Abstract
Leber’s congenital amaurosis (LCA) is a group of severe inherited retinal degenerations that are symptomatic in infancy and lead to total blindness in adulthood. Recent clinical trials using recombinant adeno-associated virus serotype 2 (rAAV2) successfully reversed blindness in patients with LCA caused by RPE65 mutations after one subretinal injection. However, it was unclear whether treatment of the second eye in the same manner would be safe and efficacious, given the potential for a complicating immune response after the first injection. Here, we evaluated the immunological and functional consequences of readministration of rAAV2-hRPE65v2 to the contralateral eye using large animal models. Neither RPE65-mutant (affected; RPE65−/−) nor unaffected animals developed antibodies against the transgene product, but all developed neutralizing antibodies against the AAV2 capsid in sera and intraocular fluid after subretinal injection. Cell-mediated immune responses were benign, with only 1 of 10 animals in the study developing a persistent T cell immune response to AAV2, a response that was mediated by CD4+ T cells. Sequential bilateral injection caused minimal inflammation and improved visual function in affected animals. Thus, subretinal readministration of rAAV2 in animals is safe and effective, even in the setting of preexisting immunity to the vector, a parameter that has been used to exclude patients from gene therapy trials.
INTRODUCTION
Leber’s congenital amaurosis (LCA) is a group of inherited retinal degenerative conditions with severe visual impairment identified in infancy. Patients typically present with nystagmus and impaired visual function, and testing reveals severely abnormal electroretinogram responses (1–4). Photoreceptor loss in LCA is progressive and results in total blindness by the third or fourth decade of life. Mutations in any one of 14 genes can cause LCA, including RPE65, the gene that encodes the 65-kD retinal pigment epithelium protein (RPE65) (5). RPE65 is an isomerohydrolase and mediates conversion of all-trans retinal back to 11-cis retinal during the visual cycle (6–9).
Three Phase 1 clinical trials have reported safety and efficacy data after subretinal delivery of the RPE65 complementary DNA (cDNA) into one eye of LCA patients with mutations in RPE65 (referred to as LCA-RPE65 subjects) using recombinant adeno-associated virus serotype 2 (rAAV2) vectors (10–16). Results of the entire trial carried out at the Children’s Hospital of Philadelphia (CHOP) demonstrate sustained improvement in retinal or visual function in all 12 of the patients (15, 16).
The successful reversal of blindness in individuals with LCA has been encouraging for the field of gene therapy as proof of the safety and efficacy of retinal gene transfer. Conservative inclusion criteria were applied with respect to immune status in the clinical trials to date, and a number of subjects were excluded from participation on the basis of serum neutralizing antibodies (NAbs) directed against AAV2 (10). However, the inclusion or exclusion criteria were borrowed from other (systemic) clinical trials and may be irrelevant to ocular gene transfer. A better understanding of the immune responses to subretinal injection will help to predict the appropriate answers to the following questions, which the teams conducting LCA-RPE65 clinical trials now face: (i) What are the immune consequences if subjects who have already received a subretinal rAAV2 injection in one eye are given a subretinal injection in their contralateral eye? (ii) Will it be safe to include individuals with high-titer antibodies to AAV2 in trials for LCA-RPE65 using rAAV2? (iii) Would these individuals have a chance of benefiting from the injection, or would transduction be blocked by an encounter with NAbs to AAV?
To address these questions, we undertook studies that explored both humoral and cellular immune responses to subretinal readministration of good manufacturing practice (GMP)–comparable rAAV2 in large animal models and in the context of preexisting serum NAbs. We chose two models for study: a canine model of LCA-RPE65, in which efficacy could be evaluated (17, 18), and an unaffected nonhuman primate (NHP) model. Both models have eyes comparable in size to human eyes and thus allow a similar surgical approach. The NHP eye is also similar anatomically to the human eye, as only primates have maculas. NHPs, like humans, are natural hosts for wild-type AAV and undergo immune conversion in response to subclinical infection (19), affording a potential model for predicting the immune responses of vector administration in humans. Here, we characterize immune responses to serial subretinal readministration of high-dose AAV2-hRPE65v2. We then compare baseline antibody concentrations in sera and intraocular fluid with those normally found in humans so that predictions can be made as to the seropositivity for AAV2 in the general population. Finally, we evaluate the effects of repeat subretinal administration of AAV2-hRPE65v2 on transgene expression, stability, efficacy with respect to restoration of visual function, and histopathology.
RESULTS
The effects of bilateral subretinal readministration of AAV2-hRPE65v2 in affected dogs and NHPs
The six affected dogs were 3 to 4 months old at the onset of the study and were naïve to AAV2. The four normal-sighted NHPs (two cynomolgus and two rhesus monkeys) were 10 to 14 years old and had been enrolled in studies that involved delivery of a series of research-grade rAAVs via intranasal, intravenous, and/or intramuscular routes. The research-grade rAAVs had been prepared by the University of Pennsylvania Vector Core in the laboratory of Dr. James Wilson, who also generously made these animals available.
AAV2-hRPE65v2 was delivered accurately to the subretinal space, first in the right eye and then later (table S1) in the left eye of each of the dogs and NHPs. Within 16 hours (the first postoperative evaluation), all media were clear, retinas were flat, and the animals had normal food and water intake, playfulness, and interactions with other animals and staff. All animals had unremarkable ophthalmological exams save for a postoperative endophthalmitis noted 4 days after injection in the left eye of NHP AP9X. A break in sterile field at the time of surgery had been reported. The infection cleared within 3 days after intravitreal injection of 200 μg of ciprofloxacin.
