Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a serious, painful and dose-limiting side effect of cancer drugs that target microtubules. The mechanisms underlying the neuronal damage are unknown, but may include disruption of fast axonal transport, an essential microtubule-based process that moves cellular components over long distances between neuronal cell bodies and nerve terminals. This idea is supported by the “dying back” pattern of degeneration observed in CIPN, and by the selective vulnerability of sensory neurons bearing the longest axonal projections. In this study, we test the hypothesis that microtubule-targeting drugs disrupt fast axonal transport using vesicle motility assays in isolated squid axoplasm and a cell-free microtubule gliding assay with defined components. We compare four clinically-used drugs, eribulin, vincristine, paclitaxel and ixabepilone. Of these, eribulin is associated with a relatively low incidence of severe neuropathy, while vincristine has a relatively high incidence. In vesicle motility assays, we found that all four drugs inhibited anterograde (conventional kinesin-dependent) fast axonal transport, with the potency being vincristine = ixabepilone > paclitaxel = eribulin. Interestingly, eribulin and paclitaxel did not inhibit retrograde (cytoplasmic dynein-dependent) fast axonal transport, in contrast to vincristine and ixabepilone. Similarly, vincristine and ixabepilone both exerted significant inhibitory effects in an in vitro microtubule gliding assay consisting of recombinant kinesin (kinesin-1) and microtubules composed of purified bovine brain tubulin, whereas paclitaxel and eribulin had negligible effects. Our results suggest that (i) inhibition of microtubule-based fast axonal transport may be a significant contributor to neurotoxicity induced by microtubule-targeting drugs, and (ii) that individual microtubule-targeting drugs affect fast axonal transport through different mechanisms.
Keywords: Peripheral neuropathy, Axonal transport, Microtubule, Kinesin, Dynein, Chemotherapy drugs, CIPN
1. Introduction
Microtubules are a major component of the cytoskeleton. They are dynamic polymers composed of tubulin dimers arranged end to end in linear protofilaments that align to form a hollow cylinder. In addition to playing essential roles in migration and mitosis (Jordan and Wilson, 2004), microtubules are critical for intracellular transport, serving as tracks for “motor” proteins that carry vesicular cargo. Neurons are especially dependent on intracellular transport because of their complex elongated cellular architecture. In neuronal axons, the motor protein conventional kinesin transports various cargoes from the cell body toward distal terminals (anterograde fast axonal transport), supplying essential new materials for synapse function and maintenance (Morfini et al., 2011). On the other hand, cytoplasmic dynein transports cargo in the opposite direction (retrograde fast axonal transport), clearing toxic components from nerve terminals (e.g., misfolded proteins, damaged organelles), and delivering survival factors to the cell body. The importance of fast axonal transport to neuronal function is highlighted by multiple lines of evidence linking deficits in this process to neurodegeneration (Millecamps and Julien, 2013; Morfini et al., 2009; Perlson et al., 2010).
Many cancer chemotherapeutic agents target microtubule structure and function (Jordan and Wilson, 2004), and a frequently dose-limiting side effect of these drugs is chemotherapy-induced peripheral neuropathy (CIPN). Sensory neurons are preferentially affected, and symptoms typically appear first in distal extremities (Argyriou et al., 2012; Carlson and Ocean, 2011; Windebank and Grisold, 2008), indicating increased vulnerability of neurons with the longest axons. In affected neurons, the fine axonal fibers innervating the skin are lost (Pachman et al., 2011; Siau et al., 2006), consistent with a “dying back” pattern of degeneration (Argyriou et al., 2012). The incidence of severe neuropathy varies among different microtubule targeting drugs, with vincristine ranking among the highest (Carlson and Ocean, 2011). In contrast, eribulin has a relatively low incidence of severe neuropathy (Cortes et al., 2012).
The mechanisms by which microtubule-targeting drugs cause neuropathy are unknown. Mitochondrial dysfunction, changes in gene expression and membrane excitability, and inflammation have all been proposed (Jaggi and Singh, 2012; Pachman et al., 2011; Xiao et al., 2011). However, none of these mechanisms explains the selective vulnerability of long sensory neurons. An alternative hypothesis consistent with the “dying back” pattern of degeneration produced by microtubule-targeting drugs is that these drugs disrupt fast axonal transport (Argyriou et al., 2012; Komlodi-Pasztor et al., 2011; Windebank and Grisold, 2008). This hypothesis is supported by studies in cultured cells (Carbonaro et al., 2012; Shemesh and Spira, 2010; Theiss and Meller, 2000) and sciatic nerves (Nakata and Yorifuji, 1999; Sahenk et al., 1987). However, in these experimental systems it is difficult to distinguish effects on fast axonal transport from effects on other cellular processes.
Here, we directly measured effects of four clinically important microtubule-targeting drugs on fast axonal transport using two simplified experimental systems. These drugs, eribulin, vincristine, paclitaxel and ixabepilone, represent four distinct drug classes (i.e. halichondrins, Vinca alkaloids, taxanes, and epothilones) and induce peripheral neuropathy to varying extents. Using vesicle motility assays in isolated squid axoplasm, all four drugs inhibited anterograde transport, with varying potencies. In contrast, retrograde axonal transport was not affected by eribulin or paclitaxel, but was inhibited by vincristine and ixabepilone. In an independent, cell-free assay, the drugs displaying the strongest inhibitory effects in the axoplasm assay, vincristine and ixabepilone, also inhibited kinesin-1-driven gliding of microtubules assembled from purified bovine brain tubulin. In contrast, eribulin and paclitaxel had no significant effect on microtubule gliding. Our results indicate that inhibition of fast axonal transport may contribute significantly to the neurotoxicity induced by microtubule-targeting drugs, and that eribulin's lower incidence of severe peripheral neuropathy may result, at least in part, from its milder effects on fast axonal transport relative to other microtubule-targeting drugs and from the low doses at which eribulin is clinically effective.
2. Methods
2.1. Proteins and chemicals
Drugs sources were as follows: eribulin mesylate (Eisai Inc.), vincristine sulfate (Sigma), paclitaxel (Molecular Probes for vesicle motility assays in axoplasm; Sigma for microtubule gliding assays), and ixabepilone (Bristol-Myers Squibb). Stocks were prepared at 10 mM concentrations in DMSO. For microtubule gliding assays, tubulin was isolated from bovine brain and purified by phosphocellulose chromatography (Miller and Wilson, 2010). Rhodamine-labeled tubulin was prepared as described (Peck et al., 2011). Guanosine-5′-[(α,β)-methyleno]triphosphate sodium salt (GMPCPP) was purchased from Jena Bioscience. The recombinant kinesin construct used in microtubule gliding assays, K560-His, was a kind gift from Dr. Ron Vale at the University of California, San Francisco. It corresponds to the first 560 amino acids of human conventional kinesin (KIF5B, GenBank Accession NP_004512.1) fused to a 6×-histidine tag (located at the carboxy terminus) to facilitate nickel-based affinity purification. It was expressed in bacteria and purified as described (Peck et al., 2011).
