Abstract
Heart muscle is activated by Ca2+ to generate force and shortening, and the signaling pathway involves allosteric mechanisms in the thin filament. Knowledge about the structure-function relationship among proteins in the thin filament is critical in understanding the physiology and pathology of the cardiac function, but remains obscure. We investigate the conformation of the cardiac troponin (Tn) on the thin filament and its response to Ca2+ activation and propose a molecular mechanism for the regulation of cardiac muscle contraction by Tn based uniquely on information from in situ protein domain orientation. Polarized fluorescence from bifunctional rhodamine is used to determine the orientation of the major component of Tn core domain on the thin filaments of cardiac muscle. We show that the C-terminal lobe of TnC (CTnC) does not move during activation, suggesting that CTnC, together with the coiled coil formed by the TnI and TnT chains (IT arm), acts as a scaffold that holds N-terminal lobe of TnC (NTnC) and the actin binding regions of troponin I. The NTnC, on the other hand, exhibits multiple orientations during both diastole and systole. By combining the in situ orientation data with published in vitro measurements of intermolecular distances, we construct a model for the in situ structure of the thin filament. The conformational dynamics of NTnC plays an important role in the regulation of cardiac muscle contraction by moving the C-terminal region of TnI from its actin-binding inhibitory location and enhancing the movement of tropomyosin away from its inhibitory position.
Abbreviations: BR, bifunctional rhodamine; CTnC, C-terminal lobe of TnC; CTnI, C-terminus of TnI; IT arm, coiled-coil formed by the TnI and TnT chains; ME, maximum entropy; NTnC, N-terminal lobe of TnC; NTnI, N-terminus of TnI; NTnT, N-terminus of TnT; Tn, troponin
Keywords: Cardiac muscle, Troponin, Thin filament, Contractile protein structure, Polarized fluorescence
Highlights
-
•
In situ conformational changes of troponin in myocardium were investigated.
-
•
A model for the cardiac thin filament was constructed based on the in situ data.
-
•
The IT arm of cardiac troponin acts as a scaffold that holds the regulatory domain.
-
•
The regulatory domain of cardiac troponin moves dynamically during activation.
-
•
The dynamics of regulatory domain is important in cardiac muscle regulation.
1. Introduction
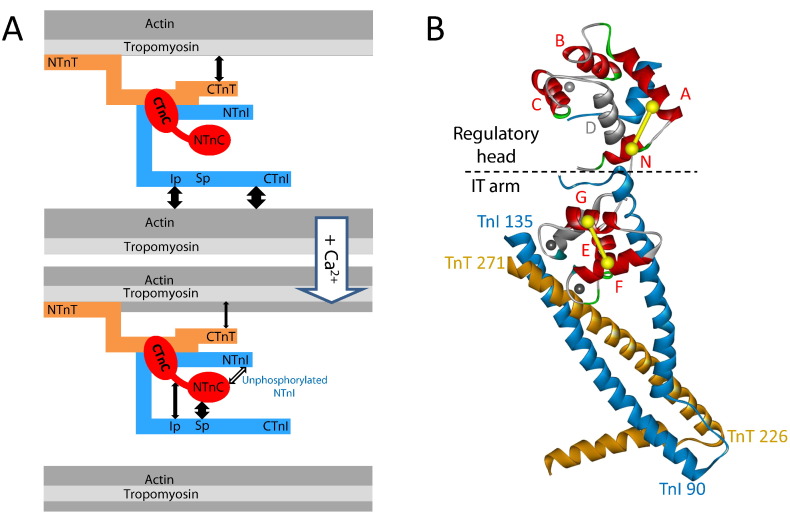
Contraction of cardiac and skeletal muscle is triggered by Ca2+ binding to the regulatory protein Tn in the actin-containing thin filaments, leading to an azimuthal movement of the tropomyosin around the filament that uncovers myosin binding sites and allows myosin motors to interact with actin and generate force [1], [2], [3]. Tn consists of three subunits, troponin I (TnI), troponin T (TnT) and troponin C (TnC). At low [Ca2+], troponin docks on to the thin filament by binding of TnT to tropomyosin [4], [5] and the C-terminus of TnI (CTnI) to actin [6], [7]. Except for the N-terminus of TnT (NTnT, residues 1-158), the interactions of these troponin components with actin and tropomyosin are sensitive to the Ca2+ level (Fig. 1A). The removal of CTnI from its actin binding site by binding of Ca2+ to TnC and the consequent shifting of equilibrium position for tropomyosin are key steps in the regulation of muscle contraction. The crystal structures of the troponin core complex [8], [9] have provided a platform for building molecular hypotheses about this steric blocking mechanism, but these are limited by uncertainties about the position and conformation of the troponin components in the filament and the structures of some key regulatory regions of Tn outside the core complex. Until recently, studies on determining the organization of the Tn in the thin filament using a variety of techniques produced inconclusive and contradictory results [10], [11], [12].
Fig. 1.
A schematic representation of the interactions between components of cardiac thin filament (A) and the Ca2+-bound form of the troponin core complex from cardiac muscle (B). A, The Ca2+-dependent interactions between TnI, TnC, TnT and actin are shown in solid double-headed arrows. Ip, TnI inhibitory peptide; Sp, TnI switch peptide. The interaction between cardiac specific NTnI and NTnC in the presence of Ca2+ occurs when Ser23/Ser24 of NTnI are unphosphorylated. B, structure of the cardiac Tn core complex contains TnC (red, grey) and parts of TnI (blue) and TnT (gold). Bifunctional rhodamine (BR) probes cross-linked cysteines along either the N, A, B, C, E, F or G helix of TnC (red). BR probes cross-linking cysteines across either N and A, or F and G helices are shown in yellow dumbbells. D helix which is inaccessible is in grey.
