Abstract
MicroRNAs guide many aspects of development in all metazoan species. Frequently, microRNAs are expressed during a specific developmental stage to perform a temporally defined function. The C. elegans mir-35-42 microRNAs are expressed abundantly in oocytes and early embryos and are essential for embryonic development. Here, we show that these embryonic microRNAs surprisingly also function to control the number of progeny produced by adult hermaphrodites. Using a temperature-sensitive mir-35-42 family mutant (a deletion of the mir-35-41 cluster), we demonstrate three distinct defects in hermaphrodite fecundity. At permissive temperatures, a mild sperm defect partially reduces hermaphrodite fecundity. At restrictive temperatures, somatic gonad dysfunction combined with a severe sperm defect sharply reduces fecundity. Multiple lines of evidence, including a late embryonic temperature-sensitive period, support a role for mir-35-41 early during development to promote subsequent sperm production in later larval stages. We further show that the predicted mir-35 family target sup-26 (suppressor-26) acts downstream of mir-35-41 in this process, suggesting that sup-26 de-repression in mir-35-41 deletion mutants may contribute to temperature-sensitive loss of fecundity. In addition, these microRNAs play a role in male fertility, promoting proper morphogenesis of male-specific mating structures. Overall, our results demonstrate that robust activity of the mir-35-42 family microRNAs not only is essential for embryonic development across a range of temperatures but also enables the worm to subsequently develop full reproductive capacity.
Keywords: fertility, maternal effect, germline, sperm, male fertility
microRNAs are a class of endogenous 22-23-nucleotide RNAs that repress expression of complementary target mRNAs to govern diverse developmental and physiological processes in essentially all complex eukaryotes. In most cases, mature microRNAs are generated from much longer transcripts through a series of nucleolytic cleavages and subsequently loaded into complexes with Argonaute proteins (Ketting 2011). Together with the effector protein GW182, the microRNA-loaded Argonaute forms the RNA-induced silencing complex (miRISC), which inhibits the translation and/or stability of complementary target mRNAs. The seed region (nucleotides 2–7) of a microRNA is the most important for determining target specificity (Bartel 2009). microRNAs that share the same seed sequence are classified as a “family” because they can potentially bind and redundantly regulate the same set of target mRNAs.
The C. elegans mir-35 family of microRNAs is abundantly expressed in oocytes and early embryos, and is essential for embryonic development (Lau et al. 2001; Wu et al. 2010; Alvarez-Saavedra and Horvitz 2010). This microRNA family consists of eight members (mir-35-42) that reside in two loci (mir-35-41 and mir-42-44) (Figure 1A). Deletion of all eight mir-35-42 microRNA genes results in slowed embryonic development culminating in completely penetrant embryonic or early larval lethality (Alvarez-Saavedra and Horvitz 2010). Strains that carry a deletion that only affects seven out of eight family members, mir-35-41(nDf50) or mir-35-41(gk262), and hence express only mir-42, display a partially penetrant embryonic lethality (Alvarez-Saavedra and Horvitz 2010; Liu et al. 2011; Massirer et al. 2012). For populations of mir-35-41(nDf50) or mir-35-41(gk262) embryos, the penetrance of lethality depends on the temperature at which the animals are grown, with lower frequency of lethality at 15° or 20° and nearly complete lethality at 25° (Alvarez-Saavedra and Horvitz 2010; Massirer et al. 2012). Thus, the mir-35-41 deletion genotype (with only mir-42 intact) can be considered hypomorphic for mir-35 family function at permissive temperatures (15° or 20°) and a more severe loss of mir-35 family function at a restrictive temperature (25°).
Figure 1.
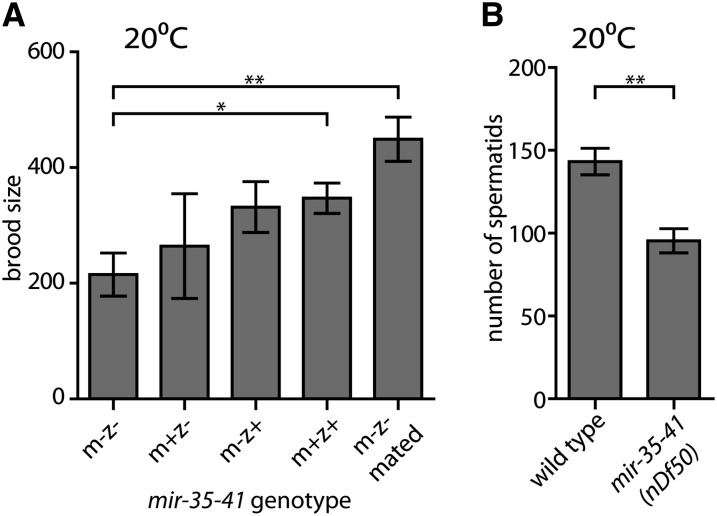
The mir-35 family promotes hermaphrodite fecundity. (A) Mature microRNA sequences of all members of the mir-35-42 seed family. Seed sequence is in bold. (B) Brood size (y-axis) represents the number of progeny produced per hermaphrodite, including dead embryos and larvae, as well as living progeny. Wild-type or mir-35-41(nDf50) animals were grown at the temperature indicated. Mean and SE are plotted. *p-value < 0.05, **p-value < 0.01, two-tailed Student’s t-test.
Because mir-35-41 deletion mutants can bypass embryonic lethality at 15° or 20°, postembryonic phenotypes have also been characterized for animals that escape lethality at these permissive temperatures. These viable mir-35-41 mutant animals display reduced proliferation of the intestine and the mitotic germline, which may result from de-repression of lin-23 and gld-1, respectively (Liu et al. 2011). Interestingly, mir-35-41 animals are also enhanced in their response to exogenous RNAi, an effect that depends on an indirect downregulation of lin-35/Rb (Massirer et al. 2012).
Here, we examined the effect of mir-35-41 deletion on hermaphrodite fecundity. We show that the mir-35 family acts in multiple processes, in both the germline and the soma, to promote full reproductive capacity. In particular, we provide evidence that these embryonically expressed microRNAs act early during development to promote spermatogenesis in subsequent larval stages. We also show that suppressor-26 (sup-26), a predicted mir-35 family target gene encoding an RNA-binding protein, acts downstream of mir-35-41 in this context.
