Abstract
Objectives
To characterize HIV/HBV coinfection in the ACTG Longitudinal Linked Randomized Trials (ALLRT) cohort and compare long-term HBV outcomes between regimens with one (MONO) or two (DUAL) anti-HBV agents.
Design
A retrospective study of coinfected ALLRT subjects who received regimens containing anti-HBV agent(s).
Methods
Stored samples at baseline and weeks 16, 32, 48, 144, and 240 were tested for HBV DNA, HBeAg, HBeAb, and HDV antibody. Resistance and genotype were tested in samples with HBV DNA >600 IU/ml. MONO vs. DUAL analyses were limited to HBV treatment-naïve subjects (Naïve-MONO, Naïve-DUAL).
Results
Of 150 study subjects, median age was 40 years, 96% were male; 57% White, 26% Black, 13% Hispanic. Baseline median CD4 was 224 cells/mm3, HIV RNA 4.48 log10 copies/ml, HBV DNA 6.30 log10 IU/ml; 59% HBeAg positive and 65% HBeAb negative; HBV genotypes A=69%, G=18%, D=7%, <2% for A/G, B, C, F, H. Coinfection with HDV was 2%. There were 49 Naïve-MONO (lamivudine) and 22 Naïve-DUAL (11 lamivudine+tenofovir, 11 emtricitabine+tenofovir) with detectable HBV DNA. In the 240-week follow-up, HBV DNA suppression was not significantly higher in Naïve-DUAL (p=0.14); lower baseline HBV DNA (p<0.01) was associated with suppression. Among 32 Naïve-MONO subjects with detectable HBV DNA at baseline and results at week 48, 41% suppressed; among such 15 Naïve-DUAL subjects, 53% suppressed. HBeAg and HBeAb analyses showed similar trends.
Conclusions
While consistent trends toward increased HBV DNA suppression, HBeAg loss and HBeAb seroconversion were observed in Naïve-DUAL compared to Naïve-MONO, they were not statistically significant. Overall, HDV coinfection was low.
Keywords: coinfection, HIV, HBV, HDV, mono-therapy, dual-therapy, lamivudine, tenofovir
INTRODUCTION
Coinfection with human immunodeficiency virus (HIV) and hepatitis B virus (HBV) is associated with increased risk of liver-related morbidity and mortality 1–4. Current guidelines to treat HIV/HBV coinfected persons recommend antiretroviral treatment (ART) that includes agents with activity against both HIV and HBV, preferably tenofovir (TDF) and emtricitabine (FTC), regardless of the level of HBV DNA 5–9.
While combination therapy is well-established in the treatment of HIV and is the current standard of care, few studies have reported on combination of two or more anti-HBV agents in HBV and are further limited in HIV/HBV coinfection. Studies conducted to date have examined various outcomes comparing ART regimens but are often limited by study design, sample size, short follow-up or heterogeneity of treatment regimens and experience 10–19. Because response to antiviral therapy may be slower in HBV compared to HIV and HCV 20, long-term studies are needed to assess clinically relevant changes.
Our primary objectives were to compare HBV virologic suppression, loss of HBV e antigen (HBeAg) and development of e antibody (HBeAb) between those who received one (MONO) and those who received two (DUAL) anti-HBV agents as part of their initial ART in the AIDS Clinical Trials Group (ACTG), with follow-up out to 240 weeks. In addition, we sought to characterize HIV/HBV coinfected participants and also assessed coinfection with hepatitis D virus (HDV). While the updated recommendations list HDV testing as essential in those with HIV/HBV coinfection, HDV testing has not been performed routinely as part of standard of care in the US and, to our knowledge, has not been described previously in ACTG participants with HIV/HBV coinfection.
METHODS
Study Design
ACTG Longitudinal Linked Randomized Trials (ALLRT) is a prospective, observational cohort study of HIV-infected subjects from selected clinical trials in the ACTG, with study visits every 16 weeks. ART-naïve and -experienced subjects enrolled in ACTG clinical trials (parent studies) in the US, who had been randomly assigned to antiretroviral therapies or strategies for HIV interventions, were eligible to enroll into ALLRT. Specifics regarding ALLRT have been described elsewhere 21.
Our study included HBV-coinfected ALLRT subjects who received antiviral regimens that contained anti-HBV agent(s) during the parent study participation: lamivudine (3TC), tenofovir (TDF), emtricitabine (FTC) and adefovir (ADV). Treatment for HBV was considered mono-therapy if there was only one anti-HBV agent in the regimen, and dual-therapy if more than one. MONO vs. DUAL analyses were limited to subjects without prior treatment with anti-HBV agents receiving HBV mono-therapy (Naïve-MONO) or HBV dual-therapy (Naïve-DUAL). Characterization of the study cohort also included subjects with prior exposure to HBV treatment receiving HBV mono-therapy (Exp-MONO) or HBV dual-therapy (Exp-DUAL). HBV coinfection was established by a reported positive HBsAg during ALLRT. If HBsAg results by baseline (initiation of anti-HBV agents as part of ART) were not available, then baseline HBV DNA, HBeAg and HBeAb results were used to confirm baseline status. Combined with subsequent results, all were assessed as infected with chronic HBV.
Adherence analyses were conducted using standardized self-report adherence forms for the ART regimen administered as part of the parent and/or ALLRT protocol(s). To avoid potential bias related to varying number of forms submitted by each participant, analysis was conducted on subjects with all 4 adherence forms during the first 48 weeks. As a summary over time, if all the forms during the first 48 weeks indicated 100% adherence (complete adherence to medications in the past 4 days), then subject was considered 100% adherent overall; otherwise, if adherence was reported as <100% at any visit, then <100% adherent overall.