Crystal structures of the troponin core complex [8], [9] have focused on the less dynamic components of the complex in the muscle regulatory system. The ‘regulatory head’ of Tn is the N-terminal lobe of the TnC (NTnC) which contains the regulatory Ca2+ site. The ‘IT-arm’ is a rigid domain and has an arrowhead shape containing a long coiled-coil formed by helices from TnI and TnT, plus the C-terminal lobe of TnC (CTnC) (Fig. 1B). The backbone fold of the IT-arm does not depend on the presence of Ca2+ in the regulatory site. Understanding of interactions between these proteins and their structural changes underlying Ca2+-control of heart muscle contraction is critical for the broad strategy and the detailed molecular design of therapeutic interventions in heart disease in which cardiac contractility is altered.
Here, we investigated the conformation of the cardiac Tn on the thin filament and its response to binding of Ca2+ to elucidate the molecular mechanism of the regulation of contraction. The conformation of the Tn core domain in the intact cardiac muscle sarcomere was determined by a fluorescence-based approach. We attached bifunctional fluorescent probes (BR) to pairs of surface-accessible cysteines of TnC. The probe attachment sites were chosen using the high resolution structures of isolated Tn components, and engineered by expressing mutants of TnC with cysteines at the chosen sites (i.e., 10–15 Å apart on α-helices with their β-carbons at surface-exposed positions) (Fig. 1B). The bifunctional attachment constrains the orientation of the probe dipoles with respect to the protein backbone, so that the polarization of the fluorescence from a heart muscle cell containing such a labelled TnC gives information about the in situ orientation of the probe, and thus the vector joining the cysteine pair. We chose four cysteine pairs for the CTnC in the IT arm of Tn and five cysteine pairs for the NTnC in the regulatory head domain of Tn (Fig. 1B). Data from sets of cysteine pairs were combined to estimate the orientation of troponin domains with respect to the thin filament axis.
By combining polarized fluorescence data from each set of the labeled TnCs with in vitro structures of the Tn domains, we estimated the orientation of the IT arm and the regulatory head domain in ventricular trabeculae, and their orientation changes associated with binding of Ca2+ and of myosin heads during contraction. Finally, by combining the data on the in situ orientation of both the IT arm and the regulatory head domain of Tn and on the proximity of the relevant regions of Tn and actin, we constructed an model of the thin filament that suggests a plausible molecular mechanism for the Ca2+ regulation of heart muscle involving the conformational dynamics of the Tn regulatory head domain.
2. Materials and methods
2.1. Preparation of BR labeled TnC
Nine double cysteine mutants of human cardiac TnC (D3C/E10C, E10C/L17C, E15C/A22C, K39C/R46C and E55C/D62C for the NTnC; E95C/R102C, E115C/Q122C, D131C/K138C and K118C/E134C for the CTnC) (Fig. 1B) were produced by site-directed mutagenesis, expressed in E.coli and purified as described previously [13], [14]. Each pair of introduced cysteines was cross-linked with a bifunctional rhodamine probe (BR).
2.2. Reconstitution of TnC into ventricular trabeculae
Ventricular trabeculae from rat right ventricle were prepared as previously described [14]. Native TnC was partially replaced by incubation of trabeculae in relaxing solution containing 30 μmol/L BR-TnC overnight at 4 °C. The fraction of TnC replaced by BR-TnC was estimated as 80 ± 2% (mean ± SE, n = 8) based on SDS-PAGE and immunoblot analysis (see Fig. S1). Following incubation, the demembranated trabeculae were mounted via aluminium T-clips between a force transducer and a fixed hook in a 60 μl trough containing relaxing solution. The sarcomere length was set to 2.1 μm. The experimental temperature was 20–22 °C.
Each trabecular activation was preceded by a 1-min incubation in pre-activating solution. Isometric force and fluorescence intensities were measured after steady-state force had been established. The maximum Ca2+-activated force above the passive tension was 42.2 ± 2.4 mN mm−2 (mean ± SE, n = 50 preparations) in the present study, which was comparable to the maximum force recorded in our previous study before incorporation of labeled TnC into trabeculae (37.9 ± 1.6 mN mm−2, n = 14) [14]. When calculated separately for the nine BR-TnCs, none of the average forces was statistically different from these two values (P > 0.1; Table S1). Our previous study also showed that the introduction of double-cysteine and BR probe on the C- or E-helix of TnC reduced the Ca2+ affinity of the regulatory site of TnC (by about 0.3 units of pCa) with no significant effect on the steepness of the force-Ca2+ relationship [14]. Since the exchange of TnC itself using previous extraction-reconstitution protocol causes the reduced Ca-sensitivity of force-Ca2+ relationship [13], [14], it is reasonable to assume that the present TnC exchange protocol without the extraction step should produce a milder effect. However, it was impractical to test the trabeculae mechanically before an over-night exchange of TnC to assess its impact.
The long incubation of BR labeled TnC for replacing endogenous TnC might increase the probability for non-specific binding of exogenous TnC. The sarcomeric localization of BR-TnC exchanged into trabeculae was checked using confocal microscopy. The confocal image showed that the BR labeled TnC was highly localized in the thin filament, as expected for specific replacement of the native TnC (Fig. S2).