Materials and Methods
C. elegans culture and phenotypic characterization
C. elegans were cultured on NGM seeded with HB101. Strains were maintained at 15° or 20° for 72 hr or 25° for 48 hr prior to beginning experiments conducted at the respective temperatures. For quantification of brood size, single L4 hermaphrodites were placed on individual 3-cm NGM plates for approximately 24 hr. Animals were moved to a fresh plate each day until progeny were no longer produced. Approximately 24 hr after removal of the parent, larvae and embryos were counted on each plate. For brood size quantification when mating with wild-type males, five males and one L4 hermaphrodite were added to a 3-cm NGM plate and transferred to a fresh plate each day until progeny were no longer produced.
Mating efficiency of mir-35-41(nDf50);him-8(e1489) males was assessed by placing one male with one fog-2(q71) L4 female on a 3-cm NGM plate. Plates containing progeny after 5 d were counted as successful matings. Mating efficiency of mir-35-41(nDf50);him-8(e1489) was normalized to him-8 mating efficiency. Sperm dissection and in vitro activation were performed as described (Singaravelu et al. 2011).
The mIn1 balancer marked with mIs14 (myo2::GFP, pes-10::GFP, F2B7.9::GFP) was used to balance mir-35-41(nDf50). To generate mir-35-41(m+z-) animals, GFP-negative animals were segregated from mir-35-41(nDf50)/mIn1 mothers. To generate mir-35-41(m-z+) animals, mir-35-41(nDf50) hermaphrodites were crossed to males containing mIn1, and GFP-positive progeny were isolated. The balancer qC1 marked with qIs26 (rol-6(su1006), lag-2::GFP) was used to balance sup-26(lf) alleles in the mir-35-41(nDf50) background. To generate mir-35-41(nDf50);sup-26(m+zlf) animals, non-Rol progeny that segregated from mir-35-41(nDf50);sup-26(lf)/qC1 mothers were isolated. To generate mir-35-41(nDf50);sup-26(mlfz+) animals, mir-35-41(nDf50);sup-26(lf) hermaphrodites were crossed to mir-35-41(nDf50) males; after the appearance of male cross progeny, hermaphrodite larval progeny were picked.
For determining the temperature-sensitive period of mir-35-41(nDf50) fecundity, a mixed-stage population of mir-35-41(nDf50) animals containing a lag-2::GFP reporter (qIs56) marking the distal tip cells was shifted to 25° for 12 hr. At the end of that period, individual animals were isolated and their developmental stages were determined by scoring the positions of the GFP-marked distal tip cells; accordingly, for each animal the approximate period of larval development spent at 25° was inferred from previously described rates of C. elegans gonadal development (Byerly et al. 1976). The brood of each animal was subsequently quantified during the adult stage.
For quantifying endomitotic oocytes, animals were maintained at 25° for least 48 hr before picking late L4 larvae or young (pre-gravid) adults. DAPI staining was performed the next day. Whole animals were fixed in 95% ethanol containing 500 ng/ml DAPI for 3 min at room temperature. For counting total spermatids, adults were harvested either at a pre-gravid stage or at an early gravid stage (14 hr after selection as late L4 larvae at 25°). After DAPI staining, Z-stacks (0.4-µm sections) were acquired of whole spermathecae. Spermatids were counted manually on 3D reconstructions of the spermathecae using the 4D viewer in MetaMorph Image Analysis Software (Sunnyvale, California).
qPCR and 3′ RACE
For sup-26 mRNA qPCR, embryo RNA samples were prepared by growing strains on egg media on NGM plates. Strains were shifted to 25° for 24 hr prior to isolating embryos by bleaching. 3′ RACE was performed from staged RNA samples using primers designed according to the SMART protocol from Clontech.
Generation of transgenics
A 1.5-kb fragment of sequence upstream of the sup-26 coding sequence was amplified using the primers CCTGGGTAGCTATTTCGTACGTAGTC and AAGATGCGTTCATTCTTGAATTATTATG tagged with an attB4 or attB1r site, respectively. The sup-26 3′ UTR was amplified with the following primers: ATGGACAGGACAACGTCTTCACTCCAC and AAAACTGCAAGACCAATCAGCGATTC tagged with attB2r or attB3 sites, respectively. The PCR products were cloned into pCFJ210 using MultiSite Gateway cloning (Life Technologies, Green Island, NY). Quickchange mutagenesis was performed on the entry clone containing the sup-26 3′ UTR using the primers CATCCACCGTTCCGTCATCGTCG and CTGCCGAGGAAAGGAGAATGAGTG to mutate the putative mir-35 family binding site. Single-copy transgenes were generated as described (Frøkjaer-Jensen et al. 2008).
Results
mir-35-41 promotes spermatogenesis
We noticed that mir-35-41(nDf50) hermaphrodites lay many unfertilized oocytes, a phenotype that can be symptomatic of reduced fecundity because of a sperm defect (Argon and Ward 1980). To measure fecundity of the mir-35-41(nDf50) strain, we counted the number of progeny produced by each hermaphrodite throughout its lifetime, including both live progeny and dead embryos and larvae. At permissive temperatures (15° or 20°), mir-35-41(nDf50) hermaphrodites produce fewer progeny than wild-type animals (Figure 1B). When animals were raised at 25°, mir-35-41(nDf50) hermaphrodites produced dramatically fewer progeny than at 20° or wild-type animals raised at 25° (Figure 1B). Therefore, mir-35-41(nDf50) has a moderate effect on hermaphrodite fecundity at a permissive temperature (20°) and a severe effect at a restrictive temperature (25°).
First, we further characterized the nature of the fecundity phenotype at a permissive temperature. Previous work demonstrated that mir-35 family function in embryonic viability can be rescued by either maternal or zygotic mir-35 family expression (Alvarez-Saavedra and Horvitz 2010). Similarly, we observed that fecundity of mir-35-41(nDf50) mutant hermaphrodites was partially rescued by maternal mir-35-41 expression and fully rescued by zygotic expression at 20° (Figure 2A).