Characterization of the immune response on readministration of AAV2-hRPE65v2 in RPE65−/− dogs
None of the dogs had detectable antibody titers to AAV2 or to the RPE65 protein at baseline as measured by enzyme-linked immunosorbent assay (ELISA). Subretinal delivery of AAV2-hRPE65v2 to the right eye, followed by readministration of the vector to the left eye 2 weeks later, resulted in an increase in serum antibodies to the AAV2 capsid but not to the RPE65 protein. Antibody subtyping suggested a primary T helper cell 2 (TH2) response to the AAV2 capsid (fig. S1). NAbs to AAV2 peaked at >1:1000 in three of six animals and at a slightly lower titer in the remaining three animals (Table 1). In six of six animals, the serum NAb titer decreased over time. No detectable NAbs were measured in anterior chamber (AC) fluid samples before rAAV delivery (Table 1); NAb titers in the AC and vitreous increased after vector administration and remained elevated in the five dogs in which these were measured (>20 months). The ratio of antibodies in AC to serum, the Goldmann-Witmer (G-W) ratio, was elevated in 5 of the 10 eyes studied (Table 1). Vitreous, which could be collected only at termination of the study, had Nab titers higher than those in terminal sera or AC.
Table 1.
AAV2 NAb titers in affected dogs. Day, days after first injection; day 0, baseline measurement; gray boxes, no sample collected; NA, not analyzed; ND, not done (animal is still under study).
| Animal ID | Sample | Day 0 |
Day 15 |
Day 45 |
23.5 to 27 months |
G-W ratio (terminal) |
|---|---|---|---|---|---|---|
| Right eye injected | Left eye injected | Serum collected | Necropsy | |||
| RM | Serum | <1:3.16 | 1:10–1:31.6 | 1:1,000–1:3,160 | 1:316–1:1,000 | |
| Left AC | NA | 1:100–1:316 | 0.3 | |||
| Right AC | <1:3.16 | 1:100–1:316 | 0.3 | |||
| Left vitreous | >3,160 | |||||
| Right vitreous | >3,160 | |||||
| NY | Serum | NA | <1:3.16 | 1:316–1:1,000 | 1:31.6–1:100 | |
| Left AC | <1:3.16 | 1:316–1:1,000 | 10.0 | |||
| Right AC | <1:3.16 | 1:316–1:1,000 | 10.0 | |||
| Left vitreous | >1:3,160 | |||||
| Right vitreous | >1:3,160 | |||||
| NE | Serum | <1:3.16 | 1:10–1:31.6 | 1:100–1:316 | 1:31.6–1:100 | |
| Left AC | <1:3.16 | 1:316–1:1,000 | 10.0 | |||
| Right AC | <1:3.16 | 1:316–1:1,000 | 10.0 | |||
| Left vitreous | 1:1,000–1:3,160 | |||||
| Right vitreous | 1:1,000–1:3,160 | |||||
| SE | Serum | <1:3.16 | 1:31.6–1:100 | 1:1,000–1:3,160 | 1:100–1:316 | |
| Left AC | NA | 1:31.6–1:100 | 0.3 | |||
| Right AC | NA | 1:31.6–1:100 | 0.3 | |||
| Left vitreous | 1:1,000–1:3,160 | |||||
| Right vitreous | >3,160 | |||||
| VE | Serum | <1:3.16 | <1:3.16 | 1:3,160–1:10,000 | 1:316–1:1,000 | |
| Left AC | NA | <1:3.16 | ND | |||
| Right AC | NA | ND | ||||
| Left vitreous | ND | |||||
| Right vitreous | ND | |||||
| PE | Serum | <1:3.16 | 1:3.16–1:10 | 1:31.6–1:100 | 1:10–1:31.6 | |
| Left AC | <1:3.16 | 1:3.16–1:10 | 0.3 | |||
| Right AC | <1:3.16 | 1:100–1:316 | 10.0 | |||
| Left vitreous | 1:31.6–1:100 | |||||
| Right vitreous | >1:3,160 |
To measure T cell responses, we performed an enzyme-linked immunosorbent spot (ELISpot) assay on peripheral blood mononuclear cells (PBMCs) (table S1). Cytokine secretion was measured in response to the AAV2 capsid or to the RPE65 protein; interferon-γ (IFN-γ) and interleukin-10 (IL-10) were used to monitor TH1 and TH2 responses, respectively. No T cell response was detectable at any time in PBMCs. Thus, bilateral administration of AAV2-hRPE65v2 in RPE65−/− dogs results in a humoral immune response to the AAV2 capsid in the absence of T cell activation.
Visual improvements after readministration of AAV2-hRPE65v2 in RPE65−/− dogs
Within 2 weeks of the first AAV2-hRPE65v2 injection, animals exhibited significantly improved navigation and pupillary responses. Further improvement was noted after the second eye was injected. Comparison of the number of obstacles avoided before injection versus after injection ~2 years later revealed a significant improvement in navigation (P ≤ 0.0001, χ2 analysis). At baseline, one dog (RM) had very high frequency (3 to 5 Hz) nystagmus in both eyes. By 3 weeks after injection, frequency of the nystagmus had diminished to 1 Hz, and the nystagmus was absent at the 2-year time point.