2.3. Determination of the orientation of Tn domains
Polarized fluorescence from ventricular trabeculae reconstituted with BR-TnC was collected both in-line with the illumination beam and perpendicular to the illumination beam and the trabecular axis [14]. The intensities of the parallel and perpendicular polarization components of each collected beam were used to calculate the order parameters given in Supplementary Table S1. The order parameters describe the orientation of the BR dipole with respect to the trabecular or thin filament axis.
The in situ orientation of the Tn domains was calculated by combining the order parameters from multiple probes using a model-free maximum entropy (ME) formalism [15]. The resulting ‘ME distribution’ is the smoothest distribution that is consistent with the order parameters measured in each experimental condition. The orientations of the NTnC and the CTnC together with the IT arm of the ternary complex were calculated separately from BR-TnCs for the corresponding domains.
The ME analysis for the orientation of the IT arm was based on the crystal structure of the Tn core complex from human cardiac muscle [8] for both relaxed trabeculae and during active contraction. The angular distribution of the IT arm with respect to the trabecular axis was described in terms of the angle β between the coiled-coil formed by TnI and TnT and the trabecular axis and γ, the rotation of the IT arm around the IT coiled-coil.
The angular distribution of the NTnC with respect to the trabecular axis was described in terms of β, the angle between the D-helix axis and the trabecular axis, and γ, which describes rotation of the domain around the D-helix axis. The ME analysis for relaxed trabeculae was based on a NMR-derived solution structure (1SPY) [16]. ME analysis of active trabeculae data was performed using crystal (1J1D and 1J1E) [8] and NMR structures (1MXL) [17] of cardiac TnC in the Ca-state.
3. Results
3.1. In situ orientation of the IT arm
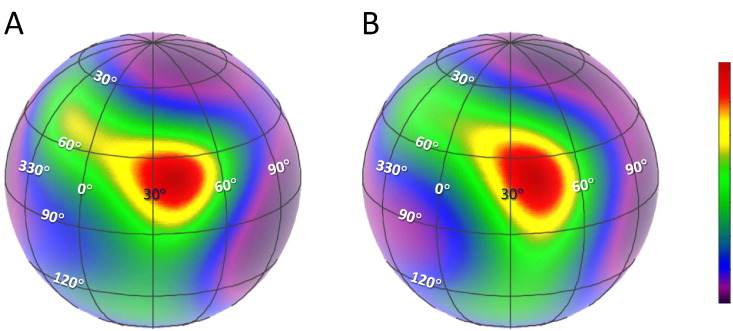
The orientation of the IT arm in a heart muscle cell was estimated from the order parameters for the four BR-TnCs in the CTnC with BR along the E-, F- and G-helix and the one with BR cross-linking the F- and G-helices. It was expressed as the tilt angle β between the IT coiled-coil and trabecular axis and the twist angle γ describing rotation of the IT arm around the IT coiled-coil (see Material and Methods for full definitions). The ME distributions for the orientation of the IT arm calculated from the present data using the average structure of 1J1D/1J1E [8] are shown in Fig. 2 with hotter colors denoting more probable orientations. The most probable orientation, denoted by the red area, was near (β = 70°, γ = 40°) in a trabecula, with (β = 69°, γ = 39°) during relaxation (diastole) and (β = 66°, γ = 40°) for active contraction (systole) (Fig. 2 A and B). The orientation (β, γ) of the IT arm determined here suggested that the IT arm did not move more than a few degrees, although the shape of distribution was changed slightly from diastole to systole.
Fig. 2.
ME distributions of the orientation of the IT arm. The latitude and longitude of the sphere correspond to the tilt (β) and twist (γ) angles, respectively. Orientation distributions are normalized to peak intensity, and hotter colors in the contour plots denote a greater probability of that orientation. A, diastole; B, systole. In both conditions, the most probable orientations are near (β = 70°, γ = 40°). The different colors of numerical labeling are for the clarity of illustration.
3.2. In situ orientation of the regulatory head
The orientation of the N-terminal lobe of TnC in situ was investigated using five probes in the NTnC. Four of the probes were with BR attached along the N-, A-, B- and C-helix of the TnC, respectively, and one with BR cross-linking two cysteines on N- and A-helices (Fig. 1B). The in situ orientation of the regulatory head of Tn was determined by combining polarized fluorescence data from the five BR-TnC probes in trabeculae with in vitro data from X-ray crystallographic and NMR studies. It was expressed as the tilt angle β between the D helix of TnC and trabecular axis and the twist angle γ describing rotation of the NTnC around the D helix (see Materials and Methods for full definitions).
3.2.1. Orientation of the regulatory head of Tn in relaxed heart muscle cells
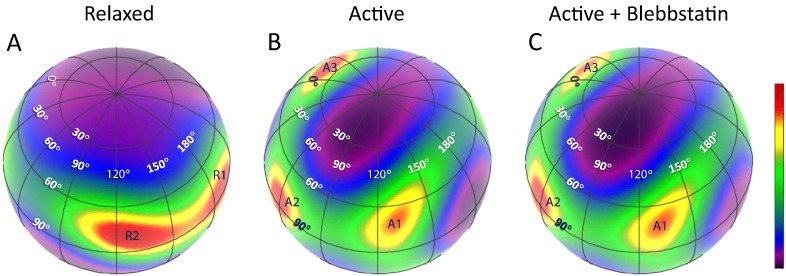
The orientational distribution of the NTnC in relaxed trabeculae based on the NMR-derived solution structure of APO TnC [16] is shown in the spherical contour plot of β and γ in Fig. 3A. The ME map showed a preference for β around 80° with a wide spread of γ. Within the broad distribution, there are two regional peaks, at R1 (β = 85°, γ = 190°) and R2 (β = 80°, γ = 130°), (hot spots in Fig. 3A), suggesting that there are two interchangeable populations of NTnC in the relaxed heart muscle cells, both with the D helix of TnC is approximately perpendicular to the actin filament axis, but with different rotations of the NTnC around the D helix.
