Abstract
The abdominal appendages on male Themira biloba (Diptera: Sepsidae) are complex novel structures used during mating. These abdominal appendages superficially resemble the serially homologous insect appendages in that they have a joint and a short segment that can be rotated. Non-genital appendages do not occur in adult pterygote insects, so these abdominal appendages are novel structures with no obvious ancestry. We investigated whether the genes that pattern the serially homologous insect appendages have been co-opted to pattern these novel abdominal appendages. Immunohistochemistry was used to determine the expression patterns of the genes extradenticle (exd), Distal-less (Dll), engrailed (en), Notch, and the Bithorax Complex in the appendages of T. biloba during pupation. The expression patterns of Exd, En, and Notch were consistent with the hypothesis that a portion of the patterning pathway that establishes the coxopodite has been co-opted to pattern the developing abdominal appendages. However, Dll was only expressed in the bristles of the developing appendages and not the proximal–distal axis of the appendage itself. The lack of Dll expression indicates the absence of a distal domain of the appendage suggesting that sepsid abdominal appendages only use genes that normally pattern the base of segmental appendages.
Keywords: Appendage, Sepsidae, Abdomen, Innovation, Co-option
Introduction
The evolution of novel structures has been a mystery to biologists since the time of Darwin. Mayr (1960) recognized that, although the basis for gradual changes in phenotype was beginning to be understood, the origin of novel structures had been neglected. Novelties are defined as structures with no known homology (Müller and Wagner 1991), meaning that there is no corresponding structure in a related taxon. Of course, no structure evolves de novo and must necessarily use pre-existing tissues and genes for elaboration. It has been proposed that the evolution of organismal form repeatedly deploys the same developmental toolkit to elaborate new structures (Ganfornia and Sanchez 1999; True and Carroll 2002). This process, also known as co-option, does not require the evolution of new genes or developmental processes, but instead relies on pre-existing mechanisms for the development and evolution of novel features. Although the underlying mechanisms at the gene level are co-opted, they are elaborated and integrated into a different developmental environment, and this makes the structure novel. The co-option of genes and genetic pathways is believed to be central to the evolution of morphological novelty.
Abdominal appendages are complex novel structures that have evolved within the fly family Sepsidae (Hennig 1949; Pont 1979; Meier 1995; Meier 1996). Most species in the Sepsidae have a sexually dimorphic sternite on the fourth abdominal segment. The extent of the dimorphism differs between species; in some species males have a slight increase in bristle number and a subtle difference in sternite shape relative to females, and in other species males have a radically modified sternite bearing an appendage with many long bristles (Eberhard 2001). This appendage is a short segment attached to the body wall via a joint with musculature that allows movement in multiple directions (Eberhard 2001; Bowsher and Nijhout 2007). Males use the long bristles on these appendages to caress the female’s abdomen during copulation, leading to the hypothesis that abdominal appendages are the result of sexual selection (Eberhard 2001; Eberhard 2003). In the sepsid Themira biloba, the abdominal appendages develop during pupation from the ventral histoblast cells, which also form the rest of the ventral abdominal epidermis (Bowsher and Nijhout 2007).
To the extent that sepsid abdominal appendages have joints and a musculature that allows rotational movement, they resemble the serially homologous insect appendages. Despite this general resemblance, sepsid abdominal appendages cannot be classified as serially homologous to insect appendages because they do not share a common evolutionary history with other insect appendages. The segmental appendages of the insect—antenna, mouthparts, walking legs, and genitalia—are serial homologs reiterated across body segments (Snodgrass 1935; Roth 1988). The homology of insect appendages is apparent not only in their morphology (Boxshall 2004) but also in the patterning genes underlying their development, which are generally shared among different arthropod lineages (reviewed in Nagy and Williams 2001; Angelini and Kauffman 2005). The abdominal appendages of sepsids evolved in a region of the abdomen that has been devoid of appendages since the origin of the pterygote Insecta (Snodgrass 1935). The superficial similarity of these abdominal appendages to other appendages suggests that their evolution may have been possible via co-option of the developmental pathways that pattern the serially homologous appendages.
The arthropod ancestor of the insects is believed to have had appendages on every segment, but appendage development is repressed on the first seven abdominal segments of the insect abdomen. The suppression of appendage formation on the insect abdomen evolved via repression of the Distal-less (Dll) gene by genes in the Bithorax Complex (BX-C). In hemimetabolous insects such as Schistocerca americana, and in some holometabolous insects such as Tribolium castaneum, abdominal-A (abd-A) alone is responsible for abdominal appendage suppression (Palopoli and Patel 1998; Lewis et al. 2000), whereas in the more derived Drosophila melanogaster, Ultrabithorax (Ubx) and abd-A both repress Dll in the abdominal segments (Vachon et al. 1992). In insect groups that have larval abdominal prolegs or pleuropodia, the outgrowth of these appendages is achieved in one of three ways: by developing appendages on the first abdominal segment where abd-A is not expressed (Palopoli and Patel 1998; Lewis et al. 2000), or by repression of the BX-C genes, which allows for Dll expression (Warren et al. 1994), or by outgrowth that is independent of Dll expression (Suzuki and Palopoli 2001). We will test the second and third of these scenarios in T. biloba by examining BX-C and Dll expression during pupal development.
We hypothesized that genes involved in the development of the serially homologous appendages were co-opted during the evolution of abdominal appendages of the sepsid T. biloba. We tested this hypothesis by examining the expression of genes known to be involved in the patterning of serially homologous insect appendages. Conservation of appendage patterning in insects has been particularly well demonstrated in walking legs (reviewed in Angelini and Kauffman 2005). Although the genes that pattern insect appendages are numerous and diverse, the subset that set up the proximal–distal axis (Mardon et al. 1994; Panganiban et al. 1994; Gonzalez-Crespo and Morata 1995; Rauskolb et al. 1995; Panganiban et al. 1997; Abu-Shaar and Mann 1998; Schoppmeier and Damen 2001) and the anterior–posterior axis (Lawrence and Struhl 1996) are particularly well studied, and the regions that they pattern are well defined. We chose exd, Dll, en, and Notch because they represent different aspects of appendage patterning. Examining the expression of exd and Dll tests the presence of proximo–distal axis. Expression of en would delineate any anterior–posterior subdivision of the abdominal appendage and Notch expression indicates joint formation. In addition to describing the patterning of the abdominal appendages, we examine how outgrowth is possible on the abdomen by investigating BX-C expression (Ubx and abd-A). Examining the expression of these appendage patterning genes together allows a comparison of the novel sepsid abdominal appendages with the serially homologous insect appendages. Our results demonstrate that the expression patterns of en, exd, and Notch in the abdominal appendages are consistent with their role in appendage patterning. However, an absence of Dll expression, and the presence of Ubx/abd-A expression indicate that these abdominal appendages achieve outgrowth independent of Dll.