Laboratory Tests
Stored serum or plasma samples at baseline and weeks 16, 32, 48, 144, 240 were tested for HBV DNA (Roche Taqman, lower limit of detection of 29 IU/mL), HBeAg and HBeAb. Resistance and genotype (Quest Diagnostics) were tested in samples with HBV DNA >600 IU/mL, including information on 15 codons: 11 polymerase regions (L180, M204, V207, T184, M250, V173, S202, I233, N236, A181, A194), 2 precore (pre-C) regions (G1896, C1858) and 2 basal core promoter (BCP) regions (A1762, G1764). HDV antibody tests were conducted on available samples, not necessarily at the time of our study baseline. HIV-1 RNA viral load (VL), CD4+T-cell count and HCV antibody results were obtained from the ALLRT database 21.
Statistical Analysis
HBV DNA suppression was defined as 29 IU/mL. Loss of HBeAg was determined by the change from reactive to non-reactive, and HBeAb seroconversion by the change from non-reactive to reactive. Logistic regression models were developed to assess conversion by week 48 using offset on follow-up time. The offset time was defined as the time of suppression (HBV DNA) or seroconversion (HBeAg and HBeAb), or the follow-up time. Time-to-event methods were applied to generate Kaplan-Meier plots, conduct log-rank tests and develop Cox proportional hazards models on time to conversion using all available data out to week 240. The log-log transformation method was used to produce 95% pointwise confidence intervals in the Kaplan-Meier plots. Comparisons of binary or categorical variables were based on the Fisher’s exact tests, including analyses on week 48 HBV DNA suppression, HBeAg and HBeAb seroconversion and adherence. Tests for trend were conducted using the Cochran-Armitage Exact Trend test. Continuous variables were compared using the Wilcoxon rank-sum test. All tests were two-sided; a p-value cutoff of 0.05 was used to determine statistical significance. Analyses were conducted using SAS 9.2.
RESULTS
Coinfected Study Population and Baseline Characteristics
Of the 3413 ALLRT subjects in the June 2008 database and 1217 additions as of June 2009, we identified 178 participants with positive HBsAg in the database. Of these, 162 had confirmed HBV infection at the time of ART initiation (baseline); 12 of these subjects were on ART that did not contain any anti-HBV agent. Therefore, we report on 150 HBV coinfected subjects who received antiviral regimens containing at least one anti-HBV agent at baseline. There were 65 Naïve-MONO, 27 Naïve-DUAL, 52 Exp-MONO and 6 Exp-DUAL subjects enrolled at 45 sites between March 1997 and October 2006. Median age was 40 years, 57% were non-Hispanic whites, 26% non-Hispanic blacks, and 4% were Asian. There were only 6 females (4%). All but one in Naïve-MONO received 3TC as part of the ART; in Naïve-DUAL, 56% received 3TC+TDF and 44% received FTC+TDF. Table 1 presents baseline characteristics, including HIV-related measures, for all study groups.
Table 1.
Baseline characteristics
| Total N=150* |
Naïve-MONO N=65* |
Naïve-DUAL N=27* |
Exp-MONO N=52* |
Exp-DUAL N=6* |
|
|---|---|---|---|---|---|
|
| |||||
| Anti-HBV agents | |||||
| 3TC | 97 (64.7%) | 64 (98.5%) | 0 | 33 (63.5%) | 0 |
| ADV | 16 (10.7%) | 1 (1.5%) | 0 | 15 (28.8%) | 0 |
| TDF | 4 (2.7%) | 0 | 0 | 4 (7.7%) | 0 |
| 3TC+TDF | 18 (12.0%) | 0 | 15 (55.6%) | 0 | 3 (50.0%) |
| FTC+TDF** | 15 (10.0%) | 0 | 12 (44.4%) | 0 | 3 (50.0%) |
|
| |||||
| Age (years) | |||||
| Median (Q1, Q3) | 40 (35, 46) | 39 (35,42) | 44 (33,51) | 41 (37,46) | 48 (35,55) |
|
| |||||
| Sex | |||||
| Male | 144 (96.0%) | 62 (95.4%) | 26 (96.3%) | 50 (96.2%) | 6 (100%) |
| Female | 6 (4.0%) | 3 (4.6%) | 1 (3.7%) | 2 (3.8%) | 0 (0%) |
|
| |||||
| Race/Ethnicity | |||||
| White non-Hispanic | 85 (56.7%) | 29 (44.6%) | 14 (51.9%) | 39 (75.0%) | 3 (50.0%) |