Fig. 3.
ME distributions of the orientation of the NTnC. The latitude and longitude of the sphere correspond to the tilt (β) and twist (γ) angles, respectively. Orientation distributions are normalized to peak intensity, and hotter colors in the contour plots denote more probable orientations. A, relaxation; B, active contraction; C, active contraction in the presence of blebbistatin. The different colors of numerical labeling are for the clarity of illustration.
3.2.2. Orientation of the regulatory head in a cardiac trabecula during active contraction
Previous studies on the structures of isolated cardiac TnC have shown that the NTnC stays in a ‘closed’ conformation even in the Ca2+-bound state [18] and subsequent binding of TnI switch peptide (cardiac TnI residues 147–163) to the hydrophobic cleft of NTnC in the presence of Ca2+ stabilizes NTnC in an open conformation [17].
For calculating the orientational distributions of NTnC in maximally Ca2+-activated trabeculae, the ME analysis was based on two in vitro structures of Ca-NTnC, one from an X-ray crystallographic study (1J1D/1J1E) [8], and the other from a NMR solution study (1MXL) [17]. Since these are the only Ca2+ structures of cardiac NTnC that contain the switch peptide without any external non-Tn components, they are likely to mimic most closely the in situ conformation of the Ca2+-NTnC. ME analysis of data from contracting trabecula was repeated using the two structures of Ca2+-NTnC. However, the crystal structure, 1J1D/1J1E, was inconsistent with the polarized fluorescence data. That is, there was no distribution of β and γ that could reproduce the observed order parameters for the five NTnC probes in trabeculae during cardiac systole. Therefore the fold/structure of NTnC in a heart is different from that in the crystal structure.
The orientation distributions of the NTnC in active contracting heart muscle cells based on the NMR structure, shown in Fig. 3B, were unexpectedly complicated. There were three preferred orientations, denoted by the red areas, centred near A1 (β = 70°, γ = 130°), A2 (β = 85°, γ = 55°) and A3 (β = 40°, γ = -20°), respectively. These three orientational distributions of NTnC were distinct from each other, with differences up to 45° in β and 150° in γ. On the other hand, the orientation distribution A1 overlaps with R2 (β = 80°, γ = 130°) for the relaxed state.
To determine how actively cycling crossbridges contribute to the formation of the multiple ME distributions during active contraction, we used 25 μmol/L blebbistatin to abolish active force. Blebbistain binds specifically to the actin-binding cleft of cardiac myosin and prevents strong binding of myosin to actin [19], [20]. It can be seen from Fig. 3C that inhibition of active force resulted in the weakening of the orientation distribution for A3 only; the peak value of A3 was reduced by approximately 10%. These results showed that the three populations of NTnC orientation exist in the presence of Ca2+, and active force-generating myosin heads alters the balance between the three orientational distributions.
4. Discussion
The results presented above have shown that, in cardiac muscle cells, CTnC and hence the IT arm of Tn does not move during activation and acts like a scaffold that holds NTnC and the actin binding regions of troponin I. The NTnC, on the other hand, exhibits multiple orientations during both relaxation and activation. The present polarized fluorescence approach provided the in situ orientations (β, γ) of the IT arm and regulatory domain of Tn. Together with in vitro structures of the component protein domains, we developed in situ structures by assembly of protein domains with the correct interdomain angles.
4.1. Structural models for the thin filament in cardiac muscle
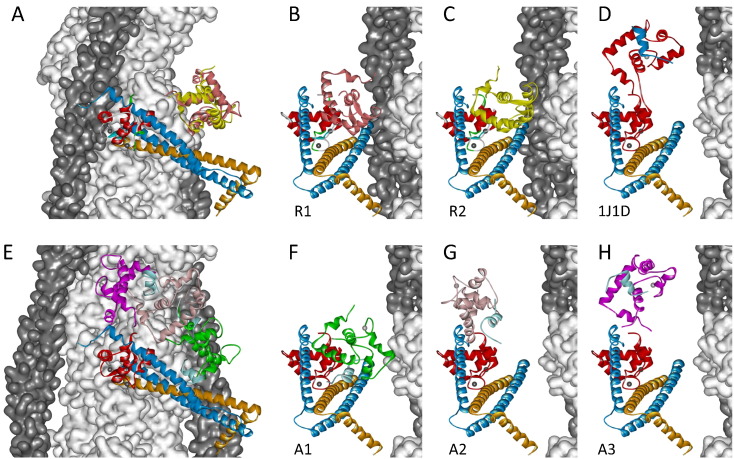
The structural models for the thin filament in cardiac muscle are shown graphically in Figs. 4 and S3. These models were assembled from various component protein domains of the thin filament. The atomic models of skeletal filamentous actin [21] and tropomyosin [22] were used since the detailed structures for the corresponding cardiac isoforms were not available. For the IT arm, the crystal structure of 1J1D [8] was used for models in both relaxation and active contraction; for the regulatory head, the NMR solution structures of 1SPY [16] and 1MXL [17] were used in the absence and presence of Ca2+, respectively. The orientations (β, γ) of the IT arm and regulatory head were determined from the present results. The azimuthal angle (α) and the axial/radial/azimuthal positions of the IT arm were estimated from previous crosslinking and fluorescence resonance energy transfer studies of isolated thin filament as described in our previous study [12]. The NTnC was then ligated to the IT arm via the link between the D- and E-helices of TnC using its (β, γ) orientation. The α of NTnC was estimated by avoiding clash with other components of the thin filament and proximity to the filament surface.
