Abstract
Transient global ischemia induces selective, delayed neuronal death in the hippocampal CA1 and delayed cognitive deficits. Estrogen treatment ameliorates hippocampal injury associated with global ischemia. Although much is known about the impact of estrogen on neuronal survival, relatively little is known about its impact on functional outcome assessed behaviorally. We investigated whether long-term estradiol (21-day pellets implanted 14 days prior to ischemia) or acute estradiol (50 μg infused into the lateral ventricles immediately after ischemia) attenuates ischemia-induced cell loss and improves visual and spatial working memory in ovariectomized female rats. Global ischemia significantly impaired visual and spatial memory, assessed by object recognition and object placement tests at 6–9 days. Global ischemia did not affect locomotion, exploration, or anxiety-related behaviors, assessed by an open-field test at 6 days. Long-term estradiol prevented the ischemia-induced deficit in visual working memory, maintaining normal performance in tests with retention intervals of up to 1 h. Long-term estradiol also prevented ischemia-induced deficits in spatial memory tests with short (1 and 7 min), but not longer (15 min), retention intervals. Acute estradiol significantly improved visual memory assessed with short retention intervals, but did not prevent deficits in spatial memory. Acute estradiol significantly increased the number of surviving CA1 neurons, assessed either at 7 days after ischemia or after the completion of behavioral testing 9 days after ischemia. In contrast, chronic estradiol did not reduce CA1 cell death 9 days after ischemia. Thus, long-term estradiol at near physiological levels and acute estradiol administered after ischemic insult improve functional recovery after global ischemia. These findings have important implications for intervention in the neurological sequellae associated with global ischemia.
Keywords: Spatial memory, Visual memory, Estrogen, Hippocampus, Female, Neuroprotection
Introduction
Transient global ischemia induces selective, delayed cell death of pyramidal neurons in the CA1 hippocampus (Jover et al., 2002; Pulsinelli and Brierley, 1979) and causes cognitive deficits in rodents, primates, and humans (Block and Schwarz, 1997; Tanabe et al., 1999; Volpe et al., 1985; Whishaw et al., 1994; Zola-Morgan et al., 1992). An important question is whether agents that protect CA1 neurons from ischemia-induced cell death also reduce functional deficits in cognition. One putative neuroprotective agent is the ovarian steroid hormone, estradiol. Estradiol and related estrogens inhibit the activation of apoptotic pathways (Bagetta et al., 2004; Jover et al., 2002; Rau et al., 2003; Wen et al., 2004), activate anti-apoptotic pathways (Chiueh et al., 2003; Rau et al., 2003), reduce excitotoxicity (Singer et al., 1996), and interact with growth factors (Azcoitia et al., 1999) and downstream signaling cascades (Kuroki et al., 2000) to increase neuronal survival in vivo and in vitro (Sudo et al., 1997).
Estrogens are neuroprotective in animal models of ischemia. Estrogen administered before induction of focal ischemia reduces the infarct size in intact and gonadectomized male (Toung et al., 1998) and ovariectomized (OVX) female rats (Dubal et al., 1998). In global ischemia models, both estradiol and estrogen receptor agonists administered before the ischemic insult reduce hippocampal cell loss in male and female gerbils (Jover et al., 2002; Shughrue and Merchenthaler, 2003), rats (Bagetta et al., 2004; He et al., 2002; Miller et al., 2005; Sudo et al., 1997), and mice (Carswell et al., 2004; Horsburgh et al., 2002). Estrogen administration before induction of ischemia also increases the number of intact synapses in male gerbils (Sudo et al., 1997). However, with one exception (Kondo et al., 1997), such studies have not evaluated any aspect of hippocampal function in estrogen-treated animals after global ischemia.
The goal of the present work was to study the functional outcomes after global ischemia in OVX female rats and the potential cognitive benefits of estradiol treatment. Cell number is not a direct measure of cell function, and neuron counts are not consistently correlated with cognitive performance after injury (Kondo et al., 1997; Nunn et al., 1994b). Furthermore, the effect of estrogen on hippocampal function after global ischemia has not been well studied in females, even though estrogen treatments can differentially affect memory performance and spine density in hippocampal CA1 in male and female subjects (Lee et al., 2004; Lund et al., 2001; Miranda et al., 1999). Moreover, the existing behavioral studies in rodents focus on spatial memory, whereas human studies suggest impairments in other memory processes after selective hippocampal damage (Cave and Squire, 1991).
To investigate the functional impact of estradiol treatment on ischemia-induced cognitive deficits in female rats, we utilized the object placement and object recognition tasks to assess spatial and visual memory, respectively. The first experiment investigated the role of long-term estradiol treatment (chronic) via estradiol pellets, which were implanted 2 weeks before the ischemic episode and remained in place for the week after ischemia during which the animals were tested. In view of recent clinical studies indicating risks associated with chronic estrogen replacement in postmenopausal women (Anderson et al., 2004), examination of the efficacy of estrogens administered after induction of ischemia is warranted. Estradiol administration shortly after focal ischemia can improve sensorimotor function, but cognition has not been systematically assessed (Li et al., 2004). Therefore, in the second experiment, a single estradiol treatment (acute) was administered intra-cerebroventricularly (icv) immediately after induction of ischemia.
Methods
Animals
Sprague–Dawley female rats (100–130 g at the time of ischemia induction) were purchased from Charles River (Wilmington, MA) and housed 4 per cage on a 14/10 h light/ dark cycle with ad lib access to food and water. Rats were anesthetized with inhaled halothane (Halocarbon Labs, River Edge, NJ) and N2O:O2 (70:30), administered by mask using a Vapomatic anesthetic vaporizer (CWE Inc., Ardmore, PA) and OVX 2 weeks before induction of ischemia. Behavioral tests were conducted in the light phase of the cycle, between 7:30 AM and 7:30 PM on days 6–9 after induction of ischemia. All procedures involving animals were performed in accordance with NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine.
Ischemia surgery
The four vessel occlusion model of transient global ischemia was employed essentially as described previously (Pulsinelli and Brierley, 1979). Briefly, on day 13 after OVX, the vertebral arteries were exposed through a midline occipital–suboccipital incision and coagulated with bipolar cauterization between the first and second cervical vertebral bodies. This procedure by itself has no effect on cerebral blood flow but prevents collateral circulation to the fore-brain during subsequent transient carotid artery occlusion on day 14. Twenty-four hours later, transient global ischemia was induced by temporary, bilateral occlusion of the common carotid arteries for 10 min followed by reperfusion (Fig. 1). Sham-operated rats had their vertebral arteries coagulated and underwent all other surgical procedures except for carotid artery occlusion. A rectal probe was inserted, and stable core body temperature was maintained using a heat lamp during surgical procedures. Ischemia was ensured by monitoring the loss of righting reflex and bilateral pupil dilation of each subject. A total of 13 animals, distributed among the treatment groups, died during surgery and hence did not undergo behavioral testing or histological analysis. Behavior testing began 6 days after ischemia to permit animals to recover from surgery and to make sure that testing occurred at a time when the majority of CA1 pyramidal neurons have died in ischemic animals (Ordy et al., 1993; Sugawara et al., 2002).
Fig. 1.
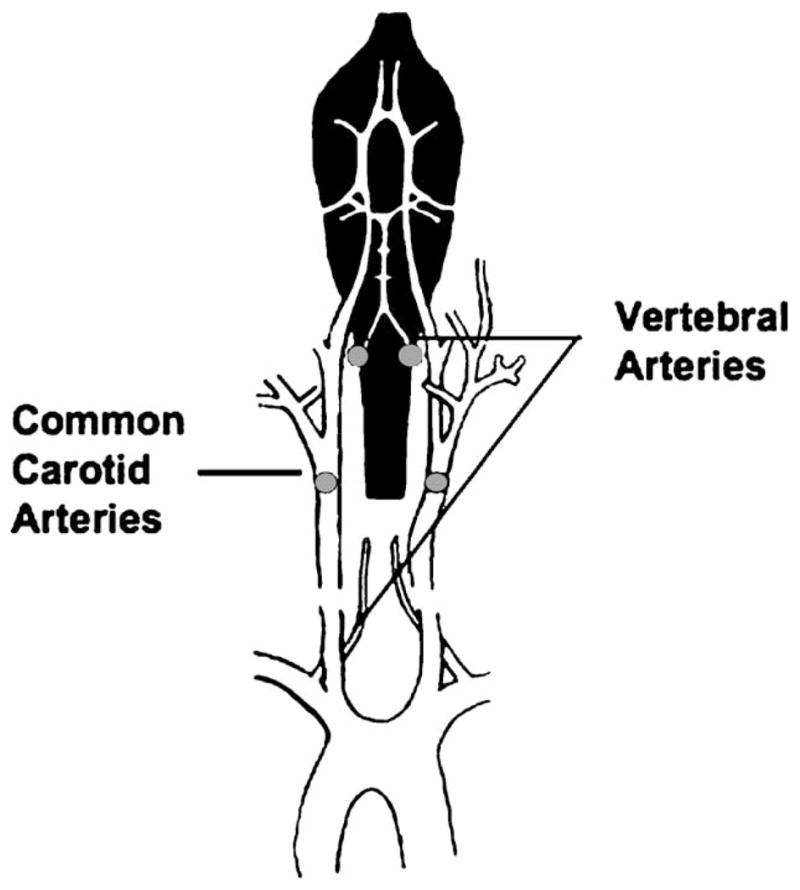
Transient global ischemia is induced in OVX female rats in a two-stage process. On day 13 after OVX, the vertebral arteries are coagulated at the position indicated by gray dots with bipolar cauterization. This procedure does not block cerebral blood flow but prevents collateral circulation to the forebrain during the carotid artery clamp on day 14. Twenty-four hours after vertebral artery coagulation, transient global ischemia is induced by occluding the common carotid arteries for 10 min followed by reperfusion. Sham-operated rats undergo vertebral artery coagulation and all other surgical procedures except for carotid artery occlusion. Figure is reprinted with permission from Zukin et al. (2004).
