Summary
Background
Arginine deiminase (ADI) is an enzyme that degrades arginine, an amino acid that is important for growth and development of normal and neoplastic cells. Melanoma cells are auxotrophic for arginine, because they lack argininosuccinatesynthetase (ASS), a key enzyme required for the synthesis of arginine.
Patients and methods
Patients with advanced melanoma were treated with 40, 80 or 160 IU/m2 ADI-PEG 20 i.m. weekly. Primary endpoints were toxicity and tumor response, secondary endpoints included metabolic response by 18FDG-PET, pharmacodynamic (PD) effects upon circulating arginine levels, and argininosuccinate synthetase tumor expression by immunohistochemistry.
Results
31 previously treated patients were enrolled. The main toxicities were grade 1 and 2 adverse events including injection site pain, rash, and fatigue. No objective responses were seen. Nine patients achieved stable disease (SD), with 2 of these durable for >6 months. Four of the 9 patients with SD had uveal melanoma. PD analysis showed complete plasma arginine depletion in 30/31 patients by day 8. Mean plasma levels of ADI-PEG 20 correlated inversely with ADI-PEG 20 antibody levels. Immunohistochemical ASS expression analysis in tumor tissue was negative in 24 patients, whereas 5 patients had <5 % cells positive.
Conclusions
ADI-PEG 20 is well tolerated in advanced melanoma patients and leads to consistent, but transient, arginine depletion. Although no RECIST responses were observed, the encouraging rate of SD in uveal melanoma patients indicates that it may be worthwhile to evaluate ADI-PEG 20 in this melanoma subgroup.
Keywords: Melanoma, Metastatic, Auxotrophy, Arginine deiminase, Argininosuccinate synthetase
Introduction
Advanced melanoma has one of the highest per-death loss of years of potential life among human malignant tumors [1]. One explanation for the poor prognosis is its resistance to most systemic therapies. Until recently, single agent dacarbazine as well as IL-2 were the FDA-approved therapies of choice, despite disappointing response rates (RR) in the 10–14 % range [2–4] for dacarbazine and 13–16 % for IL-2 [5]. Advances with targeted agents that block driver oncogenic mutations such as BRAFV600 or c-Kit and immunotherapy with co-stimulatory molecule blockade offer exciting new treatment modalities in this disease [6–10]. Nevertheless, additional, novel treatment approaches for advanced melanoma patients are still urgently needed.
The fact that some tumors are auxotrophic for non-essential amino acids provides the rationale for amino acid deprivation as a targeted treatment strategy [11], such as the use of pegylated asparaginase in the treatment of acute lymphoblastic leukemia. The majority of melanoma cells lack the enzyme argininosuccinatesynthetase (ASS), which is required for the synthesis of arginine from citrulline in the urea cycle, rendering them auxotrophic for arginine [12–14]. ADI, which is not present in mammalian cells, catabolizes arginine to citrulline and ammonia. It leads to depletion of arginine and reduced growth in cells that lack the capacity to synthesize it de novo, such as melanoma cells that are deficient in ASS. Activity of ADI against melanoma and other tumors has been documented preclinically [15–18]. Due to its non-human origin, ADI is highly antigenic; it also has a short half life. ADI-SS PEG 20,000 mw (ADI-PEG 20) constitutes ADI conjugated to polyethylene glycol (PEG) and is a compound with much reduced antigenicity and prolonged half life. Clinical benefit was seen with this agent in previous phase I and II trials in hepatocellular cancer [19–21] and melanoma [22, 23].
The rationale for our study was to confirm previous findings and to further establish the clinical efficacy and safety of ADI-PEG 20 in patients with advanced melanoma. Furthermore, we hypothesized that changes in tumor 18FDG uptake are an early indication for response to treatment with ADI-PEG 20, based on the assumption that the nutritional deprivation caused by the drug might be reflected first in metabolic activity rather than a decrease in size of tumor lesions. We also set out to examine pharmacodynamics and immunogenicity as well as to correlate tumor expression of ASS with clinical response.
Patients and methods
Patients
Patients were eligible if they were at least 18 years of age, had histologically confirmed, unresectable stage III or stage IV melanoma, measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) committee [24] 1.0 criteria, and adequate organ function. Patients with metastatic disease to the central nervous system were eligible if the lesions were treated and stable for at least 8 weeks. Exclusion criteria comprised chemotherapy, immunotherapy, or radiotherapy within 3 weeks prior to the first dose of the study agent, another malignancy that required concomitant therapy, a history of seizure disorder, HIV positivity, pregnancy and nursing. The participating institutions were Memorial Sloan Kettering Cancer Center and New York University Cancer Institute, New York, NY. The protocol was approved by the local institutional review boards at both participating institutions, and all patients provided written informed consent.