Figure 2.
mir-35-41(nDf50) hermaphrodite fecundity at permissive temperature is rescued by zygotic mir-35-41 expression or mating to wild-type males. (A) Brood size (y-axis) represents the number of progeny produced per hermaphrodite, including dead and living progeny. m = maternal mir-35-41(nDf50) genotype; z = zygotic genotype. mated = single hermaphrodite was mated to five wild-type males. (B) Number of spermatids per spermatheca in pre-gravid adult hermaphrodites of indicated genotype maintained at 20°. (A and B) Mean and SE are plotted. *p-value < 0.05, **p-value < 0.01, two-tailed Student’s t-test.
In wild-type C. elegans hermaphrodites, the number of sperm produced during larval development limits self-progeny to ∼300. However, wild-type hermaphrodites can generate up to ∼1000 progeny when mated to males, which provide additional sperm. To determine whether insufficient functional sperm could be responsible for reduced fecundity of mir-35-41(nDf50) hermaphrodites at a permissive temperature, mir-35-41(nDf50) hermaphrodites were crossed with wild-type males. At 20°, mir-35-41(nDf50) brood size was rescued by mating, indicating that a sperm defect underlies the brood size phenotype at this temperature (Figure 2A, last bar). We examined the number of sperm produced by mir-35-41(nDf50) hermaphrodites at 20° and found that mir-35-41(nDf50) hermaphrodites generate fewer spermatids than wild-type (Figure 2B). Thus, mir-35-41 promotes maximal hermaphrodite spermatogenesis at 20°.
mir-35-41 promotes male fertility and tail morphogenesis
Because mir-35-41 promotes spermatogenesis in hermaphrodites, we investigated whether mir-35-41(nDf50) males are fertile. To this end, a mutation that causes a high incidence of males (him-8(e1489)) through impaired X chromosome segregation was introduced to mir-35-41(nDf50). Spermatids produced by mir-35-41(nDf50);him-8(e1489) males are numerous, appear normal, and can be activated in vitro (Supporting Information, Figure S1A). However, the mating efficiency of mir-35-41(nDf50);him-8(e1489) males is only 60% that of him-8(e1489). We hypothesize that the low mating efficiency is largely due to defects in male-specific somatic structures. In 5% of mir-35-41(nDf50);him-8(e1489) males, tail structures essential for mating are highly abnormal (Figure S1B). Less apparent, milder tail defects may be present at higher penetrance and contribute to the large reduction in mir-35-41(nDf50);him-8(e1489) male mating efficiency. At 25°, him-8(e1489) alone exhibited temperature-sensitive phenotypes, so mir-35-41(nDf50);him-8(e1489) male phenotypes were only scored at 20°.
Functions of the somatic gonad and spermatids are impaired in mir-35-41(nDf50) hermaphrodites at a restrictive temperature
Next, we examined the nature of the severe fecundity defect of mir-35-41(nDf50) hermaphrodites at a restrictive temperature (25°). First, we determined whether maternal or zygotic loss of mir-35-41 was responsible for the temperature-sensitive fecundity phenotype. We observed that either maternal or zygotic mir-35-41 expression could partially rescue fecundity, whereas both maternal and zygotic mir-35-41 expression were required for complete rescue (Figure 3A). Notably, the maternal effect (as evidenced by partial rescue of the phenotype by maternal mir-35-41 and incomplete rescue by zygotic mir-35-41) supports a model wherein mir-35-41 acts early in the development of the hermaphrodite embryo to ensure the animal’s maximal fecundity.
Figure 3.
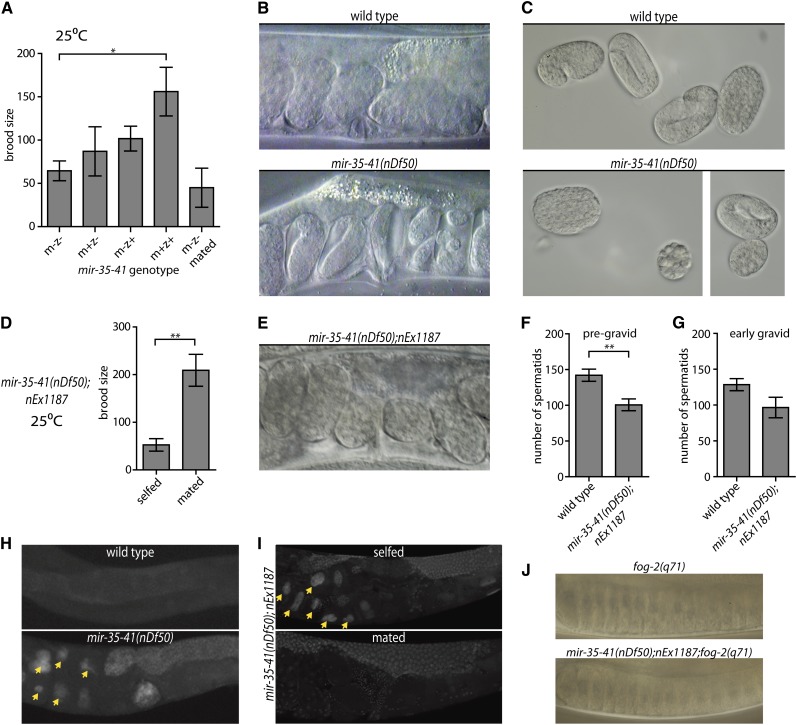
Both somatic gonad and sperm defects contribute to temperature-sensitive loss of fecundity in mir-35-41(nDf50) hermaphrodites. (A, D, F, G) Mean and SE are plotted. *p-value < 0.05, **p-value < 0.01, two-tailed Student’s t-test. (A) Brood size (y-axis) represents the number of progeny produced per hermaphrodite. m = maternal mir-35-41(nDf50) genotype; z = zygotic genotype. mated = single hermaphrodite was mated to five wild-type males. (B) Proximal uteri of wild-type or mir-35-41(nDf50) hermaphrodites grown at 25°. mir-35-41(nDf50) uteri contain very late-stage embryos, indicative of an egg-laying defective phenotype (Egl). (C) Embryos of wild-type or mir-35-41(nDf50) hermaphrodites grown at 25°. The size and shape of mir-35-41(nDf50) embryos are sometimes abnormal, indicating a defect of somatic gonad architecture. (D) Number of progeny produced by mir-35-41(nDf50) hermaphrodites containing a somatically expressed mir-35 transgene (nEx1187) when selfed or mated to wild-type males. (E) Uteri of mir-35-41(nDf50);nEx1187, indicating that somatic expression of mir-35 rescues the Egl phenotype of mir-35-41(nDf50). (F and G) Number of spermatids per spermatheca in adult hermaphrodites maintained at 25°. Adults were harvested at a (F) pre-gravid or (G) early gravid stage. (H and I) Whole mount DAPI staining in wild-type and mir-35-41(nDf50) hermaphrodites (H) or mir-35-41(nDf50) hermaphrodites containing the mir-35 transgene (nEx1187) when selfed or mated to wild-type males (I). Arrowheads indicate endomitotic oocytes. (J) Proximal gonad of virgin fog-2(q71) females that are otherwise wild-type (top) or containing mir-35-41(nDf50);nEx1187. In both genotypes, oocyte nuclei are pushed close together as oocytes accumulate and stack in the germline.