Behavioral testing
Open field
Six days after ischemia the animals were tested in an open field, consisting of a 27 × 27 in square black box with a floor divided into 9 equal squares, the sides of which were 3 ft high. After allowing the animals to acclimatize to the testing room for 30–45 min, behavior was recorded for 6 min. The behaviors measured included locomotor activity, assessed as the number of grid crosses and defined as crossing one of the grids to at least the midline of the body. The number of rears, a measure of exploratory activity, was defined as lifting of the upper body and forepaws off the ground. The amount of time spent in the center square, an indicator of anxiety levels, was defined as entering the square at least up to the midline of the body, and was measured in seconds. For this and the following behavioral measures, animals were tested in random order in a matched block design such that roughly equal numbers of animals in each treatment group were represented in each testing block. The experimenter was blind to the condition of the animals.
Object placement and object recognition tasks
During days 6–9 after ischemia induction and 1–2 h after open field testing, visual and spatial memory were assessed in the object recognition and placement task essentially as described previously (Ennaceur and Meliani, 1992). These tasks utilize the innate tendency of rats to preferentially explore novel objects, and thus do not require food or water deprivation or exposure to stressful conditions. Furthermore, because these are one-trial tasks, training before ischemia is also not required, which prevents the confounds of state-dependency and retrieval errors (Babcock and Graham-Goodwin, 1997; Blokland et al., 1998; Gaskin et al., 2003). Another advantage of these tasks is that difficulty of the task can be altered by increasing the retention interval between trials (see below). In addition, visual and spatial working memory can be assessed under similar conditions, stimulus exposures, and retention intervals.
For assessment of visual memory, rats were placed in the open field with two identical objects, and the duration of exploration of each object (sec) was recorded for 3 min (Trial 1). Exploration was defined as physical manipulation of the object (rearing toward or on object, placing paws on object, whisking or sniffing object) or orientation and looking at the object from a distance of 2 cm or less. Objects were easily distinguishable by shape and size. After a retention interval of between 1 and 60 min, rats were then placed in the same open field for 3 min (Trial 2) with one of the familiar objects and a novel object. The time spent exploring both objects (s) was recorded. These data are presented in the figures and text as an exploratory preference score, defined as the time spent exploring the novel object divided by the total time spent exploring both objects. An exploratory preference score of 50% indicates chance performance, while higher preference scores indicate better memory performance.
Spatial memory was assessed in a similar way, except that in Trial 2 the rat was exposed again to the same 2 identical objects, one of which was displaced in space. In this case, the preference score was defined as the time spent exploring the displaced object divided by the total time spent exploring both objects. Because there were no internal visual cues within the open field, care was taken to ensure that the intrinsic relationship between the objects was changed, rather than just the placement within the box.
Animals were tested in a matched block design (8–12 animals per block) with roughly equal animals in each treatment group in a series of 7 blocks. Within a single block, all animals were exposed to the same permutations of objects and placements per trial, but these were randomly varied between blocks. The order of the retention intervals was also identical for a single block, but randomly varied between blocks to ensure that neither the time after ischemia, nor practice and fatigue effects confounded assessment of memory performance. In this way, all animals underwent both spatial and visual memory testing at multiple retention intervals. Retention intervals were chosen on the basis of pilot studies (data not shown) and published data (Luine et al., 2003) demonstrating the window within which control animals can adequately perform the task. Each block of animals was given 2–3 tests (a complete test consisting of Trial 1 and Trial 2) per day with a 2 h interval between tests. Animals were excluded from the analysis of a single test if they did not explore the objects during either Trial 1 or Trial 2. Between 0 and 3 animals per treatment group per retention interval were excluded for these reasons. Chi square analysis did not show any significant effects of treatment or retention interval on exclusions (data not shown).
An exception to the matched block design was the 60 min retention interval for the visual task and the 1 min retention interval for the spatial task. These were not included in the first 4 blocks of tests. When it became evident that longer and shorter retention intervals were needed, these were added for the last 3 blocks. Thus, sample sizes for these retention intervals are smaller than the others. The number of subjects for each task, retention interval and treatment condition are shown in the relevant figures and differ due to these exclusion criteria and surgical mortality.
Estradiol assay
Blood was collected by cardiac puncture when animals were killed for histological assessment and centrifuged at 1000×g for 5 min. Sera were collected and stored at −20°C until analyzed. Samples (50 μl) were assayed for estradiol levels by fluoroimmunoassay using the DELPHIA estradiol assay (Perkin Elmer Life Sciences, Turku, Finland). All assays were performed in duplicate and the mean value reported. The sensitivity of detection is 14 pg/ml.
Effects of chronic estradiol on cognitive function after ischemia
Estradiol administration
For experiments investigating the effects of long-term estradiol treatment, experimental subjects were implanted with either 21-day sustained-release 17β-estradiol pellets (50 μg, Innovative Research, Sarasota, FL) or placebo subcutaneously on the dorsal side of the neck immediately after OVX. This form of estradiol administration results in relatively stable physiological levels of estradiol during a 3 week period after implantation. These procedures resulted in 4 treatment groups in the chronic estradiol experiments: Sham (sham surgery, placebo); Estradiol + Sham (sham surgery, estradiol); Ischemia (ischemia surgery, placebo); and Estradiol + Ischemia (ischemia surgery, estradiol).
Quantitation of pyramidal neuron survival
Chronic estradiol treatment significantly increases CA1 pyramidal neuron survival, assessed at 3–7 days after ischemia in male and OVX female gerbils (Jover et al., 2002; Shughrue and Merchenthaler, 2003) and in OVX female (He et al., 2002; Miller et al., 2005; Wang et al., 1999) and male rats (Bagetta et al., 2004). In the present study, histological analysis was performed on animals in the chronic estradiol experiment after completion of behavioral testing 9 days after ischemia. Animals were deeply anesthetized and then transcardially perfused using 0.9% saline with heparin (50–60 ml/15 min) followed by ice cold 10% phosphate buffered formalin (PBS, 200 ml for 20 min; Fisher Scientific, Pittsburgh, PA). Brains were then removed, placed in formalin at 4°C overnight, fixed in 30% sucrose in PBS at 4°C for 48 h, and then frozen. The dorsal hippocampus was coronally sectioned in a cryostat into 15 μm sections. Every fourth section was mounted and stained with toluidine blue. The CA1 region of the left and right hippocampus of 4 sections from each animal was photographed at 40× magnification, and surviving pyramidal neurons in a region of interest (or box) of 250 μm × 60 μm were counted. Data are expressed as the mean number of surviving neurons per side. Neuronal counts were restricted to pyramidal cells in the stratum pyramidale of the CA1. The stratum pyramidale is >95% pyramidal neurons; most glia and the cell bodies of inhibitory interneurons are localized to the stratum radiatum and stratum oriens. It is well established that a brief (10 min) episode of global ischemia affords highly selective, delayed death of CA1 pyramidal neurons (Herguido et al., 1999; Jover et al., 2002; Ordy et al., 1993; Pulsinelli and Brierley, 1979). In this paradigm, neurons in other brain regions are not affected, including inhibitory interneurons of the CA1 and all neurons in the nearby CA2 or transition zone, CA3 and dentate gyrus.