| Black non-Hispanic | 39 (26.0%) | 19 (29.2%) | 11 (40.7%) | 8 (15.4%) | 1 (16.7%) |
| Hispanic (any race) | 19 (12.7%) | 13 (20.0%) | 0 (0%) | 4 (7.7%) | 2 (33.3%) |
| Asian, Pacific Islander | 6 (4.0%) | 3 (4.6%) | 2 (7.4%) | 1 (1.9%) | 0 (0%) |
| More than one race | 1 (0.7%) | 1 (1.5%) | 0 (0%) | 0 (0%) | 0 (0%) |
|
| |||||
| IVD history | |||||
| Never | 134 (89.3%) | 61 (93.8%) | 27 (100%) | 42 (80.8%) | 4 (66.7%) |
| Previously | 16 (10.7%) | 4 (6.2%) | 0 (0%) | 10 (19.2%) | 2 (33.3%) |
|
| |||||
| Log10 HBV DNA (IU/mL) | [N=140] | [N=60] | [N=24] | [N=51] | [N=5] |
| Median (Q1, Q3) | 6.30 (1.65,8.52) | 8.26 (2.54, 8.92) | 7.18 (3.96, 8.59) | 4.09 (1.46***, 7.62) | 7.69 (1.46***, 8.12) |
|
| |||||
| HBeAg | [N=143] | [N=61] | [N=24] | ||
| Reactive | 84 (58.7%) | 38 (62.3%) | 14 (58.3%) | 28 (53.8%) | 4 (66.7%) |
| Non-reactive | 58 (40.6%) | 22 (36.1%) | 10 (41.7%) | 24 (46.2%) | 2 (33.3%) |
| Borderline | 1 (0.7%) | 1 (1.6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HBeAb | [N=143] | [N=61] | [N=24] | ||
| Reactive | 50 (35.0%) | 20 (32.8%) | 9 (37.5%) | 19 (36.5%) | 2 (33.3%) |
| Non-reactive | 93 (65.0%) | 41 (67.2%) | 15 (62.5%) | 33 (63.5%) | 4 (66.7%) |
|
| |||||
| HBV genotype **** | [N=103] | [N=46] | [N=23] | [N=31] | [N=3] |
| A | 71 (68.9%) | 31 (67.4%) | 16 (69.6%) | 22 (71.0%) | 2 (66.7%) |
| A/G | 2 (1.9%) | 1 (2.2%) | 1 (4.3%) | 0 (0%) | 0 (0%) |
| B | 1 (1.0%) | 1 (2.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| C | 1 (1.0%) | 0 (0%) | 1 (4.3%) | 0 (0%) | 0 (0%) |
| D | 7 (6.8%) | 3 (6.5%) | 0 (0%) | 4 (12.9%) | 0 (0%) |
| F | 1 (1.0%) | 1 (2.2%) | 0 (0%) | 0 (0%) | 0 (0%) |
| G | 18 (17.5%) | 8 (17.4%) | 4 (17.4%) | 5 (16.1%) | 1 (33.3%) |
| H | 2 (1.9%) | 1 (2.2%) | 1 (4.3%) | 0 (0%) | 0 (0%) |
|
| |||||
| ART history | |||||
| Naïve | 70 (46.7%) | 51 (78.5%) | 18 (66.7%) | 0 (0%) | 1 (16.7%) |
| Experienced | 80 (53.3%) | 14 (21.5%) | 9 (33.3%) | 52 (100%) | 5 (83.3%) |
|
| |||||
| ART regimen ***** | |||||
| 2 NRTI + 1 Boosted PI | 17 (11.3%) | 7 (10.8%) | 7 (25.9%) | 2 (3.8%) | 1 (16.7%) |
| 2 NRTI + 1 PI | 29 (19.3%) | 7 (10.8%) | 2 (7.4%) | 20 (38.5%) | 0 (0%) |
| 2 NRTI + 2 PI | 5 (3.3%) | 2 (3.1%) | 0 (0%) | 3 (5.8%) | 0 (0%) |
| 2 NRTI + 1 NNRTI | 45 (30.0%) | 26 (40.0%) | 15 (55.6%) | 4 (7.7%) | 0 (0%) |
| 2 NRTI + 1 NNRTI + 1 PI | 14 (9.3%) | 8 (12.3%) | 0 (0%) | 6 (11.5%) | 0 (0%) |
| 2 NRTI + 1 NNRTI + 2 PI | 3 (2.0%) | 0 (0%) | 0 (0%) | 3 (5.8%) | 0 (0%) |
| 3 NRTI | 10 (6.7%) | 8 (12.3%) | 1 (3.7%) | 1 (1.9%) | 0 (0%) |
| 3 NRTI + 1 Boosted PI | 3 (2.0%) | 0 (0%) | 2 (7.4%) | 1 (1.9%) | 0 (0%) |
| 3 NRTI + 1 PI | 4 (2.7%) | 0 (0%) | 0 (0%) | 4 (7.7%) | 0 (0%) |
| 3 NRTI + 1 NNRTI | 9 (6.0%) | 5 (7.7%) | 0 (0%) | 1 (1.9%) | 3 (50.0%) |
| Other | 11 (7.3%) | 2 (3.1%) | 1 (3.7%) | 7 (13.5%) | 2 (33.3%) |
|
| |||||
| Nadir CD4 (cells/mm3) | |||||
| Median (Q1, Q3) | 142 (49, 257) | 106 (49, 254) | 188.5 (43, 326) | 140 (53, 232) | 124.5 (32, 204) |
|
| |||||
| CD4 count (cells/mm3) | [N=147] | [N=65] | [N=27] | [N=49] | [N=6] |
| Median (Q1, Q3) | 223.5 (115.5, 378.5) | 185 (81.5, 322) | 229 (91, 376) | 268.5 (182.5, 479) | 192 (139, 318) |
|
| |||||
| Log10 HIV RNA (copies/mL) | [N=147] | [N=65] | [N=27] | [N=49] | [N=6] |
| Median (Q1, Q3) | 4.48 (2.84, 4.84) | 4.69 (4.33, 5.26) | 4.55 (4.11, 4.80) | 2.90 (2.53, 4.48) | 4.61 (1.70, 4.84) |
Smaller N’s [noted in brackets] for some measures based on available data. Percentages may not total 100% due to rounding.
One patient received ADV prior to study participation, and it was discontinued after the start of FTC+TDF as the antiretroviral regimen.
HBV DNA limit of detection 29 IU/mL in log10 is 1.46
HBV genotypes were done only on samples with HBV DNA > 600 IU/mL.
NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor
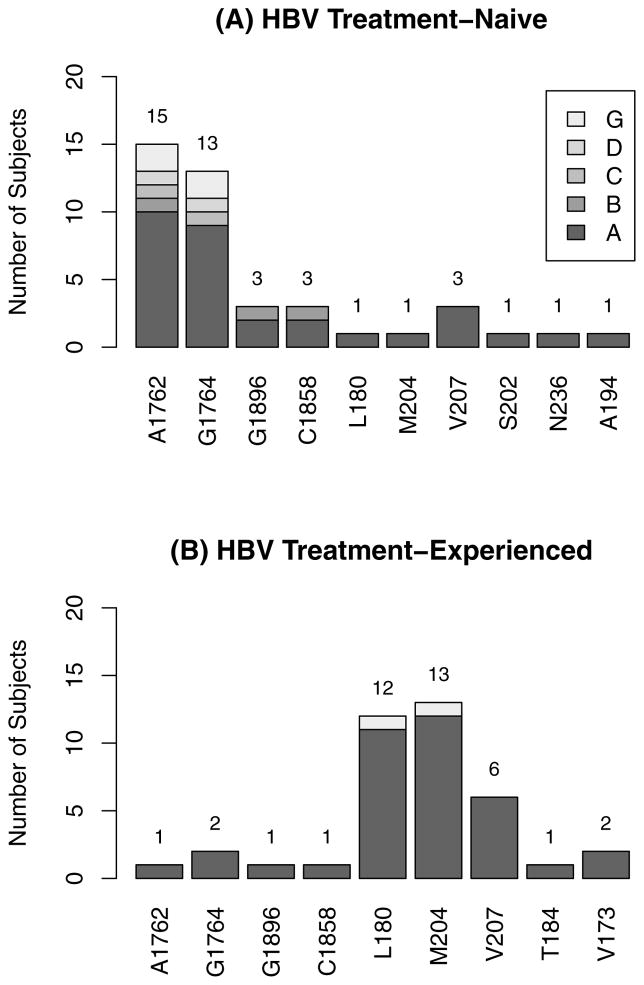
Median baseline HBV DNA levels were 8.26, 7.18, 4.09 and 7.69 log10 IU/mL in Naïve-MONO, Naïve-DUAL, Exp-MONO and Exp-DUAL groups, respectively; 59% were HBeAg reactive and 65% were HBeAb non-reactive, overall. Baseline HBV DNA levels differed significantly between those with and without prior experience with anti-HBV agents (Naïve-MONO and Naive-DUAL vs. Exp-MONO and Exp-DUAL, p<0.001). HBV DNA results were available for 140 subjects at baseline, and Table 2 shows the relationships of HBV DNA level with HBeAg and HBeAb. As expected, higher HBV DNA was associated with positive HBeAg and negative HBeAb (p<0.001). Of the 140 with baseline HBV DNA results, 88 subjects had levels >600 IU/mL and sufficient samples for HBV genotype and mutation analysis. The most common HBV was genotype A (69%), followed by G (18%) then D (7%). There were two subjects with two genotypes detected, A and G. Mutations were detected in 34 subjects: 20 among HBV treatment-naïve subjects (Naïve-MONO and Naïve-DUAL) and 14 in HBV treatment-experienced (Exp-MONO and Exp-DUAL). Figure 1 presents the number of subjects detected with each mutation. Note that one subject may have more than one mutation. Mutations in the polymerase region were detected in 20 subjects: 6 out of 60 in HBV treatment-naïve (Naïve-MONO and Naïve-DUAL) and 14 of 28 in HBV treatment-experienced (Exp-MONO and Exp-DUAL). Of the latter 28 subjects, all had received 3TC previously. Pre-c mutations were detected in 4 subjects, 3 in HBV treatment-naïve and 1 in experienced, and mutations at G1896 and C1858 occurred together. Mutations in the BCP region were detected in 17 of the 88 with results, 15 in HBV treatment-naïve and 2 in treatment-experienced; 16 of these 17 subjects had mutations at A1762 and 15 at G1764. Of the 17 with BCP mutations, 8 had positive HBeAb (3 Naïve-MONO, 4 Naïve-DUAL, 1 Exp-MONO).
Table 2.
Baseline HBV DNA levels, HBeAg and HBeAb (N=140)
| HBV DNA | HBeAg* | HBeAb* | ||
|---|---|---|---|---|
| Non-reactive (Negative) N=56 |
Reactive (Positive) ** N=84 |
Non-reactive (Negative) N=91 |
Reactive (Positive) N=49 |
|
| ≤29 IU/mL | 28 (50.0%) | 6 (7.1%) | 8 (8.8%) | 26 (53.1%) |
| >29 IU/mL to 3 log10 IU/mL | 13 (23.2%) | 0 (0.0%) | 3 (3.3%) | 10 (20.4%) |
| >3 to 5 log10 IU/mL | 8 (14.3%) | 4 (4.8%) | 5 (5.5%) | 7 (14.3%) |
| >5 to 7 log10 IU/mL | 3 (5.4%) | 13 (15.5%) | 12 (13.2%) | 4 (8.2%) |
| >7 to 9 log10 IU/mL | 4 (7.1%) | 44 (52.4%) | 46 (50.5%) | 2 (4.1%) |
| >9 log10 IU/mL | 0 (0.0%) | 17 (20.2%) | 17 (18.7%) | 0 (0.0%) |
Exact Trend test p-value < 0.001
One baseline sample that was reported as borderline is categorized as reactive in this table. This subject’s subsequent sample was reported as reactive.
Figure 1.
Baseline mutations detected in subjects with HBV DNA > 600 IU/mL
* Note that one subject may have more than one mutation.
HBV Virologic Outcome of MONO vs. DUAL HBV Regimen in HBV Treatment-Naïve
Week 48 HBV DNA Suppression
There were 47 Naïve-MONO (3TC) and 21 Naïve-DUAL subjects who had HBV DNA level >29 IU/ml at baseline and any follow-up results by week 48. These Naïve-MONO and Naïve-DUAL subjects were similar in the following characteristics: sex, age, race/ethnicity, IV drug use history, ART history, nadir and baseline CD4+ T-cell counts, HIV VL, HBV DNA levels and HBeAg and HBeAb statuses. 70% were positive for HBeAg and 75% negative for HBeAb. In addition to HBV regimen (MONO vs. DUAL), baseline log10 HBV DNA, CD4 count, log10 HIV VL and HBeAg status were considered in the analysis. Sex was not considered, because there were only 2 females in this data set. In the univariate analyses, baseline HBeAg, log10 HBV DNA and log10 HIV VL met the pre-specified criterion (p<0.10) to include as covariates in the multivariate logistic regression model to compare MONO and DUAL in HBV DNA suppression. In the multivariate model, the odds of suppression by week 48 were estimated to be higher in Naïve-DUAL compared to Naïve-MONO but without statistical significance (odds ratio [OR]=1.42, p=0.63). Lower baseline log10 HBV DNA was associated with suppression (p<0.01); other covariates were not. Among 32 Naïve-MONO and 15 Naïve-DUAL subjects with HBV DNA>29 at baseline and results at week 48, 41% suppressed in Naïve-MONO and 53% in Naïve-DUAL: 13% difference, 95% CI=(-18%,43%). Among the 26 who did not suppress, the median HBV DNA decrease was 2.66 log10 in Naïve-MONO (N=19) and 5.70 in Naïve-DUAL (N=7), with p=0.003.
