Abstract
Background
Koob's allostatic model of addiction emphasizes the transition from positive reinforcement to negative reinforcement as dependence develops. This study seeks to extend this well-established neurobiological model to humans by examining subjective response to alcohol (SR) as a biobehavioral marker of alcohol reinforcement. Specifically, this study examines (a) differential SR in heavy drinkers (HDs) vs. alcohol dependent individuals (ADs) and (b) whether HDs and ADs differ in terms of the association between SR and craving.
Methods
Data was culled from two alcohol challenge studies, totaling 91 participants (oversampled on OPRM1 Asp40 carriers). Alcohol was administered intravenously and participants completed standard measures of SR and craving at BrAC's of 0.02, 0.04, and 0.06 g/dl. SR was modeled as a multidimensional construct consisting of stimulation, sedation, and tension relief.
Results
ADs reported significantly higher sedation and craving initially and exhibited a blunted response to alcohol along escalating BrACs. ADs exhibited greater initial tension but did not differ from HDs in tension reduction across rising BrACs. Further, alcohol-induced stimulation was associated with alcohol craving to a significantly greater degree in HDs, as compared to ADs.
Conclusions
This study provides initial evidence that HDs and ADs differ in their subjective experience of alcohol and in the association between dimensions of SR and craving for alcohol. Hypotheses derived from the allostatic model were partially supported, such that, while ADs and HDs did not differ on stimulation response, there was a relative dissociation between positive reinforcement and craving in ADs as compared to HDs.
Keywords: Alcoholism, Subjective Response to Alcohol, Latent Growth Modeling, Alcohol Challenge
1. Introduction
Researchers and clinicians alike have long recognized alcohol dependence as a chronic and relapsing condition (Ray, 2012) with both animal and human theoretical models of alcoholism focusing on the biobehavioral response to alcohol, albeit from different perspectives. No studies to date have directly translated preclinical models of alcoholism to human clinical populations.
A prominent feature of several neurobiological models of alcoholism etiology is the conceptualization of the disorder in terms of a transition from positive reinforcement (i.e., alcohol use resulting in a pleasurable state, or “drinking to feel good”) to negative reinforcement (i.e., alcohol use resulting in alleviation of a negative state, or “drinking not to feel bad,” or “drinking to feel normal”). Koob and Le Moal's allostatic model (1997) conceptualizes addiction as a multifaceted construct incorporating (a) compulsions to seek and take the drug, (b) loss of control over intake limitation, and (c) emergence of negative/withdrawal state when the drug is not present. This model proposes a cycle of progressive neurobiological dysregulation, beginning with preoccupation and anticipation (reflecting positive reinforcement) and ending with withdrawal-driven alcohol use (reflecting negative reinforcement; Koob and Kreek, 2007; Koob and Le Moal, 1997; Koob and Volkow, 2010).
While clinical research to date has not directly translated this neurobiological model of addiction to humans, controlled alcohol administration in the human laboratory may allow for such translation by leveraging subjective responses to alcohol as biobehavioral markers of positive and negative reinforcement (Ray et al., 2010a). To that end, human research has demonstrated that individuals vary dramatically in their subjective response to alcohol (SR) and that these differences are in turn predictive of one's liability for alcoholism (King et al., 2011; King et al., 2013; Ray et al., 2010b; Schuckit, 1984; Schuckit and Smith, 1996). In partial agreement with the differentiator model (Newlin and Thomson, 1990), recent studies have found that lower sedative response and greater stimulant response to alcohol in the lab are associated with escalate drinking and AUD symptomatology at 2 (King et al., 2011) and even 6 years post assessment (King et al., 2013). Behavioral pharmacology studies have also established SR to be highly a reliable (Roche et al., 2013) and multi-dimensional phenotype consisting of stimulation, sedation and tension relief dimensions (Ray et al., 2009). In light of the multidimensionality of SR, the present study aims to simultaneously characterize these three key dimensions of SR (Ray et al., 2009). Thus SR, which is often in the human literature thought of as a marker of risk for future alcohol dependence, (e.g., King et al., 2011; Schuckit, 1994) will serve as a biobehavioral marker of disease progression used to test neurobiologically informed predictions regarding the association between SR and motivation for alcohol during the transition from heavy drinking to alcohol dependence.
As with subjective responses to alcohol, craving represents a clinically significant phenotype, which has been associated with loss of control over alcohol consumption. In the laboratory, a priming dose of alcohol has been associated with both increased alcohol craving (de Wit, 1996) and alcohol consumption (de Wit, 2000). Reinstatement to alcohol use from a priming dose has been demonstrated in animal studies and craving in response to priming doses of alcohol have been used for screening pharmacotherapies for alcoholism (Hutchison et al., 2001; Ray et al., 2010a, 2007) consistent with the notion that alcohol induced craving may contribute to the maintenance of alcohol dependence. As noted by Drummond et al. (2000) craving in response to priming doses versus cues may have different prognostic value, yet due to ethical limitations regarding alcohol challenge research with treatment seekers, little is known about the prognostic utility of craving in response to priming doses. In brief, human laboratory paradigms are well-suited to capture alcohol craving (Ray, 2012), including alcohol (i.e., priming) induced craving; hence this study examines SR and alcohol craving concurrently.
