Abstract
Astrocytic gap junctional communication is important in steroid hormone regulation of reproductive processes at the level of the hypothalamus, including estrous cyclicity and sexual behavior. We examined the effects of estradiol and progesterone on the abundance of the gap junctional protein, connexin 43 (CX43), which is highly expressed in astrocytes. Gonadectomized rats received hormone treatments that induce maximal sexual behavior and gonadotropin surges in females (estrogen for 48 h followed by progesterone, estrogen alone or progesterone alone). Control animals received vehicle (oil) injections. In the female rat preoptic area (POA), containing the gonadotropin-releasing hormone (GnRH) cell bodies, treatment with estrogen, progesterone or estrogen + progesterone significantly increased CX43 protein levels in immunoblots. In contrast, estrogen + progesterone significantly decreased CX43 levels in the male rat POA. This sexually dimorphic hormonal regulation of CX43 was not evident in the hypothalamus, which contains primarily GnRH nerve terminals. Treatment with estrogen + progesterone significantly decreased CX43 levels in both the male and female hypothalamus. To examine the role of CX43 in female reproductive function, we studied heterozygous female CX43 (CX43+/−) mice. Most mutant mice did not show normal estrous cycles. In addition, when compared to wild type females, CX43+/− mice had reduced lordosis behavior. These data suggest that hypothalamic CX43 expression is regulated by steroid hormones in a brain-region-specific and sexually dimorphic manner. Therefore, gap junctional communication in the POA and hypothalamus may be a factor regulating the estrous cycle and sexual behavior in female rodents.
Keywords: Preoptic area, Hypothalamus, Estrogen, Progesterone, Sex differences, Sexual behavior, Lordosis
1. Introduction
Gap junctions are membrane channels that permit direct electrical and biochemical communication between adjacent cells. The extent of intercellular coupling and the expression of gap junction proteins, known as connexins, are controlled by many factors, including neurotransmitters, protein kinases, growth factors and other endogenous compounds [1,8,51]. Gap junctional communication and connexin expression are also regulated by steroid hormones in gonadal tissue and play a role in various aspects of fertility and reproduction [16,48,53,66,67].
Hormonal regulation of gap junctions also occurs in the brain [32,43,47], suggesting that steroid hormone regulation of gap junctional communication may play a role in neural processes important for reproductive physiology and behavior. Central control of reproduction occurs in the hypothalamus, primarily via gonadotropin releasing hormone (GnRH) neurons [36]. GnRH stimulates the release of pituitary gonadotropins, which regulate the release of gonadal steroid hormones, including estradiol and progesterone. These, in turn, act in the hypothalamus to control sexual behavior, such as lordosis, in female mice and rats [12,20]. While estrogen is necessary for sexual behavior in female rats and mice, the addition of progesterone to estrogen-primed females results in maximal sexual receptivity and a full GnRH surge [12,38,54]. In contrast, male rodents do not exhibit surges of GnRH, even if primed with estrogen and progesterone [13]. Such sex differences in neuroendocrine function correlate with sex differences in hypothalamic neurons and glia, including sexually dimorphic astrocyte number and morphology [2,7,10,24].
Several lines of evidence suggest that hormonal regulation of hypothalamic gap junctional communication may be an important factor in regulating reproductive function. (1) Synchronized exocytotic bursts in cultures of immortalized GnRH neurons are blocked by inhibitors of gap junctions and are correlated with the extent of dye coupling between these cells [22,34,41,60]. (2) The high levels of estrogen and progesterone during pregnancy increase both connexin expression and dye coupling in the hypothalamus [32,43], and estrogen increases the incidence of gap junctions in the arcuate hypothalamus of female rats [47]. (3) Increases in dye coupling accompany high levels of hypothalamic peptide hormone release in lactating and pregnant animals [32,49]. (4) A mutation in a human gap junction protein can also result in hypogonadotropic hypogonadism [33].
Although these data suggest that steroid regulation of hypothalamic gap junction expression might be important for reproductive competence, there is at present no definitive ultrastructural evidence of gap junctions between GnRH neurons in adult brains. In fact, GnRH cell bodies are dispersed widely in the basal forebrain and are few in number [3]. This raises the possibility that gap junctions in non-neuronal cells, specifically astrocytes, could modulate synaptic transmission in the reproductive hypothalamus [5,15,23,37,52,69]. In fact, astrocyte morphology and protein expression in the hypothalamus are also regulated by estrogen and progesterone [23,25,59]. Furthermore, rapid changes in astrocyte morphology and protein expression occur in the hypothalamus during the estrous cycle, pregnancy and lactation [9,26,32,50,58]. This is specifically true of astrocytes that are in contact with cell bodies of GnRH neurons in the anterior hypothalamus [9], suggesting that altered astrocyte structure and function may also play a role in the preovulatory surge of GnRH [23,50,52,69].
We therefore investigated the potential role of gap junctions in neural control of reproductive function at the level of the hypothalamus by examining the effects of estradiol and progesterone on expression of connexin 43 (CX43), a gap junctional protein highly expressed in astrocytes. The hormone treatments included estradiol alone, which primes sexual behavior and the GnRH surge in ovariectomized females, estradiol + progesterone, which induce maximal sexual behavior and a full GnRH surge, or progesterone alone, which does not affect sexual behavior or induce a GnRH surge. We hypothesized that the hormonal effects on CX43 expression would be sexually dimorphic because male rats do not respond to hormone priming with lordosis behavior or a surge of GnRH. We investigated both the preoptic area (POA), which contains the majority of GnRH cell bodies, and the middle/posterior hypothalamus (HYP), containing the GnRH nerve terminals. In addition, we used heterozygous CX43 mice (CX43+/−) to determine if reduced CX43 expression affects the estrous cycle and female sexual behavior.
2. Methods
2.1. Rats
Sprague–Dawley female and male rats (150–175 g) were purchased from Taconic Farms (Germantown, NY) and housed two or three per cage on a 14/10 h light/dark cycle with ad libitum access to food and water. Rats were anesthetized with ketamine/xylazine (Henry Schein, Denver, PA) and bilaterally ovariectomized (OVX) in the case of females or castrated (Cast) in the case of males. Animals were allowed to recover for 7–10 days before injections began. All procedures involving animals were performed in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
2.2. CX43+/− mice
Mating pairs of CX43 hemizygous mice (CGJA1M1) were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained in accredited animal facilities. Genotyping was performed using PCR analysis as previously described [14]. Previously published results demonstrate an approximately 50% reduction of CX43 protein expression in CX43+/− mice when compared to wild type (WT) controls [14]. Females were 2–6 months old at the time of testing.