Week 240 HBV DNA Suppression
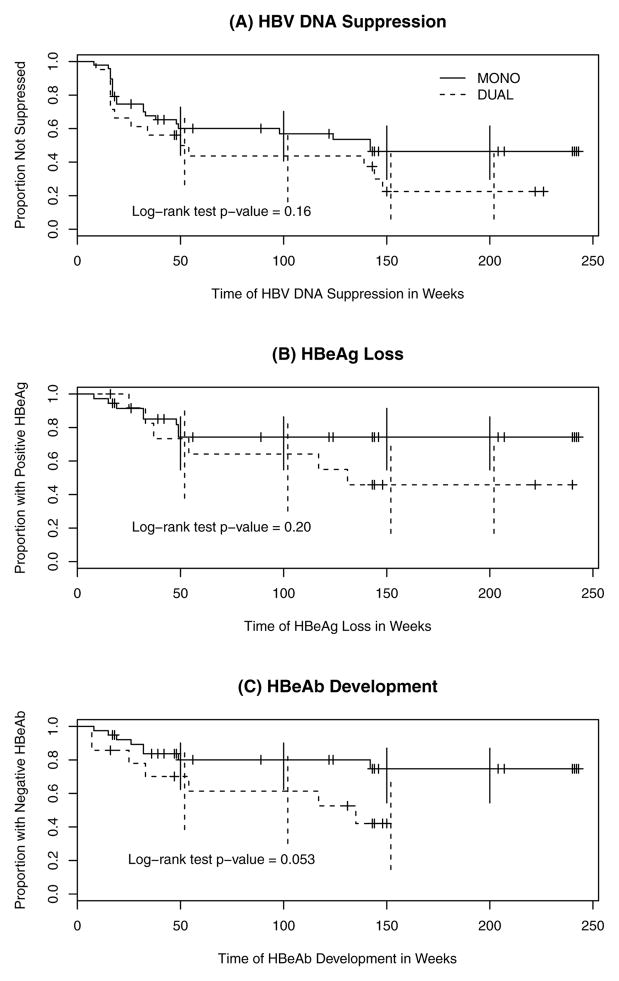
There were 49 Naïve-MONO and 22 Naïve-DUAL subjects with HBV DNA >29 IU/ml at baseline to compare time to HBV DNA suppression out to week 240 (Figure 2A). In univariate analyses, baseline log10 HBV DNA and HBeAg status were identified as significant covariates to include in the multivariate model (p<0.10). In the multivariate model, suppression was higher in Naïve-DUAL, but this was not statistically significant (hazard ratio [HR]=1.7, p=0.14). Lower baseline log10 HBV DNA was associated with suppression with statistical significance (p<0.01). Higher suppression was observed among those with negative HBeAg but without statistical significance (p=0.06).
Figure 2.
Naïve-MONO vs. Naïve-DUAL outcomes: (A) HBV DNA suppression, (B) HBeAg Loss, and (C) HBeAb development
HBV Viral Rebound and Resistance
There were 4 subjects with HBV DNA viral rebound, all receiving MONO. Changes in HBV mutations were noted in 2 subjects who had baseline BCP and pre-C mutations. One with HBV genotype D started with HBV DNA level of 8.3 log10 IU/mL with A1762T and G1764A mutations at baseline, then suppressed by week 48 on 3TC/zidovudine/efavirenz. Rebound occurred by week 144 while remaining on the ART, with HBV DNA level of 4.5 log10 IU/mL and mutations of L180M, M204V, M250L and V173L. The other subject (genotype A) started with HBV DNA level of 6.0 log10 IU/mL with mutations G1896A/G and C1858C/T. HBV DNA was suppressed by week 48 then rebounded by week 144 at 2.8 log10 IU/mL while remaining on 3TC/abacavir/zidovudine/efavirenz, with mutations L180M/L and M204M/V. The remaining 2 subjects with rebound HBV DNA also had rebound HIV RNA suggesting non-adherence.
HBV Serologic Outcomes of MONO vs. DUAL HBV Regimen in HBV Treatment-Naïve
HBeAg Loss
There were 36 Naïve-MONO and 13 Naïve-DUAL subjects with positive HBeAg at baseline and any follow-up results by week 48. All had HBV DNA >29 IU/ml at baseline. There was no statistically significant difference between MONO and DUAL in HBeAg loss by week 48 (OR=1.77, p=0.44). Baseline log10 HBV DNA, log10 HIV VL and CD4+ T-cell count were also not significantly associated with HBeAg loss by week 48. Among 21 Naïve-MONO and 6 Naïve-DUAL subjects with positive HBeAg at baseline and results at week 48,19% became HBeAg negative in Naïve-MONO and 33% in Naïve-DUAL at week 48: 14% difference, 95% CI=(-27%,56%). In Cox’s proportional hazards models on 37 Naïve-MONO and 14 Naïve-DUAL subjects with positive HBeAg at baseline and any follow-up data out to week 240, antigen loss was higher in Naïve-DUAL but not statistically significant (HR=1.96, p=0.21). Other covariates were also not significant. Figure 2B presents times to HBeAg loss for Naïve-MONO and Naïve-DUAL.
