Abstract
Background
Clozapine is an atypical antipsychotic demonstrated to be superior in the treatment of refractory schizophrenia which causes fewer movement disorders. Clozapine, however, entails a significant risk of serious blood disorders such as agranulocytosis which could be potentially fatal. Currently there are a number of newer antipsychotics which have been developed with the purpose to find both a better tolerability profile and a superior effectiveness.
Objectives
To compare the clinical effects of clozapine with other atypical antipsychotics (such as amisulpride, aripiprazole, olanzapine, quetiapine, risperidone, sertindole, ziprasidone and zotepine) in the treatment of schizophrenia and schizophrenia-like psychoses.
Search methods
We searched the Cochrane Schizophrenia Groups Register (June 2007) and reference lists of all included randomised controlled trials. We also manually searched appropriate journals and conference proceedings relating to clozapine combination strategies and contacted relevant pharmaceutical companies.
Selection criteria
All relevant randomised, at least single-blind trials, comparing clozapine with other atypical antipsychotics, any dose and oral formulations, for people with schizophrenia or related disorders.
Data collection and analysis
We selected trials and extracted data independently. For dichotomous data we calculated relative risks (RR) and their 95% confidence intervals (CI) based on a random-effects model. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate. For continuous data, we calculated mean differences (MD) again based on a random-effects model.
Main results
The review currently includes 27 blinded randomised controlled trials, which involved 3099 participants. Twelve randomised control trials compared clozapine with olanzapine, five with quetiapine, nine with risperidone, one with ziprasidone and two with zotepine. Attrition from these studies was high (overall 30.1%), leaving the interpretation of results problematic. Clozapine had a higher attrition rate due to adverse effects than olanzapine (9 RCTs, n=1674, RR 1.60 CI 1.07 to 2.40, NNT 25 CI 15 to 73) and risperidone (6 RCTs, n=627, RR 1.88 CI 1.11 to 3.21, NNT 16 CI 9 to 59). Fewer participants in the clozapine groups left the trials early due to inefficacy than risperidone (6 RCTs, n=627, RR 0.40 CI 0.23 to 0.70, NNT 11 CI 7 to 21), suggesting a certain higher efficacy of clozapine.
Clozapine was more efficacious than zotepine in improving the participants general mental state (BPRS total score: 1 RCT, n=59, MD −6.00 CI −9.83 to −2.17), but not consistently more than olanzapine, quetiapine, risperidone and ziprasidone. There was no significant difference between clozapine and olanzapine or risperidone in terms of positive or negative symptoms of schizophrenia. According to two studies from China quetiapine was more efficacious for negative symptoms than clozapine (2 RCTs, n=142, MD 2.23 CI 0.99 to 3.48).
Clozapine produced somewhat fewer extrapyramidal side-effects than risperidone (use of antiparkinson medication: 6 RCTs, n=304, RR 0.39 CI 0.22 to 0.68, NNT 7 CI 5 to 18) and zotepine (n=59, RR 0.05 CI 0.00 to 0.86, NNT 3 CI 2 to 5). More participants in the clozapine group showed decreased white blood cells than those taking olanzapine, more hypersalivation and sedation than those on olanzapine, risperidone and quetiapine and more seizures than people on olanzapine and risperidone. Also clozapine produced an important weight gain not seen with risperidone.
Other differences in adverse effects were less documented and should be replicated, for example, clozapine did not alter prolactin levels whereas olanzapine, risperidone and zotepine did; compared with quetiapine, clozapine produced a higher incidence of electrocardiogram (ECG) alterations; and compared with quetiapine and risperidone clozapine produced a higher increase of triglyceride levels. Other findings that should be replicated were: clozapine improved social functioning less than risperidone and fewer participants in the clozapine group had to be hospitalised to avoid suicide attempts compared to olanzapine.
Other important outcomes such as service use, cognitive functioning, satisfaction with care or quality of life were rarely reported.
Authors’ conclusions
Clozapine may be a little more efficacious than zotepine and risperidone but further trials are required to confirm this finding. Clozapine differs more clearly in adverse effects from other second generation antipsychotics and the side-effect profile could be key in the selection of treatment depending on the clinical situation and a patient’s preferences. Data on other important outcomes such as cognitive functioning, quality of life, death or service use are currently largely missing, making further large and well-designed trials necessary. It is also important to take into account that the large number of people leaving the studies early limits the validity and interpretation of our findings.
Medical Subject Headings (MeSH): Antipsychotic Agents [* therapeutic use], Benzodiazepines [therapeutic use], Clozapine [* therapeutic use], Dibenzothiazepines [therapeutic use], Dibenzothiepins [therapeutic use], Piperazines [therapeutic use], Randomized Controlled Trials as Topic, Risperidone [therapeutic use], Schizophrenia [* drug therapy], Thiazoles [therapeutic use]
MeSH check words: Humans
BACKGROUND
Description of the condition
Schizophrenia is a chronic and disabling severe mental disorders, which involves a complex set of disturbances, associated with abnormalities of brain structure and function, disorganised speech and behavior, delusions, and hallucinations (WHO 1998). It is sometimes called a psychotic disorder or a psychosis. Also, people with schizophrenia present dysfunction in one or more major areas of functioning e.g. social and occupational areas (Mueser 2004). The prevalence is between 0.7 - 1% of the adult population (Lehman 2004), however, due to frequent chronicity, this disease leads to high levels of social burden and cost, as well as an incalculable amount of individual pain and suffering (WHO 1998, van Os 2009).
Description of the intervention
The therapeutic arsenal for schizophrenia is wide and varied. Conventional, typical or first generation antipsychotics, such as chlorpromazine and haloperidol have been used as a first choice for treatment for over 50 years (Kane 1990). They are effective in reducing the positive and some of the negative symptoms of schizophrenia, however they could produce unpleasant adverse effects such as sedation, demotivation and movement disorders that often lead to treatment discontinuation which then may result in relapse of symptoms (Gaebel 1997). In 1959 the development of a new generation of neuroleptics, classified as atypical antipsychotics, began with clozapine (ACP 2002). Although clozapine has demonstrated to be superior to the older typical antipsychotics in the treatment of the refractory schizophrenia (Wahlbeck 1999), it can also produce severe adverse effects, particularly hypersalivation and blood disorders which restrict its use.
After clozapine, a considerable number of newer atypical antipsychotics drugs have been developed in the hope of finding new compounds with a better tolerability profile and higher efficacy (Stroup 2003). These include amisulpride, aripiprazole, olanzapine, quetiapine, risperidone, sertindole, ziprasidone, and zotepine. The effectiveness of these newer atypical antipsychotics compared to clozapine is not yet established. Some studies suggest that the newer atypical antipsychotics have a similar effectiveness to clozapine, and suggest that they may also be effective in resistant schizophrenia with a better security profile (Kane 2006, Citrome 2002).
How the intervention might work
Clozapine was the first atypical antipsychotic manufactured by Sandoz in 1959 and introduced to the market in the 1960s. Most atypical antipsychotics are antagonists at serotonin and dopamine receptors, but they have different pharmacological profiles according to their level of affinity with the different receptor subtypes (Miyamoto 2005). Clozapine has multiple sites of action such as dopaminergic, serotonergic, cholinergic and histaminergic receptors, with high affinity to D4 and 5HT2A receptors and low affinity to D1, D2 and D3 receptors. The low affinity of striatal D2 receptors and high one of 5HT2A receptors could explain its low extra-pyramidal symptoms liability, its atypical profile (Beaumont 2000, Miyamoto 2005). Clozapine differs from conventional antipsychotics for its greater efficacy in controlling positive symptoms in people with treatment-resistant illness and by inducing few extra-pyramidal effects (Kane 1988, Wahlbeck 1999). In 1975, however, sixteen people in Finland developed severe blood reactions - a substantial decline in the white blood cells (neutropenia) which made the individuals dangerously susceptible to infection (Idänpään-He.1977). From these sixteen, eight died. The drug was then largely withdrawn from the market (in the UK, Australia and USA), although the withdrawal was not worldwide (O’Brien 2004) e.g. Scandinavia, Germany and China kept the drug in the market. The cumulative experience with these patients and the subsequent studies demonstrated its superiority in patients with treatment-resistant schizophrenia and also that clozapine could be administered safely, when patients are carefully monitored (Naheed 2001,O’Brien 2004). Clozapine was reintroduced, over a decade later, for people with schizophrenia who were either resistant to typical neuroleptics or who were intolerant of the adverse effects of them (Wahlbeck 1999).
Why it is important to do this review
So far, reviews have not found any robust evidence that other atypical drugs have a clinical effect and tolerability similar to clozapine (Gilbody 2000, McEvoy 2006). This could in part be due to a lack of primary studies with good methodological quality, which measure important clinical outcomes during a prolonged time with enough statistical power (Tuunainen 2000). By systematically searching for all known randomised controlled trials of clozapine versus other atypical antipsychotics, this review should amass more data and provide robust, useful evidence.
This new review is an update of the previous review “Newer atypical antipsychotic medication vs. clozapine” which compared clozapine with all other atypical antipsychotics pooled into one group (Tuunainen 2000). Since the atypical antipsychotics are a heterogenous group with quite different pharmacological profile and the amount of data published on this topic has grown enormously during the last few years, it is now possible to explore atypical comparisons with clozapine separately. For this reason, the title and the review protocol have been modified.
OBJECTIVES
To compare the clinical effects of clozapine with other atypical antipsychotic drugs in the treatment of schizophrenia and schizophrenia-like psychoses.
METHODS
Criteria for considering studies for this review
Types of studies
All relevant randomised controlled trials that compared clozapine with other atypical antipsychotics for treatment of schizophrenia and similar psychotic mental illness. We included only the first treatment phase of randomised cross-over studies. Quasi-randomised trials were excluded. All Included trials needed to be at least single-blind (blind raters).
Types of participants
People with schizophrenia, and other types of schizophrenia-like psychoses (schizophreniform and schizoaffective disorders) diagnosed by any criteria. We included people with schizophreniform and schizoaffective disorders as there is no evidence that the schizophrenia-like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
Clozapine: oral formulation, any dose.
New atypical antipsychotics such as amisulpride, aripiprazole, olanzapine, quetiapine, risperidone, sertindole, ziprasidone and zotepine: oral formulation, any dose.
We excluded studies where participants were prescribed more than one, or combinations of atypical antipsychotics.
Types of outcome measures
We grouped outcomes by time - short term (up to 12 weeks), medium term (up to 26 weeks) and long term (more than 26 weeks). As schizophrenia is a long term disorder, short treatment studies are not clinically relevant. We decided to exclude studies lasting less than two weeks.
Primary outcomes
No clinically important response as defined by the individual studies (e.g. global impression less than much improved or less than 50% reduction on a rating scale) at long term.
Secondary outcomes
-
1.
Death
-
1.1
Suicide
-
1.2
Natural causes
-
2.
Leaving the studies early
-
2.1
Any reason
-
2.2
Specific reason (as described by individual studies)
-
3.
Global state
-
3.1
No clinically important change in global state (as defined by individual studies) at short and medium term
-
3.2
Relapse (as defined by the individual studies)
-
4.
Mental state
-
4.1
No clinically important change in general mental state at short and medium term
-
4.2
Average endpoint general mental state score
-
4.3
Average change in general mental state scores
-
4.4
No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia) at short and medium term
-
4.5
Average endpoint specific symptom score 4.6 Average change in specific symptom scores
-
5.
General functioning
-
5.1
No clinically important change in general functioning at short and medium term
-
5.2
Average endpoint general functioning score
-
5.3
Average change in general functioning scores
-
6.
Quality of life/satisfaction with treatment
-
6.1
No clinically important change in quality of life at short and medium term
-
6.2
Average endpoint quality of life score
-
6.3
Average change in quality of life scores
-
7.
Cognitive functioning
-
7.1
No clinically important change in cognitive functioning at short and medium term
-
7.2
Average endpoint cognitive functioning score
-
7.3
Average change in cognitive functioning scores
-
8.
Service use
-
8.1
Number of patients hospitalised
-
8.2
Number of patients discharged or readmitted (as defined in individual trial)
-
9.
Adverse effects - general and specific
-
9.1
Number of participants with at least one adverse effect
-
9.2
Clinically important specific adverse effects (cardiac effects, movement disorders, prolactin increase and associated adverse events, metabolic side effects (as such weight gain, hyperlipidaemia and hyperglycaemia), effects on white blood cell count)
-
9.3
Average endpoint specific adverse effects
-
9.4
Average change in specific adverse effects
Search methods for identification of studies
Electronic searches
We searched the Cochrane Schizophrenia Group’s Trials Register (June 2007) using the phrase:
[(*clozapin* OR *clozaril* OR *denzapin* OR *zaponex*) in title, abstract and index terms in REFERENCE and interventions of STUDY]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group module).
Searching other resources
1. Reference lists
We searched references of articles selected for further relevant trials.
2. Conferences
We sought studies from recent conference proceedings if available.
3. Pharmaceutical companies
We contacted companies performing trials with amisulpride, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone or zotepine directly to obtain data on unpublished trials.
4. Personal contact
We contacted the first author of each included study for information regarding unpublished trials or for missing information.
Data collection and analysis
Selection of studies
CA and KK independently inspected all reports. We resolved any disagreement by discussion, and where there was still doubt, we acquired the full article for further inspection. Once the full articles were obtained, we independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought additional information and these trials were added to the list of those awaiting assessment.
Data extraction and management
1. Data extraction
CA, KK, CR, HH, FS, SS, SL independently extracted the data from selected trials. When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, the data were not entered and we added the trial to the list of those awaiting assessment.
2. Management
We extracted the data onto standard simple forms. Where possible, we entered data into RevMan in such a way that the area to the left of the line of no effect indicated a favourable outcome for clozapine.
3. Scale-derived data
3.1 Valid scales
A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and many are not valid, or are even ad hoc. It is accepted generally that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure) (Rust 1989). Unpublished scales are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore continuous data from rating scales were included only if the measuring instrument had been described in a peer-reviewed journal. In addition, the following minimum standards for instruments were set: the instrument should either be (a) a self-report or (b) completed by an independent rater or relative (not the therapist) and (c) the instrument should be a global assessment of an area of functioning.
Assessment of risk of bias in included studies
We assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2009). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases. We would not have included studies where sequence generation was at high risk of bias or where allocation was clearly not concealed.
Measures of treatment effect
1. Binary data
We calculated the relative risk (RR) and its 95% confidence interval (CI) based on the random-effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number needed to harm (NNH) as the inverse of the risk difference, and its 95% confidence interval (CI).
Where possible, efforts were made to convert outcome measures to dichotomous data. This can be done by identifying cut-off points on rating scales and dividing participants accordingly into ‘clinically improved’ or ‘not clinically improved’. It was generally assumed that if there had been a 50% reduction in a scale-derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005a, Leucht 2005b). If data based on these thresholds were not available, we used the primary cut-off presented by the original authors.
We carried out an intention-to-treat analysis. Everyone allocated to the interventions were counted, whether they completed the follow up or not. It was assumed that those who dropped out had no change of their outcome. This rule is conservative concerning response to treatment, because it assumes that those discontinuing the studies would not have responded. It is not conservative concerning side-effects, because it assumes that those discontinuing the studies would not have developed the side-effect if they had remained in the study, but we felt that assuming that all drop-outs would have developed side-effects would overestimate the risk.
2. Continuous data
2.1 Rating scales
A wide range of instruments are available to measure mental health outcomes. These instruments vary in quality and many are not valid, or are even ad hoc. For outcome instruments some minimum standards have to be set. They were that: (i) the psychometric properties of the instrument should have been described in a peer-reviewed journal (Marshall 2000);and (ii) the instrument should either be: (a) a self report, or (b) completed by an independent rater or relative (not the therapist).
2.2 Summary statistic
For continuous outcomes we estimated a mean difference (MD) between groups. MDs were again based on the random-effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. When standard errors instead of standard deviations (SD) were presented, we converted the former to standard deviations. If both were missing we estimated SDs from P-values or used the average SD of the other studies (Furukawa 2006).
2.3 Endpoint versus change data
We combined both endpoint data and change data in the analysis, because there is no principal statistical reason why endpoint and change data should measure different effects (Higgins 2009).
2.4 Skewed data
The meta-analytic formulas applied by RevMan Analyses (the statistical programme included in RevMan) require a normal distribution of data. The software is robust towards some skew, but to which degree of skewness meta-analytic calculations can still be reliably carried out is unclear. On the other hand, excluding all studies on the basis of estimates of the normal distribution of the data also leads to a bias, because a considerable amount of data may be lost leading to a selection bias. Therefore, we included all studies in the primary analysis. In a sensitivity analysis we excluded potentially skewed data applying the following rules:
-
a)
When a scale started from the finite number zero the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, Altman 1996).
-
b)
If a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(S-Smin), where S is the mean score and Smin is the minimum score.
-
c)
In large studies (as a cut-off we used 200 participants) skewed data pose less of a problem. In these cases we entered the data in a synthesis.
-
d)
The rules explained in a) and b) do not apply to change data. The reason is that when continuous data are presented on a scale which includes a possibility of negative values, it is difficult to tell whether data are non-normally distributed (skewed) or not. This is also the case for change data (endpoint minus baseline). In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALL-STAT electronic statistics mailing list, we presented change data in RevMan Analyses in order to summarise available information. In doing this, it was assumed either that data were not skewed or that the analysis could cope with the unknown degree of skew. Without individual patient data it is impossible to test this assumption. Change data were therefore included and a sensitivity analysis was not applied.
Unit of analysis issues
1. Cluster trials
Studies increasingly employ ‘cluster randomisation’ (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a ‘unit of analysis’ error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This can cause type 1 errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non-cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a ‘design effect’. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect=1+(m-1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, synthesis with other studies would have been possible using the generic inverse variance technique.
2. Cross-over trials
A major concern of cross-over trials is the carry-over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash-out phase. For the same reason cross-over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, we will only use data of the first phase of cross-over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, the additional treatment arms were presented in comparisons. Where the additional treatment arms were not relevant, these data were not reproduced.
Dealing with missing data
Although high rates of premature discontinuation are a major problem in this field, we felt that it is unclear which degree of attrition leads to a high degree of bias. We, therefore, did not exclude trials on the basis of the percentage of participants completing them. However we addressed the drop-out problem in all parts of the review, including the abstract. For this purpose we calculated, presented and commented on frequency statistics (overall rates of leaving the studies early in all studies and comparators pooled and their ranges).
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all the included studies within any comparison to judge for clinical heterogeneity.
2. Statistical
2.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I-squared statistic
Visual inspection was supplemented using, primarily, the I-squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I-squared estimate was greater than or equal to 50% we interpreted this as indicating the presence of considerable levels of heterogeneity (Higgins 2003). If inconsistency was high and clear reasons explaining the heterogeneity were found, we presented the data separately. If not, we commented on the heterogeneity of the data.
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). We entered data from all identified and selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. A formal test for funnel-plot asymmetry was not undertaken. We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small-study effects. We did not use funnel plots for outcomes where there were ten or fewer studies, or where all studies were of similar sizes.
Data synthesis
We understand that there is a debate around the use of fixed or random-effects models. The random-effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us as we are a priori expecting some clinical heterogeneity between the patients in the different trials. Therefore, we chose the random effects model for all analyses (DerSimonian 1986). This said, we acknowledge that as a disadvantage the random effects model puts added weight onto small studies which often are the most biased ones. Depending on the direction of effect these studies can either inflate or deflate the effect size.
Subgroup analysis and investigation of heterogeneity
1. Subgroup analysis
We assessed each outcome by trial length. No other subgroup analysis was pre-specified.
2. Investigation of heterogeneity
If data were clearly heterogeneous we checked that data are correctly extracted and entered and that we had not made unit of analysis errors. If high levels of heterogeneity remained we did not undertake a meta-analyse at this point, because if there is considerable variation in results, and particularly if there is inconsistency in the direction of effects, it may be misleading to quote an average value for the intervention effect.
Sensitivity analysis
We excluded studies with potentially skewed data. A recent report showed that some of the comparisons of atypical antipsychotics may have been biased by using inappropriate comparator doses (Heres 2006). We, therefore, also analysed whether the exclusion of studies with inappropriate comparator doses changed the results of the primary outcome and the general mental state.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
For substantive descriptions of studies, please see Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Through the search strategy we found 1341 references, which includes a first search (857) in July 2006 and an update search (484) in June 2007. Two-hundred-and-twenty-six studies compared clozapine versus other atypical antipsychotics, from them only 27 studies fulfilled the review criteria.
Included studies
We selected 27 studies of which all were described as randomised. Only three studies gave details about randomisation methods and their implementation (Kumra 2008, Shaw 2006 and Wahlbeck 2000), the rest did not state methods used to generate the random allocation sequence, the allocation concealment and randomisation implementation. Twenty studies were double-blind and 7 single-blind/rater-blinded. In the studies the blindness was not assessed and no further details about it were given. Twenty-five studies were parallel clinical trials, two were cross-over studies (we included only the first phase) (Conley 2003, Lin 2003).
1. Length of trial
Twenty studies were short term studies (two up to 12 weeks), five studies belong to the medium term category (up to 26 weeks), and only two studies reported long term data (more than 26 weeks).
2. Participants
The 27 studies involved a total of 3099 participants. The comparison of clozapine versus olanzapine included 1753 participants, clozapine versus quetiapine, 306 participants, clozapine versus risperidone, 843 participants, clozapine versus ziprasidone, 146 participants, and the clozapine versus zotepine comparison, 109 participants.
Almost all studies used operationalised diagnostic criteria. Most studies included participants with diagnoses of schizophrenia or schizoaffective disorder according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) III - R or IV criteria and only one used The International Classification of Diseases, 9th Revision (ICD-9) criteria. Chinese studies used the Chinese Classification of Mental Disorders (CCMD) version 2 or 3 criteria. Two studies did not state if any operationalised diagnostic criteria were used. One of them, which compared clozapine versus ziprasidone, enrolled participants with schizophrenia who met criteria for treatment resistance (non-response in three adequate trials in past five years) and/or inability to tolerate antipsychotic treatment. The other one compared clozapine versus zotepine and reported that the participants were people with schizophrenia who have been treated with clozapine for more than five months.
Many participants were diagnosed as treatment resistant to prior antipsychotics. The criteria and definitions used varied.
Moresco 2004 defined treatment resistance as lack of satisfactory clinical response to two previous antipsychotics, with duration of at least six weeks each, given an appropriate dosage (at least 500 mg chlorpromazine equivalent).
Tollefson 2001 included participants who had a history of ‘resistance to previous antipsychotic’, defined as lack of satisfactory clinical response to at least two previous oral neuroleptic treatments, each from a different chemical class, given for a duration of at least six weeks at an appropriate daily dosage equivalent to at least 500 mg/day of chlorpromazine, or to the maximum daily dosage when intolerable side-effects had been documented.
Conley 2003 defined ‘treatment resistant’ when there was evidence of: a persistent positive psychotic symptoms: item score > or = 4 (moderate) on at least two of four positive symptoms items on the Brief Psychiatric Rating Scale (1 - 7) (BPRS); the concurrent presence of at least moderately severe illness as rated by the total BPRS score (score > or = 45 on the 18 item scale) and score of at least moderate on the Clinical Global Impression scale (CGI); two failed historical trials of antipsychotics of at least six weeks duration at doses of at least 600 mg/day chlorpromazine equivalents; and no stable period of good social and/or occupational functioning within the last five years.
McGurk 2005 included subjects who had evidence of ‘treatment resistance’ defined as at least one trial of a conventional antipsychotic at a dose equivalent to 600 mg/day of chlorpromazine, a second trial of a different conventional antipsychotic at a dose equivalent to 250 - 500 mg/day of chlorpromazine and at least a moderate severity score on one of the BPRS psychotics symptoms items or on one of the Scale for the Assessment of Negative Symptoms (SANS) global subscale.
Wahlbeck 2000 included participants with ‘resistance to previous antipsychotics’, defined as persistent psychotic symptoms for at least six months during which the participants received antipsychotic treatment from at least two different chemical classes at dosages equivalent to or greater than 1000 mg/day of chlorpromazine for a period of at least six weeks each.
Kumra 2008 included participants who had a documented treatment failure of at least two prior adequate antipsychotic trials and a baseline BPRS total score of at least 35 and a score of at least moderate on one or more psychotic items on the BPRS scale.
Breier 1999 included participants who meet the criteria for ‘partial response’ to neuroleptics, i.e. a history of residual positive and/or negative symptoms after at least a six weeks trial of a therapeutic dose of a neuroleptic agent; at least a minimum level of positive and/or negative symptoms at the time of evaluation for the study; and at least a minimum level of positive and/or negative symptoms after a prospective trial of at least two weeks with fluphenazine, 20 mg/day (with dose adjustments between 10 mg/day and 30 mg/day allowed in order to optimise outcome). The minimum positive symptoms level was a total score of at least eight for the four BPRS positive symptoms items (conceptual disorganization, hallucinations, unusual thought content, and suspiciousness). The minimum negative symptoms level was a total score on SANS of at least 20.
Volavka 2002 included participants with a history of ‘sub-optimal treatment response’ defined as
-
1)
persistent positive symptoms (hallucinations, delusions, or marked thought disorder) after at least six contiguous weeks of treatment, presented or documented in the past, with one or more typical antipsychotics at doses 600 mg/day as chlorpromazine equivalents.
-
2)
Poor level of functioning over the past two years, defined by the lack of competitive employment or enrolment in an academic or vocational program and not having age-expected interpersonal relationships with someone outside the biological family of origin with whom ongoing regular contacts were maintained.
Azorin 2001 included participants with ‘poor response to previous treatment’, i.e. the patient’s current episode had been treated continually with neuroleptic for at least the preceding 6 month without significant clinical improvement; the patients had undergone one unsuccessful trial of antipsychotic medication equivalent to 20 mg/day of haloperidol for at least six weeks (less if the patient was experiencing dose-limiting adverse events) since the onset of the concurrent episode. If several drugs have been prescribed simultaneously, the final equivalence dosage could be calculated by adding the individual equivalence and when the participants had not experienced a period of good functioning for at least 24 months despite a sufficient period of use of two antipsychotics from at least two chemical classes, or no period of good functioning for five years despite the use of three antipsychotics.
Lin 2003 included participants with partial response to clozapine, not stating the criteria.
Another six studies included participants who were treatment resistant and/or intolerant to treatment, again using various definitions:
Naber 2005 included participants who had failed to respond to at least one antipsychotic other than clozapine and olanzapine or had experienced intolerable side-effects during these prior antipsychotics treatment.
Bondolfi 1998 used a similar definition and considered participants who had previously failed to respond to or be intolerant of at least two different antipsychotic drugs given in appropriate dose for at least four weeks each.
Sacchetti 2006 defined treatment resistance as non response to three adequate trials in the past five years and/or inability to tolerate antipsychotic treatment.
Bitter 2004 included participants who had failed to adequately respond to a standard acceptable treatment with a conventional antipsychotic medication (at least one treatment trial of four to six weeks duration 400 -600 mg chlorpromazine equivalents with either insufficient effectiveness or intolerable side-effects caused by the medication).
Lindenberg 1997 included only participants who had been treated before for at least three weeks, each with two conventional neuroleptic using effective doses, without a satisfactory result or with intolerable side-effects.
Shaw 2006 included participants with ‘failure to respond to two antipsychotic medications’ (typical or atypical) used at adequate doses (>100 mg of chlorpromazine equivalents) and for adequate duration (four weeks unless terminated owing to intolerable adverse effects). ‘Failed’ was defined as insufficient response with persistent symptoms significantly impairing the child’s functioning according to child, parental, medical, and school reports or intolerable adverse effects.
Meltzer 2003 included participants who met the DMS-IV criteria for schizophrenia or schizoaffective disorder and had a high risk for suicide.
The following studies did not require treatment resistance as an inclusion criterion (Atmaca 2003, Heinrich 1994, Krakowski 2006, Li 2002, Li 2003, Liu 2004, Li 2005, Ren 2002, Wang 2002 and Zhou 2000).
Some of the studies additionally considered criteria of inclusion based on minimum scores in BPRS, PANSS, CGI or Intelligence Quotient (IQ) (Azorin 2001, Bitter 2004, Bondolfi 1998, Kumra 2008, Naber 2005, Sacchetti 2006 and Tollefson 2001).
The age of the participants ranged from 7 to 70 years old. Most participants were from 18 to 65 years old; except for two studies (Kumra 2008, Shaw 2006) which included younger people (age from 7 to 18 years old). Overall there were more men than women in the included trials.
3. Setting
Trials took place in a mixture of in patient and outpatient settings. Most of the studies were carried out with inpatients (13 studies: Atmaca 2003, Bitter 2004, Conley 2003, Heinrich 1994,Krakowski 2006, Li 2003, Li 2005, Lin 2003, Liu 2004, Moresco 2004, Shaw 2006, Volavka 2002 and Zhou 2000), followed by studies performed with in and outpatients (six studies: Azorin 2001, Kumra 2008, Li 2002, McGurk 2005, Meltzer 2003 and Wang 2002). Other studies were carried out initially with inpatients, who were later discharged (four studies: Bondolfi 1998, Naber 2005, Tollefson 2001 and Wahlbeck 2000) and finally one study was performed only with outpatients (Ren 2002). Three studies did not report the setting of the participants. Twelve of the twenty-seven studies were multicenter and the participants were recruited in diverse countries including Turkey, Canada, France, Hungary, Switzerland, USA, China, Croatia, South Africa, Italy, United Kingdom, Czech Republic, Argentina, Chile, Germany, Belgium, Denmark, Finland, Norway, Portugal, Spain, and Ireland, leading to a wide ethnic diversity.
4. Study size
In the studies that compared clozapine and olanzapine, the largest and smallest studies were Meltzer 2003 (n=980) and Conley 2003, respectively. The latter was a cross-over study, of which only the first phase was considered (n=13). The largest study that compared clozapine and risperidone was Azorin 2001 (n=273) and the smallest was Wahlbeck 2000, which is a pilot study (n=20). Li 2003 was the largest study that compared clozapine with quetiapine (n= 76) and the smallest was Atmaca 2003 (n=28). The study that compared clozapine versus ziprasidone randomised 146 participants (Sacchetti 2006), and 109 participants were randomised to the comparison of clozapine versus zotepine studies (Lindenberg 1997, Lin 2003), n=50 and n= 59 respectively).
5. Intervention and comparators
The 27 trials administered clozapine in a wide range of doses, from 207 mg/day to 642 mg/day (mean doses range). The ranges of comparator doses were wide, as well. The range of mean olanzapine doses was 16 mg/day to 30 mg/day. Conley 2003 did not state the mean dose used but mentioned an allowed dose range between 30 - 50 mg/day. For quetiapine the mean dose ranged from 362 mg/day to 536 mg/day, and one study reported a dose range from 400 -700 mg daily after the first 10 days (Liu 2004). Risperidone was used in a range from 3.2 mg/day to 12 mg/day. The mean dose of the single ziprasidone arm was 130 mg/day. Regarding clozapine versus zotepine trials, one study used a mean dose of 377 mg/day (Lin 2003) and the other one used doses from 150 to 450 mg/day (Lindenberg 1997).
6. Outcomes
6.1 Death
Death was reported in some studies that compared clozapine with olanzapine (Conley 2003; Meltzer 2003) and clozapine with risperidone (Azorin 2001; Wahlbeck 2000).
6.2 Leaving the study early
Leaving the study early was frequently reported, but in some studies it was not indicated how many participants of each group left early.
6.3 Global state
Improvement on global state was presented as dichotomous data, the criterion used was of ‘at least much improved’ using Clinical Global Impression (CGI) scales. Olanzapine (Naber 2005,Tollefson 2001) and risperidone (Heinrich 1994) trials reported this outcome. Other studies used the criterion as ‘less than common criteria’ (Li 2003) and ‘less than successfully treated and no increase on the Clinical Global Impression - Severity scale (CGI-S)’ (Lin 2003).
6.4 Mental state
6.4.1 Mental state - dichotomous
Our predefined criterion was an at least a 50% reduction of the baseline value of the BPRS or Positive and Negative Syndrome Scale (PANSS). When such a criterion was not available, the primary cut-off presented by the original author was used. Some studies also used combined criteria: at least 20% BPRS total score reduction plus CGI-S < 3 or BPRS < 35 (this was described as at least 20% BPRS reduction and ‘mildly ill or better’ in the comparison and data tables). Kumra 2008 used the criterion of at least 30% BPRS total plus ‘very much’ or ‘much improved’ on CGI. One study (McGurk 2005) that compared clozapine with risperidone used the criterion of at least 40% improvement on the BPRS psychotics cluster (cluster: hallucinations, delusions, suspiciousness and conceptual disorganization).
6.4.2 Mental state - continuous
The PANSS total was used in most studies to examine the participants overall mental state. Specific symptoms were mainly measured by the PANSS positive and negative symptoms subscores. Other studies used the Scale for the Assessment of Positive Symptoms (SAPS) or Scale for the Assessment of Negative Symptoms (SANS) scores, respectively. Only a few studies used the BPRS total score and its subscores. The data were presented as average change or average at endpoint on the score.
6.5 General functioning and social functioning
General functioning and social functioning were reported in only one study that compared clozapine with risperidone (Wahlbeck 2000). These outcomes were measured by Global Assessment of Functioning (GAF) and Social Functioning Scale (SFS) respectively. The average scores at endpoint were presented.
6.6 Quality of Life / satisfaction with treatment
Quality of life / satisfaction with treatment was measured in only one study that compared clozapine with olanzapine (Naber 2005) using the Subjective Well Being Under Neuroleptic Treatment (SWN) and Munich Life Dimension List (MLDL) respectively. Average score at endpoint was reported. Wahlbeck 2000 assessed satisfaction with the treatment by the Drug Attitude Inventory (DAI) and reported the average score at endpoint.
6.7 Cognitive functioning
Cognitive functioning was reported in only one study (Volavka 2002) that compared clozapine with risperidone and with olanzapine. The outcome was evaluated through the number of participants who presented a clinically important improvement in the neurocognitive score defined as a reduction of 0.5 SD on neurocognitive score.
In Volavka 2002 continuous data based on the PANSS cognitive subscore (average at endpoint and average change) were reported as a factor score and the neurocognitive global score at endpoint was reported as a Z score. The Z score was based on the mean and SD of each test (16 cognitive tests) at baseline. Only the variables from participants who completed each test at both baseline and follow-up, were used. This Z score result was the contribution of each test to each of the four domains chosen by the author. These variables were not useful for the analysis of this review.
6.8 Service use
Service use was missing in all studies, except one that reported the number of participants hospitalised due to risk of suicide (Meltzer 2003).
6.9 Adverse effects
Adverse effects were obtained through routine measures, e.g. blood sample, weight measure, ECG, or recorded from the clinical evaluation and spontaneously reports. Few studies used a checklist to report the adverse effects, i.e. validated questionnaires such as the Association for Methodology and Documentation in Psychiatry (AMDP) somatic scale (Heinrich 1994; Tollefson 2001), and the Udvalg for Kliniske Undersgelser (UKU) (Bondolfi 1998; Lindenberg 1997; Lin 2003), Subjective Treatment Emergent Symptoms Scale (STESS) (Shaw 2006; Kumra 2008) and Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) (Bitter 2004; Tollefson 2001). Additionally some researchers developed their own checklist specially for the study. Data were dichotomous as well as continuous.
There were some adverse effect data that could not be examined because comparator data were not reported (continuous data) or the number of patients assessed were not stated (dichotomous data) (Azorin 2001, Li 2002, Sacchetti 2006, Shaw 2006, Tollefson 2001 and Wang 2002).
Extrapyramidal effects were assessed by specific scales such as Abnormal Involuntary Movement Scale (AIMS), Barnes Akathisia Scale (BAS), Simpson Angus Scale (SAS), Hillside akathisia scale (HAS), Extrapyramidal Symptom Rating Scale (ESRS) and by means of a checklist or clinical evaluation of adverse effects.
7. Outcome scales
Details of scales that provided usable data are shown below. Reasons for data exclusion from other instruments are given under “Notes” in the “Characteristics of included studies” tables.
7.1 Global state
Clinical Global Impression Scale - CGI Scale (Guy 1972).
This scale is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardized against other people with the same diagnosis. A seven-point scoring system is usually used with low scores showing a decrease on severity and/or an overall improvement.
7.2 Mental state
7.2.1 Brief Psychiatric Rating Scale - BPRS (Overall 1962)
This scale is used to assess the severity of abnormal mental state. The original scale has 16 items, but a revised 18-item scale is commonly used. Each item is defined on a seven-point scale (0-6 or 1-7) varying from ‘not present’ to ‘extremely severe’. Scores can range from 0 to126, where high scores indicate more severe symptoms.
7.2.2 Positive and Negative Syndrome Scale - PANSS (Kay 1986)
This schizophrenia scale has 30 items, each of which can be defined on a seven-point scoring system varying from one - absent to seven - extreme. This scale can be divided into three subscales for measuring the severity of general psychopathology, positive symptoms (PANSS-P), and negative symptoms (PANSS-N). A low score indicates less severity.
7.2.3 Scale for the Assessment of Positive Symptoms - SAPS (Andreasen 1984)
This instrument covers a specific positive symptoms scale (hallucinations, delusions, thought disorder, bizarre/disorganized behavior and inappropriate affect). It is scored from 0 (not present) to 5 (very frequent) points.
7.2.4 Scale for the Assessment of Negative Symptoms - SANS (Andreasen 1984b)
This six-point scale gives a global rating of the following negative symptoms; alogia, affective blunting, avolition-apathy, anhedonia-asociality and attention impairment. Higher scores indicate more symptoms.
7.3 General Functioning
7.3.1 Global Assessment of Functioning - GAF (APA 1994)
The GAF is a clinician-rated assessment of overall functioning, which considers psychological, social, and occupational functioning on a scale 0-100. Lower scores indicate poorer functioning.
7.3.2 Social Functioning Scale - SFS (Birchwood 1990)
The SFS assesses function areas that are crucial for the community maintenance of individuals with schizophrenia. The seven areas are social engagement/withdrawal, interpersonal behavior, pro-social activities, recreation, independence-competence, independence-performance and employment/occupation. The range of total scores is from 418 (poor) to 944.5 (optimum).
7.4 Quality of Life / treatment satisfaction
7.4.1 Subjective well being under neuroleptic treatment - SWN (Naber 1995)
This scale assesses the subjective effects of antipsychotics, both benefits and burden, from the perspective of the patients. This scale is a self-rating scale, which has 38 items related to the antipsychotic treatment, each of which can be defined on six point scoring. A high score indicates better subjective well-being.
7.4.2 Munich Life Dimension List - MLDL (Heinisch 1991)
The MLDL focuses on the subjective evaluation of the quality of life and comprises 19 areas of life. The scale ranges from zero (very dissatisfied, completely unimportant) to ten (very satisfied, very important).
7.4.3 Drug Attitude Inventory - DAI-10 (Hogan 1983)
This scale is a self-report ten-item scale for assessing patient satisfaction with antipsychotic treatment. Each item is rated one (does not favour drug) or two (favour drug). The range of a total score is 10-20. Higher scores indicate a more favourable attitude towards antipsychotic drug treatment.
7.5 Side-effects
7.5.1 Abnormal Involuntary Movement Scale - AIMS (NIMH 1975)
This scale has been used to assess tardive dyskinesia, a long term, drug-induced movement disorder. The AIMS can also be used to assess some short term movement disorders such as tremor.
7.5.2 Barnes Akathisia Scale - BAS (Barnes 1989)
The scale comprises items rating the observable, restless movements that characterize akathisia, a subjective awareness of restlessness, and any distress associated with the condition. These items are rated from zero - normal to three - severe. In addition, there is an item for rating global severity (from zero - absent to five - severe). A low score indicates low levels of akathisia.
7.5.3 Extrapyramidal Symptom Rating Scale - ESRS (Chouinard 1980)
This scale consists of a questionnaire relating to parkinsonian symptoms (nine items), a physician’s examination for parkinsonism and dyskinetic movements (eight items), and a clinical global impression of tardive dyskinesia. High scores indicate severe levels of movement disorder.
7.5.4 Simpson Angus Scale - SAS (Simpson 1970)
This ten-item scale, with a scoring system from zero to four for each item, measures drug-induced Parkinsonism, a short term drug-induced movement disorder. A low score indicates low levels of Parkinsonism.
7.5.5 Hillside akathisia scale - HAS (Fleischhaker 1989)
Scale comprises two subjective items: inner restlessness and urge to move combined with a division of objective signs in three regional items: axial, upper limbs and lower limbs. Each item is rated from zero to four for each item, with separate evaluations for patient sitting, standing and lying. The global evaluation for each item can also be recorded. The full scale allows the assessment of the effect of activation; global impression items for severity and improvements under treatment are also provided with a scoring system of zero - seven points.
8. Missing outcomes
In general there were the following missing outcomes: global functioning, quality of life, cognitive functioning, service use and mortality. The studies’ principal focus was the response to the treatment, leaving the study early and adverse effects, which were not always adequately documented.
Excluded studies
We excluded 189 studies. Thirty-four were not randomised trials, including seven naturalistic, nine open label studies and one naturalistic/open label trial. One-hundred-and-thirty-one were randomised trials but the blindness was unclear. Ten were not clinical trials but rather reviews or observational studies. In another four studies the allocation was unclear. Other studies were excluded because participants or interventions did not fulfil the review criteria (three and five studies respectively). Finally, the CATIE (CATIE) and the CUtLASS (CUTLASS) studies were excluded, the first one because the clozapine trial was an open one concerning the clozapine arm. The second study was excluded because the analysis compared clozapine versus the other newer antipsychotics pooled.
Awaiting assessment
The ten studies in this category were mostly conference abstracts for which the data reported were not sufficient to decide if they fulfil the criteria to be selected. When it was possible, we contacted the relevant sources and we are waiting for some answers.
Ongoing studies
To the best of our knowledge, there are no ongoing studies during the period of the search.
Risk of bias in included studies
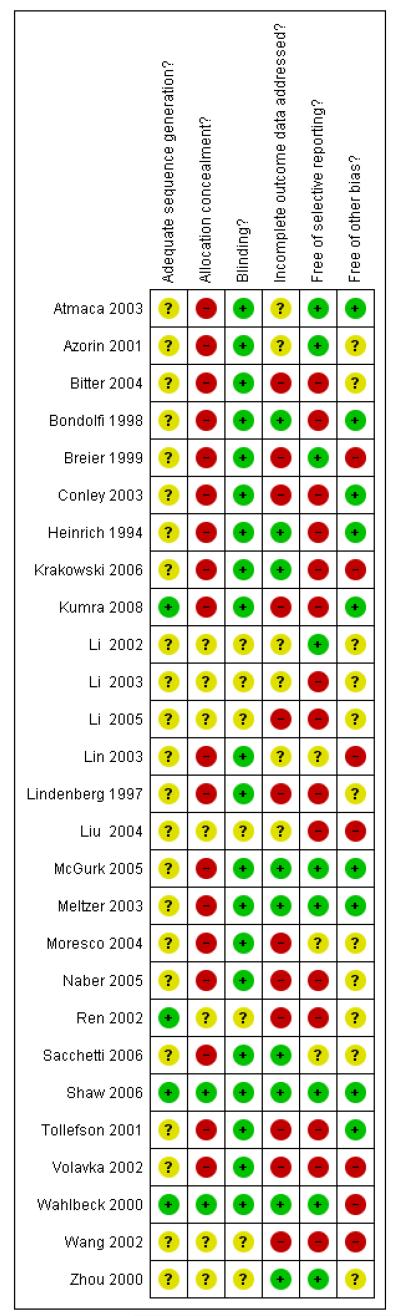
Judgement of risks are illustrated in Figure 1
Figure 1. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
Details regarding randomisation methods as well as sequence generation and allocation concealment, were largely missing. This made it difficult to judge the risk of bias. All studies included were stated to be randomised. From 27 only eight presented some details about the sequence generation, allocation concealment and restriction. Shaw 2006 used a random numbers chart and was conducted in blocks of four, numbered containers were used to implement the allocation sequence. The pharmaceutical development service generated the allocation sequence. Also Kumra 2008 and Wahlbeck 2000 stated that participants were assigned to treatment by computer generated randomisation. The following studies made some statements on restriction: Naber 2005 and Tollefson 2001 stated that they used a 1:1 allocation scheme. Krakowski 2006 used a block randomisation scheme with a block size of three and no baseline stratification. Meltzer 2003 randomised in a 1:1 ratio within blocks of four participants from each medical centre (67 medical centres). Azorin 2001 stated that the allocation was balanced by country, with block size of six. The rest of the studies did not present further details.
Blinding
Twenty studies were double-blind and seven were stated to be single-blind (rater-blinded). Some of the 27 studies gave details about people who were blinded (e.g. psychiatrics, raters, patients, nurses, investigator) (Atmaca 2003, Breier 1999, Krakowski 2006, Meltzer 2003, Moresco 2004, Shaw 2006 and Volavka 2002). Only Meltzer 2003 reported that an external entity regularly monitored the masking of the raters, however the monitoring methods were not explained. No study formally assessed the effectiveness of blinding.
Incomplete outcome data
Most findings were presented in graphs and tables. Some results were described in the text, but often these reports were incomplete (lack of case numbers or standard deviations). Descriptive statistics and statistical test were used to show similarity and equilibrium between the treatment groups and their characteristics at baseline. Often incomplete outcome data were not correctly addressed, because intention-to-treat analysis were not always applied.
Most studies reported the loss of follow up indicating the specific reason of the dropout. However, not all studies considered these data in their analysis. Intention-to-treat analysis (ITT) was applied by Azorin 2001, Bitter 2004, Bondolfi 1998, Heinrich 1994, Krakowski 2006, Kumra 2008, Meltzer 2003, Naber 2005,Sacchetti 2006, Shaw 2006 and Wahlbeck 2000. The use of this analysis was unclear in Azorin 2001 and Naber 2005 because not all randomised participants were included. Azorin 2001 established as ITT criteria all participants with at least one BPRS evaluation after treatment initiation and Naber 2005 considered as ITT population all participants with a baseline and at least one post baseline value.
Missing values were replaced by the last observed value (LOCF last observation carried forward) in Azorin 2001, Bitter 2004,Bondolfi 1998, Conley 2003, Heinrich 1994, Naber 2005,Sacchetti 2006, Shaw 2006, Tollefson 2001 and Wahlbeck 2000.
Selective reporting
Many authors reported only adverse events that occurred in at least 5% of the participants. This procedure could miss rare but important adverse events.
There is some evidence that pharmaceutical companies sometimes highlight the benefits of their compounds and tend to suppress their disadvantages (Heres 2006). Data on sponsoring of six studies were not available.
Other potential sources of bias
Eight studies were sponsored by pharmaceutical companies (Azorin 2001, Bitter 2004, Bondolfi 1998, Krakowski 2006,Lindenberg 1997, Meltzer 2003, Moresco 2004 and Naber 2005) and in Breier 1999 and Tollefson 2001 authors work for a pharmaceutical company. From eight studies, six studies did not declare if the study methods and data analysis were performed independently of the sponsor. Two of the eight studies were supported by pharmaceutical companies marketing clozapine (Azorin 2001 and Meltzer 2003), five trials were supported by pharmaceutical companies marketing the comparator substances and only one was supported by pharmaceutical companies of both clozapine and its comparator (Bondolfi 1998). Pharmaceutical companies have an inevitable conflict of interest which may well lead to bias (Heres 2006 and Leucht 2008).
Effects of interventions
1. Comparison 1. CLOZAPINE versus OLANZAPINE
Twelve studies fulfilled the review criteria. Seven were short term studies (Atmaca 2003; Krakowski 2006; Kumra 2008; Moresco 2004; Shaw 2006; Wang 2002) four medium term studies (Bitter 2004; Naber 2005; Tollefson 2001; Volavka 2002) and one was a long term study (Volavka 2002). Most of these studies were performed in America and Europe. Two studies were multinational including participants from Latin American and Africa and another one was performed in China.
1.1 Death
Only two studies reported the mortality during the trials (Conley 2003; Meltzer 2003). Deaths from any reason (1 RCT, n=980, RR 1.50 CI 0.62 to 3.64), natural causes (2 RCTs, n=993, RR 1.40 CI 0.45 to 4.38) and suicide (2 RCTs, n=993, RR 1.67 CI 0.40 to 6.94) were all similarly likely whether allocated to clozapine or olanzapine.
