Abstract
Haematopoietic stem cells (HSCs) are tissue-specific stem cells that replenish all mature blood lineages during the lifetime of an individual. Clinically, HSCs form the foundation of transplantation-based therapies for leukaemias and congenital blood disorders. Researchers have long been interested in understanding the normal signalling mechanisms that specify HSCs in the embryo, in part because recapitulating these requirements in vitro might provide a means to generate immune-compatible HSCs for transplantation. Recent embryological work has demonstrated the existence of previously unknown signalling requirements. Moreover, it is now clear that gene expression in the nearby somite is integrally involved in regulating the transition of the embryonic endothelium to a haemogenic fate. Here, we review current knowledge of the intraembryonic signals required for the specification of HSCs in vertebrates.
Haematopoietic stem cells (HSCs) are self-renewing blood and immune cell precursors capable of producing daughter cells that proliferate and mature to provide all adult blood effector cells, including erythroid, myeloid and lymphoid cells1. Clinically, these cells are the relevant component of bone marrow transplants, which are used to treat patients with leukaemia and congenital blood disorders, but the availability of immune-compatible donors remains a problem2. The advent of induced pluripotent stem cell (iPS cell) technology has raised the possibility of making HSCs from a patient’s own non-haematopoietic tissues3,4, but it is not possible so far to convert pluripotent cells to HSCs that are capable of long-term self-renewal and generation of normal distributions of the complete set of mature blood cell lineages5,6. This suggests that key specification requirements are unknown. Attempts to generate HSCs in vitro could be informed by understanding the normal in vivo mechanisms that generate these cells during embryonic development. A clear understanding of the developmental specification of HSCs might moreover provide insight into the causes of congenital diseases, such as aplastic anaemias and congenital neutropenia. Recent work in multiple species has uncovered several previously unknown signalling inputs required for HSC specification; here, we review these advances and place them in a developmental context.
Establishment of adult haematopoiesis
In all vertebrates, the establishment of self-renewing HSCs with the full set of lineage potentials is preceded during development by earlier primitive and definitive ‘waves’ of haematopoiesis (FIG. 1a), which take place in various anatomical locations (FIG. 1b). Primitive myeloid and erythroid waves (BOX 1) transiently generate limited sets of effector cells. In Xenopus laevis, the spatial separation of cells fated to generate primitive and definitive haematopoietic tissues is well defined, with primitive precursors occupying the ventral blood islands (VBIs) and definitive blood arising from the dorsal lateral plate mesoderm (DLP mesoderm)7–10; separation occurs as early as the 32-cell stage7. Interestingly, these populations retain significant plasticity with respect to their potential to adopt primitive or definitive haematopoietic fates, as transplantation of VBI cells to the DLP mesoderm and vice versa until mid-neurula stages results in cells taking on the identity specified by the site of engraftment11. These results indicate that the signalling environment has a major role in assignment of primitive versus definitive haematopoietic fates until relatively late in development.
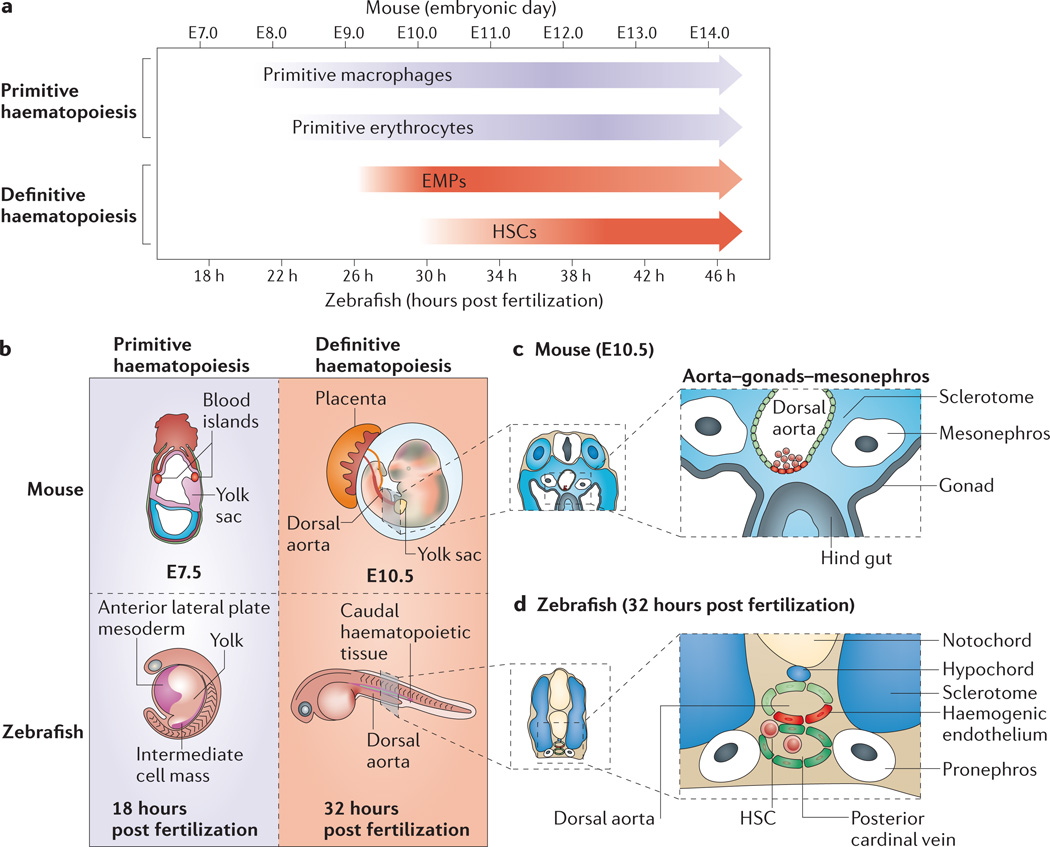
Figure 1. Haematopoietic stem cells.
a | Haematopoietic stem cell (HSC) specification is preceded by earlier waves of embryonic haematopoiesis. Primitive macrophages and erythrocytes arise first. A transient definitive erythromyeloid progenitor (EMP) population also precedes HSCs. b | Primitive blood in mouse derives from extra-embryonic tissue of the yolk sac in blood islands. In zebrafish, primitive red blood cells derive from mesoderm of the intermediate cell mass and primitive myeloid cells derive from anterior lateral plate mesoderm. EMPs are specified in the mouse yolk sac and zebrafish caudal haematopoietic tissue. c,d | HSCs (red) appear at embryonic day (E)10.5 in mouse and 32 hours post fertilization in zebrafish from haemogenic endothelium of the dorsal aorta, from where they bud into the arterial circulation (mouse; c) or the venous circulation (zebrafish; d).
Box 1. Primitive haematopoiesis.
Although haematopoietic stem cells (HSCs) sustain the long-term production of all mature blood lineages during adult life, the emergence of HSCs during development is preceded by earlier and distinct haematopoietic ‘waves’ with limited or no self-renewal capacity and restricted lineage potential. These waves have been loosely grouped into primitive and definitive waves (FIG. 1a). Primitive haematopoiesis can be further divided into two waves that produce primitive erythrocytes or primitive myeloid cells (including macrophages, as well as possibly megakaryocytes161 and neutrophils38,39,162,163). The primitive waves of haematopoiesis are distinct from definitive waves in at least four ways. First, they occur earlier than the definitive waves. Second, they occur in the absence of an identifiable self-renewing stem cell. Third, they do not yield the full distribution of adult mature blood cell lineages, notably not producing B and T cells. And fourth, primitive erythrocytes express a distinct set of globins from their adult counterparts. Primitive haematopoiesis occurs in the avian and mammalian yolk sac163, and in the intermediate cell mass and on the anterior yolk ball of the zebrafish embryo39.
Definitive haematopoiesis can be split into two waves. An earlier wave proceeds through a multipotent progenitor — known as the erythromyeloid progenitor (EMP) — with lineage potential limited to erythrocytes, mega-karyocytes and myeloid cells12–19. This wave is followed by the specification of HSCs that self-renew for the life of the individual and are capable of producing all adult lineages, including lymphocytes. That EMPs produce myeloid and erythroid lineages from a single progenitor and that murine EMPs produce erythrocytes expressing adult globins has caused some confusion, especially in in vitro assays seeking to generate HSCs, because it is impossible to distinguish EMPs and HSCs on the basis of assays with only an erythromyeloid readout. Furthermore, EMPs and HSCs cannot be distinguished by cell surface phenotype. These issues highlight the need to test haematopoietic precursors for their long-term reconstitution and lymphoid potential side-by-side with bone marrow HSCs when determining the success of in vitro HSC specification.
Anatomy of HSC specification
Across vertebrate phyla, HSCs arise from an endothelial precursor found specifically in arterial haemogenic endothelium. In mammals, there is evidence to support de novo HSC specification in the umbilical and vitel-line arteries20, placenta21,22 and, surprisingly, the head23. Some lineage tracing studies suggest that HSC specification also occurs in the yolk sac24,25. However, the signalling events involved in HSC specification in the dorsal aorta are currently the best understood, and this site of emergence for HSCs is conserved in vertebrate models such as frog and fish, which has helped to illuminate the native processes controlling specification. It is likely that many (but probably not all26,27) mammalian HSCs arise in the intraembryonic dorsal aorta20,28,29. In chick, grafting experiments have shown that adult blood is entirely embryo derived30, and in X. laevis, grafting experiments have established that adult blood comes from the tissue fated to form dorsal aorta10. Furthermore, in zebrafish31,32 and mouse explants33 the emergence of HSCs from endothelium in the ventral floor of the embryonic dorsal aorta has been directly visualized, despite differences in the directionality of budding (into the venous circulation in zebrafish compared with into the arterial circulation in mouse). This Review therefore focuses on the conserved intraembryonic signalling environment that regulates the emergence of HSCs from the dorsal aorta.