2.2. Vesicle motility assays in isolated squid axoplasm
Axoplasm from squid (Loligo pealii) giant axons was extruded from its plasma membrane as previously described (Brady et al., 1985). All drugs were diluted for perfusion in 5 mM ATP-supplemented axoplasm buffer (175 mM potassium aspartate, 65 mM taurine, 35 mM betaine, 25 mM glycine, 10 mM HEPES, 6.5 mM MgCl2, 5 mM EGTA, 1.5 mM CaCl2, 0.5 mM glucose, pH 7.2). The volume of a single axoplasm was ∼5 μl, and each axoplasm was perfused with 20 μl of buffer containing the drug of interest. Therefore, drug concentration was diminished approximately 20% by the volume of the extruded axoplasm. Motility was analyzed using a Zeiss Axiomat microscope equipped with a 100×, 1.3 N.A. objective and differential interference contrast optics. Organelle velocities were measured by matching calibrated cursor movements to the speed of vesicles moving in the axoplasm (Morfini et al., 2006), where a “match” between vesicle and cursor speed required agreement of two observers. With each axoplasm, a baseline measurement was taken of anterograde and retrograde fast axonal transport velocities prior to perfusion with drug. Following perfusion with drug, axonal transport velocities were collected over a 50 min period as described (Morfini et al., 2007).
With the exception of 10 μM ixabepilone, which was tested in a single axoplasm due to its strong effects, all drug concentrations were tested in 3–4 axoplasms. Velocity measurements of anterograde and retrograde fast axonal transport from each assay condition were pooled and plotted as a function of time. Curves were fitted in GraphPad Prism using an equation for one-phase exponential decay. To compare fast axonal transport among axoplasms of different experimental conditions, data collected between 30 and 50 min post perfusion were pooled (avg. N = 26, low N = 4, high N = 38) and then analyzed by a one-way ANOVA followed by Tukey's post-test (GraphPad Prism statistical software). All data are expressed as mean ± SEM.
2.3. Immunofluorescence microscopy-based visualization of microtubules in squid axoplasm
Squid axoplasms were extruded onto a microscope slide as described above, and a PAP pen (Sigma) was used to draw a hydrophobic oval surrounding the axoplasm. ATP-supplemented axoplasm buffer with or without drug was prepared as for vesicle motility assays, except that the total volume was increased to 30 μl to ensure that the entire axoplasm was covered. Therefore, final drug concentrations in this assay were slightly higher than in the vesicle motility assay described above. Following a 50 min incubation, the axoplasm buffer was carefully removed with a pipette and replaced with 50 μl of 4% paraformaldehyde in phosphate buffered saline (PBS), pH 7.0 (Electron Microscopy Sciences). After 1 h incubation, axoplasms were washed three times with PBS, and then blocked for 1 h in PBS + 0.1% Triton X100 (PBT) containing 1% IgG-free BSA (Jackson Immunoresearch). Axoplasms were subsequently labeled with the anti-tubulin antibody DM1A (1:500; Sigma) followed by FITC-conjugated goat anti-mouse secondary antibody (1:1000, Cappel), and then mounted using ProLong Gold antifade reagent (Invitrogen). Axoplasms were imaged at 20× magnification on a BX60 Olympus microscope equipped with a MacroFire camera (Optronics). Control experiments in which the primary antibody was excluded demonstrated that secondary antibody labeling was specific (data not shown). Images were processed in Adobe Photoshop CS5 using the Adjust Levels function in order to optimize visibility. The analysis included 4 control axoplasms and 2–4 axoplasms per drug.
2.4. Kinesin-1-driven microtubule gliding assays
In these in vitro assays, we measured the velocity of stable microtubules as they glided across glass slides coated with recombinant kinesin-1 (Peck et al., 2011). To produce stable microtubules we used GMPCPP, a very slowly hydrolyzable analog of GTP (Stumpff et al., 2007). Rhodamine-labeled tubulin and unlabeled tubulin were mixed at a ratio of 1:3 (final tubulin concentration 20 μM), and incubated with 1 mM GMPCPP in PEM80 (80 mM Pipes, pH 6.8, 1 mM EGTA, 1 mM MgSO4) for 1 h at 35 °C. The resulting microtubules were collected by centrifugation (52,000 × g for 12 min), resuspended in PEM80, and incubated on ice to promote microtubule disassembly. This initial round of assembly was performed to ensure that no GTP was present during the final microtubule preparation. Microtubules were subsequently assembled a second time with 0.5 mM GMPCPP, collected by centrifugation, and then resuspended in buffer without GMPCPP (protocol adapted from (Stumpff et al., 2007)).
Gliding assays were performed as described (Peck et al., 2011). Briefly, microscope slide chambers were coated with purified K560-His (50 μg/ml) for 3 min, and then blocked with casein (1 mg/ml, Sigma) for 1 min. GMPCPP-assembled microtubules were diluted in motility buffer (PEM80 with 5 mM ATP, 1 mg/ml casein, and an oxygen scavenging system of 1.4 ng/ml glucose oxidase (Sigma), 33 μg/ml catalase (Sigma), and 5 mg/ml glucose) containing the drug of interest (10 μM) or vehicle control (DMSO). The final concentration of DMSO was 0.1% (v/v) for all experiments. Diluted microtubules were then flowed into the assay chamber, and the chamber was sealed with paraffin wax. Images of gliding microtubules were collected at a rate of 1 frame every 4 s using a Nikon Eclipse E800 fluorescence microscope with a 60×oil-immersion lens, coupled to a Hamamatsu Orca II digital camera. Image J software with the “Manual Tracking” plug-in application was used to track individual microtubules through 10–15 frames. The tracking application determined frame-to-frame velocities, which were then averaged in Microsoft Excel to calculate a mean velocity for each microtubule (30–50 microtubules per condition). A microtubule was not tracked if any part of it left the field of view. For each experimental condition, assays were performed on at least three independent days. Data were analyzed by a one-way ANOVA followed by Dunnett's post-test (GraphPad Prism statistical software) to compare each drug condition to vehicle control, and data are shown ± SEM.
3. Results
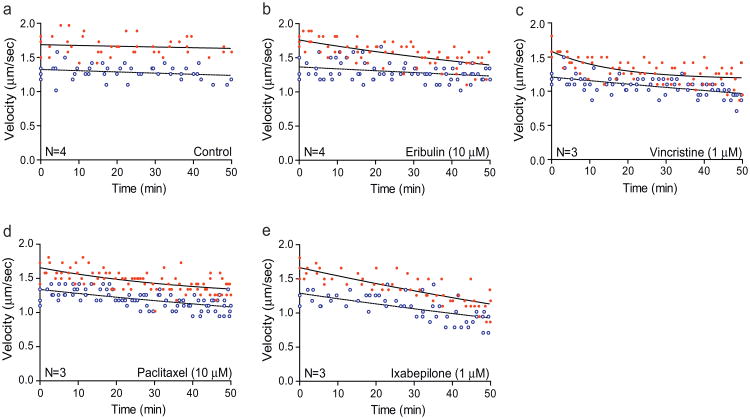
We first directly evaluated effects of the microtubule-targeting drugs on fast axonal transport by measuring the rate of vesicle motility in isolated squid axoplasm (see Section 2, also (Brady et al., 1993)). In this experimental system, anterograde and retrograde fast axonal transport of vesicles depend upon the activities of conventional kinesin (Brady et al., 1990) and cytoplasmic dynein (DeGiorgis et al., 2011), respectively. Vesicle motility assays typically analyze fast axonal transport rates over a 50-min interval, although axonal transport in this system is actually maintained for hours (Brady et al., 1985). Individual rate measurements (μm/s) are obtained at multiple time points, and these rates represent a compound value reflecting both the velocity and number of transported vesicles at any given time. Red filled circles and solid lines represent conventional kinesin-dependent anterograde fast axonal transport rates, whereas blue open circles and dotted lines show cytoplasmic dynein-dependent retrograde fast axonal transport rates (Fig. 1).
