Abstract
Objective
Theta-burst stimulation (TBS) is a repetitive transcranial magnetic stimulation (TMS) protocol, capable of enhancing or suppressing the amplitude of contralateral motor-evoked potentials (MEP) for several minutes after stimulation over the primary motor cortex. Continuous TBS (cTBS) produces a long-term depression (LTD)-like reduction of cortical excitability. The purpose of this study was to assess the test–retest reproducibility of the effects of cTBS and to investigate which neurophysiologic markers of cTBS-induced plasticity are most reproducible.
Methods
In ten healthy participants we evaluated in two different sessions the effects of cTBS (using AP–PA current direction, opposite to most commercial rTMS stimulators) on MEPs induced by single-pulse suprathreshold TMS (using AP–PA or PA current direction) over left motor cortex in the first dorsal inter-osseus (FDI) muscle.
Results
Results demonstrate that the marker of cTBS induced-plasticity with highest within-subject reproducibility is the modulation of corticospinal excitability measured 5 min after cTBS.
Conclusion
Overall the effects of cTBS modulation show limited test–retest reproducibility and some measures of the cTBS effects are more reproducible than others.
Significance
Studies comparing cTBS effects in healthy subjects and patients need to proceed with care. Further characterization of the effects of TBS and identification of the best metrics warrant future studies.
Keywords: Transcranial magnetic stimulation, Primary motor cortex, Variability, Neurophysiological markers
1. Introduction
Transcranial magnetic stimulation (TMS) has become a valuable method to non-invasively investigate the human brain, and offers opportunities to study and modulate brain functions (Chen et al., 2008; Pascual-Leone, 2006). Repetitive TMS (rTMS) can induce a lasting modulation of brain activity. A specific rTMS protocol, known as theta burst stimulation (TBS) (Huang et al., 2005) induces particularly robust physiological aftereffects despite being short and relatively low in intensity. When applied over the primary motor cortex (M1), continuous TBS (cTBS) decreases the amplitude of subsequent motor-evoked potentials (MEPs) for 10–60 min, whereas intermittent TBS (iTBS) increases it (Gentner et al., 2008; Goldsworthy et al., 2012; Huang et al., 2005; Talelli et al., 2007; Zafar et al., 2008). These protocols have been shown to be useful to assess changes in brain plasticity mechanisms related to age (Freitas et al., 2011) or pathology (Oberman et al., 2010). However, it is known that even subtle modifications of the cTBS protocol can influence its effect (for a review see Ridding and Ziemann, 2010). For example, the duration of the stimulation (Huang et al., 2005), the orientation of the coil (Suppa et al., 2008; Talelli et al., 2007), and even small changes in stimulation intensity (Doeltgen and Ridding, 2011; McAllister et al., 2009), in stimulation frequency (Goldsworthy et al., 2012), or in prior motor activity (Gentner et al., 2008) can significantly affect the TBS effect. Two studies have briefly addressed the intra-individual reproducibility of the TBS protocol. In a study by Huang et al. 7 subjects from a prior study (Huang et al., 2005) were retested, and the effects of TBS across testing days were found to be correlated (Huang et al., 2008), thus revealing that intra-individual variability was smaller than inter-individual variability. However, Di Lazzaro et al. (Di Lazzaro et al., 2008) found less reproducible effects of iTBS, with inter-individual variability being greater than an overall age effect. In the present study, we sought to further assess test–retest reliability of TBS effects in normal volunteers, and aimed to identify the most reliable markers of cTBS modulation of motor cortical excitability.
2. Materials and methods
2.1. Participants
Ten healthy, right-handed volunteers (5 women and 5 men; 33 ± 18 years old) participated in this retrospective study. All had normal neurological and medical exams, including a normal Mini Mental State Examination (MMSE). They were taking no medications and had no contraindications to TMS (Rossi et al., 2009). Demographic and clinical features are summarized in Table 1. All participated in two experimental sessions (visit A and visit B, separated by 107 ± 55 days). All gave their written informed consent to the study, which had been approved by the local Institutional Review Board.
Table 1.