1.2.Leaving the study early: 1. Any reason
Overall a high percentage of attrition for any reason were observed in both groups. The percentage of participants that discontinued the trials was 40% and 38% for clozapine and olanzapine groups, respectively. There was no significant difference between groups (11 RCTs, n=1702, RR 1.04 CI 0.93 to 1.17).
1.3. Leaving the study early: 2. Adverse effects
Leaving the study early due to adverse effects was more common in the clozapine group (10%) than in the olanzapine group (6%). This difference was statistically significant (9 RCTs, n=1674, RR 1.60 CI 1.07 to 2.40, NNT 25 CI 15 to 73).
1.4. Leaving the study early: 3. Inefficacy
Both groups showed similar attrition rates due to inefficacy (5% clozapine and 6% olanzapine) there was no significant difference between groups (10 RCTs, n=1674, RR 0.72 CI 0.40 to 1.30).
Meltzer 2003 found that in the long term clozapine was associated with less attrition due to lack of efficacy than olanzapine (1 RCT, n=980, RR 0.33 CI 0.12 to 0.91, NNT 49 CI 26 to 364).
1.5 Global state
1.5.1 No clinically important change: less than much improved
No clinically important change in global state was defined as the number of people who were not ‘at least much improved’ according to the CGI improvement rating. The frequencies in both groups were similar (clozapine: 61%; olanzapine: 54%) and not statistically significantly different (2 RCTs, n=294, RR 1.13 CI 0.93 to 1.38).
1.5.2 Relapse
Naber 2005 reported that one participant of each group suffered a relapse (2%) during the trial. Hence no significant difference between groups was found (1 RCT, n=114, RR 1.00 CI 0.06 to 15.60).
1.6 Mental state: 1. No clinically important change - various criteria
1.6.1. Less than 20% reduction on BPRS-24 (1-7) total score
The short term study Shaw 2006 found that fewer people taking clozapine (67%) than those taking olanzapine (85%) did not have an improvement on their mental state, however this difference was not statistically significant (1 RCT, n=25, RR 0.79 CI 0.50 to 1.25).
1.6.2 Less than 50% reduction on BPRS-18 (1-7) total score
The short term study Wang 2002 found that 45% of the participants of the clozapine group and 40% of the olanzapine group did not improve as this criterion. There was no significant difference between groups (1 RCT, n=61, RR 1.13 CI 0.63 to 2.03).
1.6.3 Less than 20% reduction on BPRS-24 (1-7) total score and mildly ill or better (short term)
Shaw 2006 reported that 100% of the participants from clozapine group and 92% from olanzapine group did not improve. This difference was not significant (1 RCT, n=25, RR 1.08 CI 0.87 to 1.33).
1.6.4 Less than 20% reduction on BPRS-18 (1-7) total score and mildly ill or better (medium term)
Fifty-four per cent of those participants allocated to clozapine and fifty-three per cent of those allocated to olanzapine did not improve as this criterion. There was no significant difference between groups (2 RCTs, n=327, MD 1.03 CI 0.85 to 1.25).
1.6.5 Less than 30% reduction on BPRS total score and much improved or very much improved
Even though more participants in the olanzapine group (67%) than in the clozapine group (33%) were not improved. There is some suggestion that this difference reached a borderline level of significance (1 RCT, n=39, RR 0.50 CI 0.24 to 1.03).
1.6.6 Less than 50% reduction on PANSS total
Through this criterion, Bitter 2004 and Tollefson 2001 medium term studies indicated that 82% of the people taking clozapine and 81% on olanzapine did not improve. No statistically significant difference between the groups was demonstrated (2 RCTs, n=327, RR 1.00 CI 0.92 to 1.10).
1.7 Mental state: 2a. PANSS total score
A trend in favour of olanzapine was observed at the short to medium terms, however this difference was not statistically significant (7 RCTs, n=618, MD 1.97 CI −0.71 to 4.66).
1.8 Mental state: 2b. BPRS-18 (1-7) total score
The pooled analysis showed a trend in favour of olanzapine at the short to medium terms, but this difference did not reach the conventional levels of significance (5 RCTs, n=304, MD 1.31 CI −0.30 to 2.92).
1.9 Mental state: 2c. BPRS total score (various version)
Since different versions of the BPRS score were used we present the results of the single studies separately.
1.9.1 BPRS-24 l
One short term study (Shaw 2006) assessed the mental state using the BPRS-24 (1-7) total score. The clozapine group presented a greater reduction from baseline to endpoint than the olanzapine group, but this difference was not significant (1 RCT, n=25, MD −7.00 CI −28.47 to 14.47).
1.9.2 BPRS-18 (0-6)
In one medium term study (Naber 2005) olanzapine produced a greater reduction on average change on BPRS-18 (0-6) total score compared to clozapine, however this difference was not significant (1 RCT, n=108, MD 2.80 CI −4.05 to 9.65).
1.10 Mental state: 3a. Positive symptoms: PANSS positive subscore
Two short term study and four medium term studies assessed the positive symptoms by the PANSS positive subscore. The results did not indicate that one drug was clearly more efficacious than the other one (6 RCTs, n=592, MD 0.08 CI −0.96 to 1.11).
1.11 Mental state: 3b. Positive symptoms: SAPS
One short term study Shaw 2006 found a greater SAPS decrease in the clozapine group. However this difference was not statistically significant (1 RCT, n=25, MD −9.00 CI −22.06 to 4.06).
1.12 Mental state: 3c. Positive symptoms: BPRS-18 positive subscore
1.12.1 BPRS-18 (1-7)
Positive symptoms, measured by the BPRS18 (1-7) positive subscore, were not significantly different between groups (2 RCTs, n= 189, MD −0.01 CI −1.39 to 1.37).
1.12.2 BPRS-18 (0-6)
There was no significant difference (1 RCT, n=108, MD 0.40 CI −1.57 to 2.37).
1.13 Mental state: 4a. Negative symptoms: PANSS negative subscore
There was no indication of any significant superiority of clozapine or olanzapine in this outcome (6 RCTs, n=592, MD 0.78 CI −0.21 to 1.77).
1.14 Mental state: 4b. Negative symptoms: SANS
Two short term studies assessed the negative symptoms using the SANS and the results were heterogeneous. Kumra 2008 found a trend in favour of clozapine, but this difference was not statistically significant (2 RCTs, n=39, MD −1.00 CI −3.6 to 1.6). Furthermore, in Shaw 2006 clozapine reduced the mean SANS score more than the olanzapine group (1 RCT, n=25, MD −11.00 CI −20.90 to −1.10).
1.15 Mental state: 4c. Negative symptoms: BPRS-18 negative subscore
1.15.1 BPRS-18 (1-7) negative sub-score
There was no significant difference between groups (2 RCTs, n= 189, MD −0.29 CI −1.17 to 0.60).
1.15.2 BPRS-18 (0-6) negative sub-score
One medium term study (Naber 2005) analysed this outcome but did not find a significant difference between groups (1 RCT, n= 108, MD 0.20 CI −1.29 to 1.69).
1.16 Cognitive functioning: 1. No clinically important change less than 0.5 SD reduction
Most studies did not report data about cognitive function of participants. One medium term study (Volavka 2002) defined no clinically important response as ‘less than 0.5 SD reduction on global neurocognitive score’. More people taking clozapine (80%) than people taking olanzapine (49%) met this criterion, a statistically significant difference was found (1 RCT, n=79, RR 1.64 CI 1.15 to 2.35, NNT 3 CI 2 to 9).
1.17 Quality of life: 1. SWN-38 score
In one medium term study there was no significant difference between clozapine and olanzapine in the SWN-38 score (1 RCT, n=99, MD −8.20 CI −21.67 to 5.27).
1.18 Quality of life: 2. MLDL score
There was no significant difference in the average change of the MLDL satisfaction scale (1 RCT, n=97, MD 0.00 CI −0.72 to 0.72).
1.19 Service use: Hospital re-admission
Most of studies did not provide data on service use. Only one long term study (Meltzer 2003) reported the hospitalisation for imminent risk of suicide as a rescue intervention. Significantly fewer people taking clozapine (20%) were hospitalised compared to those taking olanzapine (26%)(1 RCT, n=980, RR 0.78 CI 0.62 to 0.98, NNT 18 CI 9 to 230).
1.20 Adverse effects: 1. At least one adverse effect
Due to the high heterogeneity (I-square=81%) we did not perform the meta-analytic combination of data. Five short term studies presented the number of participants that suffered at least one adverse effect. Only one of them, Wang 2002 study, showed a statistically significant difference between treatment groups in favour of olanzapine (1 RCT, n=61, RR 3.39 CI 1.85 to 6.20, NNH 2 CI 1 to 2). All the rest reported the same trend in favour olanzapine, but the differences were not significant.
Two homogeneous medium term studies (Bitter 2004 and Naber 2005) reported that people allocated to clozapine (49%) were significantly more susceptible to experience at least one adverse effect than those in the olanzapine group (39%), (2 RCTs, n=261, RR 1.19 CI 1.02 to 1.40, NNH 10 CI 4 to infinity).
1.21 Adverse effects: 2. Cardiac problems
A lack of data about the appearance of cardiac problems was a common factor for almost all studies. Overall, a low incidence of ECG anomalies was observed in both groups.
Four per cent of the clozapine group (3 of 74 participants) showed ECG alterations, in two participants the anomalies were not specified and in the other one the alteration was a QT time prolongation. One per cent in the olanzapine group (1 of 78 participants) presented any ECG alterations. This difference was not significant (3 RCTs, n=152, RR 2.42 CI 0.38 to 15.33).
1.22 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use
A similar proportion of participants from each group (7% clozapine versus 8% olanzapine) required antiparkinson medication during the trials. The combined analysis of the studies did not show any difference between the treatment groups (6 RCTs, n=561, RR 0.87 CI 0.46 to 1.67).
1.23 Adverse effects: 3b. Extrapyramidal: various symptoms
1.23.1 At least one EPS
In a short term study (Wang 2002, n=61) none of the participants experienced ‘at least one EPS’.
1.23.2 Akathisia
Four studies reported the akathisia incidence during the trials. Overall there was no significant difference (4 RCTs, n=1320, RR 0.73 CI 0.38 to 1.41). Only the long term study by Meltzer 2003 found that more people in the olanzapine group (8%) experienced akathisia than those in the clozapine group (4%), (1 RCT, n=980, RR 0.54 CI 0.32 to 0.90, NNH 27 CI 15 to 147).
1.23.3 Dyskinesia
A trend in favour of clozapine was observed which did not reach the conventional level of significance (2 RCTs, n=327, RR 0.53 CI 0.20 to 1.43).
1.23.4 Extrapyramidal symptoms
No extrapyramidal events were reported in Moresco 2004 and Wang 2002 studies.
1.23.5 Parkinsonism
No parkinsonism events were reported in Bitter 2004 study.
1.23.6 Pseudoparkinsonism
There was no significant difference in pseudoparkinsonism symptoms between clozapine and olanzapine (1 RCT, n=180, RR 1.29 CI 0.50 to 3.30).
1.23.7 Rigor
There was no significant difference between groups (1 RCT, n= 980, RR 0.17 CI 0.02 to 1.38).
1.24 Adverse effects: 3c. Extrapyramidal: ESRS total score at end-point
A medium term study (Volavka 2002) found no significant difference in the ESRS score at endpoint (1 RCT, n=79, MD 1.30, CI −0.23 to 2.83).
1.25 Adverse effects: 3d. Extrapyramidal: SAS change or endpoint
Three short term studies and three medium term studies reported on this outcome. Overall, there was not significant difference between groups (6 RCTs, n=481, MD 0.43 CI −0.45 to 1.30).
1.26 Adverse effects: 3e. Extrapyramidal: akathisia - BARS change
A medium term study (Tollefson 2001) assessed akathisia by the BARS change from baseline to endpoint. There was no significant difference between groups (1 RCT, n=175, MD −0.10 CI −0.38 to 0.18).
1.27 Adverse effects: 3f. Extrapyramidal: Hillside Akathisia Scale
There was no significant difference between groups (1 RCT, n= 137, MD −0.40 CI −3.10 to 2.3).
1.28 Adverse effects: 3g. Extrapyramidal: tardive dyskinesia - AIMS change or endpoint
There was no significant difference between groups in one short term and two medium term studies (3 RCTs, n=352, MD 0.13, CI −0.25 to 0.51).
1.29 Adverse effects: 4a. Glucose: number of participants with significant increase
One long term study (Meltzer 2003) indicated the number of participants with significant increase of glucose levels during the study. Three per cent and four per cent of participants had an elevation of glucose levels taking clozapine and olanzapine, respectively. Hence no significant difference between clozapine and olanzapine groups was found (1 RCT, n=980, RR 0.76 CI 0.40 to 1.44).
1.30 Adverse effects: 4b. Glucose: average change or endpoint
Two short term studies showed an advantage of olanzapine (2 RCTs, n= 50, MD 9.70 CI 1.73 to 17.68). The medium term study reported an opposite result but the difference was not statistically significant (1 RCT, n=39, MD −9.9 CI −23.30 to 3.50).
1.31 Adverse effects: 5. Hypersalivation
Five studies reported that hypersalivation was more frequent in participants taking clozapine than those taking olanzapine in the short term (2 RCTs, n=64, RR 1.64 CI 1.14 to 2.38, NNH 3 CI 2 to 9), in the medium term (2 RCTs, n=289, RR 5.33 CI 1.76 to 16.68, NNH 3 CI 2 to 4), as well as in the long term (1 RCT, n=980, RR 8.18 CI 5.64 to 11.86, NNH 2, CI 2 to 3).
1.32 Adverse effects: 6a. Lipids: number of participants with significant increase
1.32.1 Increase on cholesterol total
A short term study (Shaw 2006) reported that one of twelve in clozapine group and none in the olanzapine group presented moderate hypercholesterolaemia during the trial. No significant difference between the groups was found (1 RCT, n=25, RR 3.23 CI 0.14 to 72.46).
1.32.2 Increase on triglycerides
Two short term studies provided the number of participants that presented an increase on triglycerides during the study. 17% in clozapine group and 15% in olanzapine group showed an increase on triglyceride levels, but this difference was not significant (2 RCTs, n=64, RR 1.08 CI 0.37 to 3.20).
1.33 Adverse effects:6b. Lipids: average cholesterol change or end-point
Two short term and one medium term study assessed cholesterol total levels. The two short term studies were heterogeneous showing an opposite tendency. The combined analysis of all three studies did not demonstrate any clear difference between groups (3 RCTs, n=89, MD −1.16 CI −19.85 to 17.52).
1.34 Adverse effects: 6c. Lipids: average triglycerides change
Two short term studies provided data regarding triglyceride levels. No significant difference between clozapine and olanzapine was found (2 RCTs, n=38, MD 36.07 CI −83.57 to 155.71).
1.35 Adverse effects: 7. Prolactin: average change or endpoint
1.35.1 Average change from baseline
A medium term study (Tollefson 2001) assessed prolactin levels (ng /mL). A statistically significant difference was observed between clozapine and olanzapine groups, where clozapine group showed a slightly decrease while olanzapine group had a mild increase on prolactin levels (1 RCT, n=120, MD −0.57 CI −1.05 to −0.09).
1.35.2 Average endpoint ng/ml - men only
Two heterogeneous studies (Kumra 2008 and Volavka 2002) assessed on prolactin levels in men, only one of them (Kumra 2008) showed a significant superiority of clozapine (1 RCT, n=21, MD −14.20 CI −23.8 to −5.32).
1.35.3 Average endpoint ng/ml - women only
The same study reported on prolactin levels in women. Again, the results were in favour of clozapine (1 RCT, n=18, MD −54.4, CI −86.74 to −22.06).
1.36 Adverse effects: 8. Sedation
Seven studies reported the number of participants that experienced sedation. All studies observed a tendency in favour of olanzapine. Due to high degrees of heterogeneity we did not perform a meta-analysis (I-square= 88%). Four heterogeneous short term studies reported a trend in favour of olanzapine. In Wang 2002 this difference was significant (1 RCT, n=61, RR 3.06 CI 1.42 to 6.61, NNH 3 CI 2 to 7). The studies by Shaw 2006 and Kumra 2008 did not find any significant difference (1 RCT, n=25, RR 1.08 CI 0.18 to 6.53 and 1 RCT, n=39, RR 1.10 CI 0.93 to 1.30, respectively), while in Conley 2003 study all participants experienced sedation. Two medium term studies showed the same trend in favour of olanzapine, but only Bitter 2004 reported a significant difference between groups (1 RCT, n=147, RR 5.73 CI 1.32 to 24.96, NNH 8 CI 5 to 28). The single long term study (Meltzer 2003) found a significant superiority of olanzapine in this regard (1 RCT, n=490, RR 1.86 CI 1.55 to 2.24, NNH 5 CI 4 to 7).
1.37 Adverse effects: 9. Seizures
Four studies (two short, one medium and one long term) assessed this outcome. Clozapine group participants were more likely to experience seizures than olanzapine participants (3% versus 0.4%; 4 RCTs, n=1097, RR 6.50 CI 1.73 to 24.47, NNH 39 CI 25 to 94).
1.38 Adverse effects: 10a. Weight: number of participant with weight gain
More than 50% of the studies reported the number of participants that presented a weight increase. These studies were highly heterogeneous and a meta-analysis was not undertaken.
1.38.1 Short term
20% of the participants in each group reported a weight increase according to various criteria (Wang 2002 study did not specify the criterion, Kumra 2008 used the criterion more than 7% increase of the baseline body weight), therefore there was no difference between groups (2 RCTs, n=100, RR 0.99 CI 0.45 to 2.16).
1.38.2 Medium term
Four medium term studies reported this outcome. There was no clear difference between groups (4 RCTs, n=520, RR1.03 CI 0.60 to 1.78).
1.38.3 Long term
The only long term study (Meltzer 2003) showed a significant superiority in favour of olanzapine (30% clozapine versus 52% olanzapine; 1 RCT, n=980, RR 0.57 CI 0.48 to 0.66, NNH 4 CI 3 to 6).
1.39 Adverse effects: 10b. Weight: average weight change
Three short term and four medium term studies reported on average weight change in kg. There was no significant difference between groups (7 RCTs, n=581, MD −0.04 CI −1.06 to 0.97).
1.40 Adverse effects: 11. White blood cell count: number of participants with a decrease
Four studies (one short term, two medium term and one long term) reported a higher number of participants with a decrease of the WBC in the clozapine group (6%) than in the olanzapine group (1%). This difference was statistically significant (4 RCTs, n=1264, RR 5.68 CI 2.48 to 13.00, NNH 20, CI 14 to 33).
1.41. Publication bias
Due to the small number of included studies a funnel plot analysis was not performed.
1.42. Missing outcomes
No data were reported for general and social functioning.
2. CLOZAPINE versus OLANZAPINE Sensitivity analysis
When studies with possibly skewed data were excluded the following changes were noted: Excluding Kumra 2008 from the analysis of the SANS total score clozapine was significantly more efficacious than olanzapine (1 RCT, n=25, MD −11.00 CI −20.90 to −1.10). The results of other sensitivity analyses on the PANSS total score (excluded study: Moresco 2004), the BPRS-18 (1-7) total score (excluded studies: Kumra 2008,Wang 2002), the PANSS positive subscore (excluded study: Moresco 2004) and the BPRS positive subscore (Excluded studies:Tollefson 2001) did not change to an important degree.
3. Comparison 2. CLOZAPINE versus QUETIAPINE
From five studies selected, four were carried out in China (Li 2002; Li 2003; Li 2005; Liu 2004) and one in Turkey (Atmaca 2003).
All of them were short term studies.
3.1 Leaving the studies early
Only three studies reported this outcome.
3.1.1 any reason
More people allocated to clozapine (11%) left the study early for any reason in comparison with those allocated of quetiapine (6%), however this difference was not statistically significant (2 RCTs, n=94, RR 1.51 CI 0.42 to 5.50).
3.1.2 adverse effects
Eight per cent of the participants taking clozapine and none of those taking quetiapine left the study early due to adverse effects, but this difference was not significant (1 RCT, n=72, RR 7.0 CI 0.37 to 130.82).
3.1.3 inefficacy
No participant left a study early for this reason.
3.2 Global state: no clinically important change - less than “common criteria”
One study reported the number of participants showing no clinically important change in global state according to ‘common criteria’. The author did not give further details about this criterion. There was no significant difference between groups (1 RCT, n= 76, RR 1.07 CI 0.85 to 1.35).
3.3 Mental state: 1. No clinically important change - less than 50% reduction PANSS total
Only one study (Li 2002) reported this outcome. 32% of the participants in the clozapine group and 34% in the quetiapine group did not improve. There was no significant difference between both antipsychotics (1 RCT, n=63, RR 0.94 CI 0.47 to 1.89).
3.4 Mental state: 2a. PANSS total score
Four studies reported the average PANSS total score at endpoint. There was no significant difference between clozapine and quetiapine in this regard (4 RCTs, n=232, MD 0.50 CI −1.86 to 2.85).
3.5 Mental state: 2b. BPRS-18 (1-7) total score
There was no statistical significant difference in this outcome (1 RCT, n=72, MD 0.89 CI −1.33 to 3.11).
3.6 Mental state: 3. Positive symptoms: PANSS positive subscore
There was no evidence of a significant difference between clozapine and quetiapine in this aspect (2 RCTs, n=142, MD 0.70 CI −0.68 to 2.07).
3.7 Mental state: 4. No clinically important change - less than 50% reduction SANS
One study defined no important clinical response on negative symptoms as the number of people of each treatment group who did not present ‘at least a 50% reduction on SANS’. 89% of those taking clozapine and 83% of those allocated to quetiapine did not improve as this criterion. This difference was not significant (1 RCT, n=72, RR 1.07 CI 0.89 to 1.29).
3.8 Mental state: 5a. Negative symptoms: PANSS negative subscore
People taking clozapine had a higher score on the PANSS negative subscore than those allocated to quetiapine. A statistically significant superiority of quetiapine over clozapine was found (2 RCTs, n=142, MD 2.23 CI 0.99 to 3.48).
3.9 Mental state: 5b. Negative symptoms:SANS
The study that used SANS score to assess the negative symptoms, showed a trend in favour quetiapine, however this difference did not reach the conventional levels of significance (1 RCT, n=72, MD 1.64 CI −4.66 to 7.94).
3.10 Adverse effects: 1. At least one adverse effect
In Li 2002 significantly more participants allocated to clozapine (90%) than to quetiapine (38%) experienced at least one adverse effect (1 RCT, n= 63, RR 2.41 CI 1.52 to 3.82, NNH 2 CI 2 to 5).
3.11 Adverse effects: 2. Cardiac problems
The incidence of cardiac effects was missing in almost all studies. Overall, in the two studies that reported this outcome the incidence was low.
3.11.1 ECG abnormalities
Liu 2004 showed that those taking clozapine were significantly more likely to experience ECG abnormalities than those allocated to quetiapine (1 RCT, n=72, RR 8.00 CI 1.05 to 60.72, NNH 5 CI 3 to 21), but this outcome must be interpreted with caution due to the large confidence interval.
3.11.2 Palpitation
Li 2002, reported the incidence of palpitation (45% clozapine versus 38% quetiapine). There was no significant difference in this regard (1 RCT, n=63, RR 1.20 CI 0.67 to 2.18).
3.12 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use
In Atmaca 2003 no participants (of 27) used antiparkinson medication.
3.13 Adverse effects: 3b. Extrapyramidal: various symptoms
3.13.1 akathisia
There was no significant difference (2 RCTs, n=135, RR 2.52 CI 0.50 to 12.61).
3.13.2 tremor
There was no significant difference (2 RCTs, n=135, RR 1.01 CI 0.30 to 3.43).
3.13.3 rigor
There was no significant difference (1 RCT, n=63, RR 0.52 CI 0.05 to 5.41).
3.14 Adverse effects: 4. Hypersalivation
In two studies significantly more people taking clozapine (76%) experienced hypersalivation than those taking quetiapine (1%), (2 RCTs, n=135, RR 33.91 CI 6.96 to 165.24, NNH 1 CI 1 to 2). However, this outcome must be interpreted with caution due to the large confidence interval.
3.15 Adverse effects: 5. Lipids: average triglyceride change
Clozapine group presented a significantly more pronounced increase in triglycerides levels than quetiapine (n=27, MD 24.64 CI 20.76 to 28.52).
3.16 Adverse effects: 6. Sedation
People allocated to clozapine (48%) were significantly more likely to suffer sedation than those allocated to quetiapine group (10%), (2 RCTs, n= 135, RR 4.47 CI 2.11 to 9.49, NNH 3 CI 2 to 4).
3.17 Adverse effects: 7a. Weight: number of participant with weight gain
The combined analysis of two studies suggested that weight increase was more common with clozapine than quetiapine (25% clozapine versus 13% quetiapine), however this difference was not significant (2 RCTs, n=135, RR 1.89 CI 0.90 to 3.96).
3.18 Adverse effects: 7b. Weight: average weight change
One study showed a trend in favour quetiapine regarding weight increase, but this difference did not reach conventional levels of statistical significance (1 RCT, n=27, MD 2.11 CI −0.08 to 4.30).
3.19 Adverse effects: 8. White blood cell count: number of participants with a decrease
Decrease of white blood cells (WBC) was evaluated in the Li 2002 study, where those taking clozapine (6%) were more likely to experience decrease in WBC (criterion not stated) than those allocated to quetiapine (0%). However, no statistically significant difference was found between groups (1 RCT, n=63, RR 5.16 CI 0.26 to 103.27).
3.20 Publication bias
Due to the small number of included studies a funnel plot analysis was not performed.
3.21 Missing outcomes
No data were reported for death, service use, general and social functioning, cognitive functioning, quality of life/satisfaction with treatment and some adverse effects such as increase glucose levels, increase triglycerides, increase prolactin levels and seizures.
4. CLOZAPINE versus QUETIAPINE sensitivity analysis
The results of the comparison of clozapine with quetiapine regarding the average on PANSS total score at endpoint did not change when studies with possible skewed data were excluded (Li 2002,Li 2003 and Li 2005).
5. Comparison 3. CLOZAPINE versus RISPERIDONE
Ten studies were selected according to the criterion of this review. Eight were short term studies (Atmaca 2003; Azorin 2001; Bondolfi 1998; Breier 1999; Heinrich 1994; Ren 2002; Wahlbeck 2000; Zhou 2000), one medium term (Volavka 2002) and one long term (McGurk 2005). Most studies were carried out in Europe and America and two were performed in China.
5.1 Death
Only two short term studies reported on death. There were no reports of suicides (Wahlbeck 2000). Regarding death due to natural causes, no difference was found between clozapine and risperidone (2 RCTs, n=293, RR 0.98 CI 0.06 to 15.48).
5.2. Leaving the study early: 1. Any reason
There was a similar number of participants leaving the studies early due to any reason (32% clozapine versus 35% risperidone, 7 RCTs, n=655, RR 0.92 CI 0.73 to 1.16).
5.3 Leaving the study early: 2. Adverse effects
Twelve per cent of the participants in treatment with clozapine and six per cent of those in treatment with risperidone left the study early due to adverse effects. A statistically significant difference between groups was observed (6 RCTs, n=627, RR 1.88 CI 1.11 to 3.21, NNT 16 CI 9 to 59).
5.4 Leaving the study early: 3. Inefficacy
In contrast, fewer people allocated to clozapine left the studies early due to inefficacy (5% versus 13% respectively). This difference was again statistically significant (6 RCTs, n=627, RR 0.40 CI 0.23 to 0.70, NNT 11 CI 7 to 21).
5.5 Global state: No clinically important change - less than much improved on CGI
The number of participants with no clinical improvement were all those categorized as ‘less than much improved’ on the CGI. A single, small, short term study (Heinrich 1994) found no significant difference in this regard (1 RCT, n=60, RR 0.80 CI 0.43 to 1.49).
5.6 Mental state: 1. No clinically important change - various criteria
Various criteria were used to assess an improvement of the mental state.
5.6.1 less than 20% reduction on BPRS-18 (1-7) total score
One short term study, Breier 1999, reported that 64% of the participants taking clozapine and 80% of those taking risperidone did not improve at this criterion. No statistically significant difference was observed between groups (1 RCT, n=29, RR 0.80 CI 0.50 to 1.28).
5.6.2 less than 20% reduction on BPRS-18 (1-7) total score and mildly ill or better
No significant difference was found between clozapine and risperidone in Azorin 2001 study (1 RCT, n=273, RR 0.95 CI 0.78 to 1.17).
5.6.3 less than 20% reduction on the 4-item on BPRS psychosis and no psychotic symptoms rated less than mild
This criterion was used to define ‘remission’ during the McGurk 2005 study. No significant difference between groups was observed (1 RCT, n=107, RR 0.97 CI 0.78 to 1.21).
5.6.4 less than 40% improvement on the 4-item on BPRS psychosis cluster
There was no significant difference between groups (1 RCT, n= 107, RR 0.96 CI 0.69 to 1.32).
5.6.5 less than 20% reduction on PANSS total score
There was no significant difference between clozapine (39% not improved) and risperidone (33% not improved) (2 RCTs, n=106, RR 1.18 CI 0.70 to 1.99).
5.7 Mental state: 2a. PANSS total score
Four short term studies and one medium term study provided data, but we did not combine the data due to significant heterogeneity (I-square=52%).
The short term Azorin 2001 study was the only study that showed a statistically significant difference in favour of clozapine (n=273, MD −7.60, CI −13.28 to −1.92).
Atmaca 2003 and Volavka 2002 suggested a better improvement on mental state using clozapine than risperidone, but these differences did not reach the conventional significance level (n=26, MD −1.20 CI −5.01 to 2.61 and n=81, MD −3.60 CI −13.32 to 6.12 respectively).
Contrarily, the short term Bondolfi 1998 study and Wahlbeck 2000 showed a trend in favour of risperidone, but both differences were not significant (n=86, MD 4.20 CI −5.34 to 13.74 and n= 19, MD13.00 CI −4.59 to 30.59 respectively).
5.8 Mental state: 2b. BPRS-18 (1-7) total score
Three heterogeneous studies (I-square=80%) provided data on the BPRS-18 (1-7) total score. Two homogeneous short term studies (Azorin 2001 and Breier 1999) showed a statistically significant difference in favour of clozapine (2 RCTs, n=285, MD −5.11 CI −7.99 to −2.23). The long term study, McGurk 2005, did not show any clear difference between drugs (1 RCT, n=52, MD 0.40 CI −3.52 to 4.32).
5.9 Mental state: 3a. Positive symptoms: PANSS positive subscore
There was no clear difference between clozapine and risperidone (5 RCTs, n=562, MD −0.99 CI −2.29 to 0.32).
5.10 Mental state: 3b. Positive symptoms: BPRS-18 (1-7) positive subscore
The short term Breier 1999 study showed a trend in favour of clozapine on BPRS positive subscore at endpoint, but this difference was not significant (1 RCT, n=29, MD −2.10 CI −4.76 to 0.56).
5.11 Mental state: 4a. Negative symptoms: PANSS negative subscore
Five studies reported heterogeneous results (I-square= 61%), only one of them (Wahlbeck 2000) showed a significant difference between groups in favour of risperidone (1 RCT, n=19, MD 4.00 CI 0.40 to 7.60).
5.12 Mental scale: 4b. Negative symptoms: SANS
The meta-analysis of two short term studies found no significant difference between clozapine or risperidone (2 RCTs, n=69, MD 0.62 CI −2.51 to 3.74).
5.13 General functioning: 1. GAF score
Only the short term study by Wahlbeck 2000 reported on this outcome and found no significant difference between groups (1 RCT, n=19, MD −9.00 CI −18.44 to 0.44).
5.14 Social functioning: SFS score
Again, only Wahlbeck 2000 examined social functioning and found a higher (= better) average SFS score at endpoint in the risperidone group. This result should be considered with caution due to the great variability of the scores.
5.15 Treatment satisfaction: DAI score
In the short term Wahlbeck 2000 study there was no significant difference between clozapine and risperidone regarding treatment satisfaction (1 RCT, n=19, MD 0.10 CI −2.57 to 2.77).
5.16 Cognitive Functioning: no clinically important change - less than 0.5 SD improved
The medium term study by Volavka 2002 defined ‘important clinical response in cognition’ as those who presented an ‘at least 0.5 SD reduction in neurocognitive score’. A trend in favour of risperidone was observed but this difference did not reach the conventional levels of significance (1 RCT, n=81, RR 1.26 CI 0.95 to 1.67).
5.17 Adverse effects: 1. At least one adverse effect
The proportion of participants experiencing ‘at least one adverse effect’ was reported by two short term studies. The combined analysis was impossible to perform due to the heterogeneity amongst them (I-square= 83%).
Azorin 2001 showed similar proportion of participants that experience at least one adverse effect (78% clozapine and 82% risperidone). No significant difference was observed between groups (1 RCT, n=273, RR 0.94 CI 0.84 to 1.06).
Heinrich 1994 compared one clozapine group with two risperidone groups (two different doses). To analyse this study the data of the two groups of risperidone were pooled. Significantly more people allocated to clozapine reported at least one adverse effect (75%) than those taking risperidone (48%) (1 RCT, n=60, RR 1.58 CI 1.05 to 2.39, NNH 4 CI 2 to 33).
5.18 Adverse effects: 2. Cardiac problems
Data cardiac effects were missing in most studies. The short term Heinrich 1994 study mentioned that only one participant of the risperidone group presented ECG abnormalities. There was no significant difference between groups (1 RCT, n=60, RR 0.65 CI 0.03 to 15.30). The long term McGurk 2005 study assessed the presence of myocarditis, but found that no participants experienced this cardiac alteration during the study. The combined analysis showed no significant difference between groups (2 RCTs, n= 167, RR 0.65 CI 0.03 to 15.30).
5.19 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use
Most studies reported on the use of antiparkinson medication. The meta-analysis of four short term studies and one medium term study revealed that the participants taking clozapine (13 from 142 participant) were less likely to use antiparkinson medication than those taking risperidone (37 of 162 participants). There was a statistically significant difference between groups (6 RCTs, n= 304, RR 0.39 CI 0.22 to 0.68, NNH 7 CI 5 to 18).
This tendency persisted over time and it was statistically significant in the combined analysis of the short term subcategory (5 RCTs, n=223, RR 0.39 CI 0.19 to 0.77, NNH 8 CI 5 to 32).
5.20 Adverse effects: 3b. Extrapyramidal: various symptoms
5.20.1 at least one EPS
Two short term studies reported the participants who experienced at least one EPS, but due to heterogeneity (I-square=81%) we analysed them separately. The smaller study Heinrich 1994 did not reveal any significant difference between groups, while Azorin 2001 reported a significant difference in favour of clozapine (1 RCT, n=273, RR 0.46 CI 0.28 to 0.77, NNT 7 CI 4 to 18).
5.20.2 akathisia
The short term Zhou 2000 study reported the incidence of akathisia. Only four out of twenty people in the clozapine group and none of the twenty participants in the risperidone group experienced akathisia. However, this difference was not significant (1 RCT, n=40, RR 9.00 CI 0.52 to 156.91).
5.20.3 dyskinesia
The short term Bondolfi 1998 study reported on the incidence of dyskinesia defined as a score of one or more on ESRS. There was no significant difference between the groups (1 RCT, n=86, RR 1.00 CI 0.38 to 2.61).
5.20.4 dystonia
According to the same criterion, there was no significant difference in the frequency of dystonia (1 RCT, n=86, RR 0.50 CI 0.05 to 5.31).
5.20.5 parkinsonism
Again in Bondolfi 1998 there was a higher risk to have a score of at least one in the parkinsonism item of the ESRS in people taking clozapine than those taking risperidone (63% clozapine versus 40% risperidone, 1 RCT, n=86, RR 1.59 CI 1.03 to 2.45, NNH 4 CI 2 to 37).
5.20.6 tremor
More participants in the clozapine group (40%) experienced tremor during the study than in the risperidone group (20%). There was no indication of superiority of either drug (1 RCT, n= 40, RR 2.00 CI 0.72 to 5.59).
5.21 Adverse effects: 3c. Extrapyramidal: symptom scales
5.21.1 ESRS score
Skewed data of the ESRS score did not show any difference between clozapine and risperidone (1 RCT, n=81, MD 0.30 CI −1.31 to 1.91).
5.21.2 SAS score
Pooled data from two studies (Breier 1999 and Heinrich 1994) did not reveal any significant difference between groups (2 RCTs, n=69, MD −0.81 CI −1.73 to 0.10).
5.21.3 BARS score
The long term McGurk 2005 study assessed akathisia by the BARS scale. No statistically significant difference was found (1 RCT, n= 107, MD −0.20 CI −0.50 to 0.10).
5.22 Adverse effects: 4. Glucose: average change
Both treatments produced an average increase of glucose level at medium term (14 weeks) (clozapine +4 mg/dl versus risperidone +3 mg/dl), but there was no significant difference between groups (1 RCT, n=31, MD 1.70 CI −8.64 to 12.04).
5.23 Adverse effects: 5. Hypersalivation
In the short term those receiving clozapine were significantly more likely to experience hypersalivation than those receiving risperidone (37% clozapine, 10% risperidone; 3 RCTs, n=373, RR 4.38 CI 1.86 to 10.30, NNH 4 CI 3 to 5).
5.24 Adverse effects: 6. Lipids: average change
5.24.1 average cholesterol change
The Volavka 2002 study reported a higher increase of cholesterol levels in the clozapine group than in the risperidone group, but this difference was not significant (1 RCT, n=31, MD 7.10 CI −19.81 to 34.01).
5.24.2 average triglyceride change
The short term Atmaca 2003 study measured the average change of triglyceride levels which was more pronounced in the clozapine group (1 RCT, n=26, MD 32.41 CI 29.26 to 35.46).
5.25. Adverse effects: 7a. Prolactin: associated side effects
Only one short term study (Bondolfi 1998) reported on diminished sexual drive and found no significant difference between clozapine (5%) and risperidone (10%) (1 RCT, n=86, RR 0.50 CI 0.10 to 2.59).
5.26 Adverse effects: 7b. Prolactin: average at endpoint
Two studies found a statistically significant difference in favour of clozapine, but we did not combine the results due to significant heterogeneity (I-square=72%): Breier 1999 (short term, women and men combined: 1 RCT, n= 27, MD −38.50 CI −53.70 to −23.30) and Volavka 2002 (medium term, only men: 1 RCT, n= 28, MD −20, CI −31.81 to −8.19).
5.27 Adverse effects: 8. Sedation
The combined analysis of five short term studies showed that sedation is more common in participants taking clozapine (30%) than those taking risperidone (17%). A significant difference between groups was demonstrated. (5 RCTs, n=479, RR 1.73 CI 1.24 to 2.42, NNH 8 CI 5 to 18).
5.28 Adverse effects: 9. Seizures
In two studies (one short term and one medium term) people taking clozapine were more likely to experience seizures than those in the risperidone group (9% versus 2% respectively: 2 RCTs, n= 354, RR 4.47 CI 1.43 to 14.01, NNH 14, CI 8 to 38).
5.29 Adverse effects: 10a. Weight: number of participants with weight gain
Two short term studies and one medium term study assessed the frequency of participants who reported weight gain. The pooled results were slightly heterogeneous (I-square= 53%) but the trend was the same in all studies.
The short term Bondolfi 1998 study showed that more people in the clozapine group (37%) reported weight gain than those in the risperidone group (23%), but this difference was not significant (1 RCT, n=86, RR 1.60 CI 0.82 to 3.12).
The short term Zhou 2000 study showed that 55% of the participants of the clozapine group and none of the risperidone group participants reported weight gain. This difference was significant (1 RCT, n=40, RR 23 CI 1.45 to 365.61, NNH 2 CI 1 to 3) but should be considered with caution due to the great data variability. In the medium term Volavka 2002 study 18% of participants taking clozapine and 10% of those taking risperidone reported weight increase. This difference was not significant (1 RCT, n= 81, RR 1.79 CI 0.57 to 5.66).
5.30 Adverse effects: 10b. Weight: average weight change
Three short term studies and one medium term study assessed the weight change. Again there was a consistent superiority of risperidone, although the degree of difference varied, leading to significant heterogeneity (I-square=80%).
In the short term studies by Atmaca 2003 (1 RCT, n=26, MD 5.98 CI 4.09 to 7.87) and Azorin 2001 (1 RCT, n=270, MD 2.20, CI 1.28 to 3.12) the superiority of risperidone was statistically significant, while Bondolfi 1998 found no significant difference between groups (1 RCT, n=86, MD 1.60 CI −0.03 to 3.23).
In the medium term study of Volavka 2002 clozapine again produced more weight gain than risperidone (1 RCT, n=77, MD 1.90 CI 0.17 to 3.63).
5.31 Adverse effects: 11. White blood cell count: number of participants with a decrease
Overall it was observed a low incidence on WBC decrease in the treatment groups.
Three percent (4/147) of those treated with clozapine (one person suffered from leucopenia/neutropenia, two reported only neutropenia and one agranulocytosis), and two per cent (3/147) of those treated with risperidone (three people suffered from neutropenia) experienced a decrease in WBC. This difference was not statistically significant (4 RCTs, n=294, RR 1.27 CI 0.33 to 4.99).
5.32 Publication bias
Due to the small number of included studies a funnel plot analysis was not performed.
5.33 Missing outcomes
Data on service use were not reported.
6. CLOZAPINE versus RISPERIDONE - Sensitivity analysis
When the study with potentially skewed data on the BPRS-18 (1-7) total score was excluded, there was still no significant difference between groups according to the remaining two studies (Breier 1999 and McGurk 2005). The remaining sensitivity analysis excluding possibly skewed data (PANSS positive subscore - excluded studies: Ren 2002 and Wahlbeck 2000) and PANSS negative subscore (Excluded study: Ren 2002) did not lead to an important change either.
7. Comparison 4. CLOZAPINE versus ZIPRASIDONE
Only one study (Sacchetti 2006, n=146) fulfilled the criteria of our review, it was a medium term study (18 weeks), published as a poster, which provided data on only a few outcomes.
7.1 Leaving the study early: any reason
Sacchetti 2006 reported on ‘leaving the study early’ due to any reason. 38% of the participants in each group left the study before its end (1 RCT, n=146, RR 1.00 CI 0.66 to 1.51).
7.2 Mental state: PANSS total score
There was no significant difference between clozapine and ziprasidone in the mean change from baseline (1 RCT, n=146, MD 0.50 CI −6.72 to 7.72).
7.3 Adverse effects: Cardiac problems
No participants experienced QT interval prolongation on the ECG during the trial.
7.4 Publication bias
Due to small number of included studies a funnel plot analysis was not performed.
7.5 Investigation of heterogeneity and sensitivity analysis
The reasons for the preplanned sensitivity analysis did not apply.
7.6 Missing outcomes
Data on death, leaving the study early (adverse effects and inefficacy), cognitive functioning, quality of life, service use and relevant adverse effects, other than cardiac effects, were missing.
8. Comparison 5. CLOZAPINE versus ZOTEPINE
Two studies fulfilled the criteria for this review (Lin 2003 and Lindenberg 1997). Lin 2003 was a short term study from Taiwan which has to date only been published as a poster. The Lindenberg 1997 study was from Germany. Only results on leaving the study early could be analysed, because all other outcomes were not analysed on the basis of the randomised participants.
8.1 Leaving the study early: any reason
The Lindenberg 1997 study showed a trend in favour of clozapine in this aspect, but this was not statistically significant (1 RCT, n= 50, RR 0.70 CI 0.32 to 1.54).
8.2 Global state: no clinically important change - less than successfully and no increase on CGI-S
Significantly fewer people allocated to clozapine (1/24) were considered to be not improved than those allocated to zotepine (12/35), (1 RCT, n=59, RR 0.12 CI 0.02 to 0.87, NNT 3 CI 2 to 8).
8.3 Mental state: BPRS-18 total score
The people taking clozapine had a small reduction of the mean BPRS score total, while the mean BPRS in the zotepine group increased. This difference was significant in favour of clozapine (1 RCT, n=59, MD −6.00 CI −9.83 to −2.17).
8.4. Adverse effects: 1. Extrapyramidal: antiparkinson medication use
No people allocated to clozapine used antiparkinson medication, but 13 of 35 participants on the zotepine group used it. A statistical significant difference was found (1 RCT, n=59, RR 0.05 CI 0.00 to 0.86, NNH 3 CI 2 to 5).
8.5 Adverse effects: 2. Prolactin: average change
Risperidone increased the mean prolactin level significantly more than clozapine (1 RCT, n=59, MD −33.40, CI −48.67 to −18.13).
8.6 Publication bias
Due to the small number of included studies a funnel plot analysis was not performed.
8.7 Investigation of heterogeneity and sensitivity analysis
The reasons for the preplanned sensitivity analysis did not apply.
8.8 Missing outcomes
There were no data on death, leaving the study early (adverse effects and inefficacy), cognitive functioning, quality of life, service use, and adverse effects, other than EPS and prolactin levels.
DISCUSSION
Clozapine is an atypical antipsychotic which is thought to be superior to conventional antipsychotic drugs in the treatment of refractory schizophrenia, and it causes fewer movement disorders. Clozapine, however, entails a significant risk of serious blood disorders such as agranulocytosis, which could be potentially fatal. Currently there are a number of newer antipsychotics which have been developed with the purpose to find both a better tolerability profile, and a superior effectiveness.
In the last years the number of randomised clozapine trials has dramatically increased. A previous Cochrane review comparing clozapine with newer atypical antipsychotics medication included 8 studies (Tuunainen 2000). The current review includes 27 randomised controlled trials. Nevertheless, many problems that were identified by the previous review have not been solved.
The number of participants leaving schizophrenia trials prematurely remains high. The overall attrition rate of 30% in the included studies is a threat to the validity of the findings.
Often adverse events were only reported if they had occurred in 5% of the participants or greater. This procedure results in under-reporting of rare but important adverse effects. We suggest it would be better to abandon the rules for reporting of adverse effects and that all adverse events should be reported instead, for example as online supplements that are nowadays made available by most journals.
Most trials provide data on leaving the studies early and overall efficacy. Outcomes that are possibly more important to improve the quality of life such as general functioning or satisfaction with treatment are rarely presented. Authors keep using different criteria for ‘response to treatment’ making comparison difficult, although validated suggestions for the presentation of response to treatment are available (Van Os 2006, Leucht 2005a, and Leucht 2005b).
Twenty of the included trials were categorised as ‘short term’ studies and only two were ‘long term’ studies with a length of more than 26 weeks. Schizophrenia is a chronic, often life-long disorder making more long term studies necessary.
Eight studies were sponsored by pharmaceutical companies producing either clozapine or its comparator drugs. Seven studies did not declare if data and/or implementation of the study were carried out without input from the industry. Due to the inevitable conflict of interest, industry sponsorship is a concern (Heres 2006).
Finally, most studies compared clozapine with olanzapine, risperidone and quetiapine. Fewer randomised controlled trials comparing clozapine with zotepine and ziprasidone are available, and comparisons with amisulpride, aripiprazole and sertindole are missing.
Summary of main results
The review currently includes 27 blinded randomised controlled trials, which involve 3099 participants that compare clozapine versus olanzapine, quetiapine, risperidone, ziprasidone and olanzapine. There were no studies that met the criteria for this review comparing clozapine with amisulpride, aripiprazole or sertindole. Attrition from these studies was high (30.1%), making the interpretation of the results problematic. Clozapine had a higher attrition rate due to adverse effects than olanzapine and risperidone. Contrarily, fewer participants in the clozapine groups left the trials early due to inefficacy than risperidone.
Clozapine was more efficacious than zotepine (and risperidone in one of two rating scales) in improving the participants’ general mental state. However it is necessary to replicate these findings in order to confirm these assertions with more robust evidence. Regarding olanzapine, quetiapine and ziprasidone, no differences were observed on this issue.
There was no significant difference between clozapine, olanzapine and risperidone in terms of positive or negative symptoms of schizophrenia. According to two studies from China, quetiapine was more efficacious for improving negative symptoms than clozapine. One of the most important adverse effects of clozapine is the white blood cell decrease, where it was found that only olanzapine is safer than clozapine in this regard. No differences were observed when it was compared with quetiapine and risperidone. This important side effect was not documented in the comparisons with zotepine and ziprasidone.
More participants of the clozapine group showed hypersalivation and sedation than those on olanzapine, risperidone and quetiapine, and there were more seizures than in people on olanzapine and risperidone. Also clozapine produced an important weight gain not observed with risperidone. Clozapine produced somewhat fewer extrapyramidal side-effects (use of antiparkinson medication) than risperidone and zotepine.
