Abstract
There is increasing evidence that osteocytes regulate multiple aspects of bone remodeling through bi-directional communication with osteoblasts. This is potentially mediated through cell-cell contact via osteocytic dendritic processes, through the activity of secreted factors, or both. To test whether cell-cell contact affects gene expression patterns in osteoblasts and osteocytes, we used a co-culture system where calvarial osteoblasts and IDG-SW3 osteocytes were allowed to touch through a porous membrane, while still being physically separated to allow for phenotypic characterization. Osteoblast/osteocyte cell-contact resulted in up-regulation of osteoblast differentiation genes in the osteoblasts, when compared to wells where no cell contact was allowed. Examination of osteocyte gene expression when in direct contact with osteoblasts also revealed increased expression of osteocyte-specific genes. These data suggest that physical contact mutually enhances both the osteoblastic and osteocytic character of each respective cell type. Interestingly, Gja1 (a gap junction protein) was increased in the osteoblasts only when in direct contact with the osteocytes, suggesting that Gja1 may mediate some of the effects of direct cell contact. To test this hypothesis, we treated the direct contact system with the gap junction inhibitor 18-alpha-glycyrrhetinic acid and found that Bglap expression was significantly inhibited. This suggests that osteocytes may regulate late osteoblast differentiation at least in part through Gja1. Identification of the specific factors involved in the enhancement of differentiation of both osteoblasts and osteocytes when in direct contact will uncover new biology concerning how these bone cells communicate.
Keywords: osteoblast, osteocyte, coculture, QPCR
Bone is a highly dynamic and complex tissue, requiring the contribution of several cell types to maintain tissue integrity and function. This homeostatic condition is achieved through coordinated actions and interactions of the processes of bone formation and bone resorption. During normal bone turnover, formation and resorption are coupled so that the net amount of bone remains constant. The main cell types that provide these functions are the bone-forming osteoblasts, the bone-resorbing osteoclasts, and the mechanosensing osteocytes.
In order for these cell types to maintain equilibrium between the processes of bone formation and resorption, cellular communication among these all these cell types is essential. It is well known that osteoblasts are involved in the process of osteoclast differentiation [Martin, 2013]. Likewise, recent evidence has demonstrated that osteoclasts produce soluble factors, such as Sphk1, Wnt10b, Bmp6, which influence osteoblast function [Pederson et al., 2008].
Osteocyte control of bone homeostasis is becoming increasingly recognized as an important regulator of bone metabolism. Recent evidence suggests that osteocytes play an important role in bone resorption [O’Brien et al., 2013] and that the osteocyte may in fact provide a majority of the RANKL that controls osteoclast differentiation in trabecular bone [Nakashima et al., 2011; Xiong et al., 2011]. Considerable evidence exists demonstrating that osteocytes control bone formation through the production and secretion of the Wnt inhibitor sclerostin, a soluble factor that traverses the lacuna-canalicular network, and is thought to inhibit the Wnt pathway in osteoblasts and reduce osteoblastic function [van Bezooijen et al., 2004].
In this report, we investigate the effects of osteocyte and osteoblast communication using a transwell co-culture system, whereby these cell types can physically touch through a porous membrane. We demonstrate that direct cell-cell contact between osteoblasts and osteocytes mutually enhances the osteoblastic and osteocytic character of each respective cell type. Future studies will focus on identification of the specific mechanisms and soluble factors involved in osteoblast/osteocyte communication.
Materials and Methods
Cell culture reagents
Primary calvarial osteoblasts (CalOBs) were isolated from C57BL/6 mice as previously described [Monroe et al., 2010] and cultured in αMEM growth medium (Invitrogen, Carlsbad, CA) supplemented with 1X antibiotic/antimycotic (Invitrogen) and 10% (v/v) fetal bovine serum. The mouse osteocyte cell line, IDG-SW3, was kindly provided by Dr. Lynda Bonewald (University of Missouri, Kansas City) and cultured in the same media as the CalOBs supplemented with 50 U/mL interferon-gamma (Sigma-Aldrich, St. Louis, MO) [Woo et al., 2011]. The IDG-SW3 cells were plated on rat tail collagen type 1-coated plates (BD Biosciences, Bedford, MA). Osteogenesis was induced by replacing with growth medium at confluence with fresh growth medium supplemented with 50 mg/L ascorbic acid and 10 mM β-glycerophosphate (Sigma-Aldrich). Inhibition of gap junction function was accomplished by supplementing growth medium with 10 μM 18-alpha-glycyrrhetinic acid (Sigma-Aldrich) for the 7 day coculture period.