In sum, the present study seeks to test Koob and Le Moal's (1997) well-established neurobiological model of addiction in human samples, through the application of an alcohol challenge paradigm. Specifically, this study (1) examines SR and alcohol induced craving as a function of drinking status [i.e., heavy drinkers (HDs) or alcohol dependent (ADs)], and (2) tests whether drinking status moderates the relationship between SR and alcohol craving. Taken together, this study provides an initial test of neuroscience-driven hypotheses about how chronic alcohol use may alter the subjective experience of alcohol in humans. If, as proposed by Koob and Le Moal (1997), alcohol use is maintained in HDs by positive reinforcement and in ADs by negative reinforcement, the following should be observed: (1.1) ADs should exhibit greater reductions in tension (capturing greater alleviation of negative affect or negative reinforcement). (1.2) ADs should exhibit blunted alcohol induced stimulation (capturing blunted subjective positive reinforcement from alcohol). (2.1) The association between stimulation and craving should be positive and greater in magnitude in HDs. (2.2) The association between tension and craving should be negative and greater in magnitude in ADs.
2. Methods
2.1 Participants
The present study utilized data culled from two completed alcohol challenge studies (Ray et al., 2013, 2006). Heavy drinking participants (n=49) were recruited from the Boulder Colorado community to participate in a study of subjective response to intravenous alcohol (Ray and Hutchison, 2004; Ray et al., 2006). Inclusion criteria were: (1) age 21-29, (2) score ≥ 8 on the Alcohol Use Disorders Identification Test (Allen et al., 1997) indicating heavy alcohol use, and (3) self-reported drinking frequency of at least 3 drinks (2 for women), 2 or more times a week, and (4) no self-reported history of problems with alcohol or prior attempts to quit drinking.
Alcohol dependent participants were recruited from the Los Angeles California community for a study of alcohol administration and alcohol cue reactivity (Ray et al., 2013). Inclusion criteria for the alcohol dependent group were: (1) age 21-65, (2) self-identification of alcohol related problems, (3) ≥48 drinks per month, (4) non-treatment seeking, and (5) meeting current DSM-IV criteria for alcohol dependence as determined through a structured clinical interview. Descriptive statistics of demographic and alcohol use variables are presented in Table 1.
Table 1.
Baseline differences between heavy drinking and alcohol dependent groups.
| Variable | Heavy Drinkers (n = 49) | Alcohol Dependent Individuals (n = 42) | Difference Test |
|---|---|---|---|
| Age (SD) | 21.98 (1.7) | 29.14 (9.5) | t(43.3) = -4.83; p < 0.001 |
| Education (SD) | 15.16 (1.01) | 14.62 (3.39) | t(47.3) = 1.004; ns |
| Sex (% male) | 53.06 | 73.81 | χ2(1) = 4.16; p < 0.05 |
| Ethnicity (% Caucasian)* | 89.80 | 59.52 | χ2(4) = 23.28; p < 0.001 |
| OPRM1 (% G carriers) | 39.47 | 45.24 | χ2(1) = .271; ns |
| Drinks per Drinking Day | 4.26 (1.71) | 7.11 (2.94) | t(89) = -5.756; p < 0.0001 |
| Drinking Frequency (ACQ3)** | 6.20 (1.54) | 9.00 (1.82) | t(89) = -7.93; p < 0.0001 |
Note: Ethnicity differences between groups were tested as a 5 level categorical variable and overall distribution of ethnicity was not found to differ between groups; however, for simplicity of presentation, only percent Caucasian is reported.
Note: Range of the ACQ3 is 0-11 where 6 = twice weekly and 9 = five times a week, thus average drinks per week was approximately 9 in heavy drinkers and 35 in alcohol dependent participants.
ACQ = Alcohol Consumption Questionnaire.
2.2 Screening Procedures
Complete descriptions of screening procedures are provided in Ray and Hutchison (2006) and Ray et al. (2013). Initial assessment of inclusion criteria was conducted through telephone interviews. Eligible participants then completed an in-person assessment session providing saliva samples for genotyping and completing a series of self-report measures. In both samples, participants were prospectively genotyped to over-sample for the G-allele of the A118G SNP of the µ-opioid receptor (OPRM1) gene (Ray et al., 2013; Ray and Hutchison, 2004). Participants in the alcohol dependent group also met with a trained clinician who conducted a structured clinical interview for DSM-IV (SCID; First et al., 1995) to determine current (i.e., past month) alcohol dependence. Subjects were assessed for alcohol withdrawal symptoms and those subjects experiencing serious alcohol withdrawal, as indicated by a score of 10 or higher on the Clinical Institute Withdrawal Assessment for Alcohol-Revised (CIWA-R; Puz and Stokes, 2005), were excluded from participation in the study for safety considerations. Participants deemed eligible after in-person screening and genotyping completed a physical examination to ensure medically eligibility. All participants were required to have a Breath Alcohol Concentration (BrAC) equal to 0.000 g/dl prior to each session, and participants were instructed to refrain from drinking alcohol the night before their visit.