3. Steroids and injections
The steroid hormones estradiol benzoate and progesterone were purchased from Steraloids, Inc. (Newport, RI) and dissolved in peanut oil so that each hormone injection was given in a volume of 100 μl. Gonadectomized rats received one of the following four hormone treatments. Vehicle-injected controls received 100 μl peanut oil at 0, 24 and 48 h; estradiol only rats received 2 μg estradiol benzoate at 0 and 24 h and oil at 48 h; estradiol + progesterone rats received the same estrogen treatment plus 500 μg of progesterone at 48 h; progesterone only rats received vehicle (100 μl oil) at 0 and 24 h and progesterone at 48 h (see Table 1). The animals were killed by decapitation at either 2 or 4 h after the final injection, the brain dissected and the tissue frozen on dry ice. The hypothalamus was divided into an anterior region (POA, containing the GnRH cell bodies) and a middle/posterior region (HYP, containing the GnRH nerve terminals) by cutting through the optic chiasm. The resulting POA sections thus include the suprachiasmatic nucleus and related septal regions containing GnRH cell bodies while the HYP sections include the median eminence. There were no significant differences between the 2 h and 4 h time points in females; therefore, these data are combined in the statistical analyses and figures. Males were all sacrificed 2 h after the final injection. For experiments comparing CX43 protein levels in gonadectomized and intact females, intact female rats were killed early in the morning (between 8:00–11:00) and processed as described below.
Table 1.
The time and doses of hormone injections for all treatment groups used in the Western blot evaluation (Figs. 1 and 2) of CX43 levels are shown in the first 3 columns
| Hormone condition | Time of injection
|
Sample size
|
|||
|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | Female | Male | |
| Control | Oil | Oil | Oil | 15 | 5 |
| E | 2 μg estradiol benzoate | 2 μg estradiol benzoate | Oil | 15 | 5 |
| E + P | 2 μg estradiol benzoate | 2 μg estradiol benzoate | 500 μg progesterone | 15 | 5 |
| P | Oil | Oil | 500 μg progesterone | 15 | 4 |
Sample sizes for castrated males and ovariectomized females in each treatment group are shown in the last 2 columns. Female rats were killed 2–4 h after the last injection. Males were killed 2 h after the last injection.
4. Estrous cycle determination and sexual behavior
Estrous cycle stages were determined by vaginal lavage as described previously [6]. Female mice (8 CX43+/− mice and 6 WT) were staged for 12 consecutive days. These determinations were made by an experimenter blind to the condition of the animal. Normal estrous cycle was defined as demonstrating at least of one full estrous cycle of 4–6 days, including a proestrous phase demonstrating a high number of nucleated epithelial cells.
Several measures of sexual behavior were recorded in mice with an estrous or proestrous smear starting at 1 h after lights out under red lights. These include sexual receptivity (lordosis quotient) and proceptivity (time spent in female-initiated contact with the male). Female mice were placed in a cage (19 cm × 30 cm) with a sexually active stimulus male. Stimulus males were alternated to prevent fatigue in a randomized, matched-block design such that equal numbers of WT and CX43+/− females were exposed to an individual male. The test was conducted until males had mounted an individual female 10 times. The lordosis quotient was defined as the number of times the female exhibited lordosis divided by the number of mounts multiplied by 100. Due to the low incidence of proestrus in CX43+/− females, the majority of these subjects were tested during vaginal estrus. Therefore, an equivalent proportion of WT mice were also tested during vaginal estrus. The incidence of female behaviors that were defensive or aggressive was also recorded. These included biting or attempted biting, escape behaviors, scratching and distress vocalizations. The total time spent in female-initiated contacted included any female-engendered grooming, sniffing, following or tactile stimulation.
5. Western blots
Relative abundance of CX43-immunoreactive protein was quantified in 30 μg samples after SDS-PAGE in 10% gels and standard Western blotting procedures with rabbit anti-CX43 (Zymed, South San Francisco, CA) at a 1:8000 dilution. The specificity of this antibody was confirmed by analyzing protein expression using positive controls provided by Zymed (lysate from a clonal cell line expressing CX43) and tissues known to contain high levels of CX43 (e.g., heart) or to have little or no CX43 (e.g., liver) and by comparing this antibody to a previously well-characterized antibody generously donated by Dr. E. Hertzberg (data not shown) [63]. The single 43–44 kD band was visualized after incubating with a peroxidase-conjugated anti-rabbit secondary antibody (Sigma, 1:5000) using enhanced chemoluminescence (Perkin-Elmer, Boston, MA). Bands were analyzed with Kodak 1D software and adjusted for total protein concentration based on the density of the 37 kD glyceraldehyde 3-phosphate dehydrogenase (GAPDH) band after stripping and reprobing with anti-GAPDH, 1:5000 (Chemicon, Temecula, CA). Data from Western blots are presented as a ratio to controls (gonadectomized, vehicle-injected subjects) in the relevant figures.
The experiments were done in blocks of 12–14 animals each so that approximately equal numbers of samples representing all treatment conditions were represented on a single blot. Samples were not compared among individual blots but to the controls on an individual blot within a single experimental block. Sample sizes for female HYP and POA were 15 in each hormone treatment and, for males, sample sizes were 4–5 in each hormone treatment.
In experiments comparing intact and gonadectomized rats, data are presented as a ratio to OVX females. Intact females were staged by vaginal smear through 2 full cycles to ensure that they were undergoing normal 4–5 day estrous cycles. Animals in each stage (3 diestrus, 2 proestrus and 2 estrus) were killed in the early morning (between 8 and 11 AM). At this time, rats in diestrus and estrus would have low to moderate estrogen levels, and rats in estrus would have low to intermediate levels of progesterone. Rats in proestrus would have high levels of estrogen but low progesterone because they are killed before the progesterone surge. A previous pilot study did not indicate any significant differences in CX43 expression among the stages of the estrous cycles when animals were killed at this time (data not shown).