Study design and treatment
In this phase I/II, open-label, dose-escalation study, patients received ADI-PEG 20 intramuscularly (IM) once weekly for 8 weeks. [18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) and CT scans were obtained at baseline, on day 4, and at the end of each cycle for assessment of tumor response by RECIST 1.0 criteria and assessment of tumor metabolic activity. The first cycle was 1 week longer than the subsequent 8-week cycles to allow for an at least 8-week interval from the baseline CT and FDG-PET scans, which could not be done during the same week for logistical reasons. After completion of cycle 1, treatment with ADI-PEG 20 was continued in patients with a complete (CR) or partial response (PR), stable disease (SD) or progression of existing disease if the patient was asymptomatic. If the patient developed symptoms attributable to existing disease or if new lesions developed, treatment with ADI-PEG 20 was discontinued. Cycle 2 and subsequent cycles consisted of ADI-PEG 20 given weekly for 8 weeks. Response assessments were performed at the end of each subsequent cycle according to the criteria described for cycle one. Blood samples were obtained weekly prior to each administration for correlative studies. Toxicity was assessed at every study visit using revised NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
In the phase I dose escalation part of the study, three patients were enrolled to each of three dose levels: 40 IU/m2, 80 IU/m2 and 160 IU/m2. If one of the first three patients on a dose level experienced a dose limiting toxicity (DLT), the cohort was expanded to six patients. The maximum tolerated dose (MTD) was defined as the dose level below the one at which two or more patients experienced a DLT. The patients treated at the highest tolerated dose level were included in the phase II of the trial. For the 40 IU/m2 and the 80 IU/m2 dose cohorts there was an option to expand to up to six subjects in order to obtain additional clinical information. Primary endpoints were toxicity as defined by CTCAE criteria and tumor response by RECIST 1.0 Criteria. Secondary endpoints were: 1) functional tumor response as assessed by 18FDG PET according to European Organisation for Research and Treatment of Cancer (EORTC) criteria, 2) plasma concentrations of arginine and serum concentrations of ADI following administration of ADI-PEG 20, 3) ADI-PEG 20 antibody titers, and 4) immunohistochemical analysis of tumor samples for ASS expression.
Pharmacokinetics, pharmacodynamics, immunogenicity and immunohistochemistry (IHC)
Blood for pharmacokinetics, pharmacodynamics and immunogenicity were obtained at baseline and on the day of next dosing, 8 days after the prior dose. Plasma arginine levels were determined by high-performance liquid chromatography (HPLC) at Frontage Laboratories (Shanghai, P.R. China). Plasma levels of ADI-PEG 20 were analyzed by a discontinuous fluorogenic assay, which detects released NH3 from the deimination of arginine by ADI-PEG 20 enzymatic activity at Polaris Pharmaceuticals, Inc. (San Diego, CA). Plasma ADI-PEG 20 concentrations were determined by comparing to a standard curve of known amounts of ADI-PEG 20 that were diluted into naïve plasma and assayed on the same plate. Levels of antibodies against ADI-PEG 20 were determined using an enzyme linked immunosorbant assay (ELISA) [21, 25]. Blood was collected at baseline and on day 4 as well as weekly prior to each administration of ADI-PEG 20. IHC was performed as described previously [12]
Statistical analysis
For the phase II part of the study, a Simon minimax two-stage design was utilized, in which a response rate (RR) of 10 % was considered not promising and a RR of 30 % RR was considered promising [26]. The probabilities of a type I alpha error and a type II beta error were set at 0.10 and 0.10, respectively. Sixteen patients were to be enrolled in the first stage of this protocol. If only one or no response were seen, the study was to be terminated. If two or more responses were seen, an additional nine patients were to be enrolled. The treatment was to be considered worthy of further evaluation if five responses were observed in 25 evaluable patients.
Results
Patient characteristics
Thirty-one patients were enrolled between August 2007 and March 2009. The baseline clinical characteristics are listed in Table 1. All 31 patients enrolled in the study received at least one dose of ADI-PEG 20 and were assessable for safety. Twenty-nine of the 31 patients (6/6 in cohort 1, 6/6 in cohort 2, and 17/19 in cohort 3) had at least one evaluation of tumor response by RECIST criteria on day 57. Two patients were not evaluable because they elected to not continue on the study. The numbers of cycles completed by patients in each cohort are listed in Table 2.
Table 1.
Patient demographics and disease characteristics
| Parameter | No. | % |
|---|---|---|
| Sex | ||
| Male | 17 | 55 |
| Female | 14 | 45 |
| Age, years | ||
| Median | 64 | |
| Range | 29–84 | |
| Race | ||
| Caucasian | 30 | 97 |
| Asian | 1 | 3 |
| Primary site | ||
| Cutaneous | 25 | 81 |
| Uveal | 6 | 19 |
| Stage III | ||
| IIIc | 3 | 10 |
| Stage IV | ||
| M1a | 4 | 13 |
| M1b | 4 | 13 |
| M1c | 20 | 65 |
| Metastatic sites | ||
| 1 | 9 | 29 |
| 2 | 7 | 23 |
| 3 | 9 | 29 |
| 4 | 5 | 16 |
| LDH | ||
| Within normal limits | 21 | 68 |
| Elevated | 10 | 32 |
| Prior systemic treatments for advanced disease | ||
| 1 | 8 | 26 |
| 2 | 11 | 36 |
| 3 and more | 11 | 36 |
Table 2.