Other differences in adverse effects were less documented and should be replicated, clozapine did not alter prolactin levels in contrast to olanzapine, risperidone and zotepine; compared with quetiapine, clozapine produced a higher incidence of ECG alterations and compared with quetiapine and risperidone, clozapine produce a higher increase of triglyceride levels.
Other findings that should be replicated were that clozapine improved social functioning less than risperidone and fewer participants in the clozapine group had to be hospitalised to avoid suicide attempts in comparison with those from olanzapine group.
It was observed that pharmaceutical companies generally compare newer atypical antipsychotic against a very low dose of clozapine. These doses are lower than those used in the pivotal study of Kane 1988. For this reason, it is not fully clear if this can be included in a sensitivity analysis/meta-regression, because there are only a limited number of studies that use adequate doses. Future research comparing adequate doses are needed.
1. Comparison 1: CLOZAPINE versus OLANZAPINE
1.1 Death
Only two studies assessed this outcome. Data on deaths for any reason as well as for natural causes and suicide were available. A tendency in favour of olanzapine was observed, but these differences were not statistically significant. Hence, it can be concluded that the incidence of death was generally low and similar between treatment groups.
1.2 Leaving the study early: 1. Any reason
Both groups presented a high rate of leaving the studies early for any reason. An overall attrition rate of 39% limits the interpretation of most other findings. The meta-analysis did not show any significant difference between clozapine and olanzapine which may be equally acceptable for individuals with schizophrenia.
1.3 Leaving the study early: 2. Adverse effects
In regard to leaving the study early due to adverse effects, significantly more people taking clozapine left the studies early in comparison with those taking olanzapine, suggesting that olanzapine is tolerated better than clozapine, although the lower limit of the confidence interval is located near the line ‘no difference’. The results of the Bitter 2004, Naber 2005 and Meltzer 2003 studies may have affected our result in this regard due to the low clozapine dose (less 300 mg/day) used in their studies.
1.4 Leaving the study early: 3. Inefficacy
Despite the certain heterogeneity observed on leaving the study due to inefficacy there was a trend in favour of clozapine which did not reach a conventional level of statistical significance. The heterogeneity could be due to Naber 2005, which, unlike other studies, reported a higher attrition rate in the clozapine group than in the olanzapine group.
1.5 Global state
This analysis was based on two medium term studies not finding a significant difference between clozapine and olanzapine on the CGI improvement scale. During these studies more than 50% of the participants in each group did not improve. Only Naber 2005 reported relapses and no significant difference between groups was found. The numbers of relapses was very low in both groups.
1.6 to 1.15 Mental state
Various criteria were used to evaluate a clinically significant improvement of the participants mental state, using the PANSS or the BPRS. Neither in the short term nor in the medium term was there a statistically significant superiority displayed by either group. Nevertheless it is interesting to note a slight trend in favour of clozapine in the two small studies with children (Kumra 2008; Shaw 2006).
The mean values on PANSS and BPRS total scores showed a trend in favour of olanzapine, but this difference did not reach conventional levels of statistical significance even when we excluded studies with skewed data. These results were probably affected due to the low doses of clozapine used (< 400 mg/day) and it may be the sample power was not enough to find a statistical difference between groups. New studies are necessary to assess the effect and impact of this difference, if any exists.
Observing the primary studies, both drugs produced a score decrease from baseline. However, the degree of reduction in short term studies by Krakowski 2006 and Volavka 2002 (less than 10 PANSS points) may not be clinically important. Studies with duration of more than 18 weeks showed a more pronounced reduction of symptoms (more than 10 points, Naber 2005, Bitter 2004 and Tollefson 2001).
The studies in children again showed a trend in favour of clozapine which was not statistically significant (Kumra 2008 and Shaw 2006). It could be interesting to clarify by further studies whether children respond differently to therapy than adults.
There was no significant difference between clozapine and olanzapine in the reduction of positive and negative symptoms of schizophrenia. Again, the small studies with children showed a non-significant trend in favour of clozapine in terms of the average SAPS and SANS change, which could stimulate further studies. On SANS, which excluded the study with possibly skewed data, clozapine showed a superiority over olanzapine, but this finding has a limited utility due to the low power of the sample (one study), and because the participants were children.
1.16 Cognitive functioning
This outcome was evaluated by only one study, which reported that significantly more participants in the olanzapine group than those assigned to the clozapine group presented a clinically relevant improvement, defined as a decrease of at least 0.5 standard deviation units on the neurocognitive score. More evidence on this important outcome is needed.
1.17 to 1.18 Quality of life
Subjective well being and quality of life were assessed in one study at medium term using SWN-38 score and MLDL satisfaction with treatment score, respectively. The SWN-38 showed a marked tendency in favour of olanzapine, but due to a great variability of the data the difference was not statistically significant. Furthermore, when the MLDL instead of the SWN-38 was considered, the satisfaction with clozapine and olanzapine was similar.
1.19 Service use
A long term study, Meltzer 2003, showed a significant difference, where more participants in the olanzapine group had to be hospitalised to avoid suicide attempts. The relevance of this finding is limited due to the small effect size and the CI observed, which showed that it is possible that the true RR value is likely to be very close to 1.
1.20 Adverse effects: 1. At least one adverse effects
The results on the number of participants with at least one adverse effect were statistically significantly heterogeneous and were therefore not combined. In general, all studies showed a trend indicating more people in the clozapine group had at least one adverse effect, but only one showed a significant difference between groups. Although this study presented an important effect size its generality is limited because only Chinese participants were included who might have a different idiosyncratic response to the treatment. It is possible that the biological and cultural variability between studies was the cause of the heterogeneity among them.
1.21 Adverse effects: 2. Cardiac effects
Regarding cardiac effects, a low incidence of ECG alteration was observed in both groups, even when the alteration incidence was lower in the olanzapine group, there was no statistical significant difference.
1.22 to 1.28 Adverse effects: Extrapyramidal effects
Concerning extrapyramidal side-effects there was no significant difference between clozapine and olanzapine in terms of the use of antiparkinson medication. There was also no significant difference in terms of specific extrapyramidal symptoms such as akathisia, dyskinesia, pseudoparkinsonism and rigor. All of them, except pseudoparkinsonism, showed a trend in favour of clozapine. Both antipsychotic drugs may cause some extrapyramidal effects because a number of participants needed antiparkinson medication during the trials and movement disorders occurred in both groups. Rating scales for general and specific extrapyramidal side-effects (ESRS, BARS, AIMS, SAS and HAS) did not reveal statistically significant differences.
1.29 to 1.30 Adverse effects: Glucose
There was no conclusive difference between clozapine and olanzapine in terms of glucose levels. Dichotomous data from Meltzer 2003 showed no difference regarding the incidence of diabetes mellitus (criterion not specified). Continuous glucose data were heterogeneous. Only short term studies significantly favoured olanzapine, but there was a high variability of the results and the sample sizes were small. In contrast, a medium term study showed a non-significant trend in favour of clozapine.
1.31 Adverse effects: Hypersalivation
Five studies reported on hypersalivation but as the results were heterogeneous we did not combine them in a meta-analysis. Nevertheless four studies reported a significant difference in favour of olanzapine. The results of the single studies suggest that longer study durations were associated with larger effect sizes and indeed when only studies of each duration category were pooled the results were homogeneous. It can be clearly concluded that people using clozapine are more prone to present hypersalivation than those using olanzapine.
1.32 to 1.34 Adverse effects: Lipids
Data on lipid levels were equivocal and heterogeneous. Currently there is no evidence for any superiority of one drug over the other. In order to obtain a valid conclusion further studies would be necessary.
1.35 Adverse effects: Prolactin
According to a small study, the average change in prolactin levels at medium term showed a statistically significant difference in favour of clozapine, which slightly decreased the level, while the prolactin level in the olanzapine group increased. Whether this difference is clinically important or not is questionable, but at least in specific situations (e.g. women with a history of breast cancer) it may be relevant. Others studies indicated prolactin levels by gender. The results in men were heterogeneous, possibly due to different participant ages. The study in children and adolescents found a significant difference in favour of clozapine (Kumra 2008: male only). The same study also found a significant superiority of clozapine in female participants. The effect sizes were relatively large and probably clinically meaningful, at least in female participants in whom the mean prolactin level in the olanzapine group was above normal. These results should be replicated in larger samples to corroborate the evidence base. The clinical importance of prolactin increase should also be addressed by recording associated side-effects (galactorrhoea, impotence etc).
1.36 Adverse effects: Sedation
In seven studies sedation was more frequent in participants using clozapine than in participants using olanzapine. Although all studies showed at least a trend in favour of olanzapine (irrespective of their duration), the results were heterogeneous. The heterogeneity could be caused by differences in participants age because the two studies in children and adolescents found only minimal differences between groups (Shaw 2006 and Kumra 2008). This observation suggests that children may differ in their sensitivity to sedative effects from adults but this needs to be confirmed in further trials.
1.37 Adverse effects: Seizures
Participants using clozapine presented a 6.5 times higher risk for seizures than those using olanzapine. Therefore olanzapine is more secure on this regard.
1.38 to 1.39 Adverse effects: Weight gain
Results on weight gain were inconclusive. The results in terms of ‘number of patients with weight gain’ were heterogeneous and only one study (Meltzer 2003) showed a significant difference between groups in favour of clozapine. The heterogeneity could be due to different criteria applied to define ‘weight gain’. There was also no significant difference in the mean weight gain between clozapine and olanzapine. We highlight that both drugs produced a considerable increase of weight, clozapine from 1.5 to 6.5 kg and olanzapine from 1.6 to 8.9 kg.
1.40 Adverse effects: White blood cell count
Significantly more participants in the clozapine group experienced an important reduction of the white blood cell count during the studies. The risk in the clozapine group was six times higher than in the olanzapine group. Therefore, olanzapine is a safe drug regarding this important outcome.
2. CLOZAPINE versus OLANZAPINE - Sensitivity analysis
The sensitivity analysis carried -out did not show any significant change in the results, except for the comparison average change on SANS excluding the studies with potentially skewed data. This result was difficult to interpret due to the small size sample from one study.
3. Comparison 2: CLOZAPINE versus QUETIAPINE
These studies have a limited external validity mainly for two reasons: time (only short term results) and participants’ ethnicity (80% of the studies were of oriental origin).
3.1 Leaving the study early
A trend was observed indicating that more people receiving clozapine than quetiapine left the study early due to any reason and/or adverse effects. However these differences did not reach the conventional statistical significance level. Probably due to the small size of the trial, the possibility to detect any difference between the groups, if any existed, was low. The outcome ‘leaving the study early due inefficacy’ could not be estimated since the trial that reported this result did not have any attrition due to this cause.
3.2 Global state
In practice several criteria have been used to measure the participants’ global state during the treatment. The only study that reported the number of participants with no clinical important change on the global state did not give any details regarding the criterion and only stated that a ‘common criterion’ was used. There was no evidence of any difference amongst the groups on this regard. Since the study was done in China, where criteria and medical praxis could differ from the ones used in other countries, its applicability could be limited.
3.3 to 3.9 Mental state
A similar percentage of participants in both groups (32% in clozapine versus 34% in quetiapine) did not improve their mental state using the criterion of at least 50% of reduction on PANSS at short term. The participants also had similar scores on PANSS or BPRS total at endpoint. No statistically significant evidence of superiority of either drug regarding the efficacy of them on mental state was found.
Regarding positive symptoms, both treatment groups at short term presented similar PANSS positive subscore at endpoint. Concerning the clinical improvement of negative symptoms (at least 50% diminution on SANS score), no evidence of any difference was found amongst groups. Skewed data pooled of average PANSS negative subscore at endpoint showed a quetiapine superiority over clozapine, but due to the asymmetry this result had a limited confidence level (<95%). One study that used SANS total score showed the same trend, but without reaching the conventional significance levels. Summarising both treatments were not different in efficacy on general mental state or positive and negative symptoms.
3.10 Adverse effects: At lease one adverse effects
Participants receiving clozapine presented two times more risk of at least one adverse effect than those with quetiapine treatment (NNH 2, CI 2 to 5). This NNH suggests that adverse effects were common in participants receiving clozapine.
3.11 Adverse effects: Cardiac effects
Participants on clozapine had a higher risk of presenting ECG abnormalities, but this result should be taken with precaution due to its low statistical power, which limits its interpretation (NNH 5, CI 3 to 21). The incidence of specific cardiac effects was similar in both groups.
3.12 to 3.13 Adverse effects: Extrapyramidal effects
Regarding extrapyramidal symptoms, the effect of both treatments on the use during the study of antiparkinson medication could not be estimated since only one study reported this outcome without any event amongst groups.
Evaluating the specific extrapyramidal symptoms, a trend in favour of quetiapine and clozapine was found with respect to the incidences of akathisia and rigor respectively. Regarding tremor, the trend was the same for both groups. By general rule there were no statistically significant differences between treatment groups, therefore is not possible to establish which drug was safest with regard to specific extrapyramidal adverse effects.
3.14 Adverse effects: Hypersalivation
Hypersalivation was significantly more frequent in people allocated to clozapine (NNH 1, CI 1 to 2), the NNH value indicates that this adverse effect was very common with clozapine. Although a great variability in the relative risk values, the minimum value, which is likely to include RR, was clinically important and the maximum one was overrated.
3.15 Adverse effects: Lipids
There was statistically significant evidence that participants receiving clozapine had a greater increase in triglyceride levels. This result was based only on a small study (n=25). It would be necessary to replicate this finding.
3.16 Adverse effects: Sedation
People on treatment with clozapine had 4.47 times more risk of suffering sedation (NNH 3, CI 2 to 4) than people allocated to quetiapine. This adverse effect was common in this group of participants.
3.17 to 3.18 Adverse effects: Weight
People taking clozapine were more prone to weight gain than those receiving quetiapine, the magnitude of this increase was higher in the clozapine group (+ 6.5 kg) than quetiapine group (+ 4.4 kg), however both differences were not statistically significant.
3.19 Adverse effects: White blood cell count
Concerning white blood cell decrease, there was no statistically significant difference between the treatment groups. Due to the small power of the trial it was not possible to detect any difference between the groups, if any existed. Furthermore it is necessary to have more details e.g. if the decrease was transient or conserved, the decrease severity, etc. in order to evaluate the clinical importance, as well as data from longer trials.
Overall, evaluating the results of the adverse effect analysis, it is possible to mention the existence of a trend in favour of quetiapine, but it is not statistically significant.
4. Comparison CLOZAPINE versus QUETIAPINE Sensitivity analysis
The sensitivity analysis for the comparison average PANSS total score at endpoint excluding the studies with potentially skewed data, showed no change in the results, supporting that there was no evidence to indicate a difference between clozapine and quetiapine in these aspects.
5. Comparison 3: CLOZAPINE versus RISPERIDONE
5.1 Death
Death is an outcome not usually evaluated in studies. There was no evidence of any difference between short term treatment groups with respect of the incidence of death due to natural causes. In the case of death by suicide, a small study reported that it did not occur, therefore the treatment effect was not estimable.
5.2 to 5.4 Leaving the study early
Overall, a high percentage of participants withdrew from the study in both treatments. No statistically significant difference between comparison groups was found on leaving the study early due to any reason.
More people treated with clozapine left the treatment because of adverse effects than those allocated to risperidone. Clozapine is more prone to produce important adverse effects such as sedation, seizures, hypersalivation, agranulocytosis, etc. which could explain this result. More participants allocated to risperidone left early due to the ineffectiveness compared to the people who received clozapine, suggesting a certain superior efficacy of clozapine.
5.5 Global state
This outcome is based on a single study and showed that there was no clear difference between clozapine and risperidone for CGI clinical response.
5.6 to 5.12 Mental state
A wide variety of criteria to measure the clinical improvement on mental state were used in different studies. Regardless of the criterion used either using PANSS or BPRS, studies at short and long term did not find robust evidence to demonstrate the superiority of one drug over the other.
Heterogeneous data could not be combined to estimate the treatment effects on mental state using PANSS or BPRS scale. Therefore it was difficult to obtain any conclusions on this regard. Only the short term Azorin 2001 study showed a statistically significant difference in favour of clozapine on PANSS, which was replicated in the same study by using BPRS scale.
Overall, the studies reported that mental state scale data showed a wide variability in their measurements, which could be caused by differences in sample sizes, length of the studies, clozapine doses used and methodology used in the analysis (LOCF and/or ITT). The results of combined analysis of the short term studies were strongly influenced by Azorin’s study (Azorin 2001) showing a statistically significant difference in favour of clozapine on BPRS, but the PANSS analysis did not support these results, which makes it difficult to define if there is any superiority in efficacy of either drug over the other. It would be desirable to have further studies using appropriate doses with a high quality methodological procedure, in order to include them in future versions of this review to obtain the best evidence in this regard.
There was a slight trend in favour of clozapine on the PANSS positive subscore, which was not significant. It was observed that the results of individual studies are contradictory and equivocal; this could be the cause of certain level of heterogeneity among them. Skewed data in subscores suggests the same trend on the BPRS positive, but neither was significant.
For negative symptoms, again data from PANSS were heterogeneous and equivocal, hampering the analysis. Two studies used SANS score (only short term) and did not find any statistically significant differences between groups.
5.13 General functioning
A trend in favour of risperidone is noted on GAF measures, where risperidone presented a score greater than clozapine at endpoint. The small sample size of the single available study makes any conclusions impossible.
5.14 Social functioning
Risperidone was superior to clozapine on measures of social functioning (short term). It is important to take into account the low power of the sample, which implies a high degree of variability in the measurements, therefore the clinical significance of this finding is difficult to interpret.
5.15 Treatment satisfaction
Skewed data showed no difference between clozapine and risperidone in treatment satisfaction.
5.16 Cognitive Functioning
There were no statistically significant differences between groups in terms of participants who showed a neurocognitive improvement. This study used a score composed by four neurocognitive domains performed especially for this study, therefore its application to clinical practice would require a validation procedure.
5.17 Adverse effects: At least one adverse effect
Two heterogeneous studies compared clozapine and risperidone regarding the number of people who experienced ‘at least one side effect’ during the study. One of them showed a significant difference, where more people taking clozapine suffered at least one adverse effect than those receiving risperidone. This result should be interpreted with caution due to the low sample power. The other, larger, study did not show a difference between treatment groups.
5.18 Adverse effects: Cardiac effects
There was no evidence that the treatment groups were different in terms of the incidence of cardiac alterations (ECG abnormalities - myocarditis).
5.19 to 5.21 Adverse effects: Extrapyramidal
With regard to extrapyramidal symptoms, fewer people in the clozapine group received an antiparkinson medication compared with those assigned to risperidone (NNH 6, CI 4 to 12). Two heterogeneous studies assessed the number of people who experienced at least one extrapyramidal symptom, only one of them showed a significant statistical difference in favour of clozapine. It was difficult to contrast these results with the other outcomes appraised regarding extrapyramidal symptoms since no comparison showed any statistically significant difference between clozapine and risperidone. The evaluation of the relative risk of presenting some specific extrapyramidal symptoms did not show any difference between groups with respect to akathisia, dyskinesia, tremor and dystonia. Surprisingly parkinsonism was significantly more frequent in participants with clozapine than risperidone, but the clinical significance is unclear because the criterion used was an ESRS score considering only ‘participants with score of 1 or more in ESRS’, because the mean score was not reported and because the confidence interval was very large and the lower limit very close to one (equal effects).
When extrapyramidal symptoms were evaluated by means of ESRS and BARS scores, skewed data were equivocal. Using SAS, a small study showed no difference between the treatment groups.
In general studies did not report or at least not clearly, the occurrence of extrapyramidal symptoms. In further studies a clear description of the criteria for reporting adverse effects and along with it, the way of reporting the use of antiparkinson medication during the trial, is necessary. Thus the process could systematically eliminate confusion factors. As a result it might be possible to demonstrate whether there is any difference between clozapine and risperidone in this aspect.
5.22 Adverse effects: Glycaemia
There was no evidence that the drugs were different with respect to glycaemia. Clearly, both drugs produced an average increase of glucose of 3 - 4 mg/dL on glucose level at 14 weeks (medium term). It would be interesting to know their long term effects, as well as with a bigger sample size to evaluate their clinical importance in particular situations.
5.23 Adverse effects: Hypersalivation
Hypersalivation was 4.34 times more common in participants taking clozapine than those with risperidone (NNH 4, CI 3 to 5).
5.24 Adverse effects: Lipids
With regard to lipid levels, both drugs increased the cholesterol and triglycerides levels. Although no significant differences between groups in the average change of total cholesterol at medium term was observed, a marked trend in favour of risperidone was noted, which might reflect a type II error. By contrast, there was a statistical significant difference between clozapine and risperidone in the average change on the triglycerides level, where the clozapine group showed a pronounced increase. Despite the small sample size, the precision of the measurements contributed to increase the magnitude of treatment effect, clinically and statistically significant but limited because these results were based on only one small study, and should be replicated.
5.25 to 5.26 Adverse effects: Prolactin
A single short term study reported the outcome of the diminution of sexual drive, which is a side-effect associated with an increase of prolactin levels. This side-effect presented a higher incidence in the risperidone group but without reaching the conventional levels of statistical significance. People treated with risperidone showed a high level of prolactin at endpoint (short and medium term) over the adults’ reference levels unlike those treated with clozapine who presented values inside the reference levels. Two studies reported these findings showing significant differences between groups in favour of clozapine, but these results could not be combined due to the heterogeneity amongst them. The difference between these two studies could be due to the variability of the measurements caused by the small power of the samples. Also the results in the Volavka 2002 study were biased, since the measures reported were obtained from a subgroup of male patients and not from the entire group. It would be desirable to replicate these results, in order to support with more confidence these findings. Nevertheless, it is very likely that risperidone is associated with much more prolactin increase than clozapine.
5.27 Adverse effects: Sedation
Sedation was statistically more frequent in participants using clozapine than those using risperidone (NNH 7 CI 4 to 17).
5.28 Adverse effects: Seizures
People taking clozapine were more prone to suffer seizures than those on risperidone (NNH 14 CI 8 to 38).
5.29 to 5.30 Adverse effects: Weight
The studies that reported the outcome of ‘number of participants with weight gain’ were heterogeneous. These studies showed that more participants using clozapine suffered weight gain than those using risperidone. Only a small short term study conducted in China reached levels of statistical significance in favour of risperidone, however the ample confidence interval made it difficult to conclude its clinical significance. The heterogeneity could be due to the different criteria used to define weight gain and the idiosyncratic sample variability. This trend was supported by the results of individual heterogeneous studies at short and medium term that measured average weight change, where three of four studies showed statistical significant differences between the treatment groups. Clozapine treatment produced an increase between 2 - 6 kg more than risperidone.
5.31 Adverse effects: White blood cell count
There was no evidence of difference in the incidence of white blood cell decrease between clozapine and risperidone. In general, the incidence was very low and non-fatal in both groups, neutropenia being the most frequent alteration.
6. Comparison 4: CLOZAPINE versus ZIPRASIDONE
These results are based on only one unpublished short term study including people with refractory schizophrenia.
6.1 Leaving the study early
Overall, in both groups a high number of participants left the study early due to any reason. Clozapine and ziprasidone were similar in this aspect showing a 38% rate of attrition to medium term (18 weeks). This high attrition value could affect the validity of the results.
6.2 Mental state
Both drugs produced a similar diminution, near to 24.5 points on PANSS total score after 18 weeks of treatment. Both antipsychotics drugs showed to be efficacious in decreasing schizophrenia symptoms. There was no evidence of superiority by either drug.
6.3 Adverse effects: Cardiac problems
None of participants suffered any cardiac alteration during the length of the trial, therefore both treatments were shown to be safe in this aspect at 18 weeks. Again, this result was based on a single study which has only been published as a poster.
7. Comparison 5: CLOZAPINE versus ZOTEPINE
Only two studies provided data to compare clozapine with zotepine. The Lindenberg 1997 study was based on six weeks of the following of only Caucasian participants which limited its external validity. This study provided only data for leaving the study early for any reason. The other data are based only in one short term study from Taiwan (Lin 2003). The objective of this study was to evaluate switching from clozapine to zotepine in patients with partial response to clozapine. Therefore the results presented two limitations: they are only applicable to the drug-switching situation and the external validity is limited since the study only included oriental participants.
7.1 Leaving the study early
No statistically significant difference was found between clozapine and zotepine in the rate of participants who left the study early for any reason. The specific reasons for leaving the study early were not reported.
7.2 Global state
The limited data showed a clear difference between groups, where more people on zotepine did not improve (‘less than successfully and no increase on CGI-S’) during the twelve weeks of the study in comparison with those from the clozapine group (NNT 3, CI 2 to 8). It is difficult to support this finding as results are based on one small single study.
7.3 Mental state
A statistically significant difference in favour of clozapine in the average change on BPRS total was found. The effect size was influenced by an unexpected increase of 4.7 points in the score of participants taking zotepine, while in the clozapine group only a slight decrease of 1.3 points was noted. This finding suggests that those on clozapine treatment conserved their score almost without changes and those using zotepine worsened in the twelve weeks, therefore despite clozapine being superior in comparative terms, its effect on the participants’ improvement was not clinically important.
7.4 Adverse effects: Extrapyramidal effects
The relative risk of use of antiparkinson medication was higher in the zotepine group than in the clozapine group (NNH 3, CI 2 to 5). This study reported that only participants who switched to zotepine needed medication at some point within the 12 weeks, whereas participants treated with clozapine did not require it. Due to the study features it is important to consider that participants treated with clozapine probably experienced some transient extrapyramidal symptoms before the study, therefore the conclusion obtained from this study could, in some way, be biased.
7.5 Adverse effects: Prolactin
Another adverse effect assessed in this study was increase of the prolactin level. Participants treated with zotepine presented a significant increase of the prolactin levels. Although the wide confidence interval indicates low precision of estimated effect, the increase in values over the normal levels suggest that zotepine could have some negative implications for patient health. Again, the limited data make any conclusive statement difficult and a replication is needed.
Limited data made it impossible to compare these antipsychotics regarding other important adverse effects.
Overall completeness and applicability of evidence
Data on five out of eight possible comparisons were found, hence the evidence is incomplete. Limited information on general functioning, satisfaction with the treatment or care, cognition and service use was available. Most studies were short term, which limits the applicability considering that schizophrenia is a chronic, often life-long disorder. The high attrition in the studies also limits the applicability of the evidence to practice.
Quality of the evidence
Twenty of twenty-seven studies were double-blind and seven were single-blind, and the reporting of details were limited. Without an adequate description of the methodology of the studies, it was difficult to assess their quality. Moreover, many outcomes were assessed in sample sizes that were not large enough to detect significant differences if they exist.
Potential biases in the review process
We are not aware of any obvious flaws in our review process.
Agreements and disagreements with other studies or reviews
The former Cochrane review that compared clozapine with other second generation antipsychotic drugs (Tuunainen 2000) included only eight studies, while the current report comprises 27 studies. This large increase in the evidence base makes the two reviews hardly comparable. Nevertheless, some differences in adverse events found in Tuunainen 2000 persisted in the current review making these results more robust.
AUTHORS’ CONCLUSIONS
Implications for practice
1. For people with schizophrenia
People with schizophrenia need to know that clozapine differs most clearly in adverse effects from other second generation antipsychotic drugs. It seems to produce somewhat fewer movement disorders than risperidone and zotepine, and is likely to increase prolactin levels less than olanzapine, risperidone and zotepine. On the other hand, clozapine is associated with more frequent decrease of white blood cells than olanzapine, more hypersalivation and sedation than olanzapine, quetiapine and risperidone, and more seizures than olanzapine and risperidone. Compared to risperidone, clozapine may also lead to more weight gain. Differences in efficacy are less clear, but clozapine may be slightly more efficacious than risperidone and zotepine.
2. For clinicians
The overall attrition in the included studies of this review was considerable (30%) making the interpretation of the results difficult. Furthermore, a reasonable amount of evidence is only available compared to olanzapine (12 trials) and risperidone (9 trials). Five small trials on quetiapine were conducted outside the US and Europe, limiting generalisability. Only two small studies compared clozapine with zotepine and one with ziprasidone. Randomised clinical trials comparing clozapine with other second generation antipsychotic drugs (e.g. amisulpride, aripiprazole and sertindole) are not available.
Although clozapine is usually considered to be the most efficacious antipsychotic drug available, this review could not document convincing differences in efficacy.
Adverse effects profiles are very similar, some specific differences could be crucial to selecting the more adequate treatment according to the characteristics of each patient and their expectations. Here, clozapine seems to produce more hypersalivation and sedation than olanzapine, quetiapine and risperidone; more seizures than olanzapine and risperidone; more weight gain than risperidone and more frequent leukopenia than olanzapine. On the other hand, clozapine was associated with fewer movement disorders than risperidone and zotepine, and less prolactin increase than olanzapine, risperidone and zotepine.
3. For managers/policy makers
There is insufficient information to guide the decisions of managers and policy makers. The abandonment of treatment often leads to worsening of patients’ health and therefore a greater demand for medical care. Clozapine, risperidone, olanzapine and ziprasidone show similar rates of abandonment of treatment, nearly 31%, which makes it necessary to apply efforts to improve the patient compliance. Service use was reported by only one large study which showed fewer hospitalisations to avoid suicide attempts. This evidence base is too limited for making recommendations. This review did not attempt to measure the economic impact of clozapine compared to other second generation antipsychotic drugs.
Implications for research
1. General
There is room for improvement in the conduct and reporting of randomised controlled schizophrenia trials. Rating scale derived efficacy outcomes dominate the trials and even in this regard authors keep using different definitions for response to treatment making a comparison of the results difficult. Potentially important outcomes such as satisfaction with care, functioning in the community or service use are rarely examined. Simple descriptions of the randomisation or blinding methods are usually not presented. Strict adherence to the CONSORT statement (Moher 2001) would improve the reporting and conduct of future trials.
2. Specific
Studies comparing clozapine with the second generation antipsychotic drugs amisulpride, aripiprazole and sertindole are currently completely missing and are therefore mandatory. But even the available evidence on the other comparisons was incomplete, most importantly concerning global outcomes such as satisfaction with care, death, quality of life or service use. Furthermore, only 8% of the included studies fell in the long term category. As schizophrenia is usually a chronic disorder, there is a need for further long term trials. Table 1 makes a suggestion as to how such a study could look. Limited available evidence addressed the effects of clozapine in children and adolescents with schizophrenia. As children may have a different response treatment, this evidence should be extended.