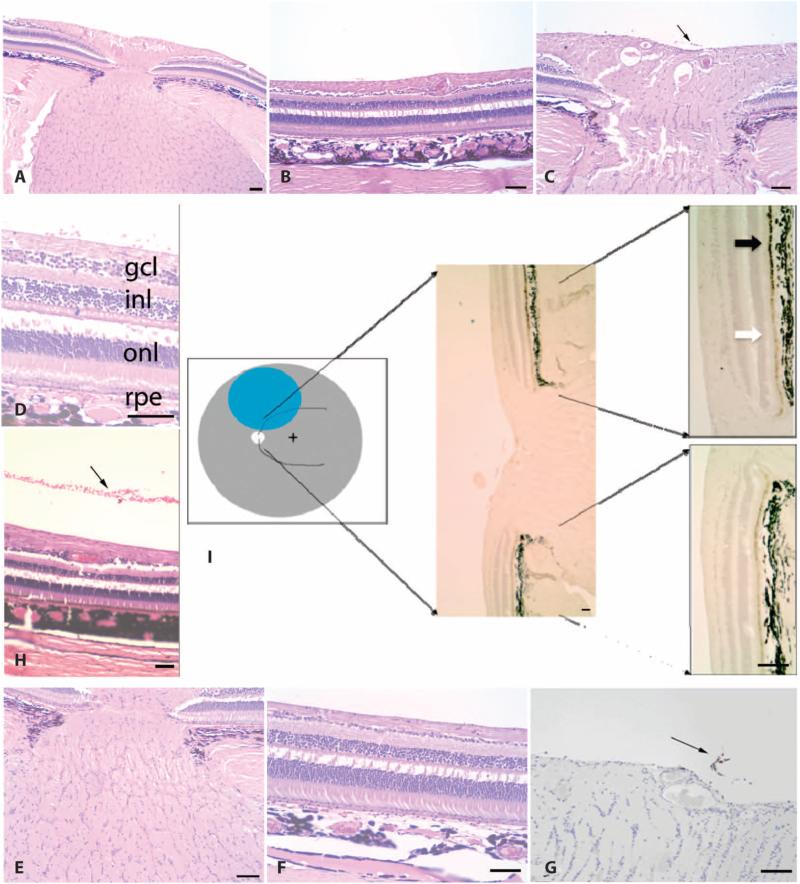
Despite the fact that the retinas of injected eyes had flattened within 16 hours after injection, the region of subretinal vector injection could be detected in the dogs throughout follow-up and after enucleation, as a result of reflective changes in the tapetum, the reflective membrane underlying the superior half of the retina in dogs (Fig. 1, A to D). Subretinal injection of AAV2-hRPE65v2 resulted in disappearance of the vacuoles in the exposed RPE cells of these animals (Fig. 1, E and F). Hematoxylin and eosin (H&E) staining revealed evidence of injury at and surrounding the retinotomy site. Regions more distant from the retinotomy showed normal retinal anatomy or layers, except for the presence of plasma cells in the vitreous and occasional lymphocytes and macrophages (Fig. 1, I to L). Immunofluorescence revealed RPE65 protein in the exposed (but not the unexposed) portions of the RPE in both eyes (Fig. 1, K and L). There were no extraocular abnormalities detected in any of the dogs at necropsy. Thus, bilateral administration of AAV2-hRPE65v2 induced stable recovery of visual function with mild histopathological changes in RPE65−/− dogs.
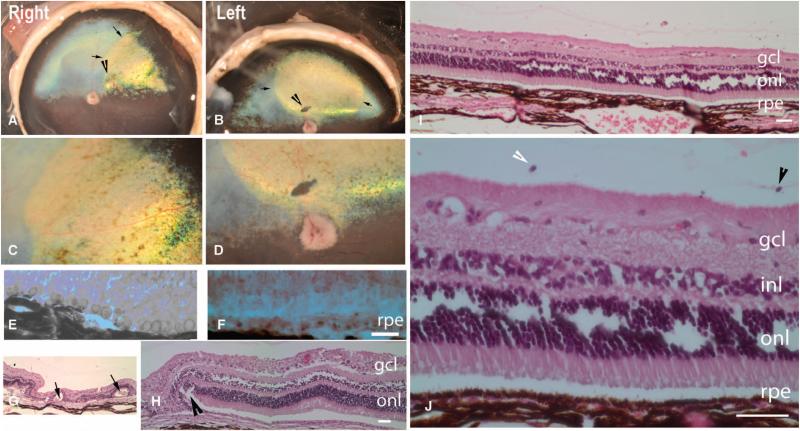
Fig. 1.
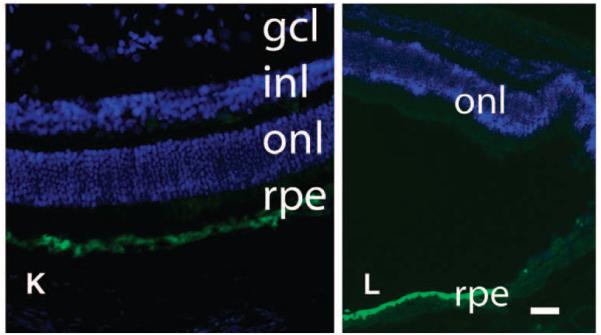
Postmortem and histopathological analyses in affected dogs. (A to D) Borders of the original retinal detachment (arrows) and the retinotomy site (arrowhead) are apparent. (C and D) Higher-magnification views of (A) and (B), respectively. Shown are the right and left retinas of dog PE 810 days after injection. (E and F) Vacuoles are present in RPE cells from untreated (E) but not treated (F) portions of the retina (shown is dog SE, left eye). (G and H) Rosettes (arrows) and scarring (arrowhead) near the retinotomy sites (dog NE, left eye). (I and J) Normal retinal anatomy or layers. Plasma cells [arrowhead, identity confirmed in high-magnification images of (I) such as that shown in (J)] in the vitreous from dog NY (right eye). (K and L) RPE65 protein immunofluorescence (green) in AAV-treated (K, left half of L) but not untreated (right half of L) RPE. (K) Dog NY (right eye). (L) Dog NY (left eye). Scale bars, 100 mm. Nuclei are stained blue. gcl, ganglion cell layer; inl, inner nuclear layer; onl, outer nuclear layer.