Recent work in multiple species has conclusively demonstrated that HSC precursors transit through an endothelial stage. Conditional labelling in mouse shows that adult HSCs were previously positive for the endothelial marker vascular endothelial (VE)-cadherin (encoded by Cdh5)34,35. Moreover, endothelial-specific deletion of the mouse runt-related transcription factor 1 (Runx1) gene (BOX 2), which is required for HSC specification, eliminates HSCs35. The process of mouse and zebrafish endothelial cells becoming haematopoietic cells has been directly visualized in real time in vitro36,37 and in vivo31–33. These results have established that HSCs derive from haemogenic endothelium of the dorsal aorta.
Box 2. Key haematopoietic factors and pathways.
RUNX1
The runt-domain transcription factor RUNX1 (also known as AML1 and CBFα) is a pivotal haematopoietic stem cell (HSC) marker. RUNX1 was originally identified as a gene that is frequently rearranged in myeloid leukaemias164. During development, it is expressed in the haematopoietic clusters in the dorsal aorta165, marks long-term HSCs166 and is required for definitive, but not primitive, haematopoiesis in mouse and zebrafish83,167,168. RUNX1 is required for the transition from endothelial cells to HSCs but is not required later for HSC maintenance35. This requirement apparently involves maintaining cell viability after emergence from the haemogenic endothelium, as live imaging in zebrafish shows that runx1-knockdown cells escape the endothelium, but quickly die32; this phenotype is consistent with the absence of RUNX1+ cells in the fetal liver of Runx1-knockout mice165. Thus Runx1 expression in the dorsal aorta during HSC specification is one of the earliest markers of prospective HSCs.
Nodal and BMP signalling
Nodals and bone morphogenetic proteins (BMPs) are members of the transforming growth factor-β (TGFβ) family of signalling molecules. They function through activin-like receptor family receptors, which homo- and heterotetramerize, cross-phosphorylate and lead to phospho-activation of downstream SMAD effector proteins165,169,170. Nodal signalling additionally requires a co-receptor of the epidermal growth factor cripto FRL cryptic (EGF-CFC) family of proteins, which includes mammalian Cripto and Criptic and zebrafish One-eyed pinhead receptors. TGFβ signals are transduced to the nucleus by the actions of SMAD proteins. Specific SMADs seem to be characteristically used by Nodal signalling (SMAD2 and SMAD3), whereas others seem to be used by BMPs (SMAD1, SMAD5 and SMAD8). SMAD4, the common SMAD, is shared by both sets. In mouse, there is a single Nodal gene, whereas in zebrafish there are two, nodal-related 1 (squint) and nodal-related 2 (cyclops).
FGF signalling
In human, mouse, and fish there are more than 20 fibroblast growth factor (FGF) ligands and five to eight FGF receptor (FGFR) or receptor-like genes. Dimeric FGFRs bind to ligand and activate multiple intracellular pathways, notably including the phosphoinositide 3-kinase (PI3K)-AKT and mitogen-activated protein kinase pathways171.
Notch signalling
The Notch pathway transmits signals between neighbouring cells and is heavily involved in embryonic patterning172,174. In mammals there are three Delta family ligands (DLL1, DLL3 and DLL4) and two Jagged ligands (Jagged 1 and Jagged 2). In zebrafish there are five Delta family ligands (Dla, Dlb, Dlc, Dld and Dll4) and three Jagged ligands (Jagged 1a, Jagged 1b and Jagged 2). During normal Notch signalling, a transmembrane ligand on a presenting cell interacts with one of the Notch receptors, Notch 1 to Notch 4 in mammals or Notch 1a, Notch 1b, Notch 2 and Notch 3 in zebrafish. Functional signalling requires ubiquitin ligases of the Mind bomb family (MIB1 in mammals; Mib and Mib2 in zebrafish). Two sequential protein cleavage events mature the receptor and release an intracellular receptor fragment, the Notch intracellular domain (NICD), which binds DNA-binding CBF1/suppressor of hairless/Lag-2 (CSL; also known as Rbpjκ), and mediates transcriptional activation in coordination with the Mastermind (Mam) co-activator. In the absence of NICD, CSL silences gene expression through the activity of co-repressors.
Canonical WNT signalling
The WNT signalling pathway regulates many aspects of development and disease175. WNT signalling has been broadly separated into two arms: the β-catenin-dependent (canonical) and the β-catenin-independent (non-canonical) pathways. The canonical pathway has been characterized in far greater detail and involves regulation of the stability of a signalling pool of the multifunctional protein, β-catenin. In the absence of WNT signalling, β-catenin is phosphorylated by a ‘destruction complex’, targeting it for degradation by the proteasome. WNT ligation of its Frizzled and LDL-receptor-related protein (LRP)-family co-receptors leads to inhibition of the destruction complex and translocation of stabilized β-catenin to the nucleus, where it can activate transcription of WNT target genes in cooperation with DNA-binding factors of the lymphocyte enhancer factor/T cell factor (LEF/TCF) family.
The dorsal aorta is part of the earliest trunk vasculature and in mouse lies in a region bounded by the primitive gonads and mesonephros, sometimes termed the aorta–gonads–mesonephros (FIG. 1b,c). The endothelium of the dorsal aorta is of mesodermal origin and derives from mesoderm termed ‘splanchnic’ in mammals or ‘lateral plate’ in anamniotes, which is initially found lateral to the somitic mesoderm. Endothelial precursors migrate axially below the developing somites either as two distinct developing endothelial tubes that merge to a single dorsal aorta (as in mouse; FIG. 1c) or as two populations of mesenchymal cells that will form a primitive vascular cord that subsequently lumenizes (as in zebrafish; FIG. 1d). Intraembryonically, only endothelial cells of the trunk dorsal aorta are competent to form HSCs, strongly suggesting that they are pre-patterned for cell-intrinsic competence to respond to later specification signals. Once HSCs have been specified from the haemogenic endothelium, they leave the dorsal aorta and move to transient proliferation tissues and finally to the adult haematopoietic organs: predominantly the bone marrow in mammals and the kidney in zebrafish7,38,39.
Early embryonic patterning
Proper specification of the body axes and primary germ layers, as well as subsequent finer compartmentalization of the mesoderm into tissues and organs, are prerequisites for haematopoietic development. In many cases, in vitro attempts to make HSCs have been structured to duplicate early embryogenesis underlying formation of the posterior trunk mesoderm that houses haemogenic endothelium3,4, so it is important to understand the signalling pathways underlying these events. Conceptually, at least five steps must occur endogenously to eventually produce HSCs: specification of mesoderm, ventralization and posteriorization of mesoderm, lateralization of mesoderm, production of trunk arterial endothelium, and specification of HSCs from haemogenic endothelium. The complex processes that generate the primary body architecture have been extensively reviewed elsewhere40–43 and only key events related to the generation of haematopoietic mesoderm are emphasized here.
Early development of lateral plate mesoderm
Mesoderm in mouse arises when cells of the epiblast ingress through the primitive streak (FIG. 2a), a process that requires Nodal44, bone morphogenetic protein (BMP)45–47, fibroblast growth factor (FGF)48,49 and WNT50 signalling (BOX 2). Similarly, zebrafish that lack all Nodal signalling have a nearly complete failure of mesoderm specification51,52. In vitro, the combined actions of WNT and Nodal signalling can produce mesodermal Brachyury-expressing primitive streak-like cells from mouse embryonic stem cells (ESCs) in embryoid bodies53.
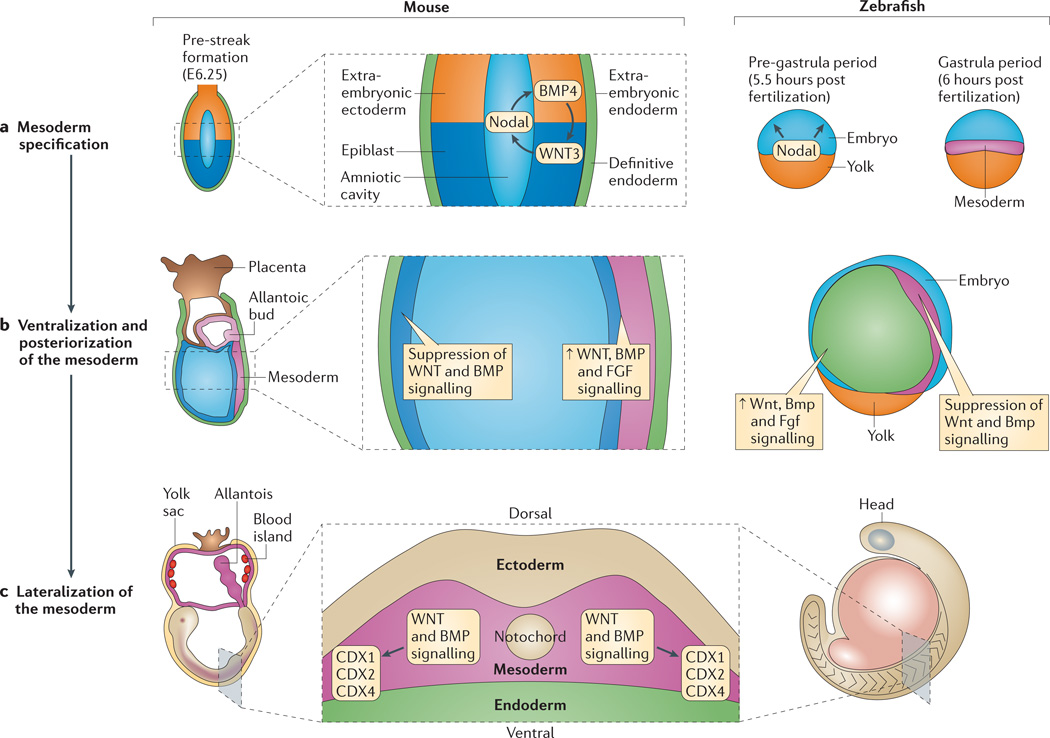
Figure 2. Early signalling regulating HSC specification.