HBeAb Seroconversion
39 Naïve-MONO and 14 Naïve-DUAL subjects had negative HBeAb at baseline and any follow-up results by week 48. All but one Naïve-MONO had HBV DNA >29 at baseline. The difference in HBeAb development by Week 48 between Naïve-MONO and Naïve-DUAL was not statistically significant (OR=3.35, p=0.093). In logistic regression models, baseline log10 HBV DNA, log10 HIV VL and CD4+ T-cell count were also not significant. Among 32 subjects with data at both baseline and week 48, 3 of 24 Naïve-MONO (12.5%) and 2 of 8 Naïve-DUAL (25.0%) had positive HBeAb at week 48: 13% difference, 95% CI=(-0.20%, 0.45%). In Cox’s proportional hazards models on 40 Naïve-MONO and 15 Naïve-DUAL subjects with negative HBeAb at baseline and any follow-up data out to week 240, seroconversion was higher in Naïve-DUAL but not statistically significant (HR=2.63, p=0.062); other covariates were also not significant. Figure 2C presents times to HBeAb seroconversion for Naïve-MONO and Naïve-DUAL; the log-rank test p-value was nearly significant at 0.053.
Serostatus Reversion
Whereas none of the 6 Naïve-DUAL subjects who were positive for HBeAg at baseline and subsequently became HBeAg negative reverted to positive HBeAg, 3 of 9 such Naïve-MONO subjects reverted by week 144. Mutations L180M and M204V were subsequently detected for 2 subjects (HBV genotype A), and there were no mutation results available for the remaining one (genotype D). For HBeAb, 2 reverted to sero-negative (by weeks 48 and 144) among the 9 Naïve-MONO subjects who were negative for HBeAb at baseline then developed antibody. (They both reverted also for HBeAg, mentioned above.) None reverted among 6 Naïve-DUAL subjects who developed HBeAb.
Treatment Adherence in HBV Treatment-Naïve
There were 66 subjects (42 Naïve-MONO, 24 Naïve-DUAL) with 4 forms completed on ART regimen adherence during the first 48 weeks of study. In Naïve-MONO, 64% were 100% adherent overall to the study regimens, and 83% of Naïve-DUAL were 100% adherent overall. The difference in the two groups was not statistically significant (p=0.16). Of these 66, 52 subjects had HBV DNA >29 at baseline to assess association between adherence and HBV DNA suppression. There was no significant association between adherence and HBV DNA suppression (p=0.76). Of the 4 HBV DNA relapsers: two were 100% adherent overall, one was <100% adherent overall, and data were not available for the remaining one.
HIV Viral Load and CD4+ T-Cell Counts
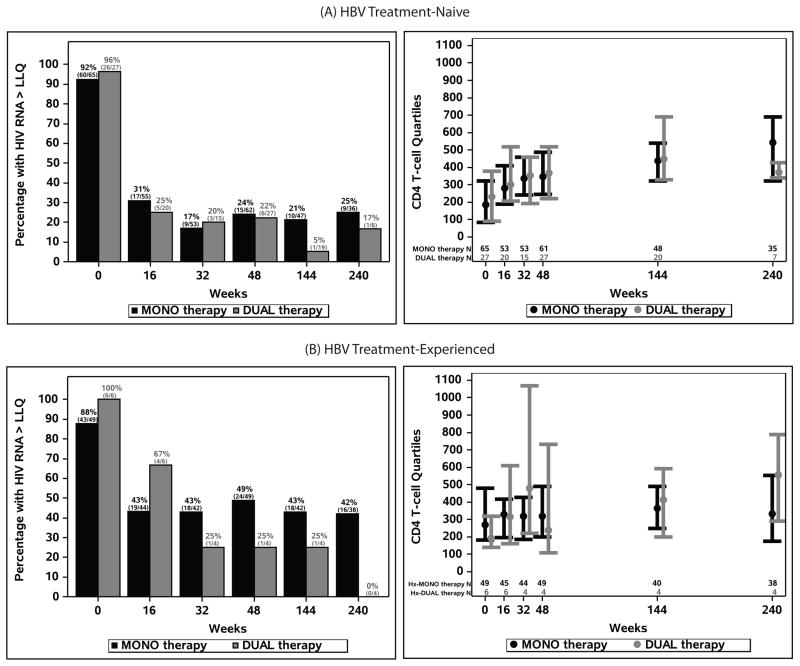
Figures 3A and 3B summarize the HIV viral loads and CD4+ T-cell counts at study weeks for the four study groups. At week 48, 76% and 78% of the MONO and DUAL subjects, respectively, had undetectable HIV RNA, and they were 75% (27 out of 36) and 83% (5 out of 6), respectively, at week 240. In the HBV treatment-experienced, 51% in Exp-MONO and 75% (3 out of 4) in Exp-DUAL suppressed HIV RNA at week 48, and 58% in Exp-MONO and 100% (all 4 subjects) in Exp-DUAL at week 240. The median CD4 increase from baseline at 48 weeks was 145 cells/mm3 for 61 Naïve-MONO subjects and 141 cells/mm3 for 27 Naïve-DUAL subjects with results at both times. By week 240, they increased to 298 cells for Naïve-MONO and 286 cells for Naïve-DUAL, but there were fewer results. The changes were more modest in the HBV treatment-experienced. Additional CD4 increase by week 240 was noted in the treatment-experienced.
Figure 3.