6. Statistics
Data from Western blots were analyzed by ANOVA followed by a post-hoc Fishers PLSD. Sample sizes for hormone and vehicle-treated female tissues are 6 at 2 h after the final injection and 9 at 4 h after the final injection in each group. For male tissues, the sample sizes is 4–5 for each hormone treatment. Sample sizes for the comparisons between gonadectomized and intact animals are 5–7 in both females and males. The incidence of normal estrous cycles in CX43+/− mice compared to WT was analyzed by a Chi square because this is the appropriate non-parametric test of statistical significance for bivariate analysis. Sexual behaviors, including lordosis quotient, defensive/aggressive behaviors and amount of time spent in female-initiated contact were analyzed by unpaired t tests.
7. Results
7.1. Connexin 43 expression is regulated in a brain-region- and sex-specific manner
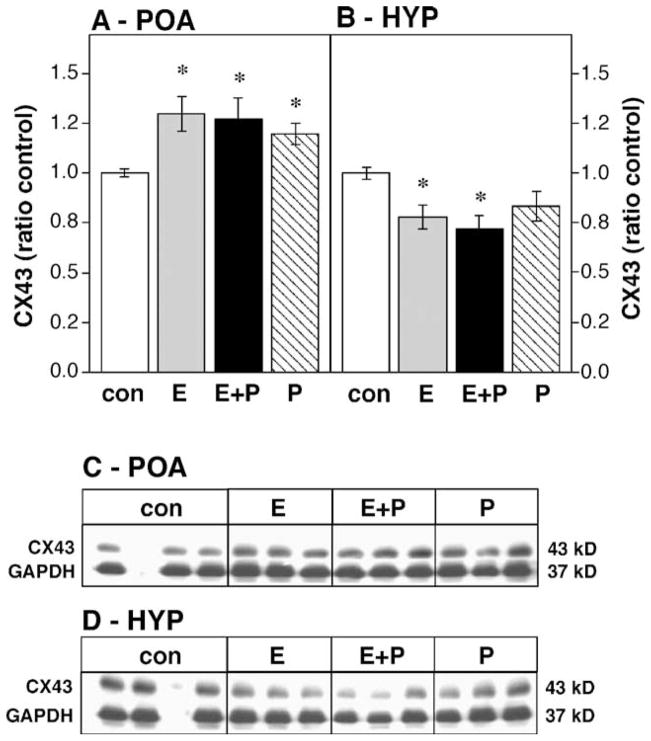
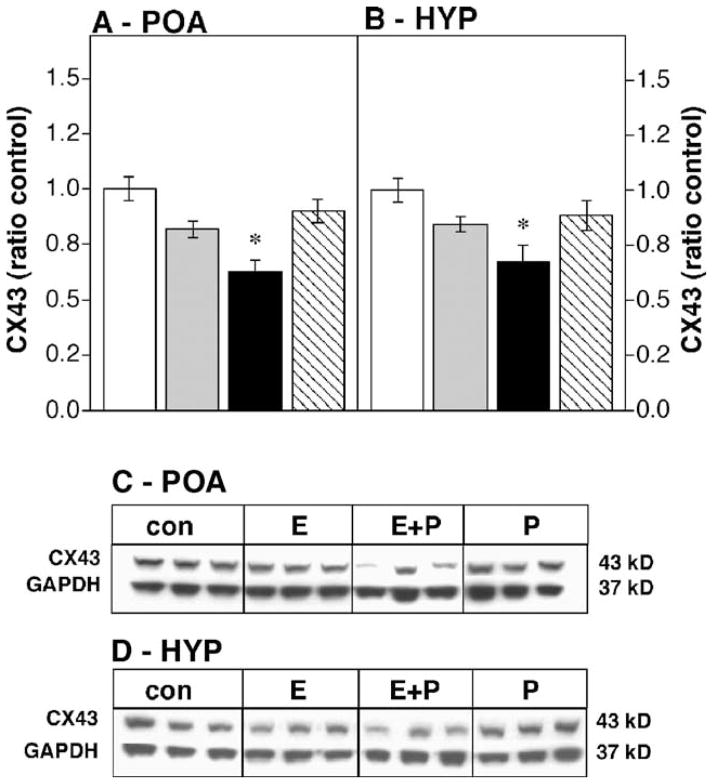
Hormone treatments significantly modified CX43 immunoreactivity in the OVX female POA (Figs. 1A and C, df 3,56; F = 4.19, P < 0.01). Progesterone alone ( P < 0.03), estradiol alone ( P < 0.005) or estradiol in combination with progesterone ( P < 0.005) increased CX43 immunoreactivity when compared to oil-injected OVX controls. Hormonal treatment also regulated CX43 expression in the male POA (Figs. 2A and C, df 3,15; F = 11.94, P < 0.01), but in contrast to the female POA, only the combination of estrogen + progesterone altered CX43 expression ( P < 0.005), decreasing CX43 protein levels.
Fig. 1.
Effects of steroid hormones on CX43 protein levels in the OVX female POA (A) and HYP (B) expressed as a ratio to oil-injected controls (con, white bars). Hormone treatments include estradiol benzoate for 48 h (E, gray bars), progesterone for 2 or 4 h (P, hatched bars) or the combination of estradiol for 48 h followed by progesterone for 2 or 4 h (E + P, black bars); n = 15 in each group. * Significantly different from control ( P < 0.05). Data are expressed as means ± standard error for this and the following graphs. (C and D) Representative Western blots showing CX43 and GAPDH bands for the female POA and HYP, respectively. The blank lane in the control condition contained molecular weight markers.
Fig. 2.
Effects of steroid hormones on CX43 protein levels in the castrated male POA (A) or HYP (B) expressed as a ratio to oil-injected controls; n = 4–5 in each group. For abbreviations, see Fig. 1. * Significantly different from control (P < 0.05). (C and D) Representative Western blots showing CX43 and GAPDH bands for the male POA and HYP, respectively.