Materials and methods
Sepsid culture conditions
Adults of the sepsid T. biloba were imported from the stocks of Rudolf Meier of the National University of Singapore (APHIS permit # 48347). Cultures were maintained in climate-control chambers at 25°C and a 16:8 h light–dark cycle. Rearing methods were adapted from Lachmann (1991). Larvae were reared in Petri dishes filled with 0.5 cm of agar mixed with soy-based infant formula (ProSobee LIPIL, Enfamil) overlaid with a centimeter thick layer of cow dung. Adults were fed dung and honey mixed with water.
Immunostaining of the abdominal epidermis
Third instar larvae that had entered the wandering stage were isolated from the feeding media and allowed to develop for 48 h, which is a third of the way through pupation, or 72 h, which is half-way through pupation in T. biloba. The pupae were removed from the puparial case and the abdomens were dissected in PEM (100 mM PIPES-disodium salt, 2.0 mM EGTA, and 1.0 mM MgSO4 anhydrous). The fat body was removed by gently flushing the abdomen with PEM using a Pasteur pipette, leaving the pupal epidermis intact. The pupal abdomens were transferred to a watch glass containing PEM-FA fixative (PEM, 1.85% formaldehyde) and fixed for 10 min.
After fixation, the abdomens were washed in phosphate-buffered saline, 0.1% Triton X-100 (PBT), and blocked at 4°C in blocking solution (PBT, 10% normal goat serum) for at least 1 h. The pupal abdomens were incubated in primary antibody at room temperature for 3 h or overnight at 4°C. The primary antibodies were used at the following concentrations: Extradenticle (B11M) 1:4 (Aspland and White 1997), Engrailed (4D9) 1:4 (Patel et al. 1989), Notch (C17.9C6) 1:10 (Fehon et al. 1990), Ubx/Abd-A (Fp6.87) 1:10 (Kelsh et al. 1994), Mab22c10 1:100 (Zipursky et al. 1984), and Distal-less 1:100 (Panganiban et al. 1994). The abdomens were washed three times fast then 6×20 min in PBT at 4°C. The abdomens were incubated overnight at 4°C in the secondary antibody. The secondary antibody, goat anti-mouse or goat anti-rabbit IgG conjugated to HRP or Cy5 (Jackson ImmunoResearch Laboratories), was diluted to a final concentration of 1:300 in blocking solution. Following incubation with the secondary antibody, abdomens were washed three times fast then 6×20 min in PBT at 4°C. For visualization of HRP, abdomens were incubated for 10 min in a solution of 3,3′-diaminobenzidine (Sigma) with NiCl2, and the stain was visualized by adding 2 μl of 0.3% H2O2. After staining was apparent, the abdomens were washed three times quickly with PBT. The abdomens were mounted in 70% glycerol. Photographs of slides were taking on a Zeiss Axioplan compound scope using a Zeiss Axiocam camera.
Cryostat sectioning and immunostaining of whole abdomens
Third instar larvae with purged guts were isolated from the feeding media and allowed to develop for 72 h. The pupae were dissected from the puparial case and fixed for 2 h in PEM-FA fixative (PEM, 3.7% formaldehyde) at room temperature. Following fixation, pupae were either stored in 100% MeOH at −20°C, or immediately rehydrated into PBT.
To prepare for embedding in the sectioning medium, pupae were equilibrated in a solution of 30% sucrose dissolved in 1× PBT at 4°C. The pupae were embedded in a gelatin matrix (1% polyvinylpyrrolidone, 10% sucrose, and 7.5% gelatin in 1× PBT) and snap frozen in liquid nitrogen. Pupae were sectioned at −25°C into 15–20-μm thick sections using a cryostat (Reichert-Jung, Cryocut 1800, Leica). The sections were mounted on glass slides (Superfrost Plus, VWR Scientific), dried at room temperature and stored at −20°C.
Sections were immunostained using the same protocol as that for the abdominal epidermis (see previous methods). Sections were mounted in 70% glycerol with 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI). Photographs of slides were taking on a Zeiss Axioplan compound scope using a Zeiss Axiocam color camera.
Results
Abdominal appendage morphology and development
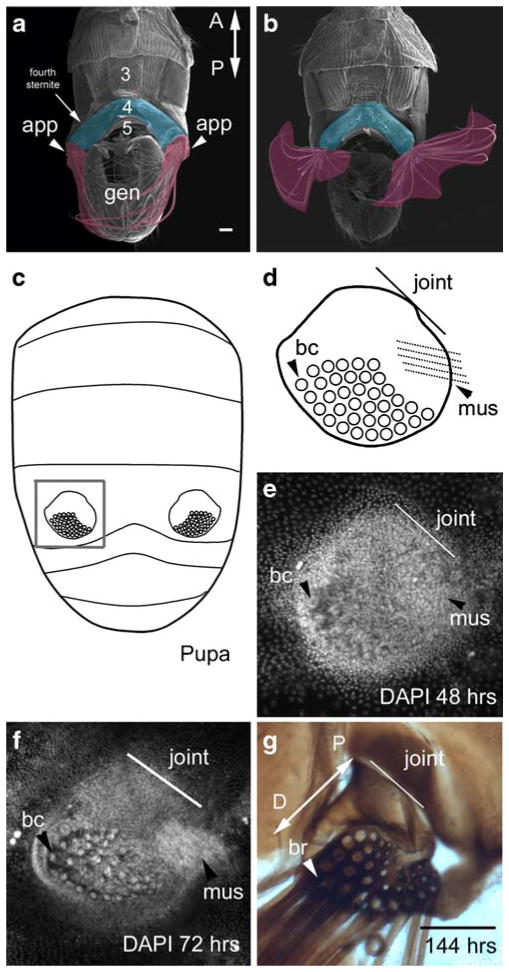
The abdominal appendages in T. biloba are extensions of the fourth ventral sternite and are attached to the sternite by a large joint, which allows the appendages to be rotated over 180° (Fig. 1a, b). The abdominal appendages are morphologically distinct from the abdominal epidermis by 48 h after puparium formation (APF; Fig. 1e), which is a third of the way through pupation in T. biloba and corresponds to P5 in D. melanogaster (Bainbridge and Bownes 1981). At this early stage, the developing bristle cells in the appendage are already visible. By 72 h APF, the ventro-lateral muscle that connects the appendage to the ventral midline, is also apparent (Fig. 1f). These features are illustrated in Fig. 1d, which will serve as guide to interpreting gene expression in later figures. When the adult male ecloses from the pupa around 144 h APF, the appendages are fully formed (Fig. 1g).
Fig. 1.