Characterization of the immune response on readministration of AAV2-hRPE65v2 in NHPs
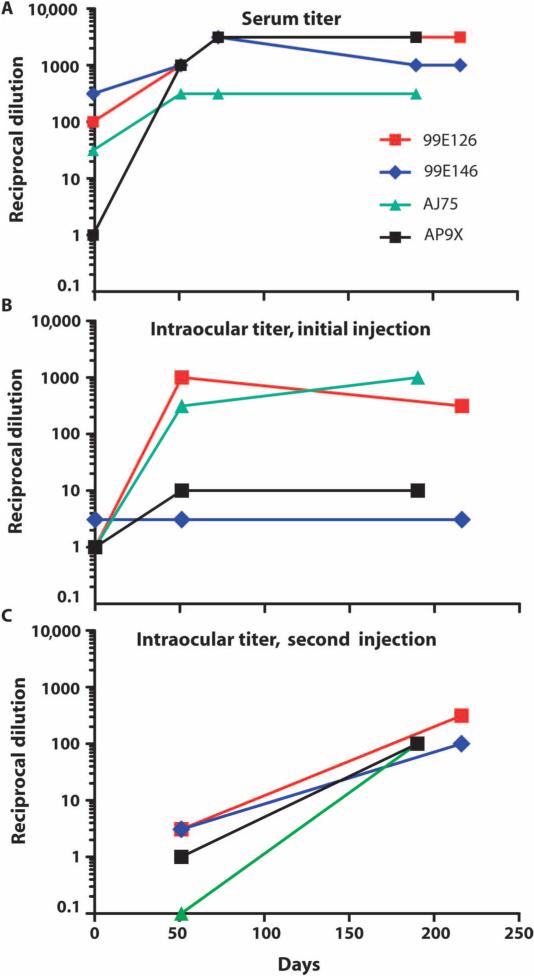
In the NHPs that participated in this study, baseline serum NAb concentrations to AAV2 ranged from negligible to high (table S1 and Fig. 2A), which was expected because the NHPs had been previously exposed to AAV2 in other studies. After the first injection of AAV2-hRPE65v2 in the right eyes, serum NAb titers rose in all animals; a further increase was observed after the second injection in three of the animals, whereas in the remaining animal no change was observed (Fig. 2A).
Fig. 2.
NAbs in four NHPs directed against the AAV2 capsid. (A to C) NAbs in serum (A), AC fluid samples from the first (right) eye that was injected (B), and AC fluid samples from the second (left) eye that was injected (C). Days, days after first injection.
Baseline NAb concentrations in the right (initially injected) eyes were negligible in all four NHPs. Fifty days after right eye injection of AAV2-hRPE65v2, NAb titers in three of the four eyes had risen above baseline amounts, whereas a fourth animal retained a low titer (Fig. 2B). Terminal measurements (210 to 217 days after right eye injection) in the right eyes showed increased NAbs in AJ75, a drop in 99E126, and no change in 99E146 or AP9X, relative to amounts measured 51 days after right eye injection (Fig. 2B). Baseline amounts of NAbs in the left (uninjected) eyes were negligible in all four animals. After left eye injection, Nab amounts in the left eyes rose in all animals (Fig. 2C). The G-W ratio was low for all eyes (including the left eye of AP9X, which had experienced the bacterial infection), except for the right eye of AJ75, in which the ratio was 2:1.
ELISAs were performed to determine whether the AAV2-hRPE65v2–injected NHPs had generated antibodies to the RPE65 protein and showed that animal 99E146 developed a serum RPE65 antibody titer of 1:100 one week after a delayed-type hypersensitivity (DTH) test (which involved administration of the AAV2 capsid and the RPE65 protein) (table S1B; Supplementary Material). The remaining animals showed no change over the study. No ocular fluid samples tested positive for antibodies to RPE65 at any time.
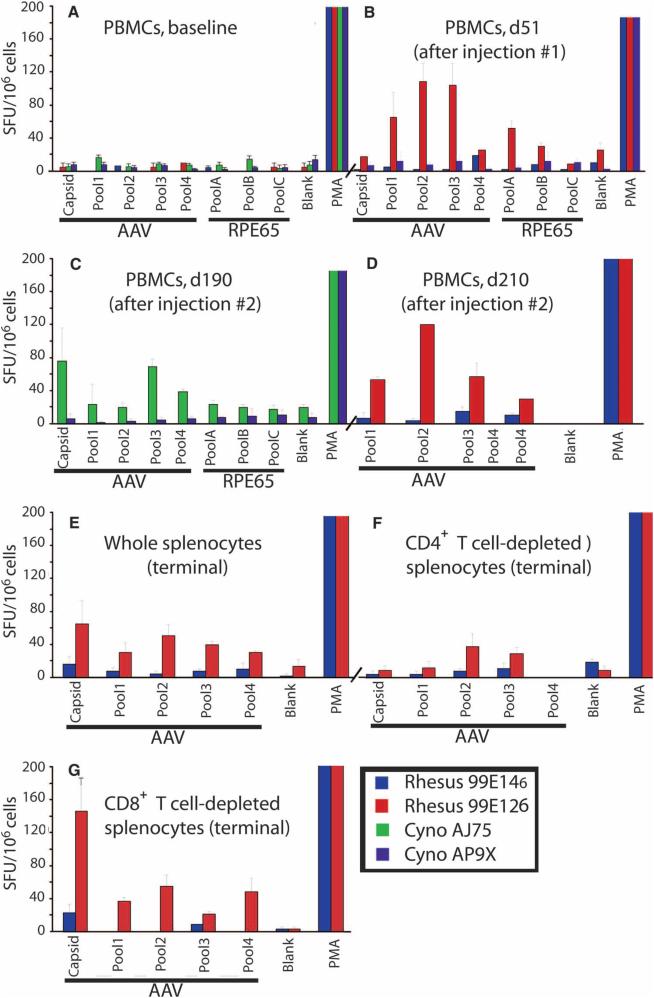
To assess T cell responses, we collected PBMCs at baseline and after each AAV2-hRPE65v2 injection and splenocytes were isolated at necropsy from monkeys 99E146 and 99E126. Cells were stimulated with the AAV2 capsid and the RPE65 protein, or AAV2 whole capsid particles, and IFN-γ secretion was measured by an IFN-γ ELISpot assay. Monkey 99E126 developed a detectable T cell response to the AAV2 capsid that increased after readministration of AAV2-hRPE65v2 (Fig. 3, B and D), and monkey AJ75 developed a T cell response 4 months after readministration (Fig. 3C).