Steroid hormones also decreased CX43 immunoreactivity in the HYP of both female (Figs. 1D and 2D, df 3,56; F = 4.11, P < 0.01) and castrated male rats (Figs. 2B and C, df 3,15; F = 11.94, P < 0.01). In the female HYP, either estrogen alone ( P < 0.02) or the combination of estrogen + progesterone ( P < 0.005) decreased CX43 expression while progesterone only injections were without effect. In the male HYP, only the combination of estrogen + progesterone significantly decreased CX43 expression ( P < 0.02).
7.2. The effect of gonadectomy on CX43 expression in male and female rats
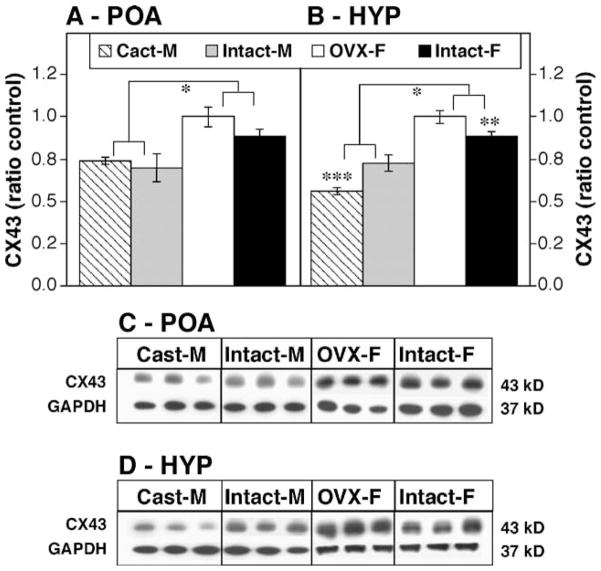
Female rats consistently had higher levels of CX43 immunoreactivity in the POA regardless of gonadal status when compared to males (Figs. 3A and C, df 1,18; Fsex = 21.35, P < 0.001). Gonadectomy in either sex did not significantly alter CX43 levels in the POA when compared to intact animals of the same sex. In contrast, there was both a main effect of sex and an interaction between sex and gonadal status in the expression of CX43 protein in the HYP (Figs. 2B and D, df 1,18 Fsex = 88.46, P < 0.001; interaction sex × gonadal status, F = 19.6, P < 0.001). Similar to the POA, females had higher levels of CX43 in the HYP than males regardless of gonadal status. In addition, intact females had lower CX43 expression than OVX females ( P < 0.01), and intact males had higher CX43 levels than castrated males ( P < 0.005).
Fig. 3.
Effects of sex and gonadectomy on CX43 levels, expressed as ratio to intact controls, in the POA (A) and HYP (B). Groups include ovariectomized females (OVX-F, white bars), intact females (Intact-F, black bars), castrated males (Cast-M, hatched bars) and intact males (Intact-M, gray bars); n = 5 – 7 in each treatment group. *Significantly different ( P < 0.05); **significantly different from OVX-F ( P < 0.05); ***significantly different from Intact-M. (C and D) Representative Western blots illustrating CX43 and GAPDH bands are shown for the POA and HYP, respectively.
7.3. CX43+/− mice have reduced sexual receptivity and proceptivity and impaired estrous cycles
Sexual receptivity was reduced in female CX43+/− mice as measured by a decreased lordosis quotient (Fig. 4A; t = 3.667, P < 0.01). A reduction in female proceptivity was also demonstrated by CX43+/− mice in that they spent significantly less time in female-initiated contact as compared to WT mice (Fig. 4B; t = 4.036, P < 0.005). Further evidence of reduced female receptivity was that female CX43+/− mice displayed a higher number of defensive or aggressive acts during the testing period (Fig. 4C; t = 2.408, P < 0.05).
Fig. 4.
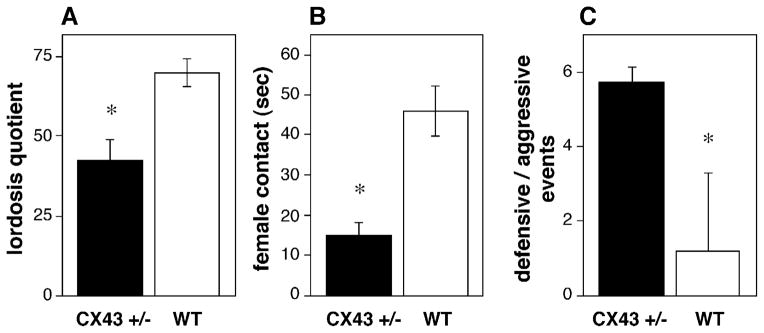
Sexual behavior is disrupted in CX43+/− mice. (A) Lordosis quotient in CX43+/− mice (black bars) compared to wild type mice (WT, white bars). (B) The total amount of female-initiated contact. (C) The incidence of aggressive or defensive events initiated by female mice during the mating test. *Significantly different from WT ( P < 0.05), n = 4 WT and n = 8 CX43+/−.
Estrous cyclicity was also impaired in female CX43+/− mice. A failure to exhibit normal estrous cycles occurred in significantly more CX43+/− mice (1 out of 8 cycling) than in WT (5 out of 6 cycling; χ2 = 7.024, P < 0.005). Furthermore, CX43+/− mice entered proestrus fewer times than WT mice during the 12-day observation period (t = 3.272, P < 0.01). CX43+/− mice exhibited three patterns of abnormal cycles. The majority of the mice were in constant estrus (5 of the 8 CX43+/− mice spent between 5–8 consecutive days in estrus). One of the CX43+/− mice was in constant diestrus (9 consecutive days) and the remaining mice alternated between estrus and diestrus, spending 2–5 days in each stage.
8. Discussion
These data demonstrate that ovarian steroid hormone regulation of CX43, a gap junctional protein highly expressed in astrocytes, is sexually dimorphic and brain-region-specific. Either estrogen or progesterone alone or the combination of estrogen and progesterone increased CX43 levels in the POA of female rats. In contrast, estrogen alone or in combination with progesterone decreased CX43 levels in the male rat POA, while progesterone was without effect. The sexually dimorphic regulation of CX43 by hormones in the POA may be important in mediating reproductive physiology, because this brain region is important in the sex-specific estrogen-dependent induction of the preovulatory GnRH surge [35,44]. In contrast to the POA, the HYP is thought to regulate the tonic pulsatile GnRH secretion that is evident in both sexes. Accordingly, the hormonal regulation of CX43 was more similar between the sexes in this brain region. The combination of estrogen and progesterone reduced CX43 levels in the HYP of both males and females. However, there were still some sex differences; estrogen alone reduced CX43 expression in the HYP of female but not male rats.