The abdominal appendages of T. biloba are located on the fourth segment of the male abdomen. a An SEM of the ventral side of the adult male abdomen shows the paired abdominal appendages (false-colored fuschia) are attached to the lateral edge fourth sternite (false-colored blue). b These abdominal appendages have a joint and musculature, which allows for 180° rotation. c The abdominal appendages first appear during pupation. The gray box indicates the abdominal appendage shown in d–g. d A cartoon of the abdominal appendage illustrates its morphological features: a large field of bristle cells (bc), a joint that connects the appendage to the body wall (line labeled ‘joint’), and a muscle (mus). e By 48 h after the beginning of pupariation, the abdominal appendage is already a distinct cluster of cells against the single-cell layer of the abdominal epidermis. The large nuclei of the bristle cells (bc) are apparent, as is the rudiment of the muscle (mus). Nuclei are stained with DAPI. f By 72 h, half-way through pupation, the morphological features or more distinct. g When the adult emerges from the pupa at 144 h, the abdominal appendage is fully formed. The long bristles (br) project toward the posterior of the abdomen. The proximal–distal axis of the appendage (double-headed arrow labeled P-D) extends from the joint where the appendage connects to the body wall (proximal) to the end of the bristle field (distal). Scale bar in a and g equals 100 μm (a adapted from Bowsher and Nijhout 2007)
Exd is expressed throughout the abdominal appendage and in the abdominal epidermis
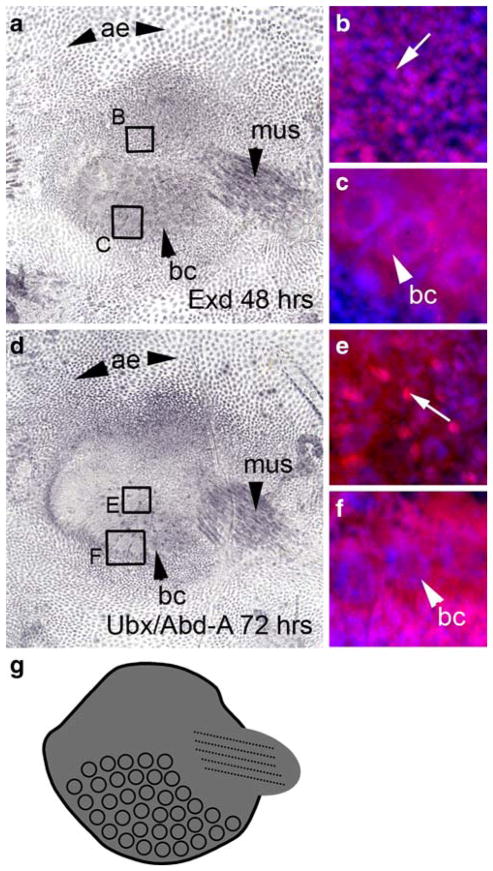
We examined the expression of Exd to determine whether it was down-regulated in the distal portion of the abdominal appendage, which is the case in the serially homologous insect appendages. However, Exd is expressed throughout the appendage at 48 and 72 h (Fig. 2). Exd is expressed in all the tissue types in the abdominal appendage (Fig. 2a, b), in the developing ventro-lateral muscle (Fig. 2a), and in the bristle cells (Fig. 2c). There was no evidence of down-regulation in any region. Exd expression in the appendage is localized to the nucleus (Fig. 2b) with the exception of the bristle cells, which have nuclear and cytoplasmic expression of Exd (Fig. 2c).
Fig. 2.
Exd and Ubx/Abd-A are expressed throughout the appendage. a At 48 h after pupariation, Exd is expressed in the abdominal epidermis (ae), the musculature (mus) of the appendage, the developing bristle cells (bc), and the appendage itself. b A close-up of the region delineated by box B shows that Exd (magenta, Cy5) is localized to the nucleus (blue, DAPI) in the body of the appendage. The arrow indicates an individual nucleus showing Exd expression. c A close-up of the region delineated by box C shows Exd is also expressed in the bristle cells, where it is expressed in both the donut-shaped nucleus (bc) and the cytoplasm. d Expression of Ubx/Abd-A at 72 h APF appears in the abdominal appendage, the abdominal epidermis (ae), the bristle cells (bc), and the musculature (mus) of the appendage. The expression of Ubx/Abd-A coincides with that of Exd, with the exception of small regions of Ubx/Abd-A expression (arrow in e) that are not localized to a nucleus. e is a close-up of the region delineated by box E. f Ubx/Abd-A is expressed in both the donut-shaped nucleus (bc) and the cytoplasm. f is a close-up of the region delineated by box F in d. g A summary of Exd and Ubx/Abd-A expression
In addition to being expressed in the abdominal appendage, Exd is also expressed in all the cells of the surrounding epidermis (Fig. 2a), which is consistent with its role in patterning the entire ventral abdomen in D. melanogaster (Gonzalez-Crespo and Morata 1995; Rauskolb et al. 1995).
Ubx/Abd-A is expressed throughout the abdominal appendage and in the abdominal epidermis
The abdominal Hox genes Ubx and abd-A repress leg formation in the abdomen of most insects. In some species with larval abdominal prolegs, Ubx and abd-A are down-regulated in the distal region of the prolegs. To determine the expression pattern of abdominal Hox genes in the abdominal appendages of T. biloba, we examined the expression of Ubx and Abd-A using an antibody that recognizes both epitopes (Kelsh et al. 1994). Ubx/Abd-A is expressed throughout the appendage, and also in the surrounding epidermis (Fig. 2d). Expression appears in body of the appendages, as well as in the muscle and bristle cells. Expression of Ubx/Abd-A overlaps that of Exd, with almost identical expression patterns. However, there are small regions of non-nuclear Ubx/Abd-A expression in the bristle field (Fig. 2e) that are not found for Exd. Despite this minor difference, the expression patterns of Exd and Ubx/Abd-A are remarkably similar. And, most significantly, both are expressed throughout the appendage with no evidence of a down-regulated region.
Because the Ubx/Abd-A antibody recognizes both Ubx and Abd-A epitopes, based on this data alone there is no way to know whether the expression seen in the abdominal appendages is due to Ubx or Abd-A. However, Ubx is not expressed in the fourth abdominal segment of D. melanogaster during pupation (Kopp and Duncan 2002), which suggests that the expression pattern seen in Fig. 2d is due the presence of Abd-A protein. A summary of Exd and Ubx/Abd-A expression can be found in Fig. 2g.