Fig. 3.
T cell responses directed against the AAV2 capsid or the RPE65 protein after subretinal readministration of AAV2-hRPE65v2 in NHPs. (A to D) IFN-γ secretion by PBMCs after stimulation with the AAV2 capsid and the RPE65 protein as measured by an IFN-γ ELISpot assay using peptide pools (see Supplementary Material). (A) Baseline. (B) Day 51 (d51). (C) Day 190 (d190). (D) Day 210 (d210). (E to G) IFN-γ ELISpot assay in whole splenocytes collected at necropsy (E), and CD4+ or CD8+ T cell–depleted splenocytes (F and G, respectively) in terminal samples from NHP 99E126. PMA, positive control. The error bars indicate SD.
To further characterize the nature of the T cell response observed in animal 99E126, we performed CD4+ or CD8+ T cell depletion on splenocytes (Fig. 3, E to G). IFN-γ ELISpots performed on the CD4−CD8+ or CD4+CD8− splenocyte populations scored negative for animal 99E146, consistent with previous results, whereas animal 99E126 showed a positive response for total, undepleted splenocytes (Fig. 3E), had a negative IFN-γ response for the CD4−CD8+ T cell fraction (Fig. 3F), but showed a strong positive response to the AAV2 capsid for the CD4+CD8− T cell population (Fig. 3G). These results indicate that the immune response to AAV2 is mediated by CD4+ T cells. This finding was confirmed by T cell activation polyfunctional analysis and was corroborated by DTH results showing a lack of cytotoxic T cell response (no induration).
Histopathological evaluations revealed mild infiltrate in each of the injected eyes of the NHPs (Fig. 4). There was a small cluster of macrophages and lymphocytes in the vitreous in 99E126 (right eye), and a few perivascular lymphocytes were visible adjacent to the optic disc of eyes AJ75 (left) and 99E126 (right) and in the vitreous overlying the disc in 99E126 (left) (Fig. 4, C and G). Leukocyte common antigen staining confirmed the identity of these trace inflammatory cells (Fig. 4G). The retina appeared histologically normal by all other parameters, even in AP9X left, the eye that had experienced endophthalmitis. Immunohistochemistry showed baseline amounts of RPE65 protein in untreated retina, and overexpression of RPE65 is in AAV-exposed RPE cells (Fig. 4I).
Fig. 4.
(A to I) Histopathological (A to H) and immunohistochemical (I) analyses in retinas of unaffected NHPs after subretinal injection. Retinal layers (A to D, F, H, and I) and optic nerves (A, C, E, G, and I) have normal thicknesses. Mild injection-related changes include inflammatory cells (C, D, and G) and, in some, red blood cells in the vitreous (D and H). (A to F and H) Stained with H&E. (G) Stained with leukocyte common antigen. (I) Cartoon corresponding to the location of the subretinal injection (blue) with representative RPE65 immunohistochemical results shown at two magnifications. Black arrow, high levels of RPE65 protein in injected portion of the retina; white arrow, endogenous levels of RPE65 protein in unexposed retina. Scale bars, 100 mm. (A, B, E, and F) NHP 99E126 (right eye). (H) NHP 99E146 (right eye). (C, D, G, and I) NHP 99E126 (left eye). (B, F, D, and H) Processing artifacts cause a break between the inner and the outer nuclear layers (inl and onl, respectively). rpe, retinal pigment epithelium.
At the time of necropsy, there were no abnormalities in any extraocular sites noted save a discoloration of a portion of the left lower lobe of the lung and an adhesion between that and the diaphragm in animal 99E146. Histopathological analysis revealed type 2 pneumocyst hyperplasia. The significance of this isolated abnormality is unknown but may be related to a previous administration of vector via the respiratory route. Thus, bilateral administration of AAV2-hRPE65v2 in unaffected NHPs results in a humoral immune response to the AAV2 capsid, CD4+ T cell activation in one of four animals, and minimal inflammatory changes in the retina.
Measurement of high-titer NAbs to the AAV2 capsid in human sera but not in intraocular fluid samples
More than one hundred paired sera and intraocular fluid samples were collected from control human subjects ages 40 to 83 years old. Antibody titers to the AAV2 capsid were detected in 41% of the sera by ELISA [total immunoglobulin G (IgG)] (20). The sera with the highest IgG titers [IgG antibody to AAV2 (>30,000 ng/ml)] were selected for analysis in conjunction with the paired intraocular fluid samples. In Table 2, antibody titers to AAV2 in the AC samples were at or near background amounts (G-W ratio of <1:100) in each of these individuals, even in the presence of very high NAb titers in sera (ranging from 1:1000 to 1:10,000).
Table 2.
Comparison of NAb titers to AAV2 in sera and AC fluid samples from human individuals with high serum IgG antibody titers to AAV2.
| Sample no. | Subject | AC fluid NAb titers | Serum NAb titers |
|---|---|---|---|
| 1 | S12 | <1:2 | 1:1,000–1:10,000 |
| 2 | S14 | 1:2–1:10 | 1:100–1:1,000 |
| 3 | S26 | <1:2 | 1:100–1:1,000 |
| 4 | S72 | <1:2 | 1:1,000–1:10,000 |
| 5 | S74 | <1:2 | 1:1,000–1:10,000 |
| 6 | S78 | <1:2 | 1:1,000–1:10,000 |
| 7 | S38 | <1:2 | 1:100–1:1,000 |
| 8 | S43 | <1:2 | 1:100–1:1,000 |
| 9 | S44 | <1:2 | 1:100–1:1,000 |
| 10 | S83 | <1:2 | 1:1,000–1:10,000 |
| 11 | S87 | <1:2 | 1:1,000–1:10,000 |
| 12 | S101 | 1:2–1:10 | 1:1,000–1:10,000 |
| 13 | S48 | <1:2 | 1:1,000–1:10,000 |
| 14 | S64 | <1:2 | 1:1,000–1:10,000 |
| 15 | S103 | <1:2 | 1:1,000–1:10,000 |
DISCUSSION
The findings reported here show a lack of ophthalmic or systemic toxicity after subretinal readministration of AAV2-hRPE65v2 in six affected dogs and four unaffected NHPs. Subretinal readministration was benign even despite previous systemic exposure to AAV (in the NHPs). There was stable RPE65 expression in exposed RPE cells in the originally targeted regions of both of the serially injected eyes using a dose of 1.5 × 10−11 vector genomes, the highest dose used in the CHOP clinical trial (15). The repeated injections resulted in improved vision in the affected dogs as judged by navigation, pupillary light reflexes, and diminution of nystagmus, similar to results reported previously after a one-time administration (20–24). Thus, sequential subretinal administration is safe and efficacious in these large animal models.