Coculture
The in vitro osteoblast and osteocyte coculture model was established using a Millicell-24 Cell Culture Insert Plate (Millipore, Billerica, MA) comprised of a polyethylene terephthalate (PET) membrane perforated with 1-μm pores, performed as previously described [Taylor et al., 2007]. Briefly, the inserts were inverted and 4 × 104 of the first cell model were seeded onto the basal surface (bottom side of insert) in 1 mL growth medium and incubated for 5 h at 37°C to permit cellular adhesion. The inserts were reverted into 6-well tissue culture plates containing 4 mL growth medium and incubated overnight at 37°C. The growth medium was then changed to osteogenic differentiation medium and changed every 2 days for a total of 21 days. Then, 4 × 104 of the second cell model was seeded on the apical surface (top side of insert) in 1 mL growth media and allowed to adhere overnight. The growth medium was again changed to osteogenic differentiation medium and changed every 2 days for a total of 7 additional days. All cultures were performed in sextuplet (n=6) replicate wells. For the “No Cell Contact” experiment, the IDG-SW3 cells were seeded on the bottom of the well instead of on the basal surface of the membrane, but were otherwise treated identically as above. The location of either the CalOBs or IDG-SW3 cells differed with each experiment (described in the Results). To maintain consistency in the coculture models and to provide a proper control, wells in which the same cell line was seeded on both the basal and apical surfaces of the membrane were established and treated identically.
RNA/cDNA isolation
Upon completion of the experiments, we harvested the RNA separately from the cells located on two surfaces of membrane by first scraping the cells on the basal surface using sterilized toothpicks and immediately placing them into 700 μL QIAzol Lysis Reagent (Qiagen, Valencia, CA). Then, the bottom sides of membrane were washed by PBS twice and wiped well by sterile gauze to remove any leftover cells. The cells on the apical surface of the membrane were then harvested in 700 μL QIAzol Lysis Reagent. The total cellular RNA was isolated using the RNeasy Mini Kit (Qiagen). Removal of contaminating genomic DNA was accomplished using an on-column RNase-free DNase solution (Qiagen). One μg of total RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and diluted 1:5 with sterile water.
Quantitative Real-time PCR Analysis (QPCR)
One μl of the cDNA was used in a 10 μl total reaction volume for QPCR using the QuantiTect SYBR Green PCR Kit (Qiagen) and the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems). Normalization for variations in input RNA was performed using a panel of 10 housekeeping genes and analyzed using the geNorm algorithm [Radonic et al., 2004; Vandesompele et al., 2002] to select the 3–4 most stable reference genes, as previously described [Modder et al., 2012]. Primer sequences for individual genes were designed using the Primer Express program (Applied Biosystems) and are available on request.
Statistical analyses
Calculations and statistical analyses were performed using Microsoft Office Excel 2003 (Microsoft Corp., Redmond, WA). The data are presented as the mean ± SE. All values of p ≤ 0.05 were considered statistically significant using Student’s t-test.
Results
Coculture design
In this report, we utilize a transwell co-culture system that uses a polyethylene terephthalate membrane (PET)-containing insert, which is perforated with 1-micron pores to facilitate cell-cell contact of two independent cell types [Taylor et al., 2007]. As is schematically described in Figure 1, this model involves seeding the basal surface (bottom side of an upside-down insert) with one cell type and allowing cellular adhesion to occur. The insert is then reverted and another cell type is seeded on the apical surface (inside of the insert), thereby allowing for direct cell-cell contact via cellular dendritic processes that extend through the pores. Importantly, the small pore size does not permit cell migration through the pore [Taylor et al., 2007]. This serves to maintain physical separation of the two cell types and facilitates isolation of each cell type for downstream analysis. In this report, we examine the effects of co-culture using primary mouse calvarial osteoblasts (CalOBs) and the IDG-SW3 osteocyte cell line [Woo et al., 2011]. In all experiments, the IDG-SW3 cells were cultured for 21 days under osteogenic conditions to fully differentiate them to osteocytes [Woo et al., 2011] before being placed into the transwell model with the CalOBs, and the cells allowed to differentiate together for an additional 7 days prior to harvest.