Effects of acute estradiol on ischemia-induced deficits in cognition
Estradiol administration
To investigate the effects of acute estradiol administration on functional recovery after ischemia, estradiol (50 μg/10 μl of 17-β-estradiol, cyclodextrin-encapsulated, Sigma) was infused icv immediately after reperfusion on the day of ischemia induction while animals were still under halothane anesthesia. Control animals were infused with 10 μl of vehicle (0.9% saline). Animals were positioned in a stereotaxic frame and a stainless steel cannula (28 gauge) was lowered into the right lateral ventricle to a position defined by the following coordinates: 0.92 mm posterior to bregma, 1.2 mm lateral to bregma, 3.6 mm below the skull surface. Estradiol or vehicle was infused by unilateral injection into the right lateral ventricle at a flow rate of 5 μl/min.
CA1 pyramidal cell survival
In the acute experiments, 2 groups of OVX animals were perfused and histology performed as in experiment 1. All animals were subjected to global ischemia and were infused acutely with estradiol or vehicle as described above. Previous studies indicate that vehicle infusions do not significantly impact cell survival (Miller et al., 2005). All rats were allowed to survive for 7 days without behavioral testing. Once cell counts confirmed that acute estradiol increased CA1 pyramidal cell survival on day 7, an additional group of animals underwent ischemia or sham surgery. Ischemic animals were infused acutely with estradiol or vehicle. All animals were killed following behavioral testing on day 9 after ischemia. Cell counts from ischemic animals receiving vehicle infusions and killed 7 days after ischemia were not statistically different than those of animals killed 9 days after ischemia (14.8 ± 2.5 vs. 16.8 ± 7.3 per 250 μm, respectively) and so were combined in the statistical analyses and figures and referred to as “Ischemia”.
Statistics
Data from behavioral experiments were analyzed using Statview® with a two-way ANOVA (hormone condition × ischemia) at each retention interval, followed by Fisher’s PLSD. Cell counts were analyzed using a two-way ANOVA with Statview®. Associations between memory performance and cell survival, based on CA1 neuron counts performed after the completion of memory tests, were analyzed using a two-way analysis of co-variance (ANCOVA) at each retention interval. For behavioral tests, sham animals were also compared to a small number of animals that did not get surgery to insure that coagulation of the vertebral arteries did not affect performance in the behavioral tasks. Because sham-operated and unoperated rats were not statistically different in any behavioral measures (see Table 1), these groups were combined in the graphs and analyses and are denoted as “sham”.
Table 1.
Neither sham surgery nor estradiol alters behavioral performance
| Sham surgery | Estradiol | N | Visual exploratory preference score (%) | Spatial exploratory preference score (%) | Total time exploring objects (s) | Grid crosses (number of events) | Mean neuronal count (CA1) |
|---|---|---|---|---|---|---|---|
| − | − | 8 | 73.4 ± 2.3 | 67.9 ± 2.8 | 13.6 ± 2.4 | 60.1 ± 7.1 | 78.1 ± 2.0 |
| − | + | 6 | 74.7 ± 4.0 | 61.9 ± 5.1 | 12.5 ± 1.8 | 76.4 ± 10.5 | 82.3 ± 5.6 |
| + | − | 8 | 72.3 ± 3.1 | 70.2 ± 4.2 | 19.1 ± 3.3 | 67.8 ± 4.5 | 78.0 ± 1.5 |
| + | + | 7 | 73.7 ± 3.3 | 68.9 ± 4.2 | 14.1 ± 2.1 | 66.6 ± 8.3 | 80.5 ± 1.6 |
Summary data of behavioral performance scores assessed in ovariectomized female rats treated for 14 days with placebo (−) or estradiol (+) and subjected to no surgical procedure (−) or to sham operation (+). Mean visual preference score is the average preference score (±SEM) across all retention intervals in the object recognition test. Mean spatial preference score is the average preference score (±SEM) across all retention intervals in the object placement test. Total time exploring both objects in Trial 1 of all memory tests is indicated in seconds (±SEM). Number of grid crosses (±SEM) in the open field during a 6-min trial (±SEM). Mean neuronal counts (±SEM) indicated that sham surgery did not significantly alter CA1 cell survival compared to no surgery.
Results
Effects of chronic estradiol treatment on visual and spatial working memory deficits induced by ischemia
Chronic estradiol treatment prevents ischemia-induced deficits in visual memory
Transient global ischemia (four vessel occlusion, 10 min) in adult rats induces selective, delayed neuronal death, primarily of the hippocampal CA1 (Jover et al., 2002; Pulsinelli and Brierley, 1979), a brain region widely demonstrated to play a role in working memory (Galani et al., 1998; Whishaw et al., 1994). To examine ischemia-induced deficits in cognition and the ability of estradiol to ameliorate these deficits, we subjected animals to behavioral assays of visual and spatial working memory. Global ischemia induced a deficit in visual memory, as assessed by the object recognition task and evident at retention times greater than 1 min (Fig. 2 and Table 2). At the 1-min retention interval, animals in all treatment groups behaved similarly. At longer retention intervals, ischemic rats exhibited significantly impaired visual memory relative to sham-operated control rats, as assessed by the object recognition task (Fig. 2). Estradiol administration did not significantly affect visual memory performance in sham-operated (control) animals, but significantly improved visual memory scores in animals subjected to global ischemia (Fig. 2). Post hoc analysis revealed that at the 1 min retention interval, all groups showed a preference for the novel object in the object recognition task with no statistical difference among the visual preference scores of any treatment group (Fig. 2). This indicates that neither ischemia nor estradiol altered the sensorimotor functions necessary to perform the task or the underlying preference for novel objects. At all retention intervals longer than 1 min, ischemic rats had deficits in visual working memory when compared to sham rats (Fig. 2, P < 0.05) and performed at chance levels (i.e., preference score ~50%). The visual working memory performance of estradiol-treated sham rats did not differ from placebo-treated sham rats at any retention time examined, demonstrating that this regime of chronic estradiol treatment did not affect performance in sham-operated control animals. In contrast, chronic treatment with estradiol improved visual preference scores in the object recognition task at 10, 20, and 60 min retention intervals in ischemic rats when compared to placebo-treated, ischemic rats (Fig. 2, P < 0.05). Thus, global ischemia induced clear deficits in visual working memory, evident at 6–9 days, which were prevented by long-term administration of estradiol. Details of ANOVAs on performance in the object recognition task are shown in Table 2.
Fig. 2.
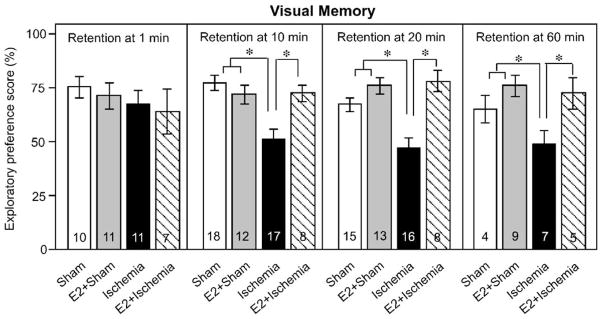
Chronic estradiol improves visual recognition memory performance in rats subjected to global ischemia. Visual working memory was assessed by the object recognition task. Data are reported as the exploratory preference score (novel object exploration / total object exploration, %; X ± SEM) for 3-min trials. At the 1 min retention interval, animals in all treatment groups behave similarly. At longer retention intervals, ischemic rats (Ischemia) exhibit significantly impaired visual memory relative to sham-operated control rats (Sham). Estradiol (E2) does not alter visual recognition memory scores in sham rats (E2 + Sham), but markedly reduces memory deficits in ischemic rats (E2 + Ischemia). Sample sizes are indicated within the bars. *P < 0.05.
Table 2.