Proportions of subjects with HIV RNA > LLQ and CD4+ T-cell count quartiles at study visits in (A) HBV treatment-naïve and (B) HBV treatment-experienced
HDV and HCV Co-Infection
HDV antibody result was not obtained in one subject, because the sample was not sufficient. There were 3 subjects who tested positive for HDV antibody, 1 in Naïve-MONO and 2 in Exp-MONO, yielding 2% HBV/HDV co-infection with HIV. HCV antibody results were collected at any time during ALLRT participation, and of the 144 subjects with results, 15 (10%) were positive. When we repeated the main analyses excluding the HCV co-infected subjects, the results were similar.
DISCUSSION
Current guidelines for the treatment of HIV/HBV coinfections recommend combination of TDF with FTC or 3TC as the nucleoside reverse transcriptase inhibitor backbone of ART 6. This is based in part on the observation that 3TC-resistant HBV is observed in approximately 40% of patients after 2 years on 3TC for chronic HBV and in approximately 90% of patients after 4 years when 3TC is used as the only active drug for HBV in coinfection 22. In this retrospective analysis of HIV/HBV coinfected ALLRT participants, HBV DNA suppression with 3TC mono-therapy at 48 weeks was higher than expected at 41%. Of note, the 3TC dose in the parent trials was 300 mg per day, which is higher than the dose typically administered in HBV mono-infection. There were only 4 HBV treatment-naïve subjects who suppressed HBV DNA then experienced HBV DNA viral rebound, and they all received MONO. Mutation results were available for two of these, and they both developed mutations in the polymerase region. Interestingly, both subjects had either BCP or pre-C mutations at baseline, which have been reported to have an increased risk of high HBV viremia after loss of HBeAg. The few relapses in HBeAg and HBeAb were also all MONO recipients, so the results are again consistent across the outcome measures, albeit in small numbers.
In this study, HBV outcomes in DUAL could not be concluded as superior compared to MONO in the treatment-naive. Various analysis approaches were taken in the present study, and the study results were consistent across the HBV virologic and serologic measures and time periods considered. All the trends pointed to better outcomes with DUAL but without statistical significance. Adherence analysis did not suggest confounding due to differential adherence to regimens in Naïve-MONO and Naïve-DUAL. Moreover, the data did not suggest association between adherence and HBV DNA suppression. Our analysis suggested that lower baseline HBV DNA level and positive HBeAg may be associated with suppression, consistent with a recent case-control study of TDF-based combination therapy in HIV/HBV coinfection 23.
We also sought to characterize HIV/HBV coinfection in the ALLRT cohort and described both the HBV treatment-naïve and –experienced. HBV genotype A was most common, followed by G. At the time of ART initiation, mutations in BCP regions were more common in the HBV treatment-naïve, and more polymerase mutations were detected in the HBV treatment-experienced, as expected. Overall, study subjects responded well to ART. Coinfection with HDV was low at 2% and with HCV was about 10% overall.
One of the limitations of the study is that no one was on TDF mono-therapy. In a Thailand trial of 36 coinfected patients randomized to 3TC, TDF or 3TC+TDF with zidovudine and efavirenz, no advantage in HBV DNA suppression was demonstrated for combination therapy at 48 weeks of follow-up 24; however 3TC alone was significantly inferior to TDF (46% vs 92% in HBV DNA decrease to <3 log). And while a recent meta-analysis of 23 studies including 550 HIV/HBV coinfected subjects 25 also found that there was no benefit of combination therapy compared with TDF alone, the one-year HBV DNA suppression was only 57%, similar to our results for DUAL therapy. Of note, resistance to TDF by HBV does not appear to be selected at a meaningful rate26,27. Our study is also limited by the small sample size; many of the analyses are descriptive where trends may be identified or supported but cannot be concluded as statistically significant. And as a retrospective study on subjects who were participating in HIV treatment clinical trials, the results may have limited generalizability.
While HIV suppression rates have greatly improved partly due to simpler and less toxic regimens, there is little data on virologic and serologic outcomes of HBV with newer therapies. Although we could not conclude that DUAL is superior, there were consistent trends suggesting increased HBV DNA suppression, HBeAg loss and HBeAb seroconversion in Naïve-DUAL, and relapses only in Naïve-MONO. Furthermore, there was a significant HBV DNA decrease in Naïve-DUAL compared to Naïve-MONO among the 26 subjects who did not suppress. When interpreting study results, the study sample size, clinical applicability and relevance, and consistency with other studies must also be considered. Despite the lack of statistically significant treatment responses in this study, the known potential for development of 3TC resistance28 warrants that caution should be taken against the interpretation that 3TC mono-therapy is a viable option for coinfected persons. As the HIV/HBV coinfected population is developing age-related decreases in glomerular filtration, there may be preference to use a kidney-sparing regimen such as abacavir (ABC) with 3TC, or the potentially new formulation of tenofovir alafenamide (TAF) if FDA approved. However, while simpler regimens may be enticing, failing to conclude that DUAL is superior to MONO with statistical significance in our study does not necessarily support that mono-therapy is non-inferior. Clinicians must continue to weigh risks and benefits of not including another drug with anti-HBV activity such as TDF or entecavir. Further strategies to explore whether TDF or entecavir can be discontinued after prolonged HBV suppression in patients on 3TC who do not require TDF for HIV virologic control may be of interest.
Supplementary Material
Acknowledgments
Funding: The study was supported by 5R21AI083106 (Aberg), AI069532 (Aberg), UM1AI068636 (ACTG) and UM1AI068634 (Statistical and Data Management Center) from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR)
We would like to thank the ALLRT study team, clinical sites and the study participants. We also thank William Meyer at Quest Diagnostics for his helpful suggestions. The study was supported by 5R21AI083106 (Aberg), AI069532 (Aberg), UM1AI068636 (ACTG) and UM1AI068634 (Statistical and Data Management Center) from the National Institute of Allergy and Infectious Diseases and supported by National Institute of Mental Health (NIMH), National Institute of Dental and Craniofacial Research (NIDCR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Part of the data were presented at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta GA, March 3–6, 2013 (Abstract P-187).