Pharmaceutical companies generally compare new drugs against a very low dose of clozapine. These doses are lower than used in the pivotal study of Kane 1988 and his collaborators. It is not fully clear if this can be included in sensitivity analysis/meta-regression, because there are only a limited number of studies that use adequate doses. This certainly indicates the need for future research using adequate doses
PLAIN LANGUAGE SUMMARY.
Clozapine versus other atypical antipsychotics for schizophrenia
This review compared the clinical effects of clozapine with the other atypical antipsychotics. Twenty-seven studies fulfilled the review’s criteria and provided data to compare clozapine with antipsychotics such as olanzapine, quetiapine, risperidone, ziprasidone and zotepine. Clozapine was somewhat more efficacious than zotepine. Also, inefficacy of treatment led more frequently to leaving the studies early in the risperidone group suggesting a certain higher efficacy of clozapine. The principal drawback of clozapine were its adverse effects which lead to significantly higher numbers of participants leaving the studies early compared to olanzapine and risperidone. Clozapine was associated with more sedation and hypersalivation than olanzapine, quetiapine and risperidone and with more seizures than olanzapine and risperidone. There was a higher incidence of white blood cell decrease in clozapine groups than olanzapine and more weight gain than in risperidone groups. On the other hand clozapine produced fewer movement disorder than risperidone and less prolactin increase than olanzapine, quetiapine and zotepine.
ACKNOWLEDGEMENTS
We would like to thank all members of the Cochrane Schizophrenia Group for their editorial assistance, and acknowledge use of the Cochrane Schizophrenia Group’s template for the Methods section which we have adapted for use in this review.
SOURCES OF SUPPORT
Internal sources
Klinikum Rechts der Isar der TU-München, Germany.
CIGES Capacitacion, Investigación y Gestion para la Salud Basada en la Evidencia, Universidad de la Frontera, Temuco, Chile.
External sources
Bundesministerium für Bildung und Forschung Nr FKZ:01KG 0606, GZ:GF-GFKG01100506, Germany.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: randomised. Blindness: single, rater-blinded. Duration: 6 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia. N=56. Age: 19-46 years (mean clozapine=31.3 years, mean olanzapine=29.6 years, mean quetiapine=30.1 years, mean risperidone=27.9 years, mean control group=32.1 years). Gender: 24 M, 29 F. Setting: inpatient. History: duration ill mean clozapine=6.6 years, mean olanzapine=6.3 years, mean quetiapine=5.9 years, mean risperidone=5.6, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental state: PANSS total score. Adverse effects: use of antiparkinson medication, extrapyramidal side effects, lipid change, weight change |
|
| Notes | There was a control group (N=11) (not randomised) receiving no pharmacologic treatment | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “The patients were randomly divided into 4 treatment group..” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes | Yes | Quote: “raters were blind to drug assignment.” “The prescribing physician was not blind to assignment” Comment: Single/rater blind study. Subjective measures may lead a source of bias. Also review authors believe that the fact the prescribing physician was not blind will introduce bias. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
Unclear | 6/62 patients were excluded (four due to requirement of additional drug and two due to intolerance). The author did not state from which group they were excluded. Another three patients did not complete the study (one clozapine / one olanzapine / one risperidone) Intention-to-treat analysis was not undertaken. |
| Free of selective reporting? | Yes | Review authors do not believe this will introduce bias. |
| Free of other bias? | Yes | Review authors have not found other sources of bias. |
| Methods | Allocation: randomised. Blindness: double. Duration: 12 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia disorganised, catatonic, paranoid, residual or un-differentiated. N=273. Age: 18-65 years (mean clozapine=37.8, mean risperidone=39.5) (of intent-to-treat population). Gender: 182 M, 74 F (of intent-to-treat population, N=256). Setting: in- and outpatient. History: duration ill mean clozapine=13.0 years, mean risperidone=15.5 years (of intent-to-treat population), age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Death. Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: PANSS total score, BPRS total score, PANSS positive and negative sub-score. Adverse effects: at least one adverse effect, extrapyramidal side-effects, sedation, seizures, weight gain, white blood cell count. Unable to use: Change of weight (no SD). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “ patients who satisfied the eligibility criteria were then randomly assigned.. (balanced by country, with a block size of six)” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “..assigned to double-blind treatment”. Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
Unclear | 38/138 missing from clozapine group. 34/135 missing from risperidone group. Analysis by intention to treat and LOCF Comment: ITT “considered patients randomly assigned with at least one post-dose BPRS evaluation” |
| Free of selective reporting? | Yes | Review authors do not believe this will introduce bias. |
| Free of other bias? | Unclear | Study supported by a grant from Novartis Pharma S.A. |
| Methods | Allocation: randomised. Blindness: double. Duration: 18 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, non-response to, or intolerance of, standard an-tipsychotic therapy. N=147. Age: 18-65 years (mean=37.6). Gender: 88 M, 59 F. Setting: inpatient. History: duration ill not reported, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: PANSS total score, PANSS positive and negative subscore. Adverse effects: at least one adverse effect, extrapyramidal side effects (akathisia, dyskinesia, parkinsonism, use of antiparkinson medication, AIMS, Hillside Akathisia Scale, SAS), sedation, weight change Unable to use: Leukopenia (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote:“This was a Phase III, 18-week, randomised.. (1:1 ratio)” Comment: Incomplete information. |
| Allocation concealment? | No | Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “This was a Phase III, 18-week, randomised, double blind, parallel study” Comment: The success of blinding was not evaluated. |
| Incomplete outcome data addressed? All outcomes |
No | 33/74 missing from clozapine and 30/76 missing from olanzapine. Analysis by intention to treat and LOCF Comment: patients analysed were all those had at least one post-baseline measurement |
| Free of selective reporting? | No | Quote: “Spontaneously reported adverse events with an incidence of 5% in either treatment group or with a statistically significant difference ( P < .05) between groups” |
| Free of other bias? | Unclear | The study was sponsored by Eli Lilly and company. |
| Methods | Allocation: randomised. Blindness: double. Duration: 8 weeks. |
|
| Participants | Diagnosis: (DSM-III-R) chronic schizophrenia, non-responders or intolerance. N=86. Age: 18-65 years (mean=37.3). Gender: 61 M, 25 F. Setting: inpatient. History: age at first hospitalisation mean clozapine=25.0, mean risperidone=26.0, age at onset mean clozapine=23.5, mean risperidone=22.4 |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental state: PANSS total score, PANSS positive and negative subscore. Adverse effects: cardiac effects (any significant cardiac effect), extrapyramidal side-effects (akinesia, dystonia, parkinsonism, use of antiparkinson medication), prolactin associated side-effects (sexual dysfunction), sedation, weight (as “weight gain” reported adverse event), white blood cell count Unable to use: Change of weight in kg (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “ Patients randomly assigned to receive risperidone or clozapine..” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “Double blind, double dummy protocol” Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
Yes | 9/43 missing from clozapine group. 9/43 missing from risperidone group Analysis by intention to treat and LOCF. |
| Free of selective reporting? | No | Quote: “Adverse events reported by 5% or more of either treatment group during the trial” |
| Free of other bias? | Yes | Study supported by a grant from industry of both study drugs. The study used low clozapine doses |
| Methods | Allocation: randomised. Blindness: double. Duration: 6 weeks. |
|
| Participants | Diagnosis: (DSM-IV) chronic schizophrenia. N=29. Age: 18-55 years (mean clozapine=37.7, mean risperidone=32.4). Gender: 19 M, 10 F. Setting: not reported. History: duration ill mean clozapine=13.9 years, mean risperidone=11.1 years, age at onset mean clozapine=23.7, mean risperidone=21.3 |
|
| Interventions |
|
|
| Outcomes | Mental state: BPRS total score, PANSS positive and negative subscore. Adverse effects: extrapyramidal side-effects (use of antiparkinson medication, SAS), prolactin (change of prolactin in ng/ml) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “All patients were then randomly assigned to treatment with clozapine or risperidone..” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “..double blind comparison trial.” Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
No | Exclusion of 5 patients after randomisation who were not considered in the total sample. Incomplete information. ITT was not performed |
| Free of selective reporting? | Yes | Review authors do not believe this will introduce bias. |
| Free of other bias? | No | Quote: “Nineteen of the 29 patients underwent a drug-free period before random assignment”. Comment: Probably performance bias. Author from Industry |
| Methods | Allocation: randomised. Blindness: double. Duration: 16 weeks (first 8 weeks observed). |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, resistance to previous treatment. N=13. Age: mean=37.58 years. Gender: 8 M, 5 F. Setting: not reported. History: duration ill not reported, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Death: natural causes, suicide. Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: BPRS total score, PANSS positive and negative subscore. Adverse effects: at least one adverse effect; cardiac effects (QTc prolongation), lipids (change on cholesterol from baseline in mg/dl), extrapyramidal side effects (akathisia, use of antiparkinson medication, SAS), glucose (change from baseline in mg/dl), sedation, seizures, weight change |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “In a randomised crossover design..” Comment: Probably not done. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “randomised double-blind 16 week..” Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
No | 0/5 missing from clozapine and 3/8 missing from olanzapine. LOCF for patients completing at least two weeks. ITT was not performed |
| Free of selective reporting? | No | Adverse events that occurred only in one person were not reported |
| Free of other bias? | Yes | Review authors have not found other sources of bias. |
| Methods | Allocation: randomised. Blindness: double. Duration: four weeks. |
|
| Participants | Diagnosis:(ICD-9) acute schizophrenia, disorganised, catatonic, paranoid, unspecified type. Schizoaffective psychosis, schizodominant type. N=59. Age: 19-65 years. Gender: 31 M, 28 F. Setting: not reported. History: duration ill not reported, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: BPRS total score, BPRS subscore. Adverse effects: at least one adverse effect, cardiac effects (pre terminal negative T-wave), extrapyramidal side-effects (extrapyramidal symptoms, use ofantiparkinson medication, Simpson-Angus Scale), sedation. Unable to use: White blood cell count: agranulocytosis (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “This is a randomised, double blind study..” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “This is a randomised, double blind study..” Tablets were identical appearance. Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
Yes | 6/20 missing from clozapine, 13/19 from risperidone 8 mg and 9/20 from risperi-done 4 mg. Intention to treat analyses and LOCF |
| Free of selective reporting? | No | There was incomplete information about some adverse events as body weight, blood pressure, ECG Adverse events considered only the most frequent spontaneously reported adverse experience |
| Free of other bias? | Yes | Review authors have not found other sources of bias. |
| Methods | Allocation: randomised - block randomisation (block size of three). Blindness: double. Duration: 12 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (N=71) or schizoaffective disorder (N=39), persistent aggression. N=110. Age: 18-60 years (mean clozapine=35.1 years, mean haloperidol=32.7 years, mean olan-zapine=35.6 years). Gender: 90 M, 20 F. Setting: inpatient. History: duration ill mean clozapine=15.7 years, mean haloperidol=13.9 years, mean olanzapine=16.8 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental state: PANSS total score, PANSS positive and negative subscore. Unable to use: Extrapyramidal Symptom Rating Scale (ESRS / EPS) (no data) ECG (no data) White cell count (no data) Adverse events (no data) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “ patients were randomly assigned to 1 of 3 treatment arm..” “The study used block randomisation scheme with a block size of 3..” Comment: Incomplete information. |
| Allocation concealment? | No | Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “The medication was administered in a double-blind fashion..” The success of blinding was not evaluated Comment: Probably done. |
| Incomplete outcome data addressed? All outcomes |
Yes | 13/37 missing from clozapine and 11/37 missing from olanzapine. Analysis by intention-to-treat was used |
| Free of selective reporting? | No | Adverse events assessed but it was not reported in the article |
| Free of other bias? | No | Quote: “The setting limits the generalis-ability of the findings” |
| Methods | Allocation: randomised - computer-generated randomisation. Blindness: double. Duration: 12 weeks. |
|
| Participants | Diagnosis: children and adolescents with (DSM-IV) schizophrenia (N=25) or schizoaffective disorder (N=14) (of intent-to-treat population), resistant to, or intolerant of, at least two antipsychotic treatments. N=40. Age: 10-18 years (mean=15.6 years). Gender: 21 M, 18 F (of intent-to-treat population). Setting: in- and outpatient. History: duration ill not reported, age at onset mean clozapine=12.7 years, mean olan-zapine=11.7 years (of intent-to-treat population) |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: BPRS total score, SANS total score. Adverse effects:at least one adverse effect, extrapyramidal side effects (AIMS, SAS), glucose (change from baseline in mg/dl), lipids (change on cholesterol from baseline in mg/dl), prolactin associated side effects (change from baseline in ng/ml - ofmen only, change from baseline in ng/ ml - of women only), sedation, weight change Unable to use: Extrapyramidal symptoms (no data) Diabetes mellitus (no data). Hyperglycemia (no data). Neutropenia (no data). |
|
| Notes | One person was excluded owing to withdrawal of parental consent after randomisation | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “…medication were assigned to their groups by research pharmacist with a computer-generated randomisation schedule.” |
| Allocation concealment? | No | Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “medication was administered under double-blind condition”. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
No | 4/19 missing from clozapine and 7/21 missing from olanzapine Quote:“…analyses were performed on the intent-to-treat population” Comment: ITT considered all patients who received at least one dose of study medication |
| Free of selective reporting? | No | Side-effects: Study reported only the participants who gained >7% of their baseline body weight |
| Free of other bias? | Yes | Review authors have not found other sources of bias. |
| Methods | Allocation: randomised. Blindness: double. Duration: 8 weeks. |
|
| Participants | Diagnosis: (CCMD-3) schizophrenia. N=63. Age: mean clozapine=30 years, mean quetiapine=28 years. Gender: not reported. Setting: in- and outpatient. History: duration ill mean clozapine=0.63 years, mean quetiapine=0.65 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Global state: CGI. Mental State: PANSS total score. Adverse effects: at least one adverse effect, cardiac effects (palpitation), extrapyramidal side effects (akathisia, rigor, tremor), sedation, weight gain, white blood cell count Unable to use: Leaving the study early due to adverse events (no data). Cardiac effects (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? All outcomes |
Unclear | Double, probably identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side-effects. This can be a problem for blinding Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. This latter, probably leads a low risk of bias |
| Incomplete outcome data addressed? All outcomes |
Unclear | The authors only mention two participants leaving the study early due to adverse events in the clozapine group. There is some doubt whether all data on leaving the study early have been presented |
| Free of selective reporting? | Yes | We did not find evidence for selective reporting. |
| Free of other bias? | Unclear | There were no data on pre study medication, therefore baseline imbalance can not be excluded |
| Methods | Allocation: randomised. Blindness: single, rater-blinded. Duration: 8 weeks. |
|
| Participants | Diagnosis: (CCMD-2) schizophrenia. N=76. Age: mean clozapine=36.2 years, mean quetiapine=34.7 years. Gender: not reported. Setting: inpatient. History: duration ill mean clozapine= 6.12 years, mean quetiapine=5.71 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Global state: CGI. Mental state: PANSS total score, PANSS positive and negative subscore Unable to use: Leaving the study early due to inefficacy (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? All outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side-effects. This can be a problem for blinding Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. This latter, probably leads a low risk of bias |
| Incomplete outcome data addressed? All outcomes |
Unclear | The authors describe only one participant in the quetiapine group who left the study early due to inefficacy. This participant was not included in the analysis. There is some doubt whether all data on leaving the study early have been presented |
| Free of selective reporting? | No | The study duration was eight weeks, but outcomes only at four weeks were available |
| Free of other bias? | Unclear | The allowed dose range was not indicated. |
| Methods | Allocation: randomised. Blindness: double. Duration: 12 weeks. |
|
| Participants | Diagnosis: (CCMD-3) schizophrenia. N=67. Age: mean=26.18 years. Gender: not reported. Setting: inpatient. History: duration ill mean clozapine=0.49 years, mean quetiapine=0.5 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental state: PANSS total score, PANSS positive and negative subscore. Unable to use: Extrapyramidal symptoms (no data). Sedation (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? All outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side-effects. This can be a problem for blinding Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. This latter, probably leads a low risk of bias |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was 9.1%. Those leaving early were only reported due to any reason. Only completer data were assessed |
| Free of selective reporting? | No | There was no reporting on adverse effects |
| Free of other bias? | Unclear | Baseline characteristics have not been presented for both groups separately. Therefore, baseline imbalance can not be excluded. Furthermore, there was no washout period |
| Methods | Allocation: randomised. Blindness: single, rater-blind. Duration: 12 weeks. |
|
| Participants | Diagnosis: schizophrenia. N=59. Age: 20-65 years. Gender: not reported. Setting: inpatient. History: BPRS >30, clozapine treatment for more than 5 months |
|
| Interventions |
|
|
| Outcomes | Global state: CGI. Mental state: BPRS total score. Adverse effects:use of antiparkinson medication. Unable to use: BAS, SAS, UKU (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “59 patients were allocated on a random..” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “.. patients were allocated on a random, rater blind basis..” Probably done. The success ofblinding was not evaluated. |
| Incomplete outcome data addressed? All outcomes |
Unclear | No information on abstract. |
| Free of selective reporting? | Unclear | No information on abstract. |
| Free of other bias? | No | Quote: “Patients taking clozapine for more 5 month were allocated on a random to two groups: one maintained on clozapine and another switched to zotepine” |
| Methods | Allocation: randomised. Blindness: double. Duration: 6 weeks. |
|
| Participants | Diagnosis:(DSM-III-R) schizophrenia catatonic, hebephrenic, paranoid or residual. N=50. Age: 18-60 years. Gender: not reported. History: BPRS >40 after washout phase, no previous treatment with either medication |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Unable to use: CGI, BPRS, SANS, adverse events: ECG, weight gain (matched samples) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “They were randomly assigned..” Comment: Incomplete information. |
| Allocation concealment? | No | Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “They were randomly assigned in a double design to clozapine or zotepine” Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
No | 7/25 missing from clozapine and 10/25 missing from zotepine. ITT was not performed |
| Free of selective reporting? | No | Quote: “Analysis refers to a sub-sample of 26 patients, (matched for age).” Comment: Data from sub sample was not used in our review. |
| Free of other bias? | Unclear | Quote: “This study was sponsored and monitored by Klinge Pharma (manufacturer of zotepine)” |
| Methods | Allocation: randomised Blindness: single, rater-blinded. Duration: 12 weeks. |
|
| Participants | Diagnosis: (CCMD-3) schizophrenia. N=72. Age: mean clozapine=37.44 years, mean quetiapine=36.86 years. Gender: not reported. Setting: inpatient. History: duration ill mean clozapine=9.36 years, mean quetiapine=8.64 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: adverse events, inefficacy. Global state: CGI. Mental state: BPRS total score, SANS total score. Adverse effects: cardiac effects (ECG abnormalities), extrapyramidal side effects (akathisia, tremor), sedation, weight gain Unable to use: Leaving the study early due to any reason (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? All outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side-effects. This can be a problem for blinding Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. This latter, probably leads a low risk of bias |
| Incomplete outcome data addressed? All outcomes |
Unclear | The authors mention five participants leaving the study early, three due to adverse events in the clozapine group and two due to unclear reasons in the quetiapine group. These five subjects were not included in the analysis. There is some doubt whether all data on leaving the study early have been presented |
| Free of selective reporting? | No | The mean doses of the medications used were not indicated. |
| Free of other bias? | No | Clozapine was titrated to 400 mg/day within 10 days. Such a fast dose increase can be accompanied by a higher rate of adverse effects |
| Methods | Allocation: randomised. Blindness: double, double-dummy protocol. Duration: 29 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n=93) or schizoaffective disorder (n=14), treatment resistance. N=107. Age: 18-60 years (mean=42 years). Gender: 84 M, 23 F. Setting: in- and outpatient. History: duration ill not reported, age at onset mean clozapine=23 years, mean risperi-done=22 years |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental state: BPRS total score. Adverse effects: white blood cell count. Unable to use: SANS (modified version). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “ random assignment trial..” Comment: Incomplete information. |
| Allocation concealment? | No | Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “ double-blind, 29 week trial..” Comment: Probably done. |
| Incomplete outcome data addressed? All outcomes |
Yes | 30/47 missing from clozapine and 32/50 risperidone. ITT was not performed |
| Free of selective reporting? | Yes | Review authors do not believe this will introduce bias. |
| Free of other bias? | Yes | Review authors have not found other sources of bias. |
| Methods | Allocation: randomised. Blindness: single, rater-blinded. Duration: 104 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (N=609) or schizoaffective disorder (N=371), high suicidal risk. N=980. Age: 18-65 years (mean=37.1 years). Gender: 602 M, 378 F. Setting: in- and outpatient. History: duration ill not reported, age at onset mean=24.7 years |
|
| Interventions |
|
|
| Outcomes | Death: any reason, suicide attempt, suicide. Leaving the study early: any reason, adverse events, inefficacy. Service use: number of participants re-hospitalised. Adverse effects: extrapyramidal side effects (akathisia, rigor), glucose (diabetes mellitus) , sedation, seizures, weight gain, white blood cell count Unable to use: ESRS (no data) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “For randomisation, patients were blocked by country and medical centre” “.. The 2 treatment groups were allocated randomly in a 1:1 ratio within blocks of 4 patients in each medical centre” Comment: Probably not done. |
| Allocation concealment? | No | No Information. Probably not done. |
| Blinding? All outcomes |
Yes | Single-open label/blind raters. Comment: Probably done. The rater’s masking was monitored by external service |
| Incomplete outcome data addressed? All outcomes |
Yes | 192/490 missing from clozapine and 187/ 490 missing from olanzapine Quote: “All data obtained was used in intent-to-treat analysis” |
| Free of selective reporting? | Yes | Review authors do not believe this will introduce bias. |
| Free of other bias? | Yes | The study sponsor was Novartis Pharmaceuticals Corp. |
| Methods | Allocation: randomised. Blindness: double. Duration: 8 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, treatment resistance to two previous antipsychotic medications, BPRS score of 27 or more. N=23. Age: 18 years or more (mean clozapine=38.3 years, mean olanzapine=34.1 years) (of completer population). Gender: 16 M, 7 F. Setting: inpatient. History: duration ill not reported, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental state: PANSS total score, BPRS total score, PANSS positive and negative sub-score. Adverse effects: at least one adverse effect, extrapyramidal side effects (SAS) Unable to use: AIMS (no useable data) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “Before randomisation..” Comment: Information incomplete. |
| Allocation concealment? | No | Probably not done. |
| Blinding? All outcomes |
Yes | Quote:“ A double blind, parallel study.” Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
No | 6/12 missing from clozapine and 2/11 missing from olanzapine ITT not performed. |
| Free of selective reporting? | Unclear | No information about form to assess the adverse events. |
| Free of other bias? | Unclear | Quote: “Study was partially supported by Eli-Lilly” |
| Methods | Allocation: randomised - computer-generated randomisation. Blindness: double. Duration: 26 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, non-response to, or intolerance of, standard an-tipsychotic therapy. N=114. Age: 18-65 years (mean=34.0 years). Gender: 69 M, 45 F. Setting: in- and outpatient, initially inpatient. History: duration ill not reported, age at onset 26.9 years. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: PANSS total score, BPRS total score, PANSS positive and negative subscore, BPRS positive and negative subscore. Quality of life: SWN. Adverse effects: at least one adverse effect, cardiac effects (QTc prolongation), extrapyra-midal side effects (use of antiparkinson medication, SAS), weight change. Unable to use: Glucose elevation (non fasting) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “This randomised double blind controlled trial..” Patients were allocated 1: 1 ratio Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “This randomised double blind controlled trial..” Comment: Probably done. Identical capsules were used.The success ofblinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
No | 35/57 missing from clozapine and 36/57 from olanzapine.Intention to treat analyses and LOCF was used Comment: ITT considered the patients with at least one post-baseline value |
| Free of selective reporting? | No | Adverse event were recorded from spontaneously report. Probably reporting bias |
| Free of other bias? | Unclear | Study funded by Lilly Deutschland GmbH. |
| Methods | Allocation: randomised - ball drawing out of box. Blindness: double. Duration: 12 weeks. |
|
| Participants | Diagnosis:(CCMD-3) schizophrenia. N=120 Age: mean clozapine=33.5 years, mean risperidone=35.4 years. Gender: not reported. Setting: outpatient. History duration ill: clozapine: 6.2 years risperidone 6.4 years |
|
| Interventions |
|
|
| Outcomes | Mental state: PANSS positive and negative subscore. | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Allocation: random, ball drawing out of a covered box. Probably yes |
| Allocation concealment? | Unclear | No further details. |
| Blinding? All outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side-effects. This can be a problem for blinding Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. This latter, probably leads a low risk of bias |
| Incomplete outcome data addressed? All outcomes |
No | Data on leaving the study early were not provided. |
| Free of selective reporting? | No | Secondary outcomes were poorly reported. |
| Free of other bias? | Unclear | The allowed dose ranges were not indicated. |
| Methods | Allocation: randomised. Blindness: double. Duration: 18 weeks. |
|
| Participants | Diagnosis: schizophrenia - treatment resistance (non-response in 3 adequate trials in past 5 years) and/or inability to tolerate antipsychotic treatment. N=146. Age: mean clozapine= 38.3 years, mean ziprasidone= 41.6 years. Gender: 101 M, 45 F. Setting: not reported. History: duration ill not reported, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental state: PANSS total score. Adverse effects: cardiac effects (QTc prolongation). Unable to use: Global state:CGI (no data). Mental state: PANSS positive and negative subscore (no data). Laboratory parameters (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “Patients completed a 3-7 day screening period before being randomised” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “Patients completed a 3-7 day screening period before being randomised, double-blind..” Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
Yes | Analysis by intention to treat and LOCF. |
| Free of selective reporting? | Unclear | No information on abstract. |
| Free of other bias? | Unclear | No information on abstract. |
| Methods | Allocation: randomised. Blindness: double. Duration: 18 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia catatonic (N=3), disorganised (N=34), paranoid (N=101), residual (N=8) or undifferentiated (N=34), previous treatment resistance. N=180. Age: 18-70 years (mean=38.6 years). Gender: 115 M, 65 F. Setting: in- and outpatient. History: duration ill not reported, age at onset mean=22.8 years |
|
| Interventions |
|
|
| Outcomes | Death: natural causes. Leaving the study early: any reason, adverse events, inefficacy. Mental state: PANSS total score, BPRS total score, PANSS positive and negative subscore, BPRS positive and negative subscore. Adverse effects: extrapyramidal side effects (akathisia, akinesia, parkinsonism, use of antiparkinson medication, AIMS, BAS, SAS), prolactin (change from baseline in ng/ml) , sedation, weight gain, white blood cell count (leukopenia) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “Random allocation used a random-numbers chart and was conducted in blocks of 4” |
| Allocation concealment? | Yes | Quote: “Numbered containers were used to implement the random allocation sequence..” |
| Blinding? All outcomes |
Yes | Double blind. Identic tablet form. Quote: “Participants and those administering and assessing the intervention and assessing outcomes were blind..” “The success of the blinding was not formally assessed” |
| Incomplete outcome data addressed? All outcomes |
Yes | No participant was loss during trial on clozapine group. 1/13 was missing from olanzapine group. Analysis by intention to treat and LOCF |
| Free of selective reporting? | Yes | Review authors do not believe this will introduce bias. |
| Free of other bias? | Yes | Review authors have not found other sources of bias. |
| Methods | Allocation: randomised. Blindness: double. Duration: 18 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia catatonic (N=3), disorganised (N=34), paranoid (N=101), residual (N=8) or undifferentiated (N=34), previous treatment resistance. N=180 Age: 18-70 years (mean=38.6 years). Gender: 115 M, 65 F. Setting: in- and outpatient. History: duration ill not reported, age at onset mean=22.8 years |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental state: clinical improvement (at least 20%BPRS reduction+CGI-S<3 or BPRS<35) ( > or = 50% reduction on PANSS total), PANSS total, positive and negative subscore, BPRS total score. Adverse effects:extrapyramidal effects (SAS, AIMS, BAS), prolactin levels, weight change |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “patients were randomly allocated in a 1:1 ratio to treatment with olanzapine 15-25 mg/day or clozapine 200-600 mg/day” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “An 18-week double-blind therapy” Comment: Probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
No | 37/90 missing from clozapine and 36/90 missing from olanzapine Quote: “All end point analyses used a last observation carried forward (LOCF) algorithm” Comment: OC technique for weekly measures of patients with at least one post-baseline measurement. ITT analysis was not performed |
| Free of selective reporting? | No | Spontaneously Reported Treatment-Emergent Adverse Events with an Incidence of ≥5% in either Treatment Group, or with a statistically significant difference (P< .05) between treatment Groups. Solicited treatment-emergent adverse events with statistically significant difference |
| Free of other bias? | Yes | Review authors have not found other sources of bias. |
| Methods | Allocation: randomised Blindness: double, identical capsules. Duration: 14 weeks. |
|
| Participants | Diagnosis: (DSM-IV) chronic schizophrenia (N=135) or schizoaffective disorder (N=22), sub optimal response to previous treatment, PANSS of 60 or more. N=167. Age: 18-60 years (mean=40.8 years) (of intent-to-treat population). Gender: 133 M, 24 F (of intent-to-treat population). Setting: inpatient. History: duration ill mean=19.5 years (of intent-to-treat population), age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental state: PANSS total score, PANSS positive and negative subscore. Cognitive functioning: Global Neurocognitive Score. Adverse effects: extrapyramidal effects (use of antiparkinson medication, ESRS), glucose (change from baseline in mg/dl), lipids levels (change on cholesterol from baseline in mg/dl), prolactin associated side effects (change from baseline in ng/ml), seizures, weight gain, white blood cell count (agranulocytosis, neutropenia) Unable to use: Quality of life scale (no data). Cognitive functioning (PANSS cognitive subscore, at endpoint and change, was reported as factor score and the neurocognitive global score at endpoint was reported as Z score, which didn’t allow its use as comparator) |
|
| Notes | The two participants with neutropenia (clozapine) are additional participants to the one with agranulocytosis | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Quote: “.. patients were randomly assigned to one of the four treatment arm..” Comment: Incomplete information. |
| Allocation concealment? | No | No information. Probably not done. |
| Blinding? All outcomes |
Yes | Quote: “In a double blind trial..” “.. all tablets looked alike” Comment: Probably done. |
| Incomplete outcome data addressed? All outcomes |
No | 18/40 missing from clozapine, 13/39 from olanzapine and 19/41 from risperidone. ITT was not performed |
| Free of selective reporting? | No | Some outcomes were reported on subgroup from the entire sample (Czobor 2002, Volavka 2004, Bilder 2001, Lindenmayer 2003).The author reported the use of the Quality ofLife Scale, but the results are not on article, (Bilder 2001) |
| Free of other bias? | No | “The olanzapine arm was added in November 1997 and required a modified randomisation procedure”…“ it entails the potential for a bias that could be manifested as a cohort effect.” |
| Methods | Allocation: randomised - computer-generated randomisation. Blindness: single, rater-blinded. Duration: 10 weeks. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, resistance to previous treatment. N=20. Age: 24-55 years (mean clozapine=35.7 years, mean risperidone=36.8 years) (of intent-to-treat population). Gender: 10 M, 9 F (of intent-to-treat population). Setting: in- and outpatient (initially inpatient). History: duration ill mean clozapine=12.6 years, mean risperidone=13.1 years (of intent-to-treat population), age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Death: natural causes, suicide. Leaving the study early: any reason, adverse events, inefficacy. Mental state: PANSS total score, PANSS positive and negative subscore. General functioning: GAF, SFS. Treatment satisfaction: DAI. Adverse effects: extrapyramidal side effects (use of antiparkinson medication), sedation, white blood cell count |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Quote: “Patients were individuallyassigned to open label treatment by computer-generated randomisation” |
| Allocation concealment? | Yes | Quote: “..the treating physician contacted the senior investigator, who provided the allocation information..” Comment: Probably done. |
| Blinding? All outcomes |
Yes | Open label/assessor blinded study. Comment: probably done. The success of blinding was not evaluated |
| Incomplete outcome data addressed? All outcomes |
Yes | 6/11 missing from clozapine and 1/9 missing from risperidone. Analysis by intention to treat and LOCF |
| Free of selective reporting? | Yes | Review authors do not believe this will introduce bias. |
| Free of other bias? | No | Quote: “Readers have to bear in mind that the low statistical power of this study may hides some clinically relevant differences in drug efficacy” |
| Methods | Allocation: randomised. Blindness: double. Duration: 8 weeks. |
|