Number of patients completing each cycle
| Cohort 1 (40 IU/m2) N (%) | Cohort 2 (80 IU/m2) N (%) | Cohort 3 (160 IU/m2) N (%) | All patients N (%) | |
|---|---|---|---|---|
| Cycle 1 | 6 (100) | 3 (50) | 13 (68.4) | 22 (71) |
| Cycle 2 | 2 (33.3) | 1 (16.7) | 3 (15.8) | 6 (19.4) |
| Cycle 3 | 1 (16.7) | 1 (16.7) | 1 (5.3) | 3 (9.7) |
| Cycle 4 | 1 (16.7) | 1 (16.7) | 1 (5.3) | 3 (9.7) |
| Cycle 5 | 1 (16.7) | 0 | 0 | 1 (3.2) |
| Cycle 6 | 1 (16.7) | 0 | 0 | 1 (3.2) |
Toxicity
In the dose-escalation phase I of the study, no DLTs were seen in the first cohort (40 IU/m2). To gain additional safety information, the cohort was expanded to six subjects. In cohort 2 (80 IU/m2), one grade 3 episode of arthralgia was observed and the cohort was expanded to six patients. Since no further DLTs were seen in the expanded cohort, enrollment to the pre-planned highest dose level of 160 IU/m2 of cohort 3 was started. No DLTs were observed in cohort 3 and enrollment onto the phase II part of the protocol (the pre-planned efficacy cohort, i.e. the group who received the highest dose of 160 IU/m2) was continued. Nineteen patients were treated at the 160 IU/m2 dose level. MTD was not reached in the study. Overall, the treatment was well tolerated (Table 3). Six treatment-related grade 3 toxicities were observed in total: in addition to the above mentioned episode of arthralgia that occurred in cohort 2, one convulsion and one grand-mal convulsion as well as skin induration at the injection site, rash, and lymphedema were seen in dose-cohort 3. In both patients who had convulsions, study drug was withdrawn because of the event. The patient who experienced convulsion was offered brain imaging to rule out brain metastases as the etiology but opted for home hospice, withdrew consent 7 days after the event, and died of disease progression 24 days after experiencing the convulsion. The patient with grand mal convulsion experienced the event approximately 30 min after treatment. The patient recovered fully from the event. Both events were considered possibly or probably related to study drug by the investigator.
Table 3.
Possibly, probably, or definitely treatment-related adverse events
| Toxicity grade | Cohort 1
|
Cohort 2
|
Cohort 3
|
|||
|---|---|---|---|---|---|---|
| Grade 1–2 n (%) |
Grade 3 | Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | |
| Constitutional | ||||||
| Fatigue | 1 (16.7) | 4 (21.1) | ||||
| Administration site conditions | ||||||
| Pain at injection site | 2 (33.3) | 1 (16.7) | 13 (61.9) | |||
| Rash at injection site | 2 (33.3) | 2 (33.3) | 4 (21.1) | |||
| Edema at injections site | 1 (16.7) | |||||
| Skin induration | 1 (5.3) | |||||
| Gastrointestinal | ||||||
| Nausea | 2 (33.3) | 2 (10.5) | ||||
| Anorexia | 1 (16.7) | 3 (15.8) | ||||
| Abdominal pain | 1 (16.7) | 3 (15.8) | ||||
| Dermatological | ||||||
| Rash | 1 (16.7) | 3 (50) | 4 (21.1) | |||
| Pruritus | 1 (16.7) | 1 (16.7) | 1 (5.3) | 1 (5.3) | ||
| Musculosceletal/vascular | ||||||
| Arthralgia | 1 (16.7) | 1 (16.7) | 3 (15.8) | |||
| Myalgia | 1 (16.7) | 1 (16.7) | 2 (10.5) | |||
| Pain in extremity | 1 (16.7) | 1 (16.7) | 1 (5.3) | |||
| Lymphedema | 1 (5.3) | |||||
| Neurological | ||||||
| Seizure | 2 (10.5) | |||||
Response by RECIST criteria
No objective responses were seen in any of the three patient cohorts. The best overall response for the evaluable population (all cohorts, 29/31 patients) was SD in nine patients (31 %). The remainder of the evaluable patients (71 %) had progressive disease at the first response assessment on day 57. In the phase II patient cohort, 4/17 (23.5 %) patients had SD. Because no tumor responses were seen in these 17 patients, further enrollment in the phase II part of the study was discontinued. Three of six patients in cohort 1 (40 IU/m2) and 2/6 patients in cohort 2 (80 IU/m2) also had SD. Two SDs lasting longer than 6 months were observed, one lasting 337 days in cohort 1 and one lasting 225 days in cohort 2. The longest duration of SD in the cohort receiving the highest dose was 169 days. Of note, both SDs >6 months and 4 of the 9 SDs observed in total occurred in patients with uveal melanoma; six patients with uveal melanoma were enrolled overall on the study, resulting in a disease stabilization rate of 66.7 % in this sub-group. The median duration of SD in the uveal melanoma patients was 141 days (range: 57–337 days). The median time to progression for the 29 evaluable patients was 57 days, whereas it was 113 days for the patients with uveal melanoma.