Dll is not expressed in the body of the abdominal appendage, but is expressed in the bristles of the abdominal appendage
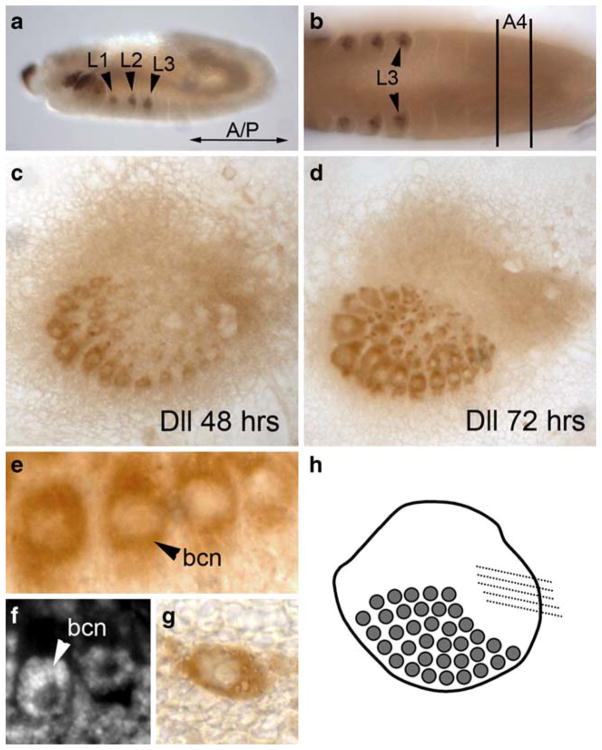
Dll is expressed in the distal portion of the serially homologous insect appendages, and in other non-appendage outgrowths from the body wall. Immunostaining with a Dll antibody (Panganiban et al. 1994) during embryogenesis demonstrates that Dll is expressed in T. biloba thoracic leg disks (Fig. 3a), consistent with a conserved function of Dll in Drosophila and T. biloba. At the time that the leg disks are being patterned, there is no visible expression of Dll in the fourth abdominal segment (Fig. 3b) in the region where the abdominal appendages will eventually develop, suggesting that Dll is not required for the specification of the abdominal appendage during embryogenesis.
Fig. 3.
Dll is expressed in the bristle cells, but not in the rest of the abdominal appendage. a During embryogenesis, Dll expression occurs in the thoracic legs (L1, L2, and L3) of T. biloba. b During embryogenesis, there is no Dll expression in the abdomen, including the ventral side of abdominal segment four (A4), where the appendages will eventually develop. c At 48 h APF, Dll is expressed in the developing bristle cells, which has been observed in Drosophila (Campbell and Tomlinson 1998), but not in the rest of the abdominal appendage. This pattern continues at 72 h APF (d) with strong Dll expression in the bristle cells but none in the surrounding appendage. A close-up of the bristle shaft cells (e) demonstrates that the expression of Dll is localized to bristle cells only, and is not in the appendage tissue surrounding the bristles. The Dll expression in the bristle cells is nuclear (bcn). f DAPI staining of the appendage at the same stage as e shows that the bristle cell nucleus (bcn) is a donut shape. Dll expression in the bristles of the appendage is the same pattern of expression seen in other bristles cells of the abdomen, such as on the fifth abdominal segment (g). h A summary of Dll expression in the abdominal appendage
Beginning as early as 48 h APF, Dll is strongly expressed in the bristle cells of the abdominal appendage (Fig. 3c, e). This expression persists through 72 h APF (Fig. 3d). This bristle-specific Dll expression is not restricted to the bristles of the abdominal appendage, but also is expressed in all abdominal bristles including those on the fifth abdominal segment (Fig. 3g), a segment that does not bear appendages. The expression of Dll in bristles has been described in Drosophila (Campbell and Tomlinson 1998), and also in the mechanoreceptors of horseshoe crabs (Mittman and Scholtz 2001) and the setae of crustaceans (Williams et al. 2002).
Although Dll is expressed in the bristle cells of the abdominal appendage, Dll is not expressed in the other abdominal appendage cells, such as those that form the joint or musculature of the appendage, or in the tissue surrounding the bristles. In particular, there appears to be no ‘distal’ region of the appendage, which is consistent with the observation that Exd and Ubx/Abd-A are expressed throughout (see Fig. 2). This suggests that the outgrowth established by Dll in the serially homologous insect appendages is not required in T. biloba abdominal appendages, or that outgrowth is established by a gene other than Dll.
En is expressed in a posterior region of the abdominal appendage and in the abdominal epidermis
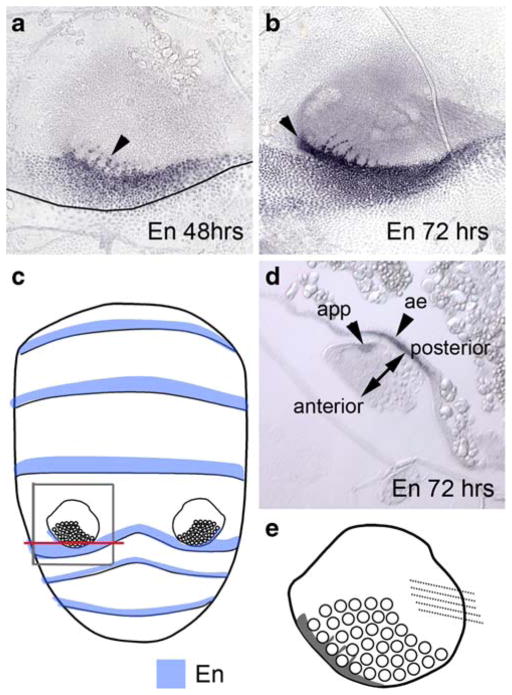
In Drosophila as in other arthropods, En expression defines the posterior region of each segment including those in the abdomen (Kopp et al. 1997). During the development of the serially homologous insect appendages, the segmental En expression extends into the appendage, becoming a separate domain that defines the posterior of the appendage. In T. biloba, En defines the posterior region of each segment, which is consistent with a conserved function of En. This segmental En expression extends into the abdominal appendage, localizing to a small region around the bristles at both 48 and 72 h (Fig. 4a, b). Nuclei expressing En are apparent around the developing bristle cells, which are much larger than the other cells of the appendage (Fig. 4a, b). Thus, En is not expressed in the bristles but in the tissue surrounding the bristle cells. By 72 h, En has established a separate domain in the abdominal appendage (Fig. 4b). This appendage-specific En expression can be seen in a cross-section of the developing appendage at 72 h (Fig. 4d). This small region of En expression has become distinct from the En domain in the body wall. We postulate that the En expressing cells in the abdominal appendage originate from the posterior of the embryonic segment and have separated from the segmental En domain due to outgrowth of the appendage from the body wall, although we have not traced the lineage of these cells. We propose the function of these cells is to define the posterior axis within the appendage. This allows us to define the anterior–posterior axis of the appendage (Fig. 2d). Thus, the orientation of the appendage is like that of a person whose arms are resting at his sides.
Fig. 4.