Fig 1.

Schematic diagram of the procedure for seeding the two independent cell types in the direct cell-cell contact co-culture system.
Direct osteocyte cell contact enhances bone marker gene expression in osteoblasts
To determine whether direct cell-cell contact of osteoblasts with osteocytes affects bone marker gene expression in osteoblasts, we established co-cultures where the CalOBs were seeded on the apical surface of the membrane with IDG-SW3 cells seeded either on the basal side of the membrane (termed “Cell Contact”) or on the bottom of the well (termed “No Cell Contact”) (Fig. 2A). As shown in Figure 2B, direct cell contact between the two cell types significantly increased gene expression in the CalOBs of all the bone marker genes tested. We also found that expression of the transcript for the gap junction protein connexin 43 (aka Gja1) was increased 9-fold in CalOBs when in co-cultured in direct contact with the IDG-SW3 osteocyte cells (Fig. 2B), suggesting that intercellular communication between the cells may be enhanced. Interestingly, the expression profile of known osteocytic marker genes was also significantly increased in the CalOBs when in direct contact with the IDG-SW3 cells (Fig 2C), suggesting the adoption of an early osteocytic phenotype in the CalOBs.
Fig 2.
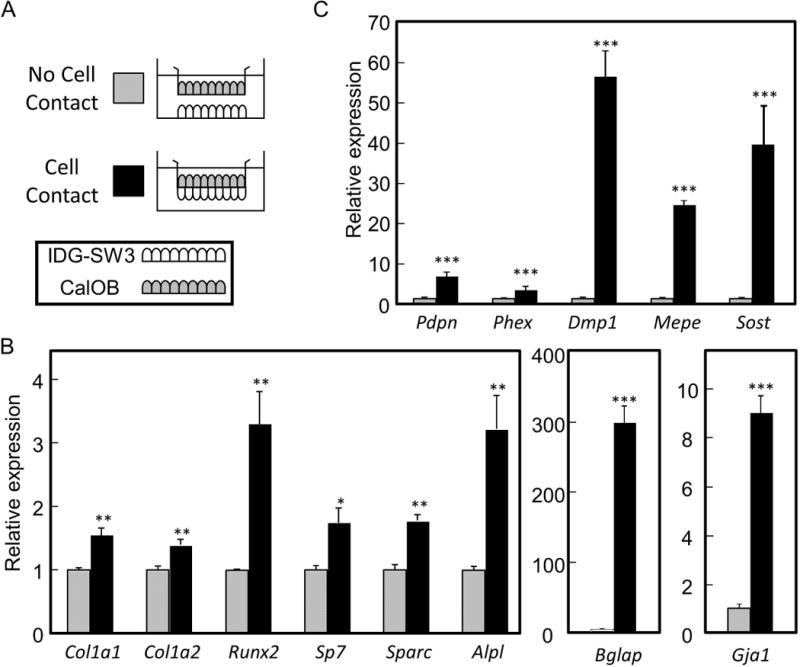
Osteoblastic gene expression is enhanced when in direct cell contact with osteocytes compared to no cell contact. A) IDG-SW3 osteocytes were seeded either on the bottom of the well (“No Cell Contact”) or on the basal surface of the membrane (“Cell Contact”) and allowed to differentiate in osteogenic media for 21 days. CalOB osteoblasts were then seeded on the apical surface of the membrane and both cell types allowed to differentiate for an additional 7 days. B) QPCR analysis of the CalOBs when either in “No Cell Contact” or “Cell Contact” with the IDG-SW3 cells. Data are expressed as mean ± standard error (SE), n=6, *p<0.05, **p<0.01, ***p<0.001.