Tumor metabolic response
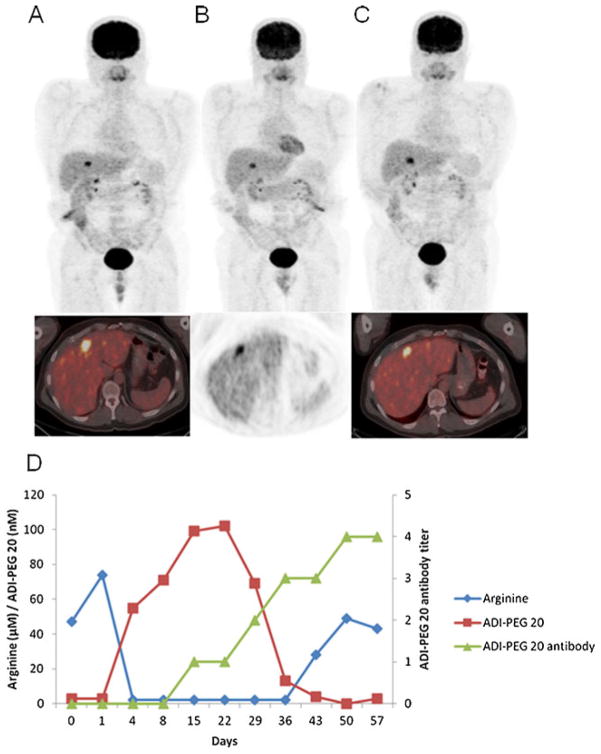
Twenty nine of 31 patients (93.5 %) were evaluable for assessment of changes in FDG uptake with PET on day 4. Eight patients (27.6 %, one in cohort 1 and 7 in cohort 3) had a partial metabolic response (PMR) at this early time point after treatment initiation. This early PMR was not indicative of a PR by RECIST criteria on day 57 (no PRs were seen) nor did a PMR on day 4 translate into SD by RECIST criteria, as only 3 of the 8 patients with PMR had SD by RECIST criteria on day 57. On day 50, 7 days prior to response assessment by RECIST criteria on day 57, one of 25 patients evaluable (4 %, cohort 2) had a PMR. Stable disease as measured by PET was seen in 12 of 29 (41.4 %) evaluable patients on day 4 and in 12 of 25 (48 %) of evaluable patients on day 50. Of note, one patient who had PD by RECIST criteria on day 169, but PMR by PET was continued on treatment and found to have SD at the next evaluation on day 225. An example of FDG PET scans of a patient with a PMR on day 4 with corresponding arginine and ADI-PEG 20 levels as well as ADI-PEG 20 antibody titers is shown in Fig. 1.
Fig 1.
NY 10- MIP images (upper panel) and transaxial images (lower panel) of FDG PET scans on a patient with metastatic disease in the liver. The baseline study (a) shows intense uptake in a liver lesion (SUV 10.1); a 4-day post-treatment scan (b) shows decrease in the uptake (SUV 7.9) with persistent uptake in the day 50 scan c with an SUV of 8.5
Arginine levels
Arginine levels were completely depleted by ADI-PEG 20 in 27/30 (90 %) patients by day 4 and in 30/31 (96.8 %) by day 8. In 17 of the 25 patients (68 %) who completed or nearly completed cycle #1, arginine levels returned to baseline (BL) levels during this cycle. In 12/17 (70.6 %) of these patients, the levels returned from complete depletion to BL or near BL levels within 1 week.
ADI-PEG 20 concentration
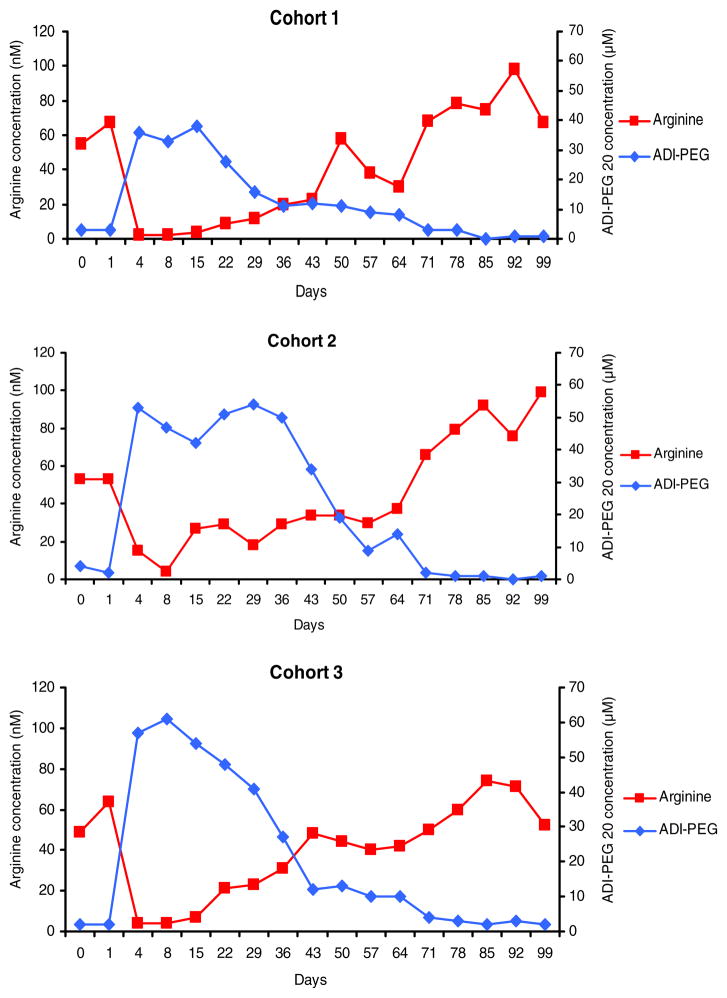
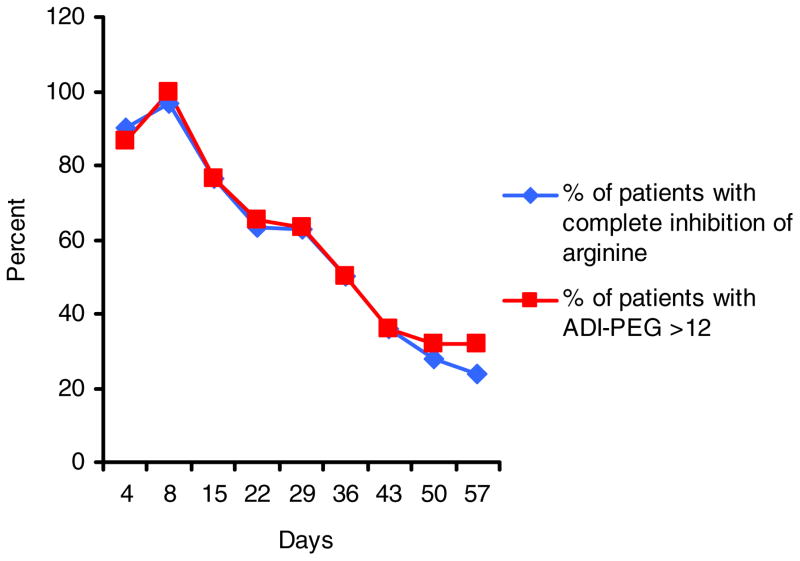
Peak levels of ADI-PEG 20 concentrations were reached within the first week of treatment (between day 4 and 8) in 16/31 (51.6 %) of the patients. Mean ADI-PEG 20 concentration levels also peaked during the first 2 weeks in all cohorts (Fig. 2). By day 36, ADI-PEG 20 levels had peaked in all patients and by day 71, none of the nine patients who were still on study had measurable ADI-PEG 20 levels. The absolute maximal ADI-PEG 20 concentrations achieved were heterogeneous among patients and ranged from 0 to 161. High ADI-PEG 20 concentrations did not result in more effective complete depletion of arginine levels in individual patients than lower concentrations; a level of 12 nM or above was sufficient to result in complete depletion for almost all patients and time points. This is evident by the striking correlation between the rate of patients with completely depleted arginine levels (<2 nM) and ADI-PEG 20 concentrations above the threshold of 12 nM (Fig. 3).