Demographic data.
| Participants | Age [years] | Gender | Time* |
Interval between visit** [days] | AMT |
RMT |
|||
|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | ||||
| 1 | 22 | M | 10:00 | 15:25 | 74 | 56 | 49 | 67 | 58 |
| 2 | 21 | M | 11:00 | 13:50 | 38 | 38 | 39 | 43 | 42 |
| 3 | 53 | F | 10:00 | 10:50 | 52 | 49 | 48 | 52 | 51 |
| 4 | 67 | F | 13:30 | 14:40 | 29 | 41 | 43 | 47 | 46 |
| 5 | 22 | F | 10:00 | 15:05 | 27 | 39 | 31 | 39 | 37 |
| 6 | 21 | F | 9:30 | 10:00 | 46 | 31 | 30 | 35 | 34 |
| 7 | 22 | M | 10:00 | 8:30 | 63 | 34 | 32 | 45 | 47 |
| 8 | 54 | M | 14:00 | 12:30 | 81 | 38 | 37 | 54 | 52 |
| 9 | 22 | M | 8:00 | 10:00 | 56 | 41 | 40 | 53 | 49 |
| 10 | 24 | F | 10:00 | 15:30 | 607 | 48 | 30 | 42 | 33 |
| 33 ± 18 | 5F/5 M | 10:36 ± 1:49 | 12:38 ± 2:37 | 107 ± 177 | 41.5 ± 7.5 | 37.9 ± 7.2 | 47.7 ± 9.2 | 44.9 ± 8.3 | |
M: Male; F: Female
Time: time of testing
Visit: difference in days from second visit to first visit; RMT: Resting motor threshold; AMT: Active motor threshold (measured with MagPro) and ±SD: Standard deviation.
2.2. Experimental set-up
All participants underwent a brain MRI to rule out structural brain lesions and to generate a high-resolution, anatomical brain image to guide the TMS. A 3-Tesla GE scanner was used for image acquisition. The stimulation setup consists in stimulators attached to figure-of-eight coils. For single-pulses, we used a Nexstim stimulator delivering biphasic pulses with a current flowing in the brain with an antero-posterior and then a postero-anterior (AP–PA) direction (Nexstim Ltd, Helsinki, Finland) or, for the subject number 10, a Magstim stimulator delivering monophasic pulses with a current flowing in the brain with a PA direction (Magstim Ltd, Withland, Wales, UK). For repetitive TMS, i.e. cTBS, we used a MagPro stimulator (MagVenture A/S, Farum, Denmark) delivering biphasic pulses with a current flowing in the brain with an AP–PA direction. Note that this direction is opposite to most commercial rTMS stimulators. The Nexstim eXimia Neuronavigation system was used to ensure that the exact same cortical location was targeted within each study session as defined by each individual's brain MRI. For each individual tested, the same system was used for the two sessions; however, the experimenter was not necessarily the same.
During stimulation, surface electromyography (EMG) was recorded and monitored continuously on-line. Active electrodes were attached to the skin overlying the first dorsal interosseus (FDI) muscle. Reference electrode was placed over the metacarpophalangeal joint. A ground electrode was placed over the wrist bone or the ipsilateral forearm. The EMG signals were filtered (8–500 Hz), amplified, displayed and stored for off-line analysis. The TMS system delivered triggered pulses that synchronize the TMS and EMG systems. The participants were also monitored for drowsiness and asked to keep their eyes open throughout the experiment. Relaxation of the measured muscle was controlled by continuous visual EMG monitoring.
2.3. TMS measurements
Participants were seated in a comfortable chair, with a head rest, and with their elbows flexed at approximately 90° and their hands resting on their laps. The optimal scalp location for activation of the right FDI using TMS was determined as the location from which TMS-induced motor evoked potentials (MEPs) of maximum peak-to-peak amplitude were detected in the right FDI. Once the optimal location was identified, a marker was placed in the MRI scan to which the individual participant was registered using the eXimia navigated brain stimulation (NBS) system. This allowed the TMS to coil to be placed systematically in the same location and with the same orientation and tilt throughout each session.
Motor threshold (MT) was determined according to the recommendations of the International Federation for Clinical Neurophysiology (Rossini et al., 1994). Single TMS pulses were delivered over the optimal scalp position at supra-threshold intensity and gradually reduced by decrements of 2% of stimulator output. Resting MT (RMT) was defined, with the Nexstim or Magstim stimulator used for single-pulse TMS, as the lowest stimulus intensity capable of inducing MEPs of P50 lV peak-to-peak amplitude in at least 5 of 10 consecutive trials. EMG monitoring was used to assure that the target muscle was at rest. Prior to cTBS, active MT (AMT) was determined, with the MagPro stimulator used for the cTBS, as the minimum single-pulse TMS intensity required to produce MEPs of ≥200 μV in at least 5 of 10 consecutive trials while participants contracted the target muscle (contralateral FDI) at approximately 20% of the maximal voluntary contraction. It has been shown that prior, concurrent, or post motor activation modulates cTBS outcome (Gentner et al., 2008; Huang et al., 2008; Iezzi et al., 2008). In order to control for prior motor contraction during the measurement of AMT, the participants were asked to contract the FDI muscle about 2 s prior to each TMS pulse and to relax it about 1 s after each TMS pulse, for at least 3 s. The cTBS protocol was applied about 1 min after the end of the AMT measurement procedures; the relaxation of hand muscles was monitored continuously by the experimenters during and after the stimulation.