En is expressed in the abdominal appendage and abdominal epidermis. a En expression at 48 h APF is between the bristle cells (arrowhead) as well as in the posterior margin of the fourth segment. The boundary between the fourth and fifth abdominal segment is marked with a line. b At 72 h the appendage-specific expression is apparent in the posterior region of the appendage (arrowhead). c En (blue) is expressed in a posterior region of each abdominal segment (data not shown). The gray box delineates the region of the abdomen shown in a, b, and d. The red line indicates the location of the transverse cross-section in d. d Transverse cross-section of the appendage at 72 h shows that there are two independent regions of expression: one in the abdominal epidermis (ae) and one in the appendage (app). En expression defines the anterior–posterior axis of the appendage e a cartoon summary of En expression in the abdominal appendage
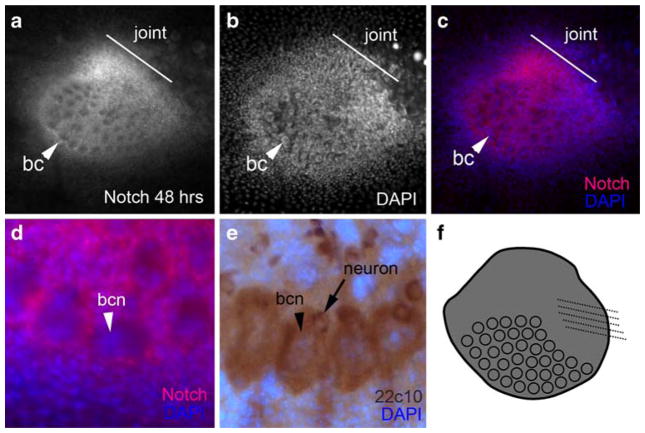
Notch is expressed in the abdominal appendage
Notch signaling defines the joints of the serially homologous insect appendages. In the abdominal appendages of T. biloba, strong expression is visible by 48 h in the joint of the appendage, the body of the appendage and the appendage bristles (Fig. 5a–d). The strongest Notch expression was in the developing joint region (Fig. 5c), which showed high levels of Notch compared to the rest of the appendage and compared to the number of cells in the joint region. Although Notch is expressed extensively throughout the abdominal appendage, we were not able to find cells in which Notch was localized to the nucleus, a requirement of active Notch signaling. However, it is possible that there are cells with nuclear Notch expression that we were not able to see due to the density and overlap of cells in the abdominal appendage. For example, Notch is expressed in all bristle precursor cells in Drosophila, but shows nuclear localization in only a subset of these cells, in which Notch signaling is activated. The socket cell and sheath cell have nuclear Notch expression, whereas the shaft cell and bristle neuron have non-nuclear Notch (Lai 2004). Antibody staining with the mab22c10 antibody, which labels the shaft cell and the bristle neuron in Drosophila (Hartenstein and Posakony 1989), identifies the corresponding cells in T. biloba (Fig. 4e). Notch is expressed in the shaft cells of the abdominal appendage, but is not localized to the nucleus (Fig. 4d) as is expected based on expression in D. melanogaster. We were not able to locate the socket cell and the sheath cell of the abdominal appendage bristle at this time.
Fig. 5.
Notch is expressed in the abdominal appendage. a At 48 h APF, Notch is strongly expressed in the developing joint (line), the body of the appendage, and the bristle cells (bc). b A DAPI image of a. c An overlay of a and b shows the strong expression of Notch in the joint region compared to the number of nuclei. d A close-up of the bristles shows Notch expression (red) occurs in the bristle shaft cell, but not in the nucleus of that cell (bcn, blue). Expression of 22c10 (e) labels both the bristle shaft cell and the bristle neuron. f a summary of Notch expression in the abdominal appendage
The Notch expression in the joint region and bristles of the abdominal appendage corresponded to the expected expression based on Drosophila. Contrary to initial expectations, Notch was also strongly expressed in the body of the appendage, a region of expression that has unknown function at this time. Additionally, a very low level of non-nuclear Notch expression was detected in the surrounding abdominal epidermis. This epidermal expression was exceedingly weak when compared to the expression in the appendage, thus, in Fig. 5a, there appears to be no expression in the surrounding epidermis.
Discussion
Sepsid abdominal appendages are complex novel structures that superficially resemble the serially homologous insect appendages in that they are jointed and have a short segment that can be rotated. Appendages have been absent from the insect abdomen since the origin of the Pterygota several hundred million years ago, thus these abdominal appendages are novel structures not homologous to other insect appendages. Previous work has demonstrated that sepsid abdominal appendages develop from histoblast cells, and not from imaginal disks like other dipteran appendages (Bowsher and Nijhout 2007). Considering this developmental difference between sepsid abdominal appendages and other dipteran appendages, we wished to determine whether the same genetic patterning processes were shared between these two types of appendages. Co-option of pre-existing pattering genes is thought to be a common event in the evolution of novel structures (Ganfornia and Sanchez 1999; True and Carroll 2002). The expression patterns of exd, Dll, en, and Notch indicated that at least part of the pathway that patterns other insect appendages also patterns these novel abdominal appendages (Fig. 6). However, the absence of Dll expression indicates a difference in patterning between the sepsid abdominal appendages and most serially homologous insect appendages.
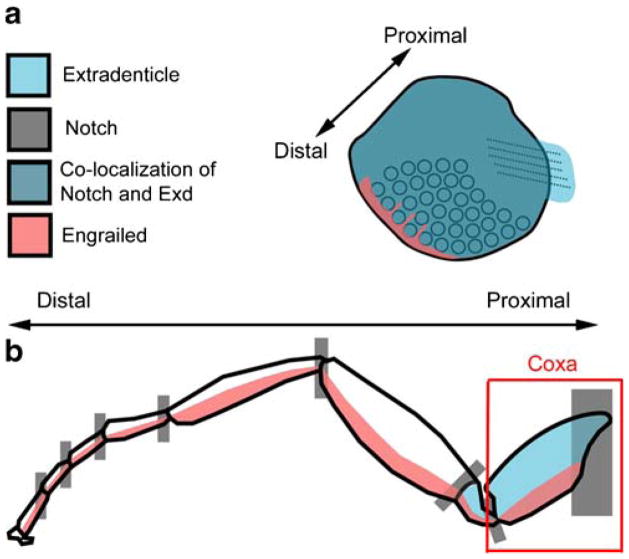
Fig. 6.
a Gene expression in the abdominal appendage of T. biloba resembles that found at the base of the serially homologous insect appendages. b In the insect walking leg, the expression of Notch, Exd, and En all overlap in the coxa and trochanter, which are the most proximal segments of the appendage. In the abdominal appendages of T. biloba (a), Exd is expressed throughout the appendage and Dll is not expressed, indicating the absence of a distal portion
Sepsid abdominal appendages are patterned like the base of an appendage
The expression patterns of En, Notch, and Exd support the hypothesis that the part of the appendage patterning pathway has been co-opted in the development of sepsid abdominal appendages. In T. biloba, En is expressed in the posterior compartment of each abdominal segment, consistent with its role in the D. melanogaster abdomen (Kopp et al 1997; Kopp and Duncan 2002). In the fourth abdominal segment, the band of En expression extends into the developing abdominal appendage. By the middle of the pupal period, En has acquired a distinct domain in the abdominal appendage. The presence of a separate En domain indicates that the abdominal appendages have an anterior–posterior axis, which is positioned in a similar location to that of a serially homologous insect appendage.
In the serially homologous insect appendages, Notch is strongly expressed in the joint region (de Celis et al. 1998; Bishop et al. 1999; Rauskolb 2001; Mirth and Akam 2002). We observe a similar pattern of expression in the abdominal appendages of T. biloba, where Notch is strongly expressed in the joint region where the appendage meets the body wall. This joint expression is consistent with the conclusion that the abdominal appendages of T. biloba are separate structures from the body wall. In D. melanogaster, Notch signaling functions in many tissues including bristle cells (Posakony 1994; Lai 2004) and neurons (Artavanis-Tsakonas et al. 1995). As expected, we observed Notch expression in the bristle cells. Although the strongest expression of Notch was in the appendage joint, Notch was also expressed throughout the body of the appendage. The function of Notch expression in the body of the appendage is unknown at this time.