Fig 2.
The relationship of mean arginine and ADI-PEG 20 plasma concentrations over time is illustrated for each dose cohort (cohort 1: n=6; cohort 2: n=6; cohort 3: n=19)
Fig 3.
The proportion of patients in which arginine levels were completely depleted (<2 μM, blue line) and percentages of patients with ADI-PEG concentration of >12 nM (red line) are shown for the first treatment cycle plasma levels below a critical threshold around 12 nM. The observation that there is a threshold of ADI-PEG 20 which leads to complete arginine depletion, measured on day 8 after the prior injection, is consistent with the findings in the prior US and Italian ADI-PEG 20 melanoma studies, which
ADI-PEG 20 antibodies
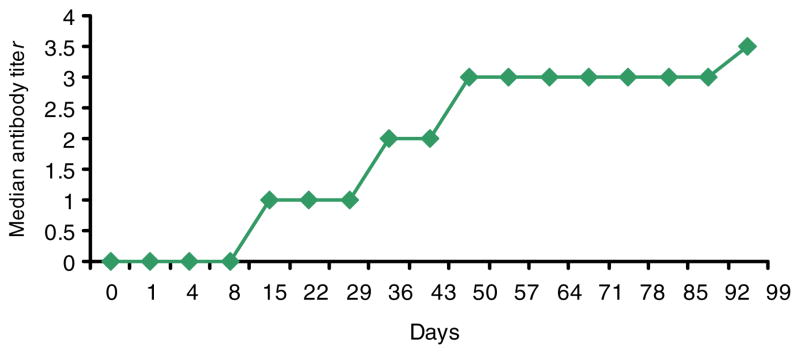
Patients had measurable ADI-PEG 20 antibodies at low titers (1:10) either prior to treatment (10/31, 32.3 %) or developed antibodies to ADI-PEG 20 after treatment initiation (Fig. 4). After the 2nd week of treatment, 22/29 (75.9 %) of patients tested positive for ADI-PEG 20 antibodies (1:10 or higher); by the end of the first cycle, 29/31 (93.5 %) of patients had ADI-PEG 20 antibodies at some point prior or during the treatment cycle. Longer exposure to ADI-PEG 20 lead to increasing ADI-PEG 20 antibody titers (Fig. 4). There was no clear correlation between onset of antibody development or antibody titers and timing of plasma ADI-PEG 20 decrease or plasma levels of ADI-PEG 20 in individual patients. Nevertheless, mean plasma ADI-PEG 20 levels were inversely correlated with ADI-PEG 20 antibody levels in all dose cohorts (Fig. 2).
Fig 4.
Median ADI-PEG 20 plasma antibody titers of the entire study population (31 patients) over time are demonstrated
Expression of ASS in tumor tissue
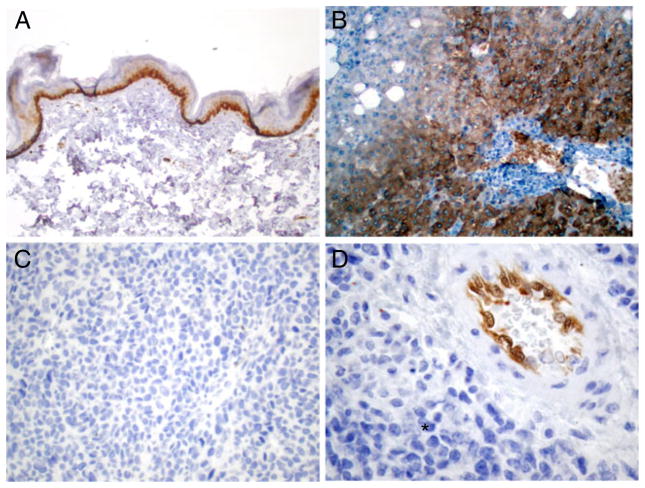
For 29 of the 31 patients, tumor tissue was available for immunohistochemical analysis of ASS expression. Examples of HC staining for ASS expression in normal and melanoma tissues are shown in Fig. 5a–d. In 24 patients, ASS was not detectable, whereas in 5 patients, <5 % of tumor cells were positive for the enzyme. The limited number of patients with SD and the absence of ASS expression in the vast majority of samples preclude conclusions about a correlation of ASS expression and outcome.
