseling. 2002;46(4):243–251. doi: 10.1016/s0738-3991(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 47.Felix-Aaron KL, Bone LR, Levine DM, Rubin HR. Using participant information to develop a tool for the evaluation of community health worker outreach services. Ethn Dis. 2002;12:87–96. [PubMed] [Google Scholar]
- 48.CAHPS. [Accessed November 16, 2011.];About the CAHPS Cultural Competence Item Set. http://cahps.ahrq.gov/clinician_group/cgsurvey/aboutculturalcompetenceitemset.pdf.
- 49.CAHPS. [Accessed November 16, 2011.];About the CAHPS Item Set for Addressing Health Literacy. http://cahps.ahrq.gov/clinician_group/cgsurvey/aboutitemsetaddressinghealthliteracy.pdf.
- 50.CAHPS. [Accessed November 16, 2011.];About the CAHPS Patient-Centered Medical Home (PCMH) Item Set. http://cahps.ahrq.gov/clinician_group/cgsurvey/aboutpatientcenteredmedicalhomeitemset.pdf.
- 51.National Heart, Lung, and Blood Institute. [Accessed May 26, 2013.];Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) http://www.nhlbi.nih.gov/guidelines/cholesterol/.htm.
- 52.American Diabetes A. Clinical Practice Recommendations 2007. Diabetes Care. 2007;30 (Supplement 1):S4–S41. [Google Scholar]
- 53.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Kidney Foundation. [Accessed 2002/01/18.];National Kidney Foundation K/DOQI Clinical Practice Guidelines. 2000 http://www.kidney.org/professionals/doqi/kdoqi/p4_class_g1.htm.
- 55.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 56. [Accessed March 15, 2012.];PROMIS Network Measures. http://www.nihpromis.org/measures/measureshome.
- 57.Rose M, Bjorner JB, Becker J, Fries JF, Ware JE. Evaluation of a preliminary physical function item bank supports the expected advantages of the Patient–Reported Outcomes Measurement Information System (PROMIS) J Clin Epidemiol. 2008;61(1):17–33. doi: 10.1016/j.jclinepi.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention. [Accessed November 15, 2011.];National Health Interview Survey. http://www.cdc.gov/nchs/nhis.htm.
- 59.Mayfield DG, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiat. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 60.Dhalla S, Kopec JA. The CAGE questionnaire for alcohol misuse: a review of reliability and validity studies. Clin Invest Med. 2007;30(1):33–41. doi: 10.25011/cim.v30i1.447. [DOI] [PubMed] [Google Scholar]
- 61.Smith PC, Schmidt SM, Allensworth-Daview D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170:1155–1160. doi: 10.1001/archinternmed.2010.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention. [Accessed March 1, 2012.];National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- 63.Block G. [Accessed December 2, 2011.];Berkeley Nutrition Services Fruit, Vegetable, and Fiber Screener. www.nutritionquest.com/fv_screener.html.
- 64.Block G. [Accessed December 2, 2011.];Berkeley Nutrition Services Fat Screener. http://www.nutritionquest.com/fat_screener.html.
- 65.Centers for Disease Control and Prevention. Measuring Healthy Days. Atlanta, Georgia: CDC; 2002. [Accessed January 20, 2012.]. http://www.cdc.gov/hrqol/pdfs/mhd.pdf. [Google Scholar]
- 66.Wright JA, Wallston K, Elasy TA, et al. Development and results of a kidney disease knowledge survey given to patients with chronic kidney disease. Am J Kidney Dis. 2011;57:387–395. doi: 10.1053/j.ajkd.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Connor AM, Rostom A, Fiset V, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. Brit Med J. 1999;319:731–734. doi: 10.1136/bmj.319.7212.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cranny A, O’Connor AM, Jacobsen MJ, et al. Development and pilot testing of a decision aid for postmenopausal women with osteoporosis. Patient Educ Couns. 2002;47:245–255. doi: 10.1016/s0738-3991(01)00218-x. [DOI] [PubMed] [Google Scholar]
- 69.O’Connor AM, Graham ID. Implementing shared decision making in diverse health care systems: the role of patient decision aids. Patient Educ Couns. 2005;57:247–249. doi: 10.1016/j.pec.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 70.Hill-Briggs F. Problem solving in diabetes self-management: a model of chronic illness self-management behavior. Ann Behav Med. 2003;25(3):182–193. doi: 10.1207/S15324796ABM2503_04. [DOI] [PubMed] [Google Scholar]
- 71.Hill-Briggs F, Gemmell L, Kulkarni B, Klick B, Brancati FL. Associations of patient health-related problem solving with disease control, emergency department visits, and hospitalizations in HIV and diabetes clinic samples. JGIM. 2007;22:649–54. doi: 10.1007/s11606-006-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40(6 Pt 1):1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 74.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 75.Wagnild GM, Young HM. Development and psychometric evaluation of the Resilience Scale. Journal of Nursing Measurement. 1993;1(2):165–178. [PubMed] [Google Scholar]
- 76.Wagnild GM. A review of the resilience scale. Journal of Nursing Measurement. 2009;17:105–113. doi: 10.1891/1061-3749.17.2.105. [DOI] [PubMed] [Google Scholar]
- 77.Brugha TS, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand. 1990;82(1):77–81. doi: 10.1111/j.1600-0447.1990.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 78.Bickel G, Nord M, Price C, Hamilton W, Cook J. Guide to measuring household food security. Washington, DC: United States Department of Agriculture, Food and Nutrition Service Office of Nutrition, Analysis and Evaluation; 2000. [Google Scholar]
- 79.Barnes J, Sampson RJ, Kindlon D, et al. A community survey approach to ecological assessment: results from a pilot survey. In: Earls F, Buka SL, editors. Project on Human Development in Chicago Neighborhoods (Report no NCJ 163495) Washington, DC: National Institute of Justice, US Department of Justice; 1997. pp. 34–45. [Google Scholar]
- 80.Sampson RJ, Raudenbush SW, Earls F. Neighborhoods and violent crime: a multilevel study of collective efficacy. Science. 1997;227:918–924. doi: 10.1126/science.277.5328.918. [DOI] [PubMed] [Google Scholar]
- 81.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–67. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- 82.Williams DR, Yu Y, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health: Socioeconomic Status, Stress, and Discrimination. J Health Psychol. 1997;2(3):335–351. doi: 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- 83.Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med. 2005;61(7):1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 84.Taylor TR, Kamarck TW, Shiffman S. Validation of the Detroit area study discrimination scale in a community sample of older African American adults: the Pittsburgh healthy heart project. Int J Behav Med. 2004;11:88–94. doi: 10.1207/s15327558ijbm1102_4. [DOI] [PubMed] [Google Scholar]
- 85.Adler N, John D, Catherine T. [Accessed November 11, 2011.];MacArthur Research Network on Socioeconomic Status and Health. http://www.macses.ucsf.edu/Research/Psychosocial/notebook/subjective.html.