Haematopoietic stem cells (HSCs) derive from haemogenic endothelium of the dorsal aorta, which is of mesodermal origin (pink). a | In mouse, Nodal, WNT3 and bone morphogenetic protein 4 (BMP4) are required for primitive streak formation (not shown) and mesoderm specification. In zebrafish, Nodal is required for mesoderm specification. b | HSCs derive specifically from ventroposterior mesoderm, specification of which requires WNT ligands, BMPs and fibroblast growth factors (FGFs). WNT and BMP signalling are actively suppressed in the anterior region by secreted antagonists, whereas WNT, BMP and FGF signalling cooperate to specify ventroposterior mesoderm. c | HSCs derive from lateral plate mesoderm. Mesoderm is lateralized by WNT and BMPs, which regulate the expression of caudal-type homeobox 1 (CDX1), CDX2 and CDX4.
Mesoderm and other germ layer specification is integrated with dorsal–ventral and anterior–posterior patterning. Blood specification relies on this patterning as the dorsal aorta, and hence HSCs, develop from ventro-posterior lateral plate mesoderm. In anamniotes, specification of the earliest dorsoventral axis involves the Wnt pathway-dependent specification of a dorsal organizer and ventral Bmp signalling, which set up self-reinforcing genetic programmes that loosely partition the embryo42,43. Across species, in subsequent anterior–posterior patterning, WNT signalling is actively suppressed in the anterior region by secreted antagonists, whereas WNT ligands and BMPs cooperate to specify ventroposterior mesoderm40–43 (FIG. 2b). FGF signalling is also required for specification of the posterior mesoderm48,49,54–56. At this point, ventroposterior mesoderm contains the precursors for a fairly broad but finite number of tissues, including notochord, somite, pronephros, vasculature and blood, so further regulatory interactions must pattern these individual tissue fates.
HSCs develop from the dorsal aorta of the trunk mesoderm, and multiple lines of evidence indicate that trunk mesoderm is distinct from both head and tail mesoderm. Although all trunk mesodermal tissues are absent in zebrafish embryos lacking Nodal signalling, a few somites in the posterior do develop, which suggests that there are distinct mechanisms specifying the most posterior mesoderm51,52,57,58. Similarly, the zebrafish cloche mutant, which lacks trunk endothelium and HSCs59, nevertheless expresses multiple vascular markers in the ventral tail and later recovers limited blood marker expression specifically in this region60–62. This differential patterning indicates that trunk mesoderm might carry forward distinct cell-intrinsic determinants of competence to respond to haematopoietic signalling.
The dorsal aorta and subsequently HSCs derive from lateral plate (splanchnic) mesoderm that is lateral to the axial, paraxial and somitic mesoderm (FIG. 2c). Thus, ventroposterior mesoderm must be ‘lateralized’ by the combined actions of WNT and BMP signalling. Ectopic expression of Wnt ligands, Nodal and Bmps in zebrafish can induce the formation of ectopic trunk and tail structures that contain lateral mesoderm, but not axial mesoderm or neural ectoderm, which indicates that these factors have a role in the induction of lateral plate mesoderm63. In mouse as well, BMP signalling is required for distinguishing non-axial mesoderm, and embryos with mosaic deletion of BMP receptor 1A (Bmpr1a) form ectopic somitic tissue at the expense of lateral plate mesoderm64.
But what are the WNT- and BMP-inducible factors that might mediate the haematopoietic competence of the lateral plate mesoderm? Studies in both mouse and zebrafish show that the cooperative actions of WNT3A, WNT8, BMP2B and BMP4 induce the expression of caudal-type homeobox (CDX) genes, including Cdx1, Cdx2 and Cdx4 (REFS 46,65–68), which regulate homeobox (HOX) gene expression (FIG. 2c). Key downstream HOX genes probably include Hoxa9a and Hoxb6b, which have been shown to be required for haematopoietic programming in vivo and in vitro69–73. Although overexpression and in vitro data support a cell-autonomous role for these genes in regulating haematopoietic competence, their apparent lack of expression in haematopoietic precursors in vivo69,70 makes it important to consider that they might be required non-cell-autonomously (for example, by regulation of a relay signal).
Formation of the haemogenic endothelium
The step immediately preceding HSC emergence is the development of the haemogenic endothelium that lies in the ventral floor of the primitive dorsal aorta, which expresses the vascular endothelial growth factor (VEGF) receptor KDR (also known as FLK1 and VEGFR2; Kdrl in zebrafish)31,32,74,75. In mouse and chick, bilateral lumenized endothelial aortae converge to the midline and fuse before the emergence of HSCs76. Before fusion, BMP antagonists, including chordin (CHRD) and noggin (NOG), prevent the aortae from migrating to the midline77,78. Maintenance of the axial ‘avascular space’ is relieved by downregulation of these BMP antagonists, signalling the onset of fusion79. By contrast, in anamniotes such as frog and fish, unlumenized, mesenchymal angioblasts migrate to the midline, where they form a primitive vascular cord, which subsequently lumenizes to form the early dorsal aorta80. This migration happens despite axial expression of multiple Bmp inhibitors81,82. In either case, HSCs do not appear until the formation of a single lumenized aorta at the midline, which indicates that trigger signals might be received at this point.
Work in zebrafish and frogs shows that Sonic hedgehog (Shh) and VegfA are involved in regulating the medial convergence of haemangioblasts (which contain future endothelium and HSCs) in the lateral plate mesoderm (FIG. 3). In all vertebrates, Shh is expressed in specific axial tissues, namely in the floorplate of the neural tube and in the notochord. In mouse and chick, Shh is also expressed in the gut endoderm at the relevant times. Pre-haematopoietic kdrl-expressing mesoderm exhibits altered midline convergence in zebrafish embryos with gain or loss of Shh signalling83–85, and one of the X. laevis Vegfa isoforms is able to regulate angioblast migration86. Thus, early on, Shh and VegfA are required for arteriovenous and HSC specification by regulating the convergence of precursors to the midline. In some cases of perturbation in the levels of Shh, although haemangioblast convergence is delayed, many cells do eventually reach the midline but fail to appropriately turn on haematopoietic markers83–85, so conceivably the precursor population must arrive in time to experience an as yet unknown transient specification signal.
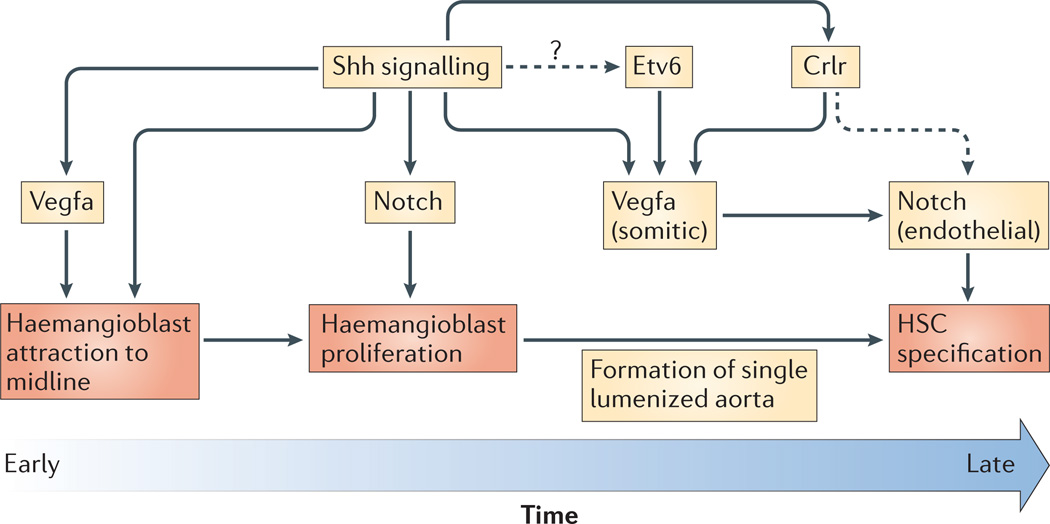
Figure 3. Processes regulated by Sonic hedgehog.
Sonic hedgehog (Shh) and vascular endothelial growth factor A (Vegfa) function as attractive cues to haemangioblasts converging to the midline in zebrafish. Shh induces Notch |- dependent proliferation of haemangioblasts. Vegfa is required for the expression of Notch receptors in haemogenic endothelium. Shh regulates vegfa expression directly and through calcitonin receptor-like receptor (Crlr). The Ets transcription factor Etv6 (also known as Tel1) also regulates vegfa expression in Xenopus laevis. HSC, haematopoietic stem cell.
HSCs derive particularly from endothelium of the primitive dorsal aorta20. This observation suggests the possibility that distinguishing artery from vein may be a prerequisite for initiation of the HSC specification programme. However, in at least one highly artificial situation, HSC markers are expressed by endothelium without arterial gene expression87. Nevertheless, many of the pathways that are required for HSC specification, such as Shh, Vegfa and Notch signalling (BOX 2), are also required for the preceding arteriovenous specification (see below), raising the possibility that the requirement for these signals in HSC specification might, in part, reflect their requirement in arteriovenous specification.