Fig 5.
Immunohistochemical expression analysis for ASS: epidermis with ASS-positive basal keratinocytes (a), ASS positive hepatocytes in the peripheral areas of a liver lobule (b), ASS-negative melanoma, low magnification (c), ASS-negative melanoma cells (asterisk), ASS-positive endothelia in blood vessel of tumor stroma (interal positive control), high magnification (d)
Discussion
The primary objectives of this trial were the assessment of toxicity and clinical efficacy of ADI-PEG 20 in patients with advanced melanoma. Consistent with earlier reports in patients with hepatocellular carcinoma (HCC) and melanoma, the drug was generally well tolerated at all three dose levels. No objective responses were seen; however, nine patients achieved stable disease with two patients having stability >6 months. Nevertheless, these data suggest that ADI-PEG 20 has limited clinical activity as a single agent at the doses tested in the patient population treated in this trial. The complete absence of responses in any of the patients is in contrast to a previous phase I/II study in 24 stage IV melanoma patients conducted in Italy, which demonstrated a RR of 25 %, including one CR [22]. Interestingly, in the Italian study, SD was also observed at 40 and 80 IU/m2; objective responses were observed in 6 subjects and 3/6 of those who were initially treated for 1 month with either 320 or 640 IU/m2 and then continued treatment at 160 IU/m2. Thus, higher doses of ADI-PEG 20, at least initially, may result in more objective responses in melanoma patients. The majority of evaluable patients in the current study (19/31) was treated at a dose of 160 IU/m2. The dosing was capped at 160 IU/m2 since this dose had previously been established as the optimal biologic dose for ADI-PEG 20, based on arginine depletion 1 week after initial dosing and because of a concern for increased immunogenicity at higher doses. It is intriguing that PMRs were seen as assessed by PET in 8 of 29 patients on day 4, very early after treatment initiation. Twenty-seven of thirty (90 %) of all patients and 7 of the 8 metabolic responders had complete depletion of arginine at this time point. By day 50, the 2nd PET scan, only 1/25 patients had a PMR and the proportion of patients with complete arginine depletion had come down from 90 % to 28 %. These data suggest that the decrease in metabolic activity reflects biologic activity of ADI-PEG 20 at this early time point in a subset of patients. However, our hypothesis that a metabolic response on PET scan is an early indicator of a response by RECIST criteria was not confirmed, since none of the eight metabolic responders had a PR.
A consistent finding across clinical studies with ADI-PEG 20 reported to date is the brief period of peak ADI-PEG 20 plasma levels independent of the dose and concomitant short interval of complete arginine depletion measured 1 week after treatment [20–22, 25]. However, arginine levels were not measured in this study or in these other studies at interim time points between the weekly blood testing for pharmacodynamics and pharmacokinetics that occurred prior to ADI-PEG 20 dosing. It has been shown that arginine levels may significantly drop yet return towards baseline by day 8 [19]. Thus there may be significant arginine starvation during this time. The striking association between an ADI-PEG 20 plasma level above 12 nM and complete depletion of arginine seen in our study indicates that the lack of persistent complete arginine depletion is most likely due to reported complete arginine depletion in all dose cohorts that were largely independent of absolute ADI-PEG 20 plasma levels [22]. Since no objective responses by RECIST criteria were seen in our trial, it is not known whether there is a correlation between successful complete arginine depletion and objective response. None of the patients with SD had ongoing complete arginine depletion beyond cycle one, suggesting that persistent and complete depletion of arginine production may not be essential for disease stabilization.
It was recently reported that ASS expression of melanoma may predict for a clinical benefit (PR or SD) in patients treated with ADI-PEG 20 [27]. In our study, the majority of the melanoma tumors (5/29) were negative for ASS expression; one of the 5 patients with ASS positive tumors and 7/24 patients with ASS negative tumors experienced SD.
It has been suggested that the emergence of ADI-PEG 20 antibodies may be responsible for the return to baseline plasma levels of ADI-PEG 20 despite the persistent administration of the drug. All patients who were treated beyond one cycle eventually developed antibody titers and antibody titers increased with continued treatment in most patients. The inverse correlation between mean levels of ADI-PEG 20 and ADI-PEG 20 antibody levels (Fig. 2) is consistent with previous studies and suggestive of immunogenicity of ADI-PEG 20. ADI-PEG 20 in combination with an immune suppressing agent may therefore result in greater clinical efficacy. In addition to the emergence of antibodies, which likely negatively affects the levels and function of ADI-PEG 20, re-induction of ASS under arginine deprivating conditions has been described as a resistance mechanism of ADI-PEG 20 therapy [28, 29]. This ASS induction has been attributed to the positive transcriptional regulators c-Myc and Sp4, which counteract HIF-1α in resistant melanoma cell lines [30, 31].