Similar to the previously reported studies in naïve animals (20, 22, 25, 26), except for one study that used mice (27), all animals in the present study showed an increase in serum NAb titers to AAV2 after subretinal administration of high-titer rAAV2. Serum antibody concentrations increased even more after rAAV2 was readministered to the contralateral eye. There has been little attention in previous studies to intraocular antibodies. It was known that intraocular antibodies to AAV2 can increase after subretinal injection of rAAV2 (22), but it was not clear whether this reflected an increase in serum titer (that is, that this increase resulted from passive diffusion of the NAbs from the serum). Here, we show that each eye is autonomous with respect to antibody titers to AAV2. Notably, the G-W ratio was high in half of the 10 dog eyes injected with AAV2-hRPE65v2 and in one of the eight NHP eyes evaluated terminally. Similar ratios are found in certain human ophthalmic disease states. For example, antibodies can be detected in AC fluid, but not always in serum, from people with uveitis that resulted from certain infectious agents (Toxoplasma gondii, Varicella zoster, Herpes simplex, and others) (28–30). The fact that more of the dog eyes than NHP eyes had high G-W ratios may be due to the inherited disease in the affected dogs or to differences between the species. Regardless, the data indicate that antibodies to AAV2 (but not antibodies to RPE65) are produced locally in the eye after subretinal injection of AAV2-hRPE65v2. The benign nature of the T cell responses is consistent with previous reports (20–22, 31).
Whereas the affected dogs used in this study were naïve to AAV, the NHPs had a range of preexisting AAV2 NAb titers from exposure in the wild and/or administration of research-grade AAV before the start of this study. This mimics the situation found in humans, in whom up to 32% have humoral evidence of previous exposure to AAV2 (32, 33). The incidence of serum AAV2 NAbs increases with age, going from ~4% in children ≤14 years old to ~24% in individuals aged 14 to 39 years (34). We found that the incidence of serum NAbs directed against AAV2 is even higher (41%) in a middle-aged or senior citizen population. Because even those individuals with high serum Nab titers to AAV2 lacked antibodies to AAV2 in their intraocular fluid, similar to the dogs and NHPs at baseline, it is likely that subretinal administration of rAAV2 will allow efficacious delivery of its cargo to individuals with high serum titers.
Because each eye is autonomous with respect to antibody positivity to AAV2 and because all uninjected eyes lacked NAbs, we conclude that it should be possible to efficaciously and safely readminister rAAVs to the contralateral eyes of humans who have already received one subretinal injection. The large-animal data reported here suggest that additional (stable) transduction events should occur. Whether readministration of AAV2 in contralateral eyes of LCA-RPE65 patients is safe and effective will depend on how well the large animal models predict the human immune response. The record on this point is somewhatmixed; studies in dogs and NHPs failed to predict a T cell-mediated response that was associated with loss of transgene expression in an AAV-mediated, liver-directed gene therapy trial for hemophilia B (35, 36). However, for subretinal administration of AAV, studies in dogs to date have been fully predictive of results in humans (2–22).
An issue that has not been addressed is the consequence of readministration of rAAV2 to the initially injected eye. Further experiments are needed to determine whether intraocular NAbs generated after the initial injection could prevent additional transduction events and/or whether this procedure would elicit harmful cell-mediated responses. Until data are generated with respect to this matter, it would be safest to limit readministration in humans to the contralateral eye.
MATERIALS AND METHODS
Study guidelines and statistical analyses
All animal, recombinant DNA, and human studies were in compliance with local and federal guidelines and were approved. Statistical significance was evaluated by paired Student’s t test (human samples prevalence of NAb to AAV2 in serum versus ocular fluid) using GraphPad software. P values of <0.05 were considered significant. For NHP ELISpots, SEMs were calculated as the SD of the readings divided by the square root of the number of readings. χ2 Analysis was used to evaluate changes in mobility between baseline and postinjection time points.
Animals
Vector administration
AAV2-hRPE65v2 contains the hRPE65 cDNA under the control of a constitutive promoter (10, 15, 20) and was manufactured by the Center for Cellular and Molecular Therapeutics (CCMT) at CHOP with a process identical to the one used for production of the GMP lot used in human subjects (10, 15). The injection procedure was similar to that used in humans (10, 15). Afterwards, a subconjunctival injection of 0.15 ml of kenalog solution (40 mg/ml) was delivered. The ocular surface was dressed with ~1 inch of PredG (prednisolone acetate–gentamicin, 0.3%/0.6%; Allergan) ointment.
Treatment and collection schedule
Ophthalmoscopic examinations were carried out at all time points. AC fluid and blood samples were collected at baseline (before subretinal injection of the right eye) and at the designated postinjection and readministration time points (table S1). An additional AC tap was performed on the left eye of NHP AP9X on day 54 when pus was found in this eye (see Results). Phlebotomy was again performed after subretinal readministration. Samples of AC and vitreous fluids, PBMCs, and sera (and splenocytes from 99E146 and 99E126) were obtained terminally (table S1). PBMCs were stored in the vapor phase of liquid nitrogen, and AC fluid, sera, and vitreous were frozen at −80°C until testing. Eyes were collected after euthanasia, and a gross necropsy was performed in which the external body surface, all orifices, and the cranial, thoracic, and abdominal cavities (including brain, stomach, intestine, heart, lungs, liver, kidney, spleen, pancreas, and gonads) were examined.