Fig. 4.
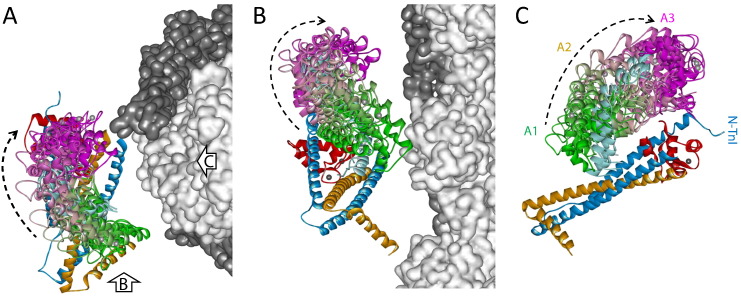
Structure of the cardiac thin filament in relaxation (A - C) and active contraction (E - H). Actin filament (light gray), with tropomyosin (dark gray) positions determined by EM-based reconstruction. Pointed end (towards M-line in a sarcomere) of the actin filament faces up. Orientations and positions of IT arm and NTnC are calculated as described in the text. Structure of IT arm is from 1J1D; CTnC is in red, TnI in blue and TnT in gold. Structure of NTnC in A - C is from 1SPY, and is in pink and yellow for orientations R1 (B) and R2 (C), respectively. Structure of NTnC in E - H is from 1MXL with orientations A1 to A3 shown in green (F), light purple (G) and magenta (H), respectively. B-C and F-H are a 90° azimuthal rotation compared with A and E, respectively. D, the Tn core complex of 1J1D is orientated according to the IT arm orientation. Note that no attempt has been made to estimate the azimuthal movement of the Tn on the filament surface because the present results give no information about the azimuthal angle.
In the resulting model, the arrowhead motif of the Tn complex points to the bottom right (Figs. 4 A and E), which is broadly consistent with a previous model for the skeletal muscle thin filament (Model 2 of Knowles et al.) [12] and the recently published model of cardiac thin filament in the relaxed state using a single-particle reconstruction technique [23]. It is also in general agreement with previous models of the thin filament based on EM and FRET data in which the arrowhead-shaped IT complex points towards the barbed end of the actin filament [10], [24], [25], but is in contrast to an earlier model based on single-particle analysis of reconstructed thin filament [11]. The acute angle between the IT coiled coil of Tn and the thin filament axis is 70°; the TnI135/TnT271 end of the coiled coil is close to the actin filament surface and also closer to the pointed end of the filament (the top in Fig. 4). The overall orientations (β, γ) of the IT arm do not change on activation (Fig. 2), which is consistent with the IT arm acting like a static scaffold that holds NTnC and the C-terminal region of TnI in positions on the filament surface allowing the Ca2+ regulation of TnI-actin interaction. The present results do not constrain the azimuthal (α) position of the IT arm, and we have assumed that this angle does not change on activation. It is possible that the whole or part of Tn complex moves azimuthally as does tropomyosin during activation. However, this will not change the present results on the orientation of IT arm.
In the present model, NTnC is connected to the rest of Tn complex by a flexible linker between the D- and E-helices and has two conformations in the relaxed state (Figs. 4 A-C, 3A). For both orientations of NTnC, the D helix is quite perpendicular to the actin filament (β = 80 - 85°), but γ decreased by 60° from R1 to R2, corresponding to a counter-clockwise rotation of NTnC around the D helix (Figs. 4 B and C). Previous study on the dynamics of TnC in the Tn complex has shown that NTnC moves independently from the rest of the complex in the absence of Ca2+ [26]. Such a dynamic movement of the regulatory head may increase the probability of it interacting with the TnI switch peptide when Ca2+ is bound, and thus compensate for the initial small structural change of cTnC upon Ca2+ binding alone [17], [18]. The broad orientational distribution of NTnC in the relaxed state may be an indication that some molecules are in the open conformatiom state even in the absence of Ca2+ [27] and may partly contribute to the lower density observed in electron microscopy reconstructions [23]. Thus the present finding suggests conformational dynamics of Tn crucial to its function, but difficult to detect experimentally.