| Participants | Diagnosis: (CCMD-3) schizophrenia. N=61. Age: mean clozapine=30 years, mean olanzapine=25.8 years. Gender: 29 M, 32 F. Setting: in- and outpatient. History: duration ill mean= 4.2 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Mental state: BPRS total score. Adverse effects: at least one adverse effect, extrapyramidal side effects (extrapyramidal symptoms), sedation, weight gain Unable to use: White blood cell countleukopenia (no usable data). Leaving the study early -adverse events (no usable data). Hypersalivation (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? All outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side-effects. This can be a problem for blinding Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. This latter, probably leads a low risk of bias |
| Incomplete outcome data addressed? All outcomes |
No | Data on leaving the study early were not provided. |
| Free of selective reporting? | No | Data were not available for all of the predefined adverse effect outcomes |
| Free of other bias? | No | The sponsor was unclear. The upper dose range limit of clozapine was 400 mg/day which was reached rather quickly (10 days), which could mean a disadvantage for cloza-pine in terms of side-effects |
| Methods | Allocation: randomised Blindness: single, rater-blinded. Duration: 8 weeks. |
|
| Participants | Diagnosis: (CCMD-2) schizophrenia. N=40. Age: mean clozapine=27.6 years, mean risperidone=29 years. Gender: 23 M, 17 F. Setting: inpatient History: duration ill mean clozapine=2.7 years, mean risperidone=3.2 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Mental state: SANS. Adverse effects: extrapyramidal side-effects (akathisia, tremor), sedation, weight gain |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? All outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side-effects. This can be a problem for blinding Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding. This latter, probably leads a low risk of bias |
| Incomplete outcome data addressed? All outcomes |
Yes | No participant left the study early. |
| Free of selective reporting? | Yes | No clear evidence for selective reporting. |
| Free of other bias? | Unclear | The description of blinding differed between the abstract (double-blind) and the method section (single-blind) |
Scales:
AIMS: Abnormal Involuntary Movement Score
BAS: Barnes Akathisia Scale
BPRS: Brief Psychiatric Rating Scale.
CGI: Clinical Global impression.
ESRS: Extrapyramidal Symptoms Rating Scale
GAF: Global Assessment of Functioning
HAS: Hillside Akathisia Scale
MLDL: Munich Life Dimension List
PANSS: Positive and Negative Syndrome Scale for Schizophrenia
SANS: Scale for the Assessment of Negative Symptoms
SAPS: Scale for the Assessment of Positive Symptoms
SAS: Simpson Angus scale
SFS: Social Functioning scale.
SWN: Subjective Well Being under Neuroleptic Treatment - 38 Items
Diagnostic Tools:
DSM -III-R: Diagnostic and Statistical Manual of Mental Disorders, third edition, revised.
DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, fourth edition.
ICD-9: International Classification of Disease.
CCMD-2: Chinese Classification of Mental Disorders, Second Edition.
CCMD-3: Chinese Classification of Mental Disorders, Third Edition.
Others:
ECG: electrocardiogram
ITT: intent- to- treat.
mg: milligram.
SD: standard deviation.
N: number
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Agelink 2001 | Allocation: not randomised. |
| Allison 2001 | Allocation: unclear |
| Altamura 1999 | Allocation: not randomised (naturalistic). |
| Anonymous 1994 | Allocation: unclear. |
| Ascher-Svanum 2006 | Allocation: not randomised (naturalistic). |
| Baumann 1993 | Allocation: unclear. |
| Beasley 2001 | Allocation: unclear. |
| Bondolfi 1996 | Allocation: not applicable (review) . |
| Cai 2000 | Allocation: randomised, no blinding. |
| CATIE | Allocation: randomised, open label trial for clozapine, double-blind for other atypicals. Raters were blinded only to the newer, not for clozapine |
| Cavazzoni 2002 | Allocation: unclear (analysis of extracted data from olanzapine clinical trial database) |
| Cha 2002 | Allocation: randomised, no blinding. |
| Chen 2002 | Allocation: randomised, no blinding. |
| Chen 2003 | Allocation: randomised, no blinding. |
| Chen 2003b | Allocation: randomised, no blinding. |
| Chen 2004 | Allocation: randomised, no blinding. |
| Chen 2005 | Allocation: randomised, no blinding. |
| Chen 2005b | Allocation: randomised, no blinding. |
| Cheng 2004 | Allocation: randomised, no blinding. |
| Chou 1999 | Allocation: randomised, no blinding. |
| Chouinard 1994 | Allocation: not randomised (case reports). |
| Conley 1999a | Allocation:unclear, open label. |
| Cui 2002 | Allocation: randomised, no blinding. |
| CUTLASS | Allocation: randomised. Participants: people with schizophrenia, treatment-resistant. Intervention: clozapine vs. other pooled newer antipsychotics |
| Dai 2004 | Allocation: randomised, no blinding. |
| David 1999 | Allocation: not randomised (review). |
| Deng 2000 | Allocation: randomised, no blinding. |
| Ding 2005 | Allocation: randomised, no blinding. |
| Du 2003 | Allocation: randomised, no blinding. |
| Du 2003b | Allocation: randomised, no blinding. |
| Du 2004 | Allocation: randomised, no blinding. |
| Du 2005 | Allocation: randomised, no blinding. |
| Earnst 1999 | Allocation: not randomised (naturalistic). |
| Ellis 2000 | Allocation: randomised. Participants: people with Parkinson’s disease and psychosis, not schizophrenia |
| Fan 2003 | Allocation: randomised, no blinding. |
| Fang 2005 | Allocation: randomised, no blinding. |
| Feng 2004 | Allocation: randomised, no blinding. |
| Fleming 1999 | Allocation: not randomised. |
| Flynn 1997 | Allocation: not randomised. |
| Fu 2005 | Allocation: randomised, no blinding. |
| Gaertner 1999 | Allocation: not randomised (retrospective study). |
| Gallhofer 1995 | Allocation: not randomised. |
| Gallhofer 1996 | Allocation: not randomised. |
| Ganguli 2005 | Allocation: randomised. Participants: people with schizophrenia, schizoaffective disorder. Intervention: behaviour therapy vs. standard care. |
| Gao 2003 | Allocation: randomised, no blinding. |
| Goldberg 2000 | Allocation: not randomised. |
| Green 2001 | Allocation: randomised. Participants: people with schizophrenia and cannabis use disorder |
| Guan 2005 | Allocation: randomised, no blinding. |
| Guo 2001 | Allocation: randomised, no blinding. |
| Guo 2003 | Allocation: randomised, no blinding. |
| Han 2005 | Allocation: randomised, no blinding. |
| Hang 2000 | Allocation: randomised, no blinding. |
| He 2005 | Allocation: randomised, no blinding. |
| Hu 2000 | Allocation: randomised, no blinding. |
| Hu 2005 | Allocation: randomised, no blinding. |
| Huang 2001 | Allocation: randomised, no blinding. |
| Huang 2003 | Allocation: randomised, no blinding. |
| Kelemen 2006 | Allocation: randomised, no blinding. |
| Kong 2001 | Allocation: randomised, no blinding. |
| Konrad 1996 | Allocation: not randomised (naturalistic). |
| Kufferle 1997 | Allocation: not randomised. |
| Lee 1995 | Allocation: unclear, open trial. |
| Lei 2002 | Allocation: randomised, no blinding. |
| Lei G 2004 | Allocation: randomised, no blinding. |
| Li 2003b | Allocation: randomised, no blinding. |
| Li 2001 | Allocation: randomised, no blinding. |
| Li 2003 | Allocation: randomised, no blinding. |
| Li 2004 | Allocation: randomised, no blinding. |
| Liang 2002 | Allocation: randomised, no blinding. |
| Liang 2005 | Allocation: randomised, no blinding. |
| Lindenmayer 1996 | Allocation: unclear. |
| Liu 1999 | Allocation: randomised, no blinding. |
| Liu 2001 | Allocation: randomised, no blinding. |
| Liu 2003 | Allocation: randomised, no blinding. |
| Liu 2003b | Allocation: randomised, no blinding. |
| Liu 2004 | Allocation: randomised, no blinding. |
| Liu 2004b | Allocation: randomised, no blinding. |
| Liu 2004c | Allocation: randomised, no blinding. |
| Liu 2005 | Allocation: randomised, no blinding. |
| Liu 2005b | Allocation: randomised, no blinding. |
| Liu 2005c | Allocation: randomised, no blinding. |
| Louwerens 1996 | Allocation: not randomised. |
| Lu 2002 | Allocation: randomised, no blinding. |
| Lu 2005 | Allocation: randomised, no blinding. |
| Luo 2005 | Allocation: randomised, no blinding. |
| Ma 1999 | Allocation: randomised, no blinding. |
| McKenna 2004 | Allocation: randomised. Participants: people with schizophrenia or psychotic disorders. Intervention: risperidone augmentation therapy of clozapine. |
| Meehan 2000 | Allocation: not randomised (data report from 8 different trials) |
| Mei 2001 | Allocation: randomised, no blinding. |
| Mulqueen 2000 | Allocation:not randomised. |
| Nan 2001 | Allocation: randomised, no blinding. |
| Ni 2001 | Allocation: randomised, no blinding. |
| Opjordsmoen 2000 | Allocation: not randomised (naturalistic). |
| Pajonk 1998 | Allocation: not randomised. |
| Pan 2006 | Allocation: randomised, no blinding. |
| Pao 2004 | Allocation: randomised, no blinding. |
| Peng 2001 | Allocation: randomised, no blinding. |
| Qian 2004 | Allocation: randomised, no blinding. |
| Qin 2005 | Allocation: randomised, no blinding. |
| Rapoport 1997 | Allocation: not randomised. |
| Ren 2000 | Allocation: randomised, no blinding. |
| Ren 2004 | Allocation: randomised, no blinding. |
| Rettenbacher 2004 | Allocation: not randomised. |
| Saletu 1987 | Allocation: randomised. Participants: healthy people. |
| Scherer 2004 | Allocation: not randomised. |
| Schlogelhofer 2006 | Allocation: not randomised. |
| Schuld 2000 | Allocation: not randomised. |
| Shao 1999 | Allocation: randomised, no blinding. |
| Sharma 2002 | Allocation: not randomised (naturalistic). |
| Sheng 2003 | Allocation: randomised, no blinding. |
| Sheng 2005 | Allocation: randomised, no blinding. |
| Shi 2000 | Allocation: randomised, no blinding. |
| Shi 2001 | Allocation: randomised, no blinding. |
| Smith 2004 | Allocation: randomised. Participants: people with schizophrenia. Intervention: addition of pioglitazone or placebo to clozapine and olanzapine |
| Speer 1997 | Allocation: not randomised. |
| Su 1996 | Allocation: not randomised. |
| Sun 2001 | Allocation: randomised, no blinding. |
| Swanson 2006 | Allocation: non applicable (observational study) |
| Tandon 2004 | Allocation: randomised, open label study. |
| Tang 2003 | Allocation: randomised, no blinding. |
| Tang 2005 | Allocation: randomised, no blinding. |
| Tang 2005b | Allocation: randomised, no blinding. |
| Tian 2005 | Allocation: randomised, no blinding. |
| Tong 2005 | Allocation: randomised, no blinding. |
| Trichard 1998 | Allocation: not randomised. |
| Turrone 2002 | Allocation: not randomised. |
| Vaughan 2000 | Allocation: not randomised. |
| Wang 2002b | Allocation: randomised, no blinding. |
| Wang 2003 | Allocation: randomised, no blinding. |
| Wang 2003b | Allocation: randomised, no blinding. |
| Wang 2003c | Allocation: randomised, no blinding. |
| Wang 2004 | Allocation: randomised, no blinding. |
| Wang 2004b | Allocation: randomised, no blinding. |
| Wang 2005 | Allocation: randomised, no blinding. |
| Wang 2005b | Allocation: randomised, no blinding. |
| Wang 2005c | Allocation: randomised, no blinding. |
| Wang 2005d | Allocation: randomised, no blinding. |
| Wang 2005e | Allocation: randomised, no blinding. |
| Wang 2005f | Allocation: randomised, no blinding. |
| Weickert 2003 | Allocation: not randomised. |
| Weng 1998 | Allocation: randomised, no blinding. |
| Wirshing 1999 | Allocation: not randomised (cross-sectional study). |
| Wu 2002 | Allocation: randomised, no blinding. |
| Wu 2004 | Allocation: randomised, no blinding. |
| Wudarsky 1999 | Allocation: not randomised (data report from different studies) |
| Xiang 2005 | Allocation: randomised, no blinding. |
| Xiao 2000 | Allocation: randomised, no blinding. |
| Xie 2005 | Allocation: randomised, no blinding. |
| Xin 2001 | Allocation: randomised, no blinding. |
| Xu 2001 | Allocation: randomised, no blinding. |
| Xu 2002 | Allocation: randomised, no blinding. |
| Yagdiran 2000 | Allocation: non applicable (observational study). |
| Yang 1998 | Allocation: randomised, no blinding. |
| Yang 2002 | Allocation: randomised, no blinding. |
| Yang 2004 | Allocation: randomised, no blinding. |
| Yang L 2004 | Allocation: randomised, no blinding. |
| Yang X 2004 | Allocation: randomised, no blinding. |
| Ye 2005 | Allocation: randomised, no blinding. |
| Yin 2004 | Allocation: randomised, no blinding. |
| Yu 2002 | Allocation: randomised, no blinding. |
| Yuan 2002 | Allocation: randomised, no blinding. |
| Yuan 2005 | Allocation: randomised, no blinding. |
| Yuo 1999 | Allocation: randomised, no blinding. |
| Zelaschi 2000 | Allocation: not randomised. |
| Zelaschi 2006 | Allocation: not randomised (naturalistic). |
| Zhan 2002 | Allocation: randomised, no blinding. |
| Zhang 2002 | Allocation: randomised, no blinding. |
| Zhang 2002b | Allocation: randomised, no blinding. |
| Zhang 2004 | Allocation: randomised, no blinding. |
| Zhang 2005 | Allocation: randomised. Participants: people with schizophrenia + obsessive compulsive symptoms |
| Zhang 2005b | Allocation: randomised, no blinding. |
| Zhang 2005c | Allocation: randomised, no blinding. |
| Zhang 2005d | Allocation: randomised, no blinding. |
| Zhang 2005e | Allocation: randomised, no blinding. |
| Zhang 2005f | Allocation: randomised, no blinding. |
| Zhang 2005g | Allocation: randomised, no blinding. |
| Zhao 2005 | Allocation: randomised, no blinding. |
| Zheng 2001 | Allocation: randomised, no blinding. |
| Zheng 2003 | Allocation: randomised, no blinding. |
| Zheng X 2001 | Allocation: randomised, no blinding. |
| Zhong 2003 | Allocation: randomised, no blinding. |
| Zhou 2003 | Allocation: randomised, no blinding. |
| Zhou 2003b | Allocation: randomised, no blinding. |
| Zhou 2005 | Allocation: randomised, no blinding. |
| Zhu 1999 | Allocation: randomised, no blinding. |
| Zhu 2002 | Allocation: randomised, no blinding. |
| Zhu 2003 | Allocation: randomised, no blinding. |
| Zhu Q 2003 | Allocation: randomised, no blinding. |
| Zoccali 2003 | Allocation: not randomised. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | Diagnosis:chronic, severe schizophrenia. |
| Interventions |
|
| Outcomes | |
| Notes | No abstract. |
| Methods | |
| Participants | Diagnosis:schizophrenia who have not responded to olanzapine or risperidone. N= 60 |
| Interventions |
|
| Outcomes | |
| Notes | Abstract incomplete. |
| Methods | Allocation:randomised. Binding: double. Duration:29 week. |
| Participants | Diagnosis:Moderately treatment refractory. N=180. Setting: inpatients. |
| Interventions |
|
| Outcomes | Cognition. Psychosocial functioning. Adverse effects. Psychopathology |
| Notes | Protocol abstract available. |
| Methods | Allocation: randomised. Blinding: not stated. Duration:17 weeks. |
| Participants | Diagnosis: (ICD-10) resistant schizophrenia. N=60. Age:15 - 60 years. Gender: 45 M - 15 F. History:duration of illness clozapine:6.92±5.07 and risperidone:18±4.38 years |
| Interventions |
|
| Outcomes | Leaving the study early: any reason, adverse effects, inefficacy. Clinically important change: at least 20% improvement on PANSS. Mental state: PANSS total, BPRS modified score, PANSS positive and negative subscore. Adverse effects: cardiac (tachycardia), extrapyramidal (akatisia), hypersalivation, sedation, weight change |
| Notes | Full text available. |
| Methods | Allocation:randomised-dose (cross-over trial). Blinding: single-blind. Duration:12 weeks. |
| Participants | Diagnosis:(DSM-III-R) chronic schizophrenia or schizoaffective disorder. N=20. Age: mean: 33.8 years (range=22-51). Gender: 7M-13F. Setting:19 outpatients and 1 inpatient. History:mean age at onset of psychosis was 22.7 years (range=15-32) |
| Interventions |
|
| Outcomes | Leaving the study early: adverse effects. Global state: severity of illness sub scale of the CGI. Mental state: PANSS total score and positive and negative subscore. dverse effects: extrapyramidal (antiparkinson medication use), sedation, weight gain |
| Notes | No report of first arm outcomes (6 weeks). |
| Methods | Allocation:randomised. Blinding: open label. |
| Participants | Diagnosis: resistant schizophrenia. N=22 |
| Interventions |
|
| Outcomes | Mental state: PANSS. Adverse effects: Dimascio and UKU Side Effect Scale. |
| Notes | Abstract available. No outcome information. |
| Methods | Allocation: randomised. Blinding: double. Duration:12 weeks. |
| Participants | Diagnosis: treatment resistant schizophrenia. N=224. |
| Interventions |
|
| Outcomes | Global functioning: CGI. Mental state: PANSS total score. Social functioning: NOSIE. Quality of life: Quality of Life Interview. Adverse effects: extrapyramidal: ESRS. |
| Notes | Only abstract available. |
| Methods | Allocation: randomised. Blinding: not stated. |
| Participants | Diagnosis:paranoid schizophrenia (first-episode). N:39. Age:18 - 29 years. |
| Interventions |
|
| Outcomes | Mental state: PANSS total score. |
| Notes | Abstract available. |
| Methods | Allocation: unclear Blinding: double |
| Participants | Diagnosis: (DSM-III-R) several psychotic conditions (schizophrenia, schizoaffective disorder, and psychotic disorders not otherwise specified).who have not responded to at least two prior typical neuroleptics. Age:6 to 18 years History: onset of psychosis by age 12. |
| Interventions |
|
| Outcomes | |
| Notes | Abstract available. |
| Methods | Allocation: randomised. Blinding: not stated. Duration:8 weeks. |
| Participants | Diagnosis:(DSM-IV) resistant schizophrenia or other psychotic disorder. N:40 |
| Interventions |
|
| Outcomes | Mental state: PANSS total score, positive and negative subscore |
| Notes | Abstract available. |
DATA AND ANALYSES
Comparison 1. CLOZAPINE versus OLANZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Any reason | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 1.5 [0.62, 3.64] |
| 1.2 Natural causes | 2 | 993 | Risk Ratio (M-H, Random, 95% CI) | 1.4 [0.45, 4.38] |
| 1.3 Suicide | 2 | 993 | Risk Ratio (M-H, Random, 95% CI) | 1.67 [0.40, 6.94] |
| 2 Leaving the study early: 1. Any reason | 11 | 1702 | Risk Ratio (M-H, Random, 95% CI) | 1.04 [0.93, 1.17] |
| 2.1 short term | 6 | 202 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.65, 1.74] |
| 2.2 medium term | 4 | 520 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.88, 1.26] |
| 2.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.88, 1.20] |
| 3 Leaving the study early: 2. Adverse effects | 10 | 1674 | Risk Ratio (M-H, Random, 95% CI) | 1.60 [1.07, 2.40] |
| 3.1 short term | 5 | 174 | Risk Ratio (M-H, Random, 95% CI) | 3.30 [0.95, 11.47] |
| 3.2 medium term | 4 | 520 | Risk Ratio (M-H, Random, 95% CI) | 1.83 [0.80, 4.17] |
| 3.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 1.30 [0.84, 2.02] |
| 4 Leaving the study early: 3. Inefficacy | 10 | 1674 | Risk Ratio (M-H, Random, 95% CI) | 0.72 [0.40, 1.30] |
| 4.1 short term | 5 | 174 | Risk Ratio (M-H, Random, 95% CI) | 0.52 [0.16, 1.64] |
| 4.2 medium term | 4 | 520 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.53, 2.00] |
| 4.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 0.33 [0.12, 0.91] |
| 5 Global state | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 5.1 no clinically important change: less than much improved - medium term | 2 | 294 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.93, 1.38] |
| 5.2 relapse - medium term | 1 | 114 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.06, 15.60] |
| 6 Mental state: 1. No clinically important change - various criteria | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 6.1 less than 20% BPRS reduction (short term) | 1 | 25 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.50, 1.25] |
| 6.2 less than 50% BPRS reduction (short term) | 1 | 61 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.63, 2.03] |
| 6.3 less than 20% BPRS reduction and mildly ill or better (short term) | 1 | 25 | Risk Ratio (M-H, Random, 95% CI) | 1.08 [0.87, 1.33] |
| 6.4 less than 20% BPRS reduction and mildly ill or better (medium term) | 2 | 327 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 6.5 less than 30% BPRS reduction and much improved or very much improved (short term) | 1 | 39 | Risk Ratio (M-H, Random, 95% CI) | 0.5 [0.24, 1.03] |
| 6.6 less than 50% PANSS reduction (medium term) | 2 | 327 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.92, 1.10] |
| 7 Mental state: 2a. PANSS total score (high = poor) | 7 | 618 | Mean Difference (IV, Random, 95% CI) | 1.97 [−0.71, 4.66] |
| 7.1 short term | 3 | 115 | Mean Difference (IV, Random, 95% CI) | 1.97 [−1.48, 5.42] |
| 7.2 medium term | 4 | 503 | Mean Difference (IV, Random, 95% CI) | 1.99 [−2.29, 6.27] |
| 8 Mental state: 2b. BPRS-18 (1-7) total score (high = poor) | 5 | 304 | Mean Difference (IV, Random, 95% CI) | 1.31 [−0.30, 2.92] |
| 8.1 short term | 4 | 128 | Mean Difference (IV, Random, 95% CI) | 0.89 [−2.02, 3.79] |
| 8.2 medium term | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 1.20 [−3.03, 5.43] |
| 9 Mental state: 2c. BPRS total score, various versions (high = poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.1 BPRS - 24 - only short term | 1 | 25 | Mean Difference (IV, Random, 95% CI) | −7.0 [−28.47, 14.47] |
| 9.2 BPRS - 18 (0-6) - only medium term | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 2.80 [−4.05, 9.65] |
| 10 Mental state: 3a. Positive symptoms: PANSS positive subscore (high = poor) | 6 | 592 | Mean Difference (IV, Random, 95% CI) | 0.08 [−0.96, 1.11] |
| 10.1 short term | 2 | 89 | Mean Difference (IV, Random, 95% CI) | −0.63 [−2.27, 1.00] |
| 10.2 medium term | 4 | 503 | Mean Difference (IV, Random, 95% CI) | 0.54 [−0.78, 1.87] |
| 11 Mental state: 3b. Positive symptoms: SAPS - short term (high = poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | −9.0 [−22.06, 4.06] |
| 12 Mental state: 3c. Positive symptoms: BPRS positive subscore (high = poor) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 BPRS-18 (1-7) | 2 | 189 | Mean Difference (IV, Random, 95% CI) | −0.01 [−1.39, 1.37] |
| 12.2 BPRS-18 ( 0-6) - only medium term | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 0.40 [−1.57, 2.37] |
| 13 Mental state: 4a. Negative symptoms: PANSS negative subscore (high = poor) | 6 | 592 | Mean Difference (IV, Random, 95% CI) | 0.78 [−0.21, 1.77] |
| 13.1 short term | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 1.32 [−0.42, 3.05] |
| 13.2 medium term | 4 | 503 | Mean Difference (IV, Random, 95% CI) | 0.52 [−0.68, 1.72] |
| 14 Mental state: 4b. Negative symptoms: SANS - short term (high = poor) | 2 | 64 | Mean Difference (IV, Random, 95% CI) | −4.81 [−14.33, 4.71] |
| 15 Mental state: 4c. Negative symptoms: BPRS negative subscore -(high = poor) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 BPRS – 18 (1-7) | 2 | 189 | Mean Difference (IV, Random, 95% CI) | −0.29 [−1.17, 0.60] |
| 15.2 BPRS-18 (0-6) | 1 | 108 | Mean Difference (IV, Random, 95% CI) | 0.20 [−1.29, 1.69] |
| 16 Cognitive functioning: 1. No clinically important change - less than 0.5 SD improved - medium term | 1 | 79 | Risk Ratio (M-H, Random, 95% CI) | 1.64 [1.15, 2.35] |
| 17 Quality of Life: 1. SWN-38 total score - medium term (high= good) | 1 | 99 | Mean Difference (IV, Random, 95% CI) | −8.2 [−21.67, 5.27] |
| 18 Quality of Life: 2. MLDL total score - medium term (high = good) | 1 | 97 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 19 Service use: Hospital readmission - long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.62, 0.98] |
| 20 Adverse effects: 1. At least one adverse effect | 7 | 422 | Risk Ratio (M-H, Random, 95% CI) | 1.39 [1.03, 1.89] |
| 20.1 short term | 5 | 161 | Risk Ratio (M-H, Random, 95% CI) | 1.56 [0.85, 2.85] |
| 20.2 medium term | 2 | 261 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [1.02, 1.40] |
| 21 Adverse effects: 2. Cardiac problems | 1 | 152 | Risk Ratio (M-H, Random, 95% CI) | 2.42 [0.38, 15.33] |
| 21.1 short term | 6 | 38 | Risk Ratio (M-H, Random, 95% CI) | 2.17 [0.22, 20.94] |
| 21.2 medium term | 1 | 114 | Risk Ratio (M-H, Random, 95% CI) | 3.00 [0.12, 72.13] |
| 22 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use | 6 | 561 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.46, 1.67] |
| 22.1 short term | 2 | 41 | Risk Ratio (M-H, Random, 95% CI) | 0.50 [0.02, 10.34] |
| 22.2 medium term | 4 | 520 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.42, 1.86] |
| 23 Adverse effects: 3d. Extrapyramidal: SAS change or endpoint (high = poor) | 6 | 481 | Mean Difference (IV, Random, 95% CI) | 0.43 [−0.45, 1.30] |
| 23.1 short term | 3 | 66 | Mean Difference (IV, Random, 95% CI) | −0.47 [−1.69, 0.76] |
| 23.2 medium term | 3 | 415 | Mean Difference (IV, Random, 95% CI) | 0.92 [−0.17, 2.01] |
| 24 Adverse effects: 3c. Extrapyramidal: ESRS score at endpoint - medium term (high = poor) | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 1.30 [−0.23, 2.83] |
| 25 Adverse effects: 3b. Extrapyramidal: various symptoms | 6 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 25.1 at least one EPS - only short term | 6 | 1 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 25.2 akathisia | 4 | 1320 | Risk Ratio (M-H, Random, 95% CI) | 0.73 [0.38, 1.41] |
| 25.3 dyskinesia | 2 | 327 | Risk Ratio (M-H, Random, 95% CI) | 0.53 [0.20, 1.43] |
| 25.4 extrapyramidal symptoms | 2 | 84 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 25.5 parkinsonism | 1 | 147 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 25.6 pseudoparkinsonism - only medium term | 1 | 180 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.50, 3.30] |
| 25.7 rigor - only long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 0.17 [0.02, 1.38] |
| 26 Adverse effects: 3e. Extrapyramidal: akathisia - BARS change - medium term (high = poor) | 1 | 175 | Mean Difference (IV, Random, 95% CI) | −0.10 [−0.38, 0.18] |
| 27 Adverse effects: 3f. Extrapyramidal: Hillside Akathisia Scale - medium term (high=poor) | 1 | 137 | Mean Difference (IV, Random, 95% CI) | −0.40 [−3.10, 2.30] |
| 28 Adverse effects: 3g. Extrapyramidal: tardive dyskinesia - AIMS change or endpoint - (high = poor) | 3 | 352 | Mean Difference (IV, Random, 95% CI) | 0.13 [−0.25, 0.51] |
| 28.1 short term | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 0.30 [−0.23, 0.83] |
| 28.2 medium term | 2 | 314 | Mean Difference (IV, Random, 95% CI) | −0.05 [−0.60, 0.49] |
| 29 Adverse effects: 4a. Glucose: number of participants with significant increase - long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.40, 1.44] |
| 30 Adverse effects: 4b. Glucose: average change or endpoint (high = poor) | 3 | 89 | Mean Difference (IV, Random, 95% CI) | 2.62 [−11.09, 16.34] |
| 30.1 short term | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 9.70 [1.73, 17.68] |
| 30.2 medium term | 1 | 39 | Mean Difference (IV, Random, 95% CI) | −9.9 [−23.30, 3.50] |
| 31 Adverse effects: 5. Hypersalivation | 5 | 1333 | Risk Ratio (M-H, Random, 95% CI) | 3.87 [1.49, 10.05] |
| 31.1 short term | 2 | 64 | Risk Ratio (M-H, Random, 95% CI) | 1.64 [1.14, 2.38] |
| 31.2 medium term | 2 | 289 | Risk Ratio (M-H, Random, 95% CI) | 5.33 [1.76, 16.08] |
| 31.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 8.18 [5.64, 11.86] |
| 32 Adverse effects: 6a. Lipids: number of participants with significant increase | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 32.1 increase cholesterol | 1 | 25 | Risk Ratio (M-H, Random, 95% CI) | 3.23 [0.14, 72.46] |
| 32.2 increase triglycerides | 2 | 64 | Risk Ratio (M-H, Random, 95% CI) | 1.08 [0.37, 3.20] |
| 33 Adverse effects: 6b. Lipids:average cholesterol change or endpoint (high = poor) | 3 | 89 | Mean Difference (IV, Random, 95% CI) | −1.16 [−19.85, 17. 52] |
| 33.1 short term | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 6.83 [−35.03, 48.69] |
| 33.2 medium term | 1 | 39 | Mean Difference (IV, Random, 95% CI) | −3.80 [−25.70, 18.10] |
| 34 Adverse effects: 6c. Lipids: average triglyceride change - short term (high = poor) | 2 | 38 | Mean Difference (IV, Random, 95% CI) | 36.07 [−83.57, 155.71] |
| 35 Adverse effects: 7. Prolactin: average change or endpoint (high=poor) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 35.1 average change from baseline ng/ml | 1 | 120 | Mean Difference (IV, Random, 95% CI) | −0.57 [−1.05,−0.09] |
| 35.2 average endpoint ng/ml - men only | 2 | 47 | Mean Difference (IV, Random, 95% CI) | −8.65 [−20.55, 3.26] |
| 35.3 average endpoint ng/ml - women only | 1 | 18 | Mean Difference (IV, Random, 95% CI) | −54.4 [−86.74, −22. 06] |
| 36 Adverse effects: 8. Sedation | 7 | 1445 | Risk Ratio (M-H, Random, 95% CI) | 1.65 [1.05, 2.58] |
| 36.1 short term | 4 | 138 | Risk Ratio (M-H, Random, 95% CI) | 1.33 [0.76, 2.31] |
| 36.2 medium term | 2 | 327 | Risk Ratio (M-H, Random, 95% CI) | 2.67 [0.92, 7.78] |
| 36.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 1.86 [1.55, 2.24] |
| 37 Adverse effects: 9. Seizures | 4 | 1097 | Risk Ratio (M-H, Random, 95% CI) | 6.50 [1.73, 24.47] |
| 37.1 short term | 2 | 38 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 37.2 medium term | 1 | 79 | Risk Ratio (M-H, Random, 95% CI) | 8.78 [0.49, 157.85] |
| 37.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 6.0 [1.35, 26.67] |
| 38 Adverse effects: 10a. Weight: number of participants with weight gain | 7 | 1600 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.55, 1.42] |
| 38.1 short term | 2 | 100 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.45, 2.16] |
| 38.2 medium term | 4 | 520 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.60, 1.78] |
| 38.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 0.57 [0.48, 0.66] |
| 39 Adverse effects: 10b. Weight: average weight change (high = poor) | 7 | 581 | Mean Difference (IV, Random, 95% CI) | −0.04 [−1.06, 0.97] |
| 39.1 short term | 3 | 64 | Mean Difference (IV, Random, 95% CI) | −1.70 [−3.69, 0.28] |
| 39.2 medium term | 4 | 517 | Mean Difference (IV, Random, 95% CI) | 0.41 [−0.56, 1.37] |
| 40 Adverse effects: 11. White blood cell count: number of participants with a decrease | 4 | 1264 | Risk Ratio (M-H, Random, 95% CI) | 5.68 [2.48, 13.00] |
| 40.1 short term | 1 | 25 | Risk Ratio (M-H, Random, 95% CI) | 2.17 [0.22, 20.94] |
| 40.2 medium term | 2 | 259 | Risk Ratio (M-H, Random, 95% CI) | 5.57 [1.00, 31.14] |
| 40.3 long term | 1 | 980 | Risk Ratio (M-H, Random, 95% CI) | 7.0 [2.47, 19.81] |
Comparison 2. CLOZAPINE versus OLANZAPINE - Sensitivity Analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1a. PANSS total score, excluding possibly skewed data (high = poor) | 5 | 577 | Mean Difference (IV, Random, 95% CI) | 2.16 [−1.23, 5.54] |
| 1.1 short term | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 2.44 [−3.10, 7.98] |
| 1.2 medium term | 4 | 503 | Mean Difference (IV, Random, 95% CI) | 1.99 [−2.29, 6.27] |
| 2 Mental state: 1b. BPRS-18 (1-7) total score, excluding possibly skewed data (high = poor) | 3 | 204 | Mean Difference (IV, Random, 95% CI) | 1.12 [−3.03, 5.27] |
| 2.1 short term | 2 | 28 | Mean Difference (IV, Random, 95% CI) | 0.32 [−8.74, 9.38] |
| 2.2 medium term | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 1.20 [−3.03, 5.43] |
| 3 Mental state: 2a. PANSS positive subscore, excluding possibly skewed data (high = poor) | 5 | 577 | Mean Difference (IV, Random, 95% CI) | 0.34 [−0.77, 1.44] |
| 3.1 short term | 1 | 74 | Mean Difference (IV, Random, 95% CI) | −0.13 [−2.12, 1.86] |
| 3.2 medium term | 503 | Mean Difference (IV, Random, 95% CI) | 0.54 [−0.78, 1.87] | |
| 4 Mental state: 2b. BPRS positive subscore, excluding possibly skewed data (high = poor) | 1 | 13 | Mean Difference (IV, Random, 95% CI) | −1.11 [−4.32, 2.10] |
| 5 Mental state: 3. SANS, excluding possibly skewed data (high=poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | −11.0 [−20.90, −1.10] |
Comparison 3. CLOZAPINE versus QUETIAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early - short term | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 any reason | 2 | 94 | Risk Ratio (M-H, Random, 95% CI) | 1.51 [0.42, 5.50] |
| 1.2 adverse effects | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 7.0 [0.37, 130.82] |
| 1.3 inefficacy | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 2 Global state: No clinically important change - less than “common criteria” - short term | 1 | 76 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.85, 1.35] |
| 3 Mental state: 1. No clinically important change - less than 50% reduction PANSS total -short term | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.47, 1.89] |
| 4 Mental state: 2a. PANSS total score - short term (high = poor) | 4 | 232 | Mean Difference (IV, Random, 95% CI) | 0.50 [−1.86, 2.85] |
| 5 Mental state: 2b. BPRS-18 (1-7) total score - short term (high = poor) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.89 [−1.33, 3.11] |
| 6 Mental state: 3. Positive symptoms: PANSS positive subscore - short term (high = poor) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 0.70 [−0.68, 2.07] |
| 7 Mental state: 4 No clinically important change - less than 50% reduction SANS - short term | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.89, 1.29] |
| 8 Mental state: 5a. Negative symptoms: PANSS negative subscore - short term (high = poor) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | 2.23 [0.99, 3.48] |
| 9 Mental state: 5b. Negative symptoms: SANS - short term (high = poor) | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 1.64 [−4.66, 7.94] |
| 10 Adverse effects: 1. At least one adverse effect - short term | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 2.41 [1.52, 3.82] |
| 11 Adverse effects: 2. Cardiac problems - short term | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 11.1 ECG abnormalities | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 8.00 [1.05, 60.72] |
| 11.2 palpitation | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 1.20 [0.67, 2.18] |
| 12 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use - short term | 1 | 27 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 13 Adverse effects: 3b. Extrapyramidal: various symptoms - short term | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 13.1 akathisia | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 2.52 [0.50, 12.61] |
| 13.2 tremor | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 1.01 [0.30, 3.43] |
| 13.3 rigor | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 0.52 [0.05, 5.41] |
| 14 Adverse effects: 4. Hypersalivation - short term | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 33.91 [6.96, 165.24] |
| 15 Adverse effects: 5. Lipids: average triglyceride change -short term (high = poor) | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 24.64 [20.76, 28.52] |
| 16 Adverse effects: 6. Sedation -short term | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 4.47 [2.11, 9.49] |
| 17 Adverse effects: 7a. Weight: number of participant with weight gain - short term | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 1.89 [0.90, 3.96] |
| 18 Adverse effects: 7b. Weight: average weight change - short term (high = poor) | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 2.11 [−0.08, 4.30] |
| 19 Adverse effects: 8. White blood cell count: number of participant with a decrease - short term | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 5.16 [0.26, 103.27] |
Comparison 4. CLOZAPINE versus QUETIAPINE - Sensitivity Analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1. PANSS total score - excluding possibly skewed data - short term (high = poor) | 1 | 27 | Mean Difference (IV, Random, 95% CI) | −0.18 [−4.47, 4.11] |
Comparison 5. CLOZAPINE versus RISPERIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death - short term | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1.1 Natural causes | 2 | 293 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.06, 15.48] |
| 1.2 Suicide | 1 | 20 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 2 Leaving the study early: 1. Any reason | 7 | 655 | Risk Ratio (M-H, Random, 95% CI) | 0.92 [0.73, 1.16] |
| 2.1 short term | 5 | 467 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.60, 1.54] |
| 2.2 medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.60, 1.56] |
| 2.3 long term | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.57, 1.18] |
| 3 Leaving the study early: 2. Adverse effects | 6 | 627 | Risk Ratio (M-H, Random, 95% CI) | 1.88 [1.11, 3.21] |
| 3.1 short term | 4 | 439 | Risk Ratio (M-H, Random, 95% CI) | 1.49 [0.82, 2.70] |
| 3.2 medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 4.1 [0.93, 18.14] |
| 3.3 long term | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | 7.13 [0.91, 56.00] |
| 4 Leaving the study early: 3. Inefficacy | 6 | 627 | Risk Ratio (M-H, Random, 95% CI) | 0.40 [0.23, 0.70] |
| 4.1 short term | 4 | 439 | Risk Ratio (M-H, Random, 95% CI) | 0.32 [0.11, 0.96] |
| 4.2 medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.15, 6.93] |
| 4.3 long term | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.19, 0.80] |
| 5 Global state: No clinically important change - less than much improved on CGI - short term | 1 | 60 | Risk Ratio (M-H, Random, 95% CI) | 0.8 [0.43, 1.49] |
| 6 Mental state: 1. No clinically important change - various criteria | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 6.1 less than 20% reduction on BPRS-18 (1-7) total score -short term | 1 | 29 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.50, 1.28] |
| 6.2 less than 20% reduction on BPRS and mildly ill or better - short term | 1 | 273 | Risk Ratio (M-H, Random, 95% CI) | 0.95 [0.78, 1.17] |
| 6.3 less than 20% reduction on 4-item BPRS psychosis and no psychotic symptoms rated less than mild - long term | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.78, 1.21] |
| 6.4 less than 40% improvement on the 4-item BPRS psychosis cluster - long term | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.69, 1.32] |
| 6.5 less than 20% reduction on PANSS total - short term | 2 | 106 | Risk Ratio (M-H, Random, 95% CI) | 1.18 [0.70, 1.99] |
| 7 Mental state: 2a. PANSS total score (high = poor) | 5 | 468 | Mean Difference (IV, Random, 95% CI) | −1.49 [−6.42, 3.44] |
| 7.1 short term | 4 | 387 | Mean Difference (IV, Random, 95% CI) | −0.75 [−6.85, 5.35] |
| 7.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −3.60 [−13.32, 6.12] |
| 8 Mental state: 2b. BPRS-18 (1-7) total score - short term (high = poor) | 3 | 337 | Mean Difference (IV, Random, 95% CI) | −2.98 [−6.93, 0.97] |
| 8.1 short term | 2 | 285 | Mean Difference (IV, Random, 95% CI) | −5.11 [−7.99, −2.23] |
| 8.2 long term | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.40 [−3.52, 4.32] |
| 9 Mental state: 3a. Positive symptoms: PANSS positive subscore (high = poor) | 5 | 562 | Mean Difference (IV, Random, 95% CI) | −0.99 [−2.29, 0.32] |
| 9.1 short term | 4 | 481 | Mean Difference (IV, Random, 95% CI) | −0.89 [−2.59, 0.81] |
| 9.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −0.40 [−3.58, 2.78] |
| 10 Mental state: 3b. Positive symptoms: BPRS-18 (1-7) positive subscore - short term (high = poor) | 1 | 29 | Mean Difference (IV, Random, 95% CI) | −2.10 [−4.76, 0.56] |
| 11 Mental state: 4a. Negative symptoms: PANSS negative subscore (high = poor) | 5 | 562 | Mean Difference (IV, Random, 95% CI) | 0.13 [−1.71, 1.96] |
| 11.1 short term | 4 | 481 | Mean Difference (IV, Random, 95% CI) | 0.55 [−1.71, 2.80] |
| 11.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −1.40 [−4.22, 1.42] |
| 12 Mental state: 4b. Negative symptoms: SANS - short term (high = poor) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 0.62 [−2.51, 3.74] |
| 13 General functioning: GAF score - short term (high = good) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | −9.0 [−18.44, 0.44] |