2.4. cTBS protocol
Continuous TBS was applied using parameters similar to those used Huang et al. (Huang et al., 2005): three pulses at 50 Hz, with an interval of 200 ms between the last pulse of a triplet and the first pulse of a triplet, for a total number of 600 pulses. The intensity was fixed at 80% of AMT. Due to limitations in our experimental set-up, the interstimulus interval was 240 ms compared to the interstimulus interval of 200 ms in the original paradigm introduced by Huang et al. Thus, in our cTBS paradigm, the triplet repetition rate was about 4.17 Hz instead of 5 Hz, both frequencies being included in the theta band.
To establish a baseline prior to cTBS, two to three batches of ten to thirty MEPs were recorded and measured in response to stimulation over the optimal FDI location, at an intensity of 120% of RMT and a rate of approximately 0.1 Hz (a random jitter of ±1 s was introduced to avoid any train effects). Following cTBS, similar batches of MEPs were measured at 5, 10, 20, 30, 40, 50, 60, 75 and 90 min following cTBS to track changes in amplitude over time.
2.5. Data analysis
To determine the MEP amplitude of the contralateral FDI muscle in response to TMS over left M1, continuous EMG was sampled to epochs starting 50–100 ms before a TMS pulse and ending 300–400 ms after. MEPs were analyzed using MegaWin (Mega Electronics Ltd, Kuopio, Finland) or Scope software (ADInstruments, Colorado Springs, USA). The latencies and peak-to-peak amplitudes were marked automatically and then checked by visual inspection. Baseline MEP amplitude was defined as the average peak-to-peak amplitude of the MEPs recorded prior to cTBS. MEPs’ amplitude at time t after cTBS was defined as the average peak-to-peak amplitude of the MEPs recorded during the corresponding batch; this value was then expressed as a ratio to baseline: (mean MEPs amplitude at time t) – (mean amplitude at baseline)/(mean amplitude at baseline). Thus, negative values reflect suppression of MEPs after cTBS.
We assessed the reproducibility across visits of baseline excitability as characterized by RMT, AMT and MEP amplitude. Then, in order to find the best parameters to reliably characterize brain responses to cTBS, we assessed the reproducibility of several physiological markers that have been used in previous studies (Di Lazzaro et al., 2005, 2008; Freitas et al., 2011; Gentner et al., 2008; Huang et al., 2005; Oberman et al., 2010). A spline interpolation was determined for each participant. As an index of the duration of the TBS-induced modulation of cortico-spinal excitability, we defined, for each participant, the “time to baseline” as the time when the spline interpolation crossed the baseline value line. Some participants did not show suppression at the first measured time point (T5); for these participants, time to baseline was estimated to be 5 min (qualitatively similar reproducibility was estimated if the time to baseline was estimated to be 0 in those cases, or even if these data were removed from the analysis). This happened for 2 participants at both visits (one male of 21 years old and one female of 53 years old) and 1 participant at visit B (one female of 67 years old). At the other end, time to baseline was set at 90 min for participants for whom all measures of excitability remained below baseline level. This happened for 3 participants at their visit B (2 females of 21 and 22 years old and one male of 54 years old). As a global measure of suppression, we calculated for each participant the “area of suppression,” i.e. the area under the spline curve between 5 min and time to baseline (see Fig. 1A for an illustration at the group level). In consequence, the area of suppression of the subjects with a time to baseline of 5 min was 0 (again, qualitatively similar reproducibility was estimated if these data were removed from the analysis). In addition, we examined the amount of modulation at the following time points following cTBS: T5, T10, T20 and T30. We also defined the “maximal suppression” as the smallest average MEP amplitude at any given time point in the 90 min time window after TBS. This measure can reflect the minimum facilitation in case the participant shows no suppression at all.