2.3 Alcohol Administration Paradigm
The two studies share identical alcohol administration methods. Alcohol was administered intravenously in order to assess participants' biobehavioral response to alcohol as distinct from learned responses to alcohol cues, and to allow for precise experimental control over BrAC (Li et al., 2001; Plawecki et al., 2008). Participants were seated in a recliner chair with an IV placed in their non-dominant arm. Alcohol was administered using a 5% alcohol solution. Participants were infused at a rate of 0.166 ml/min × body weight in kilograms (0.126 ml/min × body weight for females). The alcohol infusion started at half target rate which was escalated to the full rate after 5 minutes of monitoring. BrAC was measured via breathalyzer every three to five minutes. Target BrACs were 0.02, 0.04, and 0.06 g/dl. Upon reaching each target BrAC, infusion rates were reduced by half to maintain BrAC during testing. Participants took an average of 19.9 minutes to reach a BrAC of 0.02 (and complete the assessments), 26.1 minutes to go from a BrAC of 0.02 to 0.04, and, 33.2 minutes to reach the last target BrAC of 0.06. Participants were maintained at each target BrACs for approximately 5-7 minutes while they completed self-reports of SR and craving. Timing of the alcohol administration paradigm did not differ between HDs and ADs (ps > 0.10).
2.4 Measures
2.4.1 Baseline Measures
Demographic data was collected for all participants including, age, years of education, ethnicity, and sex during the in-person screening visit. Drinking frequency in the past year was assessed through drinks per drinking day and an 11-point Likert scale of drinking frequency ranging from “I didn't drink any alcohol” to “daily drinking” adapted from the Alcohol Consumption Questionnaire (Giovannucci et al., 1991).
2.4.2 Subjective Response Measures
Participants completed the Biphasic Alcohol Effects Scale the Profile of Mood States and the Alcohol Urge Questionnaire at baseline and at each target BrAC. These measures were selected based upon previous research which has validated the use of these measures in alcohol administration studies and provided empirical support for a three-factor model of SR (Ray et al., 2009).
2.4.2.1 Biphasic Alcohol Effects Scale (BAES)
The BAES was used to capture self-reported feelings of stimulation and sedation in response to alcohol. Each subscale (stimulation and sedation) on the BAES has seven items (e.g., Down, Elated, Energized) rated on a 0 to 10 Likert scale. The BAES has been shown to be a reliable and valid measure of SR (Erblich and Earleywine, 1995; Martin et al., 1993; Roche et al., 2013).
2.4.2.2 Profile of Mood States (POMS)
The POMS has four dimensions; positive mood, vigor, depression and tension. Sample items in the tension subscale include “Nervous,” and “Uneasy.” The POMS has been shown to be valid in the context of alcohol administration at the doses examined in this study (Ray et al., 2009) with the tension subscale representing the principle component of a tension-relieving dimension of SR.
2.4.2.3 Alcohol Urge Questionnaire (AUQ)
The AUQ is comprised of eight items rated on a 7 point Likert scale with items related to subjective feelings of alcohol craving. The AUQ has demonstrated high reliability in experimental studies of state alcohol craving (Bohn et al., 1995; MacKillop, 2006).
2.5 Data Analytic Strategy
In order to simultaneously characterize SR and alcohol-induced craving along rising BrACs, latent growth curve (LGC) modeling was employed using EQS version 6.2 for Windows (Hu and Bentler, 1995). Robust estimation procedures were utilized in light of the sample size and significant multivariate kurtosis. Model fit was assessed via Yuan-Bentler scaled χ2 (Yuan and Bentler, 1997), Comparative Fit Index (CFI; Bentler, 1990) and root means square error of approximation (RMSEA; Browne et al., 1993). CFI values greater than 0.90 indicate reasonable fit (Bentler, 1990), and a stringent RMSEA upper limit of 0.07 was used to represent adequate fit (Steiger, 2007). Significant covariances as assessed through multivariate Lagrange Multiplier (LM) tests were also included in order to improve model fit. No specific error covariances were hypothesized a priori.
To test the impact of drinking status on SR (aim 1) LGC models were constructed wherein SR was modeled as a multidimensional construct consisting of stimulant, sedative, and tension-relieving domains, as measured by the BAES stimulation, BAES sedation, and POMS tension scales respectively. Alcohol craving, as captured by the AUQ, was also characterized in this model. Estimates of intercept (value of the construct when the slope parameter path is set to 0; i.e., BrAC = 0.02) and slope (change parameter over rising BrACs) were generated for each domain and a binary drinking status variable was allowed to predict latent growth parameters (HDs = 0, ADs = 1). Models were conducted including baseline scores, however due to the categorical difference between the one pre-alcohol assessment and the three post-alcohol assessments, a linear progression across time-points was not found to adequately fit the data (CFI = 0.743; RMSEA = 0.092; Yuan-Bentler χ2 (118) = 209, p < 0.0001), thus limiting the interpretability of this ill-fitting model. Hence, the pre-alcohol time point was removed from subsequent models.
In order to assess for moderation of the relationship between dimensions of SR and alcohol craving by drinking status (aim 2), parallel models were constructed for each drinking status group in which SR intercepts were allowed to predict alcohol craving intercept, and SR slopes and the craving intercept were allowed to predict craving slope. Group analyses were then conducted wherein path coefficients for the parallel models were constrained to be equal for HDs and ADs and Lagrange Multiplier Test for Releasing Constraints were run to determine which paths differ significantly between the two groups. Incremental LM test χ2 values were calculated for each constrained path and used for hypothesis testing. In sum, two separate modeling procedures were implemented in order to address the two unique yet inter-related hypotheses concerning (1) groups differences in SR and (2) group differences in the association between SR and alcohol craving (i.e., alcohol-induced craving).