To assess whether a sperm defect is responsible for the reduced fecundity of mir-35-41(nDf50) hermaphrodites at 25°, mutant hermaphrodites were grown at 25° and mated to wild-type males. In contrast to the fecundity phenotype at a permissive temperature, which was rescued by mating (see above), mating did not rescue the brood size of hermaphrodites grown at 25° (Figure 3A, last bar). Therefore, at 25°, additional sperm-independent defects, not present at 20°, contribute to the reduced fecundity of mir-35-41(nDf50) hermaphrodites.
We hypothesized that the inability to rescue fecundity at 25° by mating mir-35-41(nDf50) hermaphrodites could be due to dysfunction of the somatic gonad. Consistent with such somatic gonad dysfunction, mir-35-41(nDf50) hermaphrodites grown at 25° display an egg-laying defective phenotype and occasionally produce misshapen eggs (Figure 3, B and C) (Greenstein et al. 1994; Kovacevic and Cram 2010). Expression of mir-35 from a transgenic extrachromosomal array (nEx1187) (Alvarez-Saavedra and Horvitz 2010) rescued both the Egl phenotype and the ability to produce large numbers of cross-progeny when mated (Figure 3, D and E). However, nEx1187 did not rescue mir-35-41(nDf50) hermaphrodite self-fecundity at 25° (Figure 3F). Fecundity of mir-35-41(nDf50); nEx1187 hermaphrodites could only be restored by mating to wild-type males. Therefore, mir-35-41(nDf50) hermaphrodites at 25° display a somatic gonad defect (which is rescued by nEx1187) and also a severe sperm defect (which is not rescued by nEx1187). Our interpretation of these results is that the sperm defect is due to germline loss of mir-35, and thus cannot be rescued by nEx1187 because expression from high-copy extrachromosomal arrays is silenced in the germline (Kelly et al. 1997); however, other interpretations for the failure of nEx1187 to rescue the sperm defect are also possible.
To further characterize the sperm defect, we examined the number of spermatids produced by mir-35-41(nDf50) hermaphrodites at 25°. Although we observed a reduced number of spermatids in mir-35-41(nDf50) hermaphrodites compared with wild-type, the quantity was similar to that observed at 20°, and thus cannot account for the more severe loss of fecundity at 25° (Figure 3F). Therefore, we examined whether sperm activation might be affected in mir-35-41(nDf50) hermaphrodites at 25°.
A hallmark of all sperm activation mutants is the rapid loss of spermatids from the spermatheca. Hermaphrodite spermatids are stored in the spermatheca and activated when they are pushed into the uterus by ovulation of the first oocyte (L’Hernault 2006). Activation results in the formation of a pseudopod, which allows mature spermatozoa that fail to fertilize the oocyte to “swim upstream” back to the spermatheca after each round of ovulation. Un-activated spermatids cannot return to the spermatheca and are thus pushed outside the body as embryos are laid. When spermatids were quantified from wild-type or mir-35-41(nDf50);nEx1187 hermaphrodites after the first few rounds of ovulation, a dramatic loss of spermatids from mir-35-41(nDf50);nEx1187 spermathecae was not observed (Figure 3G). Thus, mir-35-41(nDf50);nEx1187 sperm do not fail to activate at 25°. Therefore, the severely reduced fecundity at 25° may be due to inefficient fertilization by mir-35-41(nDf50);nEx1187 spermatozoa.
Another characteristic of sperm-defective mutants is the appearance of oocytes with abnormally high DNA content and distended nuclei (Ward and Miwa 1978). These endomitotic oocytes result when oocytes exit diakinesis and replicate their DNA in the absence of fertilization or cytokinesis (Greenstein et al. 1994; Iwasaki et al. 1996). Consistent with the sperm-defective phenotype, we observed endomitotic oocytes in the uteri of both mir-35-41(nDf50) (47.8% of uteri on the first day of gravidity, n = 113) and mir-35-41(nDf50);nEx1187 (57.1%, n = 42) hermaphrodites grown at 25° (Figure 3, H and I). Importantly, endomitotic oocytes were completely absent from the germlines of 25° mir-35-41(nDf50);nEx1187 hermaphrodites mated to wild-type males (0%, n = 40) (Figure 3I). Thus, endomitotic oocytes are a phenotypic trait of the sperm defect in mir-35-41(nDf50) and mir-35-41(nDf50);nEx1187 hermaphrodites at 25°.