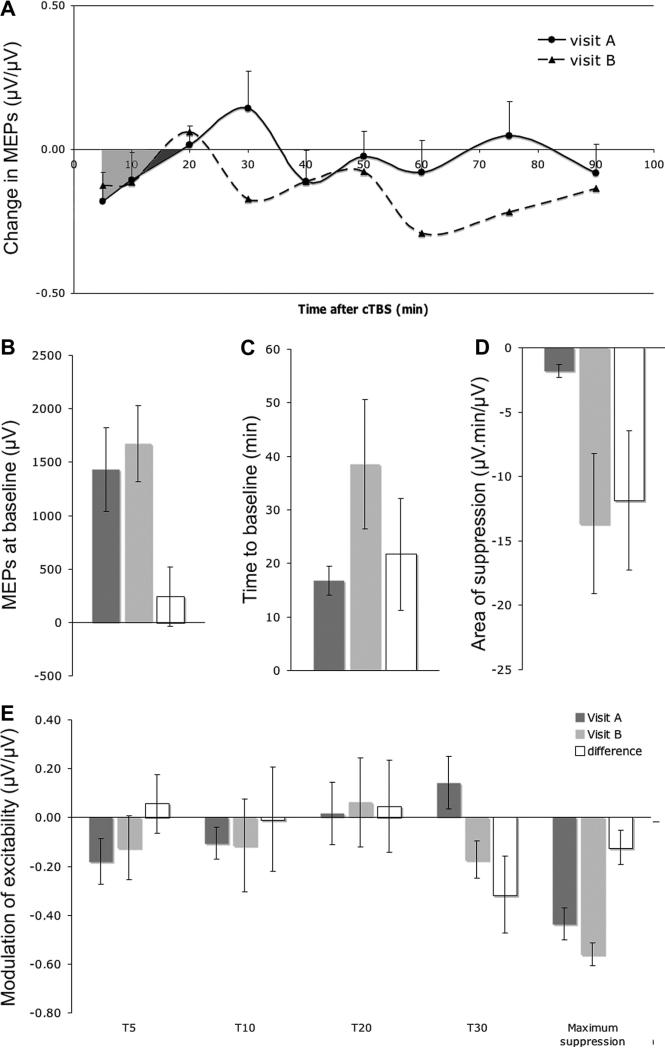
Fig. 1.
(A) Group-averaged evolution of excitability at visits A and B measured 5, 10, 20, 30, 40, 50, 60, 75 and 90 min after cTBS. For visualization are drawn the spline interpolations and areas of inhibition (visit A: dark and light grey areas; visit B: dark grey area). (B–D) Group-averaged for visit A and visit B and group-averaged difference between visits (visit B – visit A) of: B. MEP at baseline; C. time to baseline; D. area of suppression; E. modulation at T5, T10, T20, T30 and maximum suppression. (Mean ± SE).
Individual data for each calculated value was compared between the two visits (A and B) using non-parametric tests (Wilcoxon sign rank test). In addition, intra-individual variability between sessions was compared to the inter-individual variability within session (average of the two sessions values) using a one-sample t-test.
Gender influence on reproducibility was explored with un-paired t-test for comparing male versus female participants. The influence of time between visits and variability in RMT or AMT on reproducibility was explored with Pearson's correlation analysis.
Outliers (>1.5 interquartile range) were removed from statistical analysis and statistical significance for all analyses was set at p < 0.05.
3. Results
Fig. 1A shows the effects of cTBS on MEP amplitude for the group of participants for visit A and visit B. On average, cTBS caused suppression of MEPs. However this was not the case for all participants. For most, a clear suppression of MEPs was measured after cTBS, but two participants in one visit (a male of 22 years and a female of 67 years) and another participant at both visits (a female of 53 years) showed the opposite modulation.
3.1. Group-averaged comparisons between visit A and visit B
Fig. 1B–E show the group-averaged baseline and cTBS measures at visit A (dark grey bars) and visit B (light grey bars) as well as the group averaged difference between visits (visit B–visit A, white bars). The average MEP amplitude at baseline was 1.43 ± 1.24 mV for visit A and 1.67 ± 1.12 mV for visit B. There was no significant difference between visits on baseline MEP amplitude (p = 0.63; Fig. 1B).