3. Results
3.1 Baseline Comparisons
The AD group was significantly older, had a greater percentage of males, and was more ethnically diverse than the HD group (Table 1). In light of these baseline differences, age, sex, and ethnicity, were initially entered as covariates in all hypotheses testing models. Groups were not found to differ from each other with respect to OPRM1 A118G SNP genotype status (p > 0.10). As expected, ADs consumed more drinks per drinking day (p < 0.0001) and drank more frequently (p < 0.0001) than HDs, supporting the a-priori distinction between the two groups on the basis of alcohol exposure.
As an additional validity check, a covariates only model was run, in which sex was significantly associated with tension intercept, craving intercept, and craving slope (ps < 0.05), and age was associated with tension and craving intercepts (ps < 0.05). However, when drinking status was entered into the model these effects were no longer significant. Furthermore, addition of these covariates to drinking status models did not improve model fit or substantially impact the magnitude or significance of the results presented below, thus demographic covariates were not retained in the final models. The addition of OPRM1as a covariate did not improve model fit or alter the significance of the results presented. OPMR1 genotype was associated with only stimulation slope (β = 0.319, p < 0.05), a robust effect that has previously been reported for these data (Ray et al., 2013; Ray and Hutchison, 2004).
Mean, standard deviation, and Pearson bivariate correlations for all variables in the structural equation models are presented for all subjects in Table 2 and separately for HDs and ADs in Supplementary Table S11.
Table 2.
Means, standard deviations and zero-order person correlations for all variables in the final latent growth curve models presented.
| Mean | Std Dev | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| 1 | Group | 0.46 | 0.50 | 1.000 | |||||||||||
| 2 | .02_Stim | 20.03 | 13.63 | -0.122 | 1.000 | ||||||||||
| 3 | .04_Stim | 23.60 | 15.76 | -0.107 | 0.762 | 1.000 | |||||||||
| 4 | .06_Stim | 24.30 | 15.51 | -0.105 | 0.588 | 0.819 | 1.000 | ||||||||
| 5 | .02_Sed | 13.92 | 12.08 | 0.368 | 0.123 | 0.051 | 0.055 | 1.000 | |||||||
| 6 | .04_Sed | 15.64 | 12.67 | 0.159 | 0.138 | 0.007 | -0.068 | 0.727 | 1.000 | ||||||
| 7 | .06_Sed | 16.42 | 11.71 | 0.185 | 0.153 | 0.099 | 0.015 | 0.711 | 0.815 | 1.000 | |||||
| 8 | .02_Tens | 11.70 | 6.10 | 0.648 | 0.017 | 0.103 | 0.052 | 0.442 | 0.237 | 0.228 | 1.000 | ||||
| 9 | .04_Tens | 10.93 | 6.06 | 0.695 | -0.001 | 0.003 | -0.011 | 0.317 | 0.212 | 0.157 | 0.824 | 1.000 | |||
| 10 | .06_Tens | 10.94 | 6.37 | 0.725 | 0.057 | 0.041 | 0.057 | 0.375 | 0.203 | 0.197 | 0.780 | 0.907 | 1.000 | ||
| 11 | .02_AUQ | 16.62 | 11.12 | 0.411 | 0.195 | 0.301 | 0.300 | 0.335 | 0.256 | 0.235 | 0.474 | 0.385 | 0.413 | 1.000 | |
| 12 | .04_AUQ | 20.47 | 11.97 | 0.259 | 0.277 | 0.359 | 0.356 | 0.255 | 0.155 | 0.208 | 0.340 | 0.243 | 0.258 | 0.849 | 1.000 |
| 13 | .06_AUQ | 21.76 | 12.05 | 0.232 | 0.155 | 0.279 | 0.341 | 0.256 | 0.176 | 0.171 | 0.329 | 0.205 | 0.203 | 0.811 | 0.883 |
|
|
|||||||||||||||
Stim= BAES stimulation subscale, Sed = BAES sedation subscale, Tens = POMS tension subscale, AUQ = Alcohol Urge Questionnaire.
3.2 Measurement Reliability
The BAES, POMS tension subscale and AUQ were found to be reliable in this sample. Cronbach's alpha was computed for the first time point for all measures in both the full sample and separately in each drinking status group and all scales had high reliability estimates (full sample: α's ≥ 0.91, separately: α's ≥ 0.70).
3.3 Drinking Status and Subjective Response to Alcohol
The LGC model simultaneously assessing domains of SR and alcohol induced craving was found to fit the data well (CFI = 0.966; RMSEA = 0.048; Yuan-Bentler χ2(57) = 72.56, p = 0.13). Covariances between stimulation intercept and slope errors and between stimulation and craving intercept errors were estimated in the final model (ps < 0.05). In this model, drinking status was significantly related to both sedation intercept (β = 0.385, p < 0.05) and sedation slope (β = -0.298, p < 0.05). Furthermore, drinking status was associated with tension intercept (β = 0.728, p < 0.05), but not tension slope (β = 0.281, p > 0.05). Lastly, drinking status was significantly associated with both craving intercept (β = 0.417, p < 0.05) and slope (β = -0.268, p < 0.05). Drinking status was not associated with stimulation intercept or slope (βs = -0.111 and -0.008 respectively, ps > 0.05). The final model with standardized path coefficients is presented in Figure 1. These effects were such that ADs experienced greater sedation, craving and tension than HDs at the start of the infusion, yet the increases in sedation and craving over the course of the alcohol infusion were attenuated in ADs (i.e., flatter positive slope) as compared to HDs.