It is difficult to test the functional significance of steroid hormone regulation of hypothalamic CX43 levels in rats. Some classes of gap junctional inhibitors, such as 18β-glycyrrhetinic acid, octanol and halothane [18], are unsuitable for prolonged (i.e., at least 48 h) in vivo use because of their additional actions at chloride channels. Furthermore, the majority of gap junction inhibitors lack specificity for individual connexins [56,57]. Effective use of peptide inhibitors or antisense to suppress CX43 expression is also hampered by the wide distribution and small number of GnRH neurons, making it difficult to ensure accurate targeting of a designated cell population.
An alternate means of assessing the functional outcomes of reduced hypothalamic CX43 levels is the use of heterozygous CX43 mice. The murine CX43 gene also seems to be modulated by estrogen [29,39]. We show here that female CX43+/− mice, in which CX43 levels are roughly half that of WT mice [14], have irregular estrous cycles and deficits in sexual behavior. We cannot rule out the possibility that extrahypothalamic CX43 expression also regulates reproductive physiology and behavior in these mice. Although hormone levels were not assessed in CX43+/− mice, the altered estrous cycles and reduced receptivity/proceptivity in the CX43+/− mice with estrous or proestrous smears would be consistent with altered steroid hormone levels. Abnormal steroid hormone synthesis might reflect altered hypothalamic drive to the pituitary and/or reduced pituitary or gonadal hormone release, any of which could reduce sexual behavior. In fact, sexual behavior may be a more sensitive assay of hypothalamic function than estrous cycle disruption, because even the CX43+/− animals with normal cycles had lower lordosis quotients (roughly 40) and lower levels of contact with males than WT females. In addition, when they do mate, female CX43+/− mice have normal litter sizes and healthy pups (data not shown), suggesting that there are no gross alterations in the pituitary, ovaries and uterus. Therefore, while not discounting a role for CX43 in pituitary or gonadal function, our present data indicate the importance of CX43 in gap junctional communication in hypothalamic regulation of reproductive physiology and behavior.
Indeed, there is evidence documenting the participation of astrocytes in the regulation of hypothalamic neuroendocrine function. Astrocytes facilitate sexual development (puberty) in part by stimulating the release of GnRH [45]. Adult female mice that express mutant tyrosine kinase receptors targeted specifically to astrocytes also have disrupted estrous cycles and reduced gonadotropin levels [40], indicating that astrocytes are important not only in the onset of puberty, but also for maintaining normal reproductive function in adults. In fact, it has been suggested that non-neuronal elements may be important in regulating estrogen sensitivity of the hypothalamus [15,62,69].
Neuronal–glial interactions in the hypothalamus may control neuroendocrine function by several different mechanisms. Astrocytes may mediate hormonal effects on hypothalamic neuronal connectivity by regulating the amount of neuronal membrane accessible to synaptic contacts and afferent input [19,49,62]. Furthermore, astrocytes may act as barriers to peptide hormone release by preventing access to the portal circulation [37,64]. Astrocytes may also regulate synaptic efficacy in the hypothalamus by altering the rates of glutamate clearance [30,46,49] or by releasing soluble factors, such as glutamate and growth factors [42,65], which promote neural plasticity and alter the sensitivity of hypothalamic neurons to their afferent inputs [19,45,49,62]. Gap junctional communication between astrocytes could potentially synchronize the release of such neuroactive compounds. Alternatively, hormone-induced alterations in the efficacy of gap junction function could engender rapid, hormone-dependent morphological changes in astrocytes via the rapid transfer of second messengers, such as calcium and cAMP [4,21,28,61]. We can also not rule out a role of CX43 in hypothalamic cells other than astrocytes, because CX43 is also expressed in other cell types, including endothelial cells.
The sex dependence of hormonal regulation of hypothalamic CX43 expression has not previously been reported. However, our data are consistent with studies demonstrating that astrocyte number, morphology and sensitivity to stimulation by a range of compounds are sex-dependent in rodents [2,7,27]. There is also a sex difference in the ability of estrogen to stimulate signaling pathways, such as the MAP kinase pathway [68], and to regulate growth factor receptors [17] in hypothalamic astrocytes. Our data are also consistent with reports that several sexually dimorphic features of the hypothalamus correlate with the inability of male rats and mice to respond to ovarian hormones with a surge of GnRH [2,10,24]. Such sex differences are evident in both neurons and glia and include sexually dimorphic astrocyte number and morphology [2,7,10,24].
Indeed, our present findings show that hypothalamic CX43 protein levels in rats differ between the sexes, with females demonstrating higher CX43 levels in both regions of the hypothalamus than males regardless of gonadal status. The effects of gonadectomy were also sex-dependent in the HYP. The increased CX43 levels in the HYP of OVX compared to intact females agree with our finding that treatment with estrogen or the combination of estrogen and progesterone conversely reduced CX43 levels in this brain region. A similar increase of CX43 levels after OVX was not observed in the female POA, perhaps because the high levels of GnRH release caused by the loss of estrogen negative feedback maintain high CX43 levels in the POA of OVX rats. The decreased levels of CX43 in the castrated male HYP compared to intact males are probably due to the loss testicular androgens and are consistent with studies indicating that testosterone increases gap junctional communication in the male hypothalamus and pituitary [11,31,55].
In summary, these data demonstrate sex differences in CX43 protein levels in the HYP and POA of adult rats and show that ovarian hormone regulation of CX43 levels is brain-region-specific and sexually dimorphic. Furthermore, the fact that female CX43+/− mice rarely display normal estrous cycles or sexual behavior suggests that gap junctions may be important factors in hypothalamic regulation of normal reproductive physiology.
Acknowledgments
Many thanks to Dr. David Spray and colleagues, Drs. Elliot Hertzberg and Alberto Pereda for provision of mice, antibodies and advice. This work was supported by DHHS Grants RO1 HD29856, R37 MH41414 and T32 DK07513 and NS41282.