The average time to baseline was 16.8 ± 8.6 min for visit A and 38.5 ± 38.2 min for visit B. The average area of suppression was −1.80 ± 1.60 μV.min/μV for visit A and −13.63 ± 17.22 μV.min/μV for visit B. There was no statistically significant difference in time to baseline between visits (p = 0.11; Fig. 1C) and only a trend for the area of suppression to be larger at visit B (p = 0.08; Fig. 1D). The difference between the two visits is explained by three subjects not returning to baseline at their visit B, thus increasing their time to baseline and area of suppression. These three subjects were a 22 year old female, tested 27 days apart, one time in the morning and one time in the afternoon; a 21 years old female, tested 46 days apart, both times in the morning; and a 54 years old male, tested 81 days apart, both times in the afternoon. There were no obvious epidemiologic or methodological factors identified to account for the drastic change in TBS effects in session B in these subjects.
The amount of cTBS-induced modulation in MEP amplitude at T5, T10, T20 and T30 was −0.18 ± 0.30, −0.11 ± 0.21, 0.02 ± 0.41 and 0.14 ± 0.34 μV/μV for visit A, and −0.12 ± 0.42, −0.11 ± 0.60, 0.06 ± 0.58 and −0.17 −0.17 ± 0.24 μV/μV for visit B. There was no significant difference between visits at any of these time points (all the p > 0.05; Fig. 1E). However, there was a trend toward significance at T30 (p = 0.08). The average of the maximum suppression after cTBS was −0.44 ± 0.21 μV/μV for visit A and −0.56 ± 0.15 μV/μV for visit B. There was only a trend toward significance for change in maximum suppression between visits (p = 0.08, Fig. 1E).
3.2. Intra-subject variability across sessions
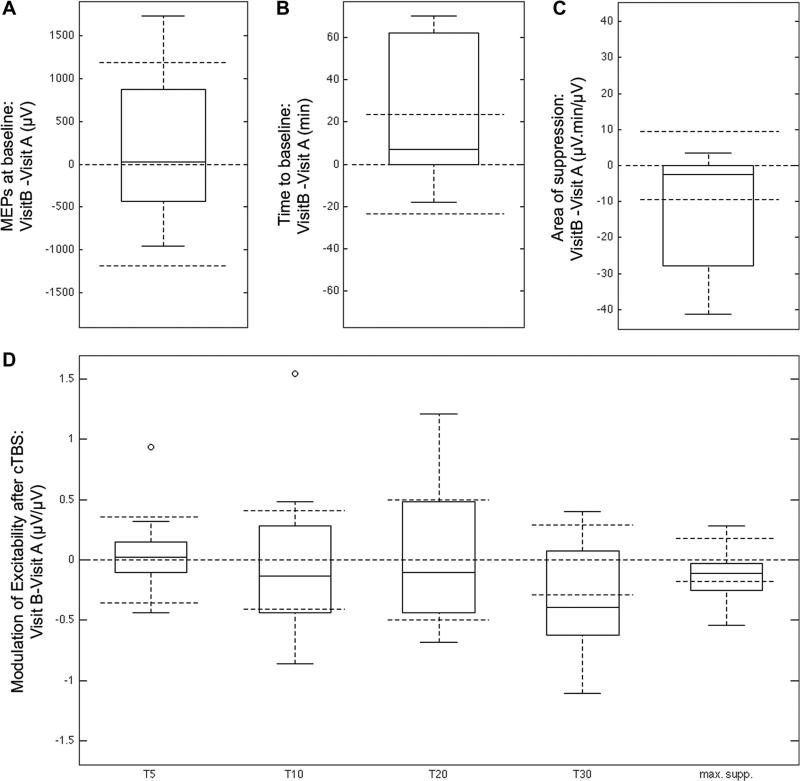
Fig. 2 shows box plots of the individual difference between the two visits for MEP amplitude at baseline, time-to baseline, area of suppression, modulation at T5, T10, T20 and T30 and maximum suppression. Observation of Fig. 2 indicates that some parameters are probably more reproducible than others. Indeed, parameters for which the difference between visits has a distribution relatively aggregated around 0 (e.g. MEPs at baseline, modulation at T5 and maximum suppression) are expected to be more reproducible than parameters for which the distribution is more widespread, especially with values higher than the inter-subject variability.
Fig. 2.
Box plots of the individual difference between the two visits for: A. MEP at baseline; B. time to baseline; C. area of suppression; D. modulation at T5, T10, T20 and T30 and maximum suppression. The box horizontal line is the median individual differences between visits; the tops and bottoms of each box are the 25th and 75th percentiles; the whiskers extend to the further observations beside outliers. Open dots are outliers, more than 1.5 times the interquartile range (i.e. distance top–bottom) away from the top or bottom of the box. Dashed horizontal lines are zero, plus and minus the inter-individual variability (averaged between the two visits).