NTnC undergoes a transition over the physiological ranges of [Ca2+] from a ‘closed’ Ca2+-free to a more ‘open’ Ca2+-bound conformation; the completion of this opening also requires the binding of TnI switch peptide to the hydrophobic cleft [17], [18], [28]. As a consequence, the binding of the TnI switch peptide to NTnC initiates the release of the two adjacent regions of TnI switch peptide from their actin binding sites (Fig. 1A). The ME analysis revealed that there were three orientation distributions or populations of NTnC during Ca2+ activation. All three NTnC orientations were well fitted in the present model without clashing with other components of the thin filament; the three in situ orientations of NTnC (A1-A3 in Fig. 3B) shown in Figs. 4 F-H in green, light purple and magenta, respectively. It can be seen from Figs. 3 (A and B) and 4 (C and F) that the orientations of NTnC in R2 and A1 are similar, and the D helix is roughly perpendicular to the thin filament axis. Thus, the main differences between these two structures are the opening of the NTnC and the binding of TnI switch peptide in A1 (Figs. 4 C and F). The conformational transition of NTnC from closed to open states is highly dynamic as reported in previous studies using various techniques [27], [28], [29], [30]; the transition between R1, R2 and A1 in the present study is likely to correspond to such a conformational equilibrium. For the other two orientations in the Ca2+ state, A2 and A3, NTnC undergoes the major movements of rotating around its D helix by 150° and tilting against the filament axis by 45° that makes it bend over towards the CTnC (Figs. 4 F-H). This in turn lifts TnI switch peptide (cyan helix in Fig. 4) together with its two flanking actin binding regions of TnI, the TnI inhibitory peptide (residues 137-148) and the CTnI (residues 169-210), away from the actin filament surface. (The TnI inhibitory peptide and the CTnI are not present in the crystal structures and are not shown in the present models.) When the Tn core complex of 1J1D [8] is orientated according to the ME distribution of IT arm, its NTnC domain is nearly superimposable on the A3 conformation (Figs. 4 D and H). Thus, the relative conformation between the regulatory domain and the IT arm in the Tn complex of 1J1D closely resembles one of those in situ.
As mentioned earlier, ME analysis avoids a priori assumptions about the shape of the orientational distribution and gives the smoothest/broadest distribution that is consistent with the order parameters measured under each experimental condition [15]. The resulting ME distribution is an exact but not necessarily unique fit to the experimental data and can be considered as a low-resolution representation on the real orientation distribution. It should also be noted that a smaller number of probes would limit the ability to distinguish multiple populations. For the multiple orientation distributions or populations determined by ME analysis, it is likely that NTnC is moving smoothly or fluctuating between different orientations in equilibrium. In relaxed muscle, such a movement involves mainly the rotation around the D helix by about 60°. However, during Ca2+ activation and, there are big changes in both the twist angle γ (150°) and tilt angle β (45°) between the A1, A2 and A3 conformations. Thus in these models, the continuous movement from A1 to A2 and to A3 does not cause any steric clash with other components of the thin filament; in Fig. 5, (four) additional orientations of NTnC each were calculated either between A1 and A2 or between A2 and A3. The smooth transition of orientations shown in Fig. 5 may represent a dynamic transition for the Tn regulatory domain, and orientation A2 can be considered intermediate between A1 and A3. The continuous transition of NTnC involves mainly the azimuthal swing around the actin filament surface (Fig. 5A, viewed from the pointed-end of actin filament), lifting the TnI switch peptide away from the filament (Fig. 5B). This can be seen more clearly in Fig. 5C when the NTnC is positioned behind the actin filament.
Fig. 5.
Orientational transition of NTnC during active contraction of cardiac muscle. A, viewed from the pointed-end of actin filament. Arrows in A indicate the viewing positions for B and C. Colors as in Fig. 4. Note that no attempt has been made to estimate the azimuthal movement of the Tn on the filament surface.
4.2. Structural basis for the regulation of cardiac muscle contraction by Tn
Contraction of heart muscle, and of striated muscle in general, is initiated by Ca2+ binding to the regulatory domain of Tn, the NTnC, that triggers a cascade of structural changes in the thin filament and leads to an azimuthal movement of tropomyosin around the filament that allow myosin to interact with actin and generate force [1], [2], [3]. At low [Ca2+], Tn docks on to the thin filament by binding of TnT to tropomysin [4], [5] and CTnI to actin [6], [7]. Except for NTnT (residues 1-158), the interactions of these Tn components with actin and tropomyosin are sensititive to the Ca2+ level; that is, only NTnT of the Tn complex binds to tropomyosin during both relaxation and contraction of the muscle (Fig. 1A). In the present model, the IT arm, that anchors to the thin filament through interaction between NTnT and tropomyosin, acts primarily as a scaffold to hold the Ca2+ regulatory domain of TnC and CTnI; The movement of the IT arm is unlikely to play an important role in regulation.
At low [Ca2+] during diastole of the heart, the Ca2+ regulatory domain of Tn, NTnC, rotates around its central D helix searching for its binding partner, TnI switch peptide. Upon activation when Ca2+ binds to its regulatory site, the subsequent TnI switch peptide binding to its hydrophobic cleft stabilizes NTnC in an open conformation [17]. By moving away from their initial location (A1 → A2 → A3, Fig. 4, Fig. 5), NTnC together with the TnI switch peptide bends over towards CTnC resulting in removal of the flanking regions of TnI from their actin-binding inhibitory locations. This in turn enhances the movement of tropomyosin from its inhibitory site and facilitates the actin-myosin interaction, and thus force generation by myosin crossbridges. Therefore, the transition of NTnC from A1 to A3 orientations would be expected to couple with the increased Ca2+ affinity to its regulatory site. The bending of NTnC towards CTnC during activation in cardiac muscle is in contrast to a rotation of NTnC around D-helix found in skeletal muscle [13], [31]. This might be related to the possibility that the link between D- and E helices in skeletal TnC is less flexible than in the cardiac isoform [32].