The observation that both subjects who had SD for >6 months (225 and 337 days, respectively) had uveal melanoma and that 4 of the 6 patients on the study with uveal melanoma had some disease stabilization is noteworthy given the limited treatment options for this patient population and suggests that this melanoma subtype might be more sensitive to arginine deprivation than cutaneous melanoma. To the best of our knowledge, similar findings have not been reported in advanced uveal melanoma patients treated with ADI-PEG 20 to date. ADI-PEG 20 was shown to be synergistic with temozolomide and cisplatin in vitro by other investigators [32] and our own preliminary observations in a A375 human melanoma xenograft model showed synergy of ADI-PEG 20 with cisplatin in vivo. In light of the signal for clinical activity in patients with uveal melanoma seen in the current study, a combination trial of ADI-PEG 20 and cisplatin seems a potentially worthwhile undertaking.
In conclusion, monotherapy with ADI-PEG 20 in unselected patients with advanced melanoma is generally well-tolerated, but at the doses used had limited clinical activity. The duration of complete arginine depletion (when measured 8 days after the prior dose) appears to be correlated with a decrease in plasma ADI-PEG 20 and the emergence of ADI-PEG 20 antibodies. The observation of SD in 4 out of 6 patients with uveal melanoma is intriguing and may be explored further in a clinical trial, potentially in combination with cisplatin, which has shown synergy with ADI-PEG 20 pre-clinically [33] or with an immunosuppressive agent.
Acknowledgments
Funding Ludwig Institute for Cancer Research
Footnotes
Financial disclosures Eric W. Hoffman is the executive officer of the Ludwig Institute for Cancer Research, which has a royalty agreement with Polaris Pharmaceuticals for ADI-PEG20. He received honoraria from Polaris for presentations and participation in investor meetings. Jedd Wolchok is an advisory board member at Polaris Pharmaceuticals. Bor-Wen Wu and John Bomalaski are employees of Polaris Group, the maker of ADI-PEG 20, and hold stock and employee stock options in Polaris Group. All remaining authors have declared no conflicts of interest.
Contributor Information
Patrick A. Ott, Email: Patrick_Ott@dfci.harvard.edu, Department of Medical Oncology, New York University School of Medicine, New York, NY, USA, Melanoma Disease Center, Dana-Farber Cancer Institute, Harvard Medical School, 450 Brookline Ave, Boston, MA 02215-5450, USA
Richard D. Carvajal, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Neeta Pandit-Taskar, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Achim A. Jungbluth, Ludwig Institute for Cancer Research, Branch at Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Eric W. Hoffman, Ludwig Institute for Cancer Research, Branch at Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Bor-Wen Wu, Polaris Pharmaceuticals, San Diego, CA, USA.
John S. Bomalaski, Polaris Pharmaceuticals, San Diego, CA, USA
Ralph Venhaus, Ludwig Institute for Cancer Research, Branch at Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Linda Pan, Ludwig Institute for Cancer Research, Branch at Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Lloyd J. Old, Ludwig Institute for Cancer Research, Branch at Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Anna C. Pavlick, Department of Medical Oncology, New York University School of Medicine, New York, NY, USA
Jedd D. Wolchok, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY, USA, Ludwig Institute for Cancer Research, Branch at Memorial Sloan-Kettering Cancer Center, New York, NY, USA
References
- 1.Albert VA, Koh HK, Geller AC, Miller DR, Prout MN, Lew RA. Years of potential life lost: another indicator of the impact of cutaneous malignant melanoma on society. J Am Acad Dermatol. 1990;23(2 Pt 1):308–310. doi: 10.1016/0190-9622(90)70214-3. [DOI] [PubMed] [Google Scholar]
- 2.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey P, Kirkwood JM, Haluska FG. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24(29):4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 3.Chapman PB, Einhorn LH, Meyers ML, Saxman S, Destro AN, Panageas KS, Begg CB, Agarwala SS, Schuchter LM, Ernstoff MS, Houghton AN, Kirkwood JM. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol. 1999;17(9):2745–2751. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 4.Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18(1):158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 5.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 6.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur AG. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Thomas L, Bondarenko I, O’Day S, Webber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH, Jr, Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, Wolchok JD. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 10.Carvajal RD, Antonescu CR, Wolchok JD, Chapman PB, Roman RA, Teitcher J, Panageas KS, Busam KJ, Chmielowski B, Lutzky J, Pavlick AC, Fusco A, Cane L, Takebe N, Vemula S, Bouvier N, Bastian BC, Schwartz GK. KIT as a therapeutic target in metastatic melanoma. JAMA: J Am Med Assoc. 2011;305(22):2327–2334. doi: 10.1001/jama.2011.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasut G, Sergi M, Veronese FM. Anti-cancer PEG-enzymes: 30 years old, but still a current approach. Adv Drug Deliv Rev. 2008;60 (1):69–78. doi: 10.1016/j.addr.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer. 2004;100(4):826–833. doi: 10.1002/cncr.20057. [DOI] [PubMed] [Google Scholar]