The statistical analysis confirmed that the intra-individual variability of the MEPs at baseline and that modulation at T5 was lower than the inter-individual variability (p < 0.05 and p < 0.005 respectively). The intra-individual variability of all the other parameters (except modulation at T30) was in the range of the inter-individual variability (all the p > 0.05). Finally, the reproducibility of modulation at T30 was particularly poor as the intra-individual variability was superior to the inter-individual variability (p > 0.05).
A similar analysis made on the reproducibility of AMT and RMT showed that the measure of RMT and AMT were both reproducible, i.e. the intra-individual variability of was lower than the inter-individual variability. This difference was significant for RMT (p < 0.001); for AMT there was a tendency toward significance (p = 0.052).
3.3. Gender, time between visits and intensity of stimulation do not explain variability
Gender was not found to have any effect on reproducibility. Differences, in absolute value, between visit A and visit B for MEPs’ amplitude at baseline, time to baseline, area of suppression, modulation at T5, T10, T20 and T30, and maximum suppression in male (N = 5, 28 ± 14 years old) and female (N = 5, 37 ± 21 years old) participants were not statistically significant (all p > 0. 05).
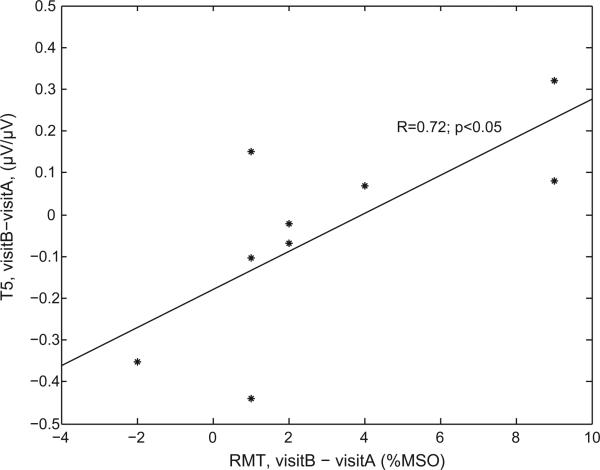
With one exception, the difference between visits A and B of any parameters (MEPs’ amplitude at baseline, time to baseline, area of suppression, modulation at T5, T10, T20 and T30, and maximum suppression) was not correlated with time between visits (all the p > 0.05), variability of RMT or variability of AMT (all the p > 0.05). The only exception was that the difference between visits of T5 was significantly correlated with RMT (Fig. 3, p < 0.05). However, such correlation does not resist correction for multiple comparisons.
Fig. 3.
Correlation between the variability of RMT and the variability of modulation at T5.
4. Discussion
As far as we know, this is the first study on the reproducibility of continuous theta burst stimulation (cTBS) and we found that the effects of cTBS to the motor cortex, as applied by the present protocol (with neuronavigation), are reasonably repeatable over an extended period of time in normal participants. We further found that one marker of TBS modulation, the degree of MEP modulation at time T5 shows the highest within-subject reproducibility. This reproducibility is similar to the reproducibility of M1 excitability (baseline MEPs, RMT). These findings are of relevance for longitudinal studies with serial assessments of TBS-induced plasticity and for studies comparing TBS-induced plasticity in healthy subjects and patients with neuropsychiatric disorders (Pascual-Leone et al., 2011).
There are at least three parameters that can be adjusted in order to maximize the cTBS effects: the temporal pattern of stimulation, the motor contraction, and the current direction of the stimulation. Concerning the temporal pattern of stimulation, we used, in this study, a paradigm of cTBS slightly modified from the original one introduced by Huang et al. (50 Hz triplets repeated with a frequency of about 4.17 Hz instead of 5 Hz). It is known that cTBS effects are influenced by the exact frequency of stimulation. Indeed, 30 Hz triplets repeated with a frequency of 6 Hz induced greater and longer-lasting effect than the standard 50 Hz triplets repeated with a frequency of 5 Hz (Goldsworthy et al., 2012). Secondly, prior, concurrent and post motor contraction influence cTBS effects (Gentner et al., 2008; Huang et al., 2008; Iezzi et al., 2008). In particular, the duration and the exact procedure to measure AMT might interact with cTBS effects. Finally, the direction of the current seems play a role, even if biphasic pulses are used. Indeed, whereas AP–PA seems to be the optimal direction for eliciting MEPs, the reverse pattern (PA–AP) seems to be the optimal direction for inducing after effects after 5 Hz stimulation (Sommer et al., 2012, but see Hamada et al., 2012 for weak TBS effects induced with PA–AP current). One might speculate that a better reproducibility could be obtained with a PA–AP current; however, it is important to also assess reproducibility with AP–PA current as the MagPro stimulator delivering AP–PA current has been used for cTBS protocols in several laboratories (e.g. Di Lazzaro et al., 2005; Freitas et al., 2011). In conclusion, our cTBS protocol might not have been optimal and we might expect higher reproducibility with other cTBS protocols. It was nevertheless able to induce at the group-level the expected inhibition.