Statistical analysis of the impact of long-term estradiol and ischemia on visual and spatial working memory
| Factors | Visual task retention interval
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 min
|
10 min
|
20 min
|
60 min
|
|||||
| F value | P value | F value | P value | F value | P value | F value | P value | |
| Hormone condition (+ or − estradiol) | 0.41 | 0.53 | 1.64 | 0.20 | 24.71 | 0.01 | 10.54 | 0.01 |
| Surgical condition (+ or − ischemia) | 0.84 | 0.37 | 6.24 | 0.02 | 6.06 | 0.02 | 4.78 | 0.05 |
| Hormone condition − surgical condition | 0.05 | 0.95 | 6.93 | 0.01 | 8.80 | 0.01 | 2.70 | 0.12 |
| Factors | Spatial task retention interval
|
|||||
|---|---|---|---|---|---|---|
| 1 min
|
7 min
|
15 min
|
||||
| F value | P value | F value | P value | F value | P value | |
| Hormone condition (+ or − estradiol) | 5.56 | 0.02 | 3.93 | 0.05 | 0.08 | 0.78 |
| Surgical condition (+ or − ischemia) | 0.14 | 0.91 | 9.23 | 0.01 | 8.14 | 0.01 |
| Hormone condition × surgical condition | 3.98 | 0.05 | 5.05 | 0.03 | 0.55 | 0.47 |
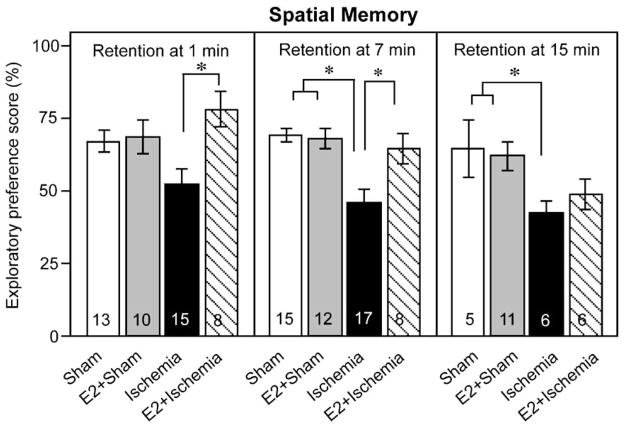
Chronic estradiol treatment reduces ischemic deficits in spatial memory at short but not longer retention intervals
It is well-established that the CA1 region of the hippocampus plays a role in spatial working memory (Broadbent et al., 2004) and that global ischemia causes loss of CA1 pyramidal neurons and spatial memory (Block and Schwarz, 1997; Jover et al., 2002). However, little is known about the ability of estradiol to ameliorate ischemia-induced deficits in spatial memory. We next subjected estradiol- and placebo-treated animals to global ischemia and assessed spatial working memory in the object placement task. Global ischemia induced a pronounced deficit in spatial working memory (Fig. 3). Long-term estradiol treatment did not significantly alter spatial memory scores in sham-operated rats at any retention interval examined, but significantly improved spatial memory scores in animals subjected to global ischemia when tested at short (1 and 7 min), but not long (15 min), retention intervals (Fig. 3). Post hoc analysis revealed that when tested with a 1-min retention interval, placebo-treated rats subjected to global ischemia performed at chance level (Fig. 3, left panel). All other groups showed a preference for the displaced object after the 1-min retention interval (Fig. 3). Chronic treatment with estradiol significantly improved performance of ischemic animals compared to that of placebo-treated, ischemic rats (Fig. 3, P < 0.05) at both the 1- and 7-min retention intervals, but not at the 15-min retention interval. Although ischemic rats did not significantly differ in their performance scores relative to sham rats when assessed with the 1-min retention interval, they exhibited significant deficits in spatial memory assessed with retention intervals of 7 and 15 min (Fig. 3, P < 0.05). Thus, global ischemia produces deficits in spatial working memory at retention times longer than 1 min, and chronic estradiol treatment improves spatial working memory, assessed at shorter (1- and 7-min) retention intervals. Details of ANOVAs for spatial preference scores are shown in Table 2.
Fig. 3.
Chronic estradiol improves spatial working memory performance in rats subjected to global ischemia. Spatial working memory was assessed by the object placement task. Data are reported as the exploratory preference score (displaced object exploration / total object exploration, %; X ± SEM) for 3-min trials. Ischemic rats (Ischemia) show impaired spatial memory relative to sham-operated rats (Sham). Chronic estradiol does not significantly alter spatial working memory scores in sham rats (E2 + Sham), but markedly reduces memory deficits in ischemic rats (E2 + Ischemia) at 1 and 7 min retention intervals. Estradiol-treated ischemic rats perform at sham levels at 1 and 7 min retention intervals. *P < 0.05.
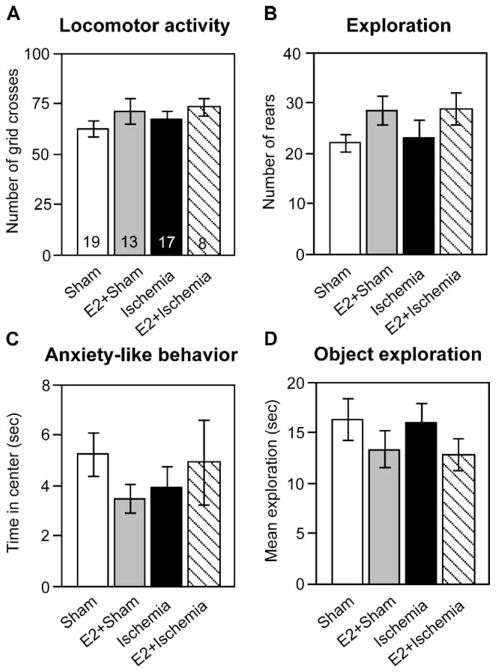
Sensorimotor function and anxiety-related behavior are not altered by chronic estradiol treatment or ischemia
Both chronic estradiol treatment and ischemic damage to the hippocampus could conceivably alter behaviors not specifically related to memory, such as locomotor activity or anxiety-related behaviors, which could confound assessment of memory performance. We therefore tested animals in the open field and recorded the extent of object exploration in Trial 1 of the memory tasks. There was a significant main effect of hormone treatment on the number of rears ( F = 5.423, P < 0.05), with estradiol-treated animals rearing more often, but post hoc analysis did not reveal any significant differences as a function of ischemia. Neither total locomotor activity (grid crosses) nor anxiety-like behavior (time spent in the center) was significantly different among treatment groups (Fig. 4). The mean total time spent exploring both objects in Trial 1 was also not significantly different among treatment groups (Fig. 4). Therefore, neither estradiol treatment nor ischemia surgery significantly affects sensorimotor function, anxiety, or object exploration.
Fig. 4.
Neither global ischemia nor chronic estradiol alters sensorimotor performance in the open field. (A) Locomotor activity was assessed as the number of grid crosses. (B) Exploration of the novel arena was assessed as the number of rears. (C) Anxiety-like behavior was assessed as the time (s) spent in the center of the open field. (D) Total time in exploration was assessed as the average time (s) spent exploring both objects in Trial 1 of the object placement and object recognition tests. Open field tests were of 6-min duration, and trials in the object placement and object recognition tasks were of 3-min duration. Neither global ischemia nor estradiol significantly altered sensorimotor performance in any of the four tests. Samples sizes are shown within the bars in panel A and are the same for all panels in this figure. All data are expressed as X ± SEM.
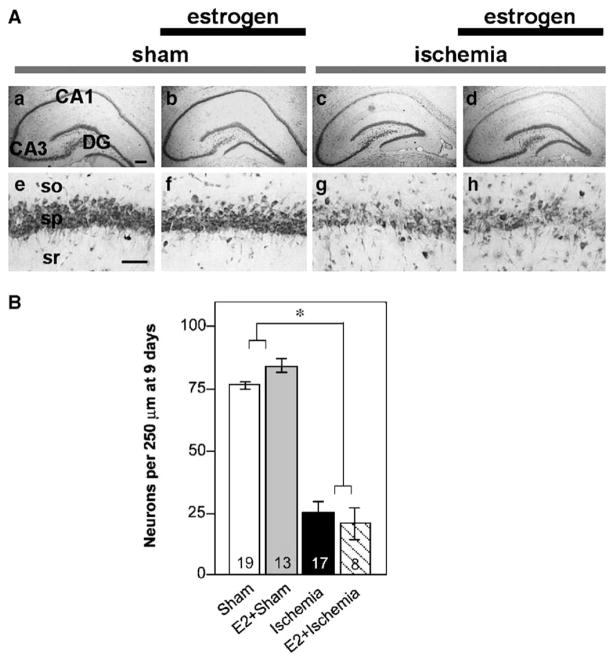
CA1 pyramidal cell survival
Long-term estradiol administration significantly reduces global ischemia-induced loss of CA1 neurons, assessed at 3 to 7 days after insult (Bagetta et al., 2004; He et al., 2002; Jover et al., 2002; Miller et al., 2005; Shughrue and Merchenthaler, 2003; Wang et al., 1999). However, little is known about the duration of estradiol neuroprotection. Cell counts performed after completion of behavioral testing on day 9 revealed that chronic estradiol did not significantly increase CA1 pyramidal neuron survival (Fig. 5). Ischemic animals treated with placebo and with estradiol exhibited similar decreases in CA1 pyramidal cell number compared to sham-operated rats treated with placebo or estradiol ( F = 266.25, P < 0.0001). There was no main effect of hormone condition and no interaction between surgical condition and estradiol. Although cell counts and memory performance were correlated with surgical condition (sham or ischemia), the number or surviving neurons (assessed by an ANCOVA) did not correlate with either visual or spatial memory performance at any retention interval within the ischemia group. ANOVA comparing sham and unoperated animals revealed no significant effects of sham surgery on CA1 cell survival (Table 1).
Fig. 5.