Although endomitotic oocytes arise due to relatively rare, stochastic ovulation in the absence of functional sperm (Ward and Miwa 1978; McCarter et al. 1997), the phenotype may be exacerbated if mir-35-41 also contributes to the inhibition of oocyte meiotic maturation in the absence of sperm (Greenstein et al. 1994; Govindan et al. 2006, 2009). If aberrant oocyte maturation contributes to the endomitotic oocyte phenotype observed in mir-35-41(nDf50);nEx1187 at 25°, then oocytes in mir-35-41(nDf50);nEx1187 females (which lack sperm) would aberrantly mature and fail to accumulate or “stack” in the proximal gonad. To generate females, we introduced a feminization-of-germline mutation (fog-2(q71)) into the mir-35-41(nDf50);nEx1187 background. In mir-35-41(nDf50);nEx1187;fog-2(q71) females, oocytes stack similarly to fog-2(q71) females (Figure 3J). Therefore, the inhibition of oocyte meiotic maturation is normal in mir-35-41(nDf50);nEx1187;fog-2(q71), and a defect in this inhibition is thus unlikely to contribute to the endomitotic oocyte phenotype observed in mir-35-41(nDf50);nEx1187 at 25°.
mir-35-41 acts early during hermaphrodite development to promote sperm function
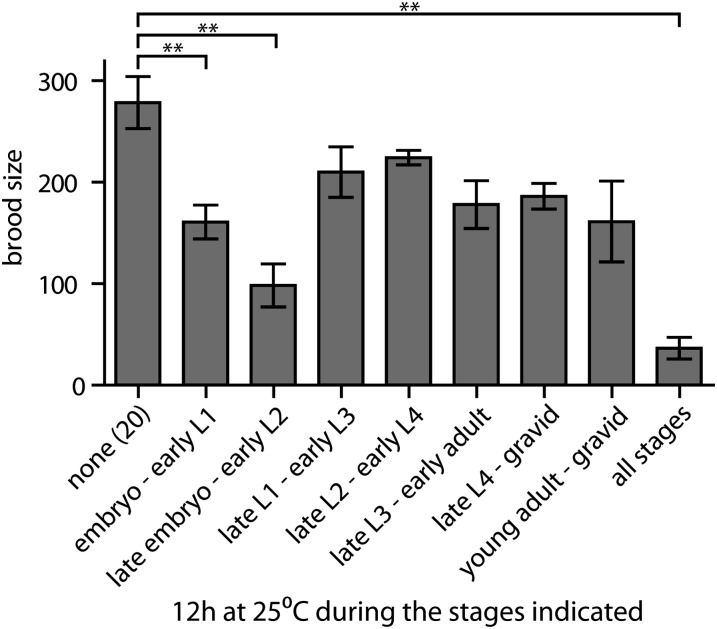
We observed that the self-fecundity of mir-35-41(nDf50) hermaphrodites at restrictive temperature depends on contributions from both their mother’s genotype and from their own zygotic genotype (Figure 3A). This suggests that that the spermatogenesis defect of mir-35-41(nDf50) hermaphrodites could be caused (at least in part) by reduction of mir-35 family function early in development. To further test this model, we determined the temperature-sensitive period of mir-35-41(nDf50) self-fecundity. Animals were shifted to 25° for a period of 12 hr at various stages of development. All animals that spent late embryogenesis at 25° displayed severely reduced fecundity (Figure 4). A much milder impact on fecundity was observed in animals shifted to 25° at any stage after L1. Therefore, mir-35-41 acts early in development—perhaps during embryogenesis—to ensure the maximal fecundity of the animal when it matures to the adult. This is consistent with the temporal expression pattern of the mir-35 family, primarily in oocytes and early embryos.
Figure 4.
The temperature-sensitive period of mir-35-41(nDf50) hermaphrodite fecundity is prior to the L2 stage. Number of progeny from mir-35-41(nDf50) hermaphrodites shifted to 25° for 12 hr at the indicated larval stages. Mean and SE are plotted. **p-value < 0.01, two-tailed Student’s t-test.
SUP-26 contributes to the temperature-sensitive sperm defect in mir-35-41(nDf50)
Next, we looked for predicted mir-35 family target genes that might play a role in hermaphrodite fecundity downstream of mir-35-41. Interestingly, the top prediction on Targetscan, sup-26 (also known as tag-310), was previously implicated in sex-specific development (Jan et al. 2011). SUP-26 is an RNA-binding protein that promotes masculine sex determination by inhibiting translation of tra-2 (transformer-2) mRNA in somatic tissues (Manser et al. 2002; Mapes et al. 2010). In the germline of developing hermaphrodite larvae, tra-2 translational status controls when sperm or oocytes are generated. Stage-specific inhibition of germline tra-2 translation by the RNA binding proteins FOG-2 and GLD-1 (defective in GermLine Development-1) drives spermatogenesis during the third and fourth larval stages (Ellis and Schedl 2007). The role of SUP-26 in the germline has not been examined. However, the previously described somatic role of SUP-26 in controlling tra-2 translation makes sup-26 an interesting candidate mir-35 family target gene in the context of hermaphrodite fecundity. Multiple 3′ UTRs have been annotated for the sup-26 mRNA (Mangone et al. 2010; Jan et al. 2011), only one of which contains the mir-35 family target site (Figure S2A). For sup-26 to be a direct mir-35 family target, the longest 1146-bp 3′ UTR must be used. We performed 3′ Rapid Amplification of cDNA Ends (RACE) to determine which sup-26 3′ UTRs are present throughout development. The 1146-bp 3′ UTR was amplified from all samples and confirmed by sequencing, whereas products corresponding to the other annotated 3′ UTRs were not observed (Figure S2B). In addition, sup-26 mRNA was significantly enriched in pull-down of the RISC effector proteins ALG-1-Interacting-1 (AIN-1) and AIN-2 (Zhang et al. 2007; Hammell et al. 2008). Thus, endogenous sup-26 mRNA contains a highly conserved mir-35 family target site and associates with miRISC.