Direct osteoblast cell contact also enhances bone marker gene expression in osteocytes
To determine whether this co-culture method also enhanced the osteocytic character of the osteocytes when placed in direct contact with osteoblasts, we cultured CalOBs and IDG-SW3 cells in direct co-culture and compared with IDG-SW3 cells in direct co-culture with themselves, as depicted in Figure 3A. Expression of the osteocyte marker genes in the IDG-SW3 cells were increased when placed in direct contact with CalOBs (Fig. 3B). Expression of the early osteocytic marker Pdpn (−2.9-fold) was decreased.
Fig 3.
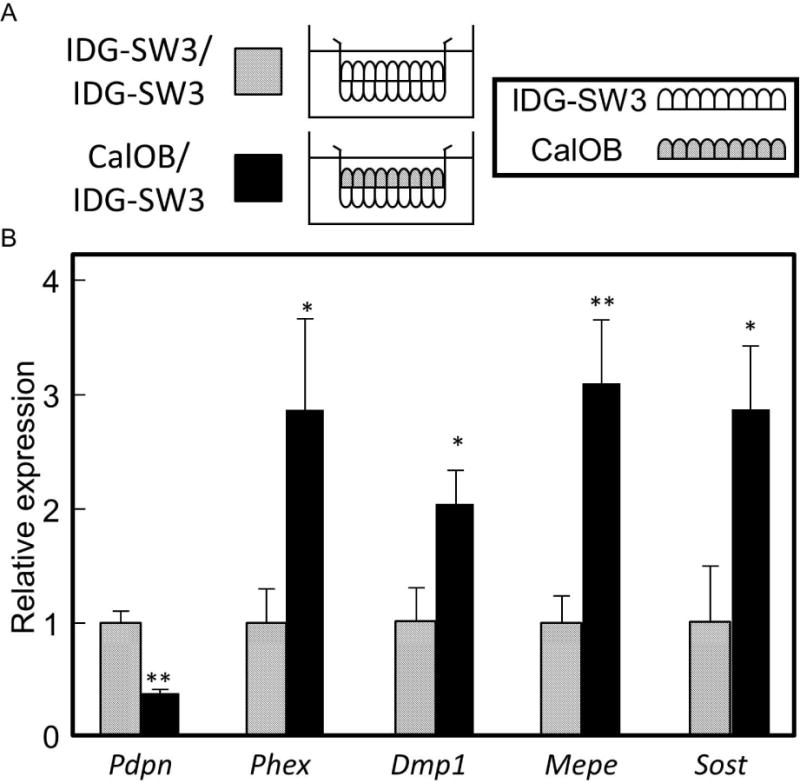
Osteocytic gene expression is enhanced when in direct cell contact with osteoblasts, compared to direct cell contact with the osteocytes themselves. A) IDG-SW3 osteocytes were seeded on the basal surface of the membrane for both conditions, and either IDG-SW3 cells or CalOBs seeded on apical surface. Seeding and differentiation conditions were identical as described in Fig. 1. B) QPCR analysis of the IDG-SW3 cells when either in direct contact with other IDG-SW3 cells (“IDG-SW3/ IDG-SW3”) or the CalOB cells (“CalOB/IDG-SW3”). Data are expressed as in Fig 1., *p<0.05, **p<0.01.
Gap junction inhibition decreases Bglap expression
Since expression of gap junction protein Gja1 was increased with CalOB/IDG-SW3 coculture (Fig. 2B), and since Gja1 is recognized as an important mediator of cellular communication in osteoblasts [Schiller et al., 2001], we used 18-alpha-glycyrrhetinic acid (AGA) to inhibit gap junction function in our co-culture model and examined the osteoblast and osteocyte marker gene profiles in the CalOBs (Fig. 4A). Interestingly, only Bglap (−2.3-fold) expression was decreased, with no significant changes observed in any of the other genes tested (Fig. 4B). This suggests that although gap junction communication may be important for the regulation of Bglap expression, other mechanisms likely also contribute to the enhancement of osteoblast differentiation.
Fig 4.