Notch signalling is iteratively involved in patterning the early trunk vasculature of vertebrates, including the dorsal aorta that contains haemogenic endothelium. Before the formation of a primitive vascular cord in fish, Notch signalling downstream of Shh regulates the proliferation (FIG. 3) of lateral plate haemangioblasts positive for friend leukaemia integration (fli; also known as fli1a) and ets1-related protein (etsrp; also known as etv2)87–93,87. Later, Notch signalling controls arteriovenous specification94 (FIG. 4a,b). Loss of Notch 1 or of Notch 1 and Notch4 (REF 95), or of other Notch pathway components required for Notch activity (such as mind bomb (Mib)94,96,97 and CBF1/suppressor of hairless/Lag-2 (Csl; also known as Rbpjκ)94,98) leads to loss of arterial-specific marker expression in both mouse and zebrafish, as does loss of specific downstream effectors of Notch signalling such as hairy/enhancer-of-split related with YRPW motif 2 (Hey2; also known as Herp1, Herp2, Hesr2 and Hrt2)99–101. Notch signalling leads to the expression of arterial-specific genes including ephrin B2 (Efnb2) (FIG. 4b), which has been identified as a direct target of Notch 1 in endothelial cell culture102. Efnb2 is a ligand for Eph receptor b4 (Ephb4), which is expressed by venous cells, with reciprocal signalling partially mediating differential adhesion and arteriovenous segregation72,103,104. After the initial patterning of the dorsal aorta and posterior cardinal vein, Notch signalling mediated by Delta-like 4 (DLL4) regulates angiogenic growth of the intersegmental vessels105,106. Thus Notch signalling is a conserved integral part of maturation of the arterial endothelium from which HSCs derive.
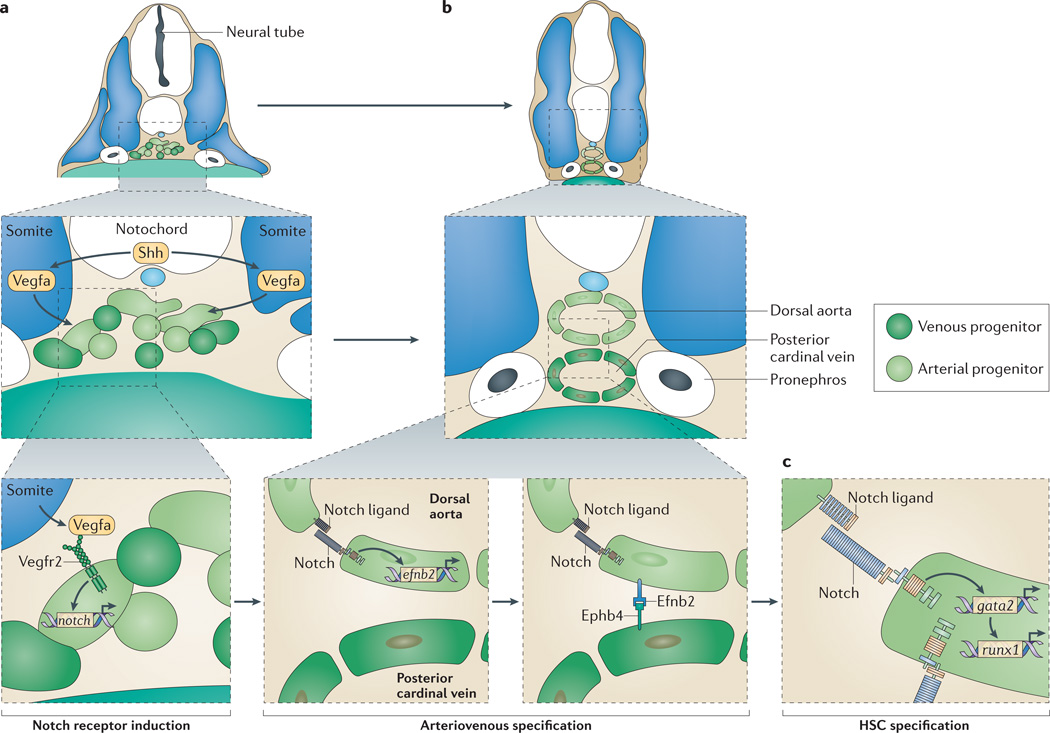
Figure 4. Processes regulated by Notch.
Arteriovenous specification in zebrafish. a | Sonic hedgehog (Shh) produced by the notochord induces somite expression of vascular endothelial growth factor A (Vegfa), which, in turn, through binding its receptor Vegfr2, induces expression of notch in haemogenic endothelial cells. b | Notch regulates arteriovenous specification by regulating ephrin B2 (efnb2) expression. Arterial cells (light green) express Efnb2 and segregate from venous cells (dark green), which express the Efnb2 receptor Eph receptor b4 (Ephb4). c | Notch ligands including Jagged 1 expressed by neighbouring cells activate Notch receptors to specify haematopoietic stem cells (HSCs) by activating gata2 expression, which in turn activates runx1 expression. The relevant runx1 enhancer does not contain Notch-responsive binding elements, which indicates that Notch does not directly regulate runx1 expression.
The question of when the first cells of the dorsal aorta are specified relative to the posterior cardinal vein is crucial to understanding the signalling underlying specification of HSCs, because HSCs only derive from arterial endothelium. If the artery is specified earlier than at the midline, then HSC programming could occur when the vessels are en route to their axial destination. In mouse and chick, the endothelium of the early bilateral aortae derives from splanchnopleural mesoderm, whereas the bilateral veins come from somitic mesoderm. In chick, somite-derived endothelium later completely replaces the original aortic endothelium, but this second-wave endothelium is not haemogenic, and it is not clear whether this trait is conserved in other vertebrates. However, in both mouse and chick, arteriovenous segregation clearly occurs before aortic fusion at the midline, and HSC patterning signals might therefore be presented before fusion. Nevertheless, the timing of HSC appearance, after the formation of a single lumenized aorta at the midline, supports the likelihood of at least some signals for HSC specification being presented at the midline.
In fish, presumptive arterial and venous angioblasts are morphologically indistinguishable during their convergence. Experimental evidence as to when these cells are specified as artery or vein is mixed. Fate mapping99 and live imaging84 studies imply that individual lateral plate mesoderm cells ‘know’ their fate before arrival at the midline. By contrast, another study has shown that the first venous cells sprout from the primitive aorta, suggesting that they are mixed until then103. One potential resolution of these data is that intermingled cells of the primitive vascular cord may already know their fate, and that specified venous cells sort (rather than divide) away from arterial cells in a ventral migration. It is also possible that different mechanisms operate in parallel: some mixed but pre-specified arteriovenous cells might first populate the primitive vascular cord before distinct lateral plate mesoderm cells migrate directly to the posterior cardinal vein region. Venous cells of the mixed cord might then sprout off and contribute to the existing posterior cardinal vein rudiment. In any case, it seems likely that arteriovenous specification is underway before mesoderm cells reach the midline in fish.
HSC specification from haemogenic endothelium
HSCs are ultimately specified from haemogenic endothelium in the ventral floor of the dorsal aorta at the midline. Several immediately proximal signalling events trigger this final specification step.
Notch signalling
As described above, Notch signalling is required for vascular patterning, particularly vascular morphogenesis and arteriovenous specification. Notch signalling is additionally required for HSC specification. Zebrafish and mouse embryos with a mutation or targeted deletion in Mib, which encodes a ubiquitin ligase required for Notch signalling, lack HSCs107,108. Similarly, mice with a targeted deletion of the Notch pathway component Csl have a decreased number of HSCs109. At least one Notch ligand, Jagged 1, has been found to contribute to the HSC specification process, as Jagged1-knockout mice have decreased numbers of Gata2- and Runx1 -expressing HSCs in the dorsal aorta at embryonic day (E)10.5–E11 (REF. 110). Interestingly, these animals represent one of the few circumstances in which arteriovenous and HSC specification can be separated, as Jagged 1 -knockout animals retain expression of the arterial marker EFNB2 (REF 110). Of the Notch family receptors, Notch 1 is required for definitive haematopoietic development111 upstream of Runx1, which when ectopically expressed can rescue haematopoietic gene expression in cultured para-aortic splanchnopleural cells deficient for Notch1 (REF. 112). Notch 1-mediated activation of Runx1 has been posited to work indirectly through GATA2, as the Runx1 promoter does not contain Notch-responsive elements. Importantly, the requirement for Notch 1 is cell-autonomous; in other words, HSC precursors must experience a Notch 1-mediated signal at some point to become HSCs, as shown by the fact that chimeric mice generated from wild-type and Notch1-deleted cells show no contribution of Notch 1-deficient cells to the adult haematopoietic system113. Together these findings have led to a model wherein Notch ligands, including Jagged 1, either in the endothelium of the dorsal aorta itself or in nearby cells, signal through Notch 1 for cellular commitment to an HSC fate (FIG. 4c). However, as noted above, Notch signalling is also required for arteriovenous specification, and it has not yet been established whether HSC specification through Notch signalling is a distinct process. Interestingly, reception of a Notch signal (or signals) is one of the few things that distinguishes HSCs from EMPs111,114.