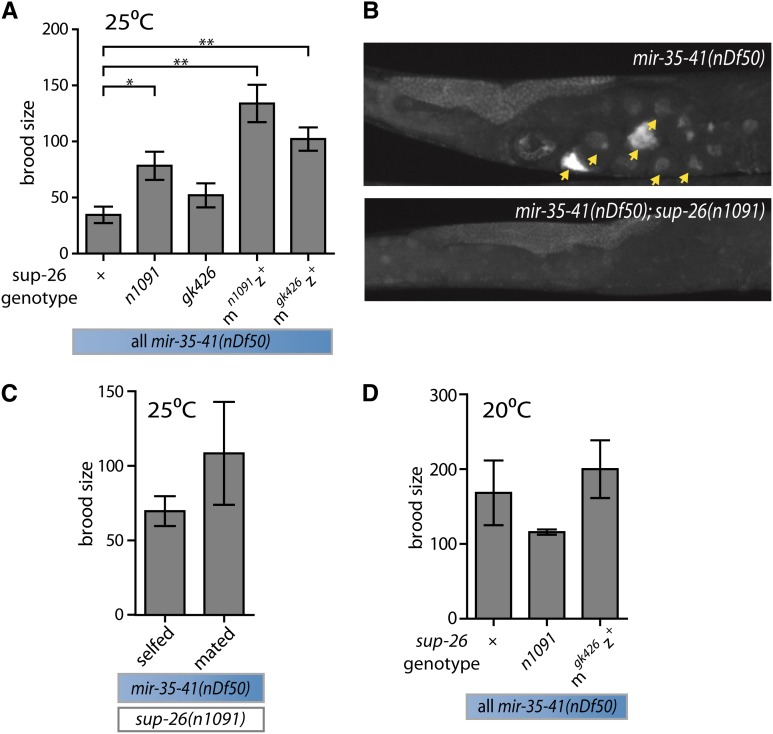
If sup-26 is a mir-35 family target gene whose de-repression in mir-35-41(nDf50) contributes to loss of fecundity, then sup-26(lf) might suppress one or more aspects of the mir-35-41(nDf50) fecundity phenotype. mir-35-41(nDf50);sup-26(lf) animals exhibit incompletely penetrant embryonic lethality, so we assessed the fecundity of escaper mir-35-41(nDf50);sup-26(lf) hermaphrodites that survived to adulthood. Strikingly, a weak (n1091) or strong (gk426) allele of sup-26 partially suppressed the temperature-sensitive fecundity of mir-35-41(nDf50) at 25°, indicated by an increased number of hermaphrodite self-progeny in mir-35-41(nDf50);sup-26(lf) (Figure 5A).
Figure 5.
sup-26 contributes to the temperature-sensitive sperm defect of mir-35-41(nDf50), but not other aspects of mir-35-41(nDf50) reduced fecundity. (A) Number of progeny produced per hermaphrodite (grown at 25°). m = maternal; z = zygotic sup-26 genotype. (B) DAPI staining. Arrowheads indicate endomitotic oocytes. (C) Number of self- or cross-progeny produced by mir-35-41(nDf50);sup-26(n1091) hermaphrodite at 25°. Wild-type sperm do not significantly increase fecundity. (D) Number of self-progeny produced per hermaphrodite (grown at 20°). Sup-26(lf) does not robustly affect mir-35-41(nDf50) fecundity at 20°. (A, C, D) Mean and SE are plotted. *p-value < 0.05, **p-value < 0.01, two-tailed Student’s t-test with Welch’s correction for unequal variance.
Because the number of self-progeny was increased in mir-35-41(nDf50);sup-26(lf) compared with mir-35-41(nDf50), we hypothesized that the temperature-sensitive sperm defect was rescued by sup-26(lf). Consistent with this, sup-26(lf) also suppressed the appearance of endomitotic oocytes in mir-35-41(nDf50);sup-26(lf) (Figure 5B). Only 6.8% of uteri in mir-35-41(nDf50);sup-26(n1091) contained endomitotic oocytes on the first day of gravidity (n = 74), compared with 47.8% in mir-35-41(nDf50). Therefore, the predicted mir-35 family target gene sup-26 functions downstream of mir-35-41 in regulating spermatogenesis at restrictive temperature.
Because the mir-35-41(nDf50) sperm phenotype at restrictive temperature is attributable, at least in part, to maternal loss of mir-35-41, we postulated that mir-35-41 might act in the oocyte and early embryo to repress the maternal load of sup-26 mRNA. If this is the case, then loss of maternal sup-26 function would be predicted to suppress the mir-35-41(nDf50) self-fecundity defect at restrictive temperature. Removing the maternal contribution of wild-type sup-26 mRNA (sup-26(mlfz+)) rescues the brood size of mir-35-41(nDf50) animals at 25°, at least as well as (and, in fact, better than) complete sup-26 loss-of-function (sup-26(mlfzlf)) (Figure 5A). The rescue of mir-35-41(nDf50) by sup-26(mlfz+) to nearly wild-type fecundity suggests that the primary effect of mir-35-41 on hermaphrodite fecundity is upstream of the maternal contribution of sup-26. This suggests that mir-35-41 promotes sperm function by acting in the maternal germline and/or early embryo to limit the expression of SUP-26 from maternally supplied sup-26 mRNA. Interestingly, sup-26(mlfz+) appeared to suppress mir-35-41(nDf50) brood size at 25° better than sup-26(mlfzlf) (Figure 5A), suggesting a second function for sup-26 (in this case zygotically expressed) in promoting fecundity of mir-35-41(nDf50) embryos.
Because examining the translational status of maternally supplied sup-26 mRNA (without detecting the zygotic contribution of the same transcript) is technically difficult, we examined total embryonic sup-26 mRNA for evidence of mir-35-41–dependent regulation. Neither qPCR of endogenous sup-26 nor GFP reporter transgenes showed evidence of mir-35-41–dependent or sup-26 3′ UTR–dependent control of mRNA abundance or translation in whole embryos (Figure S2, C and D). Thus, although we were unable to examine the maternal sup-26 transcript alone, the bulk of zygotically transcribed sup-26 mRNA does not appear to be subject to mir-35-41 regulation (see Discussion).
In addition, we examined whether sup-26 plays a role in the other aspects of mir-35-41(nDf50) loss of fecundity. In contrast to the sperm defect at a restrictive temperature, function of the somatic gonad is not rescued in mir-35-41(nDf50);sup-26(lf) hermaphrodites, because they do not reproducibly produce large numbers of cross progeny when mated to wild-type males (Figure 5C). Furthermore, loss of sup-26 function does not suppress the mir-35-41(nDf50) fecundity phenotype at 20° (Figure 5D). Therefore, only the defect in sperm function in mir-35-41(nDf50) at a restrictive temperature, but not the defect in spermatogenesis observed at a permissive temperature, depends on sup-26.