- 13.Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA. Pegylated arginine deiminase (ADI-SS PEG20,000 mw) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res. 2002;62(19):5443–5450. [PubMed] [Google Scholar]
- 14.Sugimura K, Ohno T, Kimura Y, Kimura T, Azuma I. Arginine deiminase gene of an AIDS-associated mycoplasma, Mycoplasma incognitus. Microbiol Immunol. 1992;36(6):667–670. doi: 10.1111/j.1348-0421.1992.tb02069.x. [DOI] [PubMed] [Google Scholar]
- 15.Philip R, Campbell E, Wheatley DN. Arginine deprivation, growth inhibition and tumour cell death: 2. Enzymatic degradation of arginine in normal and malignant cell cultures. Br J Cancer. 2003;88 (4):613–623. doi: 10.1038/sj.bjc.66006816600681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen LJ, Shen WC. Drug evaluation: ADI-PEG-20—a PEGylated arginine deiminase for arginine-auxotrophic cancers. Curr Opin Mol Ther. 2006;8(3):240–248. [PubMed] [Google Scholar]
- 17.Szlosarek PW, Klabatsa A, Pallaska A, Sheaff M, Smith P, Crook T, Grimshaw MJ, Steele JP, Rudd RM, Balkwill FR, Fennell DA. In vivo loss of expression of argininosuccinate synthetase in malignant pleural mesothelioma is a biomarker for susceptibility to arginine depletion. Clin Cancer Res. 2006;12(23):7126–7131. doi: 10.1158/1078-0432.CCR-06-1101. [DOI] [PubMed] [Google Scholar]
- 18.Yoon CY, Shim YJ, Kim EH, Lee JH, Won NH, Kim JH, Park IS, Yoon DK, Min BH. Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int J Cancer. 2007;120(4):897–905. doi: 10.1002/ijc.22322. [DOI] [PubMed] [Google Scholar]
- 19.Curley SA, Bomalaski JS, Ensor CM, Holtsberg FW, Clark MA. Regression of hepatocellular cancer in a patient treated with arginine deiminase. Hepatogastroenterology. 2003;50(53):1214–1216. [PubMed] [Google Scholar]
- 20.Glazer ES, Piccirillo M, Albino V, Di Giacomo R, Palaia R, Mastro AA, Beneduce G, Castello G, De Rosa V, Petrillo A, Ascierto PA, Curley SA, Izzo F. Phase II study of pegylated arginine deiminase for nonresectable and metastatic hepatocellular carcinoma. J Clin Oncol. 2010;28(13):2220–2226. doi: 10.1200/JCO.2009.26.7765. [DOI] [PubMed] [Google Scholar]
- 21.Izzo F, Marra P, Beneduce G, Castello G, Vallone P, De Rosa V, Cremona F, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA, Ng C, Curley SA. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22(10):1815–1822. doi: 10.1200/JCO.2004.11.120JCO.2004.11.120. [DOI] [PubMed] [Google Scholar]
- 22.Ascierto PA, Scala S, Castello G, Daponte A, Simeone E, Ottaiano A, Beneduce G, De Rosa V, Izzo F, Melucci MT, Ensor CM, Prestayko AW, Holtsberg FW, Bomalaski JS, Clark MA, Savaraj N, Feun LG, Logan TF. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23(30):7660–7668. doi: 10.1200/JCO.2005.02.0933. [DOI] [PubMed] [Google Scholar]
- 23.Feun LG, Savaraj N, Marini A, Wu C, Robles C, Herrera C, Spector S, Luedemann K, Moffat F, Bomalaski J. Phase II study of pegylated arginine deiminase (ADI-PEG20), a novel targeted therapy for melanoma. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. 2006;24(18S) June 20 Supplement:8045. [Google Scholar]
- 24.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Nat Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Yang TS, Lu SN, Chao Y, Sheen IS, Lin CC, Wang TE, Chen SC, Wang JH, Liao LY, Thomson JA, Wang-Peng J, Chen PJ, Chen LT. A randomised phase II study of pegylated arginine deiminase (ADI-PEG 20) in Asian advanced hepatocellular carcinoma patients. Br J Cancer. 2010;103(7):954–960. doi: 10.1038/sj.bjc.6605856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 27.Feun LG, Marini A, Walker G, Elgart G, Moffat F, Rodgers SE, Wu CJ, You M, Wangpaichitr M, Kuo MT, Sisson W, Jungbluth AA, Bomalaski J, Savaraj N. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J Cancer. 2012 doi: 10.1038/bjc.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feun L, Savaraj N. Pegylated arginine deiminase: a novel anticancer enzyme agent. Exp Opin Investig Drugs. 2006;15(7):815–822. doi: 10.1517/13543784.15.7.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen LJ, Lin WC, Beloussow K, Shen WC. Resistance to the anti-proliferative activity of recombinant arginine deiminase in cell culture correlates with the endogenous enzyme, argininosuccinate synthetase. Cancer Lett. 2003;191(2):165–170. doi: 10.1016/s030-43835(02)00693-6. [DOI] [PubMed] [Google Scholar]
- 30.Tsai WB, Aiba I, Lee SY, Feun L, Savaraj N, Kuo MT. Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther. 2009;8(12):3223–3233. doi: 10.1158/1535-7163.MCT-09-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo MT, Savaraj N, Feun LG. Targeted cellular metabolism for cancer chemotherapy with recombinant arginine-degrading enzymes. Oncotarget. 2010;1(4):246–251. doi: 10.18632/oncotarget.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des. 2008;14(11):1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You M, Savaraj N, Wu C, Wangpaichitr M, Kuo MT, Dinh V, Feun LG. Enhancing melanoma deprivation therapy in melanoma by combining with cisplatin. American Associationf for Cancer Research, Annual Meeting.2010. [Google Scholar]