The gap junction inhibitor 18-alpha-glycyrrhetinic acid (AGA) inhibits Bglap expression in osteoblasts when in direct contact with osteocytes. IDG-SW3 cells were seeded on the basal surface of the membrane and CalOBs seeded on the apical surface (as in Figs. 1–3). 10 μM AGA (or vehicle control) was added to the well for the entire 7 day coculture period. QPCR analysis of osteoblast (A) and osteocyte (B) marker genes in the CalOBs, when in direct contact with the IDG-SW3 cells treated −/+ AGA, was performed. Data are expressed as in Fig. 1., *p<0.05.
Discussion
In the current study, we examined the effects of direct cell-cell contact on expression of cellular differentiation markers in CalOB osteoblasts and IDG-SW3 osteocytes, using a co-culture system described previously [Taylor et al., 2007]. In this system, osteoblasts and osteocytes are seeded on opposing sides of a porous membrane, allowing the cells to contact, and therefore communicate via dendritic processes extending through the pore. The advantage to this system is that cellular migration through the pores is not possible (due to their small 1-micron size) and therefore clean isolation of each respective cell type following the experimental manipulation can be achieved.
Osteocytes exist embedded in the bone matrix and are connected to other cells (i.e. other osteocytes, osteoblasts, bone lining cells, osteoclasts, stromal cells) in the bone microenvironment via extension of their dendritic processes through the lacuna-canalicular network [Bonewald, 2011]. This provides a physical framework in which cells can potentially communicate and influence each other’s activity. Indeed, we found that our direct co-culture system, which is a simplified model of the direct cell-cell contact observed in vivo, results in a not only enhanced osteoblastic character of the CalOBs, but also enhanced osteocytic character of the IDG-SW3 cells. This suggests that bidirectional communication between the osteoblasts and osteocytes is occurring.
The mechanism of this bidirectional communication between osteoblasts and osteocytes is unclear, but may involve mediators of cell-cell contact such as gap junction proteins. Gap junctions are critical in mediating the connection between cells and provide a mechanism that permits the diffusion of small signaling molecules such as calcium ions, inositol 1,4,5-triphosphate and cyclic AMP [Bonewald and Johnson, 2008; Buo and Stains, 2014; Jiang et al., 2007; Taylor et al., 2007]. Connexin 43 (Gja1) hemichannels have been identified as an important mediator of cellular communication in bone through direct cell-cell contact [Plotkin and Bellido, 2013; Ren et al., 2013]. We showed that the Gja1 inhibitor AGA inhibited the increased expression of Bglap in the osteoblasts. This suggests that at least a portion of the enhanced osteogenic signal in our direct co-culture system may be due to Gja1-mediated cell communication. However, other classical bone marker genes were not affected by the AGA inhibitor. The reason for this is unclear, however may be due to incomplete Gja1 inhibition over the 7 day co-culture timeframe. Future studies are needed further clarify how osteoblasts and osteocytes communicate when in direct co-culture.
Birmingham and colleagues found that osteoblasts and osteocytes increased the differentiation capacity of mesenchymal stem cells when co-cultured in vitro using either conditioned media or indirect co-culture methodology [Birmingham et al., 2012]. Our data extend this finding by demonstrating that this osteogenic signal is further enhanced utilizing a system where osteoblasts and osteocytes are in direct contact. Interestingly, expression of known osteocytic gene markers was also significantly increased in the osteoblasts when in direct contact with osteocytes. This suggests that not only does direct co-culture enhance the osteogenic phenotype of osteoblasts, but also that the osteoblasts start adopting the phenotypic characteristics of early osteocytes. It is certainly possible that osteoblast-osteocyte cell contact may be an important signal which influences late-stage osteoblasts to become osteocytes, although this is only speculation and needs to be addressed by carefully controlled experiments.
A particularly interesting finding is the increase in Sost transcripts in osteoblasts that are in direct contact with osteocytes. While increased Sost is a well-recognized marker of the osteocytic phenotype [van Bezooijen et al., 2004], the sclerostin protein is an inhibitor of Wnt signaling and would inhibit bone formation. A potential explanation is that increased sclerostin in late osteoblastic or early osteocytic cells serves to limit the amount of bone formed in the bone remodeling unit, thereby protecting against excess bone formation in cells at this stage of differentiation.