In vitro immunologic assays
NAb assay and ELISA antibody to AAV2
NAb titer to AAV was determined as previously described with a β-galactosidase colorimetric assay (37). G-W ratios were calculated for each eye using the maximum titers measured.
ELISpot assays
Assays on PBMCs were performed as previously described (20, 36). Both IFN-γ and IL-10 ELISpots were performed with the dog samples, and for NHPs, IFN-γ ELISpots were performed (see “Methods S1” in Materials and Methods). For the ELISpot assay, response to an antigen was considered positive when the number of spot-forming unit (SFU) per million PBMCs (average of triplicate testing) was higher than 50 SFUs per million PBMCs and three or more times the SFU per million PBMCs measured for the medium control (blank). For splenocyte studies, 8E6 splenocytes were divided into three fractions; 2E6 splenocytes were used for direct testing on ELISpot and 4E6 splenocytes each were used for CD8+ and CD4+ cell depletion studies. Depletion of CD4+ or CD8+ T cell populations was performed as previously described (36). The CD4+CD8− or the CD4−CD8+ T cell fraction, as well as untouched splenocytes, was used in an IFN-γ ELISpot assay. Efficiency of depletion (>95%) was confirmed by surface antibody staining and flow cytometry. All experiments were repeated two times.
ELISA for antibodies to RPE65
Antibody titer to RPE65 was determined with an ELISA by modification of previously described methods (10, 15, 20). The titer was defined as the reciprocal of the highest sample dilution such that the mean optical density for the test antigen was at least three times that for the control antigen and at least three times the background level.
T cell activation polyfunctional analysis
The T cell activation assay was performed as previously described (36). Splenocytes were treated with 1 mg of CD28 (BD Biosciences) and 1 mg of CD49d (BD Biosciences) and then either stimulated with 4 ml of test peptide or 1 ml of staphylococcal enterotoxin B (Sigma), or left untreated as a negative control. Cells were stained for surface markers (CD3, CD4, CD8, CD14, CD16, and CD19) and intracellularly for T cell activation and effector function markers (IL-2, tumor necrosis factor–α, and IFN-γ). Samples were analyzed with flow cytometry using a FACSCanto II flow cytometer and FACSDiva (version 6.0, BD Biosciences) and FlowJo (TreeStar) software.
Sample preparation for histology
Eyes were fixed in 4% paraformaldehyde–2% glutaraldehyde in phosphate-buffered saline (PBS) before paraffin embedding and sectioning. Immunohistochemistry was carried out using the PETLET antibody to RPE65. Additional canine ocular samples and rhesus lung and spleen biopsy samples were fixed in 4% paraformaldehyde in PBS, paraffin-sectioned, and then stained with H&E. For studies that evaluated the presence of leukocyte common antigen, sections were stained using a monoclonal mouse antibody to CD45 (Dako) and then assessed with Dako Link using the Dako Flex Visualization system.
Human subjects
Patients at the Scheie Eye Institute, University of Pennsylvania scheduled to have cataract surgery gave informed consent for collection of ~0.1 ml of AC fluid, normally discarded at the time of surgery, and a blood sample by phlebotomy. AC fluid samples and sera were stored at −80°C until evaluation for NAbs to AAV2 (see above).
Acknowledgments
We thank F. Wright for providing the reference vector; E. Furth for histopathological expertise; T. M. Redmond for providing the PETLET antibody to RPE65; and F. Saponara, B. Johnson, D. Chung, M. Toure, K. Bhatt, and K. Brint for technical support. Funding: CCMT at CHOP, Foundation Fighting Blindness–sponsored CHOP-PENN Pediatric Center for Retinal Degenerations, NIH grant 1F30AG030961-01, Research to Prevent Blindness (J.B., Scheie Eye Institute, University of Iowa, and Mason Eye Institute), Howard Hughes Medical Institute, Hope for Vision, Paul and Evanina Mackall Foundation Trust at Scheie Eye Institute, and F. M. Kirby Foundation.
Footnotes
Competing interests: A.M.M. and J.B. are co-inventors of a pending patent for a method to treat or slow the development of blindness, but both waived any financial interest in this technology in 2002. J.B. served on a scientific advisory board for Ceregene in 2006 to 2008 and presented a seminar at Novartis in 2009. F.M. has served as consultant for Arthrogen, BV, but there was no retinal research involved. K.A.H. has served as a consultant for Tacere and as a scientific advisory board member for Amsterdam Molecular Therapeutics on extraocular targets, has been a speaker at the invitation of Genzyme, a company with a research program in AAV, and is the Director of CCMT at CHOP, which sponsors a clinical trial using AAV2-hRPE65v2. The other authors declare that they have no competing interests.
SUPPLEMENTARY MATERIAL
www.sciencetranslationalmedicine.org/cgi/content/full/2/21/21ra16/DC1
Materials and Methods
Table S1. Procedure schedules showing the time (days) relative to baseline sampling.