(A) Representative photomicrographs from OVX female rats treated with estradiol or placebo for 14 days and subjected to global ischemia or sham operation. At 9 days after reperfusion, surviving neurons were counted in the hippocampal CA1. So, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum. Scale bars: lower magnification (a to d, 4× magnification), 400 μm; higher magnification (e to h, 40× magnification), 60 μm. (B) Chronic estradiol did not increase cell survival assessed at 9 days after ischemia. Cell counts in 15 μm sections at the level of the dorsal hippocampus are presented as the average number of pyramidal neurons in the right and left halves of a 250 μm length of the CA1 region. Sample sizes are indicated at the base of the bars. Data are expressed as X ± SEM.
Estradiol levels
Mean serum estradiol levels assayed after the completion of behavioral testing in chronically implanted animals were significantly higher in OVX rats with estradiol pellets (26 ± 3.7 pg/ml) than in rats implanted with placebo (16.9 ± 2.0 pg/ ml, t = 3.522, P < 0.001). However, they are substantially lower than the levels we normally find in chronically estradiol-treated animals killed 7 days after ischemia (33–58 pg/ml; see Miller et al., 2005).
The effects of acute estradiol administration on ischemia-induced deficits in spatial and visual working memory
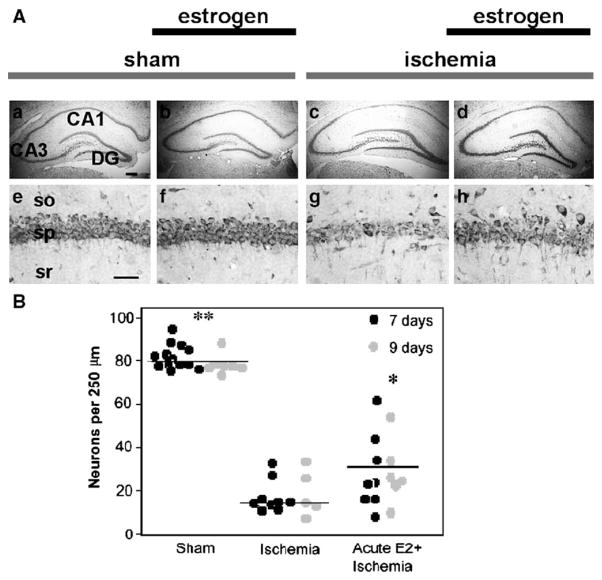
Acute estradiol significantly increases CA1 cell survival 7 days after ischemia
The data presented above demonstrate that treatment with estradiol both before and for an extended period after ischemia improves cognitive deficits in visual and spatial working memory apparent 6–9 days after ischemia. To date, few studies have examined the neuroprotective effects of a single dose of estradiol administered shortly after global ischemia. In the present study, we first showed that a high dose of estradiol administered acutely into the lateral ventricle immediately after reperfusion significantly increased CA1 cell survival 7 days after ischemia (Fig. 6, P < 0.05). In addition, acute estradiol afforded protection of CA1 pyramidal neurons, assessed after completion of behavioral testing on day 9 after ischemia (Fig. 6, P < 0.05) compared to vehicle-injected, ischemic rats. Ischemic rats injected with either vehicle or estradiol exhibited significantly fewer surviving neurons than sham rats (Fig. 6, P < 0.05).
Fig. 6.
(A) Representative photomicrographs from OVX female rats subjected to global ischemia or sham surgery, followed by acute estradiol or vehicle infusion immediately after ischemia. At 7 or 9 days after reperfusion, surviving neurons were counted in the hippocampal CA1. So, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum. Scale bars: lower magnification, 400 μm; higher magnification, 60 μm. (B) Acute estrogen treatment increases CA1 pyramidal cell survival in ischemic animals at 7 and 9 days after ischemia. One group of rats was subjected to sham surgery or ischemia, followed by placebo or estradiol treatment and was killed 7 days after surgery. A separate group was subjected to the ischemia, followed by placebo or estradiol administration, underwent subsequent behavioral testing, and was killed at 9 days after surgery. Ischemic rats exhibit similar pyramidal neuron counts in the CA1 at the two time points; thus, data from the two groups are pooled in the figure and denoted as Ischemia. Global ischemia induced a dramatic cell loss vs. sham-operated animals (Sham). Estradiol did not significantly alter neuronal survival in sham-operated animals, but afforded marked protection against ischemia-induced cell death, assessed at 7 days (black circles) or 9 days (gray circles) after surgery. Horizontal bars indicate the mean. Significant differences between Ischemia and Acute E2 + Ischemia are indicated by *P < 0.05 and between Acute E2 + Ischemia and sham are indicated by **P < 0.05.
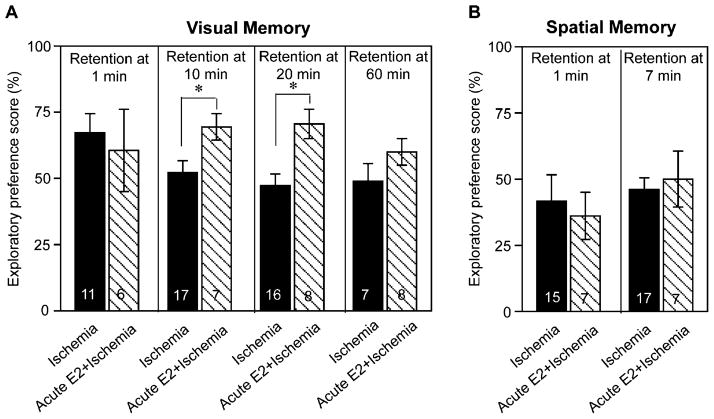
Acute estradiol treatment ameliorates ischemic deficits in visual but not spatial memory
Because acute estradiol treatment significantly improved neuronal survival after ischemia, we tested whether acute estradiol would reduce cognitive deficits in visual and spatial working memory in rats subjected to global ischemia. Acute estradiol infusion significantly improved visual working memory in rats subjected to global ischemia relative to that of vehicle-treated ischemic rats, assessed by object recognition scores at the 10- and 20-min retention intervals (Fig. 7A, P < 0.05), but not at the 60 min retention interval. In contrast, acute post-ischemic estradiol treatment did not improve spatial preference scores in animals subjected to global ischemia, assessed at any retention interval (Fig. 7B); both vehicle-treated and estradiol-treated animals subjected to global ischemia performed at chance levels at all retention intervals examined. The number of surviving pyramidal neurons did not correlate with visual or spatial memory performance at any retention interval when analyzed by ANCOVA. Details of statistics for memory tests in ischemic rats treated acutely with either vehicle or estradiol are shown in Table 3. These findings indicate that acute estradiol ameliorates ischemic deficits in visual, but not spatial memory.
Fig. 7.
The effects of acute estradiol treatment on ischemia-induced deficits in visual working memory (A) and spatial working memory (B). Data are reported as the exploratory preference score as defined in Figs. 1 and 2. (A) At retention times greater than 1 min, ischemic rats (Ischemia) perform at chance (i.e., 50%). A single icv infusion of 17β-estradiol (50 μg, Acute E2 + Ischemia) markedly improved visual preference scores in ischemic rats, assessed at the 10- and 20-but not at the 60-min retention intervals. (B) In contrast, acute estradiol treatment did not significantly improve spatial working memory in ischemic rats (compare Acute E2 + Ischemia with Ischemia). Sample sizes are denoted on individual bars. *P < 0.05.
Table 3.
Statistical analysis of the impact of estradiol administered acutely after ischemia on visual and spatial working memory
| Visual task retention interval
|
Spatial task retention interval
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 min
|
10 min
|
20 min
|
60 min
|
1 min
|
7 min
|
||||||
| F value | P value | F value | P value | F value | P value | F value | P value | F value | P value | F value | P value |
| 0.22 | 0.64 | 4.84 | 0.04 | 10.43 | 0.01 | 1.91 | 0.19 | 1.047 | 0.32 | 2.541 | 0.13 |
F and P values for visual and spatial exploratory preference scores at different retention intervals were calculated by a one-way ANOVA. Data are from Fig. 6. Acute estrogen administration markedly improved visual memory performance assessed at the 10 and 20 min retention intervals. Ischemic rats perform at chance levels in the visual test at all retention intervals longer than 1 min and in the spatial test at all retention intervals.