In this model, binding of myosin to actin would stabilize the tropomyosin away from its initial inhibitory position and, would therefore be expected to suppress the return of CTnI to its actin binding site and enhance the structural transition of NTnC towards the A3 conformation. The finding that inhibition of active force by blebbistatin weakened the orientation distribution of A3 (Fig. 3C) is consistent with this role of active force-generating myosin heads in the structural transition of NTnC during heart muscle activation. It has been reported recently that the extent of NTnC opening was reduced when force was inhibited [33]. Therefore, it cannot be ruled out that the different orientations of NTnC may also correspond to the degrees of NTnC opening. Previous work also showed that inhibition of active force reduced the Ca2+ sensitivity of TnC structural changes [14], [33]. Thus, the inhibition of active force weakened the transition of NTnC from A1 to A3 that was associated with the myofilament Ca2+ sensitivity. Taken together, it is plausible that, in the presence of Ca2+, the interaction of NTnC with TnI shifts the equilibrium between orientations of NTnC (A1↔A2↔A3) towards A3 state and the consequent interaction between myosin and actin pushes it further towards A3. More generally, such a dynamic equilibrium of NTnC orientations is in agreement with the transitions between blocked, closed and open states of thin filament in the three-state model of muscle regulation [34] and, therefore, supports the hypothesis that there is a positional ambiguity of tropomyosin on actin [35].
In summary, our in situ polarized fluorescence data from heart muscle cells reveal conformational dynamics of the regulatory domain of Tn, NTnC, during both diastole and systole. The derived models of cardiac thin filament provide new insight into mechanism of cardiac muscle regulation. During diastole, NTnC rotates around searching for TnI switch peptide. Upon activation, NTnC together with TnI switch peptide can move away from its initial location and bend over towards the CTnC resulting in removal of the flanking regions of TnI away from their actin-binding inhibitory locations. The conformational dynamics of NTnC during systole can play an important role in the regulation of cardiac muscle contraction. The IT arm of cardiac Tn, on the other hand, acts as a scaffold holding NTnC and the actin binding regions of TnI.
Acknowledgments
This study was supported by the British Heart Foundation and by a studentship from KCL BHF Centre of Research Excellence (to I.S.). We thank Malcolm Irving, David Trentham, Brian Sykes, Mathias Gautel and Ian Robertson for comments on the manuscript.
Disclosures
None.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.yjmcc.2014.07.015.
Appendix A. Supplementary data
Supplementary Materials.
References
- 1.Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969;4:351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- 2.Greaser M.L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971;246:4226–4233. [PubMed] [Google Scholar]
- 3.Gordon A.M., Homsher E., Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 4.Pearlstone J.R., Smillie L.B. Effects of troponin-I plus-C on the binding of troponin-T and its fragments to alpha-tropomyosin. Ca2+ sensitivity and cooperativity. J Biol Chem. 1983;258:2534–2542. [PubMed] [Google Scholar]
- 5.Morris E.P., Lehrer S.S. Troponin-tropomyosin interactions. Fluorescence studies of the binding of troponin, troponin T, and chymotryptic troponin T fragments to specifically labeled tropomyosin. Biochemistry. 1984;23:2214–2220. doi: 10.1021/bi00305a018. [DOI] [PubMed] [Google Scholar]
- 6.Tripet B., Van Eyk J.E., Hodges R.S. Mapping of a second actin-tropomyosin and a second troponin C binding site within the C terminus of troponin I, and their importance in the Ca2+-dependent regulation of muscle contraction. J Mol Biol. 1997;271:728–750. doi: 10.1006/jmbi.1997.1200. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y., Wu J.L., Li B., Langsetmo K., Gergely J., Tao T. Photocrosslinking of benzophenone-labeled single cysteine troponin I mutants to other thin filament proteins. J Mol Biol. 2000;296:899–910. doi: 10.1006/jmbi.1999.3495. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S., Yamashita A., Maeda K., Maeda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 9.Vinogradova M.V., Stone D.B., Malanina G.G., Karatzaferi C., Cooke R., Mendelson R.A. Ca2+-regulated structural changes in troponin. Proc Natl Acad Sci U S A. 2005;102:5038–5043. doi: 10.1073/pnas.0408882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirani A., Vinogradova M.V., Curmi P.M., King W.A., Fletterick R.J., Craig R. An atomic model of the thin filament in the relaxed and Ca2+-activated states. J Mol Biol. 2006;357:707–717. doi: 10.1016/j.jmb.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 11.Paul D.M., Morris E.P., Kensler R.W., Squire J.M. Structure and orientation of troponin in the thin filament. J Biol Chem. 2009;284:15007–15015. doi: 10.1074/jbc.M808615200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knowles A.C., Irving M., Sun Y.-B. Conformation of the troponin core complex in the thin filaments of skeletal muscle during relaxation and active contraction. J Mol Biol. 2012;421:125–137. doi: 10.1016/j.jmb.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson R.E., Sun Y.