Abstract
The thalamic reticular nucleus (TRN) is hypothesized to regulate neocortical rhythms and behavioral state. Using optogenetics and multi-electrode recording in behaving mice, we found that brief selective drive of TRN switched thalamocortical firing mode from tonic to bursting and generated state-dependent neocortical spindles. These findings provide causal support for the TRN in state-regulation in vivo and introduce a new model for addressing the role of this structure in behavior.
Thalamocortical (TC) neurons, the primary output neurons of the thalamus, fire in two distinct modes: tonic and bursting1,2,3. These modes are invoked in theories of dynamic regulation of sensory information processing in behaving animals, with contrasting views arguing for the optimal transmission of information by tonic firing2 or bursts1,3. Further, transition from wakefulness to sleep is demarcated by an increase in the propensity for thalamic bursting, and is associated with expression of ‘spindles’, sustained 1–2 second epochs of 7–15 Hz oscillations in neocortex.
Burst firing, mediated by T-type Ca2+ channels, can be triggered by synaptic inhibition4. The thalamic reticular nucleus (TRN) provides the major source of inhibition to thalamocortical relay neurons (TC), and has been implicated in attention5 and sleep regulation6. TRN neurons are thought to generate spindle oscillations7, which may play important roles in sensory filtering8 and sleep-dependent memory consolidation9. The link between TRN activity and thalamic relay nucleus firing state is based on in vitro10 or on correlative in vivo data7. Causal examination of the impact of TRN in vivo has primarily relied on sustained manipulations, such as chronic deafferentation7 or genetic disruption. Systemic alteration of TRN neuron membrane properties impacts generation of neocortical spindles and disrupts sleep behavior6.
In this study, we sought to selectively and causally test the role of the TRN in thalamic bursting and the generation of neocortical spindles. Several thalamocortical and corticothalamic fibers pass through the TRN, making selective electrical stimulation of this structure impossible. To manipulate TRN function in vivo, we used a transgenic animal in which the light-activated ion channel, channelrhodospin-2 (ChR2), was expressed under the control of the vesicular GABA transporter (VGAT2). Histological characterization of brain sections derived from VGAT-ChR2 animals revealed expression of ChR2 in GABAergic neurons of several brain regions. Somatosensory thalamic expression of ChR2 was limited to TRN (Fig. S1A–C) and was several-fold higher than that in the neocortex (p < 0.001) (Fig. S1D–E). When recording extracellularly from putative TRN units in these mice, we observed short latency, high-reliability spiking in response to brief pulses of 473 nm light(Fig. S2).
We isolated 11 TC neurons, and recorded cortical local field potential (LFP) and surface electroencephalography (EEG) across six sessions in three freely behaving VGAT-ChR2 mice using integrated optical fiber/dual site multielectrode implants (Fig. 1a). Thalamic stereotrodes yielded well-isolated putative TC single units (Fig. S2), whose firing was altered by delivering 20 msec blue laser pulses to the somatosensory section of the TRN (Fig. 1b, c). Though the overall firing rate in the 1 second period post stimulation did not significantly change (pre vs. post, p = 0.4131), following a brief suppression period, TC units exhibited “bursts” (Fig 1d, green arrows) characterized by smaller interspike intervals (ISI) than the pre-stimulus period (Fig. 1e). Enhanced probability of bursting (as defined by two or more spikes of ISI < 4 msec, preceded by 100 msec of silence11) was sustained for hundreds of milliseconds following stimulation, across all units (Fig. 1f, Fig. S3; pre vs. post, p<0.0028, Wilcoxon signed-rank test). The probability of bursting in the second following a light pulse for single units was 26% (vs. 9% in the second prior to the light pulse; p < 0.05, Wilcoxon signed-rank test; Fig. S4 shows the distribution across cells). The probability that at least one neuron in our recorded sample (either 3 or 4 units/session) would burst following each light pulse was 72% (55–89%; N = 4 sessions). Burst probability peaked in the bin 100–200 msec post-stimulation, consistent with ‘rebound’ bursting of TC cells following inhibition generated by TRN activation.
Figure 1. Optical drive of TRN generates thalamocortical burst firing.