Fig. 1.
Inhibition of fast axonal transport by microtubule-targeting drugs in squid axoplasm. Isolated axoplasm was perfused with (a) control buffer, (b) eribulin (10 μM), (c) vincristine (1 μM), (d) paclitaxel (10 μM), or (e) ixabepilone (1 μM). Vesicle transport velocities were measured in the anterograde (red filled circles, solid upper lines) and retrograde (blue open circles, dotted lower lines) direction over a 50 min period. Each plot includes pooled data from 3 to 4 axoplasms (N). (For interpretation of the references to color in figure legend, the reader is referred to the web version of the article.)
We evaluated the effects of two drugs that can promote microtubule disassembly (eribulin and vincristine) and two that can promote microtubule assembly (paclitaxel and ixabepilone). For each axoplasm, we first measured baseline anterograde and retrograde fast axonal transport velocities, and then perfused the axoplasm with either 1 μM or 10 μM drug and measured vesicle velocities for an additional 50 min after perfusion. The drug concentrations we chose are higher than those required in cell culture medium to inhibit cell proliferation because microtubule-targeting drugs are generally taken up and concentrated in intact cells. Indeed, drugs added to cell culture media at nanomolar concentrations often achieve micromolar concentrations within cells (Jordan and Kamath, 2007; Oroudjev et al., 2010). Thus, we sought to assess the effects of drugs in plasma membrane-free axoplasm at their approximate intracellular concentrations.
3.1. Effects of drugs on fast axonal transport
In axoplasms perfused with control buffer, transport rates are maintained for over 1 h with little or no reduction (Brady et al., 1985). Consistent with previous results (Brady et al., 1993), anterograde transport rates in control axoplasms varied between 1.42 and 1.97 mm/s over the 50 min observation period with a mean of 1.66 ± 0.02 μm/s, and retrograde transport rates varied between 1.02 and 1.58 μm/s with a mean of 1.29 ± 0.02 μm/s (Fig. 1a). When axoplasms were perfused with eribulin, anterograde fast axonal transport rates decreased with time, while retrograde rates remained unchanged (Fig. 1b). To quantify these effects, we pooled velocity measurements taken between 30 and 50 min post-perfusion and compared them with velocities obtained from control axoplasms during the same time window (Fig. 2, Table 1). In control axoplasms, the mean anterograde axonal transport rate in this window was 1.65 ± 0.03 μm/s, and the mean retrograde axonal transport rate was 1.25 ± 0.03 μm/s (Fig. 2 and Table 1).
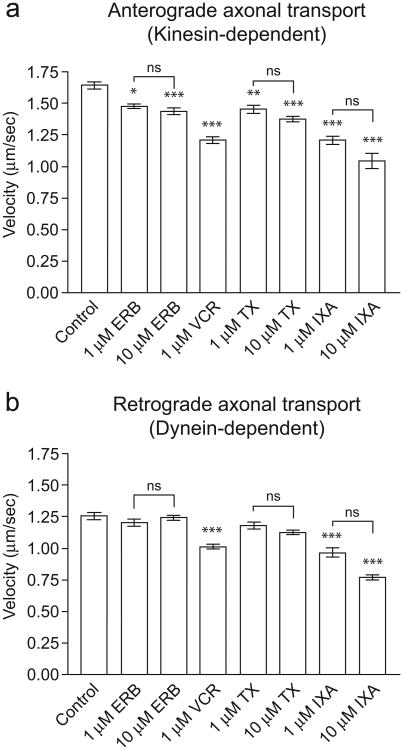
Fig. 2.
Effects of microtubule-targeting on anterograde and retrograde transport in squid axoplasm. Measurements were taken between 30 and 50 min after perfusion. Graphs show pooled data from 1 to 4 axoplasms (4–38 observations per condition). Error bars are SEM. Asterisks indicate a significant difference vs. control, where *P < 0.05, **P < 0.01, ***P < 0.001. No significant differences were found between different drug concentrations (N/S = not significant).
Table 1.
Axonal transport velocities in isolated squid axoplasm in the presence and absence of microtubule-targeting drugs.
| Condition | Anterograde velocity (±SEM) | Anterograde % change | Retrograde velocity (±SEM) | Retrograde % change | N |
|---|---|---|---|---|---|
| Control | 1.65 ±0.03 μm/s | – | 1.25 ±0.03 μm/s | – | 4 |
| Eribulin (1 μM) | 1.48 ±0.02 μm/s* | −10% | 1.20 ±0.03 μm/s | −4% | 3 |
| Eribulin (10 μM) | 1.44 ±0.03 μm/s*** | −13% | 1.24 ±0.02 μm/s | −1% | 4 |
| Vincristine (1 μM) | 1.21 ±0.03 μm/s*** | −27% | 1.01 ±0.02 μm/s*** | −19% | 3 |
| Paclitaxel (1 μM) | 1.45 ±0.03 μm/s** | −12% | 1.18 ±0.03 μm/s | −6% | 3 |
| Paclitaxel (10 μM) | 1.37 ±0.02 μm/s*** | −17% | 1.12 ±0.02 μm/s | −10% | 3 |
| Ixabepilone (1 μM) | 1.21 ±0.03 μm/s*** | −27% | 0.97 ±0.04 μm/s*** | −22% | 3 |
| Ixabepilone (10 μM) | 1.04 ±0.06 μm/s*** | −37% | 0.77 ±0.02 μm/s*** | −38% | 1 |
N: number of axoplasms; 4–38 values per condition.
ANOVA (P <0.0001) followed by Tukey's post-test, P <0.05.
ANOVA (P <0.0001) followed by Tukey's post-test, P <0.01.
ANOVA (P <0.0001) followed by Tukey's post-test, P <0.001.
Eribulin inhibited anterograde axonal transport by 10% (to 1.48 ± 0.02 μm/s, P < 0.05) and 13% (to 1.44 ± 0.03 μm/s, P < 0.001) at concentrations of 1 μM and 10 μM, respectively. Importantly, its effects on retrograde axonal transport were not significant at either concentration. In contrast to eribulin, vincristine (1 μM) had strong inhibitory effects on bidirectional fast axonal transport (Figs. 1c and 2 and Table 1), reducing both anterograde and retrograde transport rates by 27% (to 1.21 ± 0.03 μm/s, P < 0.001) and 19% (to 1.01 ± 0.02 μm/s, P < 0.001), respectively. Because vincristine is a potent microtubule depolymerizer at concentrations ≥1 μM, and since its effects at 1 μM were so pronounced, we did not test vincristine at higher concentrations. Interestingly, 1 μM vincristine induced a rapid decrease in anterograde transport within the first 20 min of perfusion, after which the rate of decrease leveled off. The inhibitory effects of vincristine on retrograde transport were more gradual (Fig. 1c).
