Abstract
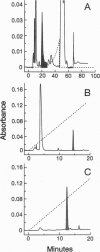
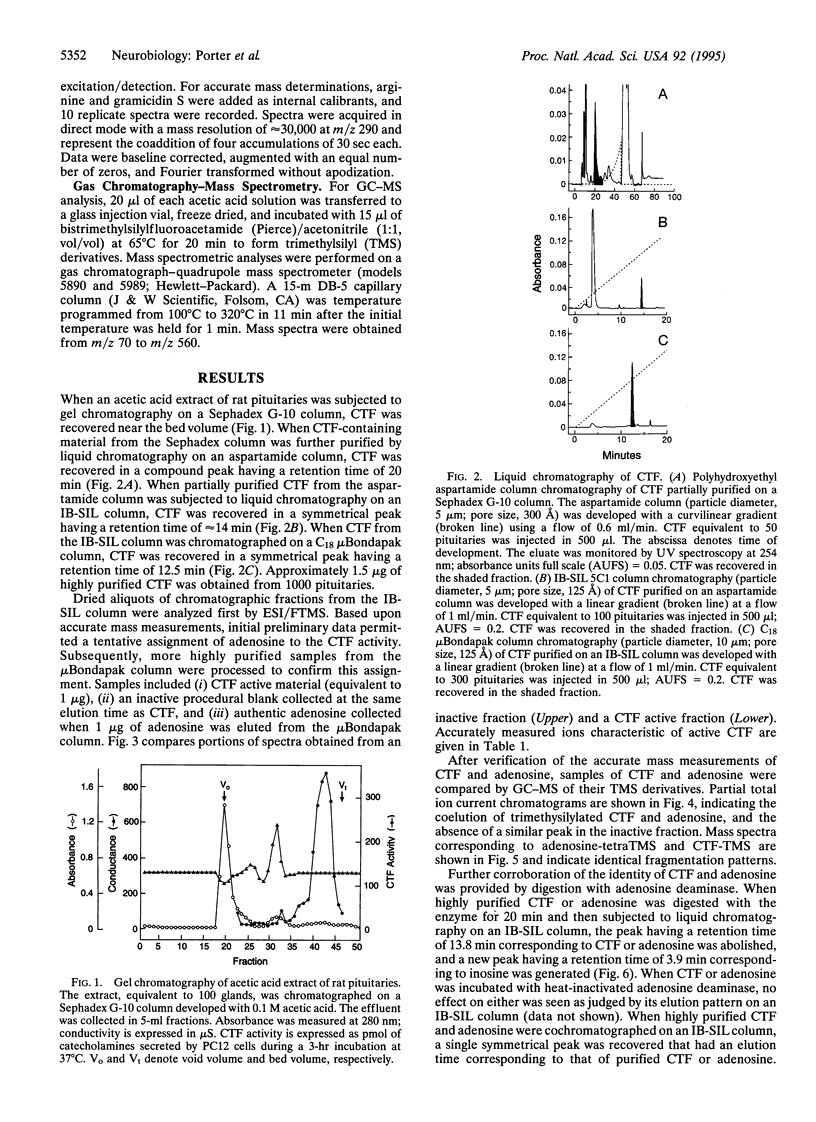
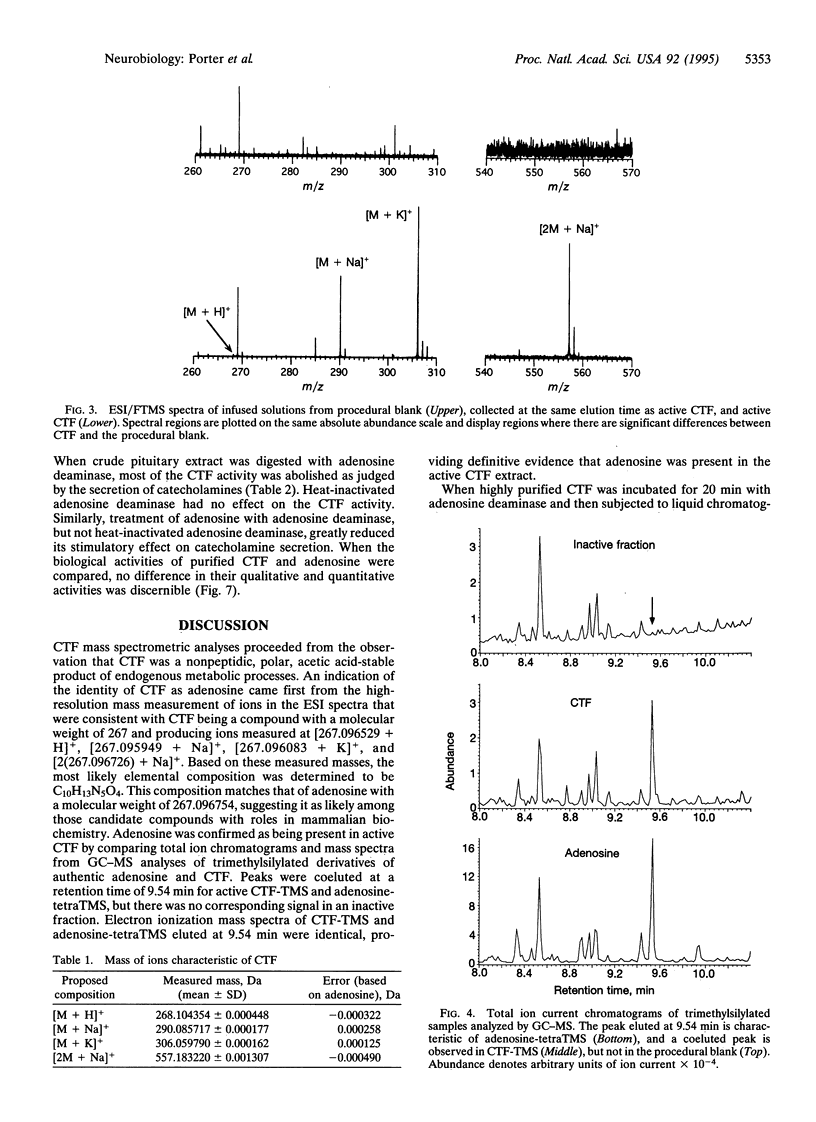
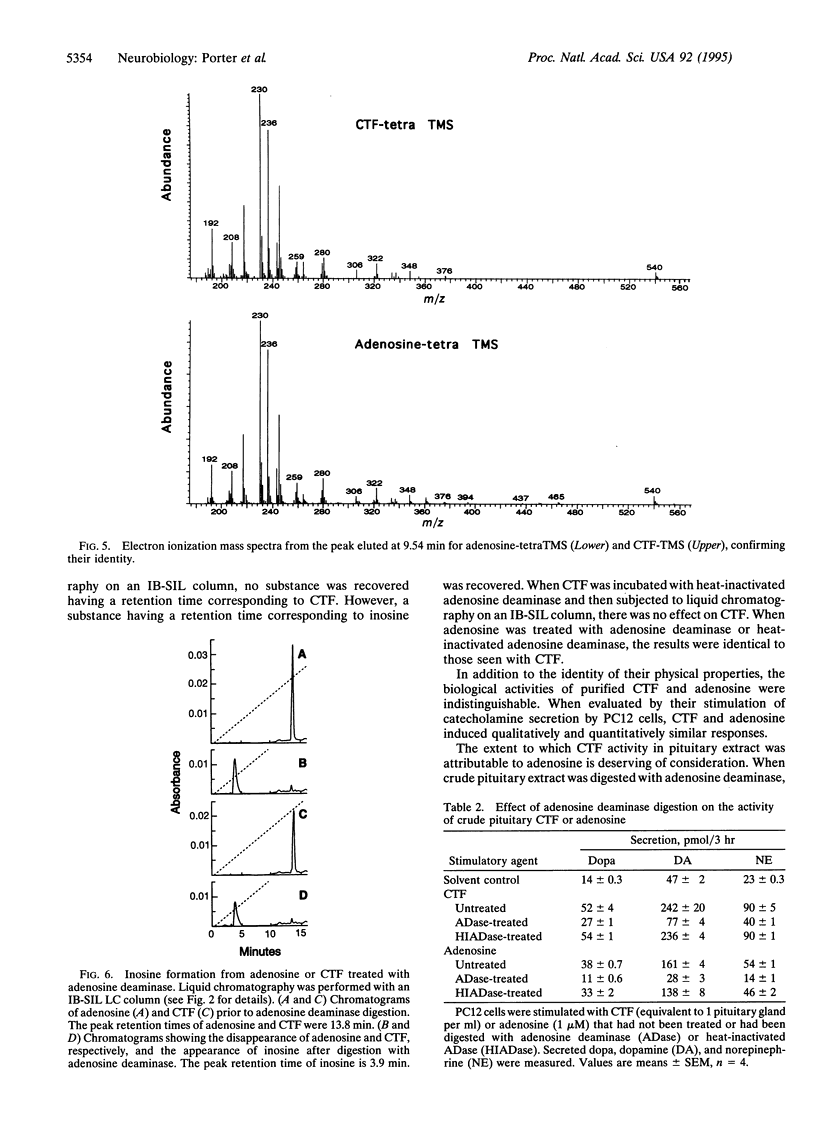
It has been shown that the pituitary contains a cytotropic factor (CTF) that stimulates the secretion of catecholamines by dopaminergic neurons of the hypothalamus. In the present study, CTF was purified from rat pituitaries and found by means of mass spectrometric analysis to be adenosine. This finding was corroborated by the observations that CTF behaves identically to adenosine when subjected to liquid chromatography, is inactivated and converted to inosine by adenosine deaminase, and is qualitatively and quantitatively indistinguishable from adenosine in its biological activity. It is concluded that pituitary adenosine is a trophic factor for hypothalamic dopaminergic neurons.