Conflicts: Dr. Aberg serves on AbbVie, Janssen and Merck scientific advisory boards; Dr. Aberg has moved affiliation to the ICAHN School of Medicine at Mount Sinai.
References
- 1.Soriano V, Barreiro P, Sherman KE. The Changing Epidemiology of Liver Disease in HIV Patients. AIDS reviews. 2013 Jan-Mar;15(1):25–31. [PubMed] [Google Scholar]
- 2.Puoti M, Moioli MC, Travi G, Rossotti R. The burden of liver disease in human immunodeficiency virus-infected patients. Seminars in liver disease. 2012 May;32(2):103–113. doi: 10.1055/s-0032-1316473. [DOI] [PubMed] [Google Scholar]
- 3.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection--a global challenge. The New England journal of medicine. 2012 May 10;366(19):1749–1752. doi: 10.1056/NEJMp1201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iser DM, Lewin SR. The pathogenesis of liver disease in the setting of HIV-hepatitis B virus coinfection. Antiviral therapy. 2009;14(2):155–164. [PubMed] [Google Scholar]
- 5.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009 Apr 10;58(RR-4):1–207. [PubMed] [Google Scholar]
- 6.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2013. [Accessed July 23, 2013]. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 7.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America; 2013. [Accessed July 23, 2013]. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. [Google Scholar]
- 8.EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. Journal of hepatology. 2012 Jul;57(1):167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 9.EASL Clinical Practice Guidelines: management of hepatitis C virus infection. Journal of hepatology. 2011 Aug;55(2):245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Avihingsanon A, Lewin SR, Kerr S, et al. Efficacy of tenofovir disoproxil fumarate/emtricitabine compared with emtricitabine alone in antiretroviral-naive HIV-HBV coinfection in Thailand. Antiviral therapy. 2010;15(6):917–922. doi: 10.3851/IMP1645. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou Y, Fleury H, Trimoulet P, et al. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006 Mar;43(3):548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 12.Dore GJ, Cooper DA, Pozniak AL, et al. Efficacy of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. The Journal of infectious diseases. 2004 Apr 1;189(7):1185–1192. doi: 10.1086/380398. [DOI] [PubMed] [Google Scholar]
- 13.Engell CA, Pham VP, Holzman RS, Aberg JA. Virologic Outcome of Using Tenofovir/Emtricitabine to Treat Hepatitis B in HIV-Coinfected Patients. ISRN gastroenterology. 2011;2011:405390. doi: 10.5402/2011/405390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain MK, Comanor L, White C, et al. Treatment of hepatitis B with lamivudine and tenofovir in HIV/HBV-coinfected patients: factors associated with response. Journal of viral hepatitis. 2007 Mar;14(3):176–182. doi: 10.1111/j.1365-2893.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- 15.Lada O, Gervais A, Branger M, et al. Long-term outcome of primary non-responders to tenofovir therapy in HIV/HBV-co-infected patients: impact of HBV genotype G. Liver Int. 2012 Jan;32(1):93–101. doi: 10.1111/j.1478-3231.2011.02601.x. [DOI] [PubMed] [Google Scholar]
- 16.Lok AS, Trinh H, Carosi G, et al. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naive patients with chronic hepatitis B. Gastroenterology. 2012 Sep;143(3):619–628. e611. doi: 10.1053/j.gastro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Nunez M, Ramos B, Diaz-Pollan B, et al. Virological outcome of chronic hepatitis B virus infection in HIV-coinfected patients receiving anti-HBV active antiretroviral therapy. AIDS research and human retroviruses. 2006 Sep;22(9):842–848. doi: 10.1089/aid.2006.22.842. [DOI] [PubMed] [Google Scholar]
- 18.Peters MG, Andersen J, Lynch P, et al. Randomized controlled study of tenofovir and adefovir in chronic hepatitis B virus and HIV infection: ACTG A5127. Hepatology. 2006 Nov;44(5):1110–1116. doi: 10.1002/hep.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Bommel F, Wunsche T, Mauss S, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004 Dec;40(6):1421–1425. doi: 10.1002/hep.20464. [DOI] [PubMed] [Google Scholar]
- 20.Soriano V, Perelson AS, Zoulim F. Why are there different dynamics in the selection of drug resistance in HIV and hepatitis B and C viruses? The Journal of antimicrobial chemotherapy. 2008 Jul;62(1):1–4. doi: 10.1093/jac/dkn175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV clinical trials. 2008 Jul-Aug;9(4):269–282. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999 Nov;30(5):1302–1306. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 23.Childs K, Joshi D, Byrne R, et al. Tenofovir based combination therapy for HIV/HBV co-infection: Factors associated with a partial HBV virological response in patients with undetectable HIV viraemia. AIDS. Feb 21; doi: 10.1097/QAD.0b013e32836011c2. [DOI] [PubMed] [Google Scholar]
- 24.Matthews GV, Avihingsanon A, Lewin SR, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naive individuals in Thailand. Hepatology. 2008 Oct;48(4):1062–1069. doi: 10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 25.Price H, Dunn D, Pillay D, et al. Suppression of HBV by Tenofovir in HBV/HIV Coinfected Patients: A Systematic Review and Meta-Analysis. PloS one. 2013;8(7):e68152. doi: 10.1371/journal.pone.0068152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitrinos KM, Corsa A, Liu Y, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2013 Aug 12; doi: 10.1002/hep.26686. [DOI] [PubMed] [Google Scholar]
- 27.Hamers RL, Zaaijer HL, Wallis CL, et al. HIV-HBV coinfection in Southern Africa and the effect of lamivudine- versus tenofovir-containing cART on HBV outcomes. Journal of acquired immune deficiency syndromes. 2013 Oct 1;64(2):174–182. doi: 10.1097/QAI.0b013e3182a60f7d. [DOI] [PubMed] [Google Scholar]
- 28.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007 Feb;45(2):507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.