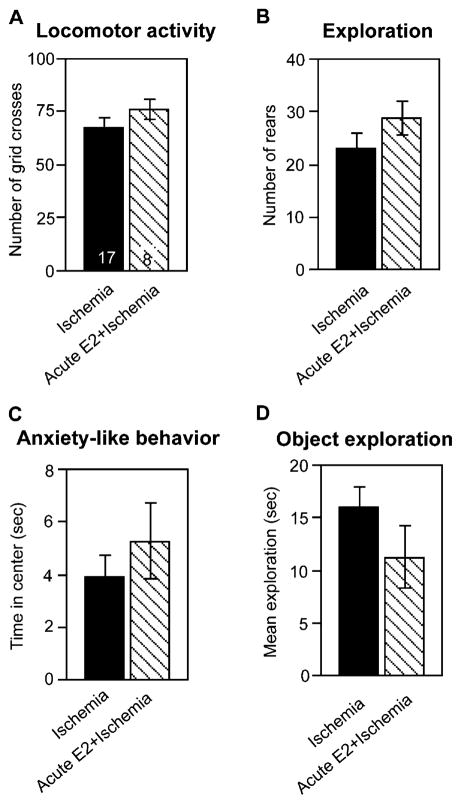
Sensorimotor performance is not altered by acute estradiol treatment
Vehicle- and estradiol-infused ischemic rats behaved similarly in tests of locomotor activity (grid crosses), exploration of the open field (rears), and anxiety-like behavior (time spent in the center), indicating that acute estradiol does not significantly affect sensorimotor function or anxiety-like behavior (Fig. 8). The treatment groups did not significantly differ in the mean total time spent exploring both objects in Trial 1 (Fig. 8).
Fig. 8.
Acute estradiol treatment does not alter sensorimotor performance. (A) Locomotor activity was assessed as the number of grid crosses. (B) Exploration was assessed as the number of rears. (C) Anxiety-like behavior was assessed as the time (s) spent in the center of the open field. (D) Object exploration was assessed as the average time (s) spent exploring both objects in Trial 1 of the object placement and object recognition tests. Open field tests were of 6-min duration, and trials in the object placement and object recognition tasks were of 3-min duration. Estradiol did not significantly alter sensorimotor performance in any tests examined. Samples sizes are denoted on individual bars in panel A and are the same for panels A–D. Data are expressed as X ± SEM.
Discussion
Transient global ischemia induces selective, delayed neuronal death in the hippocampal CA1 and may cause delayed cognitive deficits. Whereas there is considerable evidence that estradiol treatment can enhance neuronal survival after global ischemia (Bagetta et al., 2004; He et al., 2002; Jover et al., 2002; Miller et al., 2005; Shughrue and Merchenthaler, 2003; Wang et al., 1999), relatively little is known about its impact on functional outcome assessed behaviorally. Here, we document the novel finding that near physiological levels of estradiol, when present for a prolonged period both before and after ischemia, significantly protected female rats from ischemia-induced deficits in visual and spatial working memory. Moreover, a supraphysiological dose of estradiol administered immediately after induction of ischemia significantly improved functional outcome in visual, but not spatial, memory. These findings thus have important implications for intervention in the neurological sequellae associated with cardiac arrest or stroke.
Our findings underscore the specificity of the cognitive deficits induced by global ischemia and support the interpretation that the behavioral protection afforded by estradiol is not attributable to non-specific effects of estradiol on sensorimotor function, motivation, or anxiety-like behavior. Neither surgical nor hormone treatments affected locomotion or anxiety-like behavior, assessed in the open field at 6 days. It is also notable that all subjects exhibited a preference for investigating the novel object in the visual task at the 1 min retention interval. Thus, neither ischemia nor hormone treatment altered the underlying motivation to explore novel objects or produced perceptual or sensorimotor deficits that significantly affect the capacity of the animal to perform the task. The behavioral protection by estradiol demonstrated in this study is also unlikely to be confounded by non-specific effects of estradiol on performance or motivation, because acute estradiol treatment also reduced cognitive deficits induced by ischemia. In this case, estradiol is no longer present in the brain of animals subjected to behavioral testing at 6 to 9 days after ischemia and acute estradiol administration. The present study is consistent with the sole existing report that high levels of estradiol can improve behavioral deficits after global ischemia (Kondo et al., 1997). The present study extends the earlier study in that it shows for the first time that estradiol at physiological levels improves functional outcome in global ischemia. Moreover, our methodology affords several advantages. Kondo et al. (1997) reported that estradiol improved spatial memory after global ischemia, assessed in the Morris water maze. However, the high dose of estradiol used in that study affected locomotion, thus complicating interpretation of the results. Our method is less dependent on strenuous locomotor activity and permits us to assess visual and spatial memory performance in the same subjects under comparable conditions.
Surprisingly, neither spatial nor visual memory performance appears related to the number of surviving CA1 pyramidal cells or the presence of estradiol at the time of behavioral testing. Long-term estradiol was much more effective in maintaining visual and spatial working memory than was acute estradiol, but only animals treated with acute estradiol had significantly more surviving CA1 pyramidal neurons after 9 days than ischemic controls. The acute estradiol treatment modestly but significantly increased hippocampal cell survival, assessed at 7 and 9 days after ischemia, whereas the neuroprotective effects of chronic estradiol previously observed 7 days after ischemia (Jover et al., 2002; Miller et al., 2005) were no longer evident 9 days after ischemia. There were poor correlations between memory performance and CA1 pyramidal cell counts at this time point, especially in the animals given chronic estradiol. These animals exhibited considerable preservation of working memory, especially in the visual task, even though they did not have significantly higher numbers of surviving CA1 neurons at the time of sacrifice than ischemic females treated with placebo. Thus, one possible interpretation of these data is that preservation of intact, functional circuits between a few cells may be more important for maintaining cognitive performance than the absolute number of surviving cells.
Our data provide yet another example of the absence of a strict linear relationship between cell number and function in neurodegenerative disorders. Quantitative estimates of neuronal loss are poor predictors of cognitive function in Alzheimer’s patients (von Gunten et al., 2005; West et al., 2004), for example. The literature is also inconsistent regarding the relationship between cell survival and cognitive performance after ischemia, with reports of robust correlations (Kiyota et al., 1991; Olsen et al., 1994) or no correlation (Kondo et al., 1997; Nunn et al., 1994b; Poignet et al., 1989) between performance and CA1 cell number after ischemia despite the fact that these studies generally report comparable levels of cell loss after ischemia and cell survival after treatment with various neuroprotective agents. There are several possible explanations for these discrepancies. Linear correlation analyses are biased toward finding correlations between performance and cell number when sham cell counts are included, but these correlations tend to have predictive power only at either end of the distribution (e.g., very high or very low cell counts) (Nunn et al., 1994a). A less biased way to analyze this type of data is by ANCOVA. In our hands, ANCOVA did not reveal covariation between cell counts and performance except between sham and ischemic animals. Linear correlation analyses detected positive correlations when sham cell counts were included, but not when sham cell counts were excluded (data not illustrated).
There is still limited information on the cellular targets mediating the protective effects of estradiol on CA1 cell survival or hippocampal function after global ischemia. Estrogen could act both indirectly and directly on the selectively vulnerable CA1 region to improve ischemia-induced deficits in working memory. Estrogen induces synapse formation in the CA1 (Leranth et al., 2002; MacLusky et al., 2005; Woolley and McEwen, 1994; Yankova et al., 2001). However, hippocampal circuits outside CA1 are also known targets of estrogen (Kadish and Van Groen, 2002; Liu et al., 2001; Tanapat et al., 1999; Veliskova et al., 2000). Impaired long-term potentiation in the dentate gyrus is evident after transient global ischemia (Aoyagi et al., 1998). This feature of hippocampal circuitry is also important for cognitive function and is regulated by estrogen (Gupta et al., 2001; Gureviciene et al., 2003; Miller et al., 2002). Theta rhythm is also an important characteristic of intact hippocampal function which is impaired by ischemic stroke and may be related to cognitive deficits (Elwan et al., 1994; Mariucci et al., 2003; Monmaur et al., 1990; Monmaur et al., 1986), and could also be affected by estrogen (Leranth et al., 1999; Saletu et al., 1995). Furthermore, estrogen can alter acetylcholine release in the hippocampus as well as the morphology and excitatory threshold of septo-hippocampal cholinergic terminals (Gibbs et al., 1997; Kadish and Van Groen, 2002; Lam and Leranth, 2003; Marriott and Korol, 2003; Rudick et al., 2003), which are known to play a role in memory formation (Daniel and Dohanich, 2001; Marriott and Korol, 2003). Thus, estrogen could attenuate ischemia-induced deficits in hippocampal-dependent behaviors not only by affecting CA1 cell survival, but also by influencing other properties of hippocampal circuits.
Another possible interpretation of these data is that estradiol may globally improve memory performance rather than specifically reducing ischemic damage. This hypothesis would predict that estradiol administration should also improve performance in the estradiol-treated sham animals relative to vehicle-treated sham rats. Although chronic estradiol did not improve memory performance in sham rats, others have demonstrated that estradiol administration to OVX female rats can improve working memory in both the object recognition and object placement tasks (Luine et al., 2003) and in other spatial and visual memory tests (Gibbs, 2002; Luine and Rodriguez, 1994). However, the duration of the retention interval is a critical factor in detecting effects of estradiol in these tasks, with improvement only evident after comparatively long delay times (4 h in the object placement and recognition task) (Luine and Rodriguez, 1994; Luine et al., 2003). We may not have been able to detect effects of estradiol on memory formation in sham animals within the retention intervals we employed. Further studies that include longer retention intervals will resolve this issue.