| 14 Social functioning: SFS score - short term (high = good) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | −47.0 [−93.55, −0.45] |
| 15 Treatment satisfaction: DAI score - short term (high = good) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.10 [−2.57, 2.77] |
| 16 Cognitive functioning: No clinically important change -less than 0.5 SD improved -medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 1.26 [0.95, 1.67] |
| 17 Adverse effects: 1. At least one adverse effect - short term | 2 | 333 | Risk Ratio (M-H, Random, 95% CI) | 1.17 [0.71, 1.95] |
| 18 Adverse effects: 2. Cardiac problems | 2 | 167 | Risk Ratio (M-H, Random, 95% CI) | 0.65 [0.03, 15.30] |
| 18.1 short term | 1 | 60 | Risk Ratio (M-H, Random, 95% CI) | 0.65 [0.03, 15.30] |
| 18.2 long term | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 19 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use | 6 | 304 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.22, 0.68] |
| 19.1 short term | 5 | 223 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.19, 0.77] |
| 19.2 medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.15, 1.00] |
| 20 Adverse effects: 3b. Extrapyramidal: various symptoms - short term | 4 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 20.1 at least one EPS | 2 | 333 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.26, 2.28] |
| 20.2 akathisia | 1 | 40 | Risk Ratio (M-H, Random, 95% CI) | 9.00 [0.52, 156.91] |
| 20.3 dyskinesia | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.38, 2.61] |
| 20.4 dystonia | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 0.5 [0.05, 5.31] |
| 20.5 parkinsonism | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 1.59 [1.03, 2.45] |
| 20.6 tremor | 1 | 40 | Risk Ratio (M-H, Random, 95% CI) | 2.0 [0.72, 5.59] |
| 21 Adverse effects: 3c. Extrapyramidal: symptom scales (high = poor) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 21.1 ESRS score | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.30 [−1.31, 1.91] |
| 21.2 SAS score | 2 | 69 | Mean Difference (IV, Random, 95% CI) | −0.81 [−1.73, 0.10] |
| 21.3 BARS score | 1 | 106 | Mean Difference (IV, Random, 95% CI) | −0.20 [−0.42, 0.02] |
| 22 Adverse effects: 4. Glucose: average change - medium term (high = poor) | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 1.70 [−8.64, 12.04] |
| 23 Adverse effects: 5. Hypersalivation -short term | 3 | 373 | Risk Ratio (M-H, Random, 95% CI) | 4.38 [1.86, 10.30] |
| 24 Adverse effects: 6. Lipids: average change (high = poor) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 24.1 average cholesterol change | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 7.10 [−19.81, 34.01] |
| 24.2 average triglyceride change | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 32.41 [29.26, 35.56] |
| 25 Adverse effects: 7a. Prolactin: associated side effects - short term | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 25.1 disminished sexual drive | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 0.5 [0.10, 2.59] |
| 26 Adverse effects: 7b. Prolactin: average at endpoint (high = poor) | 2 | 55 | Mean Difference (IV, Random, 95% CI) | −28.61 [−46.69, −10. 52] |
| 26.1 short term | 1 | 27 | Mean Difference (IV, Random, 95% CI) | −38.5 [−53.70, −23. |
| 26.2 medium term | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 30] −20.0 [−31.81, −8.19] |
| 27 Adverse effects: 8. Sedation -short term | 5 | 479 | Risk Ratio (M-H, Random, 95% CI) | 1.73 [1.24, 2.42] |
| 28 Adverse effects: 9. Seizures | 2 | 354 | Risk Ratio (M-H, Random, 95% CI) | 4.47 [1.43, 14.01] |
| 28.1 short term | 1 | 273 | Risk Ratio (M-H, Random, 95% CI) | 3.91 [1.13, 13.56] |
| 28.2 medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 9.22 [0.51, 165.87] |
| 29 Adverse effects: 10a. Weight: number of participants with weight gain | 3 | 207 | Risk Ratio (M-H, Random, 95% CI) | 2.28 [0.80, 6.46] |
| 29.1 short term | 2 | 126 | Risk Ratio (M-H, Random, 95% CI) | 4.65 [0.24, 88.47] |
| 29.2 medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 1.79 [0.57, 5.66] |
| 30 Adverse effects: 10b. Weight: average weight change (high=poor) | 4 | 459 | Mean Difference (IV, Random, 95% CI) | 2.84 [1.17, 4.50] |
| 30.1 short term | 3 | 382 | Mean Difference (IV, Random, 95% CI) | 3.16 [0.92, 5.41] |
| 30.2 medium term | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 1.90 [0.17, 3.63] |
| 31 Adverse effects: 11. White blood cell count: number of participants with a decrease | 4 | 294 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.33, 4.99] |
| 31.1 short term | 2 | 106 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.10, 8.58] |
| 31.2 medium term | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 1.54 [0.27, 8.72] |
| 31.3 long term | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
Comparison 6. CLOZAPINE vs. RISPERIDONE - Sensitivity Analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1. BPRS-18 (1-7) total score, excluding possibly skewed data (high = poor) | 1 | 256 | Mean Difference (IV, Random, 95% CI) | −5.5 [−8.78, −2.22] |
| 2 Mental state: 2. PANSS positive subscore, excluding possibly skewed data (high = poor) | 3 | 423 | Mean Difference (IV, Random, 95% CI) | −0.85 [−2.91, 1.21] |
| 2.1 short term | 2 | 342 | Mean Difference (IV, Random, 95% CI) | −0.66 [−4.20, 2.87] |
| 2.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −0.40 [−3.58, 2.78] |
| 3 Mental state: 3. PANSS negative subscore, excluding possibly skewed data (high = poor) | 4 | 442 | Mean Difference (IV, Random, 95% CI) | −0.14 [−2.31, 2.02] |
| 3.1 short term | 3 | 361 | Mean Difference (IV, Random, 95% CI) | 0.39 [−2.59, 3.37] |
| 3.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −1.40 [−4.22, 1.42] |
Comparison 7. CLOZAPINE versus ZIPRASIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: any reason - medium term | 1 | 146 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.66, 1.51] |
| 2 Mental state: PANSS total score - medium term (high = poor) | 1 | 146 | Mean Difference (IV, Random, 95% CI) | 0.5 [−6.72, 7.72] |
| 3 Adverse effects: 1. Cardiac problems | 1 | 146 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
Comparison 8. CLOZAPINE versus ZOTEPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early: any reason | 1 | 50 | Risk Ratio (M-H, Random, 95% CI) | 0.7 [0.32, 1.54] |
| 2 Global state: no clinically important change - less than successfully and no increase on CGI-S - short term | 1 | 59 | Risk Ratio (M-H, Random, 95% CI) | 0.12 [0.02, 0.87] |
| 3 Mental state: BPRS-18 total score - short term (high=poor) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | −6.0 [−9.83, −2.17] |
| 4 Adverse effects: 1. Extrapyramidal: antiparkinson medication use - short term | 1 | 59 | Risk Ratio (M-H, Random, 95% CI) | 0.05 [0.00, 0.86] |
| 5 Adverse effects: 2. Prolactin: average change - short term (high=poor) | 1 | 59 | Mean Difference (IV, Random, 95% CI) | −33.4 [−48.67, −18.13] |
Analysis 1.1. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 1 Death.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 1 Death
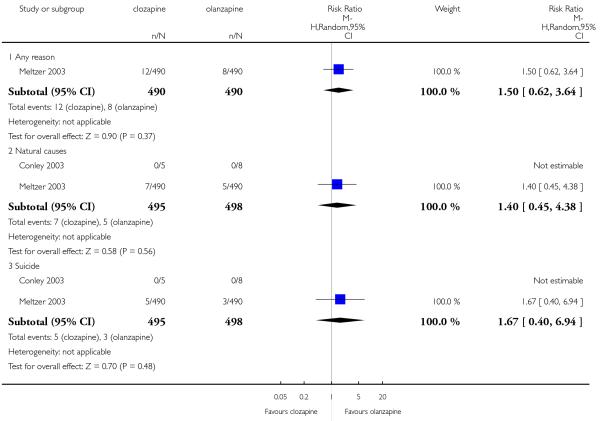 |
Analysis 1.2. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 2 Leaving the study early: 1. Any reason.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 2 Leaving the study early: 1. Any reason
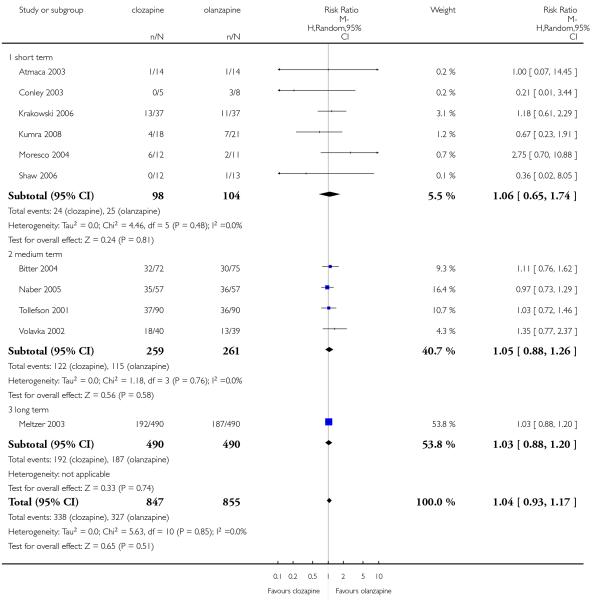 |
Analysis 1.3. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 3 Leaving the study early: 2. Adverse effects.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 3 Leaving the study early: 2. Adverse effects
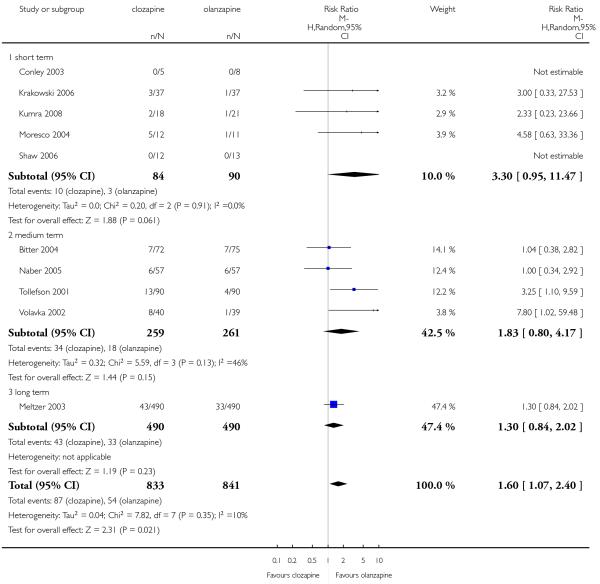 |
Analysis 1.4. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 4 Leaving the study early: 3. Inefficacy.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 4 Leaving the study early: 3. Inefficacy
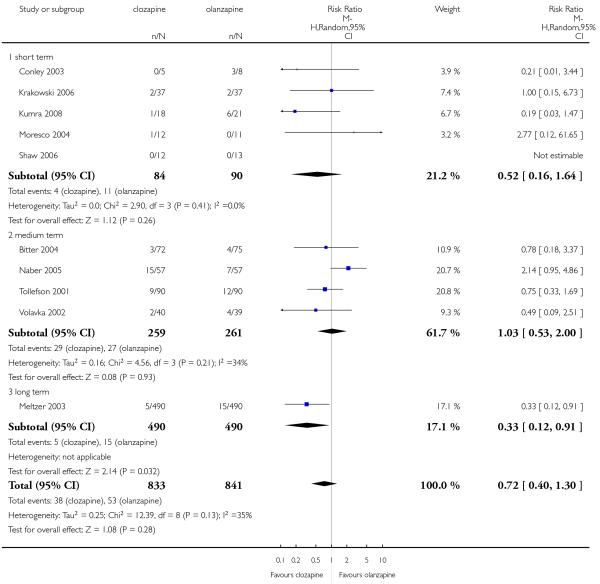 |
Analysis 1.5. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 5 Global state.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 5 Global state
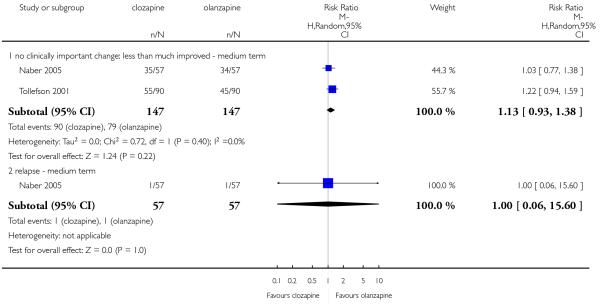 |
Analysis 1.6. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 6 Mental state: 1. No clinically important change - various criteria.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 6 Mental state: 1. No clinically important change - various criteria
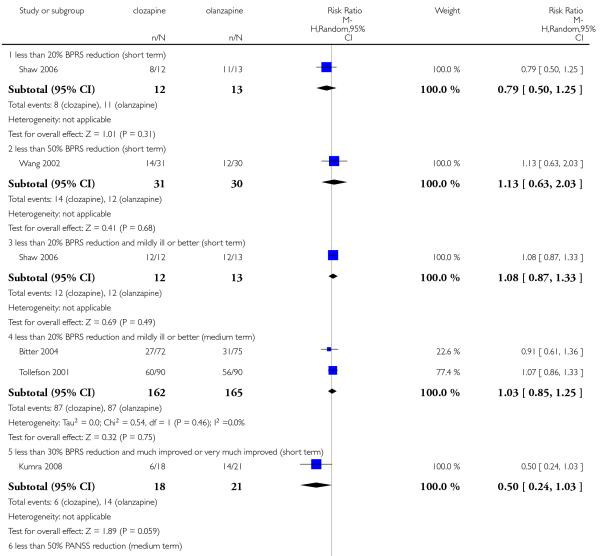 |
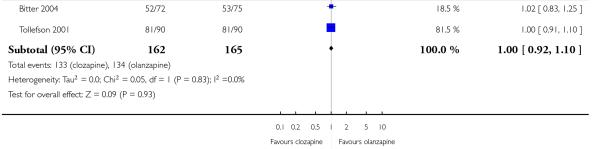 |
Analysis 1.7. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 7 Mental state: 2a. PANSS total score (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 7 Mental state: 2a. PANSS total score (high = poor)
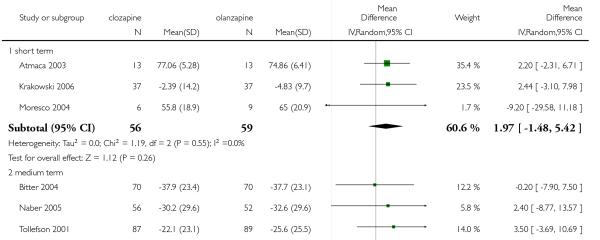 |
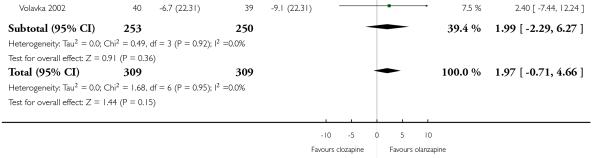 |
Analysis 1.8. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 8 Mental state: 2b. BPRS-18 (1- 7) total score (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 8 Mental state: 2b. BPRS-18 (1-7) total score (high = poor)
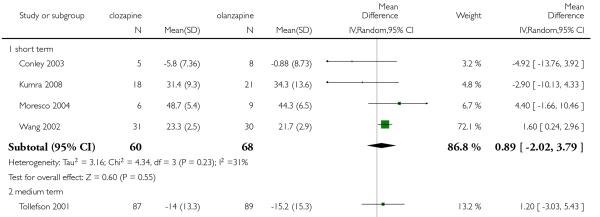 |
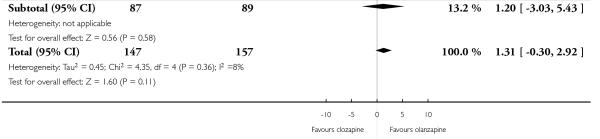 |
Analysis 1.9. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 9 Mental state: 2c. BPRS total score, various versions (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 9 Mental state: 2c. BPRS total score, various versions (high = poor)
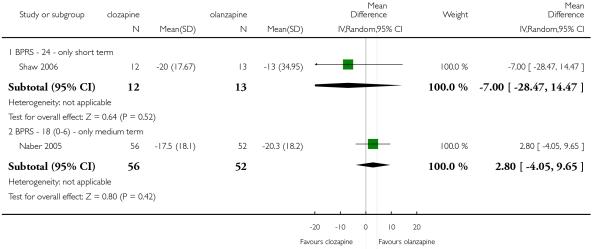 |
Analysis 1.10. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 10 Mental state: 3a. Positive symptoms: PANSS positive subscore (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 10 Mental state: 3a. Positive symptoms: PANSS positive subscore (high = poor)
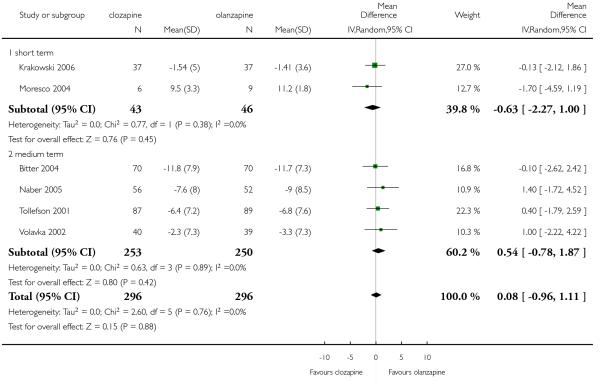 |
Analysis 1.11. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 11 Mental state: 3b. Positive symptoms: SAPS - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 11 Mental state: 3b. Positive symptoms: SAPS - short term (high = poor)
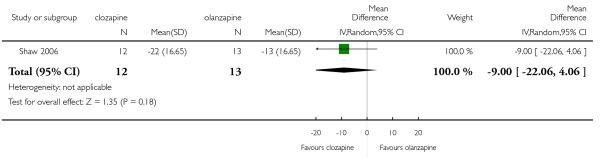 |
Analysis 1.12. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 12 Mental state: 3c. Positive symptoms: BPRS positive subscore (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 12 Mental state: 3c. Positive symptoms: BPRS positive subscore (high = poor)
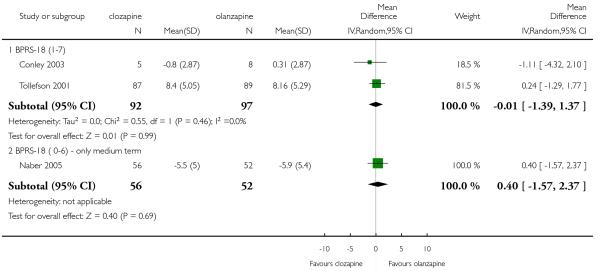 |
Analysis 1.13. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 13 Mental state: 4a. Negative symptoms: PANSS negative subscore (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 13 Mental state: 4a. Negative symptoms: PANSS negative subscore (high = poor)
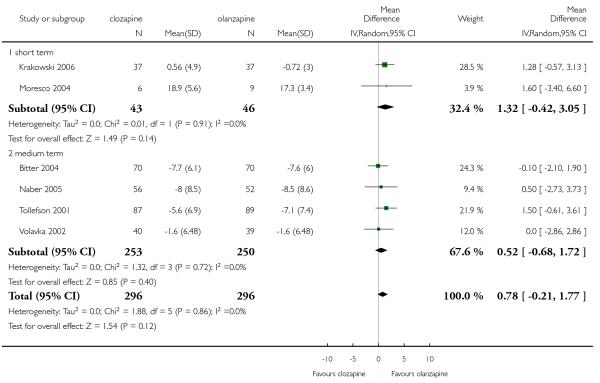 |
Analysis 1.14. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 14 Mental state: 4b. Negative symptoms: SANS - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 14 Mental state: 4b. Negative symptoms: SANS - short term (high = poor)
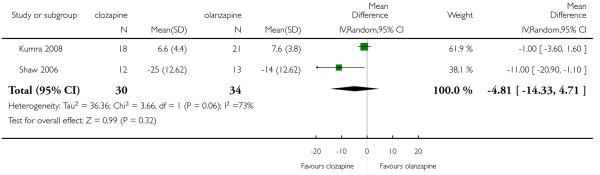 |
Analysis 1.15. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 15 Mental state: 4c. Negative symptoms: BPRS negative subscore -(high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 15 Mental state: 4c. Negative symptoms: BPRS negative subscore -(high = poor)
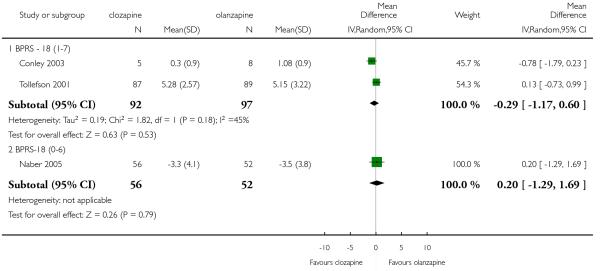 |
Analysis 1.16. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 16 Cognitive functioning: 1. No clinically important change - less than 0.5 SD improved - medium term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 16 Cognitive functioning: 1. No clinically important change - less than 0.5 SD improved - medium term
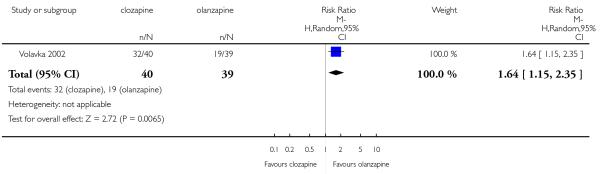 |
Analysis 1.17. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 17 Quality of Life: 1. SWN-38 total score - medium term (high= good).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 17 Quality of Life: 1. SWN-38 total score - medium term (high= good)
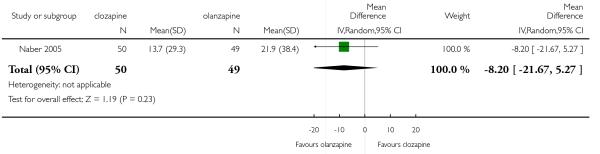 |
Analysis 1.18. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 18 Quality of Life: 2. MLDL total score - medium term (high = good).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 18 Quality of Life: 2. MLDL total score - medium term (high = good)
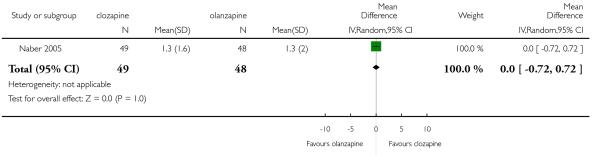 |
Analysis 1.19. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 19 Service use: Hospital readmission - long term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 19 Service use: Hospital readmission - long term
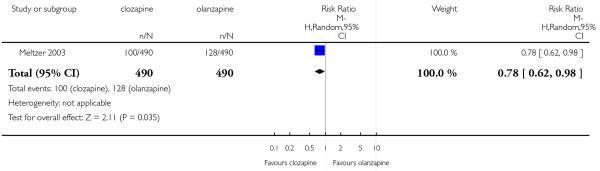 |
Analysis 1.20. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 20 Adverse effects: 1. At least one adverse effect.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 20 Adverse effects: 1. At least one adverse effect
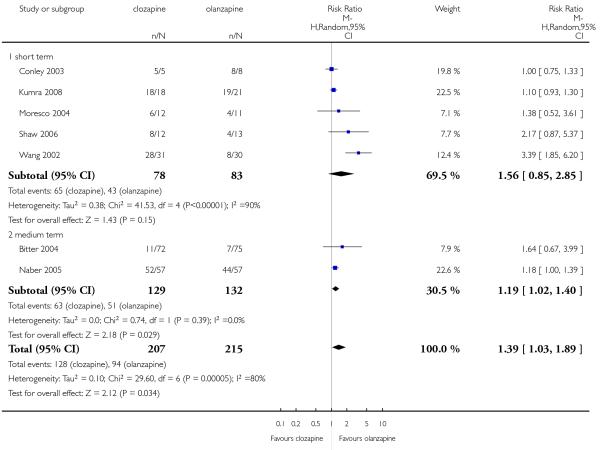 |
Analysis 1.21. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 21 Adverse effects: 2. Cardiac problems.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 21 Adverse effects: 2. Cardiac problems
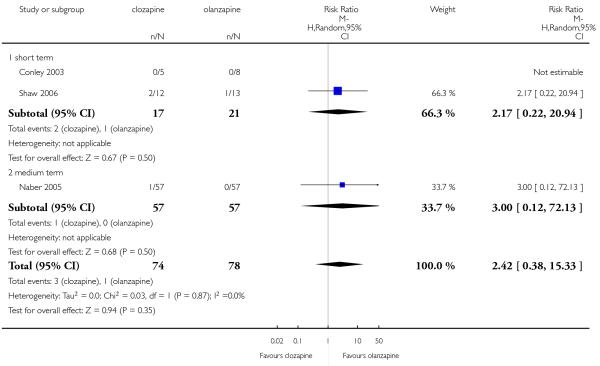 |
Analysis 1.22. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 22 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 22 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use
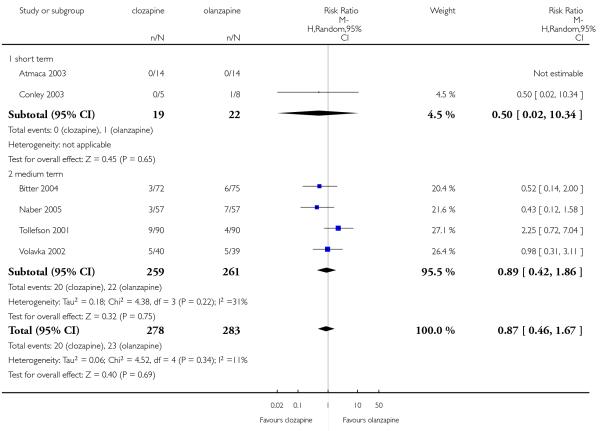 |
Analysis 1.23. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 23 Adverse effects: 3d. Extrapyramidal: SAS change or endpoint (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 23 Adverse effects: 3d. Extrapyramidal: SAS change or endpoint (high = poor)
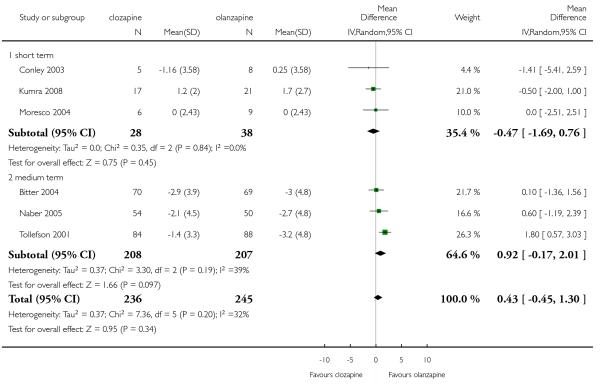 |
Analysis 1.24. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 24 Adverse effects: 3c. Extrapyramidal: ESRS score at endpoint - medium term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 24 Adverse effects: 3c. Extrapyramidal: ESRS score at endpoint - medium term (high = poor)
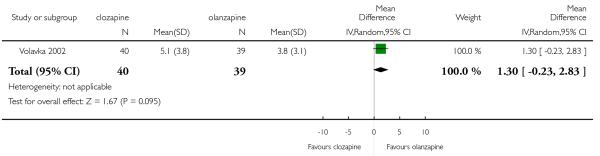 |
Analysis 1.25. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 25 Adverse effects: 3b. Extrapyramidal: various symptoms.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 25 Adverse effects: 3b. Extrapyramidal: various symptoms
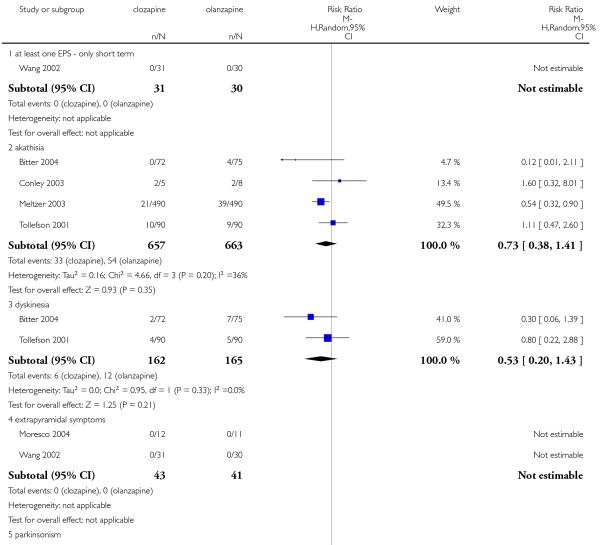 |
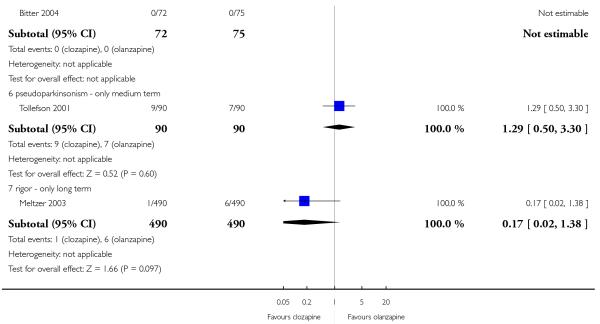 |
Analysis 1.26. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 26 Adverse effects: 3e. Extrapyramidal: akathisia - BARS change - medium term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 26 Adverse effects: 3e. Extrapyramidal: akathisia - BARS change - medium term (high = poor)
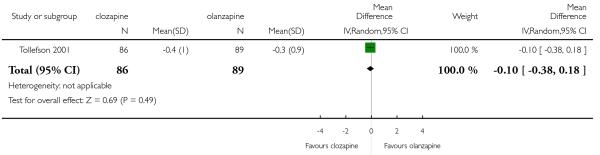 |
Analysis 1.27. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 27 Adverse effects: 3f. Extrapyramidal: Hillside Akathisia Scale - medium term (high=poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 27 Adverse effects: 3f. Extrapyramidal: Hillside Akathisia Scale - medium term (high=poor)
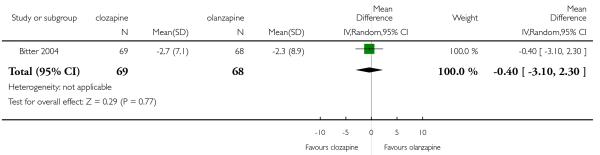 |
Analysis 1.28. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 28 Adverse effects: 3g. Extrapyramidal: tardive dyskinesia - AIMS change or endpoint - (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 28 Adverse effects: 3g. Extrapyramidal: tardive dyskinesia - AIMS change or endpoint - (high = poor)
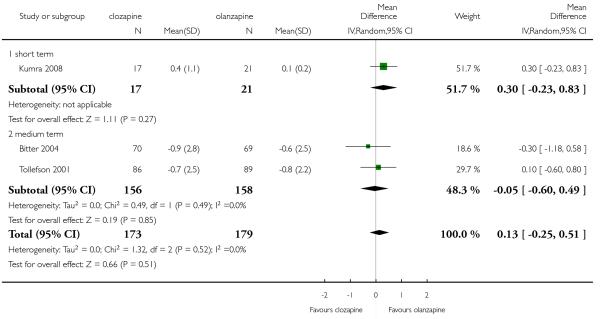 |
Analysis 1.29. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 29 Adverse effects: 4a. Glucose: number of participants with significant increase - long term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 29 Adverse effects: 4a. Glucose: number of participants with significant increase - long term
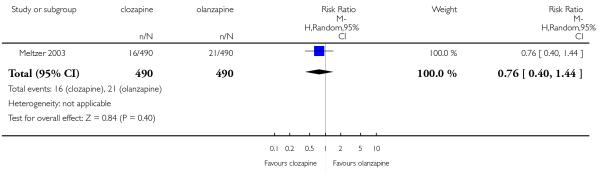 |
Analysis 1.30. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 30 Adverse effects: 4b. Glucose: average change or endpoint (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 30 Adverse effects: 4b. Glucose: average change or endpoint (high = poor)
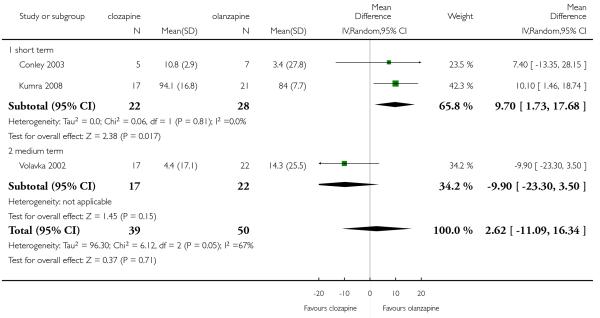 |
Analysis 1.31. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 31 Adverse effects: 5. Hypersalivation.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 31 Adverse effects: 5. Hypersalivation
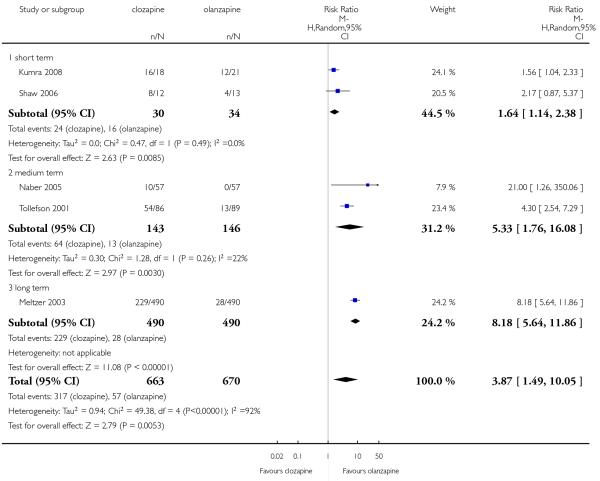 |
Analysis 1.32. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 32 Adverse effects: 6a. Lipids: number of participants with significant increase.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 32 Adverse effects: 6a. Lipids: number of participants with significant increase
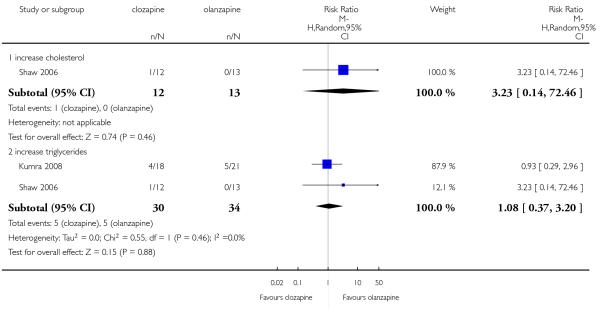 |
Analysis 1.33. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 33 Adverse effects: 6b. Lipids:average cholesterol change or endpoint (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 33 Adverse effects: 6b. Lipids:average cholesterol change or endpoint (high = poor)
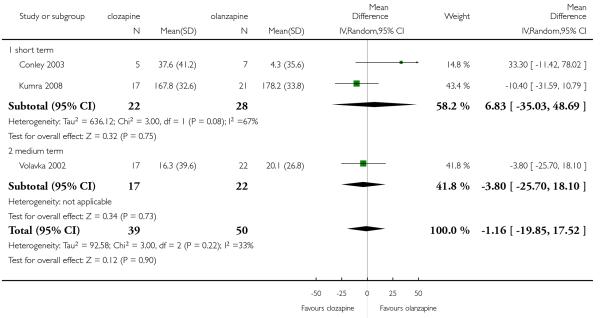 |
Analysis 1.34. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 34 Adverse effects: 6c. Lipids: average triglyceride change -short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 34 Adverse effects: 6c. Lipids: average triglyceride change -short term (high = poor)
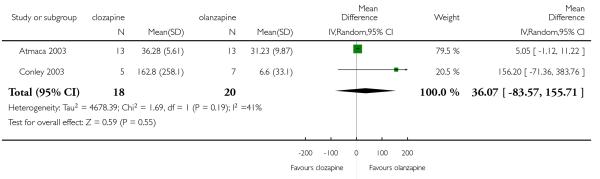 |
Analysis 1.35. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 35 Adverse effects: 7. Prolactin: average change or endpoint (high=poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 35 Adverse effects: 7. Prolactin: average change or endpoint (high=poor)
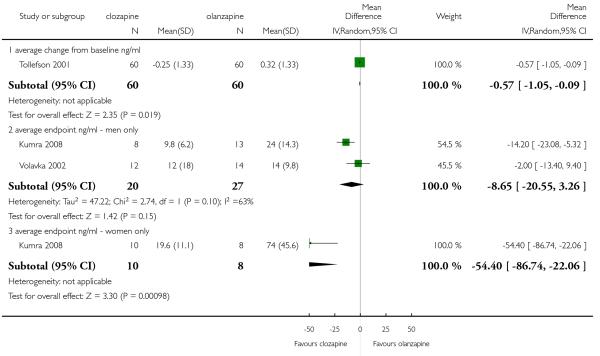 |
Analysis 1.36. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 36 Adverse effects: 8. Sedation.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 36 Adverse effects: 8. Sedation
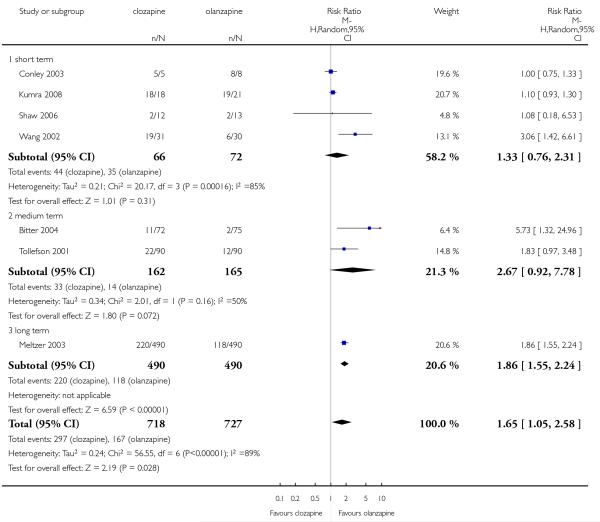 |
Analysis 1.37. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 37 Adverse effects: 9. Seizures.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 37 Adverse effects: 9. Seizures
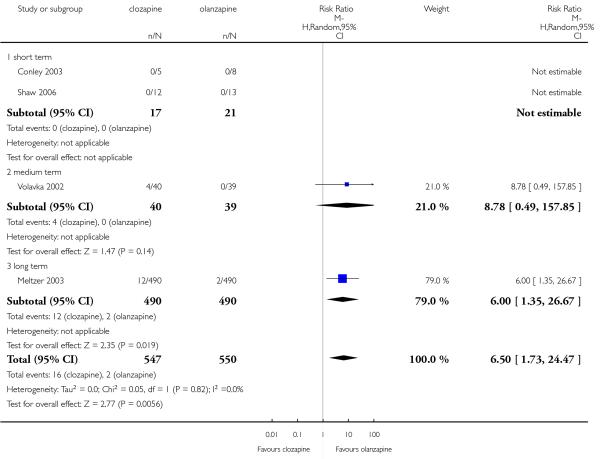 |
Analysis 1.38. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 38 Adverse effects: 10a. Weight: number of participants with weight gain.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 38 Adverse effects: 10a. Weight: number of participants with weight gain
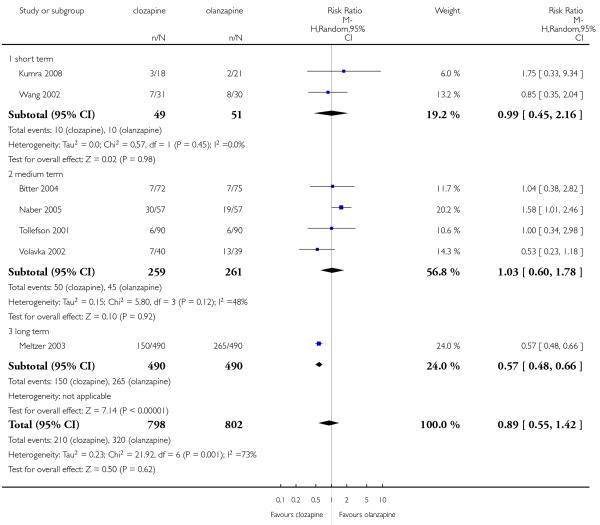 |
Analysis 1.39. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 39 Adverse effects: 10b. Weight: average weight change (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 39 Adverse effects: 10b. Weight: average weight change (high = poor)
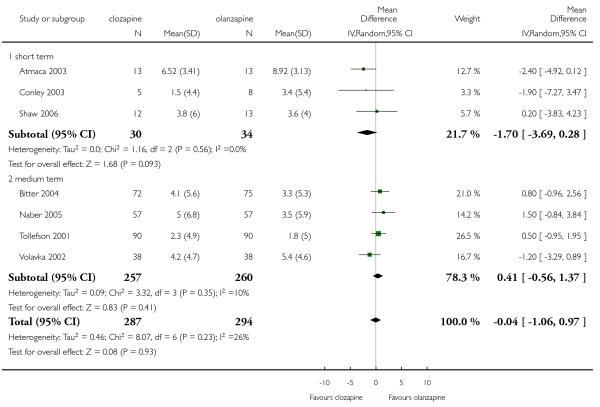 |
Analysis 1.40. Comparison 1 CLOZAPINE versus OLANZAPINE, Outcome 40 Adverse effects: 11.White blood cell count: number of participants with a decrease.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 CLOZAPINE versus OLANZAPINE
Outcome: 40 Adverse effects: 11. White blood cell count: number of participants with a decrease
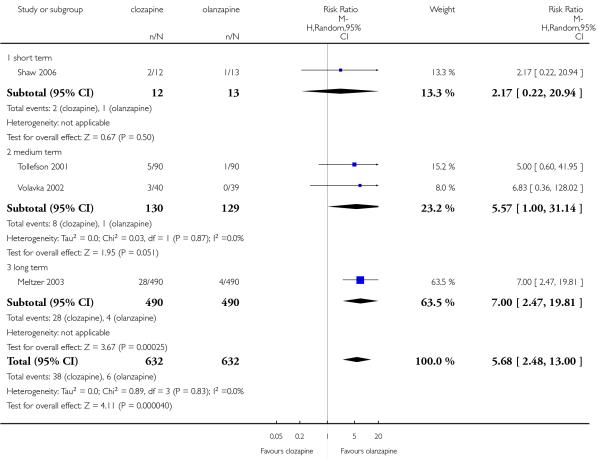 |
Analysis 2.1. Comparison 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis, Outcome 1 Mental state: 1a. PANSS total score, excluding possibly skewed data (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis
Outcome: 1 Mental state: 1a. PANSS total score, excluding possibly skewed data (high = poor)
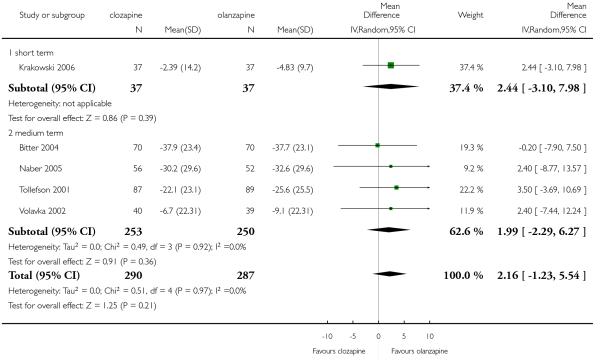 |
Analysis 2.2. Comparison 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis, Outcome 2 Mental state: 1b. BPRS-18 (1-7) total score, excluding possibly skewed data (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis
Outcome: 2 Mental state: 1b. BPRS-18 (1-7) total score, excluding possibly skewed data (high = poor)
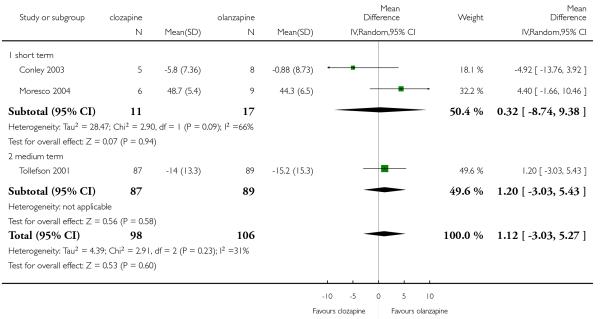 |
Analysis 2.3. Comparison 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis, Outcome 3 Mental state: 2a. PANSS positive subscore, excluding possibly skewed data (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis
Outcome: 3 Mental state: 2a. PANSS positive subscore, excluding possibly skewed data (high = poor)
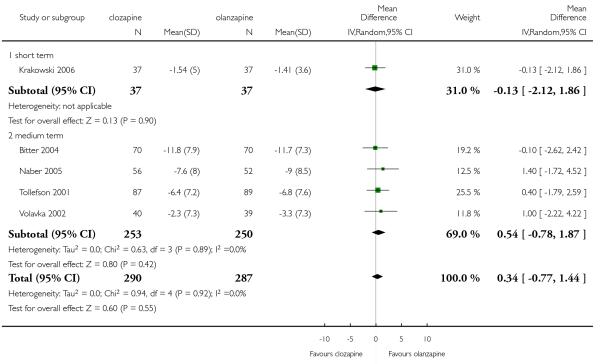 |
Analysis 2.4. Comparison 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis, Outcome 4 Mental state: 2b. BPRS positive subscore, excluding possibly skewed data (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis
Outcome: 4 Mental state: 2b. BPRS positive subscore, excluding possibly skewed data (high = poor)
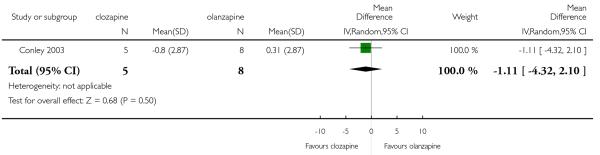 |
Analysis 2.5. Comparison 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis, Outcome 5 Mental state: 3. SANS, excluding possibly skewed data (high=poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 CLOZAPINE versus OLANZAPINE - Sensitivity Analysis
Outcome: 5 Mental state: 3. SANS, excluding possibly skewed data (high=poor)
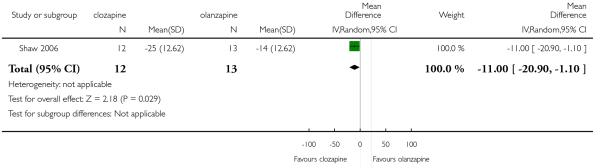 |
Analysis 3.1. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 1 Leaving the study early - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 1 Leaving the study early - short term
 |
Analysis 3.2. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 2 Global state: No clinically important change - less than “common criteria” - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 2 Global state: No clinically important change - less than “common criteria” - short term
 |
Analysis 3.3. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 3 Mental state: 1. No clinically important change - less than 50% reduction PANSS total - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 3 Mental state: 1. No clinically important change - less than 50% reduction PANSS total - short term
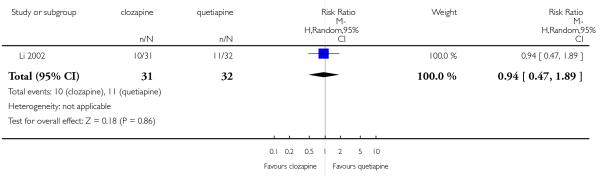 |
Analysis 3.4. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 4 Mental state: 2a. PANSS total score - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 4 Mental state: 2a. PANSS total score - short term (high = poor)
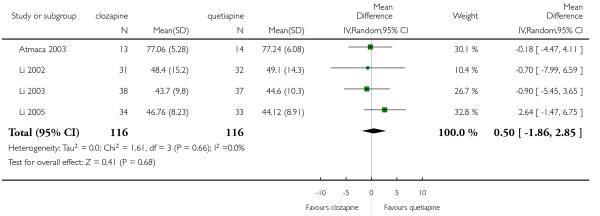 |
Analysis 3.5. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 5 Mental state: 2b. BPRS-18 (1-7) total score - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 5 Mental state: 2b. BPRS-18 (1-7) total score - short term (high = poor)
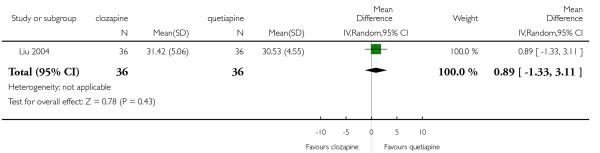 |
Analysis 3.6. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 6 Mental state: 3. Positive symptoms: PANSS positive subscore - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 6 Mental state: 3. Positive symptoms: PANSS positive subscore - short term (high = poor)
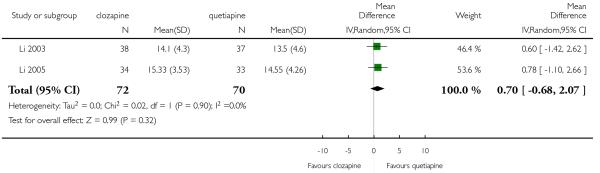 |
Analysis 3.7. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 7 Mental state: 4 No clinically important change - less than 50% reduction SANS - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 7 Mental state: 4 No clinically important change - less than 50% reduction SANS - short term
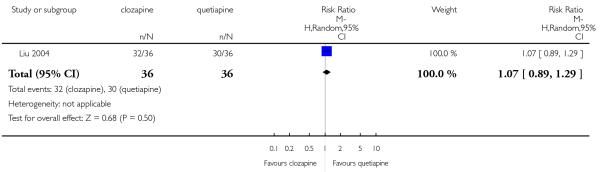 |
Analysis 3.8. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 8 Mental state: 5a. Negative symptoms: PANSS negative subscore - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 8 Mental state: 5a. Negative symptoms: PANSS negative subscore - short term (high = poor)
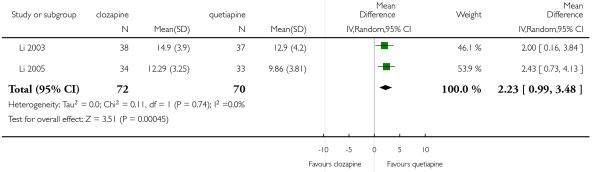 |
Analysis 3.9. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 9 Mental state: 5b. Negative symptoms: SANS - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 9 Mental state: 5b. Negative symptoms: SANS - short term (high = poor)
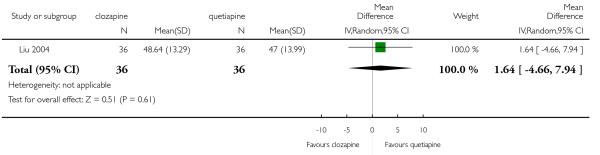 |
Analysis 3.10. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 10 Adverse effects: 1. At least one adverse effect - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 10 Adverse effects: 1. At least one adverse effect - short term
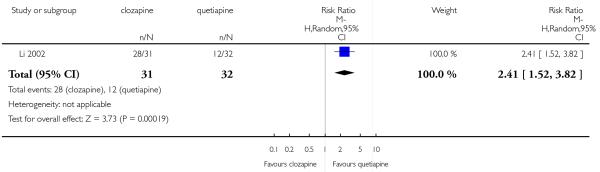 |
Analysis 3.11. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 11 Adverse effects: 2. Cardiac problems - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 11 Adverse effects: 2. Cardiac problems - short term
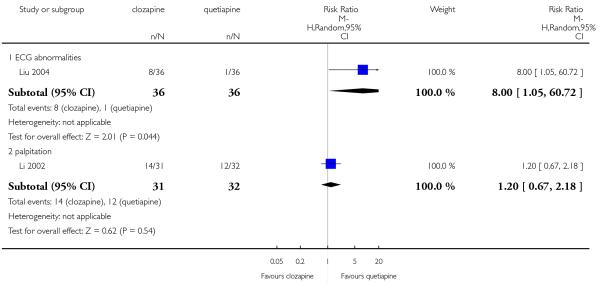 |
Analysis 3.12. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 12 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 12 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use - short term
 |
Analysis 3.13. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 13 Adverse effects: 3b. Extrapyramidal: various symptoms - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 13 Adverse effects: 3b. Extrapyramidal: various symptoms - short term
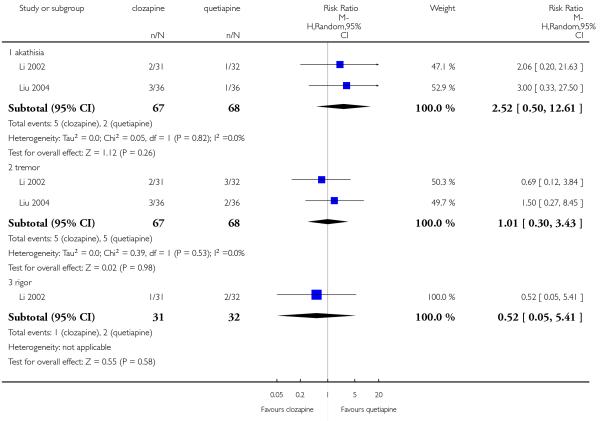 |
Analysis 3.14. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 14 Adverse effects: 4. Hypersalivation - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 14 Adverse effects: 4. Hypersalivation - short term
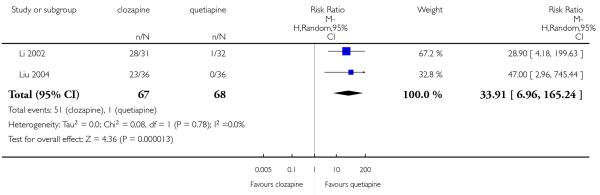 |
Analysis 3.15. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 15 Adverse effects: 5. Lipids: average triglyceride change - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 15 Adverse effects: 5. Lipids: average triglyceride change - short term (high = poor)
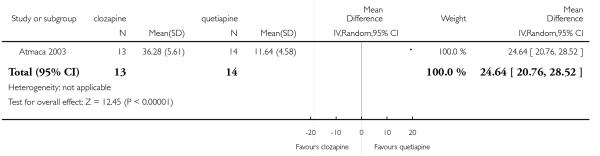 |
Analysis 3.16. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 16 Adverse effects: 6. Sedation - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 16 Adverse effects: 6. Sedation - short term
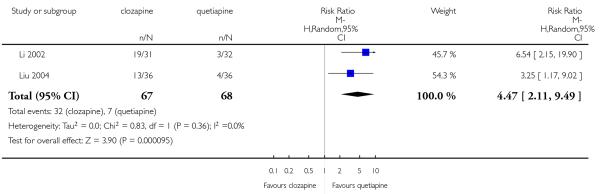 |
Analysis 3.17. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 17 Adverse effects: 7a.Weight: number of participant with weight gain - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 17 Adverse effects: 7a. Weight: number of participant with weight gain - short term
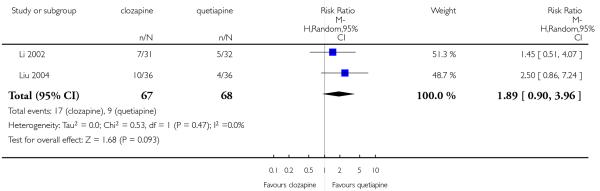 |
Analysis 3.18. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 18 Adverse effects: 7b.Weight: average weight change - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 18 Adverse effects: 7b. Weight: average weight change - short term (high = poor)
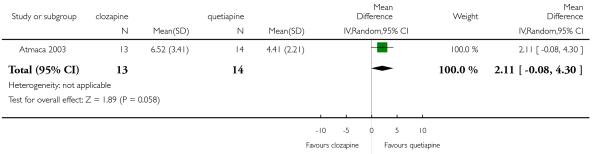 |
Analysis 3.19. Comparison 3 CLOZAPINE versus QUETIAPINE, Outcome 19 Adverse effects: 8.White blood cell count: number of participant with a decrease - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 CLOZAPINE versus QUETIAPINE
Outcome: 19 Adverse effects: 8. White blood cell count: number of participant with a decrease - short term
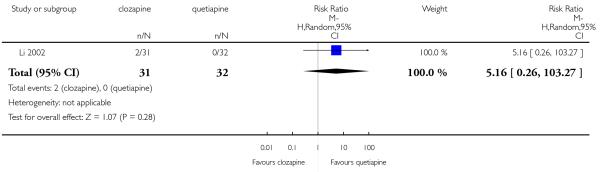 |
Analysis 4.1. Comparison 4 CLOZAPINE versus QUETIAPINE - Sensitivity Analysis, Outcome 1 Mental state: 1. PANSS total score - excluding possibly skewed data - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 CLOZAPINE versus QUETIAPINE - Sensitivity Analysis
Outcome: 1 Mental state: 1. PANSS total score - excluding possibly skewed data - short term (high = poor)
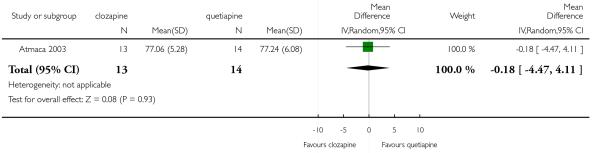 |
Analysis 5.1. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 1 Death - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 1 Death - short term
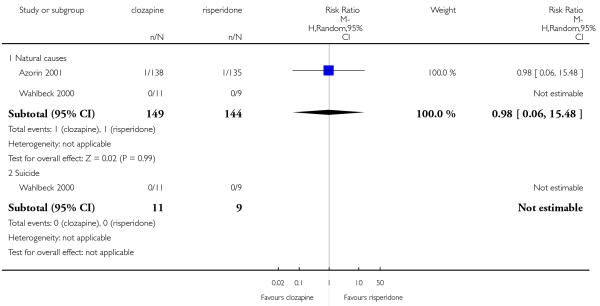 |
Analysis 5.2. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 2 Leaving the study early: 1. Any reason.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 2 Leaving the study early: 1. Any reason
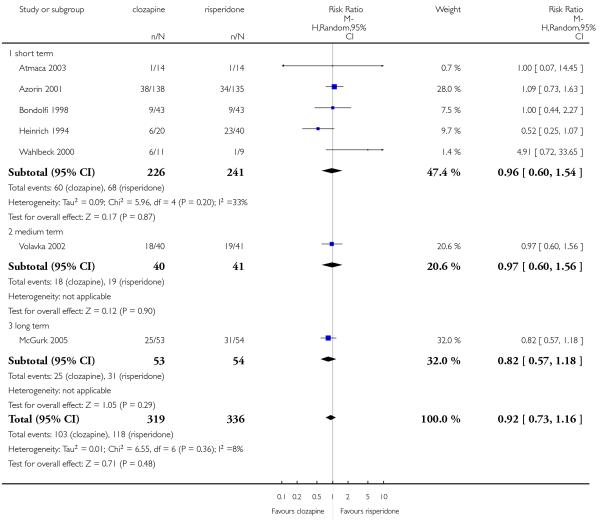 |
Analysis 5.3. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 3 Leaving the study early: 2. Adverse effects.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 3 Leaving the study early: 2. Adverse effects
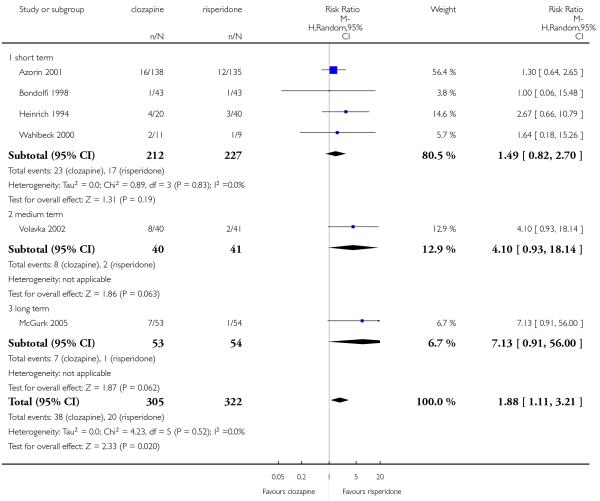 |
Analysis 5.4. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 4 Leaving the study early: 3. Inefficacy.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 4 Leaving the study early: 3. Inefficacy
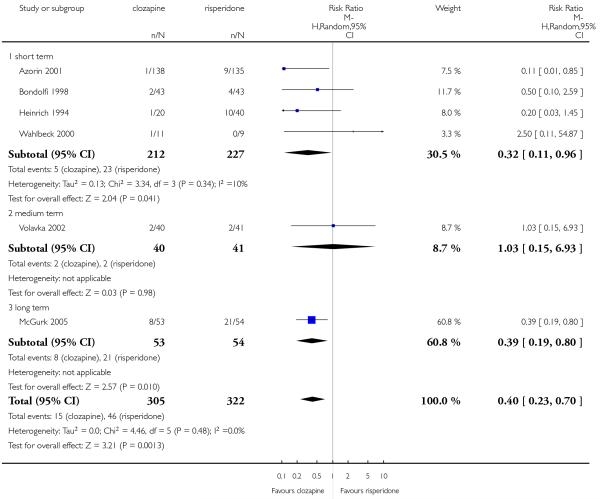 |
Analysis 5.5. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 5 Global state: No clinically important change - less than much improved on CGI - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 5 Global state: No clinically important change - less than much improved on CGI - short term
 |
Analysis 5.6. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 6 Mental state: 1. No clinically important change - various criteria.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 6 Mental state: 1. No clinically important change - various criteria
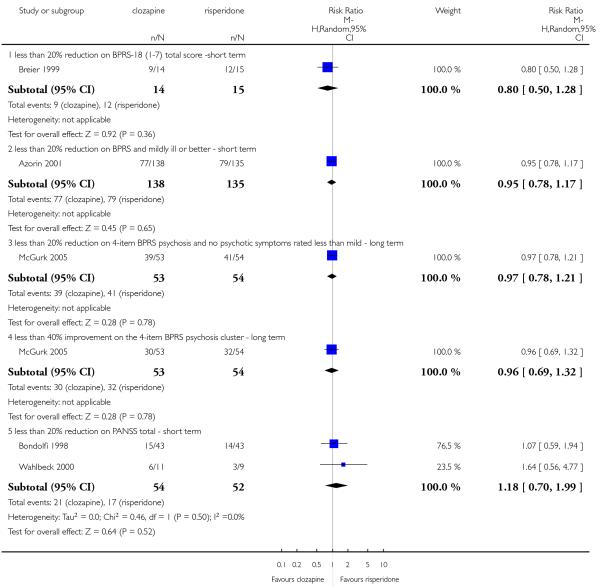 |
Analysis 5.7. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 7 Mental state: 2a. PANSS total score (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 7 Mental state: 2a. PANSS total score (high = poor)
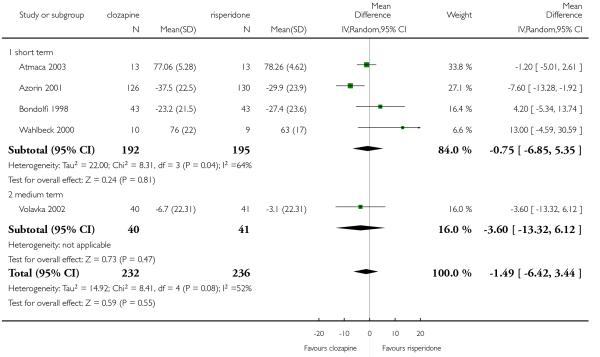 |
Analysis 5.8. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 8 Mental state: 2b. BPRS-18 (1- 7) total score - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 8 Mental state: 2b. BPRS-18 (1-7) total score - short term (high = poor)
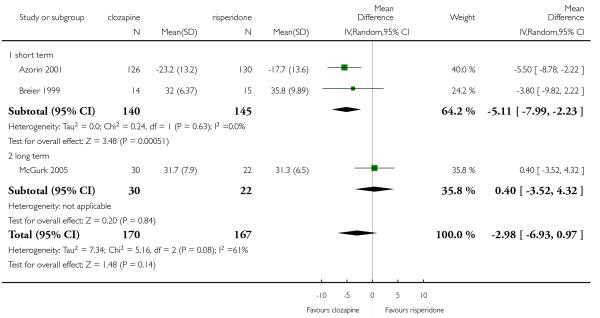 |
Analysis 5.9. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 9 Mental state: 3a. Positive symptoms: PANSS positive subscore (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 9 Mental state: 3a. Positive symptoms: PANSS positive subscore (high = poor)
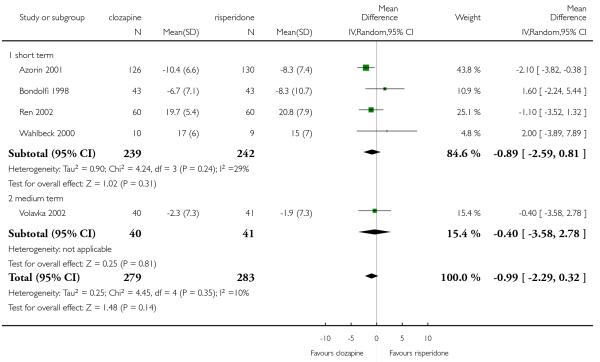 |
Analysis 5.10. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 10 Mental state: 3b. Positive symptoms: BPRS-18 (1-7) positive subscore - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 10 Mental state: 3b. Positive symptoms: BPRS-18 (1-7) positive subscore - short term (high = poor)
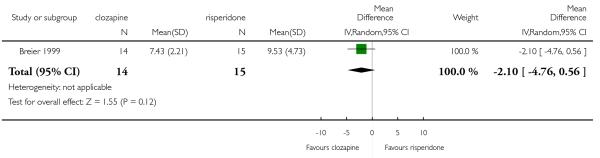 |
Analysis 5.11. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 11 Mental state: 4a. Negative symptoms: PANSS negative subscore (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 11 Mental state: 4a. Negative symptoms: PANSS negative subscore (high = poor)
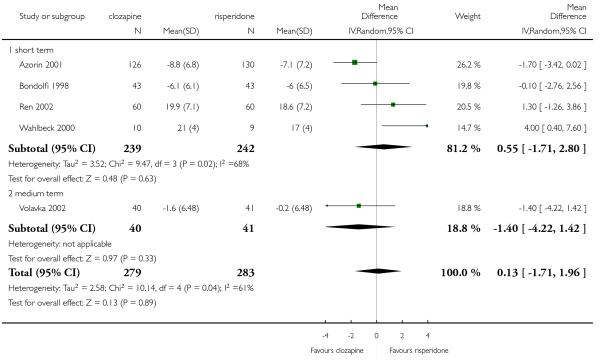 |
Analysis 5.12. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 12 Mental state: 4b. Negative symptoms: SANS - short term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 12 Mental state: 4b. Negative symptoms: SANS - short term (high = poor)
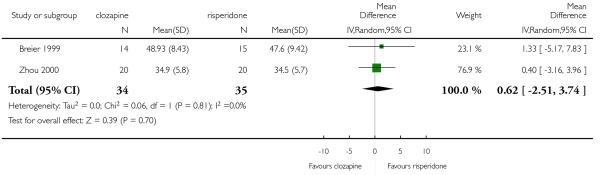 |
Analysis 5.13. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 13 General functioning: GAF score - short term (high = good).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 13 General functioning: GAF score - short term (high = good)
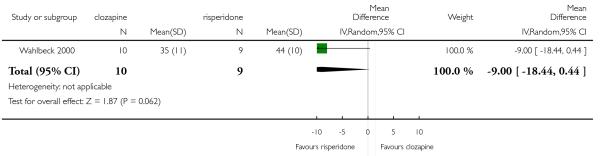 |
Analysis 5.14. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 14 Social functioning: SFS score - short term (high = good).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 14 Social functioning: SFS score - short term (high = good)
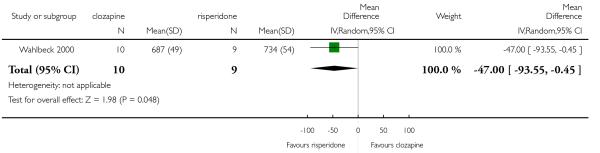 |
Analysis 5.15. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 15 Treatment satisfaction: DAI score - short term (high = good).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 15 Treatment satisfaction: DAI score - short term (high = good)
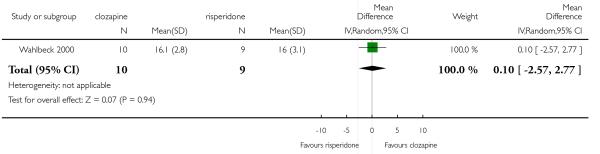 |
Analysis 5.16. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 16 Cognitive functioning: No clinically important change - less than 0.5 SD improved - medium term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 16 Cognitive functioning: No clinically important change - less than 0.5 SD improved - medium term
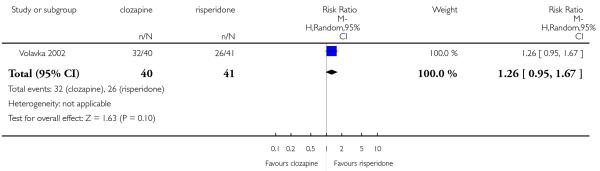 |
Analysis 5.17. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 17 Adverse effects: 1. At least one adverse effect - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 17 Adverse effects: 1. At least one adverse effect - short term
 |
Analysis 5.18. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 18 Adverse effects: 2. Cardiac problems.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 18 Adverse effects: 2. Cardiac problems
 |
Analysis 5.19. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 19 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 19 Adverse effects: 3a. Extrapyramidal: antiparkinson medication use
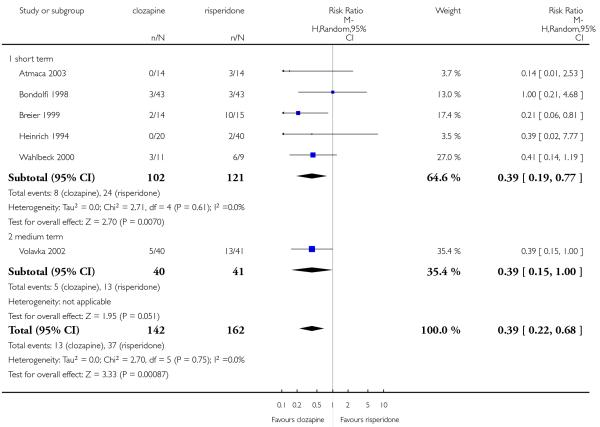 |
Analysis 5.20. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 20 Adverse effects: 3b. Extrapyramidal: various symptoms - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 20 Adverse effects: 3b. Extrapyramidal: various symptoms - short term
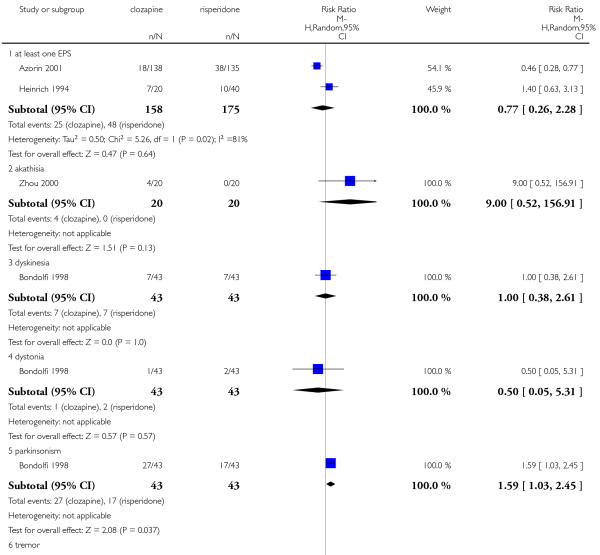 |
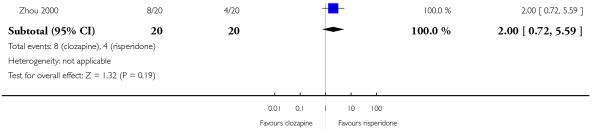 |
Analysis 5.21. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 21 Adverse effects: 3c. Extrapyramidal: symptom scales (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 21 Adverse effects: 3c. Extrapyramidal: symptom scales (high = poor)
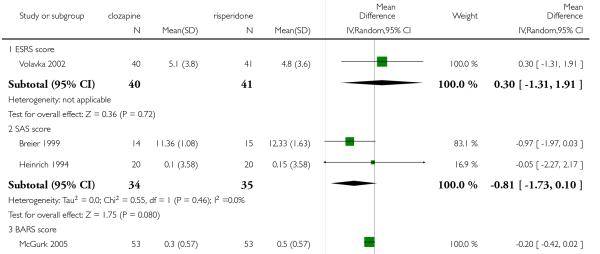 |
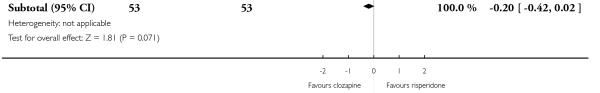 |
Analysis 5.22. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 22 Adverse effects: 4. Glucose: average change - medium term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 22 Adverse effects: 4. Glucose: average change - medium term (high = poor)
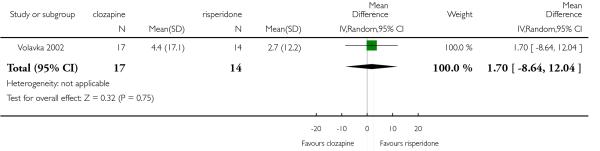 |
Analysis 5.23. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 23 Adverse effects: 5. Hypersalivation -short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 23 Adverse effects: 5. Hypersalivation -short term
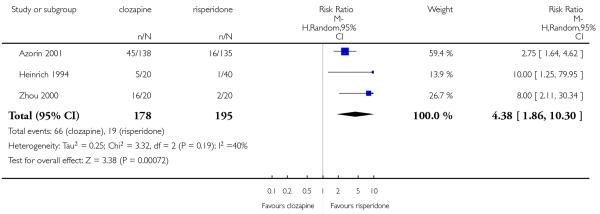 |
Analysis 5.24. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 24 Adverse effects: 6. Lipids: average change (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 24 Adverse effects: 6. Lipids: average change (high = poor)
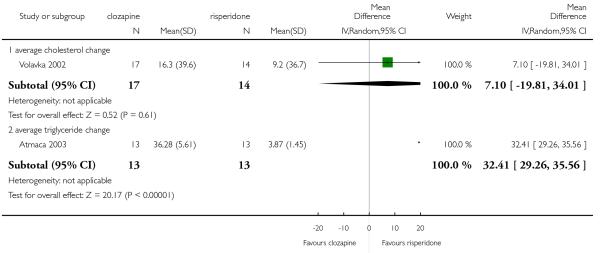 |
Analysis 5.25. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 25 Adverse effects: 7a. Prolactin: associated side effects - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 25 Adverse effects: 7a. Prolactin: associated side effects - short term
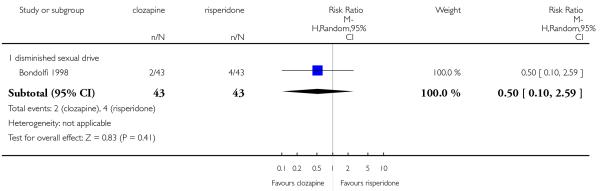 |
Analysis 5.26. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 26 Adverse effects: 7b. Prolactin: average at endpoint (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 26 Adverse effects: 7b. Prolactin: average at endpoint (high = poor)
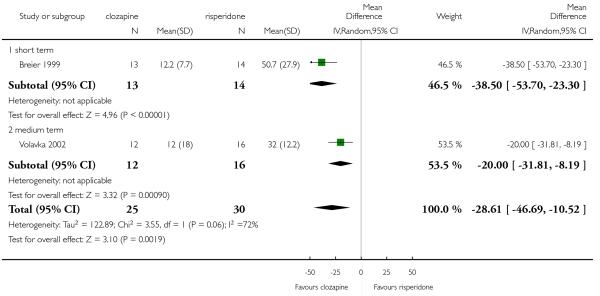 |
Analysis 5.27. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 27 Adverse effects: 8. Sedation - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 27 Adverse effects: 8. Sedation - short term
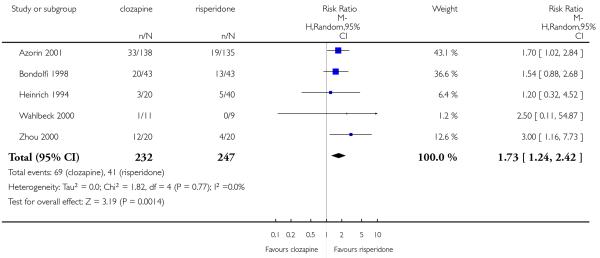 |
Analysis 5.28. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 28 Adverse effects: 9. Seizures. Review: Clozapine versus other atypical antipsychotics for schizophreni.
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 28 Adverse effects: 9. Seizures
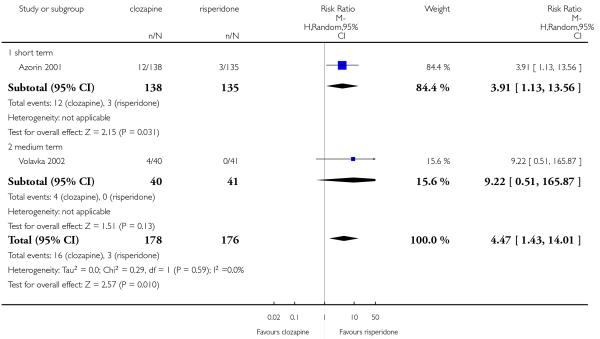 |
Analysis 5.29. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 29 Adverse effects: 10a. Weight: number of participants with weight gain.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 29 Adverse effects: 10a. Weight: number of participants with weight gain
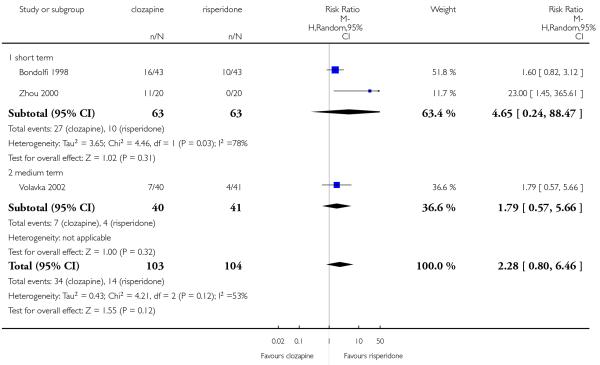 |
Analysis 5.30. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 30 Adverse effects: 10b. Weight: average weight change (high=poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 30 Adverse effects: 10b. Weight: average weight change (high=poor)
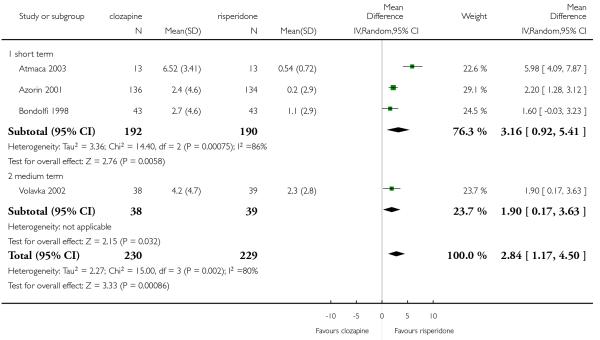 |
Analysis 5.31. Comparison 5 CLOZAPINE versus RISPERIDONE, Outcome 31 Adverse effects: 11.White blood cell count: number of participants with a decrease.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 CLOZAPINE versus RISPERIDONE
Outcome: 31 Adverse effects: 11. White blood cell count: number of participants with a decrease
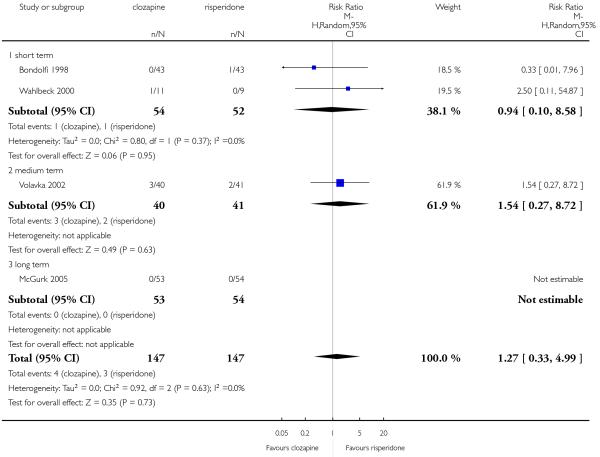 |
Analysis 6.1. Comparison 6 CLOZAPINE vs. RISPERIDONE - Sensitivity Analysis, Outcome 1 Mental state: 1. BPRS-18 (1-7) total score, excluding possibly skewed data (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 6 CLOZAPINE vs. RISPERIDONE - Sensitivity Analysis
Outcome: 1 Mental state: 1. BPRS-18 (1-7) total score, excluding possibly skewed data (high = poor)
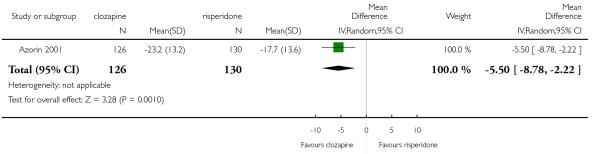 |
Analysis 6.2. Comparison 6 CLOZAPINE vs. RISPERIDONE - Sensitivity Analysis, Outcome 2 Mental state: 2. PANSS positive subscore, excluding possibly skewed data (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 6 CLOZAPINE vs. RISPERIDONE - Sensitivity Analysis
Outcome: 2 Mental state: 2. PANSS positive subscore, excluding possibly skewed data (high = poor)
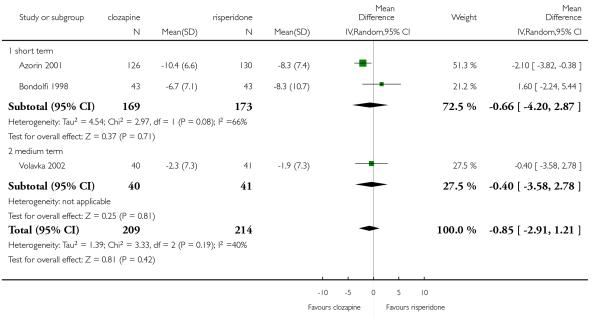 |
Analysis 6.3. Comparison 6 CLOZAPINE vs. RISPERIDONE - Sensitivity Analysis, Outcome 3 Mental state: 3. PANSS negative subscore, excluding possibly skewed data (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 6 CLOZAPINE vs. RISPERIDONE - Sensitivity Analysis
Outcome: 3 Mental state: 3. PANSS negative subscore, excluding possibly skewed data (high = poor)
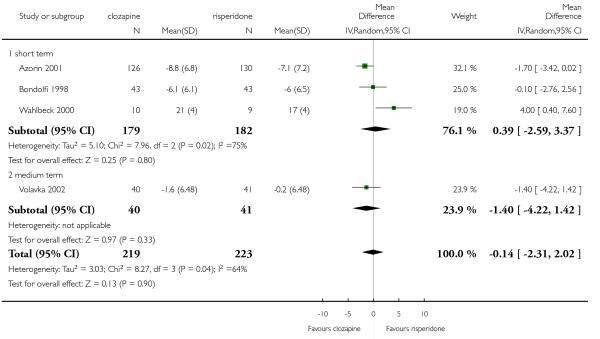 |
Analysis 7.1. Comparison 7 CLOZAPINE versus ZIPRASIDONE, Outcome 1 Leaving the study early: any reason - medium term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 7 CLOZAPINE versus ZIPRASIDONE
Outcome: 1 Leaving the study early: any reason - medium term
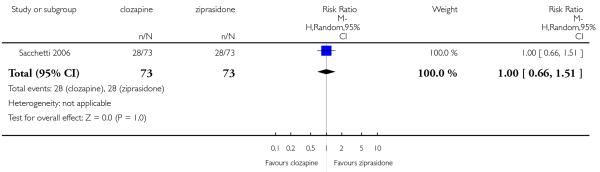 |
Analysis 7.2. Comparison 7 CLOZAPINE versus ZIPRASIDONE, Outcome 2 Mental state: PANSS total score - medium term (high = poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 7 CLOZAPINE versus ZIPRASIDONE
Outcome: 2 Mental state: PANSS total score - medium term (high = poor)
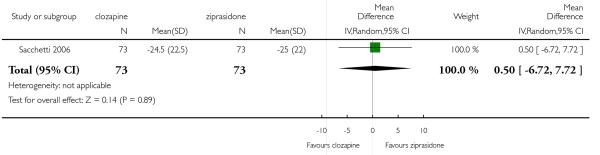 |
Analysis 7.3. Comparison 7 CLOZAPINE versus ZIPRASIDONE, Outcome 3 Adverse effects: 1. Cardiac problems.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 7 CLOZAPINE versus ZIPRASIDONE
Outcome: 3 Adverse effects: 1. Cardiac problems
 |
Analysis 8.1. Comparison 8 CLOZAPINE versus ZOTEPINE, Outcome 1 Leaving the study early: any reason.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 8 CLOZAPINE versus ZOTEPINE
Outcome: 1 Leaving the study early: any reason
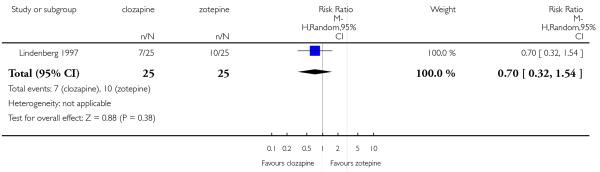 |
Analysis 8.2. Comparison 8 CLOZAPINE versus ZOTEPINE, Outcome 2 Global state: no clinically important change - less than successfully and no increase on CGI-S - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 8 CLOZAPINE versus ZOTEPINE
Outcome: 2 Global state: no clinically important change - less than successfully and no increase on CGI-S - short term
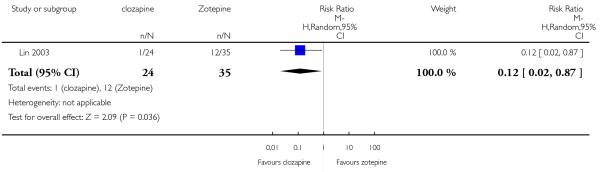 |
Analysis 8.3. Comparison 8 CLOZAPINE versus ZOTEPINE, Outcome 3 Mental state: BPRS-18 total score - short term (high=poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 8 CLOZAPINE versus ZOTEPINE
Outcome: 3 Mental state: BPRS-18 total score - short term (high=poor)
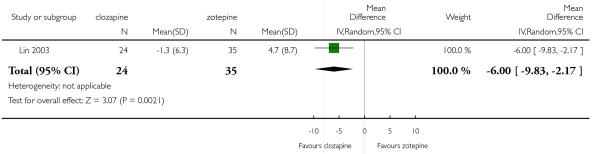 |
Analysis 8.4. Comparison 8 CLOZAPINE versus ZOTEPINE, Outcome 4 Adverse effects: 1. Extrapyramidal: antiparkinson medication use - short term.
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 8 CLOZAPINE versus ZOTEPINE
Outcome: 4 Adverse effects: 1. Extrapyramidal: antiparkinson medication use - short term
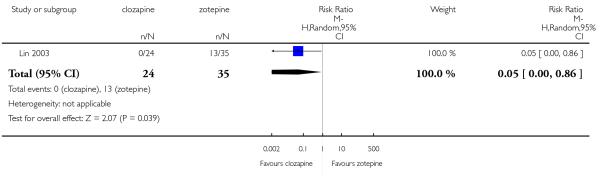 |
Analysis 8.5. Comparison 8 CLOZAPINE versus ZOTEPINE, Outcome 5 Adverse effects: 2. Prolactin: average change - short term (high=poor).
Review: Clozapine versus other atypical antipsychotics for schizophrenia
Comparison: 8 CLOZAPINE versus ZOTEPINE
Outcome: 5 Adverse effects: 2. Prolactin: average change - short term (high=poor)
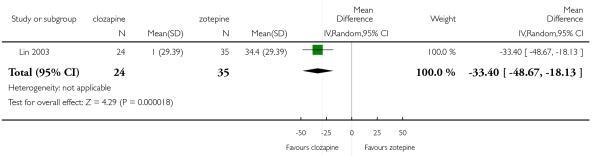 |
ADDITIONAL TABLES
Table 1. Suggested design of future study.
| Methods | Allocation:randomised - clearly described generation of sequence and concealment of allocation. Blinding: double - described and tested. Duration: 6 months minimum. |
| Participants | Diagnosis: schizophrenia (operational criteria). N=2700.* Age: any. Sex: both. History: any. |
| Interventions |
|
| Outcomes | Leaving study early (any reason, adverse events, inefficacy). Service outcomes: hospitalised, time in hospital, attending out patient clinics. Global impression: CGI**, relapse. Mental state: PANSS. Adverse events: UKU. Employment, family satisfaction, patient satisfaction. |
Power calculation suggested 300/group would allow good chance of showing a 10% difference between groups for primary outcome.
Primary outcome.