References
- 1.Aberg ND, Blomstrand F, Aberg MA, Bjorklund U, Carlsson B, Carlsson-Skwirut C, Bang P, Ronnback L, Eriksson PS. Insulin-like growth factor-I increases astrocyte intercellular gap junctional communication and connexin43 expression in vitro. J Neurosci Res. 2003;74:12–22. doi: 10.1002/jnr.10734. [DOI] [PubMed] [Google Scholar]
- 2.Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett-Clarke C, Joseph SA. Immunocytochemical distribution of LHRH neurons and processes in the rat: hypothalamic and extra-hypothalamic locations. Cell Tissue Res. 1982;221:493–504. doi: 10.1007/BF00215698. [DOI] [PubMed] [Google Scholar]
- 4.Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92:255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- 5.Bruzzone R, Giaume C. Connexins and information transfer through glia. Adv Exp Med Biol. 1999;468:321–337. doi: 10.1007/978-1-4615-4685-6_26. [DOI] [PubMed] [Google Scholar]
- 6.Camano L. Histochemical study of mucus of the vaginal epithelium of rats (Rattus norvegicus var. albinus, rodentia, Mammalia), during the estrus cycle, pregnancy and postpartum period. Matern Infancia (Sao Paulo) 1969;28:31–66. [PubMed] [Google Scholar]
- 7.Cambiasso MJ, Colombo JA, Carrer HF. Differential effect of oestradiol and astroglia-conditioned media on the growth of hypothalamic neurons from male and female rat brains. Eur J Neurosci. 2000;12:2291–2298. doi: 10.1046/j.1460-9568.2000.00120.x. [DOI] [PubMed] [Google Scholar]
- 8.Cameron SJ, Malik S, Akaike M, Lerner-Marmarosh N, Yan C, Lee JD, Abe J, Yang J. Regulation of epidermal growth factor-induced connexin 43 gap junction communication by big mitogen-activated protein kinase1/ERK5 but not ERK1/2 kinase activation. J Biol Chem. 2003;278:18682–18688. doi: 10.1074/jbc.M213283200. [DOI] [PubMed] [Google Scholar]
- 9.Cashion AB, Smith MJ, Wise PM. The morphometry of astrocytes in the rostral preoptic area exhibits a diurnal rhythm on proestrus: relationship to the luteinizing hormone surge and effects of age. Endocrinology. 2003;144:274–280. doi: 10.1210/en.2002-220711. [DOI] [PubMed] [Google Scholar]
- 10.Chen WP, Witkin JW, Silverman AJ. Sexual dimorphism in the synaptic input to gonadotropin releasing hormone neurons. Endocrinology. 1990;126:695–702. doi: 10.1210/endo-126-2-695. [DOI] [PubMed] [Google Scholar]
- 11.Cobbett P, Yang QZ, Hatton GI. Incidence of dye coupling among magnocellular paraventricular nucleus neurons in male rats is testosterone dependent. Brain Res Bull. 1987;18:365–370. doi: 10.1016/0361-9230(87)90014-1. [DOI] [PubMed] [Google Scholar]
- 12.Couzinet B, Schaison G. The control of gonadotrophin secretion by ovarian steroids. Hum Reprod. 1993;8(Suppl 2):97–101. doi: 10.1093/humrep/8.suppl_2.97. [DOI] [PubMed] [Google Scholar]
- 13.Crowley WR. Sex differences in the responses of hypothalamic luteinizing hormone-releasing hormone and catecholamine systems to ovarian hormones and naloxone: implications for sexual differentiation of luteinizing hormone secretion in rats. Brain Res. 1988;461:314–321. doi: 10.1016/0006-8993(88)90261-2. [DOI] [PubMed] [Google Scholar]
- 14.Dermietzel R, Gao Y, Scemes E, Vieira D, Urban M, Kremer M, Bennett MV, Spray DC. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 15.Dhandapani KM, Mahesh VB, Brann DW. Astrocytes and brain function: implications for reproduction. Exp Biol Med (Maywood) 2003;228:253–260. doi: 10.1177/153537020322800303. [DOI] [PubMed] [Google Scholar]
- 16.Di WL, Lachelin GC, McGarrigle HH, Thomas NS, Becker DL. Oestriol and oestradiol increase cell to cell communication and connexin43 protein expression in human myometrium. Mol Hum Reprod. 2001;7:671–679. doi: 10.1093/molehr/7.7.671. [DOI] [PubMed] [Google Scholar]
- 17.Duenas M, Luquin S, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Gonadal hormone regulation of insulin-like growth factor-I-like immunoreactivity in hypothalamic astroglia of developing and adult rats. Neuroendocrinology. 1994;59:528–538. doi: 10.1159/000126702. [DOI] [PubMed] [Google Scholar]
- 18.Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Galaz MC, Morschl E, Chowen JA, Torres-Aleman I, Naftolin F, Garcia-Segura LM. Role of astroglia and insulin-like growth factor-I in gonadal hormone-dependent synaptic plasticity. Brain Res Bull. 1997;44:525–531. doi: 10.1016/s0361-9230(97)00238-4. [DOI] [PubMed] [Google Scholar]
- 20.Flanagan-Cato LM, Calizo LH, Daniels D. The synaptic organization of VMH neurons that mediate the effects of estrogen on sexual behavior. Horm Behav. 2001;40:178–182. doi: 10.1006/hbeh.2001.1679. [DOI] [PubMed] [Google Scholar]
- 21.Fujita K, Nakanishi K, Sobue K, Ueki T, Asai K, Kato T. Astrocytic gap junction blockage and neuronal Ca2+ oscillation in neuron-astrocyte cocultures in vitro. Neurochem Int. 1998;33:41–49. doi: 10.1016/s0197-0186(05)80007-5. [DOI] [PubMed] [Google Scholar]
- 22.Funabashi T, Suyama K, Uemura T, Hirose M, Hirahara F, Kimura F. Immortalized gonadotropin-releasing hormone neurons (GT1–7 cells) exhibit synchronous bursts of action potentials. Neuroendocrinology. 2001;73:157–165. doi: 10.1159/000054632. [DOI] [PubMed] [Google Scholar]