Full text
PDF
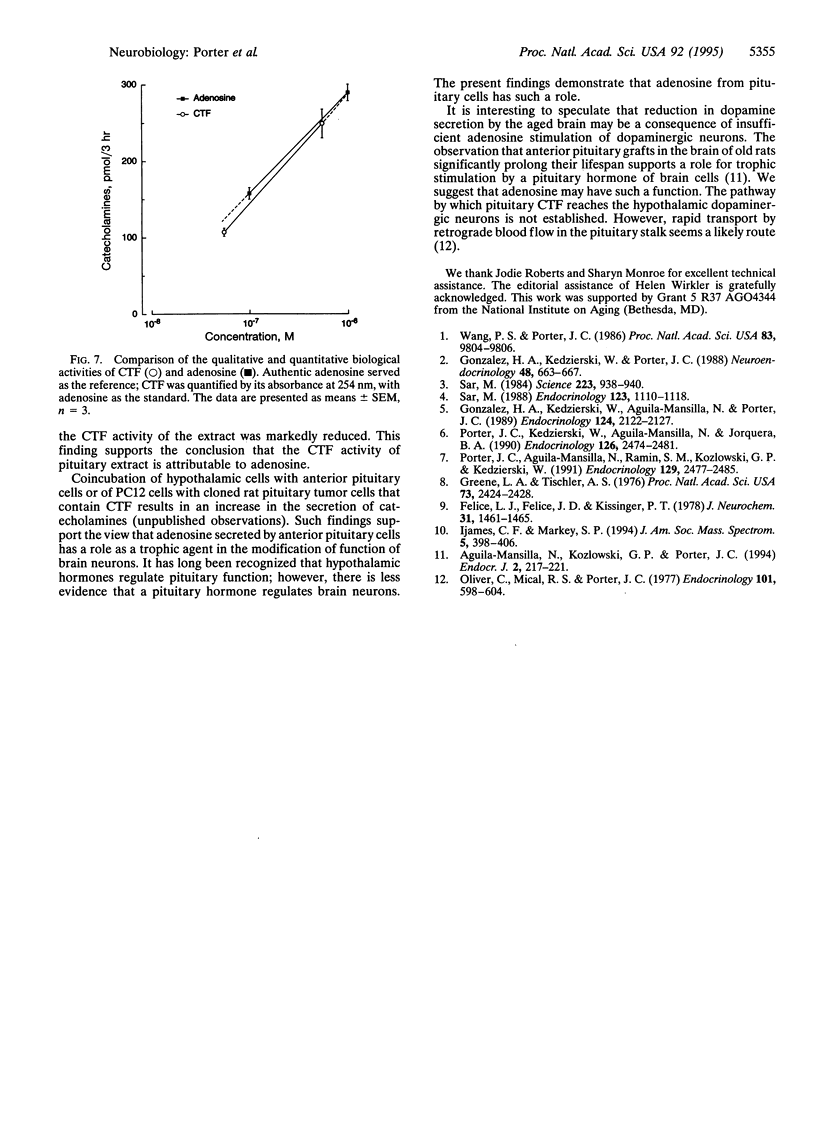
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Felice L. J., Felice J. D., Kissinger P. T. Determination of catecholamines in rat brain parts by reverse-phase ion-pair liquid chromatography. J Neurochem. 1978 Dec;31(6):1461–1465. doi: 10.1111/j.1471-4159.1978.tb06573.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez H. A., Kedzierski W., Aguila-Mansilla N., Porter J. C. Hormonal control of tyrosine hydroxylase in the median eminence: demonstration of a central role for the pituitary gland. Endocrinology. 1989 May;124(5):2122–2127. doi: 10.1210/endo-124-5-2122. [DOI] [PubMed] [Google Scholar]
- Gonzalez H. A., Kedzierski W., Porter J. C. Mass and activity of tyrosine hydroxylase in tuberoinfundibular dopaminergic neurons of the aged brain. Control by prolactin and ovarian hormones. Neuroendocrinology. 1988 Dec;48(6):663–667. doi: 10.1159/000125079. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C., Mical R. S., Porter J. C. Hypothalamic-pituitary vasculature: evidence for retrograde blood flow in the pituitary stalk. Endocrinology. 1977 Aug;101(2):598–604. doi: 10.1210/endo-101-2-598. [DOI] [PubMed] [Google Scholar]
- Porter J. C., Kedzierski W., Aguila-Mansilla N., Jorquera B. A. Expression of tyrosine hydroxylase in cultured brain cells: stimulation with an extractable pituitary cytotropic factor. Endocrinology. 1990 May;126(5):2474–2481. doi: 10.1210/endo-126-5-2474. [DOI] [PubMed] [Google Scholar]
- Sar M. Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science. 1984 Mar 2;223(4639):938–940. doi: 10.1126/science.6141639. [DOI] [PubMed] [Google Scholar]
- Wang P. S., Porter J. C. Hormonal modulation of the quantity and in situ activity of tyrosine hydroxylase in neurites of the median eminence. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9804–9806. doi: 10.1073/pnas.83.24.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]