WHAT’S NEW
Last assessed as up-to-date: 14 October 2008.
| Date | Event | Description |
|---|---|---|
| 10 November 2010 | Amended | Contact details updated. |
HISTORY
Protocol first published: Issue 3, 2007
Review first published: Issue 11, 2010
| Date | Event | Description |
|---|---|---|
| 6 October 2010 | New citation required and conclusions have changed | This review is an update of the review “Newer atypical antipsychotic medication vs. clozapine” which compared clozapine with all other atypical antipsychotics pooled into one group. Since the atypical antipsychotics are a heterogenous group with quite different pharmacological profile and the amount of data published on this topic has grown enormously during the last few years, it is now possible to explore atypical comparisons with clozapine separately. For this reason, the title and the review protocol have been modified |
| 15 October 2008 | Amended | Converted to new format |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The review was adapted to new functions and formatting available in Review Manager 5, notably the risk of bias tables.
Footnotes
DECLARATIONS OF INTEREST
Claudia Asenjo L: none known.
Katja Komossa: participated in investigator meetings for clinical trials sponsored by Astra Zeneca, Pfizer, Sanofi Aventis, Servier.
Christine Rummel: received lecture honoraria and travel grants to attend scientific meetings from AstraZeneca, Janssen-Cilag, EliLilly and Pfizer.
Heike Hunger: none
Franziske Schmidt: none.
Sandra Schwarz: none.
Stefan Leucht: received speaker/consultancy honoraria from SanofiAventis, BMS, EliLilly, Janssen, Lundbeck and Pfizer. He received research support from SanofiAventis and EliLilly.
References to studies included in this review
- Atmaca 2003 {published data only} .Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64(5):598–604. doi: 10.4088/jcp.v64n0516. [DOI] [PubMed] [Google Scholar]
- Azorin 2001 {published data only} .Azorin JM, Spiegel R, Remington G, Vanelle JM, Pere JJ, Giguere M, Bourdeix I. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. American Journal of Psychiatry. 2001;158(8):1305–13. doi: 10.1176/appi.ajp.158.8.1305. [DOI] [PubMed] [Google Scholar]
- Bitter 2004 {published data only} .Bitter I, Brook S, Dossenbach M, Janka Z, Banki CsM, Selemani S, Grundy S, Martenyi F. Olanzapine versus clozapine in patients non-responsive or intolerant to standard acceptable treatment of schizophrenia. Journal of the European College of Neuropsychopharmacology. 1999;9:S288. [Google Scholar]
- Bitter I, Dossenbach MRK, Brook S, Feldman PD, Metcalfe S, Gagiano CA, Furedi J, Bartko G, Janka Z, Banki CM, Kovacs G, Breier A. Olanzapine versus clozapine in treatment-resistant or treatment-intolerant schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:173–80. doi: 10.1016/j.pnpbp.2003.09.033. [DOI] [PubMed] [Google Scholar]
- *; Bitter I, Slabber M, Pretorius J, Bartko GY, Danics Z, Dossenbach M, Martenyi F. Olanzapine versus clozapine in patients non responsive or intolerant to standard acceptable treatment of schizophrenia. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S141. [Google Scholar]
- Dossenbach M, Bitter I, Slabber M, Pretorius J, Bartko GY, Banics Z, Martenyi F. Olanzapine versus clozapine in patients nonresponsive or intolerant to standard acceptable treatment for schizophrenia; Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]
- Dossenbach M, Bitter I, Slabber M, Pretorius J, Bartko GY, Banics Z, Martenyi F. Olanzapine versus clozapine in patients nonresponsive or intolerant to standard acceptable treatment for schizophrenia; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Dossenbach M, Slabber M, Martenyi F, Bartko G, Bitter I. Olanzapine vs. clozapine in patients non responsive or intolerant to standard acceptable treatment of schizophrenia; Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. p. 148. [Google Scholar]
- Bondolfi 1998 {published data only} .Bondolfi G, Baumann P, Dufour H. Treatment-resistant schizophrenia - clinical experience with new antipsychotics. European Neuropsychopharmacology. 1996;6(Suppl 2):S21–5. doi: 10.1016/0924-977x(96)00012-0. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Baumann P, Patris M, May J, Billeter U, Dufour H, De Smedt G, Lemmens P. A randomized double-bind trial of risperidone versus clozapine for treatment-resistant chronic schizophrenia; Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30 - Oct 4.1995. [Google Scholar]
- Bondolfi G, Baumann P, Patris M, May JP, Billeter U, Dufour H. A randomised double-blind trial of risperidone versus clozapine for treatment-resistant chronic schizophrenia; Proceedings of the 148th Annual Meeting of the American Psychiatric Association; Miami, Florida, USA. 1995 May 20-25.1995. [Google Scholar]
- Bondolfi G, Baumann P, Patris M, May JP, Billeter U, Dufour H. A randomized double-blind trial of risperidone versus clozapine for treatment-resistant chronic schizophrenia; Proceedings of the Workshop on Critical Issues in the Treatment of Schizophrenia; Florence, Italy. 1995 Mar 10-12.1995. pp. 174–5. [Google Scholar]
- *; Bondolfi G, Dufour H, Patris M, May JP, Billeter U, Eap CB, Baumann P. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomized double-blind study. The Risperidone Study Group. American Journal of Psychiatry. 1998;155(4):499–504. doi: 10.1176/ajp.155.4.499. [DOI] [PubMed] [Google Scholar]
- Breier 1999 {published data only} .*; Breier AF, Malhotra AK, Su TP, Pinals DA, Elman I, Adler CM, Lafargue RT, Clifton A, Pickar D. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. American Journal of Psychiatry. 1999;156(2):294–8. doi: 10.1176/ajp.156.2.294. [DOI] [PubMed] [Google Scholar]
- Conley 2003 {published data only} .*; Conley RR, Kelly DL, Richardson CM, Tamminga CA, Carpenter WT., Jr The efficacy of high-dose olanzapine versus clozapine in treatment-resistant schizophrenia: a double-blind, crossover study. Journal of Clinical Psychopharmacology. 2003;23(6):668–71. doi: 10.1097/01.jcp.0000096246.29231.73. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR, Richardson CM, Tamminga CA, Carpenter WT., Jr Adverse effects and laboratory parameters of high-dose olanzapine vs. clozapine in treatment-resistant schizophrenia. Annals of Clinical Psychiatry. 2003;15(3-4):181–6. doi: 10.1023/b:acli.0000008171.90644.f8. [DOI] [PubMed] [Google Scholar]
- Heinrich 1994 {published data only} .Heinrich K. Risperidone versus clozapine in acute schizophrenia; Proceedings of the 1st International Risperidone Investigators’ Meeting; Paris, France. 1992 Mar 9-10.1992. pp. 27–28. [Google Scholar]
- Heinrich K, Klieser E, Lehmann E, Kinzler E. Experimental comparison of the efficacy and compatibility of clozapine and risperidone in acute schizophrenia. Clinical Neuropharmacology. 1992;15(Suppl 1 (Pt B)):375B. [Google Scholar]
- Heinrich K, Klieser E, Lehmann E, Kinzler E. Experimental comparison of the efficacy and compatibility of risperidone and clozapine in acute schizophrenia; Proceedings of the 17th Collegium Internationale Neuro-Psychopharmacologicum Congress; Kyoto, Japan. 1990 Sep 10-14.1991. pp. 37–9. [Google Scholar]
- *; Heinrich K, Klieser E, Lehmann E, Kinzler E, Hruschka H. Risperidone versus clozapine in the treatment of schizophrenic patients with acute symptoms: a double blind, randomized trial. Progress in Neuropsychopharmacology and Biological Psychiatry. 1994;18(1):129–37. doi: 10.1016/0278-5846(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Klieser E, Kinzler E. Risperidone versus clozapine in the treatment of schizophrenic patients with acute symptoms: a double-blind, randomised trial. Clinical report. 1991 doi: 10.1016/0278-5846(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Klieser E, Lehmann E, Kinzler E, Wurthmann C, Heinrich K. Randomized, double-blind, controlled trial of risperidone versus clozapine in patients with chronic schizophrenia. Journal of Clinical Psychopharmacology. 1995;15(1 Suppl 1):S45–51. doi: 10.1097/00004714-199502001-00008. [DOI] [PubMed] [Google Scholar]
- Klieser E, Rappard F. Randomized, double-blind, controlled trial of risperidone versus clozapine in patients with acute exacerbations of schizophrenia. European Psychiatry. 1994;9(Suppl 1):154S. [Google Scholar]
- Krakowski 2006 {published data only} .Krakowski MI. [accessed 19th February 2001];Clozapine and olanzapine in violent schizophrenics. https://www-commons.cit.nih.gov/crisp/index.html. CRISP database.
- *; Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Archives of General Psychiatry. 2006;63(6):622–9. doi: 10.1001/archpsyc.63.6.622. [DOI] [PubMed] [Google Scholar]
- Kumra 2008 {published and unpublished data} .Kumra S, Kranzler H, Gerbino-Rosen G, Kester HM, DeThomas C, Kafantaris V, Correl CU, Kane JM. Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: a 12 weeks randomized and double-blind comparison. Biological Psychiatry. 2008;63(5):524–29. doi: 10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Li 2002 {published data only} .Li Yan, Wang CH, Zhang DH. A study of quetiapine and clozapine in treatment of the first episode schizophrenia. Chinese Journal of Nervous and Mental Diseases. 2002;28(3):219–20. [Google Scholar]
- Li 2003 {published data only} .Li CH. A study of quetiapine and clozapine in treatment of schizophrenia. Chinese Journal of Nervous and Mental Diseases. 2003;29(4):306–07. [Google Scholar]
- Li 2005 {published data only} .Li Y-F, Feng Y-G. A double blind comparing study between the effects of quetiapine and clozapine on the life quality of the patients with schizophrenia. Medical Journal of Chinese People Health. 2005;17(6):262–4. [Google Scholar]; *
- Lin 2003 {published data only} .Lin C-C, Bai Y-M, Chen J-Y, Wang Y-C, Liou Y-J, Chao C-H, Lai I-C, Tsai K-Y, Chiu H-J. Switching from clozapine to zotepine in schizophrenic patients: a randomized, single-blind controlled study. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S318. [Google Scholar]
- Lin CC, Chen JY, Bai YM, Chen TT, Wang YC, Liou YJ, Lin WK. Remission of tardive dyskinesia following the switch from clozapine to zotepine: 2-year follow-up. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S410. [Google Scholar]
- Lin CC, Chen JY, Bai YM, Wang YC, Liou YJ, Lin WK, Chen TT. Long-term outcome of switching from clozapine to zotepine in refractory schizophrenia: a 2-year follow-up study. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):S500. [Google Scholar]
- Lindenberg 1997 {published data only} .Gallhofer B, Gruppe H, Bauer U. Zotepine versus clozapine: A comparison of the impact on the cognitive dysfunction syndrome in schizophrenia (a double blind trial) Pharmacopsychiatry. 1995;28:179. [Google Scholar]
- Gallhofer B, Ulferts J, Bauer U, Gruppe H. Cognitive dysfunction in schizophrenia: a comparison of the impact of zotepine versus clozapine on the syndrome (a double blind trial) Schizophrenia Research. 1996;18(2-3):220. [Google Scholar]
- Meyer-Lindenberg A, Bauer U, Lis S, Krieger S, Gallhofer B. Improvement of cognitive function in schizophrenic patients receiving clozapine or zotepine: Results from a double blind trial. Pharmacopsychiatry. 1997;30(2):35–42. doi: 10.1055/s-2007-979481. [DOI] [PubMed] [Google Scholar]; *
- Liu 2004 {published data only} .Liu Y, Xu M, Chen X. A controlled study of quetiapine and clozapine in the treatment of schizophrenia with predominantly negative symptoms. Shandong Archives of Psychiatry. 2004;17(1):6–8. [Google Scholar]; *
- McGurk 2005 {published data only} .Bellack AS, Schooler NR, Marder SR, Kane JM, Brown CH, Yang Y. Do clozapine and risperidone affect social competence and problem solving? American Journal of Psychiatry. 2004;161(2):364–7. doi: 10.1176/appi.ajp.161.2.364. [DOI] [PubMed] [Google Scholar]
- Marder SR, Schooler NR, Kane JM, Petrides G, Chengappa KN, Wirshing WC, Wirshing DA, Umbricht D, Parapelli H. Tolerability of clozapine and risperidone during a twenty nine week trial. Schizophrenia Research. 2003;60(1):293–4. [Google Scholar]
- McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H, Schooler NR, Kane JM. The effects of clozapine and risperidone on spatial working memory in schizophrenia. American Journal of Psychiatry. 2005;162(5):1013–6. doi: 10.1176/appi.ajp.162.5.1013. [DOI] [PubMed] [Google Scholar]; *
- McGurk SR, Goldman RS, Carter C, Green MF, Schooler N, Marder SR, Kane J. A double-blind comparison of clozapine and risperidone on spatial working memory in treatment-resistant schizophrenia; Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]
- McGurk SR, Goldman RS, Carter C, Green MF, Schooler R, Marder SR, Kane JM. A double-blind comparison of clozapine and risperidone on spatial working memory in treatment resistant schizophrenia. Schizophrenia Research. 2001;49(1-2 Suppl):238. [Google Scholar]
- Schooler N, Marder S, Kane J, Chengappa KNR, Wirshing W, Umbricht D, Parepally H, Wirshing D, Petrides G. Clozapine and risperidone: a 29-week randomized clinical trial. Schizophrenia Research. 1999;1, 2 & 3:296. [Google Scholar]
- Meltzer 2003 {published data only} .Alphs L, Anand R, Islam MZ, Meltzer HY, Kane JM, Krishnan R, Green AI, Potkin S, Chouinard G, Lindenmayer JP, Kerwin R. The International Suicide Prevention Trial (InterSePT): Rationale and design of a trial comparing the relative ability of clozapine and olanzapine to reduce suicidal behavior in schizophrenia and schizoaffective patients. Schizophrenia Bulletin. 2004;30(3):577–86. doi: 10.1093/oxfordjournals.schbul.a007102. [DOI] [PubMed] [Google Scholar]
- Bourgeois M, Swendsen J, Young F, Amador X, Pini S, Cassano GB, Lindenmayer JP, Hsu C, Alphs L, Meltzer HY, InterSePT Study Group Awareness of disorder and suicide risk in the treatment of schizophrenia: results of the international suicide prevention trial. American Journal of Psychiatry. 2004;161(8):1494–6. doi: 10.1176/appi.ajp.161.8.1494. [DOI] [PubMed] [Google Scholar]
- Glick ID, Zaninelli R, Hsu C, Young FK, Weiss L, Gunay I, Kumar V. Patterns of concomitant psychotropic medication use during a 2-year study comparing clozapine and olanzapine for the prevention of suicidal behavior. Journal of Clinical Psychiatry. 2004;65(5):679–85. doi: 10.4088/jcp.v65n0513. [DOI] [PubMed] [Google Scholar]
- Kane J, Alphs LD, Meltzer HY, Green AI, Hsu C, Anand R, Young F, Fahy T. The relationship between clinical response and changes in weight for clozapine and olanzapine-treated patients. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S171. [Google Scholar]
- Meltzer H. Decreasing suicide in schizophrenia: the intersept study. European Psychiatry. 2002;17(Suppl 1):26. [Google Scholar]
- Meltzer HY. An evidence-based approach to reducing the risk of suicide in schizophrenia. Journal of the European College of Neuropsychopharmacology. 2004;14(Suppl 3):S145. [Google Scholar]
- Meltzer HY. Reducing risk of suicide in schizophrenia. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S163. [Google Scholar]
- Meltzer HY. Reducing suicidality in schizophrenia and schizoaffective disorder; Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]
- Meltzer HY. Suicide and schizophrenia: clozapine and the InterSePT study. Journal of Clinical Psychiatry. 1999;60(Suppl 12):47–50. [PubMed] [Google Scholar]
- Meltzer HY. Treatment of suicidality in schizophrenia. Annals of the New York Academy of Sciences. 2001;932:44–58. doi: 10.1111/j.1749-6632.2001.tb05797.x. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Alphs L, Anand AR, Islam MZ, Potkin SG. The intersept trial: reduced suicidality with clozapine treatment; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Meltzer HY, Alphs L, Greem AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer J-P, Potkin S, InterSePT Study Group Clozapine treatment for suicidality in schizophrenia. Archives of General Psychiatry. 2003;60:82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]; *
- Meltzer HY, Baldessarini RJ. Reducing the risk for suicide in schizophrenia and affective disorders. Journal of Clinical Psychiatry. 2003;64(9):1122–9. doi: 10.4088/jcp.v64n0920. [DOI] [PubMed] [Google Scholar]
- Mortimer A. A prospective, randomised, international, parallel group comparison of Clozaril/Leponex vs Zyprexa in the reduction of suicidality in patients with schizophrenia or schizoaffective disorder who are at risk of suicide. Vol. 3. National Research Register; 2001. [Google Scholar]
- Mortimer A. Randomised prospective parallel group comparison of clozapine vs. olanzapine in the reduction of suicidality in patients with schizophrenia/schizoaffective disorder. Vol. 1. National Research Register; 2002. [Google Scholar]
- Novartis Pharmaceuticals Group . Archives of General Psychiatry. Novartis Pharmaceuticals Corporation; New Jersey: 2003. New treatment for suicidal behavior in schizophrenia or schizoaffective disorder. International suicide prevention trial (InterSePT): reduced suicidality in schizophrenia with clozapine treatment [CLO-8176] [Google Scholar]
- Potkin SG, Alphs L, Hsu C, Krishn n K, Rama Ranga, Anand R, Young FK, Meltzer H, Green A. Predicting suicidal risk in schizophrenic and schizoaffective patients in a prospective two-year trial. Biological Psychiatry. 2003;54(4):444–52. doi: 10.1016/s0006-3223(03)00178-1. [DOI] [PubMed] [Google Scholar]
- Young F, Meltzer H, Alphs L, Anand R, Green A, Potkin S, Kane J, Krishnan R, Islam Z, Hsu C, Martin S. The Intersept study: reduced suicidal behavior in schizophrenic clozapine-treated patients. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S77. [Google Scholar]
- Moresco 2004 {published data only} .Moresco RM, Cavallaro R, Messa C, Bravi D, Gobbo C, Galli LLG, Colombo C, Rizzo G, Velona I, Smeraldi E, Fazio F. Cerebral D2 and 5-HT2 receptor occupancy in schizophrenic patients treated with olanzapine or clozapine. Journal of Psychopharmacology. 2004;18(3):355–65. doi: 10.1177/026988110401800306. [DOI] [PubMed] [Google Scholar]; *
- Naber 2005 {published data only} .Bender S, Dittmann-Balcar A, Schall U, Klimke A, Riedel M, Vorbach U, Kuehn KU, Lambert M, Dittmann RW, Naber D. Effects of olanzapine versus clozapine on executive functions in schizophrenia. Schizophrenia Research. 2002;53(3 Suppl 1):194. [Google Scholar]
- Bender S, Dittmann-Balcar A, Schall U, Wolstein J, Klimke A, Riedel M, Vorbach E-U, Kuhn K-U, Lambert M, Dittmann RW, Naber D. Influence of atypical neuroleptics on executive functioning in patients with schizophrenia: a randomized, double-blind comparison of olanzapine vs clozapine. International Journal of Neuropsychopharmacology. 2006;9(2):135–45. doi: 10.1017/S1461145705005924. [DOI] [PubMed] [Google Scholar]
- Dittmann-Balcar A, Bender S, Schall U, Klimke A, Mueller N, Vorbach U, Kuehn KU, Dittmann RW, Naber D. Effects of olanzapine versus clozapine on executive functions in schizophrenia. Schizophrenia Research. 2003;60(1):131. [Google Scholar]
- Naber D, Bandelow B, Bender S, Klimke A, Kuehn K, Lambert M, Lemmer W, Dittmann M, Riedel ER. Subjective well-being under neuroleptic treatment with olanzapine versus clozapine: first results from a double-blind clinical trial using the SWN self-rating scale. Schizophrenia Research. 2001;49(1, 2):240. [Google Scholar]
- Naber D, Degner D, Bender S, Klimke A, Kuhn KU, Lambert M, Lemmer W, Riedel M, Vorbach EU, Dittmann RW. Olanzapine vs. clozapine: findings on subjective well-being from a double-blind clinical trial. Schizophrenia Research. 2002;53(3 Suppl 1):176. [Google Scholar]
- Naber D, Riedel M, Klimke A, Vorbach E-U, Lambert M, Kühn K-U, Bender S, Bandelow B, Lemmer W, Moritz S, Dittmann RW. Randomized double blind comparison of olanzapine vs. clozapine on subjective well-being and clinical outcome in patients with schizophrenia. Acta Psychiatrica Scandinavica. 2005;111(2):106–15. doi: 10.1111/j.1600-0447.2004.00486.x. [DOI] [PubMed] [Google Scholar]; *
- Ren 2002 {published data only} .Ren Q, Lu Y. Comparison of quality of life of schizophrenic outpatients treated with risperdal or clozapine. Chinese Mental Health Journal. 2002;16(3):198–9. [Google Scholar]; *
- Sacchetti 2006 {published data only} .Sacchetti E, Galluzzo A, Valsecchi P, Romeo F, Gorini B, Warrington L. Efficacy and safety of ziprasidone and clozapine in treatment refractory schizophrenic patients: results of a randomized, double-blind, 18-week trial. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S374. [Google Scholar]; *
- Shaw 2006 {published data only} .Shaw P, Sporn A, Gogtay N, Overman GP, Greenstein D, Gochman P, Tossell JW, Lenane M, Rapoport JL. Childhood-onset schizophrenia: a double blind, randomized clozapine-olanzapine comparison. Archives of General Psychiatry. 2006;63(7):721–30. doi: 10.1001/archpsyc.63.7.721. [DOI] [PubMed] [Google Scholar]; *
- Tollefson 2001 {published data only} .Beasley CM, Beuzen JN, Birkett MA. Olanzapine versus clozapine: an international double-blind study in the treatment of patients with treatment-resistant schizophrenia; Proceedings of the 39th Annual Meeting of the New Clinical Drug Evaluation Unit; Boca Raton, Florida, USA. 1999 Jun 1-4.1999. [Google Scholar]
- Beasley CM, Beuzen JN, Birkett MA, Kiesler GM, Tollefson GD, Wood AJ. Olanzapine versus clozapine: an international double-blind study of the treatment of resistant schizophrenia; Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]
- Beuzen JN, Birkett M, Kiesler G, Wood A. Olanzapine vs. clozapine in resistant schizophrenic patients - results of an international double-blind randomised clinical trial; Proceedings of the 21st Collegium Internationale Neuro-Psychopharmacologicum Congress; Glasgow, UK. 1998 Jul 12-16.1998. [Google Scholar]
- Beuzen JN, Birkett MA, Kiesler GM. An investigation of subgroup effects in a study of olanzapine versus clozapine in the treatment of resistant schizophrenic patients; Proceedings of the 11th European College of Neuropsychopharmacology Congress; Paris, France. 1998 Oct 31 - Nov 4.1998. [Google Scholar]
- Beuzen JN, Wood AJ, Kiesler GM, Birkett M, Tollefson GD. Olanzapine vs. Clozapine: an international double-blind study in the treatment of patients with treatment-resistant schizophrenia; Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. [Google Scholar]
- David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):s140. [Google Scholar]
- Dossenbach M, Bitter I, Slabber M, Pretorius J, Bartko GY, Banics Z, Martenyi F. Olanzapine versus clozapine in patients nonresponsive or intolerant to standard acceptable treatment for schizophrenia; Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois. 2000 May 13-18.2000. [Google Scholar]
- Dossenbach M, Bitter I, Slabber M, Pretorius J, Bartko GY, Banics Z, Martenyi F. Olanzapine versus clozapine inpatients nonresponsive or intolerant to standard acceptable treatment for schizophrenia; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Dossenbach M, Slabber M, Martenyi F, Bartko G, Bitter I. Olanzapine vs. clozapine in patients non responsive or intolerant to standard acceptable treatment of schizophrenia. World Psychiatry. 1999;2:148. [Google Scholar]
- Jean-Noel B, Wood AJ, Kiesler GM, Birkett M, Tollefson GD. Olanzapine vs. clozapine: an international double blind study in the treatment of resistant schizophrenia. World Psychiatry. 1999;2:143. [Google Scholar]
- Tollefson GD, Birkett MA, Kiesler GM, Wood AJ, Lilly Resistant Schizophrenia Study Group Double-blind comparison of olanzapine versus clozapine in schizophrenic patients clinically eligible for treatment with clozapine. Biological Psychiatry. 2001;49(1):52–63. doi: 10.1016/s0006-3223(00)01026-x. [DOI] [PubMed] [Google Scholar]; *
- Volavka 2002 {published data only} .Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 2002;159(6):1018–28. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment-resistant patients with schizophrenia and schizoaffective disorder. European Neuropsychopharmacology. 2001;11(3):256. [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy J, Kunz M, Chakos M, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment-resistant patients with schizophrenia and schizoaffective disorder. Schizophrenia Research. 2002;53(3 Suppl 1):194. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatric Services. 2001;52(11):1510–4. doi: 10.1176/appi.ps.52.11.1510. [DOI] [PubMed] [Google Scholar]
- Citrome LL, Volavka J, Czobor P, Nolan K, Lieberman JA, Lindenmayer J-P, Sheitman BB. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol; Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]
- Citrome LL, Volavka J, Czobor P, Sheitman BB, Lindenmayer J-P, McEvoy JP, Lieberman JA. Atypical antipsychotics and hostility in schizophrenia: a double-blind study; Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. Marathon Multimedia. 2001 May 5-10.2001. [Google Scholar]
- Citrome LL, Volavka J, Czobor P, Sheitman BB, Lindenmayer J-P, McEvoy JP, Lieberman JA. Atypical antipsychotics and hostility in schizophrenia: a double-blind study; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Czobor P, Volavka J, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Antipsychotic-induced weight gain and therapeutic response: a differential association. Journal of Clinical Psychopharmacology. 2002;22(3):244–51. doi: 10.1097/00004714-200206000-00003. [DOI] [PubMed] [Google Scholar]
- De Luca V, Vincent JB, Muller DJ, Hwang R, Shinkai T, Volavka J, Czobor P, Sheitman BB, Lindenmayer J-P, Citrome L, McEvoy JP, Lieberman JA, Kennedy JL. Identification of a naturally occurring 21 bp deletion in alpha 2c noradrenergic receptor gene and cognitive correlates to antipsychotic treatment. Pharmacological Research. 2005;51(4):381–4. doi: 10.1016/j.phrs.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Changes in glucose and cholesterol levels in schizophrenia patients treated with typical and atypical antipsychotics. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S169. [Google Scholar]
- Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. American Journal of Psychiatry. 2003;160(2):290–6. doi: 10.1176/appi.ajp.160.2.290. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Czobor P, Volavka J, Citrome LL, Sheitman BB, McEvoy JP, Cooper TB. Changes in glucose and cholesterol in schizophrenia treated with atypicals; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Lindenmayer JP, Czobor P, Volavka J, Lieberman JA, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M. Effects of atypical antipsychotics on the syndromal profile in treatment-resistant schizophrenia. Journal of Clinical Psychiatry. 2004;65(4):551–6. doi: 10.4088/jcp.v65n0416. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Czobor P, Volavka J, Lieberman JA, Citrome LL, Sheitman BB, McEvoy JP. Effects of atypicals on the syndromal profile in treatment-resistant schizophrenia; Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]
- Lindenmayer J-P, Czobor P, Yolayka J, Lieberman JA, McEvoy JP, Citrome LL, Sheitman BB. Do atypicals change the syndrome profile in treatment-resistant schizophrenia?; Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. Marathon Multimedia. 2001 May 5-10.2001. [Google Scholar]
- Lindenmayer J-P, Volavka J, Lieberman JA, Citrome LL, Sheitman B, McEvoy JP, Cooper T. Do atypicals change the syndromal profile in treatment-resistant schizophrenia?; Proceedings of the155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Mohr P, Volavka J, Lieberman JA, Czobor P, McEnvoy J, Lindenmayer J-P, Citrome L, Sheitman B. Clozapine, olanzapine, risperidone, and haloperidol in refractory schizophrenia. European Psychiatry. 2000;15(Suppl 2):284S. [Google Scholar]
- Nolan KA, Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, Citrome LL, McEvoy J, Lieberman JA. Aggression and psychopathology in treatment-resistant inpatients with schizophrenia and schizoaffective disorder. Journal of Psychiatric Research. 2005;39(1):109–15. doi: 10.1016/j.jpsychires.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Cooper TB, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Lieberman JA. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychiatry. 2004;65(1):57–61. doi: 10.4088/jcp.v65n0109. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome LMJP, Cooper TB, Lieberman JA. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychopharmacology. 2004;24(2):225–8. doi: 10.1097/01.jcp.0000117424.05703.29. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy J, Cooper TB, Chakos M, Kline JALN. Clozapine, olanzapine, risperidone, and haloperidol in refractory schizophrenia; Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; San Juan, Puerto Rico. 2000 Dec 10-14.2000. [Google Scholar]
- Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. American Journal of Psychiatry. 2002;159(2):255–62. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]; *
- Wahlbeck 2000 {published data only} .Cheine MV, Wahlbeck K, Tuisku K, Joffe G. Risperidone versus clozapine in the treatment of neuroleptic-refractory/intolerant schizophrenia patients: a single-blind randomized trial: Preliminary report of an on-going study. Nordic Journal of Psychiatry. 1997;51:99. [Google Scholar]
- Wahlbeck K, Cheine M, Tuisku K, Ahokas A, Joffe G, Rimon R. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. Progress in Neuropsychopharmacology and Biological Psychiatry. 2000;24(6):911–22. doi: 10.1016/s0278-5846(00)00118-4. [DOI] [PubMed] [Google Scholar]; *
- Wahlbeck K, Cheine M, Tuisku K, Ahokas A, Joffe G, Rimón R. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):S122. doi: 10.1016/s0278-5846(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Cheine M, Tuisku K, Joffe G. Risperidone versus clozapine in treatment resistant schizophrenia: a randomised pilot trial. Schizophrenia Research. 2000;41(1):197. doi: 10.1016/s0278-5846(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Wang 2002 {published data only} .Wang C, Feng Y, Wang L. A double-blind randomized controlled study of olanzapine and clozapine on treatment of schizophrenia. Shanghai Archives of Psychiatry. 2002;14(3):143–5. [Google Scholar]; *
- Zhou 2000 {published data only} .Zhou Z, Shu Z, Yu Y. A control study of risperidone and clozapine in the treatment of the negative symptoms of schizophrenia. Aerospace Medicine. 2000;11(2):69–71. [Google Scholar]; *
References to studies excluded from this review
- Agelink 2001 {published data only} .Agelink MW, Majewski T, Wurthmann C, Lukas K, Ullrich H, Linka T, Klieser E. Effects of newer atypical antipsychotics on autonomic neurocardiac function: a comparison between amisulpride, olanzapine, sertindole, and clozapine. Journal of Clinical Psychopharmacology. 2001;21(1):8–13. doi: 10.1097/00004714-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Allison 2001 {published data only} .Allison D, Cavazzoni P, Beasley C, Holcombe J, Buse J. Analysis of random glucose concentration data from patients with schizophrenia treated with typical and atypical agents during double-blind, randomized, controlled clinical trials; Proceedings of the 7th World Congress of Biological Psychiatry; Berlin, Germany. 2001 Jul 1-6.2001. [Google Scholar]
- Allison DB, Cavazzoni P, Beasley CM, Jr, Berg PH, Mukhopadhyay N, Mallinckrodt C, Baker RW, Holcombe J, Taylor CC, Breier A, Buse JB. Random blood glucose levels in patients with schizophrenia treated with typical and atypical antipsychotic agents: an analysis of data from double-blind, randomized, controlled clinical trial. European Neuropsychopharmacology. 2001;11(3):280. [Google Scholar]
- Altamura 1999 {published data only} .Altamura AC, Cao A, La Croce ML, Serri L, Soddu A, Laddomada A, Percudani M. Are atypical antipsychotics less depressogenic than typical compounds? Journal of the European College of Neuropsychopharmacology. 1999;9:S168. [Google Scholar]
- Anonymous 1994 {published data only} .Anonymous Risperidone vs clozapine. Biological Therapies in Psychiatry. 1994;17(12):47–8. [Google Scholar]
- Ascher-Svanum 2006 {published data only} .Ascher-Svanum H, Zhu B, Faries D, Landbloom R, Swartz M, Swanson J. Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia. BMC Psychiatry. 2006;6:8. doi: 10.1186/1471-244X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann 1993 {published data only} .Baumann P, Billeter U, Kasas A, Blajev B, Juvet P, Souche A, Bondolfi G, Meylan C, Dufour H. Pharmacogenetic investigations about risperidone and clozapine in schizophrenic patients; Proceedings of the 9th World Congress of Psychiatry; Rio de Janeiro, Brazil. Switzerland. 1993 Jun 6-12.1993. p. 76. [Google Scholar]
- Beasley 2001 {published data only} .Beasley CM, Berg PH, Dananberg J, Kwong KC, Taylor CCM, Breier A. Treatment emergent potential impaired glucose tolerance and potential diabetes with olanzapine compared to other antipsychotic agents and placebo. emergent potential impaired glucose tolerance and potential diabetes with olanzapine compared to other antipsychotic agents and placebo emergent potential impaired glucose tolerance and potential diabetes with olanzapine compared to other antipsychotic agents and placebo. Biological Psychiatry. 2001;49(8):121S. [Google Scholar]
- Bondolfi 1996 {published data only} .Bondolfi G, Baumann P, Dufour H. Treatment-resistant schizophrenia - clinical experience with new antipsychotics. European Neuropsychopharmacology. 1996;6(Suppl 2):S21–5. doi: 10.1016/0924-977x(96)00012-0. [DOI] [PubMed] [Google Scholar]
- Cai 2000 {published data only} .Cai C, Wan C, Cheng F. The controlled investigation of risperidone and clozapine in the treatment of schizophrenia. Health Psychology Journal. 2000;8(2):152–4. [Google Scholar]
- CATIE {published data only} .Lieberman JA, Schneider LS, McEvoy J, Pariot P, Stroup S, Adiao J, Lebowitz BD. Effectiveness trials of antipsychotic drugs; Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10; 2001. Marathon Multimedia. [Google Scholar]
- Lieberman JA, Schneider LS, McEvoy J, Pariot P, Stroup S, Adiao J, Lebowitz BD. Effectiveness trials of antipsychotic drugs; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Lieberman JA, Stroup S. Schizophrenia, VI. The CATIE Project Protocol. American Journal of Psychiatry. 2003;160(10):1748. doi: 10.1176/appi.ajp.160.10.1748. [DOI] [PubMed] [Google Scholar]
- McEvoy JP. Comparison of clozapine versus other atypical drugs in prospectively defined, unresponsive patients; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American Journal of Psychiatry. 2006;163(4):600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Stroup TS, McEvoy JP, Swartz MS, Byerly MJ, Glick ID, Canive JM, McGee MF, Simpson GM, Stevens MC, Lieberman JA. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: Schizophrenia trial design and protocol development. Schizophrenia Bulletin. 2003;29(1):15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- Cavazzoni 2002 {published data only} .Cavazzoni P, Berg PH, Millikan M, Carlson C, Beasley CM. An integrated analysis of treatment-emergent extrapyramidal syndrome in schizophrenic patients during olanzapine clinical trials versus placebo, haloperidol, risperidone or clozapine. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):S164. [Google Scholar]
- Cavazzoni P, Berg PH, Millikan M, Carlson C, Beasley CM. An integrated analysis of treatment-emergent extrapyramidal syndrome in schizophrenic patients during olanzapine clinical trials versus placebo, haloperidol, risperidone or clozapine. Schizophrenia Research. 2002;53(3 Suppl 1):171. doi: 10.4088/jcp.v64n0807. [DOI] [PubMed] [Google Scholar]
- Cha 2002 {published data only} .Cha A, Xu Y, Zhu XH. The curative effect comparison of risperidone and clozapine to treat recurrent schizophrenia. Chinese Medical Journal of Metallurgical Industry. 2002;19(3):143–4. [Google Scholar]
- Chen 2002 {published data only} .Chen SZ, Chi XZ, Li QH, Jiang YQ. A study of risperidone and clozapine in treatment of resistant schizophrenia. Heath Psychology Journal. 2002;10(1):44–46. [Google Scholar]
- Chen 2003 {published data only} .Chen F, Liang L, Zhu XH. A control study of elderly patients with schizophrenia treated with olanzapine or clozapine. Journal of Clinical Psychological Medicine. 2003;13(5):298–99. [Google Scholar]
- Chen 2003b {published data only} .Chen CQ. A study of risperidone and clozapine in treatment of first episode schizophrenia. HuaXia Medicine. 2003;27:578–79. [Google Scholar]
- Chen 2004 {published data only} .Chen YH, Li YM. A study of risperidone with haloperidol and clozapine in treatment of positive symptoms in schizophrenia. Suzhou University Journal of Medical Science. 2004;24(6):912–17. [Google Scholar]
- Chen 2005 {published data only} .Chen J, Li Z. A controlled study of olanzapine versus clozapine in schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(3):217–8. [Google Scholar]
- Chen 2005b {published data only} .Chen B, Liu X, Zhang X. A study of risperidone combination with clozapine in the treatment of the schizophrenia. Health Psychology Journal. 2005;13(2):116–7. [Google Scholar]
- Cheng 2004 {published data only} .Cheng Q, Huang CF. The influence of risperidone and clozapine in the quality of life of schizophrenia. Chinese Journal of Clinical Rehabilitation. 2004;8(18):3640–41. [Google Scholar]
- Chou 1999 {published data only} .Chou X, Chen Q, Li J. A clinical controlled study between risperidone and clozapine in the treatment of chronic schizophrenia. Sichuan Mental Health. 1999;12(4):244–6. [Google Scholar]
- Chouinard 1994 {published data only} .Chouinard G, Vainer JL, Belanger MC, Turnier L, Beaudry P, Roy JY, Miller R. Risperidone and clozapine in the treatment of drug-resistant schizophrenia and neuroleptic-induced supersensitivity psychosis. Progress in Neuropsychopharmacology and Biological Psychiatry. 1994;18(7):1129–41. doi: 10.1016/0278-5846(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Conley 1999a {published data only} .Conley J, Goldman RS, Bilder RM, Bates J, Reiter G, Pappadopulos E, Robinson D, Alvir JMA, Lieberman J, Schooler N. A comparison of the neurocognitive effects of treatment with typical and atypical neuroleptics in first-episode schizophrenia. Schizophrenia Research. 1999;36(1-3):128. [Google Scholar]
- Cui 2002 {published data only} .Cui B, Shen J, Tian H, Wang Y, Guo X. A comparative study of the effects of risperidone, chlorpromazine and clozapine to patients with schizophrenia by the electrocardiograms. Tianjin Pharmacy. 2002;3:54–5. [Google Scholar]
- CUTLASS {published data only} .Brownlee MM. The CUTLASS study: a multi-centre randomised controlled trial of atypical anti-psychotic drugs in severe schizophrenia. National Research Register; 2000. [Google Scholar]
- Jones PB. Cost utility of the latest antipsychotics in severe schizophrenia (CUtlASS) Vol. 3. National Research Register; 2001. [DOI] [PubMed] [Google Scholar]
- Kerwin R. Cost utility of the latest antipsychotics in severe schizophrenia (CUTLASS) - a multi-centre, randomised controlled trial. National Research Register; 2000. [Google Scholar]
- Lewis S. Cost utility of the latest antipsychotics in severe schizophrenia (CUTLASS): a multi-centre, randomised, controlled trial. National Research Register; 2000. [Google Scholar]
- Lewis S. The CUTLASS study: a multi-centre randomised controlled trial of atypical anti-psychotic drugs in severe schizophrenia. National Research Register; 2000. [Google Scholar]
- Lewis S, Davies L, Jones P, Barnes T, Murray R, Kerwin R, Taylor D, Hayhurst K, Markwick A, Lloyd H, Dunn G. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Draft final report for NCCHTA. 2003 doi: 10.3310/hta10170. [DOI] [PubMed] [Google Scholar]
- Lewis SW, Barnes TRE, Davies L, Murray RM, Dunn G, Hayhurst KP, Markwick A, Lloyd H, Jones PB. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophrenia Bulletin. 2006;32(4):715–23. doi: 10.1093/schbul/sbj067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, Taylor D, Hayhurst KP, Markwick A, Lloyd H, Dunn G. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment. 2006;10(17):III–182. doi: 10.3310/hta10170. [DOI] [PubMed] [Google Scholar]
- Marshall M. Cost utility of the latest anti psychotics in severe schizophrenia (CUTLASS) National Research Register; 2000. [Google Scholar]
- Spurrell M. Cost utility of the latest antipsychotics in severe schizophrenia (CUTLASS) Vol. 1. National Research Register; 2002. [Google Scholar]
- Dai 2004 {published data only} .Dai J-P, Zhao Z-H, Mai G-Y. Comparative study on the influence of olanzapine and clozapine on efficacy and quality of life in schizophrenia. Chinese Journal of Behavioral Medical Science. 2004;13(4):396–98. [Google Scholar]
- David 1999 {published data only} .David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):s140. [Google Scholar]
- David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. Journal of the European College of Neuropsychopharmacology. 1999;9:S292. [Google Scholar]
- Deng 2000 {published data only} .Deng H, Zheng H, He Z. A comparative trial of olanzapine versus clozapine in the treatment of schizophrenia. Shanghai Archives of Psychiatry. 2000;12(3):143–45. [Google Scholar]
- Ding 2005 {published data only} .Ding Z, Yu Z, Xu G. Control studies of increase of blood sugar induced by neo-antipsychotics. Journal of Clinical Psychosomatic Diseases. 2005;11(4):309–10. [Google Scholar]
- Du 2003 {published data only} .Du JF, Zhou T, Xiong LH, Tian DF. A study of quetiapine and clozapine in treatment of chronic schizophrenia. Journal of Luzhou Medical College. 2003;26(6):511–12. [Google Scholar]
- Du 2003b {published data only} .Du WJ, Yu JL, Zhen HB, Zhong XQ, Ma C. The influence of chlorpromazine, clozapine and risperidone on the cognitive function of schizophrenia. International Medicine & Health Guidance News. 2003;9(16):86–8. [Google Scholar]
- Du 2004 {published data only} .Du Z-H. A study on the change of serum thyroxin treated with Risperdal or clozapine. Journal of Practical Medical Techniques. 2004;11(68):1020–21. [Google Scholar]
- Du 2005 {published data only} .Du Z, Wu H. Effects of clozapine and risperidone on the glucose metabolism in schizophrenic patients. Qilu Journal of Medical laboratory Sciences. 2005;16(1):8–9. [Google Scholar]
- Earnst 1999 {published data only} .Earnst KS, Taylor SF, Smet IC, Goldman RS, Tandon R, Berent S. The effects of typical antipsychotics, clozapine, and risperidone on neuropsychological test performance in schizophrenia. Schizophrenia Research. 1999;40(3):255–6. doi: 10.1016/s0920-9964(99)00064-x. [DOI] [PubMed] [Google Scholar]
- Ellis 2000 {published data only} .Ellis T, Cudkowicz ME, Sexton PM, Growdon JH. Clozapine and risperidone treatment of psychosis in parkinson’s disease. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12(3):364–9. doi: 10.1176/jnp.12.3.364. [DOI] [PubMed] [Google Scholar]
- Fan 2003 {published data only} .Fan C, Leiying Wang K. The effect of clozapine and risperidone on blood sugar of patients with schizophrenia. Shandong Archives of Psychiatry. 2003;16(3):131–2. [Google Scholar]
- Fang 2005 {published data only} .Fang JH, Li WJ. A study of quetiapine in treatment of the dominant negative symptoms in schizophrenia. Sichuan Mental Health. 2005;18(3):178. [Google Scholar]
- Feng 2004 {published data only} .Feng Y. The influence of clozapine and risperidone on the weight. Journal of Clinical Psychological Medicine. 2004;14(2):101. [Google Scholar]
- Fleming 1999 {published data only} .Fleming K, Potkin SG, Alva G, Carreon D. Dissociation of improvement in neurocognition and negative symptomatology with olanzapine and clozapine conference abstract. Schizophrenia Research. 1999;1, 2 & 3:279. [Google Scholar]
- Flynn 1997 {published data only} .Flynn SW, MacEwan GW, Altman S, Kopala LC, Smith GN, Honer WG. A comparison of two atypical antipsychotics in treatment resistant schizophrenia. Schizophrenia Research. 1997;24(1, 2):185. [Google Scholar]
- Fu 2005 {published data only} .Fu H, Yu H, Huo J. A comparative study of quetiapine vs clozapine in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(4):313–4. [Google Scholar]
- Gaertner 1999 {published data only} .Gaertner I, Gaertner HJ, Lampe D. Therapeutic drug monitoring of clozapine and olanzapine. Similarities and differences. Psychopharmakotherapie. 1999;6(3):105–9. [Google Scholar]
- Gallhofer 1995 {published data only} .Gallhofer B, Gruppe H, Bauer U. Zotepine versus clozapine: A comparison of the impact on the cognitive dysfunction syndrome in schizophrenia (a double blind trial) Pharmacopsychiatry. 1995;28:179. [Google Scholar]
- Gallhofer 1996 {published data only} .Gallhofer B, Bauer U, Lis S, Krieger S, Gruppe H. Cognitive dysfunction in schizophrenia: comparison of treatment with atypical antipsychotic agents and conventional neuroleptic drugs. European Neuropsychopharmacology. 1996;6(Suppl 2):S13–20. doi: 10.1016/0924-977x(96)00010-7. [DOI] [PubMed] [Google Scholar]
- Ganguli 2005 {published data only} .Ganguli R, Brar JS. Prevention of weight gain, by behavioral interventions, in patients starting novel antipsychotics. Schizophrenia Bulletin. 2005;31:561–62. [Google Scholar]
- Gao 2003 {published data only} .Gao CZ, Gao Z. A study of quetiapine in the treatment of first-onset schizophrenia. Journal of Clinical Psychological Medicine. 2003;13(4):221–2. [Google Scholar]
- Goldberg 2000 {published data only} .Goldberg TE, Dodge M, Aloia M, Egan MF, Weinberger DR. Effects of neuroleptic medications on speech disorganization in schizophrenia: biasing associative networks towards meaning. Psychological Medicine. 2000;30(5):1123–30. doi: 10.1017/s0033291799002639. [DOI] [PubMed] [Google Scholar]; *
- Green 2001 {published data only} .Green AI. [accessed 19th February 2001];Cannabis and schizophrenia-clozapine vs risperidone. https://www-commons.cit.nih.gov/crisp/index.html. CRISP database.