The expression of Exd in the abdominal appendage is consistent with its role in patterning the proximal part of appendages. In the serially homologous insect appendages, expression of exd is restricted to the proximal region of the appendages, also called the coxopodite (Rauskolb et al. 1995; Abu-Shaar and Mann 1998). Snodgrass (1935) divided the leg into two regions, coxopodite and telopodite, based on morphological comparisons between arthropod groups. The coxa and trochanter constituted the coxopodite with the more distal segments comprising the telopodite. This subdivision of the leg is supported by expression of exd, which functions in the coxopodite and body wall but not in the telopodite (Abu-Shaar and Mann 1998). In D. melanogaster leg development, exd is restricted to the proximal part of the appendage by the upstream regulators of Dll (Abu-Shaar and Mann 1998). Yet, in T. biloba, Exd expression was ubiquitous in the abdominal appendage and not absent from the distal domain as it is in serially homologous appendages. Expression of Exd throughout the abdominal appendage rudiment implies that a distal region is not established in the abdominal appendages of T. biloba.
To determine whether a distal domain exists in the abdominal appendage, we investigated the expression of Dll. Dll has been shown to be critical for outgrowth of the serially homologous insect appendages (Panganiban et al. 1994). In animals, most projections from the body wall express Dll, including structures that are not normally classified as appendages, such as sea urchin podia and spines, caterpillar prolegs, and beetle horns (Lowe and Wray 1997; Panganiban et al 1997; Moczek et al 2006). Contrary to expectations, the abdominal appendages do not express Dll in their distal portion. However, Dll was expressed in the walking legs of T. biloba, supporting a conserved role of Dll in sepsids. Although the absence of Dll expression in the body of the abdominal appendages is surprising, it is consistent with the expression of Exd, which is normally excluded from the distal portion of appendages by the upstream regulators of Dll (Abu-Shaar and Mann 1998), but is expressed throughout the abdominal appendages of T. biloba.
In the insect abdomen, Ubx and abd-A repress Dll expression (Vachon et al. 1992; Palopoli and Patel 1998; Lewis et al. 2000), which explains the absence of abdominal appendages in most insects. Thus, a constraint on the development of abdominal appendages is the repression of Dll by the BX-C. The rarity of abdominal appendages in insects suggests that it must be difficult to break this constraint. We found that Ubx/abd-A is expressed throughout the abdominal appendages of T. biloba, and we hypothesize that this expression represses the expression of Dll.
The absence of a genetically defined distal domain in sepsid abdominal appendages is surprising, but it is not without precedent. Although the majority of the serially homologous insect appendages express Dll, the mandibles of insects are an exception to this rule (Popadic et al. 1998; Scholtz et al. 1998). The absence of Dll expression in insect mandibles has been interpreted as evidence that the mandible represents only the base of the appendage or coxopodite, and that the telopodite has been lost (Popadic et al. 1998). Interestingly, a similar pattern of gene expression is found in the larval abdominal prolegs of sawflies, which express Ubx/abd-A and Exd, but not Dll (Suzuki and Palopoli 2001). Sawfly prolegs have also been interpreted as the base of an appendage (Suzuki and Palopoli 2001). The same interpretation could be applied to the abdominal appendages of T. biloba (Fig. 6). Because a molecular marker specific to the coxopodite has not been identified, it is impossible to tell definitively whether these three types of appendages are coax-like. However, the molecular similarity between three different types of appendages suggests that a simple way to make an appendage-like structure without Dll expression is to use the genes that pattern the base of the appendage only.
Although we provide evidence for the co-option of the appendage patterning pathway, this co-option is only partial without the presence of Dll expression. Although the expression of En, Exd, and Notch in the abdominal appendages is consistent with what has been observed in the insect mandible and sawfly prolegs, the exact extent to which these appendages share a pattering network is still unclear. Although we did not observe Dll expression in the abdominal appendages, other genes that establish the proximal–distal axis in insect legs may be expressed, such as dachshund (dac). If dac were expressed, then the patterning similarity between the insect mandible and abdominal appendages of T. biloba would be strengthened, because insect mandibles express dac (Abzhanov and Kaufman 2000; Prpic et al 2001).
Co-option: old patterning genes in a new tissue
Although the abdominal appendages use some of the genes expressed during imaginal leg disk patterning, they develop from histoblast cells and not imaginal disks (Bowsher and Nijhout 2007). The co-option of the appendage patterning pathway in a non-disk imaginal tissue might seem surprising from the perspective of D. melanogaster development, in which all appendages develop from imaginal disks. However, many holometabolous insects develop adult appendages from tissues that are unlike canonical imaginal disks (Svácha 1992). Abdominal histoblasts are an imaginal cell type that is unique to dipterans, but their behavior during the larval stage and metamorphosis is not unlike that of the imaginal cells of the Manduca sexta leg (Tanaka and Truman 2005). The evolution of imaginal tissue and metamorphosis is thought to be a key-innovation in generating morphology diversity in insects. In this context, the co-option of some appendage patterning genes in the histoblast cells of T. biloba represents a mechanism by which the process of evolution can generate novelty. Gene co-option of genes into imaginal tissues may be a powerful mechanism for generating morphological novelty across insects.
Genes and morphology: where’s the homology?
When comparing gene expression across different morphological structures and different taxa, questions of homology naturally arise (Dickinson 1995; Abouheif 1997). Although similar pattering genes are used in the development of abdominal appendages in T. biloba and the serially homologous insect appendages, that similarity does not necessarily imply homology. The arthropod ancestor to the insects had appendages on every segment. These paired appendages are considered serially homologous because they share the same developmental and evolutionary origin. With the evolution of the insects, appendages were lost on the first through seven segments of the abdomen. This loss of appendages is thought to be the result of the repression of Dll by the abdominal Hox genes. Considering the abdominal appendages of the insect ancestor, one could imagine that the appendage pattering pathway is poised to be expressed in the insect abdomen, with only a few genetic changes being necessary for its deployment. From this perspective, does the appearance of appendages on the T. biloba abdomen represent co-option of the appendage patterning pathway to a new function, or is it simply a reactivation of a pre-existing pathway? The distinction between co-option and reactivation is the following: co-option is the use of the same gene in two non-homologous structures, whereas reactivation is the re-evolution of a lost homologous trait. So, could the evolution of sepsid abdominal appendages be considered reactivation and not co-option because these abdominal appendages are essentially homologous to other insect appendages?