Shh–Vegfa signalling
From work in zebrafish, it is clear that Notch pathway-mediated activation of HSC specification (and arterial fate) is directly regulated by an Shh–VegfA signalling axis (FIGS 3,4a). Shh signalling regulates somitic expression of vegfa; Vegfa is a ligand for the kdrl gene product Vegfr2 (REF. 115). Vegfa in turn regulates endothelial cell expression in the presumptive arterial endothelium of arterial markers such as efnb2a, as well as expression of the Notch receptor notch3 (previously known as notch5)71,115. Shh, Vegfr2 and Notch signalling are all required for HSC specification83. Taken together, these results suggest a model wherein Shh signalling from the floorplate, notochord and hypochord induces vegfa expression in the somites. Vegfa, in turn, activates expression of Notch receptor genes in the arterial endothelium, thereby potentiating the ability to receive the Notch signals that are required for HSC specification. There are still some pieces of information missing to fully validate this model. For example, the specific Notch receptor thought to receive the requisite HSC-specifying Notch signal is Notch 1 (REF. 113); however, although Shh regulates expression of arterial notch1b84, no studies have shown that Vegfa is required for expression of either of the notch1 paralogues in zebrafish.
Multiple additional factors also influence the Shh– Vegfa–Notch pathway (FIG. 3b). The Ets transcription factor Etv6 (also known as Tel1) was recently shown to be required non-cell-autonomously for HSC specification by activating expression of vegfa in the somites of X. laevis embryos116. Knockdown of etv6 results in a failure of arterial specification, including loss of notch4 expression. Whether or not etv6 is regulated by Shh remains to be determined. Another player in this pathway is the calcitonin receptor-like receptor (Crlr). In zebrafish, Shh positively regulates expression of crlr117, and Crlr is required for vegfa and arterial gene expression85,117. Interestingly, under gain-of-function Shh signalling conditions, both Crlr and Vegfa seem to independently regulate arterial specification, as both must be knocked down to abrogate arterial gene expression, and Notch is required for the effects of both85. As Crlr overexpression is unable to produce ectopic arterial gene expression on its own85, these results suggest that Shh gain of function is able to upregulate as-yet-unknown Crlr cofactors that can cooperate to specify arterial gene expression independently of Vegfa.
BMP signalling
BMP signalling, as described earlier, is essential for patterning the ventroposterior mesoderm of the developing embryo in conjunction with WNT family members. Recently, Bmp4 signalling has been shown to be directly required to trigger HSC specification from haemogenic endothelium of the dorsal aorta in zebrafish82. Secreted Bmp antagonists, including Chd and Nog, are expressed in the axial tissue surrounding the forming dorsal aorta until shortly before the appearance of HSC precursors marked by runx1. Immediately before the onset of runx1 expression, expression of these Bmp antagonists is downregulated and the proteins are degraded by the pro-Bmp molecule Tolloid82,118. Interestingly, although HSC specification fails in animals with conditional abrogation of Bmp signalling, arterial gene expression was left intact, demonstrating that arterial and HSC fate specification can be separated. A similar requirement for BMP signalling in mouse has been suggested by the identification of BMP4 as a factor expressed by stromal cells of the aorta–gonads–mesonephros that augments HSC potential119.
Canonical WNT signalling
The effects of canonical WNT signalling on the homeostasis of the adult haematopoietic system have been highly controversial because of conflicting results based on gain of function120–125 and loss of function126–130 experiments involving central components of the pathway. Although increased canonical WNT signalling has significant effects on HSC self-renewal and lineage maturation, deletion of key WNT factors in mouse, such as β-catenin and γ-catenin, has in some cases produced no effect on HSC homeostasis126–128. However, in these studies, residual WNT activity was observable, indicating that the deletions produced hypomorphic alleles or that alternative factors transduced WNT activity in the absence of β-catenin and γ-catenin. Using alternative approaches, abrogating canonical WNT signalling does produce HSC and lineage maturation effects124,130. Recent studies have shown that the haematopoietic system is highly sensitive to the dosage of WNT signalling, for example by enhancing HSC self-renewal or skewing lineage distribution125,131,132.
During development, WNT signalling is crucial for the initial specification and/or very early maintenance of HSCs in multiple ways. The requirement for WNT factors in patterning the ventroposterior mesoderm that gives rise to HSCs has been discussed above. In addition, it seems that WNT factors have a more direct role in signalling to early HSCs or HSC precursors. Wnt3α-knockout mice have decreased numbers of HSCs in the fetal liver and an impaired ability to support serial transplantation129. Interestingly, the deleterious effects of a failure to experience early WNT3A signalling cannot be rescued by transplantation of those HSCs that do develop into wild-type hosts, which indicates that knockout cells might ‘remember’ their early environment, perhaps through epigenetic modifications125,129. These results indicate a crucial requirement for WNT3A signalling in the early maintenance and proliferation of HSCs. Direct effects of WNT3A on the original specification of HSCs in the haemogenic endothelium of the dorsal aorta have not been reported, but conditional deletion of the central effector of the canonical WNT pathway, β-catenin, in endothelial cells results in a strong loss of haematopoietic potential, but does not otherwise affect formation of endothelium in, for example, the liver133. These results indicate that WNT–β-catenin signalling is required for haematopoietic commitment from endothelium.
Prostaglandins
An important role for prostaglandin E2 (PGE2) in HSC specification or early maintenance was uncovered in a chemical screen for molecules that alter the number of HSCs in zebrafish at 36 hours post fertilization134, about 13 hours after HSC precursors first appear as a runx1-expressing group of cells in the ventral floor of the dorsal aorta. In these studies, PGE2 treatment increased HSC numbers, whereas an inhibitor of PGE2, indomethacin, led to loss of HSCs. In follow-up studies135, this group showed that PGE2 mediates cAMP-directed stimulation of cAMP-dependent protein kinase (also known as PKA) activity, which augments Wnt-mediated inhibition of glycogen synthase kinase 3 (Gsk3). These conditions amplify β-catenin levels, resulting in greater canonical Wnt signal transduction, which in turn results in increased HSC numbers. Subsequent preclinical studies have verified the ability of PGE2 to augment the transplantation potential of human cord blood total and CD34+ cells in xenotransplants to mice, as well as of mobilized peripheral blood stem cells in autologous transplantation of non-human primates136.
Non-canonical WNT signalling
Multiple β-catenin-independent (non-canonical) WNT signalling pathways have been described137–139. In some cases, these pathways make use of Frizzled receptors, whereas in others they seem to function through atypical receptors such as members of the receptor tyrosine kinase-like kinase (RYK) and the receptor tyrosine kinase-like orphan receptor (ROR) families. These pathways activate diverse cellular responses and signal transduction pathways, from cytoskeletal changes to alterations in calcium signalling to antagonism of canonical WNT signalling.
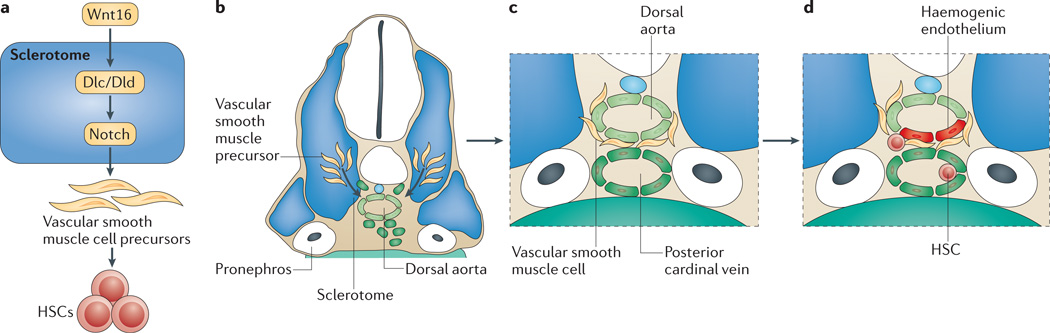
We recently showed that non-canonical Wnt signalling by the conserved Wnt16 ligand is required for HSC specification in zebrafish140 (FIG. 5). Wnt16 does not act directly on HSC precursors, but rather acts in the adjacent somites to activate expression of two Notch family ligand genes, deltac (dlc) and deltad (dld), which are required in combination for HSC specification (FIG. 5a). In the absence of either ligand, HSC numbers are decreased, and in the absence of both ligands, HSC specification is abrogated. As described above, Notch signalling is required cell-autonomously for HSC specification in mouse, but expression of Notch reporter transgenes and arteriovenous specification remain intact in wnt16-knockdown zebrafish, which indicates that HSC precursors in the dorsal aorta are still experiencing Notch signals. Moreover, rescue experiments in which Notch activation in HSC precursors was enforced in the absence of Wnt16 show that the Notch signalling event controlled by Wnt16–Dlc and Wnt16–Dld is earlier than and distinct from the cell-autonomous requirement for Notch signalling. Thus somitic Dlc-and Dld-mediated Notch signalling does not seem to act directly on HSC precursors. These results suggest that Wnt16–Dlc and Wnt16–Dld activate expression of a relay signal or are required for a morphogenetic event that potentiates HSC specification.
Figure 5. Model for indirect Notch-mediated haematopoietic stem cell specification in zebrafish.
a | Schematic of a model for Wnt16-mediated regulation of haematopoietic stem cells (HSCs) through Notch patterning of the ventromedial compartment of the somite, the sclerotome. Wnt16 regulates somitic expression of the Delta family ligand genes dlc and dld. Both Wnt16 and the combined actions of Dlc and Dld are required for the specification of sclerotome, which contains vascular smooth muscle cell precursors. These precursors emigrate from the somite (b), sheathe the dorsal aorta (c) and signal to haemogenic endothelium to trigger HSC specification (d).