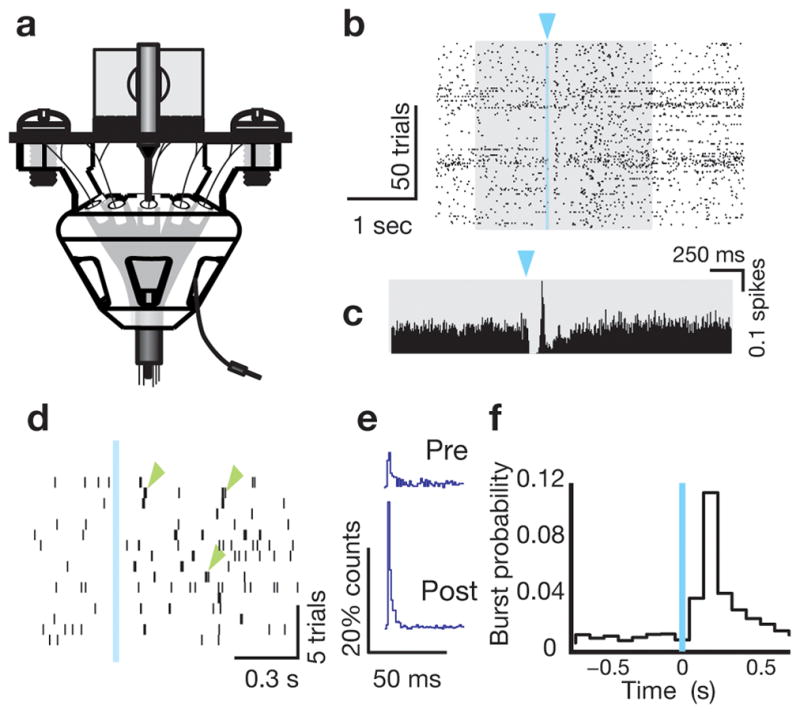
(a) The multi-electrode plus fiber-optic implant used for in vivo experiments. (b) A raster plot of a single TC unit’s spike times showing impact of a 20 msec laser pulse (blue triangle) delivered to neighboring TRN. (c) A PSTH of the same unit, gray background is matched to that in b. (d) Example of a TC unit where a single 20 msec laser pulse resulted in switching the firing mode from tonic to bursting (green arrow). (e) ISI histogram of the same cell in d, showing a robust change in ISIs < 4msec. (f) Histogram of burst probability across all cells shows a sustained increase in bursting for the 800 msec period post stimulation. All experiments were conducted according to the Institutional Animal Care and Use Committee guidelines at MIT.
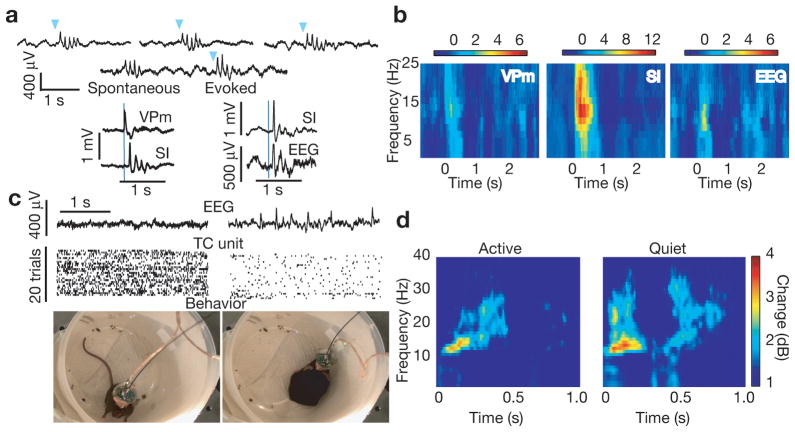
Single 20 msec optical pulses delivered to the TRN were occasionally followed by neocortical spindle oscillations that were indistinguishable from spontaneous spindle oscillations recorded in the same animals (Fig. 2a). Evoked spindles were detected at the level of neocortical LFP and EEG and were preceded by thalamic activity (Fig. 2a). Spectrograms of thalamic LFP, cortical LFP and surface EEG showed a pronounced increase in power following TRN stimulation that was maximal in the spindle frequency range (Fig. 2b). Spontaneous and evoked neocortical spindles were not time-locked to bursting or LFP activity in the spindle range at the site of recording electrodes in thalamus (Fig. 2a, Fig. S5). These results in naturally sleeping mice are in apparent contrast to prior observations of sustained spindles and widely synchronous bursting in thalamic relay centers during neocortical spindles in barbiturate anesthetized cats12, but are in agreement with recent intracranial human recordings in sleep showing localized spindle expression13.
Figure 2. Optical drive of TRN generates neocortical spindle oscillations.