There is no reason to believe that sepsid abdominal appendages are homologous to other insect appendages because, since the origin of the Insecta some 300 million years ago, there have not been any appendage-like structures on the fourth abdominal segment. The fact that a structure that looks like an appendage, with a joint and moves, has evolved on the sepsid abdomen does not make it homologous to pre-existing appendages. The development of appendages in this novel location depends on the deployment of a subset of the appendage patterning genes in a novel anatomical and cellular context, and thus constitutes a true co-option of part of a pre-existing pathway.
Acknowledgments
We would like to thank Rudolf Meier for sharing his culture of T. biloba, and Bill Eberhard for sharing his knowledge of sepsid behavior and rearing. Thank you to Rob White, Nipam Patel, and Sean Carroll for providing antibodies. We would like to thank Lisa Nagy, Yui Suzuki, Robin Smith, and two anonymous reviewers for their comments on the manuscript. Laura Grunert provided critical technical assistance. We would also like to thank Maple View Farm in Chapel Hill, NC and the University of Arizona Agricultural Center for providing cow dung. This work was funded by the Department of Biology at Duke University (JHB and HFN), National Science Foundation grant IBN-0315897 (HFN), and the Center for Insect Science at the University of Arizona through the National Institute of Health Training Grant 1K12 GM000708 (JHB).
Footnotes
Communicated by P. Simpson
Contributor Information
Julia H. Bowsher, Email: jhb4@email.arizona.edu, Center for Insect Science, University of Arizona, 1007 E. Lowell St., Tucson, AZ 85721, USA
H. Frederik Nijhout, Department of Biology, Duke University, PO Box 90338, Durham, NC 27708, USA.
References
- Abouheif E. Developmental genetics and homology: a hierarchical approach. TREE. 1997;12:405–408. doi: 10.1016/s0169-5347(97)01125-7. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Kaufman TC. Homologs of Drosophila appendage genes in the patterning of arthropod limbs. Dev Biol. 2000;227:673–689. doi: 10.1006/dbio.2000.9904. [DOI] [PubMed] [Google Scholar]
- Abu-Shaar M, Mann RS. Generation of multiple antagonistic domains along the proximodistal axis during Drosophila leg development. Development. 1998;125:3821–3830. doi: 10.1242/dev.125.19.3821. [DOI] [PubMed] [Google Scholar]
- Angelini DR, Kauffman TC. Insect appendages and comparative ontogenetics. Dev Biol. 2005;286:57–77. doi: 10.1016/j.ydbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Aspland SE, White RAH. Nucleocytoplasmic localization of extradenticle protein is spatially regulated throughout development in Drosophila. Development. 1997;124:741–747. doi: 10.1242/dev.124.3.741. [DOI] [PubMed] [Google Scholar]
- Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- Bishop SA, Klein T, Arias AM, Couso JP. Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development. 1999;126:2993–3003. doi: 10.1242/dev.126.13.2993. [DOI] [PubMed] [Google Scholar]
- Bowsher JH, Nijhout HF. Evolution of novel abdominal appendages in a sepsid fly from histoblasts, not imaginal discs. Evol Dev. 2007;9(4):347–354. doi: 10.1111/j.1525-142X.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- Boxshall GA. The evolution of arthropod limbs. Biol Rev. 2004;79:253–300. doi: 10.1017/s1464793103006274. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. The roles of the homeobox genes aristaless and Distal-less in patterning the legs and wings of Drosophila. Development. 1998;125:4483–4493. doi: 10.1242/dev.125.22.4483. [DOI] [PubMed] [Google Scholar]
- de Celis JF, Tyler DM, de Celis J, Bray SJ. Notch signaling mediates segmentation of the Drosophila leg. Development. 1998;125:4617–4626. doi: 10.1242/dev.125.23.4617. [DOI] [PubMed] [Google Scholar]
- Dickinson WJ. Molecules and morphology: where’s the homology. Trends Genet. 1995;11:119–121. doi: 10.1016/s0168-9525(00)89015-0. [DOI] [PubMed] [Google Scholar]
- Eberhard WG. Multiple origins of a major novelty: moveable abdominal lobes in male sepsid flies (Diptera: Sepsidae), and the question of developmental constraints. Evol Dev. 2001;3:206–222. doi: 10.1046/j.1525-142x.2001.003003206.x. [DOI] [PubMed] [Google Scholar]
- Eberhard WG. Sexual behavior and morphology of Themira minor (Diptera: Sepsidae) males and the evolution of male sternal lobes and genitalic surstyli. Can Entomol. 2003;135:569–581. [Google Scholar]
- Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MAT, Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Ganfornia MD, Sanchez D. Generation of evolutionary novelty by functional shift. BioEssays. 1999;21:432–439. doi: 10.1002/(SICI)1521-1878(199905)21:5<432::AID-BIES10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Crespo S, Morata G. Control of Drosophila adult pattern by extradenticle. Development. 1995;121:2117–2125. doi: 10.1242/dev.121.7.2117. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Posakony JW. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 1989;107:389–405. doi: 10.1242/dev.107.2.389. [DOI] [PubMed] [Google Scholar]
- Hennig W. Sepsidae. In: Lindner E, editor. Die Fliegen der Palaearktischen Region. E Schweizerbart’sche Verlagsbuchhandlung; Stuttgart, Germany: 1949. pp. 1–91. [Google Scholar]
- Kelsh R, Weinzierl ROJ, White RAH, Akam M. Homeotic gene expression in the locust Schistocerca: an antibody that detects conserved epitopes in ultrabithorax and abdominal-A proteins. Dev Gen. 1994;15:19–31. doi: 10.1002/dvg.1020150104. [DOI] [PubMed] [Google Scholar]
- Kopp A, Muskavitch MAT, Duncan I. The roles of hedgehog and engrailed in patterning adult abdominal segments of Drosophila. Development. 1997;124:3703–3714. doi: 10.1242/dev.124.19.3703. [DOI] [PubMed] [Google Scholar]
- Kopp A, Duncan I. Anterioposterior patterning in adult abdominal segments of Drosophila. Dev Biol. 2002;242:15–30. doi: 10.1006/dbio.2001.0529. [DOI] [PubMed] [Google Scholar]
- Lachmann A. Vergleichende Untersuchung zum Lebenszyklus der kuhdungbewohnenden Sphaeroceridenarten Chaetopodella scutellaris (Haliday, 1836) und Coproica lugubris (Haliday, 1836) Deutsche Entomologische Zeitschrift. 1991;38:197–210. [Google Scholar]
- Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131(5):965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from Drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Lewis DL, DeCamillis M, Bennett RL. Distinct roles of the homeotic genes Ubx and abd-A in beetle embryonic abdominal appendage development. Proc Natl Acad Sci USA. 2000;97(9):4504–4509. doi: 10.1073/pnas.97.9.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CJ, Wray GA. Radical alterations in the roles of homeobox genes during echinoderm evolution. Nature. 1997;389:718–721. doi: 10.1038/39580. [DOI] [PubMed] [Google Scholar]