Figure 1.
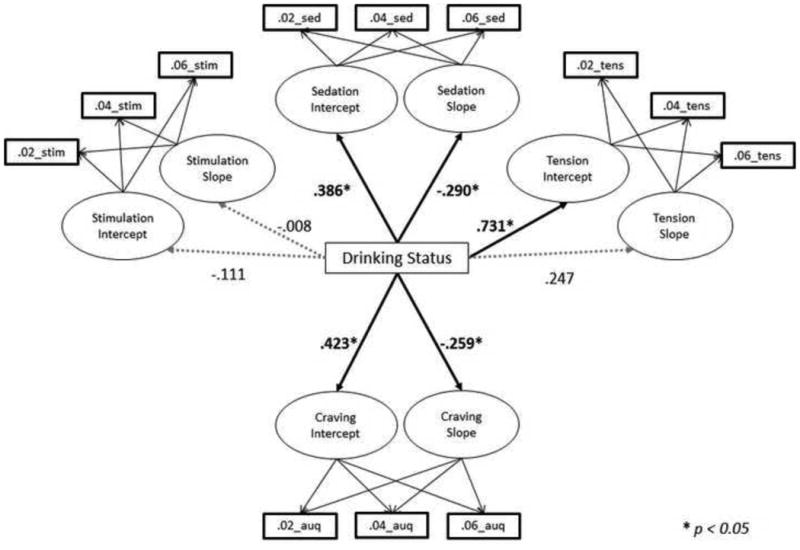
Drinking Status (0 = Heavy Drinking [n=49]; 1 = Alcohol Dependent [n=42]) predicting domains of subjective response to alcohol, as assessed by the BAES Stimulation and Sedation subscales (Stim and Sed respectively), the Tension subscale of the POMS (Tens), and alcohol craving as assessed by the AUQ. For ease of presentation, factor loadings for latent growth parameters, covariances between independent variables, and error terms are not depicted.
3.4 Associations between Dimensions of Subjective Response and Alcohol Craving
The model testing the relationship between dimensions of SR and alcohol craving in HDs demonstrated good fit (CFI = 0.976; RMSEA = 0.064; Yuan-Bentler χ2(50) = 59.71, p = 0.16); see Figure 2. A significant covariance between tension intercept and slope was included in this model (p < 0.05). Only stimulation intercept was significantly related to alcohol craving intercept in HDs (β = 0.479, p < 0.05; sedation and tension p's > 0.05). Furthermore, only stimulation slope was significantly associated with craving slope (β = 0.718, p < 0.05; sedation and tension p's > 0.05).
Figure 2.
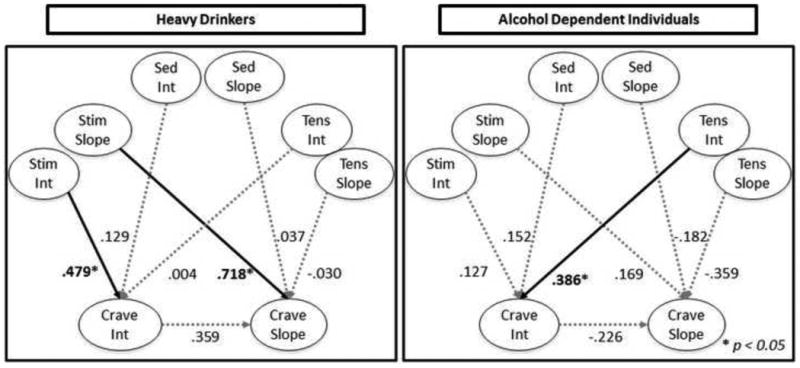
Dimensions of subjective response predicting alcohol craving intercept and slope in Heavy Drinkers vs. Alcohol Dependent Individuals. The path from stimulation slope to craving slope was found to differ significantly between alcohol groups (χ2 = 3.919, p < 0.05) such that increases in stimulation along rising BrAC were more strongly associated with increased craving for alcohol in heavy drinkers as compared to alcohol dependent individuals. Stim= BAES stimulation subscale, Sed = BAES sedation subscale, Tens = POMS tension subscale, Crave = alcohol craving assessed by the AUQ, Int = latent intercept parameter, Slope = latent slope parameter. For ease of presentation only latent constructs are depicted.
A parallel model was simultaneously generated for ADs, which demonstrated good fit (CFI = 0.965, RMSEA = 0.068; Yuan-Bentler χ2(50) = 59.60, p = 0.17); see Figure 2. Significant covariance was observed between stimulation and tension intercepts (p < 0.05). In this model, only tension intercept was significantly associated with craving intercept (β = 0.386, p < 0.05), such that higher tension at the start of the infusion was associated with greater craving concurrently. Contrary to the findings in HDs, among ADs no dimension of SR slope was significantly related to craving slope.