Animals with wnt16 knockdown or dlc and dld knockdown also have defects in the ventromedial compartment of the somite, the sclerotome. Therefore, sclerotomal specification or morphogenesis could be required for HSC specification. The sclerotome has been shown to give rise to multiple adult tissues, including the vascular smooth muscle cells (VSMCs) that sheath the dorsal aorta141–144. In chick and mouse, VSMC precursors emigrate from the somite and surround the dorsal aorta to make mature vasculature (FIG. 5b,c). Notch signalling has been shown to control the emigration of a population of somitic cells that joins the dorsal aorta in chick, although it was not determined whether these cells are indeed future VSMCs145. A single model that explains all these events is that Wnt16–Dlc and Wnt16–Dld signalling direct VSMC precursors to carry an HSC-specification relay signal to the dorsal aorta (FIG. 5d). Testing this model will require the generation of transgenic animals to determine the timing, origin and signalling capacity of VSMC precursors.
Blood flow, shear force and nitric oxide signalling
The effects of blood flow and pressure on the endothelium have an important role in both patterning the developing cardiovascular system and remodelling adult vasculature146. Recent work indicates that blood flow is also required for the early maintenance of HSCs147–149. A chemical screen for modifiers of HSC development in zebrafish showed that multiple compounds that regulate heartbeat and blood flow had effects on HSC numbers in developing zebrafish148. Many of these effects can be explained by a lack of blood flow, as embryos mutant for the cardiac troponin T2 (tnnt2) gene, which lack a heartbeat, also had large decreases in the number of HSCs at 36 hours post fertilization. Similarly, less than a day after the initiation of blood flow, the E9.25 aorta–gonads–mesonephros region of mouse sodium/calcium exchanger 1 (Ncx1; also known as Slc8a1)−/− embryos (which also have no heartbeat) had less-abundant expression of HSC markers and decreased haematopoietic potential in vitro compared with wild-type147. Subjecting embryoid bodies to artificial shear stress in vitro augmented haematopoietic potential, even in Ncx1−/− mouse-derived tissue147. It is likely, however, that blood flow maintains the HSC programme rather than initiating it, because although the expression of HSC genes — for example, Runx1 — was ultimately decreased in Ncx1−/− mice147, initiation of runx1 and even cmyb expression occurs fairly normally in zebrafish with tnnt2 knockdown149.
Nitric oxide signalling has a key role in transmitting the effects of blood flow. Nitric oxide has well-established effects on the vascular system and endothelial cells150, and loss of function of specific nitric oxide synthase genes in both mouse and zebrafish resulted in notable decreases in HSC numbers148. Pharmacological manipulation of nitric oxide signalling also had strong effects on haematopoietic potential in vitro147. Enforced nitric oxide signalling could rescue HSC specification in embryos with no heartbeat, and transplanted cells with knockdown of nitric oxide synthase genes could not contribute to HSCs148. These results indicate that nitric oxide signalling cell-autonomously mediates the haemogenic effects of blood flow.
Catecholamines and GATA3
Signalling by the sympathetic nervous system to the dorsal aorta is also likely to regulate HSC specification. In a screen for genes that are expressed in the mouse aorta–gonads–mesonephros, Gata3 was found to be upregulated151. Gata3 is expressed by numerous tissues, including embryonic HSCs13, kidney152 and the sympathetic nervous system153. By various criteria, fewer HSCs are specified during development in Gata3−/− mice154. Surprisingly, however, HSC precursors do not normally express Gata3, which indicates that the effects of GATA3 are non-cell-autonomous. Several tissues near HSCs in the dorsal aorta express Gata3, including the subaortic mesenchyme and neurons of the sympathetic nervous system derived from recently migrated neural crest. Gata3 is required for generation of the sympathetic nervous system155–158, which produces signalling molecules such as catecholamines. Exogenous catecholamines can rescue HSC defects when added to dissected aorta–gonads–mesonephros in ex vivo explant cultures154, suggesting that the requirement for GATA3 for HSC specification reflects a contribution by signalling from neurons of the developing sympathetic nervous system.
Conclusion
HSCs form the foundation of the adult haematopoietic system. The production of HSCs during development is a key step in making a healthy organism, and it involves multiple regulatory interactions during embryogenesis. The in vitro production of HSCs from iPS cells could provide a source of transplantable cells for regenerative medicine and the treatment of sickle cell anaemia, thalassaemia, leukaemia and other diseases with autologous, genetically corrected cells. In vitro-generated HSCs would also provide material for patient-specific disease studies. Although the generation of specific types of haematopoietic cells for specific applications is now becoming feasible, generating robust numbers of bona fide HSCs with normal lineage potential that are capable of generating erythrocytes expressing adult globins so far remains impossible5,6. Understanding how HSC specification occurs natively during embryonic development and trying to carefully reproduce these events is one clear means of trying to develop improved techniques for directed differentiation protocols.
Current protocols for the haematopoietic differentiation of pluripotent cells involve treating cell populations with signalling factors in vitro for defined periods of time in order to induce a stepwise progression towards the desired fate outcome. These protocols necessarily involve selecting desirable cell fate intermediates from the total pool for further differentiation by treatment with additional factors over defined times, rather than the more difficult goal of directing a homogeneous cell fate. In some cases, cells have been modified to carry genes that augment their differentiation potential, but lasting genetic modification has unpredictable consequences and is therefore undesirable for protocols for therapeutic purposes. A clearer understanding of native embryonic signalling and morphogenetic processes might enable the selection of a preferable intermediate or suggest additional molecular factors that could make the differentiation process more robust, efficient and realistic, and therefore come closer to generating large numbers of true HSCs. For example, the recent recognition that HSCs derive from arterial haemogenic endothelium suggests that one obvious step is to attempt to reproduce the formation of this tissue159,160.
These advances highlight the fact that work in non-human vertebrates as diverse as mouse and zebrafish can productively inform strategies for human haematopoietic programming owing to the conservation of mechanisms between species. We hope that in the future, the complete set of native signals involved can be defined and incorporated into existing protocols to realize the ultimate goal of producing HSCs in vitro.
Acknowledgements
This work was supported in part by US National Institutes of Health (NIH)/National Heart, Lung and Blood Institute grant R00 HL097150 to W.K.C., as well as a California Institute for Regenerative Medicine (CIRM) New Faculty Award (RN1-00575), NIH/National Institute of Diabetes and Digestive and Kidney Diseases grant R01 DK074482-06 and an American Heart Association (AHA) Innovative Science Award (12PILT12860010) to D.T.
Glossary
- Induced pluripotent stem cell
(iPS cell). An adult cell reprogrammed by one of several protocols to ‘pluripotency’, a state competent to form all embryonic tissues.
- Ventral blood islands
A ventroposterior region of the embryo in Xenopus laevis and other species that houses primitive haematopoiesis, in particular erythropoiesis.
- Dorsal lateral plate mesoderm
(DLP mesoderm). Trunk mesoderm in Xenopus laevis that is lateral to the more axial notochord and somitic mesoderm. The DLP mesoderm contains tissue fated to form haemogenic endothelium (and thus haematopoietic stem cells) and is equivalent to zebrafish lateral plate mesoderm and mammalian splanchnic mesoderm.
- Mid-neurula
In Xenopus laevis, a time in the middle of formation of the neural plate that occurs between the end of gastrulation (formation of the germ layers) and the initiation of somitogenesis.
- Haemogenic endothelium
Endothelial cells found most notably in the ventral floor of the dorsal aorta that give rise to haematopoietic stem cells.
- Yolk sac
An extra-embryonic tissue derived from the fertilized egg in mammals that ultimately surrounds the embryo and at early times functions as a signalling centre. The yolk sac is also the site of primitive and transient definitive haematopoiesis.
- Dorsal aorta
The earliest trunk vessel and precursor to the adult descending aorta. Endothelium of the dorsal aorta forms from splanchnic mesoderm and contains the haemogenic endothelium that generates the first haematopoietic stem cells.
- Aorta–gonads– mesonephros
The embryonic region in the embryonic day 9.5–11.0 mouse containing the primitive dorsal aorta and bounded by the mesonephros and gonads, where haematopoietic stem cells are specified.
- Lateral
Anatomical adjective defining the direction away from the midline.
- Anamniotes
An informal grouping of vertebrates (including frogs and fish) that develop external to the mother in eggs without an amnion, in contrast to birds and mammals, which have amnions.
- Somites
Primitive mesodermal tissue found lateral to the notochord and axial to the lateral plate or splanchnic mesoderm. This tissue is segmented and contains precursors for adult skeleton, skeletal muscle, vascular smooth muscle and dermis.
- Epiblast
In mouse and chick, the embryonic portion of the embryo before formation of the primary germ layers (ectoderm, mesoderm and endoderm).
- Primitive streak
The site in the epiblast of primary ingression of cells that give rise to mesoderm.
- Dorsal–ventral
Anatomical adjectives defining the back and belly directions, respectively.
- Anterior–posterior
Anatomical adjectives defining the head and tail directions, respectively.
- Dorsal organizer
A group of cells acting as a signalling centre that patterns local tissue to a specific fate, in this case dorsal and anterior. Sometimes known as ‘Spemann’s organizer’.
- Intermediate cell mass
A region in the axial trunk of the 23–28 hours post fertilization zebrafish embryo that is ventral to the notochord. Primitive haematopoiesis occurs here, as well as formation of the haemogenic endothelium.
- Yolk ball
An extra-embryonic region of the zebrafish embryo that contains nutrients that sustain early development before feeding.
- Serial transplantation
A procedure to test the longevity and self-renewal potential of putative populations of haematopoietic stem cells by transplanting into primary, secondary, tertiary and higher-order recipients.
- Sclerotome
A ventromedial compartment of each somite containing a variety of tissue precursors, including vertebral column cells, breast bone cells and vascular smooth muscle cells.