Discussion
We have demonstrated that the mir-35 family acts at multiple levels to promote hermaphrodite fecundity. By examining the phenotype of mir-35-41(nDf50) at multiple temperatures and the effects of a somatic mir-35 rescue, we have delineated at least four ways in which mir-35-41 promotes fecundity. In mir-35-41(nDf50);him-8(e1489) males, mating is impaired by abnormal development of the male-specific copulatory apparatus. In mir-35-41(nDf50) hermaphrodites, a moderate defect in spermatogenesis reduces the number of progeny produced at a permissive temperature. At a restrictive temperature, dysfunction of the somatic gonad and a severe defect in sperm function further reduce mir-35-41(nDf50) hermaphrodite fecundity. We propose that while the number of spermatids produced at both temperatures is reduced, inefficient fertilization further hampers the function of spermatids produced at restrictive temperature. Interestingly, sup-26(lf) only suppresses the low fecundity observed at restrictive temperature, indicating that sup-26 may play a role in sperm function but not in sperm production.
The fact that adult fecundity is affected by loss of mir-35-41 is intriguing in light of the expression pattern of the mir-35 family: primarily in oocytes and early embryos. Here, we show that loss of mir-35-41 causes early developmental defects that result in the observed adult phenotypes. In particular, our studies on the temperature-sensitive sperm defect support this model. First, zygotic mir-35 expression does not fully rescue this phenotype, indicating a maternal effect of mir-35-41 on fecundity of the adult. Second, the temperature-sensitive period of this phenotype is early in development (approximately late embryogenesis). Third, loss of the maternal contribution of sup-26 rescues the fecundity of mir-35-41(nDf50) adults at a restrictive temperature. Together, these results strongly support the model that the molecular basis of this phenotype arises in early development.
The suppression of mir-35-41(nDf50) loss of self-fecundity at 25° by loss of maternal sup-26 strongly suggests that mir-35-41 ensures fecundity by repressing maternal sup-26 in the oocyte or early embryo. When examining zygotic sup-26 endogenous transcripts and reporter transgenes, we did not see clear evidence of mir-35-41–dependent regulation. One possibility is that the maternal load of sup-26 is at a sufficiently low concentration to be subject to mir-35 family regulation, while strong zygotic transcription increases sup-26 mRNA abundance beyond the threshold of repression. This is consistent with the expression pattern of sup-26, and also with that of mir-35-42, which is most abundant in oocytes and early embryos, decaying in levels throughout embryogenesis (Wu et al. 2010; Stoeckius et al. 2009). Alternatively, mir-35-41 may act upstream of sup-26 in an unknown indirect manner.
We do not yet understand how sup-26 affects hermaphrodite fecundity downstream of mir-35-41. Sup-26 is thought to modulate sex determination by inhibiting translation of tra-2 mRNA in the soma (Manser et al. 2002; Mapes et al. 2010). Here, we show that sup-26 can also affect germline development. If the germline effect of sup-26 were also via translational inhibition of tra-2, then ectopic de-repression of maternal sup-26 in mir-35-41(nDf50) would be expected to extend the period of tra-2 inhibition, thus increasing rather than decreasing sperm production (Kuwabara et al. 1998). One possibility is that SUP-26 interacts with a different complement of proteins in the soma and the germline to cause tra-2 activation in the germline and tra-2 inhibition in the soma. It is also possible that SUP-26 binds and regulates additional unidentified RNA targets other than tra-2, and these interactions may account for the deleterious effect of SUP-26 on sperm function that we observe here. Identification of other target genes through an approach such as affinity purification of SUP-26 followed by deep sequencing of associated RNAs would be a key advance in our understanding of SUP-26 biology.
This work describes two new temperature-sensitive phenotypes of mir-35-41(nDf50), in addition to the previously described temperature-sensitive embryonic lethality (Alvarez-Saavedra and Horvitz 2010). Although the basis of temperature sensitivity of these phenotypes is not fully understood, we hypothesize that microRNA target repression (at least by mir-35 family microRNAs) is less efficient at 25° than at 20°. In this scenario, a single mir-35-42 family member (mir-42) could be sufficient to repress the mir-35-42 target genes at 20°, but insufficient at 25°. This model is consistent with the observation that the embryonic lethality phenotype of the mir-35-42 deletion strain is completely penetrant at all temperatures (Alvarez-Saavedra and Horvitz 2010). Thus, this model provides a sound framework for conceptualization of the temperature-sensitive phenotypes observed in the mir-35-41 deletion strain. The mir-35 microRNA family may provide a fascinating setting in which to explore the effects of natural in vivo temperature changes on microRNA target recognition and/or repression.
The mir-35 family is unique among microRNAs across diverse species for its strong maternal effect. In vertebrates, microRNAs that are essential for embryogenesis, such as miR-430 in zebrafish and miR-290-295 in mice, are expressed abundantly only after the onset of zygotic transcription (Giraldez et al. 2006; Medeiros et al. 2011). Thus, the mir-35 family may represent a novel paradigm for microRNA control of embryogenesis. The maternal and early embryonic expression of mir-35-42 suggests that its targets may be repressed at the earliest stages of embryogenesis and later released from repression. Our studies of sup-26 are consistent with this model. Thus, future studies of mir-35 family function may have broad implications for our understanding of the reversibility of microRNA target repression and the full complement of microRNA functions during development.