The present data also raise interesting questions about the relationship between estrogen and cell survival after global ischemia. Previous studies indicate that chronic estrogen significantly increases survival of CA1 cells assessed at 3–7 days after ischemia (Bagetta et al., 2004; He et al., 2002; Jover et al., 2002; Miller et al., 2005; Shughrue and Merchenthaler, 2003; Wang et al., 1999). In the present study, acute estradiol improved cell survival assessed at either 7 or 9 days after ischemia. Currently, we do not know whether long-term estradiol affords permanent protection or merely delays neuronal death in CA1. The important issue of sustained neuronal survival and/or cognitive function at times longer than 1 week after global ischemia has not been addressed systematically. Perhaps threshold levels of estrogen are required during a critical time period in order to maintain cell survival. In this context, it is noteworthy that Harukuni et al. (2001) reported deleterious effects of chronic estrogen on CA1 cell survival using pellets at roughly half the dose used in the present study. Under the present regime of long-term estradiol treatment, plasma estradiol was very low 9 days after ischemia. Further experiments are needed to determine whether estrogen withdrawal caused the reduced cell survival at time points longer than 1 week after global ischemia, and if so, whether there is a critical window within which estrogen levels must be maintained in order to durably protect against cell loss.
This study also implicates the hippocampal CA1 in visual working memory. Ischemia-induced neuronal death, which is clearly limited to the CA1 region of hippocampus in this protocol, produced significant visual memory deficits. This result is consistent with observations that the hippocampus is activated by visual presentation of novel objects (Pihlajamaki et al., 2004) and that several different methods of inducing specific hippocampal damage produce deficits in visual working memory (Gaskin et al., 2003; Zola et al., 2000; Zola-Morgan and Squire, 1986). Despite this evidence, the role of the hippocampus in visual working memory is controversial. This lack of consensus may reflect the fact that the retention intervals and the specific tasks utilized are crucial factors in assessing visual memory deficits after hippocampal damage. Delayed matching to sample and delayed non-matching to sample tasks are frequently used for this purpose, and several investigators have reported normal visual memory after hippocampal damage within the short retention intervals (<5 min) typically used in these tasks (Duva et al., 1997; Mumby et al., 2002). However, others have demonstrated impaired visual memory after hippocampal damage with longer retention intervals (Hammond et al., 2004). In our hands, visual memory was intact in ischemic animals at retention intervals shorter than 10 min, but deteriorated significantly at longer intervals.
In conclusion, the present results demonstrate that physiological levels of estradiol, when present both before and after injury, can dramatically improve cognitive performance after global ischemia in young adult female rats. This is an issue not addressed by the recent Women’s Health Initiative (Anderson et al., 2004). This report examined the incidence of stroke and cardiac arrest after estradiol administration in postmenopausal women, but did not study cognitive function after such events. Our study also indicates potential benefits of acute estradiol, administered shortly after induction of ischemia, on both cell survival and cognitive function. This regime of estradiol treatment produced more moderate cognitive improvements than did the chronic treatment, but is likely to have broader therapeutic potential.
Acknowledgments
We thank L.F. Jacome for generous help with the behavioral tasks and M. Ceide for technical assistance. This work was supported by Grants RO1 NS045693, R37 MH41414, T32 DK07513, and AHA 0335285N.
References
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291 (14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Aoyagi A, Saito H, Abe K, Nishiyama N. Early impairment and late recovery of synaptic transmission in the rat dentate gyrus following transient forebrain ischemia in vivo. Brain Res. 1998;799(1):130–137. doi: 10.1016/s0006-8993(98)00465-x. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58(6):815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Babcock AM, Graham-Goodwin H. Importance of preoperative training and maze difficulty in task performance following hippocampal damage in the gerbil. Brain Res Bull. 1997;42(6):415–419. doi: 10.1016/s0361-9230(96)00330-9. [DOI] [PubMed] [Google Scholar]
- Bagetta G, Chiappetta O, Amantea D, Iannone M, Rotiroti D, Costa A, Nappi G, Corasaniti MT. Estradiol reduces cytochrome c translocation and minimizes hippocampal damage caused by transient global ischemia in rat. Neurosci Lett. 2004;368(1):87–91. doi: 10.1016/j.neulet.2004.06.062. [DOI] [PubMed] [Google Scholar]
- Block F, Schwarz M. Correlation between hippocampal neuronal damage and spatial learning deficit due to global ischemia. Pharmacol Biochem Behav. 1997;56(4):755–761. doi: 10.1016/s0091-3057(96)00484-4. [DOI] [PubMed] [Google Scholar]
- Blokland A, Prickaerts J, Honig W, de Vente J. State-dependent impairment in object recognition after hippocampal NOS inhibition. NeuroReport. 1998;9 (18):4205–4208. doi: 10.1097/00001756-199812210-00037. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101(40):14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective oestrogen receptor β agonist in a mouse model of global ischaemia. Am J Physiol: Heart Circ Physiol. 2004;287(4):H1501–H1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- Cave CB, Squire LR. Equivalent impairment of spatial and nonspatial memory following damage to the human hippocampus. Hippocampus. 1991;1 (3):329–340. doi: 10.1002/hipo.450010323. [DOI] [PubMed] [Google Scholar]
- Chiueh C, Lee S, Andoh T, Murphy D. Induction of antioxidative and antiapoptotic thioredoxin supports neuroprotective hypothesis of estrogen. Endocrine. 2003;21 (1):27–31. doi: 10.1385/endo:21:1:27. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21(17):6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18(11):1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Duva CA, Floresco SB, Wunderlich GR, Lao TL, Pinel JP, Phillips AG. Disruption of spatial but not object-recognition memory by neurotoxic lesions of the dorsal hippocampus in rats. Behav Neurosci. 1997;111(6):1184–1196. doi: 10.1037//0735-7044.111.6.1184. [DOI] [PubMed] [Google Scholar]
- Elwan O, Hashem S, Helmy AA, el Tamawy M, Abdel Naseer M, Elwan F, Madkour O, Abdel Kader A, el Tatawy S. Cognitive deficits in ischemic strokes: psychometric, electrophysiological and cranial tomographic assessment. J Neurol Sci. 1994;125(2):168–174. doi: 10.1016/0022-510x(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Meliani K. A new one-trial test for neurobiological studies of memory in rats: III. Spatial vs. non-spatial working memory. Behav Brain Res. 1992;51(1):83–92. doi: 10.1016/s0166-4328(05)80315-8. [DOI] [PubMed] [Google Scholar]
- Galani R, Weiss I, Cassel JC, Kelche C. Spatial memory, habituation, and reactions to spatial and nonspatial changes in rats with selective lesions of the hippocampus, the entorhinal cortex or the subiculum. Behav Brain Res. 1998;96(1–2):1–12. doi: 10.1016/s0166-4328(97)00197-6. [DOI] [PubMed] [Google Scholar]
- Gaskin S, Tremblay A, Mumby DG. Retrograde and anterograde object recognition in rats with hippocampal lesions. Hippocampus. 2003;13 (8):962–969. doi: 10.1002/hipo.10154. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42(3):245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res. 1997;749(1):143–146. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888(2):356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Gureviciene I, Puolivali J, Pussinen R, Wang J, Tanila H, Ylinen A. Estrogen treatment alleviates NMDA-antagonist induced hippocampal LTP blockade and cognitive deficits in ovariectomized mice. Neurobiol Learn Mem. 2003;79(1):72–80. doi: 10.1016/s1074-7427(02)00012-6. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82(1):26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Harukuni I, Hurn PD, Crain BJ. Deleterious effect of β-estradiol in a rat model of transient forebrain ischemia. Brain Res. 2001;900(1):137–142. doi: 10.1016/s0006-8993(01)02278-8. [DOI] [PubMed] [Google Scholar]
- He Z, He YJ, Day AL, Simpkins JW. Proestrus levels of estradiol during transient global cerebral ischemia improves the histological outcome of the hippocampal CA1 region: perfusion-dependent and -independent mechanisms. J Neurol Sci. 2002;193(2):79–87. doi: 10.1016/s0022-510x(01)00648-7. [DOI] [PubMed] [Google Scholar]
- Herguido MJ, Carceller F, Roda JM, Avendano C. Hippocampal cell loss in transient global cerebral ischemia in rats: a critical assessment. Neuroscience. 1999;93 (1):71–80. doi: 10.1016/s0306-4522(99)00163-3. [DOI] [PubMed] [Google Scholar]
- Horsburgh K, Macrae IM, Carswell H. Estrogen is neuroprotective via an apolipoprotein E-dependent mechanism in a mouse model of global ischemia. J Cereb Blood Flow Metab. 2002;22(10):1189–1195. doi: 10.1097/01.wcb.0000037991.07114.4e. [DOI] [PubMed] [Google Scholar]