To test group invariance, path coefficients for the parallel models above were constrained to be equal between groups. Model fit of the constrained paths models was good (CFI = 0.966, RMSEA = 0.069; Yuan-Bentler χ2(50) = 129.7, p = 0.07). Incremental univariate comparisons of path discrepancies revealed a single significant path discrepancy, namely the association between stimulation slope and craving slope (χ2 = 3.919, p < 0.05). This effect was such that compared to HDs, ADs displayed a significantly weaker association between stimulation slope and craving slope (Figure 2).
4. Discussion
This study tested hypotheses derived from the allostatic model of alcoholism in human samples ascertained for heavy drinking or alcohol dependence. The allostatic model emphasizes the progression from positive reinforcement-driven drinking to negatively reinforced drinking (Koob and Le Moal, 1997; Koob and Volkow, 2010). To examine this transition, HDs and ADs underwent an intravenous alcohol administration to assess their SR, alcohol-induced craving, and the association between SR and craving across escalating BrACs.
In terms of SR, alcohol dependent individuals reported significantly higher sedation at the start of the alcohol administration but exhibited a blunted response along escalating BrACs (i.e., flatter positive slope). Additionally, ADs exhibited greater tension at the start of the infusion but did not differ in tension reduction from HDs. These intercept level differences in sedation and tension may reflect allostatic differences in affective set points as a result of chronic alcohol use; these are often described in the literature as protracted withdrawal symptoms (Martinotti et al., 2008). Furthermore, ADs reported greater alcohol craving initially but their craving did not increase as rapidly as HDs' along rising BrACs. This blunted craving response may indicate a ceiling effect, or it may be that craving in ADs is not dependent upon acute response to alcohol to the extent that it is in HDs. This latter possibility was supported by our second set of analyses. In addition, ADs and HDs were not found to differ on alcohol-induced stimulation (either initially or over time).
Hypotheses regarding group differences in tension response were partially supported such that there was a significant effect of drinking status on initial tension, yet we did not observe group-level differences in terms of tension reduction across rising BrACs. Likewise, the hypothesis that AD participants would have a blunted stimulation response to alcohol as compared to HDs was not supported. Together these results suggest that drinking status is selectively associated with sedation and craving response to alcohol while not impacting stimulation or tension reduction slopes. This pattern is distinct from what would be predicted by generalized tolerance syndrome, in which SR slopes are expected to be blunted across all domains (Morean and Corbin, 2008).
The second study aim was to test whether drinking status moderated the associations between SR and craving for alcohol during alcohol administration (i.e., priming induced craving). It was hypothesized that ADs would have a stronger association between tension reduction and craving and a weaker association between stimulation and craving as compared to HDs. The results partially supported these hypotheses. Specifically, stimulation slope was strongly associated with craving slope in HDs, but not in ADs suggesting greater functional significance of stimulant response to alcohol in motivating drinking, indexed by self-reported craving, in HDs as compared to ADs. Thus while ADs and HDs experienced comparable self-reported stimulation from alcohol (i.e., non-significant group differences on stimulation), these stimulant effects were relatively de-coupled from craving in ADs, consistent with the hypothesized transition away from positive reinforcement in alcohol dependence.
We also observed that tension at the start of the alcohol challenge was associated with greater craving in ADs, but not in HDs, although no effect of tension reduction from alcohol was observed. Relatedly, stimulation intercept was associated with craving at the start of the infusion in HDs, but not in ADs. These findings wherein level of initial positive hedonic response is predictive of craving in HDs only and negative affect initially is predictive of craving in ADs only are consistent with the hypothesized transition from positively to negatively reinforced alcohol use; however, caution is warranted in interpreting these results as path discrepancy analysis did not reveal significant differences between groups, which in turn may be a function of limited statistical power for higher-order interactions.
In sum, this study provides a preliminary test of translational hypotheses based on Koob's allostatic model of addiction pathophysiology (Koob and Le Moal, 1997). These results supported some of the key predictions from this model, in that a positive hedonic response to alcohol (i.e., stimulation) was more weakly associated with craving among alcohol dependent participants as compared to sub-clinical heavy drinkers. Some predictions based on the allostatic model (Koob and Le Moal, 1997) were not supported in the present analyses. First, ADs did not differ from HDs in their positive response to alcohol (i.e., stimulation). This null result may be partially explained by the fact that both studies were balanced on OPRM1 genotype, which has been linked with greater stimulation response to alcohol (Barr et al., 2007; Ray and Hutchison, 2004; Ray et al., 2010c). While, statistically controlling for OPRM1 did not alter the significance of the results presented, prospective genotyping may have biased our findings away from detecting group level differences on stimulation response. Nevertheless, while the AD group may have been genetically selected to be high stimulation responders, they still exhibited a reduced association between alcohol-induced stimulation and alcohol craving, consistent with the initial hypothesis that positive reinforcement would be less salient to ADs.
Secondly, drinking status was not predictive of tension relief, nor did it moderate the relationship between tension reduction and alcohol craving as was hypothesized. Visual inspection of the tension means across BrAC revealed that the alcohol administration did not influence the tension dimension to the same extent as other dimensions of SR in either group. This result may be a function of the artificial nature of the experimental session, limiting the generalizability to naturalistic alcohol use where tension relief is thought to be more salient (Ray et al., 2010c). The moderate dose of alcohol, coupled with the possibility of acquired tolerance in both groups might have limited our ability to detect significant group differences in tension reduction and tension-mediated craving. It is also possible that tension reduction mechanisms depend upon a host of factors beyond the pharmacological effects of alcohol (e.g., response to alcohol cues, social context), which were suppressed in the intravenous alcohol administration paradigm. Furthermore, drinkers, even alcohol dependent drinkers are known to differ in terms of relief drinking (Verheul et al., 1999).