- Guan 2005 {published data only} .Guan D-H, Song H-F. A control study of social function improvement in schizophrenia patients with risperdal and clozapine. Medical Journal of Chinese People Health. 2005;17(9):506–7. [Google Scholar]
- Guo 2001 {published data only} .Guo HR, Zhang SR, Sun FG. Clinical controlled study of risperidone and clozapine in treatment of schizophrenia. Journal of Xinxiang Medical College. 2001;18(3):174–6. [Google Scholar]
- Guo 2003 {published data only} .Guo S, Lu L, Cheng W. Effect of clozapine and risperidone on interleukin-2 on schizophrenia. Shanghai Archives of Psychiatry. 2003;15(1):35–8. [Google Scholar]
- Han 2005 {published data only} .Han Z-Y, Wang L-J, Wang J. Analysis of risperidone, clonazepam in treatment of positive symptoms in schizophrenia. Ningxia Medical Journal. 2005;27(9):631–2. [Google Scholar]
- Hang 2000 {published data only} .Hang P. A study of risperidone and clozapine in treatment of resistant schizophrenia. Journal of Jining Medical College. 2000;23(3):75. [Google Scholar]
- He 2005 {published data only} .He Y-Q, Ma S-H, Du J. A controlled study on hostility and side effects in clozapine and risperidone for treatment of patients with schizophrenia. Journal of Xinxiang Medical College. 2005;22(2):118–20. [Google Scholar]
- Hu 2000 {published data only} .Hu X-C, Tao R-F. A study of risperidone and clozapine in treatment of schizophrenia. Journal of Handan Medical College. 2000;13(2):91. [Google Scholar]
- Hu 2005 {published data only} .Hu T-S. A study of risperidone in treatment of dominant positive symptoms in schizophrenia. Chinese Journal of Behavioral Medical Science. 2005;14(5):428. [Google Scholar]
- Huang 2001 {published data only} .Huang QM, Li J, Zhu XW, Mel QY. A study of risperidone and clozapine in treatment of the behaviours in schizophrenia. Shanghai Archives of Psychiatry. 2001;13(3):152–4. [Google Scholar]
- Huang 2003 {published data only} .Huang S-P, Ma Z-W, Guo B-Y. Effectiveness of quetiapine vs clozapine in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2003;9(4):206–7. [Google Scholar]
- Kelemen 2006 {published data only} .Kelemen O, Nagy O, Máttyássy A, Kiss I, Janka Z, Kéri S. Do second-generation antipsychotics disrupt decision-making abilities in schizophrenia? Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S430. [Google Scholar]
- Kong 2001 {published data only} .Kong Q-R, Yang R-X, Li S-C, Lu Z-S, Li J-Y, Chen X. A study of clozapine with risperidone and clozapine with sulpiride in treatment of resistant schizophrenia. Shandong Archives of Psychiatry. 2001;14(2):119–20. [Google Scholar]
- Konrad 1996 {published data only} .Konrad C, Schormair C, Eikelmann B, Berger K, Rottmann P. Clozapine and risperidone in the treatment of therapy-resistant schizophrenia: a preliminary report on two ongoing clinical trials; Proceedings of the 8th Congress of the Association of European Psychiatrists; London, UK. 1996 Jul 7-12.1996. [Google Scholar]
- Konrad C, Schormair C, Knickelbein U, Ophaus P, Eikelmann B. Risperidone and clozapine in pharmaco-resistant schizophrenia. Pharmacopsychiatry. 1997;30:190. [Google Scholar]
- Konrad C, Schormair C, Ophaus P, Knickelbein U, Eikelmann B. Clozapine versus risperidone in pharmaco-refractory schizophrenia: a preliminary report; Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]
- Kufferle 1997 {published data only} .Kufferle B, Tauscher J, Asenbaum S, Vesely C, Podreka I, Brucke T, Kasper S. IBZM SPECT imaging of striatal dopamine-2 receptors in psychotic patients treated with the novel antipsychotic substance quetiapine in comparison to clozapine and haloperidol. Psychopharmacology. 1997;133:323–28. doi: 10.1007/s002130050409. [DOI] [PubMed] [Google Scholar]
- Lee 1995 {published data only} .Lee MS, Jung IK, Kwak DI. Clinical efficacy of clozapine in treatment-refractory schizophrenic patients; Proceedings of the 8th European College of Neuropsychopharmacology Congress; Venice, Italy. 1995 Sep 30 - Oct 4.1995. [Google Scholar]
- Lei 2002 {published data only} .Lei X, Liang T, Zhang X. A controlled study on risperidone and clozapine in treatment of schizophrenia. China Pharmacist. 2002;5(3):169–71. [Google Scholar]
- Lei G 2004 {published data only} .Lei G-W, Hen A-P, Qi L, Liu G-X. The influence of atypical antipsychotics on glucose, triglycerides and total cholesterol. Chinese Journal of Misdiagnostics. 2004;4(10):1665. [Google Scholar]
- Li 2003b {published data only} .Li T, Xue XM, Gong CF. A comparison study on the efficacy of risperidone vs clozapin in cognition of schizophrenia. Medical Journal of Chinese People Health. 2003;15(11):653–6. [Google Scholar]
- Li 2001 {published data only} .Li J, Dai X. A comparative study of risperidone and sulpiride in treating schizophrenia. Medical Journal of Chinese Civil Administration. 2001;13(3):136–8. [Google Scholar]
- Li 2003 {published data only} .Li C-M, Guo L. A comparison of cognitive function in the first-onset schizophrenia treated with quetiapine and clozapine. Medical Journal of Chinese People Health. 2003;15(12):718–21. [Google Scholar]
- Li 2004 {published data only} .Li Z. A comparative study of clozapine and risperidone lead to putting on weight. Heath Psychology Journal. 2004;12(5):396–97. [Google Scholar]
- Liang 2002 {published data only} .Liang S, Yu G, Ding G. Controlled study of olanzapine and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry. 2002;15(4):193–94. [Google Scholar]
- Liang 2005 {published data only} .Liang Z-Y, Huang X-S. A study of risperidone and clozapine in treatment of first episode schizophrenia. Medical Journal of Chinese People Health. 2005;17(9):509–10. [Google Scholar]
- Lindenmayer 1996 {published data only} .Lindenmayer JP, Iskander A, Apergi FS, Park M. Cognitive profile and soft signs in clozapine versus risperidone treatment; Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]
- Lindenmayer JP, Park M, Iskander A, Bark NM, Smith RM, Cooper TB. Clozapine versus risperidone in treatment refractory state psychiatric inpatients; Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, USA. 1996 Oct 2 - May 9.1996. [Google Scholar]
- Liu 1999 {published data only} .Liu Q, Man C, Li X. A control study of risperidone and clozapine to treating the positive symptoms of schizophrenia. Sichuan Mental Health. 1999;12(2):98–100. [Google Scholar]
- Liu 2001 {published data only} .Liu Q, Li X. A comparative study on the efficacy of combining risperidone and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry. 2001;14(1):28–30. [Google Scholar]
- Liu 2003 {published data only} .Liu X, Cheng J. A comparative study between quetiapine and clozapine in the treatment of schizophrenia and the relation with the plasma levels of il-2, sil-2r. an Baofu. Sichuan Mental Health. 2003;16(3):152–4. [Google Scholar]
- Liu 2003b {published data only} .Liu F, Dai M. The effects of risperidone and clozapine to patients with schizophrenia’s life quality. Sichuan Mental Health. 2003;16(4):203–4. [Google Scholar]
- Liu 2004 {published data only} .Liu W, Li H, Zheng L. A controlled study on olanzapine and clozapine in the treatment of the acute phase of schizophrenia. Shanghai Archives of Psychiatry. 2004;16(5):282–84. [Google Scholar]
- Liu 2004b {published data only} .Liu J-B, Wu J-H. The influence of clozapine, risperidone and chlorpromazine on the glucose, triglycerides, total cholesterol and the weight in first episode schizophrenia. Chinese Journal of Nervous and Mental Diseases. 2004;30(4):293–95. [Google Scholar]
- Liu 2004c {published data only} .Liu N-H, Zhang J-Y. A study of risperidone with clonazepam in treatment of schizophrenia. Journal of Hainan Medical College. 2004;10(6):394–95. [Google Scholar]
- Liu 2005 {published data only} .Liu Y. A comparative study of risperidone and clozapine in the treatment of refractory schizophrenia. Journal of Heze Medical College. 2005;17(1):13–4. [Google Scholar]
- Liu 2005b {published data only} .Liu C-X, Liu S-H, Tu Z-M, Liu B. A study of quetiapine with small dose of clozapine in treatment of resistant schizophrenia. Medical Journal of Chinese People Health. 2005;17(12):748–9. [Google Scholar]
- Liu 2005c {published data only} .Liu GF, Zhou JS. The influence of risperidone and clozapine in the quality of life of schizophrenia. Medical Journal of Chinese People Health. 2005;17(6):289–90. [Google Scholar]
- Louwerens 1996 {published data only} .Louwerens JW, Slooff CJ, Korf J, Coppens HJ, Paans AMJ. Dopamine2- and serotonine2-receptor-antagonism by antipsychotics in man. Schizophrenia Research. 1996;18(2):141. [Google Scholar]
- Lu 2002 {published data only} .Lu Y, Ren Q, Tian M. A comparison of cognitive function in the first-onset schizophrenia treated with risperidone and clozapine. Shandong Archives of Psychiatry. 2002;15(4):206–7. [Google Scholar]
- Lu 2005 {published data only} .Lu S, Zhang S-A, Ren Y. Comparative study on the psychopathology and the quality of life of schizophrenia treated with risperidone, clozapine and quetiapine. Journal of Nursing Science. 2005;20(3):57–59. [Google Scholar]
- Luo 2005 {published data only} .Luo Y, Zhang S-W, Wang A-Q. The comparative study of quetiapine and clozapine in the treatment of female schizophrene. Medical Journal of Chinese People Health. 2005;17(6):269–70. [Google Scholar]
- Ma 1999 {published data only} .Ma QM, Guan HY, Jiang L, Zhang YY, Yi XR, Guo JM, Sun SG, Liu KH, Gao SQ, Wu HR, Xu SW. A study of risperidone in treatment of chronic schizophrenia. Heath Psychology Journal. 1999;7(3):290–93. [Google Scholar]
- McKenna 2004 {published data only} .McKenna PJ. An international study of improving treatment for the most severely ill with schizophrenia. Vol. 2. National Research Register; 2004. [Google Scholar]
- Meehan 2000 {published data only} .Meehan KM, David SR, Taylor CC, Sutton VK. Change in extrapyramidal symptoms with olanzapine in comparison with other antipsychotic agents. Schizophrenia Research. 2000;41(1):192. [Google Scholar]
- Mei 2001 {published data only} .Mei Q, Zhu X, Shen J. A comparative study of social function in schizophrenic patients treated with risperidone or clozapine. Journal of Clinical Psychological Medicine. 2001;11(2):83–5. [Google Scholar]
- Mulqueen 2000 {published data only} .Mulqueen AW, Wudarsky M, Nicolson RJ, Gochman P, Hamburger S, Lenane M, Rapoport JHL. Weight gain in pediatric patients on typical and atypical antipsychotics; 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Mulqueen AW, Wudarsky M, Nicolson RJ, Gochman P, Hamburger S, Lenane M, Rapoport JHL. Weight gain in pediatric patients on typical and atypical antipsychotics; Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]
- Nan 2001 {published data only} .Nan Z, Wang J, Ji H. A controlled study of risperidone and clozapine both influences cognitive function of patients with schizophrenia. Sichuan Mental Health. 2001;14(4):198–200. [Google Scholar]
- Ni 2001 {published data only} .Ni JL, Jiiang LC, Hong X, Yang H, Zheng XP. A study of clozapine and risperidone in treatment of schizophrenia. Heath Psychology Journal. 2001;9(3):181–82. [Google Scholar]
- Opjordsmoen 2000 {published data only} .Opjordsmoen S, Brunsvik S, Melle I, Dahl A, Friis S, Haahr U, Hustoft K, Johannessen JO, Larsen TK, McGlashan TH, Simonsen E, Vaglum P. A comparison between novel and traditional antipsychotics as first-line medication in early psychosis; Proceedings of the 2nd International Conference on Early Psychosis; New York, USA. 2000 Mar 31 - Apr 2.2000. [Google Scholar]
- Pajonk 1998 {published data only} .Pajonk FG, Tiessen F, Holtzbach R, Naber D. Clozapine versus risperidone: an open comparative and prospective study on efficacy, tolerability and quality of life; Proceedings of the 21st congress of the collegium Internationale Neuro-psychopharmacologicum; Glasgow. 1998 Jul 12-16.1998. [Google Scholar]
- Pan 2006 {published data only} .Pan SM, Zhao LJ. A study of olanzapine and clozapine in treatment of schizophrenia. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2006;16(1):32–3. [Google Scholar]
- Pao 2004 {published data only} .Pao M, Zhang SQ, Zhao Z. Influence of three antipsychotic drugs on electrocardiogram in patients with schizophrenia. Journal of Xinxiang Medical College. 2004;21(5):403–4. [Google Scholar]
- Peng 2001 {published data only} .Peng H, Kuang Y, Huang X. A control study of risperidone in combination with clozapine in treating refractory schizophrenia. Journal of Modern Clinical Medical Bioengineering. 2001;7(2):100–2. [Google Scholar]
- Qian 2004 {published data only} .Qian D, Pan B, Yang G. Cost-effectiveness analysis of 3 kinds of therapeutic schemes for schizophrenia. Evaluation and Analysis of Drug-use in Hospital of China. 2004;4(2):110–11. [Google Scholar]
- Qin 2005 {published data only} .Qin Y, Kang W, Song L. A comparative study of risperidone combining alprazolam and clozapine in the treatment of patients with schizophrenia. Heath Psychology Journal. 2005;13(6):444–5. [Google Scholar]
- Rapoport 1997 {published data only} .Rapoport J, Kumra S, Jacobsen LK. The spectrum of extrapyramidal symptoms in children and young adults; Proceedings of the 150th Annual Meeting of the American Psychiatric Association; San Diego, California, USA. 1997 May 17-22.1997. [Google Scholar]
- Ren 2000 {published data only} .Ren JJ, Su JL, Li BQ. A study of risperidone and clozapine in treatment of resistant schizophrenia. Baotou Medical Journal. 2000;35(2):6–7. [Google Scholar]
- Ren 2004 {published data only} .Ren k, Zhao X, Jiang X. Effects of risperidone and clozapine on life quality of schizophrenics. Journal of Clinical Psychosomatic Diseases. 2004;10(1):3–4. [Google Scholar]
- Rettenbacher 2004 {published data only} .Rettenbacher MA, Baumgartner S, Ebenbichler C, Edlinger M, Hofer a, Hummer M, Kemmler G, Lechleitner M, Fleischhacker W. Alterations of glucose metabolism under treatment with clozapine vs. amisulpride. Schizophrenia Research. 2004;67(1):191. [Google Scholar]
- Rettenbacher MA, Hummer M, Hofer A, Baumgartner S, Ebenbichler C, Edlinger M, Kemmler G, Lechleitner M, Fleischhacker WW. Alterations of glucose metabolism during treatment with clozapine or amisulpride: results from a prospective 16-week study. Journal of Psychopharmacology. 2006;21(4):400–4. doi: 10.1177/0269881106069467. [DOI] [PubMed] [Google Scholar]
- Saletu 1987 {published data only} .Saletu B, Grunberger J, Linzmayer L, Anderer P. Comparative placebo-controlled pharmacodynamic studies with zotepine and clozapine utilizing pharmaco-EEG and psychometry. Pharmacopsychiatry. 1987;20(Suppl 1):12–27. doi: 10.1055/s-2007-1017125. [DOI] [PubMed] [Google Scholar]
- Scherer 2004 {published data only} .Scherer H, Bedard M-A, Stip E, Paquet F, Richer F, Beriault M, Rodriguez J-P, Motard J-P. Procedural learning in schizophrenia can reflect the pharmacologic properties of the antipsychotic treatments. Cognitive and Behavioral Neurology. 2004;17(1):32–40. doi: 10.1097/00146965-200403000-00004. [DOI] [PubMed] [Google Scholar]
- Schlogelhofer 2006 {published data only} .Schlogelhofer M, Amminger GP, Werneck-Rohrer S, Aschauer HN, Edwards J. Emotion recognition deficit in first-episode schizophrenic patients and atypical antipsychotics; Proceedings of the 19th European College of Neuropsychopharmacology Congress; Paris, France. 2006 Sep 16-20.2006. [Google Scholar]
- Schuld 2000 {published data only} .Schuld A, Kuhn M, Haack M, Kraus T, Hinze Selch D, Lechner C, Pollmacher T. A comparison of the effects of clozapine and olanzapine on the EEG in patients with schizophrenia. Pharmacopsychiatry. 2000;33(3):109–11. doi: 10.1055/s-2000-7976. [DOI] [PubMed] [Google Scholar]
- Shao 1999 {published data only} .Shao YQ. The extrapyramidal side effects of risperidone. Journal of Southeast China National Defense Medicinal Science. 1999;1(1):28–9. [Google Scholar]
- Sharma 2002 {published data only} .Sharma T, Kumari V, Hughes C, Soni W, Mehrotra R, Binneman B. Cognitive effects of clozapine and olanzapine in patients with chronic schizophrenia. Schizophrenia Research. 2002;53(3 Suppl 1):195. [Google Scholar]
- Sheng 2003 {published data only} .Sheng Y, Liu T, Li Y. A comparison study of the blood sugar in the treatment of schizophrenic patients with risperidone, clozapine and chlorpromazine. Qilu Journal of Medical Laboratory Sciences. 2003;14(1):11–2. [Google Scholar]
- Sheng 2005 {published data only} .Sheng Y. Effects of clozapine and risperidone on serum interleukin-2 and interleukin-6 of patients with first episode schizophrenia. Qilu Journal of Medical Laboratory Sciences. 2005;16(5):14–5. [Google Scholar]
- Shi 2000 {published data only} .Shi F, Cheng X, Zhang Y. Controlled study of risperidone and clozapine in the treatment of schizophrenia. Shanghai Archives of Psychiatry. 2000;12(1):27–9. [Google Scholar]
- Shi 2001 {published data only} .Shi XH. A study of risperidone and clozapine in treatment of schizophrenia. Medical Journal of Communications. 2001;15(6):652–3. [Google Scholar]
- Smith 2004 {published data only} .Smith R. An addition of pioglitazone or placebo to 40 patients with schizophrenia under treatment with olanzapine or clozapine. Stanley Foundation Research Programs; 2004. [Google Scholar]
- Speer 1997 {published data only} .Speer AM, Risch SC, Hamner MB, Molloy M, Ulmer HG, De Vane CL, Vincent DJ, George MS. The effect of an atypical antipsychotic (risperidone) on temporal lobe and prefrontal cortex activity in schizophrenia. Schizophrenia Research. 1997;24(1, 2):173. [Google Scholar]
- Su 1996 {published data only} .Su TP, Breier A, Coppola R, Hadd K, Elman I, Adler C, Malhotra AK, Watsky E, Gorey J, Weinberger D, Pickar D. D2 receptor occupancy in risperidone and clozapine-treated schizophrenics. Biological Psychiatry. 1996;39:513–4. [Google Scholar]
- Su TP, Malhotra AK, Hadd K, Breier A, Pickar D. D-2 dopamine receptor occupancy: a crossover comparison of risperidone with clozapine therapy in schizophrenic patients. Archives of General Psychiatry. 1997;54(10):972–3. doi: 10.1001/archpsyc.1997.01830220102017. [DOI] [PubMed] [Google Scholar]
- Sun 2001 {published data only} .Sun QZ, Liang JS, Yu GH, Huang X. A study of risperidone and clozapine in treatment of negative symptoms of resistant schizophrenia. Journal of Qiqihar Medical. 2001;22(11):1247–8. [Google Scholar]
- Swanson 2006 {published data only} .Swanson JW, Swartz MS, Van Dorn RA. Effectiveness of atypical antipsychotics for substance abuse in schizophrenia patients; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Tandon 2004 {published data only} .Octavio I, Tandon R, Stock E, Riera L, Kujawa M, Lam S, Pans M, Iwamoto T, Carson W. A naturalistic study of aripiprazole treatment in a general psychiatric setting; Proceedings of the Thematic Conference of the World Psychiatric Association on “ Treatments in Psychiatry: An Update”; Florence, Italy. 2004 Nov 10-13.2004. [Google Scholar]
- Tandon R, Marcus RN, Stock EG, Riera LC, Kostic D, Pans M, McQuade RD, Nyilas M, Iwamoto T, Crandall DT. A prospective, multicenter, randomized, parallel-group, open-label study of aripiprazole in the management of patients with schizophrenia or schizoaffective disorder in general psychiatric practice: Broad Effectiveness Trial With Aripiprazole (BETA) Schizophrenia Research. 2006;84(1):77–89. doi: 10.1016/j.schres.2005.12.857. [DOI] [PubMed] [Google Scholar]
- Tandon R, Stock E, Riera L, Kujawa M, Lam S, Pans M, Iwamoto T. A naturalistic trial with aripiprazole in a general psychiatric setting; Proceedings of the 157th Annual Meeting of the American Psychiatric Association; New York, New York, USA. 2004 May 1-6.2004. [Google Scholar]
- Tang 2003 {published data only} .Tang Y. A controlled study of schizophrenia treated with quetiapine and clozapine. Shanghai Archives of Psychiatry. 2003;15(1):27–9. [Google Scholar]
- Tang 2005 {published data only} .Tang Z-K, Xu C-M, Chen D-C. A comparative study of quetiapine and clozapine in the treatment of recurrent schizophrenia. Shandong Archives of Psychiatry. 2005;18(3):167–8. [Google Scholar]
- Tang 2005b {published data only} .Tang B, Lu Z, Zhou Z. A control study of ziprasidone vs clozapine in the treatment of refractory schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(4):303–4. [Google Scholar]
- Tian 2005 {published data only} .Tian RF. A study of risperidone and clozapine in treatment of schizophrenia in children. Journal of Clinical Psychosomatic Diseases. 2005;11(3):261. [Google Scholar]
- Tong 2005 {published data only} .Tong SN, Gan HQ, Liu YY. A study of clozapine and risperidone influencing on IQ and memory in schizophrenia. Chinese Journal of Behavioral Medical Science. 2005;14(7):634. [Google Scholar]
- Trichard 1998 {published data only} .Trichard C, Paillere Martinot ML, Attar Levy D, Recassens C, Monnet F, Martinot JL. Binding of antipsychotic drugs to cortical 5-HT(2A) receptors: A PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. American Journal of Psychiatry. 1998;155(4):505–8. doi: 10.1176/ajp.155.4.505. [DOI] [PubMed] [Google Scholar]
- Turrone 2002 {published data only} .*; Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. American Journal of Psychiatry. 2002;159(1):133–5. doi: 10.1176/appi.ajp.159.1.133. [DOI] [PubMed] [Google Scholar]
- Vaughan 2000 {published data only} .Vaughan K, McConaghy N, Wolf C, Myhr C, Black T. Community Treatment Orders: Relationship to clinical care, medication compliance, behavioural disturbance and readmission. Australian and New Zealand Journal of Psychiatry. 2000;34(5):801–8. doi: 10.1080/j.1440-1614.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- Wang 2002b {published data only} .Wang G, Chen Z, Wang H. Comparative study on quality of life of schizophrenics treated with risperidone or clozapine. Chinese Mental Health Journal. 2002;16(3):200–2. [Google Scholar]
- Wang 2003 {published data only} .Wang J, Zhao J, Wang J. A comparative study of risperidone and clozapine on life quality of schizophrenia. Shandong Archires of Psychiatry. 2003;16(3):149–50. [Google Scholar]
- Wang 2003b {published data only} .Wang JJ. A study of risperidone and clozapine with sulpiride in treatment of schizophrenia. Shandong Archives of Psychiatry. 2003;16(4):234–35. [Google Scholar]
- Wang 2003c {published data only} .Wang K, Zhang K. A study of olanzapine and clozapine in the treatment of schizophrenia. Shandong Archires of Psychiatry. 2003;16(03):141–3. [Google Scholar]
- Wang 2004 {published data only} .Wang X. Comparative study of effects of quetiapine and clozapine on quality of life of schizophrenics. Journal of Clinical Psychosomatic Diseases. 2004;10(3):167–8. [Google Scholar]
- Wang 2004b {published data only} .Wang H. Controlled study of the effect of olanzapine and clozapine on electroencephalogram of schizophrenic patients. Journal of North China Coal Medical College. 2004;6(3):289–90. [Google Scholar]
- Wang 2005 {published data only} .Wang Y, Sun M, Shao X. A comparative study between quetiapine and clozapine in the treatment of schizophrenia. Heath Psychology Journal. 2005;13(4):271–2. [Google Scholar]
- Wang 2005b {published data only} .Wang C-H, Li Y, Ma J-D, Wang L-H, Pan M, Mu J-L. Comparison of cognitive function and p300 potentials in first-episode schizophrenia treated with risperidone and clozapine. Chinese Journal of Nervous and Mental Diseases. 2005;31(4):267–71. [Google Scholar]
- Wang 2005c {published data only} .Wang J, Guo H, Yu X. Controlled studies of risperidone combined with clozapine in negative symptoms of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(3):225–7. [Google Scholar]
- Wang 2005d {published data only} .Wang SY, Liu Z, Bian J, Xu JG. The influence of risperidone in the quality of life in schizophrenia. Nervous Diseases and Mental Hygiene. 2005;5(2):153–4. [Google Scholar]
- Wang 2005e {published data only} .Wang XF, Wang WH, Wang JQ. Analysis of quetiapine in treatment of first episode schizophrenia. Sichuan Mental Health. 2005;18(4):235–6. [Google Scholar]
- Wang 2005f {published data only} .Wang Y-B, Xu H-C, Sun Y, Yang M-S, Wang X-H, Li Y-C. Effectiveness of olanzapine in treatment of schizophrenia. Journal of Clinical Psychological Medicine. 2005;15(4):224–5. [Google Scholar]
- Weickert 2003 {published data only} .Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of cognitive performances during a placebo period and an atypical antipsychotic treatment period in schizophrenia: critical examination of confounds. Neuropyschopharmacology. 2003;28(8):1491–500. doi: 10.1038/sj.npp.1300216. [DOI] [PubMed] [Google Scholar]
- Weng 1998 {published data only} .Weng Y. A controlled trial of risperidone versus clozapine in the treatment of schizophrenia. Journal of Clinical Psychological Medicine. 1998;8(2):83–5. [Google Scholar]
- Wirshing 1999 {published data only} .Wirshing DA, Perkins V, Marder SR, Wirshing WC. Sexual side effects of atypical antipsychotic medications; Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]
- Wu 2002 {published data only} .Wu L. A control study of risperidone and clozapine combination for the treatment of refractory schizophrenia. Health Psychology Journal. 2002;10(2):135–7. [Google Scholar]
- Wu 2004 {published data only} .Wu JF, Li Z. A study of risperidone and clozapine in treatment of schizophrenia. Medical Journal of Chinese People Health. 2004;16(4):213–14. [Google Scholar]
- Wudarsky 1999 {published data only} .Wudarsky M, Nicolson R, Hamburger SD, Spechler L, Gochman P, Bedwell J, Lenane MC, Rapoport JL. Elevated prolactin in pediatric patients on typical and atypical antipsychotics. Journal of Child and Adolescent Psychopharmacology. 1999;9(4):239–45. doi: 10.1089/cap.1999.9.239. [DOI] [PubMed] [Google Scholar]
- Xiang 2005 {published data only} .Xiang D-F, Liu X-L. Treatment of 31 cases of schizophrenia with quetiapine. Herald of Medicine. 2005;24(1):40–42. [Google Scholar]
- Xiao 2000 {published data only} .Xiao XM, Song XC, Jiang HY. A study of risperidone and clozapine in treatment of schizophrenia. Journal of Clinicial Psychological Medicine. 2000;10(4):229. [Google Scholar]
- Xie 2005 {published data only} .Xie B-Q, Wang Z-M, Han Q-L. A control study of risperidone and clozapine in the treatment the chronic schizophrenia. Shandong Archives of Psychiatry. 2005;18(2):76–7. [Google Scholar]
- Xin 2001 {published data only} .Xin X, Du B, Zeng Z. A controlled clinical study of risperidone and low dose of clozapine in treating schizophrenia. Herald of Medicine. 2001;8:501–2. [Google Scholar]
- Xu 2001 {published data only} .Xu C, Tang J, Zhang X. The effect of risperidone and clozapine treatment on serum interleukin 8, interleukin 15 in schizophrenia. Medical Journal of Chinese Civil Administration. 2001;13(3):132–5. [Google Scholar]
- Xu 2002 {published data only} .Xu MQ, Peng HY. A study of quetiapine in treatment of schizophrenia. Journal of Clinical Psychological Medicine. 2002;12(4):227–28. [Google Scholar]
- Yagdiran 2000 {published data only} .Yagdiran O, Krausz M. Depressive symptoms under atypical neuroleptic treatment in schizophrenia. Nervenarzt. 2000;71(Suppl 1):S135–36. [Google Scholar]
- Yagdiran O, Moritz S, Haasen C, Krausz M. Depressive symptoms among patients with schizophrenia treated with atypical antipsychotics. European Neuropsychopharmacology. 2001;11(3):244. [Google Scholar]
- Yang 1998 {published data only} .Yang Z, Xu H, Song Y. A controlled study of efficacy of risperidone and clozapine on treating schizophrenia whose predominant clinical features were negative symptoms. Sichuan Mental Health. 1998;11(3):151–2. [Google Scholar]
- Yang 2002 {published data only} .Yang M, Wu BQ. A study of risperidone and clozapine in treatment of chronic schizophrenia. Medical Journal of Chinese Civil Administration. 2002;14(6):350–51. [Google Scholar]
- Yang 2004 {published data only} .Yang B, Wang YD, Zhang L. Effects of clozapine, risperidone and haloperidol on plasma leptin in first-episode schizophrenic patients. Journal of The Fourth Military Medical University. 2004;25(14):1323–25. [Google Scholar]
- Yang L 2004 {published data only} .Yang L, Yang QN, Hou YH, Liu T, Yan HL. The influence of antipsychotics on the ECG in schizophrenia. Journal of Applied Clinical Pediatrics. 2004;19(12):1076–77. [Google Scholar]
- Yang X 2004 {published data only} .Yang XN, Mei QY. A study of risperidone with clonazepam in treatment of schizophrenia. Journal of Clinical Psychological Medicine. 2004;14(3):163. [Google Scholar]
- Ye 2005 {published data only} .Ye S-C, Zhang W-Y, Zhang Y-M. Observation of developments of the level of serum prolactin of first-episode schizophrenia patients during the treatment. Medical Journal of Chinese People Health. 2005;17(6):267–8. [Google Scholar]
- Yin 2004 {published data only} .Yin HR, Fang YM, Huang RH, Yang XQ. A study of risperidone and clozapine in treatment of schizophrenia. China Medical Engineering. 2004;12(3):87–8. [Google Scholar]
- Yu 2002 {published data only} .Yu G, Ding G, Li X. An economic burden comparison of typical and atypical antipsychotic therapies for schizophrenia. Chinese Journal of Psychiatry. 2002;35(3):177–9. [Google Scholar]
- Yuan 2002 {published data only} .Yuan GZ, Huang YP, Li X. The influence of antipsychotics on the weight and glucose. Journal of Clinical Psychological Medicine. 2002;12(6):369–70. [Google Scholar]
- Yuan 2005 {published data only} .Yuan F-J, Gao M-Z, Liu X. A contrast observation of quetiapine and clozapine in affecting electroencephalogram. Journal of Practical Medical Techniques. 2005;12(2B):482–4. [Google Scholar]
- Yuo 1999 {published data only} .Yu GH, Huang X. A study of risperidone and clozapine in treatment of resistant patients in schizophrenia. Chinese Journal of Nervous and Mental Diseases. 1999;25(6):366–67. [Google Scholar]
- Zelaschi 2000 {published data only} .Zelaschi NM, Rodriguez JL, Gaitan S, Panizzo S, Sobrera A, Lopez A, Archuby F. A follow-up study comparing clinical and endocrine effects of clozapine and risperidone; Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]
- Zelaschi 2006 {published data only} .Zelaschi NM, Sr, Rodriguez JL, Sr, Gaitan S, Palacios Vallejos ME, Zieher LM. The effects of the switch of conventional neuroleptics to atypical antipsychotics: a follow-up study of patients with chronic schizophrenia; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Zhan 2002 {published data only} .Zhan LY. Analysis of risperidone and clozapine in behavioral change in schizophrenia. Modern Rehabilitation. 2002;6(19):2943. [Google Scholar]
- Zhang 2002 {published data only} .Zhang Q. A comparison of cognitive function in the first-onset schizophrenia treated with risperidone and clozapine. Journal of Qiqihar Medical. 2002;23(8):852–3. [Google Scholar]
- Zhang 2002b {published data only} .Zhang X-Z, Yu J-L. Control studies of relapses between risperidone and clozapine in schizophrenia. Journal of Clinical Psychosomatic Diseases. 2002;8(3):145–47. [Google Scholar]
- Zhang 2004 {published data only} .Zhang HY, Lin QC, Lin JC, Guo YB. A study of olanzapine and clozapine in EEG. International Medicine & Health Guidance News. 2004;10(14):113–14. [Google Scholar]
- Zhang 2005 {published data only} .Zhang Y, Jiang Y, Zhao S. A comparative study of quetiapine combined with clomipramine in treatment of schizophrenia with obsessive-compulsive symptoms. Heath Psychology Journal. 2005;13(4):260–2. [Google Scholar]
- Zhang 2005b {published data only} .Zhang H, Zhang M, Gu W. A comparative study of social function in schizophrenia patients treated with Seroquel (quetiapine) or clozapine. Sichuan Mental Health. 2005;18(2):91–3. [Google Scholar]
- Zhang 2005c {published data only} .Zhang HY, Zhan C. Cost efficiency analysis of four atypical antipsychotics in treatment of schizophrenia. Medical Journal of Chinese People Health. 2005;17(12):756–7. [Google Scholar]
- Zhang 2005d {published data only} .Zhang J-X, Zhu F-Y, Shi X-M, Zhang X-M, Wei Li-H, Ji Z-F, Lin R-M. Controlled study of risperidone versus clozapine in treatment of refractory schizophrenia with depressive symptoms. Journal of Clinical Psychological Medicine. 2005;15(4):201–2. [Google Scholar]
- Zhang 2005e {published data only} .Zhang M, Zhang H-F, Gu W. A comparative study of quetiapine and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry. 2005;18(2):86–8. [Google Scholar]
- Zhang 2005f {published data only} .Zhang R-X, Cao Y-F. A comparative study of EKG changed by quetiapine and clozapine in the treatment of schizophrenia. Medical Journal of Chinese People Health. 2005;17(6):290–1. [Google Scholar]
- Zhang 2005g {published data only} .Zhang ZP, Xie YY. 40 cases of olanzapine in treatment of schizophrenia. Shandong Medical Journal. 2005;56:61. [Google Scholar]
- Zhao 2005 {published data only} .Zhao YH, Fang JH, Wang SH, An CG, Ma X, Li HX. A study of quetiapine and clozapine in treatment of schizophrenia. Journal of Clinical Psychological Medicine. 2005;15(4):231–2. [Google Scholar]
- Zheng 2001 {published data only} .Zheng H, Xu CT. A clinical study of clozapine and olanzapine in treatment of resistant schizophrenia. Journal of Qiqihar Medical. 2001;22(8):865. [Google Scholar]
- Zheng 2003 {published data only} .Zheng YJ, Wang GH, Cheng ZL. Effects of clozapine and risperidone on the glucose metabolism in first-episode schizophrenic patients. Chinese Journal of Psychiatry. 2003;36(4):207–10. [Google Scholar]
- Zheng X 2001 {published data only} .Zheng XX, Huang JH, Huang ZZ. A study of risperidone and clozapine in treatment of side effects on schizophrenia. Strait Pharmaceutical Journal. 2001;13(3):79–80. [Google Scholar]
- Zhong 2003 {published data only} .Zhong H, Wang H. The influence of risperidone, chlorpromazine and clozapine on ECG. Journal of Gannan Medical College. 2003;23(6):644–45. [Google Scholar]
- Zhou 2003 {published data only} .Zhou P, Gong F, Fan C. A control of treating chronic schizophrenia with seroquel or clozapine. Jiangxi Medical Journal. 2003;6:395–6. [Google Scholar]
- Zhou 2003b {published data only} .Zhou HJ, Zhu YP, Lu SL. The influence of clozapine on glucose in schizophrenia. Liaoning Journal of Practical Diabetology. 2003;11(1):21–22. [Google Scholar]
- Zhou 2005 {published data only} .Zhou B, Tang Y, Zhu L. Effects of risperidone vs clozapine on blood-lipid of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(4):315–6. [Google Scholar]
- Zhu 1999 {published data only} .Zhu X, Mei Q. A controlled study comparing drug compliance with risperidone and clozapine in treatment of schizophrenia. Journal of Clinical Psychological Medicine. 1999;9(3):151–2. [Google Scholar]
- Zhu 2002 {published data only} .Zhu H, Ma C, Hou J, Yin Q, Hu H, Guo Y. Study of serum IL-6 and a-IFN in the pre-and post-treatment of 68 patients with first episode schizophrenia. Chinese Journal of Nervous and Mental Diseases. 2002;28(5):324–6. [Google Scholar]
- Zhu 2003 {published data only} .Zhu F, Lin R, Zhang J. Risperidone versus clozapine in treatment-resistant schizophrenia:a randomized controlled study. Shanghai Archives of Psychiatry. 2003;15(3):168–71. [Google Scholar]
- Zhu Q 2003 {published data only} .Zhu QY, Zhang SW. 15 cases of risperidone in treatment of resistant schizophrenia. Herald of Medicine. 2003;6(S1):13–14. [Google Scholar]
- Zoccali 2003 {published data only} .Zoccali R, Muscatello MR, Torre DL, Malara G, Canale A, Crucitti DDC, Spina E. Lack of a pharmacokinetic interaction between mirtazepine and the newer antipsychotics clozapine, risperidone and olanzapine in patients with chronic schizophrenia. Pharmacological Research. 2003;48(4):411–4. doi: 10.1016/s1043-6618(03)00178-6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Anand 1999 {published data only} .Anand R, Alphs L, Azorin JM, Remington G, Péré J-J, Bourdeix I. Superior efficacy of clozapine in chronic, severe schizophrenia: comparison with risperidone. Journal of Clinical Psychiatry. 1999;60(Suppl 12):3–50. [Google Scholar]
- Byerly 1999 {published data only} .Byerly M, Weber M. Clozapine versus quetiapine for schizophrenia. Stanley Foundation Research Programs; 1999. [Google Scholar]
- Chengappa 2001 {published data only} .Chengappa KN. [accessed 19th February 2001];New antipsychotics-clinical trials and follow up. https://www-commons.cit.nih.gov/crisp/index.html. CRISP database.
- Chowdhury 1999 {published data only} .*; Chowdhury AN, Mukherjee A, Ghosh K, Chowdhury S, Das Sen K. Horizon of a new hope: Recovery of schizophrenia in India. International Medical Journal. 1999;6(3):181–5. [Google Scholar]
- Daniel 1996 {published data only} .Daniel D. Crossover comparison of risperidone and clozapine on clinical, cognitive, and side effect measures in treatment-resistant psychosis. Psychopharmacology Bulletin. 1994;30(4):629. [Google Scholar]
- Daniel DG, Goldberg TE, Lubick LJ, Weinberger DR, Kleinman JE, Pickar D, Williams TS. Self-reported cognitive impairment predicts patient preference between risperidone and clozapine. Schizophrenia Research. 1995;15(1, 2):147–8. [Google Scholar]
- *; Daniel DG, Goldberg TE, Weinberger DR, Kleinman JE, Pickar D, Lubick LJ, Williams TS. Different side effect profiles of risperidone and clozapine in 20 outpatients with schizophrenia or schizoaffective disorder: a pilot study. American Journal of Psychiatry. 1996;153(3):417–9. doi: 10.1176/ajp.153.3.417. [DOI] [PubMed] [Google Scholar]
- Estrella 1996 {published data only} .Estrella MH, Soria FL, Gonzalez CJC, Butron MAL, Torres JA, Escareño RR. Cost-effectiveness of clozapine vs risperidone for treatment-resistant schizophrenic patients; Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996 Aug 23-28.1996. [Google Scholar]
- Lieberman 2001 {published data only} .Lieberman JA. [accessed 19th February 2001];Risperidone and clozapine in chronic schizophrenia. https://www-commons.cit.nih.gov/crisp/index.html. CRISP database.
- Loza 2002 {published data only} .Loza B. Atypical antipsychotic treatment prognosis in first-episode paranoid schizophrenia based on syllabic and language-related dichotic listening scores. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):S298. [Google Scholar]
- Magnuson 2001 {published data only} .Magnuson WG. Childhood onset psychotic disorders: characterization and treatment with atypical neuroleptics / treatment of childhood onset psychotic disorders with olanzapine or clozapine. National Institutes of Health; 2001. [Google Scholar]
- Oliemeulen 2000 {published data only} .Oliemeulen EAP, Van Hoof JJM, Jogem-Kosterman BJM, Hulsttijn W, Tuynman-Qua HG. Is olanzapine a substitute for clozapine? The effects on psychomotor performance. Schizophrenia Research. 2000;41(1):187. [Google Scholar]
Additional references
- ACP 2002 .Carpenter WT, Thaker K. Section 13 Psychiatry (VII. Schizophrenia) In schizophrenia: management. The American College of Physicians and WebMD Professional Publishing; 2002. www.acpmedicine.com/ ACP Medicine Online. [Google Scholar]
- Altman 1996 .Altman DG, Bland JM. Detecting skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen 1984 .Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Univ. of Iowa; Iowa City: 1984. [Google Scholar]
- Andreasen 1984b .Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Univ. of Iowa; Iowa City: 1984. [PubMed] [Google Scholar]
- APA 1994 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Barnes 1989 .Barnes TR. A rating scale for drug-induced akathisia. British Journal of Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Beaumont 2000 .Beaumont G. Antipsychotics - The future of schizophrenia treatment. Current Medical Research and Opinion. 2000;16(1):37–42. doi: 10.1185/0300799009117006. [DOI] [PubMed] [Google Scholar]
- Birchwood 1990 .Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The social functioning scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. British Journal of Psychiatry. 1990;157:853–9. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- Bland 1997 .Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel 1999 .Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie. 1999;54(4):405–11. [PubMed] [Google Scholar]
- Carpenter 1994 .Carpenter WT, Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine. 1994;330:681–90. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- Chouinard 1980 .Chouinard G, Ross-Chouinard A, Annable L, Jones BD. The extrapyramidal symptom rating scale. Canadian Journal of Neurological Sciences. 1980;7:233. [Google Scholar]
- Citrome 2002 .Citrome L, Bilder RM, Volavka J. Managing treatment-resistant schizophrenia: evidence from randomized clinical trials. Journal of psychiatric practice. 2002;8(4):205–15. doi: 10.1097/00131746-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Deeks 2000 .Deeks J. Issues in the selection for meta-analyses of binary data; Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa. 2000 Oct 25-28th.2000. [Google Scholar]
- DerSimonian 1986 .DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trial. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Divine 1992 .Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians’ patient care behavior. Journal of General Internal Medicine. 1992;7:623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- Donner 2002 .Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 2002 .Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology. 2002;31(1):140–9. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- Fleischhaker 1989 .Fleischhaker W, Bergmann K, Perovich R. The hillside akathisia scale: a new rating instrument for neuroleptic-induced akathisia. Psychopharmacology Bulletin. 1989;25:222–26. [PubMed] [Google Scholar]
- Furukawa 2006 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Gaebel 1997 .Gaebel W. Towards the improvement of compliance: the significance of psycho-education and new antipsychotic drugs. International Clinical Psychopharmacology. 1997;12(Suppl 1):S37–42. [PubMed] [Google Scholar]
- Gilbody 2000 .Gilbody SM, Bagnall AM, Duggan L, Tuunainen A. Risperidone versus other atypical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews. 2000;(3) doi: 10.1002/14651858.CD002306. DOI: 10.1002/14651858.CD002306. [DOI] [PubMed] [Google Scholar]
- Gulliford 1999 .Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology. 1999;149:876–83. doi: 10.1093/oxfordjournals.aje.a009904. [DOI] [PubMed] [Google Scholar]
- Guy 1972 .Guy W. Early Clinical Drug Evaluation Unit (ECDEU) assessment manual for psychopharmacology. National Institute of Mental Health; Rockville, MD: 1976. Publication no 76-338. [Google Scholar]
- Heinisch 1991 .Heinisch M, Ludwig M, Bullinger M. Psychometric testing of the Münchner Lebensqualitäts Dimensionen Liste (MLDL) Hogrefe Verlag für Psychologie; Göttingen,Toronto, Zurich: 1991. [Google Scholar]
- Heres 2006 .Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine. American Journal of Psychiatry. 2006;163:185–94. doi: 10.1176/appi.ajp.163.2.185. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2009 .Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 5.0.2. The Cochrane Collaboration; 2009. updated September 2009. Available from www.cochrane-handbook.org. [Google Scholar]
- Hogan 1983 .Hogan TP, Awad AG, Eastwood MR. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychological Medicine. 1983;13:177–83. doi: 10.1017/s0033291700050182. [DOI] [PubMed] [Google Scholar]
- Idänpään-He.1977 .Idänpään-Heikkilä J, Alhava E, Olkinuora M, Palva IP. Agranulocytosis during treatment with clozapine. European Journal of Clinical Pharmacology. 1977;11(3):193–98. doi: 10.1007/BF00606409. [DOI] [PubMed] [Google Scholar]
- Kane 1988 .Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment resistant schizophrenic: a double-blind comparison with chlorpromazine. Archives of General Psychiatry. 1988;45(9):789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kane 1990 .Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica Supplementum. 1990;358:151–7. doi: 10.1111/j.1600-0447.1990.tb05309.x. [DOI] [PubMed] [Google Scholar]
- Kane 2006 .Kane JM, Khanna S, Rajadhyaksha S, Giller E. Efficacy and tolerability of ziprasidone in patients with treatment-resistant schizophrenia. International Clinical Psychopharmacology. 2006;21(1):21–28. doi: 10.1097/01.yic.0000182114.65134.81. [DOI] [PubMed] [Google Scholar]
- Kay 1986 .Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. Multi-Health Systems; North Tonawanda (NY): 1986. [Google Scholar]
- Lehman 2004 .Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J, American Psychiatric Association, Steering Committee on Practice Guidelines Practice guideline for the treatment of patients with schizophrenia, second edition. American Journal of Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- Leucht 2005a .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean? Schizophrenia Research. 2005;79:231–8. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Leucht 2005b .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. British Journal of Psychiatry. 2005;187:366–71. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- Leucht 2008 .Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM. A meta-analysis of head-to-head comparisons of second generation antipsychotics in the treatment of schizophrenia. American Journal of Psychiatry. 2008;166(2):152–63. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- Marshall 2000 .Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales - a major source of bias in randomised controlled trials of treatments for schizophrenia? British Journal of Psychiatry. 2000;176:249–52. doi: 10.1192/bjp.176.3.249. [DOI] [PubMed] [Google Scholar]
- McEvoy 2006 .McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American Journal of Psychiatry. 2006;163(4):600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Miyamoto 2005 .Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Molecular Psychiatry. 2005;10(1):79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Moher 2001 .Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–4. [PubMed] [Google Scholar]
- Mueser 2004 .Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363(9426):2063–72. doi: 10.1016/S0140-6736(04)16458-1. PUBMED: 15207959. [DOI] [PubMed] [Google Scholar]
- Naber 1995 .Naber D. A self-rating to measure subjective effects of neuroleptic drugs, relationships to objective psychopathology, quality of life, compliance and other clinical variables. International Clinical Psychopharmacology. 1995;10(Suppl 3):133–8. [PubMed] [Google Scholar]
- Naheed 2001 .Naheed M, Green B. Focus on clozapine. Current Medical Research and Opinion. 2001;17(3):223–9. doi: 10.1185/0300799039117069. [DOI] [PubMed] [Google Scholar]
- NIMH 1975 .National Institute of Mental Health, Psychopharmacology Research Branch Development of a dyskinetic movement scale. Early clinical drug evaluation unit. Intercom. 1975;3:3–6. [Google Scholar]
- O’Brien 2004 .O’Brien A. Starting clozapine in the community: a UK perspective. CNS Drugs. 2004;18(13):845–52. doi: 10.2165/00023210-200418130-00002. [DOI] [PubMed] [Google Scholar]
- Overall 1962 .Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Rust 1989 .Rust J, Golombok S. Modern Psychometrics. Routledge; London: 1989. [Google Scholar]
- Simpson 1970 .Simpson EN, Angus JWF. A rating scale for extrapyramidal side-effects. Acta Physiologica Scandinavica Supplementum. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Stroup 2003 .Stroup S, McEvoy J, Swartz MS, Byerly M, Glick I, Canive J, McGee MF, Simpson GM, Stevens MC, Lieberman JA. The national institute of mental health clinical antipsychotic trial of intervention effectiveness (CATIE) project: Schizophrenia trial design and protocol development. Schizophrenia Bulletin. 2003;29(1):15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- Ukoumunne 1999 .Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment. 1999;3(5):iii–92. [PubMed] [Google Scholar]
- Van Os 2006 .Van Os J, Drukker M, à Campo J, Meijer J, Bak M, Delespaul P. Validation of remission criteria for schizophrenia. American Journal of Psychiatry. 2006;163(11):2000–2. doi: 10.1176/ajp.2006.163.11.2000. [DOI] [PubMed] [Google Scholar]
- van Os 2009 .Van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–45. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Wahlbeck 1999 .Wahlbeck K, Cheine MV, Essali A. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews. 1999;(4) doi: 10.1002/14651858.CD000059. DOI: 10.1002/14651858.CD000059. [DOI] [PubMed] [Google Scholar]
- WHO 1998 .Barbato A. Schizophrenia and public health. Division of Mental Health and Prevention of Substance Abuse. World Health Organization; 1998. pp. 1–32. [Google Scholar]
References to other published versions of this review
- Tuunainen 2000 .Tuunainen A, Wahlbeck K, Gilbody SM. Newer atypical antipsychotic medication versus clozapine for schizophrenia. Cochrane Database of Systematic Reviews. 2000;(2) doi: 10.1002/14651858.CD000966. DOI: 10.1002/14651858.CD000966. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study