Supplementary Material
Acknowledgments
We thank members of the Ambros and Mello laboratories for helpful discussions, and the Walker lab for sharing use of their confocal microscope. This work was funded by NIH grants R01 GM34028 (to V.A.) and F32 GM097895 (to K.M.). Some strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Footnotes
Communicating editor: J. K. Kim
Literature Cited
- Alvarez-Saavedra, E., and H. R. Horvitz, 2010 Many families of C. elegans microRNAs are not essential for development or viability. Curr. Biol. 20: 367–373. [DOI] [PMC free article] [PubMed]
- Argon Y., Ward S., 1980. Caenorhabditis elegans fertilization-defective mutants with abnormal sperm. Genetics 96: 413–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P., 2009. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Cassada R. C., Russell R. L., 1976. The life cycle of the nematode Caenorhabditis elegans. I. Wild-type growth and reproduction. Dev. Biol. 51: 23–33 [DOI] [PubMed] [Google Scholar]
- Ellis, R., and T. Schedl, 2007 Sex determination in the germ line. WormBook 1–13. [DOI] [PMC free article] [PubMed]
- Frøkjaer-Jensen C., Davis M. W., Hopkins C. E., Newman B. J., Thummel J. M., et al. , 2008. Single-copy insertion of transgenes in Caenorhabditis elegans. Nat. Genet. 40: 1375–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez A. J., Mishima Y., Rihel J., Grocock R. J., van Dongen S., et al. , 2006. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science (New York, N.Y.) 312: 75–79 [DOI] [PubMed] [Google Scholar]
- Govindan, J. A., H. Cheng, J. E. Harris, and D. Greenstein, 2006 Galphao/i and Galphas signaling function in parallel with the MSP/Eph receptor to control meiotic diapause in C. elegans. Curr. Biol. CB 16: 1257–1268. [DOI] [PubMed]
- Govindan J. A., S. Nadarajan, S. Kim, T. A. Starich, and D. Greenstein, 2009. Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans. Development 136: 2211–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein D., Hird S., Plasterk R. H., Andachi Y., Kohara Y., et al. , 1994. Targeted mutations in the Caenorhabditis elegans POU homeo box gene ceh-18 cause defects in oocyte cell cycle arrest, gonad migration, and epidermal differentiation. Genes Dev. 8: 1935–1948 [DOI] [PubMed] [Google Scholar]
- Hammell M., Long D., Zhang L., Lee A., Carmack C. S., et al. , 2008. mirWIP: microRNA target prediction based on microRNA-containing ribonucleoprotein-enriched transcripts. Nat. Methods 5: 813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K., McCarter J., Francis R., Schedl T., 1996. emo-1, a Caenorhabditis elegans Sec61p gamma homologue, is required for oocyte development and ovulation. J. Cell Biol. 134: 699–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan C. H., Friedman R. C., Ruby J. G., Bartel D. P., 2011. Formation, regulation and evolution of Caenorhabditis elegans 3′UTRs. Nature 469: 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., Fire A., 1997. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146: 227–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketting R. F., 2011. microRNA Biogenesis and Function: An overview. Adv. Exp. Med. Biol. 700: 1–14 [DOI] [PubMed] [Google Scholar]
- Kovacevic I., Cram E. J., 2010. FLN-1/filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev. Biol. 347: 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara P. E., Okkema P. G., Kimble J., 1998. Germ-line regulation of the Caenorhabditis elegans sex-determining gene tra-2. Dev. Biol. 204: 251–262 [DOI] [PubMed] [Google Scholar]
- L’Hernault, S. W., 2006 Spermatogenesis. WormBook 1–14. [DOI] [PMC free article] [PubMed]
- Lau N. C., Lim L. P., Weinstein E. G., Bartel D. P., 2001. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294: 858–862 [DOI] [PubMed] [Google Scholar]
- Liu M., Liu P., Zhang L., Cai Q., Gao G., et al. , 2011. mir-35 is involved in intestine cell G1/S transition and germ cell proliferation in C. elegans. Cell Res. 21: 1605–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangone M., Manoharan A. P., Thierry-Mieg D., Thierry-Mieg J., Han T., et al. , 2010. The landscape of C. elegans 3′UTRs. Science 329: 432–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser, J., W. B. Wood, and M. D. Perry, 2002 Extragenic suppressors of a dominant masculinizing her-1 mutation in C. elegans identify two new genes that affect sex determination in different ways. Genesis 34: 184–195. [DOI] [PubMed]
- Mapes J., Chen J., Yu J., Xue D., 2010. Somatic sex determination in Caenorhabditis elegans is modulated by SUP-26 repression of tra-2 translation. Proc. Natl. Acad. Sci. USA 107: 18022–18027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massirer K. B., Perez S. G., Mondol V., Pasquinelli A. E., 2012. The miR-35–41 family of microRNAs regulates RNAi sensitivity in Caenorhabditis elegans. PLoS Genet. 8: e1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter J., Bartlett B., Dang T., Schedl T., 1997. Soma-germ cell interactions in Caenorhabditis elegans: multiple events of hermaphrodite germline development require the somatic sheath and spermathecal lineages. Dev. Biol. 181: 121–143 [DOI] [PubMed] [Google Scholar]
- Medeiros L. A., Dennis L. M., Gill M. E., Houbaviy H., Markoulaki S., et al. , 2011. Mir-290–295 deficiency in mice results in partially penetrant embryonic lethality and germ cell defects. Proc. Natl. Acad. Sci. USA 108: 14163–14168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singaravelu G., Chatterjee I., Marcello M. R., Singson A., 2011. Isolation and in vitro activation of Caenorhabditis elegans sperm. J. Vis. Exp. 47: 2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckius M., Maaskola J., Colombo T., Rahn H., Friedländer M. R., et al. , 2009. Large-scale sorting of C. elegans embryos reveals the dynamics of small RNA expression. Nat. Methods 6: 745–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Miwa J., 1978. Characterization of temperature-sensitive, fertilization-defective mutants of the nematode caenorhabditis elegans. Genetics 88: 285–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu E., Thivierge C., Flamand M., Mathonnet G., Vashisht A. A., et al. , 2010. Pervasive and cooperative deadenylation of 3′UTRs by embryonic microRNA families. Mol. Cell 40: 558–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ding L., Cheung T. H., Dong M., Chen J., Sewell A. K., Liu X., J. R. Yates 3rd, and M. Han, 2007. Systematic identification of C. elegans miRISC proteins, miRNAs, and mRNA targets by their interactions with GW182 proteins AIN-1 and AIN-2. Mol. Cell 28: 598–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.