However, we found that some subjects did not show any cTBS-dependent modulation in MEP size. While clinical and epidemiologic data did not reveal any differences between these individuals and the rest of the participants, it is possible that genetic differences may account for these instances. For example, a common polymorphism (BDNF Val66Met) in the brain derived neurotrophic factor gene has been shown to influence the effects of repetitive TMS (Cheeran et al., 2008) (but see Li Voti et al., 2011 for different results). We do not have genotypes in our participants, but future studies may need to consider obtaining such data. In addition, a recent study on 56 healthy individuals showed that (i) inter-individual variability of the response to TBS protocols was higher than previously reported and (ii) the variability depends on the recruitment of interneurons by single-pulse of TMS, and consequently on the early or late I-waves generated, as measured by latencies of MEPs evoked with different current directions (Hamada et al., 2012).
In the present study, in addition to the inter-individual variability, the intra-individual variability was not negligible. Several factors may account for within-participant variability. The physiological state of brain activity at the time of stimulation has a crucial influence on the magnitude and direction of any TMS-induced changes in cortical excitability. Within the same individual, TMS can produce different perceptual or behavioral outcomes that may depend on the excitability levels of specific neuronal populations (Romei et al., 2008; Silvanto and Pascual-Leone, 2008). As we mentioned earlier, in the case of TBS, motor activity has been shown to influence the direction of induced plasticity. The procedure for measurements of AMT was chosen in order to maximally control for any prior motor activity. However, we cannot exclude that other physical or mental activities could create subtle difference of brain states between sessions affecting TBS effects. We found indeed a modest correlation between change in RMT between visits and change in T5 suppression. Thus, variability in our most robust measure of plasticity was correlated with variability of excitability as measured with RMT. In future study, monitoring of baseline brain state, to assure that TBS is consistently applied across sessions, could be facilitated with the combined application of TBS with fMRI or EEG.
Another source of variability could be related to the variability of MEPs measures themselves. Firstly, trial-to-trial variability could be high enough to create fluctuation of MEPs amplitude when averaging tens of trials at each time point (measurement variability). Secondly, participants’ physiological state might fluctuate during the course of the session, for example due to fatigue (physiological variability). Future studies with sham cTBS will help to estimate how much the variability of MEPs measure can account for the variability of cTBS effects.
Finally, one should note that different experimenters could have carry out the two sessions for each subject. This could increase the variability. In clinical environment, it is expected that different technicians perform neurophysiologic assessment of patients. Thus, any reliable measures should be relatively robust regarding changes of experimenters. We expect that any parameters with a low (resp. high) reproducibility in our study are less (resp. more) likely to be useful in a clinical environment.
We found that the modulation at T5 showed the greatest within-subject reproducibility. However, the neurobiological mechanisms of TBS in humans are not fully understood. Several studies have pointed toward the role of NMDA or GABA modulation; others suggested a change in the expression of immediate early genes proteins (for a review, see Cardenas-Morales et al., 2010); and the underlying processes reflected by any marker are unclear. While in the motor cortex, these effects have been shown to reflect intracortical, rather than spinal, modulation of plasticity (Di Lazzaro et al., 2005), our study was not designed to provide greater neurobiological understanding, but simply to assess test–retest reliability and to identify the most reliable index of TBS-induced modulation.
5. Conclusion
In conclusion, TBS shows reasonable within subject test–retest reliability, even with long inter-test intervals, but some markers of TBS effects appear to be more reliable than others. Obviously, our results may not apply directly to patients with different pathologies, and to those on medications that may affect the reproducibility of TMS in general and TBS in particular. Therefore, future studies comparing TBS-induced plasticity in healthy subjects and patients should consider experimental designs that evaluate test–retest reliability.
highlights.
Estimating cortical plasticity with continuous theta-burst stimulation (cTBS) should rely on a robust within-subject reproducibility.