We next assayed paclitaxel and ixabepilone, two drugs that can promote microtubule assembly. Paclitaxel effects on fast axonal transport (Fig. 1d, Table 1) were similar to those elicited by eribulin. Paclitaxel inhibited anterograde transport by 12% (to 1.45 ± 0.03 μm/s, P < 0.01) at 1 μM, and 17% (to 1.37 ± 0.02 μm/s, P < 0.001) at 10 μM, but had no significant effect on retrograde axonal transport. Although the effects of paclitaxel were slightly stronger than eribulin at each concentration, the difference between the two drugs was not statistically significant. Ixabepilone had potent effects on both directions of fast axonal transport that were similar in potency to vincristine. When isolated axoplasms were perfused with ixabepilone (1 μM), the rate of both anterograde and retrograde fast axonal transport decreased markedly over the 50-min observation period (Fig. 1e). At 1 μM ixabepilone, anterograde transport was inhibited by 27%, to 1.21 ± 0.03 μm/s (P < 0.001), and retrograde fast axonal transport was inhibited by 22%, to 0.97 ± 0.04 μm/s (P < 0.001). At 10 μM ixabepilone, anterograde fast axonal transport was slowed 37%, resulting in a rate of 1.04 ± 0.06 μm/s (P < 0.001), and retrograde transport was slowed 38% resulting in a rate of 0.77 ± 0.02 μm/s (P < 0.001, Fig. 2, Table 1). We did not find statistically significant concentration-dependent effects for any of the four drugs, although the data trend in that direction. Therefore, we conclude that the effects are near maximal at 1 μM.
In summary, the data indicate that in isolated axoplasm, vincristine and ixabepilone had the strongest inhibitory effects upon anterograde fast axonal transport, whereas eribulin and paclitaxel had milder effects. In addition, vincristine and ixabepilone inhibited retrograde fast axonal transport, whereas eribulin and paclitaxel did not.
3.2. Effects of drugs on microtubule organization in the axoplasm interior
One potential mechanism by which drugs may interfere with fast axonal transport is through their effects on microtubule organization and integrity. Specifically, compounds like taxanes and epothilones that can promote microtubule assembly (paclitaxel and ixabepilone in this work) can induce formation of abnormal bundles of microtubules in cultured cells (Jordan and Wilson, 2004), which could inhibit fast axonal transport by restricting the movement of motor proteins and/or cargo. Conversely, compounds like the Vinca alkaloids and halichondrins (vinblastine and eribulin in this work), which can promote microtubule disassembly (Jordan et al., 1991; Jordan and Wilson, 2004; Smith et al., 2010), might be expected to disrupt fast axonal transport by depolymerizing the microtubule tracks.
To determine whether the inhibitory effects observed in our axoplasm experiments were associated with microtubule bundling or depolymerization, we visualized axonal microtubules by immunofluorescence microscopy. As for vesicle motility assays, we incubated isolated axoplasms for 50 min with eribulin (10 μM), vincristine (1 μM), paclitaxel (10 μM), or ixabepilone (10 μM). Control axoplasms were incubated for the same amount of time in the absence of any drug. Axoplasms were subsequently fixed, labeled with an anti-tubulin antibody, and visualized by immunofluorescence microscopy. The interior of the axoplasm, where measurements are taken in the vesicle motility assay, appeared similar under all conditions (Fig. 3). As observed in untreated control axoplasms, immunofluorescence imaging of axoplasms exposed to drugs revealed a dense network of microtubules, with the vast majority oriented roughly parallel to each other and to the long axis of the axoplasm. There were two exceptions to this observation, which were not quantified. First, although a few areas with disorganized microtubules could be found in all axoplasms, these were more frequent in vincristine-treated axoplasms. Second, a few areas in two of the three paclitaxel-treated axoplasms contained stellate aggregates of tubulin in addition to ordered parallel microtubules (not shown). However, as a general assessment, none of the four microtubule-targeting drugs used in this study induced detectable bundling or depolymerization of microtubules in the axoplasm interior.
Fig. 3.
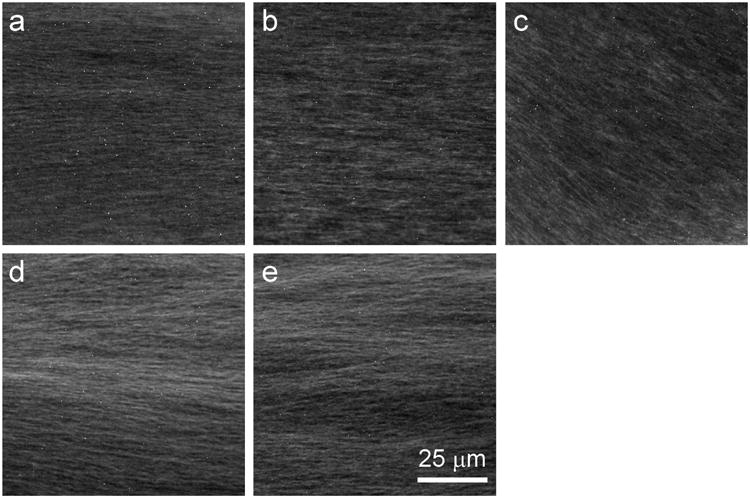
Microtubule organization in the axoplasm interior is well preserved after incubation with microtubule-targeting drugs. Axoplasm was incubated for 50 min in buffer alone (a) or in buffer containing 10 μM eribulin (b), 1 μM vincristine (c), 10 μM paclitaxel (d), or 10 μM ixabepilone (e), then fixed and processed for immunofluorescence microscopy with an anti-tubulin antibody (see Section 2). At this resolution, axoplasms incubated with drugs generally resembled untreated control axoplasms, with no evidence of extensive bundling or depolymerization observed. However, axoplasms with vincristine appear to have slightly more disorganized areas.
3.3. Effects of microtubule-targeting drugs on kinesin-1 driven microtubule gliding in a purified defined system
As presented above, all four drugs tested in squid axoplasm inhibited anterograde fast axonal transport, a process carried out by conventional kinesin. Conventional kinesin is a multisubunit motor protein. The kinesin-1 subunits of conventional kinesin contain binding domains for microtubules and ATP, and are responsible for the mechanochemical properties of the conventional kinesin holoenzyme (DeBoer et al., 2008). To assess direct effects of the drugs on kinesin-1 activity in the absence of cytoplasmic regulatory proteins such as microtubule associated proteins (MAPs), kinases, and phosphatases known to affect the functionality of this motor protein (Morfini et al., 2009), we performed microtubule gliding assays in vitro using purified, defined molecular components. Microtubules were assembled from MAP-free bovine brain tubulin and introduced into a chamber coated with purified recombinant kinesin-1. In this assay, kinesin-1 binds to the glass surface and is stationary, and thus its activity propels microtubules across the surface of the assay chamber. Drugs or other factors can be introduced into the system, and effects on microtubule gliding velocity can be quantified (Peck et al., 2011).