(a) Top: Three examples of spindle generation by brief, 20 msec laser pulses (blue triangles) delivered to TRN and example of a spontaneous spindle followed by an optically-induced spindle, showing nearly identical waveforms. Bottom left: An example trace of thalamic LFP activity onset prior to neocortical spindling following laser stimulation (blue line). Bottom right: An example of a spindle oscillation appearing in the cortical LFP (SI) and the surface EEG. (b) Average spectrograms of ventral posteromedial thalamus (VPm) LFP, primary somatosensory cortex (SI) LFP, and surface EEG showing the emergence of oscillatory activity maximal at the spindle frequency range (7–15 Hz) following laser pulses to somatosensory TRN at time zero. Example from all stimulations (N = 204) of one animal (c) Example of a VGAT-ChR2 mouse exhibiting two states of activity, delineated by visual inspection of the EEG, TC firing rate, and high resolution video recording (d) Trial sorting based on visual inspection of these measures reveals a more robust optical induction of spindle oscillations in the EEG when the animal is quiet. Spectrogram of one animal. State-dependent spindle power increase after optical stimulation of the TRN shows a more robust impact when mice are quiet (N = 3 animals; p<0.001)
Spontaneous spindle events are observed in non-rapid eye movement (NREM) sleep9. To test whether optically-driven spindle generation was state dependent, we used video recording to monitor behavior in addition to electrophysiological recording. As described for other mammalian species14, mice in this study showed state-dependent changes in TC neural firing rate that paralleled behavioral and EEG changes (Fig. 2c). Based on monitoring gross behavioral changes (activity vs. inactivity), we observed that brief optical activation of the TRN resulted in state-dependent changes in EEG spindle power increase (example session, Fig. 2d). A significant negative correlation between firing rate and probability of spindle induction was observed (Fig. S6). Using polysomnography with combined EEG and EMG in two additional VGAT-ChR2 mice to fully characterize this state-dependence, we found that spindle induction was largely limited to NREM sleep: Only 7% of all induced spindles occurred in electrographically identified wake epochs, and none occurred in REM sleep (spindles/laser trials: NREM 19.56%, Wake 1.27%, REM 0%, p<0.001; Fig. S7).
Our findings support the hypothesis that the TRN controls TC neural output by switching its firing mode (Fig. 1). Whether burst firing is used in the brain to enhance transmission of sensory input is unknown and is a key point of debate in sensory neuroscience. Several preparations across different modalities have shown that sensory drive can induce bursts, suggesting these events could differentially impact processing1. The ability to record and control thalamocortical relay cell activity described here during free behavior is a crucial step toward answering this important question. Combining selective optical drive of TRN with the precise control of sensory input afforded by head-fixed preparations can address the role of sensory-driven burst activity in stimulus detection.
Our findings provide the first demonstration that TRN activation drives neocortical spindle oscillations in the intact mouse brain (Fig. 2). These spindles were largely limited to NREM sleep, suggesting that optical stimulation was utilizing endogenous physiological mechanisms of spindle generation. Selective silencing of TRN, by optical or other means, will be required to establish a full causal relation between TRN activity and spindle generation. The ability to control spindle generation with high temporal precision will allow for causally testing the role of these oscillations in processes such as sleep-dependent memory consolidation9. Given that disrupted sleep spindles are a characteristic of diseases such as schizophrenia15, our approach can be applied to animal models of neurological and psychiatric illness to investigate diagnostic and therapeutic questions.
Supplementary Material
References
- 1.Sherman SM. A wake-up call from the thalamus. Nat Neurosci. 2001;4:344–346. doi: 10.1038/85973. [DOI] [PubMed] [Google Scholar]
- 2.Steriade M. To burst, or rather, not to burst. Nat Neurosci. 2001;4:671. doi: 10.1038/89434. [DOI] [PubMed] [Google Scholar]
- 3.Lesica NA, Weng C, Jin J, Yeh CI, Alonso JM, Stanley GB. Dynamic encoding of natural luminance sequences by LGN bursts. PLoS Biol. 2006;4:e209. doi: 10.1371/journal.pbio.0040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 5.McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Espinosa F, Torres-Vega MA, Marks GA, Joho RH. Ablation of Kv3.1 and Kv3.3 potassium channels disrupts thalamocortical oscillations in vitro and in vivo. J Neurosci. 2008;28:5570–5581. doi: 10.1523/JNEUROSCI.0747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steriade M, Domich L, Oakson G, Deschenes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 8.Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 20:R626–627. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 9.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sohal VS, Pangratz-Fuehrer S, Rudolph U, Huguenard JR. Intrinsic and synaptic dynamics interact to generate emergent patterns of rhythmic bursting in thalamocortical neurons. J Neurosci. 2006;26:4247–4255. doi: 10.1523/JNEUROSCI.3812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bezdudnaya T, et al. Thalamic burst mode and inattention in the awake LGNd. Neuron. 2006;49:421–432. doi: 10.1016/j.neuron.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Spatiotemporal patterns of spindle oscillations in cortex and thalamus. J Neurosci. 1997;17:1179–1196. doi: 10.1523/JNEUROSCI.17-03-01179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nir Y, et al. Regional slow waves and spindles in human sleep. Neuron. 2011;70:153–169. doi: 10.1016/j.neuron.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Funke K, Worgotter F, Eysel UT. Correlated variations in EEG pattern and visual responsiveness of cat lateral geniculate relay cells. J Physiol. 1999;514:857–874. doi: 10.1111/j.1469-7793.1999.857ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrarelli F, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010;167:1339–1348. doi: 10.1176/appi.ajp.2010.09121731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.