The present study indicates that the marker of cTBS induced-plasticity with highest within-subject reproducibility is modulation of corticospinal excitability measured 5 min after cTBS.
Studies comparing cTBS effects in healthy subjects and patients need to proceed with care as some measures of the cTBS effects are more reproducible than others.
Acknowledgements
This work was supported in part by grants from CIMIT and the National Center for Research Resources: Harvard Clinical and Translational Science Center (UL1 RR025758). Dr. Pascual-Leone serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Allied Mind, Neosync, and Novavision, and is listed as inventor on patents and patent applications on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). Dr. Vernet was supported by the Fyssen Foundation, France. Dr. Yoo was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund (521-2008-1-E00099). Dr. Oberman was supported by NIH fellowship F32MH080493 and 1KL2RR025757-01.
Footnotes
Conflict of interest
There was no other conflict of interest.
References
- Cardenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schonfeldt-Lecuona C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010;22:294–306. doi: 10.1007/s10548-009-0084-7. [DOI] [PubMed] [Google Scholar]
- Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–25. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, et al. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–32. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008;586:3871–9. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–50. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeltgen SH, Ridding MC. Low-intensity, short-interval theta burst stimulation modulates excitatory but not inhibitory motor networks. Clin Neurophysiol. 2011;122:1411–6. doi: 10.1016/j.clinph.2010.12.034. [DOI] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in cortical plasticity across the lifespan. Front Aging Neurosci. 2011;3:5. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex. 2008;18:2046–53. doi: 10.1093/cercor/bhm239. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Pitcher JB, Ridding MC. A comparison of two different continuous theta burst stimulation paradigms applied to the human primary motor cortex. Clin Neurophysiol. 2012;123:2256–63. doi: 10.1016/j.clinph.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The Role of Interneuron Networks in Driving Human Motor Cortical Plasticity. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs147. http://dx.doi.org/10.1093/cercor/bhs147. [DOI] [PubMed]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–70. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- Iezzi E, Conte A, Suppa A, Agostino R, Dinapoli L, Scontrini A, et al. Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. J Neurophysiol. 2008;100:2070–6. doi: 10.1152/jn.90521.2008. [DOI] [PubMed] [Google Scholar]
- Li Voti P, Conte A, Suppa A, Iezzi E, Bologna M, Aniello MS, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp Brain Res. 2011;212:91–9. doi: 10.1007/s00221-011-2700-5. [DOI] [PubMed] [Google Scholar]
- McAllister SM, Rothwell JC, Ridding MC. Selective modulation of intracortical inhibition by low-intensity Theta Burst Stimulation. Clin Neurophysiol. 2009;120:820–6. doi: 10.1016/j.clinph.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Oberman L, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, et al. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile X syndrome and autism spectrum disorder. Front Synaptic Neurosci. 2010;2:1–8. doi: 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A. Disrupting the brain to guide plasticity and improve behavior. Prog Brain Res. 2006;157:315–29. doi: 10.1016/s0079-6123(06)57019-0. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–15. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by noninvasive brain stimulation in healthy subjects. J Physiol. 2010;588:2291–304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual-Leone A, Thut G. Spontaneous fluctuations in posterior alpha-band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex. 2008;18:2010–8. doi: 10.1093/cercor/bhm229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Norden C, Schmack L, Rothkegel H, Lang N, Paulus W. Opposite optimal current flow directions for induction of neuroplasticity and excitation threshold in the human motor cortex. Brain Stimul. 2012 doi: 10.1016/j.brs.2012.07.003. http://dx.doi.org/10.1016/j.brs.2012.07.003. [DOI] [PubMed]
- Suppa A, Ortu E, Zafar N, Deriu F, Paulus W, Berardelli A, et al. Theta burst stimulation induces after-effects on contralateral primary motor cortex excitability in humans. J Physiol. 2008;586:4489–500. doi: 10.1113/jphysiol.2008.156596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Cheeran BJ, Teo JT, Rothwell JC. Pattern-specific role of the current orientation used to deliver Theta Burst Stimulation. Clin Neurophysiol. 2007;118:1815–23. doi: 10.1016/j.clinph.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Zafar N, Paulus W, Sommer M. Comparative assessment of best conventional with best theta burst repetitive transcranial magnetic stimulation protocols on human motor cortex excitability. Clin Neurophysiol. 2008;119:1393–9. doi: 10.1016/j.clinph.2008.02.006. [DOI] [PubMed] [Google Scholar]