Fig. S1. Subtyping of humoral antibody responses to AAV2 in the six affected dogs. References
REFERENCES AND NOTES
- 1.Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, Furushima M, Redmond TM, Bennett J, Palczewski K, Cideciyan AV. Impairment of the transient pupillary light reflex in Rpe65−/− mice and humans with Leber congenital amaurosis. Invest. Ophthalmol. Vis. Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz B, Gyürüs P, Preising M, Bremser D, Gu S, Andrassi M, Gerth C, Gal A. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest. Ophthalmol. Vis. Sci. 2000;41:2735–2742. [PubMed] [Google Scholar]
- 3.Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, Bianchi PE, Fossarello M, Signorini S, Bertone C, Galantuomo S, Brancati F, Valente EM, Ciccodicola A, Rinaldi E, Auricchio A, Banfi S. Clinical and molecular genetics of Leber’s congenital amaurosis: A multicenter study of Italian patients. Invest. Ophthalmol. Vis. Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- 4.Perrault I, Rozet JM, Gerber S, Ghazi I, Leowski C, Ducroq D, Souied E, Dufier JL, Munnich A, Kaplan J. Leber congenital amaurosis. Mol. Genet. Metab. 1999;68:200–208. doi: 10.1006/mgme.1999.2906. [DOI] [PubMed] [Google Scholar]
- 5.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 9.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 12.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, Flotte TR, Fishman GA, Heon E, Stone EM, Byrne BJ, Jacobson SG, Hauswirth WW. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum. Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang JJ, Roman AJ, Byrne BJ, Jacobson SG. Human RPE65 gene therapy for Leber congenital amaurosis: Persistence of early visual improvements and safety at 1 year. Hum. Gene Ther. 2009;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: A phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol. Ther. 2009 doi: 10.1038/mt.2009.277. 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narfström K, Wrigstad A, Nilsson SE. The Briard dog: A new animal model of congenital stationary night blindness. Brit. J. Ophthalmol. 1989;73:750–756. doi: 10.1136/bjo.73.9.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguirre GD, Baldwin V, Pearce-Kelling S, Narfström K, Ray K, Acland GM. Congenital stationary night blindness in the dog: Common mutation in the RPE65 gene indicates founder effect. Mol. Vis. 1998;423 [PubMed] [Google Scholar]
- 19.Muzyczka N, Berns K. In: Fields Virology. Knipe D, Howley P, editors. Vol. 2. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 2327–2359. [Google Scholar]
- 20.Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, Mingozzi F, Hui D, Chung D, Rex TS, Wei Z, Qu G, Zhou S, Zeiss C, Arruda VR, Acland GM, Dell’Osso LF, High KA, Maguire AM, Bennett J. Reversal of blindness in animal models of Leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol. Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat. Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 22.Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, Dejneka NS, Pearce-Kelling SE, Maguire AM, Palczewski K, Hauswirth WW, Jacobson SG. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol. Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs JB, Dell’Osso LF, Hertle RW, Acland GM, Bennett J. Eye movement recordings as an effectiveness indicator of gene therapy in RPE65-deficient canines: Implications for the ocular motor system. Invest. Ophthalmol. Vis. Sci. 2006;47:2865–2875. doi: 10.1167/iovs.05-1233. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs JB, Dell’Osso LF, Wang ZI, Acland GM, Bennett J. Using the NAFX to measure the effectiveness over time of gene therapy in canine LCA. Invest. Ophthalmol. Vis. Sci. 2009;50:4685–4692. doi: 10.1167/iovs.09-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand V, Chirmule N, Fersh M, Maguire AM, Bennett J. Additional transduction events after subretinal readministration of recombinant adeno-associated virus. Hum. Gene Ther. 2000;11:449–457. doi: 10.1089/10430340050015914. [DOI] [PubMed] [Google Scholar]
- 26.Bennett J, Anand V, Acland GM, Maguire AM. Cross-species comparison of in vivo reporter gene expression after recombinant adeno-associated virus-mediated retinal transduction. Methods Enzymol. 2000;316:777–789. doi: 10.1016/s0076-6879(00)16762-x. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Miller R, Han PY, Pang J, Dinculescu A, Chiodo V, Hauswirth WW. Intraocular route of AAV2 vector administration defines humoral immune response and therapeutic potential. Mol. Vis. 2008;14:1760–1769. [PMC free article] [PubMed] [Google Scholar]
- 28.Liekfeld A, Schweig F, Jaeckel C, Wernecke KD, Hartmann C, Pleyer U. Intraocular antibody production in intraocular inflammation. Graefes Arch. Clin. Exp. Ophthalmol. 2000;238:222–227. doi: 10.1007/s004170050347. [DOI] [PubMed] [Google Scholar]
- 29.Robert PY, Liekfeld A, Metzner S, Ranger-Rogez S, Adenis JP, Denis F, Hartmann C, Pleyer U. Specific antibody production in herpes keratitis: Intraocular inflammation and corneal neovascularisation as predicting factors. Graefes Arch. Clin. Exp. Ophthalmol. 2006;244:210–215. doi: 10.1007/s00417-005-0014-7. [DOI] [PubMed] [Google Scholar]
- 30.de Visser L, Rothova A, de Boer JH, van Loon AM, Kerkhoff FT, Canninga-van Dijk MR, Weersink AY, de Groot-Mijnes JD. Diagnosis of ocular toxocariasis by establishing intraocular antibody production. Am. J. Ophthalmol. 2008;145:369–374. doi: 10.1016/j.ajo.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Barker SE, Broderick CA, Robbie SJ, Duran Y, Natkunarajah M, Buch P, Balaggan KS, MacLaren RE, Bainbridge JW, Smith AJ, Ali RR. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J. Gene Med. 2009;11:486–497. doi: 10.1002/jgm.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 33.Blacklow NR, Hoggan MD, Kapikian AZ, Austin JB, Rowe WP. Epidemiology of adenovirus-associated virus infection in a nursery population. Am. J. Epidemiol. 1968;88:368–378. doi: 10.1093/oxfordjournals.aje.a120897. [DOI] [PubMed] [Google Scholar]
- 34.Chen CL, Jensen RL, Schnepp BC, Connell MJ, Shell R, Sferra TJ, Bartlett JS, Clark KR, Johnson PR. Molecular characterization of adeno-associated viruses infecting children. J. Virol. 2005;79:14781–14792. doi: 10.1128/JVI.79.23.14781-14792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 36.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, Zhou S, Wright JF, Jiang H, Pierce GF, Arruda VR, High KA. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, Ragni MV, Manno CS, Sommer J, Jiang H, Pierce GF, Ertl HC, High KA. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]