- Sympathetic nervous system
A neural crest-derived component of the autonomic nervous system that regulates tissue responses including the ‘fight-or-flight’ response. The sympathetic nervous system develops in tandem with the adrenal glands and regulates secretion of cardiovascular system regulatory hormones including the catecholamines adrenaline and noradrenaline.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
FURTHER INFORMATION
Wilson K. Clements’ homepage: http://www.stjude.org/clements
David Traver’s homepage: http://labs.biology.ucsd.edu/traver/Traver_Laboratory/Home.html
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Kondo M, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 2.Bordignon C. Stem-cell therapies for blood diseases. Nature. 2006;441:1100–1102. doi: 10.1038/nature04962. [DOI] [PubMed] [Google Scholar]
- 3.Irion S, Nostro MC, Kattman SJ, Keller GM. Directed differentiation of pluripotent stem cells: from developmental biology to therapeutic applications. Cold Spring Harb. Symp. Quant. Biol. 2008;73:101–110. doi: 10.1101/sqb.2008.73.065. [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman DS. Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells. Blood. 2009;114:3513–3523. doi: 10.1182/blood-2009-03-191304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters A, et al. Challenges and strategies for generating therapeutic patient-specific hemangioblasts and hematopoietic stem cells from human pluripotent stem cells. Int. J. Dev. Biol. 2010;54:965–990. doi: 10.1387/ijdb.093043ap. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 8.Maeno M, Tochinai S, Katagiri C. Differential participation of ventral and dorsolateral mesoderms in the hemopoiesis of Xenopus, as revealed in diploid– triploid or interspecific chimeras. Dev. Biol. 1985;110:503–508. doi: 10.1016/0012-1606(85)90108-3. [DOI] [PubMed] [Google Scholar]
- 9.Maeno M, Todate A, Katagiri C. The localization of precursor cells for larval and adult hemopoietic cells of Xenopus laevis in two regions of embryos. Dev. Growth Differ. 1985;27:137–148. doi: 10.1111/j.1440-169X.1985.00137.x. [DOI] [PubMed] [Google Scholar]
- 10.Turpen JB, Knudson CM, Hoefen PS. The early ontogeny of hematopoietic cells studied by grafting cytogenetically labeled tissue anlagen: localization of a prospective stem cell compartment. Dev. Biol. 1981;85:99–112. doi: 10.1016/0012-1606(81)90239-6. [DOI] [PubMed] [Google Scholar]
- 11.Turpen JB, Kelley CM, Mead PE, Zon LI. Bipotential primitive–definitive hematopoietic progenitors in the vertebrate embryo. Immunity. 1997;7:325–334. doi: 10.1016/s1074-7613(00)80354-4. [DOI] [PubMed] [Google Scholar]
- 12.Yokota T, et al. Tracing the first waves of lymphopoiesis in mice. Development. 2006;133:2041–2051. doi: 10.1242/dev.02349. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand JY, et al. Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc. Natl Acad. Sci. USA. 2005;102:134–139. doi: 10.1073/pnas.0402270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand JY, et al. Definitive hematopoiesis initiates through a committed erythromyeloid precursor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 16.Yoder MC, et al. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7:335–344. doi: 10.1016/s1074-7613(00)80355-6. [DOI] [PubMed] [Google Scholar]
- 17.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc. Natl Acad. Sci. USA. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokomizo T, et al. Characterization of GATA-1+ hemangioblastic cells in the mouse embryo. EMBO J. 2007;26:184–196. doi: 10.1038/sj.emboj.7601480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen MJ, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Dev. Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev. Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, et al. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell. 2012;11:663–675. doi: 10.1016/j.stem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, et al. Early ontogenic origin of the hematopoietic stem cell lineage. Proc. Natl Acad. Sci. USA. 2012;109:4515–4520. doi: 10.1073/pnas.1115828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumaravelu P, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes KE, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bruijn MF, et al. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 29.Medvinsky A, Rybtsov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 30.Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J. Embryol. Exp. Morphol. 1975;33:607–619. [PubMed] [Google Scholar]
- 31.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. doi: 10.1038/nature08761. [DOI] [PubMed] [Google Scholar]
- 33.Boisset JC, et al. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. doi: 10.1038/nature08764. [DOI] [PubMed] [Google Scholar]
- 34.Zovein AC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. The first conclusive demonstration that HSCs transit through an endothelial stage
- 35.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 37.Lancrin C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- 40.Arnold SJ, Robertson EJ. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nature Rev. Mol. Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- 41.Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- 42.Langdon YG, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu. Rev. Genet. 2011;45:357–377. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- 43.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 44.Conlon FL, et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 45.Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- 46.Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- 47.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol. Cell. Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ciruna BG, Schwartz L, Harpal K, Yamaguchi TP, Rossant J. Chimeric analysis of fibroblast growth factor receptor-1 (Fgfr1) function: a role for FGFR1 in morphogenetic movement through the primitive streak. Development. 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- 49.Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 50.Liu P, et al. Requirement for Wnt3 in vertebrate axis formation. Nature Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 51.Feldman B, et al. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- 52.Gritsman K, et al. The EGF-CFC protein one-eyed pinhead is essential for nodal signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- 53.Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- 55.Draper BW, Stock DW, Kimmel CB. Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development. 2003;130:4639–4654. doi: 10.1242/dev.00671. [DOI] [PubMed] [Google Scholar]
- 56.Griffin K, Patient R, Holder N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development. 1995;121:2983–2994. doi: 10.1242/dev.121.9.2983. [DOI] [PubMed] [Google Scholar]
- 57.Szeto DP, Kimelman D. The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains. Genes Dev. 2006;20:1923–1932. doi: 10.1101/gad.1435306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin BL, Kimelman D. Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell. 2012;22:223–232. doi: 10.1016/j.devcel.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stainier DY, Weinstein BM, Detrich HW, Zon LI, Fishman MC. Cloche, an early acting zebrafish gene, is required by both the endothelial and hematopoietic lineages. Development. 1995;121:3141–3150. doi: 10.1242/dev.121.10.3141. [DOI] [PubMed] [Google Scholar]
- 60.Liao W, et al. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- 61.Ma N, et al. Characterization of a weak allele of zebrafish cloche mutant. PLoS ONE. 2011;6:e27540. doi: 10.1371/journal.pone.0027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson MA, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 63.Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- 64.Miura S, Davis S, Klingensmith J, Mishina Y. BMP signaling in the epiblast is required for proper recruitment of the prospective paraxial mesoderm and development of the somites. Development. 2006;133:3767–3775. doi: 10.1242/dev.02552. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev. Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 66.Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech. Dev. 2001;103:27–33. doi: 10.1016/s0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- 67.Pilon N, et al. Cdx4 is a direct target of the canonical Wnt pathway. Dev. Biol. 2006;289:55–63. doi: 10.1016/j.ydbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 68.Prinos P, et al. Multiple pathways governing Cdx1 expression during murine development. Dev. Biol. 2001;239:257–269. doi: 10.1006/dbio.2001.0446. [DOI] [PubMed] [Google Scholar]
- 69.Davidson AJ, et al. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature. 2003;425:300–306. doi: 10.1038/nature01973. The first report of potential candidates for the molecular factors that contribute to haematopoietic competence of lateral plate mesoderm
- 70.Davidson AJ, Zon LI. The caudal-related homeobox genes cdx1a and cdx4 act redundantly to regulate hox gene expression and the formation of putative hematopoietic stem cells during zebrafish embryogenesis. Dev. Biol. 2006;292:506–518. doi: 10.1016/j.ydbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Lengerke C, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 72.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 73.McKinney-Freeman SL, et al. Modulation of murine embryonic stem cell-derived CD41+c-kit+ hematopoietic progenitors by ectopic expression of Cdx genes. Blood. 2008;111:4944–4953. doi: 10.1182/blood-2007-11-124644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nishikawa SI, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development. 1998;125:1747–1757. doi: 10.1242/dev.125.9.1747. [DOI] [PubMed] [Google Scholar]
- 75.Shalaby F, et al. A requirement for Flk1 in primitive and definitive hematopoiesis and vasculogenesis. Cell. 1997;89:981–990. doi: 10.1016/s0092-8674(00)80283-4. [DOI] [PubMed] [Google Scholar]
- 76.Hogan KA, Bautch VL. Blood vessel patterning at the embryonic midline. Curr. Top. Dev. Biol. 2004;62:55–85. doi: 10.1016/S0070-2153(04)62003-5. [DOI] [PubMed] [Google Scholar]
- 77.Reese DE, Hall CE, Mikawa T. Negative regulation of midline vascular development by the notochord. Dev. Cell. 2004;6:699–708. doi: 10.1016/s1534-5807(04)00127-3. [DOI] [PubMed] [Google Scholar]
- 78.Garriock RJ, Mikawa T. Early arterial differentiation and patterning in the avian embryo model. Semin. Cell Dev. Biol. 2011;22:985–992. doi: 10.1016/j.semcdb.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garriock RJ, Czeisler C, Ishii Y, Navetta AM, Mikawa T. An anteroposterior wave of vascular inhibitor downregulation signals aortae fusion along the embryonic midline axis. Development. 2010;137:3697–3706. doi: 10.1242/dev.051664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- 81.Furthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev. Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- 82.Wilkinson RN, et al. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev. Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. This study shows that BMP signalling is required for HSC specification
- 83.Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev. Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. The first demonstration that Hedgehog signalling is required for HSC specification