For optimal imaging, microtubules are diluted in buffer prior to the assay, a process that can trigger microtubule depolymerization. Therefore, microtubules in the gliding assay must be stabilized by some means (paclitaxel is often used). Microtubule stability was an especially important consideration in these experiments, since two of the four drugs under study (eribulin and vincristine) can promote microtubule disassembly. In order to generate stable microtubules without the use of paclitaxel (since that is one of the drugs being tested), we assembled microtubules using GMPCPP (Stumpff et al., 2007), a very slowly hydrolyzable analog of GTP. We then diluted the stable GMPCPP-microtubules into buffer containing one of the four microtubule-targeting drugs (10 μM) or vehicle (DMSO), and measured kinesin-1 driven microtubule gliding velocities.
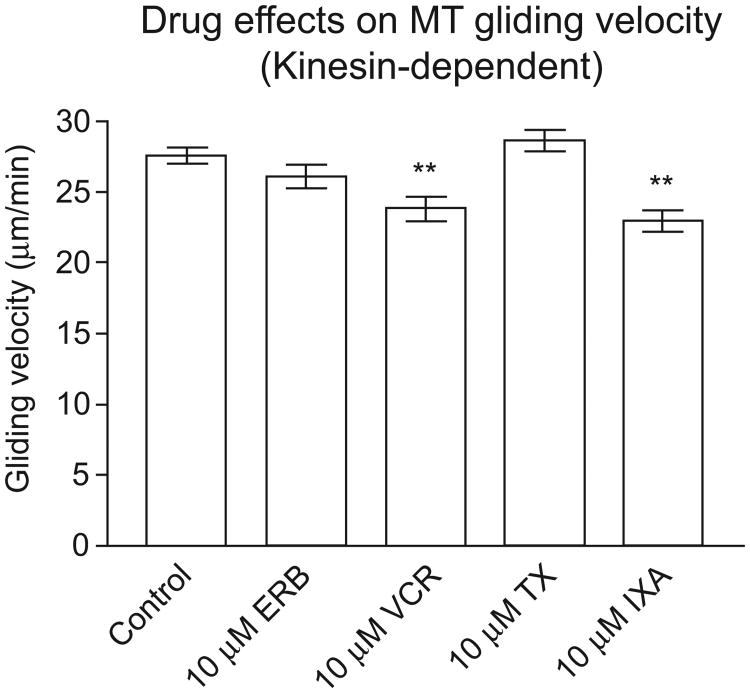
As shown in Fig. 4 and Table 2, 10 μM vincristine and ixabepilone both slowed kinesin-1 driven microtubule gliding velocity relative to vehicle control, by 13% and 17%, respectively, both of which were statistically significant at P < 0.01. In contrast, neither 10 μM paclitaxel nor 10 μM eribulin had a statistically significant effect on kinesin-driven gliding velocity. Inhibition of kinesin-1 driven gliding of stabilized microtubules by vincristine and ixabepilone paralleled the strong inhibitory effects of these drugs in axoplasm, although the in vitro effects were somewhat weaker. In contrast, eribulin and paclitaxel had no significant effect on in vitro gliding activity, consistent with the fact that they had the weakest effects on vesicle motility in axoplasm. Thus in both assays vincristine and ixabepilone were the most potent inhibitors of kinesin-mediated transport.
Fig. 4.
Effects of microtubule-targeting drugs on kinesin-1-driven gliding velocity of microtubules in vitro. Rhodamine-labeled microtubules were assembled with bovine brain tubulin and GMPCPP. The effects of 10 μM drug on the velocity of microtubules gliding on kinesin-1 coated glass slides were measured as described in Section 2. Error bars show SEM. **P < 0.01.
Table 2.
Kinesin-driven gliding velocity of GMPCPP microtubules in the presence or absence of microtubule-targeting drugs.
| Condition | Gliding velocity (±SEM) | % change | # of MTs |
|---|---|---|---|
| Control | 27.55 ± 0.56 μm/min | – | 30 |
| Eribulin (10 μM) | 26.14 ± 0.82 μm/min | −5% | 30 |
| Vincristine (10 μM) | 23.86 ± 0.87 μm/min** | −13% | 30 |
| Paclitaxel (10 μM) | 28.67 ± 0.77 μm/min | 4% | 50 |
| Ixabepilone (10 μM) | 22.99 ± 0.76 μm/min** | −17% | 30 |
ANOVA (P < 0.022) followed by Dunnett's post-test, P < 0.01.
4. Discussion
Chemotherapy-induced peripheral neuropathy can be graded on a scale of 1–4 in the clinic, where grades 3 and 4 are considered severe. Of the four drugs tested here, vincristine induces the highest levels of severe neuropathy (31%). Ixabepilone (12–20%) and paclitaxel (2–32%) are associated with the next highest incidence (Carlson and Ocean, 2011). Eribulin is associated with the lowest incidence of severe neuropathy, with 3–8% grade 3 and <1% grade 4 (Cortes et al., 2012). A similar pattern was observed when the neuropathic effects of eribulin, paclitaxel and ixabepilone were directly compared in mouse sciatic nerves, in that the effects of eribulin were significantly milder than those of either paclitaxel or ixabepilone (Wozniak et al., 2011).
In this work, we use a vesicle transport/squid axoplasm assay as well as a microtubule gliding assay to test the hypothesis that the different incidences of severe peripheral neuropathy produced by vincristine, ixabepilone, paclitaxel and eribulin relate to their differential effects on fast axonal transport. The pattern we observe in our assays is in general agreement with the relative incidence of severe peripheral neuropathy caused by these four drugs. Vincristine and ixabepilone had the most severe inhibitory effects on fast axonal transport in both assays, significantly inhibiting both anterograde and retrograde directions. In contrast, eribulin and paclitaxel had milder inhibitory effects on anterograde fast axonal transport and did not inhibit retrograde fast axonal transport. Furthermore, although eribulin and paclitaxel inhibited anterograde fast axonal transport to similar extents when compared at equal concentrations, eribulin is typically used at ∼100-fold lower concentration than paclitaxel in the clinic (1.4 mg/m2 for eribulin (Eisai Inc., 2012) vs. 175 mg/m2 for paclitaxel (Bristol-Myers Squibb Company, 2011)). Although correlative and not causative, the results from our study are completely consistent with the model that alterations in fast axonal transport contribute to peripheral neuropathy induced by microtubule-targeting drugs. Some potential mechanisms by which microtubule-targeted drugs could disrupt fast axonal transport are discussed below.
4.1. Drug binding changes microtubule structure
Paclitaxel and ixabepilone
The taxanes and epothilones have many similarities in their modes of action. Both promote microtubule assembly and suppress microtubule dynamic instability (Kamath and Jordan, 2003; Perez, 2009) (Smiyun et al., unpublished data). The epothilones bind near the taxane site on β-tubulin, located at the interior surface of the microtubule (Khrapunovich-Baine et al., 2011), and both drugs bind along the microtubule length. Taxanes and epothilones stabilize microtubules by strengthening contacts between adjacent tubulin dimers within protofilaments and also by stabilizing lateral contacts between protofilaments (Khrapunovich-Baine et al., 2011). Given these similarities, it is interesting that the ability of ixabepilone to induce severe peripheral neuropathy, as well as to inhibit fast axonal transport (this study) was greater than that of paclitaxel. The differences may be due in part to the higher binding affinity of ixabepilone relative to paclitaxel (Perez, 2009), but they may also arise from different effects on microtubule structure. For example, paclitaxel and the epothilones have drug-specific effects on microtubule protofilament number (Diaz et al., 1998; Meurer-Grob et al., 2001). In addition, both drugs also induce conformational changes in the carboxy terminus of tubulin (Khrapunovich-Baine et al., 2011), a region that plays a role in the binding of motor proteins and other MAPs (Khrapunovich-Baine et al., 2009, 2011) and in the regulation of kinesin-1 ATPase activity (Uchimura et al., 2010). Drug-specific structural changes in the tubulin carboxy terminus might therefore influence the binding and/or activity of motor proteins and MAPs.