- Mardon G, Solomon NM, Rubin GM. dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development. 1994;120:3473–3486. doi: 10.1242/dev.120.12.3473. [DOI] [PubMed] [Google Scholar]
- Mayr E. The emergence of evolutionary novelties. In: Tax S, editor. Evolution after Darwin. University of Chicago Press; Chicago: 1960. pp. 349–380. [Google Scholar]
- Meier R. Cladistic analysis of the Sepsidae (Cyclorrhapha: Diptera) based on a comparative scanning electron microscopic study of larvae. Syst Entomol. 1995;20:99–128. [Google Scholar]
- Meier R. Larval morphology of the Sepsidae (Diptera: Sciomyziodea), with a cladistic analysis using adult and larval characters. Bulletin of the AMNH. 1996;228:1–147. [Google Scholar]
- Mirth C, Akam M. Joint development in the Drosophila leg: cell movements and cell populations. Dev Biol. 2002;246:391–406. doi: 10.1006/dbio.2002.0593. [DOI] [PubMed] [Google Scholar]
- Mittman B, Scholtz G. Distal-less expression in embryos of Limulus polyphemus (Chelicerata, Xiphosura) and Lepisma saccharina (Insecta, Zygentoma) suggests a role in the development of mechanoreceptors, chemoreceptors, and the CNS. Dev Genes Evol. 2001;211:232–243. doi: 10.1007/s004270100150. [DOI] [PubMed] [Google Scholar]
- Moczek AP, Rose D, Sewell W, Kesselring BR. Conservation, innovation, and the evolution of horned beetle diversity. Dev Genes Evol. 2006;216:655–665. doi: 10.1007/s00427-006-0087-2. [DOI] [PubMed] [Google Scholar]
- Müller GB, Wagner GP. Novelty in evolution: restructuring the concept. Annu Rev Ecol Syst. 1991;22:229–256. [Google Scholar]
- Nagy LM, Williams TA. Comparative limb development as a tool for understanding the evolutionary diversification of limbs in arthropods: challenging the modularity paradigm. In: Wagner GP, editor. The character concept in evolutionary biology. Academic; San Diego: 2001. pp. 455–488. [Google Scholar]
- Palopoli MF, Patel NH. Evolution of the interaction between Hox genes and a downstream target. Curr Biol. 1998;8:587–590. doi: 10.1016/s0960-9822(98)70228-3. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Nagy L, Carroll SB. The role of the Distal-less gene in the development and evolution of insect limbs. Curr Biol. 1994;4:671–675. doi: 10.1016/s0960-9822(00)00151-2. [DOI] [PubMed] [Google Scholar]
- Panganiban G, Irvine SM, Lowe C, Roehl H, Corley LS, Sherbon B, Grenier JK, Fallon JF, Kimble J, Walker M, Wray GA, Swalla BJ, Martindale MQ, Carroll SB. The origin and evolution of animal appendages. Proc Natl Acad Sci USA. 1997;94:5162–5166. doi: 10.1073/pnas.94.10.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NM, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989;58:955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Pont AC. Sepsidae: Diptera, Cyclorrhapha, Acalyptrata. In: Fitton MG, editor. Handbooks for the identification of British insects. Royal Entomological Society of London; London: 1979. pp. 1–35. [Google Scholar]
- Popadic A, Panganiban G, Rusch D, Shear WA, Kaufman TC. Molecular evidence for the gnathobasic derivation of arthropod mandibles and for the appendicular origin of the labrum and other structures. Dev Genes Evol. 1998;208:142–150. doi: 10.1007/s004270050165. [DOI] [PubMed] [Google Scholar]
- Posakony JW. Nature versus nurture: asymmetric cell divisions in Drosophila bristle development. Cell. 1994;76:415–418. doi: 10.1016/0092-8674(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Prpic NM, Wigand B, Damen WG, Klingler M. Expression of dachshund in wild-type and Distal-less mutant Tribolium corroborates serial homologies in insect appendages. Dev Genes Evol. 2001;211:467–477. doi: 10.1007/s004270100178. [DOI] [PubMed] [Google Scholar]
- Rauskolb C. The establishment of segmentation in the Drosophila leg. Development. 2001;128:4511–4521. doi: 10.1242/dev.128.22.4511. [DOI] [PubMed] [Google Scholar]
- Rauskolb C, Smith KM, Peifer M, Wieschaus E. extradenticle determines segmental identities throughout Drosophila development. Development. 1995;121:3663–3673. doi: 10.1242/dev.121.11.3663. [DOI] [PubMed] [Google Scholar]
- Roth VL. The biological basis of homology. In: Humphries CJ, editor. Ontogeny and systematics. Columbia University Press; New York: 1988. pp. 1–26. [Google Scholar]
- Scholtz G, Mittmann B, Gerberding M. The pattern of Distal-less expression in the mouthparts of crustaceans, myriapods and insects: new evidence for the gnathobasic mandible and the common origin of Mandibulata. Int J Dev Biol. 1998;42:801–810. [PubMed] [Google Scholar]
- Schoppmeier M, Damen WGM. Double-stranded RNA interference in the spider Cupiennius salei: the role of Distal-less is evolutionarily conserved in arthropod appendage formation. Dev Genes Evol. 2001;211:76–82. doi: 10.1007/s004270000121. [DOI] [PubMed] [Google Scholar]
- Snodgrass RE. Principles of insect morphology. McGraw-Hill Book Company; New York: 1935. [Google Scholar]
- Suzuki Y, Palopoli MF. Evolution of insect abdominal appendages: are prolegs homologous or convergent traits? Dev Genes Evol. 2001;211:486–492. doi: 10.1007/s00427-001-0182-3. [DOI] [PubMed] [Google Scholar]
- Svácha P. What are and what are not imaginal discs: reevaluation of some basic concepts (Insecta, Holometabola) Dev Biol. 1992;154:101–117. doi: 10.1016/0012-1606(92)90052-i. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Truman JW. Development of the adult leg epidermis in Manduca sexta: contribution of different larval cell populations. Dev Genes Evol. 2005;215(2):78–89. doi: 10.1007/s00427-004-0458-5. [DOI] [PubMed] [Google Scholar]
- True JR, Carroll SB. Gene co-option in physiological and morphological evolution. Annu Rev Cell Dev Biol. 2002;18:53–80. doi: 10.1146/annurev.cellbio.18.020402.140619. [DOI] [PubMed] [Google Scholar]
- Vachon G, Cohen B, Pfeifle C, McGuffin ME, Bota J, Cohen SM. Homeotic genes of the Bithorax Complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- Warren RW, Nagy L, Selegue J, Gates J, Carroll S. Evolution of homeotic gene regulation and function in flies and butterflies. Nature. 1994;372:458–461. doi: 10.1038/372458a0. [DOI] [PubMed] [Google Scholar]
- Williams TA, Nulsen C, Nagy LM. A complex role for Distal-less in crustacean appendage development. Dev Biol. 2002;241:302–312. doi: 10.1006/dbio.2001.0497. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Venkatesh TR, Teplow DB, Benzer S. Neural development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984;36:15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]