In order for neurobiologically precise research utilizing animal models to contribute optimal insights into human psychopathology, such theories must be validated in clinical samples. Validation in clinical samples then permits theory driven-inferences both in terms of etiology and treatment development. For example, our results are consistent with the hypothesis that interventions targeting stimulation (such as opioid antagonism) may be better tailored for early stage alcoholism, while CRF antagonists may be better tailored interventions in later stages of addiction (Koob and Zorrilla, 2010). In this way translational studies aimed at validating preclinical models of alcoholism have the potential to inform a more complete understanding of addiction etiological and lead to more efficient treatment development and optimization.
The present findings must be interpreted in light of the study's strengths and limitations. Strengths of the study include its translational nature and multi-dimensional approach to testing SR. Additionally, the highly controlled and standardized alcohol administration paradigm represents a significant strength. Furthermore, the analytical techniques employed represent a strength in that they allow for simultaneous examination of several hypothesized associations thereby reducing multiple comparisons and providing a parsimonious and theory-driven set of tests. The primary study limitation is that we cannot definitively assert that the HDs did not meet criteria for alcohol dependence as diagnostic interviews were not conducted in the HD sample. That being said, an inclusion criteria of no self-reported history of alcohol problems or attempts to quit markedly reduces the possibility that HD subjects were alcohol dependent. Moreover, the AD group did drink significantly more than the HD group, thus establishing a meaningful difference between groups. Additionally, while analyses were conducted exploring the influence of potential demographic factors (e.g., age, ethnicity, education), it is possible that unmeasured effects explains some of the observed differences. Though statistically controlling for age did not substantively impact our results, the difference in age range between HDs and ADs represents a potential confounding factor. Additional analyses (data not shown) were conducted to compare the HD group with a subset of the AD group with identical age restrictions and the primary findings were maintained in this younger subsample. Additional study limitations include the moderate dose of alcohol and the assessment along the ascending limb only. While previous comparisons have shown the target dose of 0.06 g/dl to be adequate for modeling SR (Ray et al., 2007), additional studies using higher alcohol doses and the full BrAC curve are warranted.
Participants were also aware they were receiving alcohol and thus alcohol expectancies may have factored into the results obtained. Of note, participants were not told how much alcohol they received (e.g., BrAC), and intravenous alcohol represents a novel stimuli, thus reducing, although not eliminating, the potential influence of learned expectancies. Lastly, additional analyses were conducted on pre-alcohol levels of SR and craving in order to examine the role of alcohol expectancies. Identical to the intercept-level results presented, HDs and ADs significantly differed in terms of craving, sedation and tension (p's < 0.05), but not stimulation (p = 0.77), thus providing confidence that the interpretation of intercept-level group differences as differences in allostatic set point are likely valid, and not simply a result of differential alcohol expectancies. Lastly, this study only examined craving in response to a priming dose of alcohol, which, while representing an important factor in alcoholism etiology, may only represent a subset of craving responses maintaining alcohol misuse (e.g., cue and stress induced craving Drummond et al., 2000). Given the complexity of the study hypotheses and resulting analytical techniques, sample size was modest thereby increasing the likelihood of false negatives (type II error).
Limitations notwithstanding, this study extends the literature on SR by demonstrating that drinking status alters the subjective experience of alcohol as well as the association between SR and craving. Critical for the translation of the allostatic model, this initial study demonstrates that positive hedonic response to alcohol is more predictive of alcohol craving in HDs as compared to ADs even though absolute response did not differ significantly. Future studies are warranted to extend this translational approach and further validate, disconfirm, or refine the behavioral hypotheses derived from Koob's allostatic model in larger, more representative samples, and using measures of motivation towards alcohol use (e.g., alcohol self-administration).
Supplementary Material
Acknowledgments
The authors would like to thank Eliza Hart, Andia Heydari, Pauline Chin, James Ashenhurst, Nathasha Moallem, Molly Tartter, Belinda De La Torre, and Ryan Arellano, Erin Marshall, Heather Chamberlain, Kent Hutchison PhD and staff at the General Clinical Research Center at both the University of Colorado, Boulder and at the University of California Los Angeles for their contribution to data collection and data management for this project. The authors would also like to thank J. David Jentsch for his contribution to this study's conceptualization and interpretation.
Role of Funding Source: This study was supported by grants from ABMRF, the Foundation for Alcohol Research, the UCLA Clinical and Translational Science Institute, National Institutes of Health (M01-RR00865 to LAR), NIAAA Grants F31 AA14847 (LAR) and R01 AA12238 (Kent E Hutchison) and by Grant M01 RR00051 from the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary Table S1 provides means, standard deviations and correlations for all subjective response and craving variables at each time point in HDs and ADs separately and can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:….
Contributors: All authors have made substantial contributions warranting authorship on the current manuscript. LAR is the graduate mentor to SB and was Principle Investigator for both studies from which data was culled. SB conducted the statistical analyses. Both SB and LAR contributed to manuscript preparation and revision. Both authors have given their approval for the current version to be submitted for peer-review and publication.