Vincristine and eribulin
Vincristine (a Vinca alkaloid) and eribulin (derived from halichondrin B) suppress microtubule dynamic instability at low concentrations and promote microtubule disassembly at high concentrations (Jordan et al., 2005; Jordan and Wilson, 2004; Smith et al., 2010). Both classes of drugs bind to β-tubulin, although the binding sites do not overlap (Bai et al., 2011; Gigant et al., 2005). The Vinca alkaloids bind with high affinity to the ends of microtubules, and with lower affinity along microtubule sides at the interface between adjacent tubulin dimers (Jordan and Wilson, 2004). Vinca alkaloid binding along the length of the protofilament strengthens longitudinal contacts within the protofilament while simultaneously weakening lateral contacts between adjacent protofilaments. As a result, at high Vinca alkaloid concentrations microtubule ends splay out and protofilaments peel away in spirals (Jordan and Wilson, 2004; Rendine et al., 2010). Unlike the Vinca alkaloids, eribulin binds exclusively at microtubule ends, and binding may occur preferentially at “plus” ends (Smith et al., 2010). Eribulin is also unusual in that it suppresses microtubule growth without appreciably affecting the rate of microtubule shortening (Jordan et al., 2005; Smith et al., 2010). The stronger inhibitory effects of vincristine as compared with eribulin on fast axonal transport may result, at least in part, from their different modes of microtubule binding and action. Because vincristine binds along the length of microtubules, the conformational changes induced by vincristine along the microtubule surface may readily affect the interactions between motors and microtubules as well as the conformation of tubulin dimers. In contrast, eribulin may have fewer effects on fast axonal transport because it binds only at microtubule ends.
All four drugs reduced kinesin-dependent transport velocities in isolated axoplasm. However, only the two showing the strongest inhibitory effects, vincristine and ixabepilone, reduced kinesin-1 activity in microtubule gliding assays. Even for vincristine and ixabepilone, their inhibitory effects in the gliding assay were less pronounced than those observed in vesicle motility assays using axoplasm. While both of these assays measure key features of fast axonal transport, they do have technical differences. For example, whereas fast axonal transport in squid axoplasm relies on endogenous microtubules, our gliding assays employ GMPCPP-stabilized microtubules. Although drugs can induce structural changes in microtubules assembled with GMPCPP (Khrapunovich-Baine et al., 2009, 2011), GMPCPP itself has strong effects on microtubule structure (Meurer-Grob et al., 2001; Vale et al., 1994). Kinesin may be sensitive to GMPCPP-induced changes in microtubules, as indicated by in vitro studies demonstrating higher kinesin binding affinity (Nakata et al., 2011) and faster kinesin-driven gliding velocities (Vale et al., 1994) with GMPCPP microtubules. However, the GTP-bound tubulin conformation mimicked by GMPCPP is enriched in axons in vivo (Nakata et al., 2011), suggesting that this conformation has physiological relevance for studies addressing fast axonal transport. In addition, the gliding assay has the advantage of isolating effects of a pure kinesin-driven system without the cellular complexities of the intact axoplasm, and the reduced suppression in this in vitro assay as compared with the axoplasm may indicate that additional drug effects are at play in the axoplasm, as discussed below.
4.2. Potential effects of microtubule-targeting drugs on the organization and integrity of axonal microtubules
Microtubule-targeting drugs could disrupt fast axonal transport by bundling or depolymerizing axonal microtubules. Although these effects are easily observed by immunofluorescence microscopy with tubulin antibodies in cultured cells at high drug concentrations (Jordan et al., 1991; Jordan and Wilson, 1998, 2004; Kowalski et al., 1997), we did not observe evidence of microtubule bundling or depolymerization in isolated squid axoplasm. However, our results do not rule out subtle effects that might be revealed by higher resolution imaging methods.
In axons, microtubules are uniformly arranged with their “plus” ends oriented away from the cell body. The directionality of kinesin and dynein movement along microtubules is dependent upon this polar orientation (Morfini et al., 2011). In this study, we observed a slight trend toward more disorganized areas of microtubules in vincristine-treated axoplasms, which may contribute to the observed effects on fast axonal transport. Microtubule disorganization has also been reported in sciatic nerves bathed in vincristine, where it coincided with a reduction in the average microtubule length (Sahenk et al., 1987). Disruption of axonal microtubule polarity in cultured neurons has also been reported with drugs that promote microtubule assembly (Shemesh and Spira, 2010). Significant disruption of microtubule polarity in the squid axoplasm would be expected to affect both anterograde and retrograde fast axonal transport such that the rates of each would converge on a single value. However, the fact that we observed different anterograde and retrograde rates suggests that this was not a major factor in our analyses.
4.3. Other potential effects in axoplasm at the microtubule surface
All four microtubule-targeting drugs studied here, at relatively low concentrations, suppress microtubule dynamic instability, which may in turn influence the posttranslational modification state of the microtubules (Janke and Bulinski, 2011). Some studies suggest that kinesin-1 is sensitive to tubulin acetylation and tyrosination (Konishi and Setou, 2009; Reed et al., 2006), and changes in tubulin acetylation have also been linked to impaired axonal transport and neurodegeneration (reviewed in Millecamps and Julien, 2013). However, there is no evidence that tubulin modifications are altered in the axoplasm studies, and the enzymes needed to modify tubulin are absent from the gliding assays. Microtubule-targeting drugs could also affect fast axonal transport through their effects on the binding of non-motor MAPs to microtubules (Kar et al., 2003; Xiao et al., 2012), or by masking the motor-regulatory effects of these MAPs (Peck et al., 2011). In addition, microtubule-targeting drugs could influence fast axonal transport indirectly through effects on microtubule-associated phosphatases and kinases that regulate the activity of kinesin and cytoplasmic dynein (Morfini et al., 2009). For example, exposure of cultured cells to paclitaxel activates JNK (Figueroa-Masot et al., 2001), a kinase that negatively regulates fast axonal transport (Morfini et al., 2006).