- 84.Williams C, et al. Hedgehog signaling induces arterial endothelial cell formation by repressing venous cell fate. Dev. Biol. 2010;341:196–204. doi: 10.1016/j.ydbio.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wilkinson RN, et al. Hedgehog signaling via a calcitonin receptor-like receptor can induce arterial differentiation independently of VEGF signaling in zebrafish. Blood. 2012;120:477–488. doi: 10.1182/blood-2011-10-383729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–3914. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- 87.Ren X, Gomez GA, Zhang B, Lin S. Scl isoforms act downstream of etsrp to specify angioblasts and definitive hematopoietic stem cells. Blood. 2010;115:5338–5346. doi: 10.1182/blood-2009-09-244640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chun CZ, et al. Fli+etsrp+ hemato-vascular progenitor cells proliferate at the lateral plate mesoderm during vasculogenesis in zebrafish. PLoS ONE. 2011;6:e14732. doi: 10.1371/journal.pone.0014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin H, Xu J, Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109:5208–5214. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- 90.Liu F, Walmsley M, Rodaway A, Patient R. Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr. Biol. 2008;18:1234–1240. doi: 10.1016/j.cub.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 91.Sumanas S, et al. Interplay among Etsrp/ER71, Scl, and Alk8 signaling controls endothelial and myeloid cell formation. Blood. 2008;111:4500–4510. doi: 10.1182/blood-2007-09-110569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sumanas S, Jorniak T, Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood. 2005;106:534–541. doi: 10.1182/blood-2004-12-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lawson ND, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 95.Krebs LT, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 96.Koo BK, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development. 2005;132:3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- 97.Koo BK, et al. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PLoS ONE. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krebs LT, et al. Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 2004;18:2469–2473. doi: 10.1101/gad.1239204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- 100.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev. Biol. 2005;278:301–309. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 102.Grego-Bessa J, et al. Notch signaling is essential for ventricular chamber development. Dev. Cell. 2007;12:415–429. doi: 10.1016/j.devcel.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herbert SP, et al. Arterialvenous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ. Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 105.Leslie JD, et al. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- 106.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 107.Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yoon MJ, et al. Mind bomb-1 is essential for intraembryonic hematopoiesis in the aortic endothelium and the subaortic patches. Mol. Cell. Biol. 2008;28:4794–4804. doi: 10.1128/MCB.00436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjκ-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- 110.Robert-Moreno A, et al. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008;27:1886–1895. doi: 10.1038/emboj.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumano K, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. This study shows that Notch signalling is required for HSC specification
- 112.Nakagawa M, et al. AML1/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood. 2006;108:3329–3334. doi: 10.1182/blood-2006-04-019570. [DOI] [PubMed] [Google Scholar]
- 113.Hadland BK, et al. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104:3097–3105. doi: 10.1182/blood-2004-03-1224. This study shows that Notch 1 is cell-autonomously required for HSC specification
- 114.Bertrand JY, Cisson JL, Stachura DL, Traver D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood. 2010;115:2777–2783. doi: 10.1182/blood-2009-09-244590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 116.Ciau-Uitz A, Pinheiro P, Gupta R, Enver T, Patient R. Tel1/ETV6 specifies blood stem cells through the agency of VEGF signaling. Dev. Cell. 2010;18:569–578. doi: 10.1016/j.devcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 117.Nicoli S, Tobia C, Gualandi L, De Sena G, Presta M. Calcitonin receptor-like receptor guides arterial differentiation in zebrafish. Blood. 2008;111:4965–4972. doi: 10.1182/blood-2007-10-118166. [DOI] [PubMed] [Google Scholar]
- 118.Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- 119.Durand C, et al. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc. Natl Acad. Sci. USA. 2007;104:20838–20843. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nature Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 121.Reya T, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 122.Scheller M, et al. Hematopoietic stem cell and multilineage defects generated by constitutive β-catenin activation. Nature Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 123.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 124.Fleming HE, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luis TC, Ichii M, Brugman MH, Kincade P, Staal FJ. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26:414–421. doi: 10.1038/leu.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cobas M, et al. β-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jeannet G, et al. Long-term, multilineage hematopoiesis occurs in the combined absence of β-catenin and γ-cateninj. Blood. 2008;111:142–149. doi: 10.1182/blood-2007-07-102558. [DOI] [PubMed] [Google Scholar]
- 128.Koch U, et al. Simultaneous loss of β- and γ-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 129.Luis TC, et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. The first conclusive demonstration of a specific WNT ligand required for HSC patterning
- 130.Zhao C, et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luis TC, Naber BA, Fibbe WE, van Dongen JJ, Staal FJ. Wnt3a nonredundantly controls hematopoietic stem cell function and its deficiency results in complete absence of canonical Wnt signaling. Blood. 2010;116:496–497. doi: 10.1182/blood-2010-04-282624. [DOI] [PubMed] [Google Scholar]
- 132.Luis TC, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9:345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 133.Ruiz-Herguido C, et al. Hematopoietic stem cell development requires transient Wnt/β-catenin activity. J. Exp. Med. 2012;209:1457–1468. doi: 10.1084/jem.20120225. This study shows that canonical WNT signalling is required for HSC specification
- 134.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. The first demonstration of prostaglandin involvement in HSC patterning
- 135.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goessling W, et al. Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long-term safety in preclinical nonhuman primate transplant models. Cell Stem Cell. 2011;8:445–458. doi: 10.1016/j.stem.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Veeman MT, Axelrod JD, Moon RT. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev. Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 138.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nature Rev. Mol. Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 139.Semenov MV, Habas R, Macdonald BT, He X. SnapShot: noncanonical Wnt signaling pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 140.Clements WK, et al. A somitic Wnt16/Notch pathway specifies haematopoietic stem cells. Nature. 2011;474:220–224. doi: 10.1038/nature10107. This study indicates the presence of a non-canonical Wnt-dependent somitic pathway for HSC specification
- 141.Pouget C, Pottin K, Jaffredo T. Sclerotomal origin of vascular smooth muscle cells and pericytes in the embryo. Dev. Biol. 2008;315:437–447. doi: 10.1016/j.ydbio.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 142.Wasteson P, et al. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- 143.Wiegreffe C, Christ B, Huang R, Scaal M. Sclerotomal origin of smooth muscle cells in the wall of the avian dorsal aorta. Dev. Dyn. 2007;236:2578–2585. doi: 10.1002/dvdy.21279. [DOI] [PubMed] [Google Scholar]
- 144.Wiegreffe C, Christ B, Huang R, Scaal M. Remodeling of aortic smooth muscle during avian embryonic development. Dev. Dyn. 2009;238:624–631. doi: 10.1002/dvdy.21888. [DOI] [PubMed] [Google Scholar]
- 145.Sato Y, et al. Notch mediates the segmental specification of angioblasts in somites and their directed migration toward the dorsal aorta in avian embryos. Dev. Cell. 2008;14:890–901. doi: 10.1016/j.devcel.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 146.Jones EA. Mechanical factors in the development of the vascular bed. Respir. Physiol. Neurobiol. 2011;178:59–65. doi: 10.1016/j.resp.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 147.Adamo L, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. References 147 and 148 demonstrate a requirement for blood flow in HSC specification
- 149.Wang L, et al. A blood flow-dependent klf2a-NO signaling cascade is required for stabilization of hematopoietic stem cell programming in zebrafish embryos. Blood. 2011;118:4102–4110. doi: 10.1182/blood-2011-05-353235. [DOI] [PubMed] [Google Scholar]
- 150.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb. Exp. Pharmacol. 2006:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 151.Mascarenhas MI, Parker A, Dzierzak E, Ottersbach K. Identification of novel regulators of hematopoietic stem cell development through refinement of stem cell localization and expression profiling. Blood. 2009;114:4645–4653. doi: 10.1182/blood-2009-06-230037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated Gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development. 2006;133:53–61. doi: 10.1242/dev.02184. [DOI] [PubMed] [Google Scholar]
- 153.Pandolfi PP, et al. Targeted disruption of the G TA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nature Genet. 1995;11:40–44. doi: 10.1038/ng0995-40. [DOI] [PubMed] [Google Scholar]
- 154.Fitch SR, et al. Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell. 2012;11:554–566. doi: 10.1016/j.stem.2012.07.002. This study indicates interplay between the developing sympathetic nervous system and HSC specification
- 155.Lim KC, et al. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nature Genet. 2000;25:209–212. doi: 10.1038/76080. [DOI] [PubMed] [Google Scholar]
- 156.Moriguchi T, et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development. 2006;133:3871–3881. doi: 10.1242/dev.02553. [DOI] [PubMed] [Google Scholar]
- 157.Tsarovina K, et al. Essential role of Gata transcription factors in sympathetic neuron development. Development. 2004;131:4775–4786. doi: 10.1242/dev.01370. [DOI] [PubMed] [Google Scholar]
- 158.Tsarovina K, et al. The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J. Neurosci. 2010;30:10833–10843. doi: 10.1523/JNEUROSCI.0175-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kennedy M, et al. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 160.Choi KD, et al. Identification of the hemogenic endothelial progenitor and its direct precursor in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:553–567. doi: 10.1016/j.celrep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tober J, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Le Guyader D, et al. Origins and unconventional behavior of neutrophils in developing zebrafish. Blood. 2008;111:132–141. doi: 10.1182/blood-2007-06-095398. [DOI] [PubMed] [Google Scholar]
- 163.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nature Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rabbitts TH. Chromosomal translocations in human cancer. Nature. 1994;372:143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- 165.North T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 166.North TE, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 167.Kalev-Zylinska ML, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- 168.Wang Q, et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl Acad. Sci. USA. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- 170.Dutko JA, Mullins MC. SnapShot: BMP signaling in development. Cell. 2011;145:636. doi: 10.1016/j.cell.2011.05.001. e2. [DOI] [PubMed] [Google Scholar]
- 171.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nature Rev. Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 172.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–973. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 174.Gazave E, et al. Origin and evolution of the Notch signalling pathway: an overview from eukaryotic genomes. BMC Evol. Biol. 2009;9:249. doi: 10.1186/1471-2148-9-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]