Conflict of Interest: No conflicts declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT) Alcohol Clin Exp Res. 1997;21:613–619. [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Arch Gen Psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R, Bollen KA, Long JS. Alternative ways of assessing model fit. Sage Focus Editions. 1993;154:136–136. [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Exp Clin Psychopharmacol. 1996;4:5–10. [Google Scholar]
- de Wit H. Laboratory-based assessment of alcohol craving in social drinkers. Addiction. 2000;95(Suppl. 2):S165–169. doi: 10.1080/09652140050111735. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Litten RZ, Lowman C, Hunt WA. Craving research: future directions. Addiction. 2000;95(Suppl. 2):S247–255. doi: 10.1080/09652140050111816. [DOI] [PubMed] [Google Scholar]
- Erblich J, Earleywine M. Distraction does not impair memory during intoxication: support for the attention-allocation model. J Stud Alcohol. 1995;56:444–448. doi: 10.15288/jsa.1995.56.444. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient edition (SCID-I/P, version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Giovannucci E, Colditz G, Stampfer MJ, Rimm EB, Litin L, Sampson L, Willett WC. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM, editors. Evaluating Model Fit. Sage Publications; Thousand Oaks, CA, USA: 1995. [Google Scholar]
- Hutchison KE, Swift R, Rohsenow DJ, Monti PM, Davidson D, Almeida A. Olanzapine reduces urge to drink after drinking cues and a priming dose of alcohol. Psychopharmacology (Berl) 2001;155:27–34. doi: 10.1007/s002130000629. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.001. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr Opin Investig Drugs. 2010;11:63. [PMC free article] [PubMed] [Google Scholar]
- Li TK, Yin SJ, Crabb DW, O'Connor S, Ramchandani VA. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res. 2001;25:136–144. [PubMed] [Google Scholar]
- MacKillop J. Factor structure of the alcohol urge questionnaire under neutral conditions and during a cue-elicited urge state. Alcohol Clin Exp Res. 2006;30:1315–1321. doi: 10.1111/j.1530-0277.2006.00159.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Nicola MD, Reina D, Andreoli S, Foca F, Cunniff A, Tonioni F, Bria P, Janiri L. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst Use Misuse. 2008;43:271–284. doi: 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective alcohol effects and drinking behavior: the relative influence of early response and acquired tolerance. Addict Behav. 2008;33:1306–1313. doi: 10.1016/j.addbeh.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O'Connor SJ. Physiologically based pharmacokinetic (PBPK) models for ethanol. IEEE Trans Biomed Eng. 2008;55:2691–2700. doi: 10.1109/TBME.2008.919132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puz CA, Stokes SJ. Alcohol withdrawal syndrome: assessment and treatment with the use of the Clinical Institute Withdrawal Assessment for Alcohol-revised. Crit Care Nurs Clin North Am. 2005;17:297. doi: 10.1016/j.ccell.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ray LA. Clinical neuroscience of addiction: applications to psychological science and practice. Clinical Psychology: Science and Practice. 2012;19:154–166. [Google Scholar]
- Ray LA, Bujarski S, MacKillop J, Courtney KE, Monti PM, Miotto K. Subjective response to alcohol among alcohol-dependent individuals: effects of the mu-opioid receptor (OPRM1) gene and alcoholism severity. Alcohol Clin Exp Res. 2013;37(Suppl. 1):E116–124. doi: 10.1111/j.1530-0277.2012.01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE, Tartter M. Application of human laboratory models to pharmacotherapy development for alcohol dependence. Curr Pharm Des. 2010a;16:2149–2158. doi: 10.2174/138161210791516422. [DOI] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res. 2009;33:2154–2161. doi: 10.1111/j.1530-0277.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, Monti PM. Subjective responses to alcohol consumption as endophenotypes: advancing behavioral genetics in etiological and treatment models of alcoholism. Subst Use Misuse. 2010b;45:1742–1765. doi: 10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, McGeary J, Marshall E, Hutchison KE. Risk factors for alcohol misuse: examining heart rate reactivity to alcohol, alcohol sensitivity, and personality constructs. Addict Behav. 2006;31:1959–1973. doi: 10.1016/j.addbeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Ray LA, Meskew-Stacer S, Hutchison KE. The relationship between prospective self-rating of alcohol sensitivity and craving and experimental results from two alcohol challenge studies. J Stud Alcohol Drugs. 2007;68:379–384. doi: 10.15288/jsad.2007.68.379. [DOI] [PubMed] [Google Scholar]
- Ray LA, Miranda R, Jr, Tidey JW, McGeary JE, MacKillop J, Gwaltney CJ, Rohsenow DJ, Swift RM, Monti PM. Polymorphisms of the mu-opioid receptor and dopamine D4 receptor genes and subjective responses to alcohol in the natural environment. J Abnorm Psychol. 2010c;119:115–125. doi: 10.1037/a0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, Palmeri MD, King AC. Acute alcohol response phenotype in heavy social drinkers is robust and reproducible. Alcohol Clin Exp Res. 2013;38:844–852. doi: 10.1111/acer.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994;151:184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Understanding the limitations of global fit assessment in structural equation modeling. Person Individ Diff. 2007;42:893–898. [Google Scholar]
- Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- Yuan KH, Bentler PM. Mean and covariance structure analysis: theoretical and practical improvements. J Am Stat Assoc. 1997;92:767–774. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