4.4. A fast axonal transport framework for selective cell-type vulnerability in chemotherapy-induced peripheral neuropathy
Peripheral neuropathies induced by various unrelated microtubule-targeting drugs result from “dying back” degeneration of sensory neurons bearing the longest axons. However, some of these drugs (including vincristine) also exhibit significant motor neuron involvement (Argyriou et al., 2012; Carlson and Ocean, 2011; Windebank and Grisold, 2008). These observations suggest that, in addition to axon length, various factors might render certain neuronal subtypes selectively vulnerable to specific microtubule-targeting drugs. The present study does not address the issue of selective cell-type vulnerability. However, many neurodegenerative conditions linked to fast axonal transport abnormalities preferentially affect specific subpopulations of neurons. For example, mutations in kinesin-1A, one of three kinesin-1 isoforms (DeBoer et al., 2008), are linked to a specific form of hereditary spastic paraplegia that features “dying back” degeneration of upper motor neurons (Reid et al., 2002). Similarly, mutations in different cytoplasmic dynein subunits promote “dying back” degeneration of striatal, motor and/or sensory neurons (reviewed in (Eschbach and Dupuis, 2011)). Molecular mechanisms underlying the differential vulnerability of specific neuronal cell types to specific perturbations in fast axonal transport remain elusive, but might result from unique cell type-specific characteristics, including different metabolic demands, reliance on different transported cargoes, and differences in the compliment of proteins involved in fast axonal transport regulation, including various kinases and phosphatases (Han et al., 2010; Millecamps and Julien, 2013). Uncovering the basis of selective cell-type vulnerability in chemotherapy-induced peripheral neuropathy represents an important area for future research.
4.5. Inhibition of motor protein-dependent intracellular transport by microtubule-targeting drugs may contribute to their anticancer efficacy both in mitosis and in interphase
Microtubule-targeting drugs block mitosis of cancer cells in vitro and induce cell death. One underlying mechanism is through their inhibition of microtubule dynamic instability (Jordan and Wilson, 2004). However, motor proteins, including dynein and kinesins, also play key roles in mitosis (Sharp et al., 2000). In addition, cells in interphase rely on motor protein-dependent transport for survival and for cancer-relevant functions (Giannakakou et al., 2000; Hirokawa et al., 2009; Kardon and Vale, 2009; Thadani-Mulero et al., 2012). An important implication of our findings is that inhibition of motor protein-dependent transport by microtubule-targeting drugs may contribute significantly to their antiproliferative effects and their anticancer efficacy.
Acknowledgments
The authors would like to thank Herbert P. Miller for providing bovine brain tubulin, Alex Sturbaum, Anoopa Singh, Michaela Martin, Lisa Baker, and Dr. Sarah Ward for help with data collection, Dr. Joe DeGiorgis for a helpful technical discussion, and two anonymous reviewers for constructive feedback. We also gratefully acknowledge the Marine Biological Laboratory in Woods Hole, MA, and the NRI-MCDB Microscopy Facility at UC Santa Barbara.
Conflict of interest statement: This research was supported by funding from Eisai Inc. (MAJ, LW, SCF), the NIH (NS23868 to STB; NS066942A to GM), and the Santa Barbara Foundation (NL).
References
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Bai R, Nguyen TL, Burnett JC, Atasoylu O, Munro MH, Pettit GR, et al. Interactions of halichondrin B and eribulin with tubulin. J Chem Inf Model. 2011;51:1393–404. doi: 10.1021/ci200077t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST, Lasek RJ, Allen RD. Video microscopy of fast axonal transport in extruded axoplasm: a new model for study of molecular mechanisms. Cell Motil. 1985;5:81–101. doi: 10.1002/cm.970050203. [DOI] [PubMed] [Google Scholar]
- Brady ST, Pfister KK, Bloom GS. A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc Natl Acad Sci U S A. 1990;87:1061–5. doi: 10.1073/pnas.87.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady ST, Richards BW, Leopold PL. Assay of vesicle motility in squid axoplasm. Methods Cell Biol. 1993;39:191–202. doi: 10.1016/s0091-679x(08)60171-5. [DOI] [PubMed] [Google Scholar]
- Bristol-Myers Squibb Company. TAXOL® (paclitaxel) Injection prescribing information. 2011 [Google Scholar]
- Carbonaro M, Escuin D, O'Brate A, Thadani-Mulero M, Giannakakou P. Microtubules regulate hypoxia inducible factor-1alpha protein trafficking and activity: implications for taxane therapy. J Biol Chem. 2012;287:11859–69. doi: 10.1074/jbc.M112.345587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K, Ocean AJ. Peripheral neuropathy with microtubule-targeting agents: occurrence and management approach. Clin Breast Cancer. 2011;11:73–81. doi: 10.1016/j.clbc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Cortes J, Montero AJ, Gluck S. Eribulin mesylate, a novel microtubule inhibitor in the treatment of breast cancer. Cancer Treat Rev. 2012;38:143–51. doi: 10.1016/j.ctrv.2011.03.006. [DOI] [PubMed] [Google Scholar]
- DeBoer SR, You Y, Szodorai A, Kaminska A, Pigino G, Nwabuisi E, et al. Conventional kinesin holoenzymes are composed of heavy and light chain homodimers. Biochemistry. 2008;47:4535–43. doi: 10.1021/bi702445j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgis JA, Cavaliere KR, Burbach JP. Identification of molecular motors in the Woods Hole squid, Loligo pealei: an expressed sequence tag approach. Cytoskeleton (Hoboken) 2011;68:566–77. doi: 10.1002/cm.20531. [DOI] [PubMed] [Google Scholar]
- Diaz JF, Valpuesta JM, Chacon P, Diakun G, Andreu JM. Changes in microtubule protofilament number induced by Taxol binding to an easily accessible site. Internal microtubule dynamics. J Biol Chem. 1998;273:33803–10. doi: 10.1074/jbc.273.50.33803. [DOI] [PubMed] [Google Scholar]
- Eisai Inc. HALAVEN® (eribulin mesylate) Injection full prescribing information. 2012 [Google Scholar]
- Eschbach J, Dupuis L. Cytoplasmic dynein in neurodegeneration. Pharmacol Ther. 2011;130:348–63. doi: 10.1016/j.pharmthera.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Figueroa-Masot XA, Hetman M, Higgins MJ, Kokot N, Xia Z. Taxol induces apoptosis in cortical neurons by a mechanism independent of Bcl-2 phosphorylation. J Neurosci. 2001;21:4657–67. doi: 10.1523/JNEUROSCI.21-13-04657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. p53 is associated with cellular microtubules and is transported to the nucleus by dynein. Nat Cell Biol. 2000;2:709–17. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- Gigant B, Wang C, Ravelli RB, Roussi F, Steinmetz MO, Curmi PA, et al. Structural basis for the regulation of tubulin by vinblastine. Nature. 2005;435:519–22. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- Han I, You Y, Kordower JH, Brady ST, Morfini GA. Differential vulnerability of neurons in Huntington's disease: the role of cell type-specific features. J Neurochem. 2010;113:1073–91. doi: 10.1111/j.1471-4159.2010.06672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y, Tanaka Y, Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–96. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12:773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets. 2007;7:730–42. doi: 10.2174/156800907783220417. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4:1086–95. doi: 10.1158/1535-7163.MCT-04-0345. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Thrower D, Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991;51:2212–22. [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr Opin Cell Biol. 1998;10:123–30. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Kamath K, Jordan MA. Suppression of microtubule dynamics by epothilone B is associated with mitotic arrest. Cancer Res. 2003;63:6026–31. [PubMed] [Google Scholar]