- 23.Galbiati M, Saredi S, Melcangi RC. Steroid hormones and growth factors act in an integrated manner at the levels of hypothalamic astrocytes: a role in the neuroendocrine control of reproduction. Ann N Y Acad Sci. 2003;1007:162–168. doi: 10.1196/annals.1286.016. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Segura LM, Baetens D, Naftolin F. Sex differences and maturational changes in arcuate nucleus neuronal plasma membrane organization. Brain Res. 1985;351:146–149. doi: 10.1016/0165-3806(85)90241-x. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Segura LM, Torres-Aleman I, Naftolin F. Astrocytic shape and glial fibrillary acidic protein immunoreactivity are modified by estradiol in primary rat hypothalamic cultures. Brain Res Dev Brain Res. 1989;47:298–302. doi: 10.1016/0165-3806(89)90186-7. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Segura LM, Luquin S, Parducz A, Naftolin F. Gonadal hormone regulation of glial fibrillary acidic protein immunoreactivity and glial ultrastructure in the rat neuroendocrine hypothalamus. Glia. 1994;10:59–69. doi: 10.1002/glia.440100108. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Segura LM, Duenas M, Busiguina S, Naftolin F, Chowen JA. Gonadal hormone regulation of neuronal–glial interactions in the developing neuroendocrine hypothalamus. J Steroid Biochem Mol Biol. 1995;53:293–298. doi: 10.1016/0960-0760(95)00066-9. [DOI] [PubMed] [Google Scholar]
- 28.Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- 29.Grummer R, Hewitt SW, Traub O, Korach KS, Winterhager E. Different regulatory pathways of endometrial connexin expression: preimplantation hormonal-mediated pathway versus embryo implantation-initiated pathway. Biol Reprod. 2004;71:273–281. doi: 10.1095/biolreprod.103.024067. [DOI] [PubMed] [Google Scholar]
- 30.Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, Thorlin T, Nilsson M, Ronnback L. Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem Int. 2000;37:317–329. doi: 10.1016/s0197-0186(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 31.Hatton GI, Yang QZ, Koran LE. Effects of ovariectomy and estrogen replacement on dye coupling among rat supraoptic nucleus neurons. Brain Res. 1992;572:291–295. doi: 10.1016/0006-8993(92)90487-t. [DOI] [PubMed] [Google Scholar]
- 32.Hatton GI, Yang QZ. Peripartum interneuronal coupling in the supraoptic nucleus. Brain Res. 2002;932:120–123. doi: 10.1016/s0006-8993(02)02279-5. [DOI] [PubMed] [Google Scholar]
- 33.Houang M, Gourmelen M, Moatti L, Le BY, Garabedian EN, Denoyelle F. Hypogonadotrophic hypogonadism associated with pre-lingual deafness due to a connexin 26 gene mutation. J Pediatr Endocrinol Metab. 2002;15:219–223. doi: 10.1515/jpem.2002.15.2.219. [DOI] [PubMed] [Google Scholar]
- 34.Hu L, Olson AJ, Weiner RI, Goldsmith PC. Connexin 26 expression and extensive gap junctional coupling in cultures of GT1–7 cells secreting gonadotropin-releasing hormone. Neuroendocrinology. 1999;70:221–227. doi: 10.1159/000054480. [DOI] [PubMed] [Google Scholar]
- 35.Jarry H, Leonhardt S, Schwarze T, Wuttke W. Preoptic rather than mediobasal hypothalamic amino acid neurotransmitter release regulates GnRH secretion during the estrogen-induced LH surge in the ovariectomized rat. Neuroendocrinology. 1995;62:479–486. doi: 10.1159/000127037. [DOI] [PubMed] [Google Scholar]
- 36.Keri G, Horvath A, Nikolics K, Teplan I. On the central role of GnRH in the regulation of reproductive functions: mechanism of action and practical results. J Steroid Biochem. 1985;23:719–723. doi: 10.1016/s0022-4731(85)80007-8. [DOI] [PubMed] [Google Scholar]
- 37.King JC, Rubin BS. Dynamic changes in LHRH neurovascular terminals with various endocrine conditions in adults. Horm Behav. 1994;28:349–356. doi: 10.1006/hbeh.1994.1031. [DOI] [PubMed] [Google Scholar]
- 38.Leadem CA, Kalra SP. Stimulation with estrogen and progesterone of luteinizing hormone (LH)-releasing hormone release from perifused adult female rat hypothalami: correlation with the LH surge. Endocrinology. 1984;114:51–56. doi: 10.1210/endo-114-1-51. [DOI] [PubMed] [Google Scholar]
- 39.Lefebvre DL, Piersanti M, Bai XH, Chen ZQ, Lye SJ. Myometrial transcriptional regulation of the gap junction gene, connexin-43. Reprod Fertil Dev. 1995;7:603–611. doi: 10.1071/rd9950603. [DOI] [PubMed] [Google Scholar]
- 40.Li B, Yang Z, Hou J, McCracken A, Jennings MA, Ma MY. Compromised reproductive function in adult female mice selectively expressing mutant ErbB-1 tyrosine kinase receptors in astroglia. Mol Endocrinol. 2003;17:2365–2376. doi: 10.1210/me.2003-0023. [DOI] [PubMed] [Google Scholar]
- 41.Matesic DF, Hayashi T, Trosko JE, Germak JA. Upregulation of gap junctional intercellular communication in immortalized gonadotropin-releasing hormone neurons by stimulation of the cyclic AMP pathway. Neuroendocrinology. 1996;64:286–297. doi: 10.1159/000127130. [DOI] [PubMed] [Google Scholar]
- 42.Melcangi RC, Galbiati M, Messi E, Magnaghi V, Cavarretta I, Riva MA, Zanisi M. Astrocyte–neuron interactions in vitro: role of growth factors and steroids on LHRH dynamics. Brain Res Bull. 1997;44:465–469. doi: 10.1016/s0361-9230(97)00227-x. [DOI] [PubMed] [Google Scholar]
- 43.Micevych PE, Popper P, Hatton GI. Connexin 32 mRNA levels in the rat supraoptic nucleus: up-regulation prior to parturition and during lactation. Neuroendocrinology. 1996;63:39–45. doi: 10.1159/000126933. [DOI] [PubMed] [Google Scholar]
- 44.Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143:2284–2292. doi: 10.1210/endo.143.6.8869. [DOI] [PubMed] [Google Scholar]