- 86.PROMIS Network. [Accessed March 15, 2012.];Measures. http://www.nihpromis.org/measures/measureshome.
- 87.Hahn EA, DeVellis RF, Bode RK, Garcia SF, Castel LD, Eisen SV, Bosworth HB, Heinemann AW, Rothrock N, Cella D on behalf of the PROMIS Cooperative Group. Measuring social health in the Patient-Reported Outcomes Measurement Information System (PROMIS): Item bank development and testing. Qual Life Res. 2010;19(7):1035–44. doi: 10.1007/s11136-010-9654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smilkstein G, Ashworth C, Montano D. Validity and reliability of the Family APGAR as a test of family function. J Fam Practice. 1982;15:303–311. [PubMed] [Google Scholar]
- 89.Arozullah AM, Yarnold PR, Bennett CL, et al. Development and validation of a short-form, rapid estimate of adult literacy in medicine. Med Care. 2007;45(11):1026–1033. doi: 10.1097/MLR.0b013e3180616c1b. [DOI] [PubMed] [Google Scholar]
- 90.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514–22. doi: 10.1370/afm.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Osborn CY, Weiss BD, Davis TC, et al. Measuring adult literacy in health care: performance of the newest vital sign. Am J Health Behav. 2007;31 (Suppl 1):S36–46. doi: 10.5555/ajhb.2007.31.supp.S36. [DOI] [PubMed] [Google Scholar]
- 92.Fagerlin A, Zikmund-Fisher BJ, Ubel PA, Jankovic A, Derry HA, Smith DM. Measuring numeracy without a math test: development of the Subjective Numeracy Scale (SNS) Med Decis Making. 2007;27:672–680. doi: 10.1177/0272989X07304449. [DOI] [PubMed] [Google Scholar]
- 93.Zikmund-Fisher BJ, Smith DM, Ubel PA, Fagerlin A. Validation of the subjective numeracy scale (SNS): effects of low numeracy on comprehension of risk communications and utility elicitations. Med Decis Making. 2007;27:663–671. doi: 10.1177/0272989X07303824. [DOI] [PubMed] [Google Scholar]
- 94.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using Telephone Screening of Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 95.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 96.Centers for Disease Control and Prevention. [Accessed March 20, 2012.];National Health Interview Survey. http://www.cdc.gov/nchs/nhis.htm.
- 97.Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 professional manual. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- 98.Cooper LA, Roter DL, Bone LR, et al. A randomized controlled trial of interventions to enhance patient-physician partnership, patient adherence and high blood pressure control among ethnic minorities and poor persons: study protocol NCT00123045. Implement Sci. 2009;4:7. doi: 10.1186/1748-5908-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fahey T, Schroeder K, Ebrahim S. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2006;(4):CD005182. doi: 10.1002/14651858.CD005182.pub2. [DOI] [PubMed] [Google Scholar]
- 100.Brownstein JN, Bone LR, Dennison CR, Hill MN, Myong K, Levine DM. Community health workers as interventionists in the prevention and control of heart disease and stroke. Am J Prev Med. 2005;29(5SI):128–133. doi: 10.1016/j.amepre.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 101.Boulware LE, Daumit GL, Frick KD, Minkovitz CS, Lawrence RS, Powe NR. An evidence-based review of patient-centered behavioral interventions for hypertension. Am J Prev Med. 2001;21(3):221–232. doi: 10.1016/s0749-3797(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 102.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002 Dec 11;288(22):2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 103.Roter DL, Hall JA, Merisca R, Nordstrom B, Cretin D, Svarstad B. Effectiveness of interventions to improve patient compliance: a meta-analysis. Med Care. 1998 Aug;36(8):1138–1161. doi: 10.1097/00005650-199808000-00004. [DOI] [PubMed] [Google Scholar]