- Kar S, Fan J, Smith MJ, Goedert M, Amos LA. Repeat motifs of tau bind to the insides of microtubules in the absence of Taxol. EMBO J. 2003;22:70–7. doi: 10.1093/emboj/cdg001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–65. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khrapunovich-Baine M, Menon V, Verdier-Pinard P, Smith AB, 3rd, Angeletti RH, Fiser A, et al. Distinct pose of discodermolide in Taxol binding pocket drives a complementary mode of microtubule stabilization. Biochemistry. 2009;48:11664–77. doi: 10.1021/bi901351q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khrapunovich-Baine M, Menon V, Yang CP, Northcote PT, Miller JH, Angeletti RH, et al. Hallmarks of molecular action of microtubule stabilizing agents: effects of epothilone B, ixabepilone, peloruside A, and laulimalide on microtubule conformation. J Biol Chem. 2011;286:11765–78. doi: 10.1074/jbc.M110.162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat Rev Clin Oncol. 2011;8:244–50. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nat Neurosci. 2009;12:559–67. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- Kowalski RJ, Giannakakou P, Hamel E. Activities of the microtubule-stabilizing agents epothilones A and B with purified tubulin and in cells resistant to paclitaxel (Taxol(R)) J Biol Chem. 1997;272:2534–41. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- Meurer-Grob P, Kasparian J, Wade RH. Microtubule structure at improved resolution. Biochemistry. 2001;40:8000–8. doi: 10.1021/bi010343p. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Julien JP. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–76. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- Miller HP, Wilson L. Preparation of microtubule protein and purified tubulin from bovine brain by cycles of assembly and disassembly and phosphocellulose chromatography. In: Wilson L, Correia JJ, editors. Methods in cell biology. Amsterdam, Netherlands: Elsevier Inc.; 2010. pp. 3–15. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Mizuno N, Kikkawa M, Brady ST. Tau binding to microtubules does not directly affect microtubule-based vesicle motility. J Neurosci Res. 2007;85:2620–30. doi: 10.1002/jnr.21154. [DOI] [PubMed] [Google Scholar]
- Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady ST. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–16. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- Morfini GA, Burns M, Binder LI, Kanaan NM, LaPointe N, Bosco DA, et al. Axonal transport defects in neurodegenerative diseases. J Neurosci. 2009;29:12776–86. doi: 10.1523/JNEUROSCI.3463-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, Burns MR, Stenoien DL, Brady ST. Axonal transport. In: Brady ST, Siegel G, Albers RW, Price D, editors. Basic neurochemistry. 8th. New York, NY: Academic Press; 2011. pp. 146–64. [Google Scholar]
- Nakata T, Niwa S, Okada Y, Perez F, Hirokawa N. Preferential binding of a kinesin-1 motor to GTP-tubulin-rich microtubules underlies polarized vesicle transport. J Cell Biol. 2011;194:245–55. doi: 10.1083/jcb.201104034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Yorifuji H. Morphological evidence of the inhibitory effect of Taxol on the fast axonal transport. Neurosci Res. 1999;35:113–22. doi: 10.1016/s0168-0102(99)00074-7. [DOI] [PubMed] [Google Scholar]
- Oroudjev E, Lopus M, Wilson L, Audette C, Provenzano C, Erickson H, et al. Maytansinoid-antibody conjugates induce mitotic arrest by suppressing microtubule dynamic instability. Mol Cancer Ther. 2010;9:2700–13. doi: 10.1158/1535-7163.MCT-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachman DR, Barton DL, Watson JC, Loprinzi CL. Chemotherapy-induced peripheral neuropathy: prevention and treatment. Clin Pharmacol Ther. 2011;90:377–87. doi: 10.1038/clpt.2011.115. [DOI] [PubMed] [Google Scholar]
- Peck A, Sargin ME, LaPointe NE, Rose K, Manjunath BS, Feinstein SC, et al. Tau isoform-specific modulation of kinesin-driven microtubule gliding rates and trajectories as determined with tau-stabilized microtubules. Cytoskeleton (Hoboken) 2011;68:44–55. doi: 10.1002/cm.20494. [DOI] [PubMed] [Google Scholar]
- Perez EA. Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8:2086–95. doi: 10.1158/1535-7163.MCT-09-0366. [DOI] [PubMed] [Google Scholar]
- Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL. Retrograde axonal transport:pathways to cell death. Trends Neurosci. 2010;33:335–44. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, et al. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–72. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Reid E, Kloos M, Ashley-Koch A, Hughes L, Bevan S, Svenson IK, et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–94. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendine S, Pieraccini S, Sironi M. Vinblastine perturbation of tubulin protofilament structure: a computational insight. Phys Chem Chem Phys. 2010;12:15530–6. doi: 10.1039/c0cp00594k. [DOI] [PubMed] [Google Scholar]
- Sahenk Z, Brady ST, Mendell JR. Studies on the pathogenesis of vincristine-induced neuropathy. Muscle Nerve. 1987;10:80–4. doi: 10.1002/mus.880100115. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41–7. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- Shemesh OA, Spira ME. Paclitaxel induces axonal microtubules polar reconfiguration and impaired organelle transport: implications for the pathogenesis of paclitaxel-induced polyneuropathy. Acta Neuropathol. 2010;119:235–48. doi: 10.1007/s00401-009-0586-0. [DOI] [PubMed] [Google Scholar]
- Siau C, Xiao W, Bennett GJ. Paclitaxel- and vincristine-evoked painful peripheral neuropathies: loss of epidermal innervation and activation of Langerhans cells. Exp Neurol. 2006;201:507–14. doi: 10.1016/j.expneurol.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49:1331–7. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpff J, Cooper J, Domnitz S, Moore AT, Rankin KE, Wagenbach M, et al. In vitro and in vivo analysis of microtubule-destabilizing kinesins. Methods Mol Biol. 2007;392:37–49. doi: 10.1007/978-1-59745-490-2_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thadani-Mulero M, Nanus DM, Giannakakou P. Androgen receptor on the move: boarding the microtubule expressway to the nucleus. Cancer Res. 2012;72:4611–5. doi: 10.1158/0008-5472.CAN-12-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss C, Meller K. Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell Tissue Res. 2000;299:213–24. doi: 10.1007/s004419900120. [DOI] [PubMed] [Google Scholar]
- Uchimura S, Oguchi Y, Hachikubo Y, Ishiwata S, Muto E. Key residues on microtubule responsible for activation of kinesin ATPase. EMBO J. 2010;29:1167–75. doi: 10.1038/emboj.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale RD, Coppin CM, Malik F, Kull FJ, Milligan RA. Tubulin GTP hydrolysis influences the structure, mechanical properties, and kinesin-driven transport of microtubules. J Biol Chem. 1994;269:23769–75. [PubMed] [Google Scholar]
- Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Nomoto K, Lapidus RG, Wu Y, Carozzi V, Cavaletti G, et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Res. 2011;71:3952–62. doi: 10.1158/0008-5472.CAN-10-4184. [DOI] [PubMed] [Google Scholar]
- Xiao H, Wang H, Zhang X, Tu Z, Bulinski C, Khrapunovich-Baine M, et al. Structural evidence for cooperative microtubule stabilization by Taxol and the endogenous dynamics regulator MAP4. ACS Chem Biol. 2012;7:744–52. doi: 10.1021/cb200403x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao WH, Zheng H, Zheng FY, Nuydens R, Meert TF, Bennett GJ. Mitochondrial abnormality in sensory, but not motor, axons in paclitaxel-evoked painful peripheral neuropathy in the rat. Neuroscience. 2011;199:461–9. doi: 10.1016/j.neuroscience.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]