- Jover T, Tanaka H, Calderone A, Oguro K, Bennett MV, Etgen AM, Zukin RS. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22(6):2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish I, Van Groen T. Low levels of estrogen significantly diminish axonal sprouting after entorhinal cortex lesions in the mouse. J Neurosci. 2002;22(10):4095–4102. doi: 10.1523/JNEUROSCI.22-10-04095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota Y, Miyamoto M, Nagaoka A. Relationship between brain damage and memory impairment in rats exposed to transient forebrain ischemia. Brain Res. 1991;538(2):295–302. doi: 10.1016/0006-8993(91)90443-y. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Suzuki K, Sakuma Y. Estrogen alleviates cognitive dysfunction following transient brain ischemia in ovariectomized gerbils. Neurosci Lett. 1997;238(1–2):45–48. doi: 10.1016/s0304-3940(97)00847-1. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400(2–3):205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci. 2003;17(10):1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS. Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience. 2004;127 (4):983–988. doi: 10.1016/j.neuroscience.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Horvath TL. Estrogen receptor-alpha in the raphe serotonergic and supramammillary area calretinin-containing neurons of the female rat. Exp Brain Res. 1999;128(3):417–420. doi: 10.1007/s002210050863. [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE., Jr Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol. 2002;447(1):34–42. doi: 10.1002/cne.10230. [DOI] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187(1):94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gastard M, Verina T, Bora S, Mouton PR, Koliatsos VE. Estrogens modulate experimentally induced apoptosis of granule cells in the adult hippocampus. J Comp Neurol. 2001;441(1):1–8. doi: 10.1002/cne.1393. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62(3):230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144 (7):2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, Adlercreutz H, Lephart ED. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001;2(1):20. doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146 (1):287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- Mariucci G, Stasi MA, Taurelli R, Nardo P, Tantucci M, Pacifici L, Carminati P, Ambrosini MV. EEG power spectra changes and forebrain ischemia in rats. Can J Neurol Sci. 2003;30(1):54–60. doi: 10.1017/s0317167100002444. [DOI] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80(3):315–322. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36 (3):507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor α and β to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J Neurosci. 1999;19(9):3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monmaur P, Thomson MA, M’Harzi M. Temporal changes in hippocampal theta activity following twenty minutes of forebrain ischemia in the chronic rat. Brain Res. 1986;378(2):262–273. doi: 10.1016/0006-8993(86)90929-7. [DOI] [PubMed] [Google Scholar]
- Monmaur P, Allix M, Schoevaert-Brossault D, Houcine O, Plotkine M, Willig F. Effects of transient cerebral ischemia on the hippocampal dentate theta (theta) profile in the acute rat: a study 4–5 months following recirculation. Brain Res. 1990;508(1):124–134. doi: 10.1016/0006-8993(90)91125-z. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9(2):49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn JA, LePeillet E, Netto CA, Hodges H, Gray JA, Meldrum BS. Global ischaemia: hippocampal pathology and spatial deficits in the water maze. Behav Brain Res. 1994a;62(1):41–54. doi: 10.1016/0166-4328(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Nunn JA, LePeillet E, Netto CA, Hodges H, Gray JA, Meldrum BS. Global ischaemia: hippocampal pathology and spatial deficits in the water maze. Behav Brain Res. 1994b;62(1):41–54. doi: 10.1016/0166-4328(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Olsen GM, Scheel-Kruger J, Moller A, Jensen LH. Relation of spatial learning of rats in the Morris water maze task to the number of viable CA1 neurons following four-vessel occlusion. Behav Neurosci. 1994;108(4):681–690. doi: 10.1037//0735-7044.108.4.681. [DOI] [PubMed] [Google Scholar]
- Ordy JM, Wengenack TM, Bialobok P, Coleman PD, Rodier P, Baggs RB, Dunlap WP, Kates B. Selective vulnerability and early progression of hippocampal CA1 pyramidal cell degeneration and GFAP-positive astrocyte reactivity in the rat four-vessel occlusion model of transient global ischemia. Exp Neurol. 1993;119(1):128–139. doi: 10.1006/exnr.1993.1014. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur J Neurosci. 2004;19(7):1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Poignet H, Beaughard M, Lecoin G, Massingham R. Functional, behavioral, and histological changes induced by transient global cerebral ischemia in rats: effects of cinnarizine and flunarizine. J Cereb Blood Flow Metab. 1989;9(5):646–654. doi: 10.1038/jcbfm.1989.92. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23(36):11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23(11):4479–4490. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu B, Brandstatter N, Metka M, Stamenkovic M, Anderer P, Semlitsch HV, Heytmanek G, Huber J, Grunberger J, Linzmayer L, et al. Double-blind, placebo-controlled, hormonal, syndromal and EEG mapping studies with transdermal oestradiol therapy in menopausal depression. Psychopharmacology (Berlin) 1995;122 (4):321–329. doi: 10.1007/BF02246261. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Estrogen prevents the loss of CA1 hippocampal neurons in gerbils after ischemic injury. Neuroscience. 2003;116 (3):851–861. doi: 10.1016/s0306-4522(02)00790-x. [DOI] [PubMed] [Google Scholar]
- Singer CA, Rogers KL, Strickland TM, Dorsa DM. Estrogen protects primary cortical neurons from glutamate toxicity. Neurosci Lett. 1996;212(1):13–16. doi: 10.1016/0304-3940(96)12760-9. [DOI] [PubMed] [Google Scholar]
- Sudo S, Wen T-C, Desaki J, Matsuda S, Tanaka J, Arai T, Maeda N, Sakanaka M. β-Estradiol protects hippocampal CA1 neurons against transient forebrain ischemia in gerbil. Neurosci Res. 1997;29(4):345–354. doi: 10.1016/s0168-0102(97)00106-5. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma. 2002;19 (1):85–98. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Watanabe T, Ishibashi M, Hirano N, Tabuchi S, Takigawa H. Hippocampal ischemia in a patient who experienced transient global amnesia after undergoing cerebral angiography. Case illustration. J Neurosurg. 1999;91(2):347. doi: 10.3171/jns.1999.91.2.0347. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19(14):5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29 (8):1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Veliskova J, Velisek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41 (Suppl 6):S30–S35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Pulsinelli WA, Davis HP. Amnesia in humans and animals after ischemic cerebral injury. Ann N Y Acad Sci. 1985;444:492–493. doi: 10.1111/j.1749-6632.1985.tb37621.x. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Kovari E, Rivara CB, Bouras C, Hof PR, Giannakopoulos P. Stereologic analysis of hippocampal Alzheimer’s disease pathology in the oldest-old: evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol. 2005;193(1):198–206. doi: 10.1016/j.expneurol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Santizo R, Baughman VL, Pelligrino DA, Iadecola C. Estrogen provides neuroprotection in transient forebrain ischemia through perfusion-independent mechanisms in rats. Stroke. 1999;30 (3):630–637. doi: 10.1161/01.str.30.3.630. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain Res. 2004;1008(2):147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Stewart WF, Rudow GL, Troncoso JC. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol Aging. 2004;25 (9):1205–1212. doi: 10.1016/j.neurobiolaging.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Rod MR, Auer RN. Behavioral deficits revealed by multiple tests in rats with ischemic damage limited to half of the CA1 sector of the hippocampus. Brain Res Bull. 1994;34(3):283–289. doi: 10.1016/0361-9230(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14(12):7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci U S A. 2001;98(6):3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola SM, Squire LR, Teng E, Stefanacci L, Buffalo EA, Clark RE. Impaired recognition memory in monkeys after damage limited to the hippocampal region. J Neurosci. 2000;20(1):451–463. doi: 10.1523/JNEUROSCI.20-01-00451.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behav Neurosci. 1986;100(2):155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Rempel NL, Clower RP, Amaral DG. Enduring memory impairment in monkeys after ischemic damage to the hippocampus. J Neurosci. 1992;12(7):2582–2596. doi: 10.1523/JNEUROSCI.12-07-02582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin RS, Jover T, Yokota H, Calderone A, Simionescu M, Lau CG. Molecular and cellular mechanisms of ischemia. In: Mohr JP, Choi DW, Grotta JC, Weir B, Wolf PA, editors. Stroke, Pathophysiology, Diagnosis and Management. Churchill Livingstone; 2004. pp. 29–54. [Google Scholar]