A limitation of this study is that assessment of mineralization using Alizarin Red S staining was not possible. This is due to the fact that the cells of either side of the porous membrane could not be separated while in direct co-culture and any stain applied would leach through the membrane staining both populations. This makes assessment of mineralization using Alizarin Red staining of the each cell line not possible in this model. In any case, in our direct co-culture system the IDG-SW3 osteocytes on the basal surface of the membrane are cultured in osteogenic media for 21 days prior to seeding the CalOB (or IDG-SW3 cells in some experiments) on the apical surface of the membrane, and therefore are already in a highly differentiated and mineralized state. However, this is balanced by the major strength of this study in that the cells could be cleanly separated for RNA isolation, makes independent assessment of the gene expression patterns of each cell line possible. Potentially, this direct co-culture system could be utilized by other investigators to examine isolated osteoblast or osteocyte cell populations from any mouse model (i.e. gene deletion, transgenic expression) to assess the effects these mutations on osteoblast-osteocyte communication.
In conclusion, we provide evidence that physical cell-cell contact of osteoblasts and osteocytes mutually enhances differentiation of each respective cell type. This suggests that cellular communication between these cells in the bone microenvironment, or at least within the bone remodeling unit, is vital to maintain tissue integrity and function. Identification of the specific factors and/or cellular adhesion molecules involved in the enhancement of differentiation of both osteoblasts and osteocytes when in direct contact will uncover new biology concerning how these bone cells communicate.
Acknowledgments
The mouse osteocyte cell line, IDG-SW3, was kindly provided by Dr. Lynda Bonewald (University of Missouri, Kansas City).
Contract grant sponsor: NIH, Contract Grant number: R01 AG004875. Work was also supported by the Mayo Kogod Center on Aging.
Footnotes
The authors have no conflict of interest to declare.
References
- Birmingham E, Niebur GL, McHugh PE, Shaw G, Barry FP, McNamara LM. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater. 2012;23:13–27. doi: 10.22203/ecm.v023a02. [DOI] [PubMed] [Google Scholar]
- Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buo AM, Stains JP. Gap junctional regulation of signal transduction in bone cells. FEBS letters. 2014;588:1315–21. doi: 10.1016/j.febslet.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Frontiers in bioscience: a journal and virtual library. 2007;12:1450–62. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TJ. Historically significant events in the discovery of RANK/RANKL/OPG. World journal of orthopedics. 2013;4:186–197. doi: 10.5312/wjo.v4.i4.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modder UI, Rudnik V, Liu G, Khosla S, Monroe DG. A DNA binding mutation in estrogen receptor-a leasts to supression of Wnt signaling via b-catenin destabilization in osteoblasts. J Cell Biochem. 2012;113:2248–2255. doi: 10.1002/jcb.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe DG, Hawse JR, Subramaniam M, Spelsberg TC. Retinoblastoma binding protein-1 (RBP1) is a Runx2 coactivator and promotes osteoblastic differentiation. BMC Musculoskelet Disord. 2010;11:104. doi: 10.1186/1471-2474-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–63. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20764–9. doi: 10.1073/pnas.0805133106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Bellido T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone. 2013;52:157–66. doi: 10.1016/j.bone.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Ren J, Wang XH, Wang GC, Wu JH. 17beta estradiol regulation of connexin 43-based gap junction and mechanosensitivity through classical estrogen receptor pathway in osteocyte-like MLO-Y4 cells. Bone. 2013;53:587–96. doi: 10.1016/j.bone.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Schiller PC, D’Ippolito G, Balkan W, Roos BA, Howard GA. Gap-junctional communication is required for the maturation process of osteoblastic cells in culture. Bone. 2001;28:362–9. doi: 10.1016/s8756-3282(00)00458-0. [DOI] [PubMed] [Google Scholar]
- Taylor AF, Saunders MM, Shingle DL, Cimbala JM, Zhou Z, Donahue HJ. Mechanically stimulated osteocytes regulate osteoblastic activity via gap junctions. AM J Physiol Cell Physiol. 2007;292:C545–C552. doi: 10.1152/ajpcell.00611.2005. [DOI] [PubMed] [Google Scholar]
- van Bezooijen RL, Roelen BAJ, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CWGM. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199:805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometeric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1-0-34.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26:2634–2646. doi: 10.1002/jbmr.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’ Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]