- 45.Ojeda SR, Ma YJ, Lee BJ, Prevot V. Glia-to-neuron signaling and the neuroendocrine control of female puberty. Recent Prog Horm Res. 2000;55:197–223. (discussion 223–224) [PubMed] [Google Scholar]
- 46.Ozog MA, Siushansian R, Naus CC. Blocked gap junctional coupling increases glutamate-induced neurotoxicity in neuron–astrocyte co-cultures. J Neuropathol Exp Neurol. 2002;61:132–141. doi: 10.1093/jnen/61.2.132. [DOI] [PubMed] [Google Scholar]
- 47.Perez J, Tranque PA, Naftolin F, Garcia-Segura LM. Gap junctions in the hypothalamic arcuate neurons of ovariectomized and estradiol-treated rats. Neurosci Lett. 1990;108:17–21. doi: 10.1016/0304-3940(90)90699-a. [DOI] [PubMed] [Google Scholar]
- 48.Petrocelli T, Lye S. Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology. 1993;133:284–290. doi: 10.1210/endo.133.1.8391423. [DOI] [PubMed] [Google Scholar]
- 49.Piet R, Poulain DA, Oliet SH. Contribution of astrocytes to synaptic transmission in the rat supraoptic nucleus. Neurochem Int. 2004;45:251–257. doi: 10.1016/j.neuint.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Prevot V. Glial–neuronal–endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol. 2002;14:247–255. doi: 10.1046/j.0007-1331.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- 51.Reuss B, Unsicker K. Regulation of gap junction communication by growth factors from non-neural cells to astroglia: a brief review. Glia. 1998;24:32–38. [PubMed] [Google Scholar]
- 52.Richter TA, Keen KL, Terasawa E. Synchronization of Ca(2+) oscillations among primate LHRH neurons and nonneuronal cells in vitro. J Neurophysiol. 2002;88:1559–1567. doi: 10.1152/jn.2002.88.3.1559. [DOI] [PubMed] [Google Scholar]
- 53.Roscoe WA, Barr KJ, Mhawi AA, Pomerantz DK, Kidder GM. Failure of spermatogenesis in mice lacking connexin43. Biol Reprod. 2001;65:829–838. doi: 10.1095/biolreprod65.3.829. [DOI] [PubMed] [Google Scholar]
- 54.Rubin BS, Barfield RJ. Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrinology. 1983;113:797–804. doi: 10.1210/endo-113-2-797. [DOI] [PubMed] [Google Scholar]
- 55.Sakuma E, Herbert DC, Soji T. The effects of sex steroids on the formation of gap junctions between folliculo-stellate cells; a study in castrated male rats and ovariectomized female rats. Arch Histol Cytol. 2003;66:229–238. doi: 10.1679/aohc.66.229. [DOI] [PubMed] [Google Scholar]
- 56.Spray DC, Rozental R, Srinivas M. Prospects for rational development of pharmacological gap junction channel blockers. Curr Drug Targets. 2002;3:455–464. doi: 10.2174/1389450023347353. [DOI] [PubMed] [Google Scholar]
- 57.Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]
- 58.Theodosis DT, Poulain DA. Evidence for structural plasticity in the supraoptic nucleus of the rat hypothalamus in relation to gestation and lactation. Neuroscience. 1984;11:183–193. doi: 10.1016/0306-4522(84)90222-7. [DOI] [PubMed] [Google Scholar]
- 59.Tranque PA, Suarez I, Olmos G, Fernandez B, Garcia-Segura LM. Estradiol-induced redistribution of glial fibrillary acidic protein immunoreactivity in the rat brain. Brain Res. 1987;406:348–351. doi: 10.1016/0006-8993(87)90805-5. [DOI] [PubMed] [Google Scholar]
- 60.Vazquez-Martinez R, Shorte SL, Boockfor FR, Frawley LS. Synchronized exocytotic bursts from gonadotropin-releasing hormone-expressing cells: dual control by intrinsic cellular pulsatility and gap junctional communication. Endocrinology. 2001;142:2095–2101. doi: 10.1210/endo.142.5.8123. [DOI] [PubMed] [Google Scholar]
- 61.Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in [cAMP] and protein kinase a in mammalian oocytes. Dev Biol. 2002;246:441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- 62.Witkin JW, Ferin M, Popilskis SJ, Silverman AJ. Effects of gonadal steroids on the ultrastructure of GnRH neurons in the rhesus monkey: synaptic input and glial apposition. Endocrinology. 1991;129:1083–1092. doi: 10.1210/endo-129-2-1083. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T, Ochalski A, Hertzberg EL, Nagy JI. On the organization of astrocytic gap junctions in rat brain as suggested by LM and EM immunohistochemistry of connexin43 expression. J Comp Neurol. 1990;302:853–883. doi: 10.1002/cne.903020414. [DOI] [PubMed] [Google Scholar]
- 64.Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro–glial interaction between GnRH nerve terminals and glial endfeet in Japanese quail. Endocrinology. 2004;145:4264–4267. doi: 10.1210/en.2004-0366. [DOI] [PubMed] [Google Scholar]
- 65.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.You S, Li W, Lin T. Expression and regulation of connexin43 in rat Leydig cells. J Endocrinol. 2000;166:447–453. doi: 10.1677/joe.0.1660447. [DOI] [PubMed] [Google Scholar]
- 67.Yu W, Dahl G, Werner R. The connexin43 gene is responsive to oestrogen. Proc R Soc London, Ser B Biol Sci. 1994;255:125–132. doi: 10.1098/rspb.1994.0018. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L, Li B, Zhao W, Chang YH, Ma W, Dragan M, Barker JL, Hu Q, Rubinow DR. Sex-related differences in MAPKs activation in rat astrocytes: effects of estrogen on cell death. Brain Res Mol Brain Res. 2002;103:1–11. [PubMed] [Google Scholar]
- 69.Zwain IH, Arroyo A, Amato P, Yen SS. A role for hypothalamic astrocytes in dehydroepiandrosterone and estradiol regulation of gonadotropin-releasing hormone (GnRH) release by GnRH neurons. Neuroendocrinology. 2002;75:375–383. doi: 10.1159/000059434. [DOI] [PubMed] [Google Scholar]