-B., Mercier P., Brack A.S., Sykes B.D., Corrie J.E. In situ Orientations of Protein Domains: Troponin C in Skeletal Muscle Fibers. Mol Cell. 2003;11:865–874. doi: 10.1016/s1097-2765(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y.-B., Lou F., Irving M. Calcium- and myosin-dependent changes in troponin structure during activation of heart muscle. J Physiol. 2009;587:155–163. doi: 10.1113/jphysiol.2008.164707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Heide U.A., Hopkins S.C., Goldman Y.E. A maximum entropy analysis of protein orientations using fluorescence polarization data from multiple probes. Biophys J. 2000;78:2138–2150. doi: 10.1016/S0006-3495(00)76760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spyracopoulos L., Li M.X., Sia S.K., Gagné S.M., Chandra M., Solaro R.J. Calcium-induced structural transition in the regulatory domain of human cardiac troponin C. Biochemistry. 1997;36:12138–12146. doi: 10.1021/bi971223d. [DOI] [PubMed] [Google Scholar]
- 17.Li M.X., Spyracopoulos L., Sykes B.D. Binding of cardiac troponin-I147 -163 induces a structural opening in human cardiac troponin-C. Biochemistry. 1999;38:8289–8298. doi: 10.1021/bi9901679. [DOI] [PubMed] [Google Scholar]
- 18.Sia S.K., Li M.X., Spyracopoulos L., Gagné S.M., Liu W., Putkey J.A. Structure of cardiac muscle troponin C unexpectedly reveals a closed regulatory domain. J Biol Chem. 1997;272:18216–18221. doi: 10.1074/jbc.272.29.18216. [DOI] [PubMed] [Google Scholar]
- 19.Straight A.F., Cheung A., Limouze J., Chen I., Westwood N.J., Sellers J.R. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 20.Allingham J.S., Smith R., Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005;12:378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 21.Holmes K.C., Angert I., Kull F.J., Jahn W., Schröder R.R. Electron cryomicroscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425:423–427. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 22.Whitby F.G., Phillips G.N.J. Crystal structure of tropomyosin at 7 Å resolution. Proteins Struct Funct Genet. 2000;38:49–59. [PubMed] [Google Scholar]
- 23.Yang S., Barbu-Tudoran L., Orzechowski M., Craig R., Trinick J., White H. Three-dimensional organization of troponin on cardiac muscle thin filaments in the relaxed state. Biophys J. 2014;106:855–864. doi: 10.1016/j.bpj.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura-Sakiyama C., Ueno Y., Wakabayashi K., Miki M. Fluorescence resonance energy transfer between residues on troponin and tropomyosin in the reconstituted thin filament: modelling the troponin-tropomyosin complex. J Mol Biol. 2008;376:80–91. doi: 10.1016/j.jmb.2007.10.078. [DOI] [PubMed] [Google Scholar]
- 25.Miki M., Makimura S., Sugahara Y., Yamada R., Bunya M., Saitoh T. A three-dimensional FRET analysis to construct an atomic model of the actin-tropomyosin-troponin core domain complex on a muscle thin filament. J Mol Biol. 2012;420:40–55. doi: 10.1016/j.jmb.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Blumenschein T.M., Stone D.B., Fletterick R.J., Mendelson R.A., Sykes B.D. Calcium-dependent changes in the flexibility of the regulatory domain of troponin C in the troponin complex. J Biol Chem. 2005;280:21924–21932. doi: 10.1074/jbc.M500574200. [DOI] [PubMed] [Google Scholar]
- 27.Cordina N.M., Liew C.K., Gell D.A., Fajer P.G., Mackay J.P., Brown L.J. Effects of calcium binding and the hypertrophic cardiomyopathy A8V mutation on the dynamic equilibrium between closed and open conformations of the regulatory N-domain of isolated cardiac troponin C. Biochemistry. 2013;52:1950–1962. doi: 10.1021/bi4000172. [DOI] [PubMed] [Google Scholar]
- 28.Dong W.J., Xing J., Villain M., Hellinger M., Robinson J.M., Chandra M. Conformation of the regulatory domain of cardiac muscle troponin C in its complex with cardiac troponin I. J Biol Chem. 1999;274:31382–31390. doi: 10.1074/jbc.274.44.31382. [DOI] [PubMed] [Google Scholar]
- 29.Robinson J.M., Cheung H.C., Dong W. The cardiac Ca2+-sensitive regulatory switch, a system in dynamic equilibrium. Biophys J. 2008;95:4772–4789. doi: 10.1529/biophysj.108.131318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindert S., Kekenes-Huskey P.M., McCammon J.A. Long-timescale molecular dynamics simulations elucidate the dynamics and kinetics of exposure of the hydrophobic patch in troponin C. Biophys J. 2012;103:1784–1789. doi: 10.1016/j.bpj.2012.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genchev G.Z., Kobayashi T., Lu H. Calcium induced regulation of skeletal troponin — computational insights from molecular dynamics simulations. PLoS One. 2013;8:e58313. doi: 10.1371/journal.pone.0058313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varughese J.F., Chalovich J.M., Li Y. Molecular dynamics studies on troponin (TnI-TnT-TnC) complexes: insight into the regulation of muscle contraction. J Biomol Struct Dyn. 2010;28:159–174. doi: 10.1080/07391102.2010.10507350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieck D.C., Li K.L., Ouyang Y., Solaro R.J., Dong W.J. Structural basis for the in situ Ca2+ sensitization of cardiac troponin C by positive feedback from force-generating myosin cross-bridges. Arch Biochem Biophys. 2013;537:198–209. doi: 10.1016/j.abb.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKillop D.F., Geeves M.A. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lehman W., Galińska-Rakoczy A., Hatch V., Tobacman L.S., Craig R. Structural basis for the activation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2009;388:673–681. doi: 10.1016/j.jmb.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials.