Abstract
Phospholipid remodeling involves phospholipase activity to remove acyl chains and acyltransferases to replace acyl chains. We here describe the characterization of a lysophospholipid acyltransferase in the opportunistic fungal pathogen, Candida albicans. Expression of this gene, C.a. LPT1, complemented the lysophospholipid acyltransferase defect in Saccharomyces cerevisiae strains lacking the homologous LPT1 gene. In vitro, lysophospholipid acyltransferase activity in these strains showed acyl-CoA substrate specificity, as measured by apparent Vmax/Km ratios, to be linolenoyl-CoA > oleoyl-CoA > linoleoyl-CoA > stearoyl-CoA. To address the physiological importance of C.a. LPT1, homozygous deletion strains were generated. Lysophospholipid acyltransferase activity with amine containing lysophospholipids was dramatically reduced while lysophosphatidylinositol and lysophosphatidic acid esterification was not significantly lowered. However, C.a. LPT1 over-expression yielded an increased amount of lysophosphatidic acyltransferase activity, suggesting a role in de novo phospholipid synthesis. LPT1 deletion strains showed slightly slowed growth in standard liquid media but no phenotype in media containing three antifungals that target sterols. To assess the role of C.a. Lpt1 in phospholipid remodeling, an in vivo, pulse–chase assay utilizing polysorbitan palmitate and mass spectrometry was developed. Cellular phospholipid composition became atypical with the provision of palmitate and gradually returned to the typical distribution when palmitate was removed. Deletion of C.a. LPT1 showed a modest yet significant effect on remodeling under these conditions.
Keywords: Lysophospholipid acyltransferase, Candida albicans, Phospholipid remodeling, Mass spectrometry
1. Introduction
Membranes perform an array of physiological functions. These include providing a diffusion barrier, a milieu for cell surface proteins, a pliable surface for cell morphological changes, a dynamic surface for events such as endocytosis, and cell signaling precursors. Proper membrane function is associated with maintenance of membrane components in defined ratios [1]. Among these components are phospholipids (PLs) which contain a wealth of combinatorial diversity derived from an assortment of acyl chains and head groups. In Candida albicans, mode of growth, biofilm versus planktonic [2] or yeast versus hyphal [3] distinctly change the profile of PLs. Limiting the production of unsaturated fatty acids limited the transition from yeast to hyphal form [4,5]. In Saccharomyces cerevisiae, perturbation of the degree of acyl chain saturation in Golgi membranes alters protein quality control [6]. Mammalian cells with increased abundance of saturated acyl chains in endoplasmic reticulum (ER) PLs undergo the unfolded protein response [7,8].
PL composition can be established through a combination of de novo synthesis, hydrolysis, and remodeling. Acyl-CoA glycerol 3-phosphate acyltransferases (GPATs) and acyl-CoA lysophosphatidic acid acyltransferases (LPAATs) [EC 2.3.1.51] mediate the incorporation of acyl chains into the sn-1 and sn-2 positions, respectively, during de novo synthesis. Four genes that encode for LPAATs have been identified in S. cerevisiae: SLC1, LPT1 (aka ALE1, SLC4, LCA1), ICT1, and LOA1. Deletion of SLC1, which encodes for an ER and lipid droplet associated enzyme [9], reduces cellular LPAAT activity by 30–50% [10,11]. Concurrent deletion of SLC1 and LPT1 results in synthetic lethality [10,12,13], supporting the respective enzymes as the major LPAATs in S. cerevisiae. The two genes are not homologous. Deletion of LPT1 alone resulted in either a minor [10,14] or major [15] reduction in cellular LPAAT activity. Ict1p is a soluble LPAAT which has particular physiological importance during organic solvent stress [16]. Loa1p is a lipid droplet associated LPAAT whose deletion reduces TG synthesis without influencing PL synthesis, suggesting a specialized function in lipid droplet maturation [17].
After de novo synthesis, PL remodeling is involved in establishing PL composition [18]. In C. albicans, acyl chains with a total of 34 carbons predominate in phosphatidic acid (PA), a precursor in the synthetic pathway, while the abundance of 34 and 36 carbon acyl chains is similar in phosphatidylcholine (PC) and phosphatidylethanolamine (PE) [19]. In S. cerevisiae, cellular PC, PE, phosphatidylinositol (PI) and phosphatidylserine (PS) populations contain distinctly different acyl chain compositions [20] even though a common synthetic pathway is shared. That PI contains 53% saturated acyl chains and PC contains 16% is particularly striking. Remodeling involves removal of one or both acyl chains by a phospholipase(s) and replacement of the acyl chain(s) by an acyltransferase(s). Alteration of the sn-2 position has been more frequently observed than the sn-1.
In S. cerevisiae, a significant portion of acyl-CoA sn-1-acyl-2-lysophospholipid acyltransferase (LPLAT) activity is mediated by Lpt1p. This reaction is identical to LPAAT except lysoPLs besides lysoPA are esterified. Deletion of LPT1 dramatically reduces, if not abrogates, the in vitro esterification of lysoPC, lysoPE, lysophosphatidylglycerol (lysoPG), lysoPI, and lysoPS [10–13,21]. Slc1p may be responsible for the residual LPLAT activity toward lysoPI and lysoPS [12]. There are also other, seemingly more specialized, lysoPL acyltransferases. Psi1p mediates the incorporation of stearoyl-CoA into the sn-1 position of sn-2-acyl-1-lysoPI [22], Gup1p mediates the incorporation of a 26-carbon saturated acyl chain into the PI component of GPI anchors [23], and Taz1p mediates acyl-CoA independent lysoPL esterification with a role in cardiolipin remodeling [24]. Secreted phospholipases have also been found to catalyze lysophospholipase–transacylase activity [25].
The studies described here address the goal of extending the current understanding of PL metabolism in S. cerevisiae into C. albicans. C. albicans is an opportunistic fungal pathogen of particular concern to immunocompromised patients. Even with antifungal therapy, bloodstream infections can cause 35% mortality [26]. Systemic candidemia may be treated with three classes of antifungal drugs: echinocandins, azoles, and polyene antibiotics [27]. Echinocandins inhibit the synthesis of glucan in the yeast cell wall. Azoles inhibit ergosterol synthesis while polyene antibiotics bind plasma membrane sterol creating pores and causing cell leakage. The shared action of these drugs is compromising integrity of the cell periphery. This led us to investigate PL remodeling as a potential antifungal target. Better understanding PL metabolism in C. albicans may also enhance basic knowledge of eukaryotic cell physiology. The ability of C. albicans to produce polyunsaturated acyl-CoA species [28] provides for a complex array of PLs more similar to higher eukaryotes than S. cerevisiae which only produces monounsaturated acyl-CoA species.
2. Materials and methods
2.1. Materials
Chemicals were mainly obtained from Sigma or Fisher. Acyl-CoA and lysoPL species were obtained from Avanti Polar Lipids. Radioactive [14C]oleoyl-CoA and [14C]lysophosphatidylcholine were from Perkin Elmer.
2.2. Yeast strains and culturing
The S. cerevisiae lpt1Δ strain ODY545 (MATa ade2-1, can1-1, trp1-1, ura3-1, his3-11, 15, leu2-3, 112, lpt1Δ::URA) in the W303-1b background was described earlier [10]. The normal C. albicans strain SN152 (arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ URA3/ura3::imm434 IRO1/iro1::imm434) was kindly provided by S. Noble [29]. In SN152, both LPT1 alleles were replaced using PCR generated constructs with either C. albicans LEU2 or HIS1 autotrophic marker genes flanked by 50–60 nucleotides of C.a. LPT1 specific sequence. Molecular biology and yeast genetic procedures were performed according to conventional protocols [30]. Transformants were selected on SCD–Leu or SCD–His agar plates and genotyped by PCR. Primers used in PCR are in supplemental Table 1.
Yeast were cultured in YPD (2% (w/v) peptone, 1% (w/v) yeast extract, 2% (w/v) glucose), YPDT (YPD + 0.2% (v/v) Tween-40 (polysorbitan palmitate)), SCD (synthetic complete (MP Biomedicals) with 2% (w/v) dextrose) lacking certain amino acids (e.g. SCD–Leu), or RPMI-1640 (Mediatech) containing 165 mM MOPS, pH 7. Temperature was 37 °C unless otherwise noted. Doubling times were determined by diluting a stationary-phase culture to 1 × 106 cells/ml in 25 ml of YPD and culturing at 30 or 37 °C. Cell density, as Abs600, was measured every hour with extra measurements during log phase. Log phase routinely occurred between 3 and 8 h in culture. Doubling time = (Time2 – Time1) * log 2/log (Abs2/Abs1).
2.3. Generation of a C.a. LPT1 expression plasmid and transformation into S. cerevisiae
Site directed mutagenesis involving two rounds of PCR was used to change four CUG codons in C.a. LPT1 to UCG (universally serine). In the first round, five products were formed which contained, respectively, the start codon and mutation 1, 1 and 2, 2 and 3, 3 and 4, 4 the stop codon. The five products were then included in one reaction with the far 5′ and 3′ primers. This generated a 2123 bp product that was digested with SpeI, SacI and ligated next to the Gal 1, 10 promoter in pRS423GP which contains a 2 μ origin and HIS3 gene. pRS423GP/C.a. LPT1 (4 CUG to UCG) or empty vector was transformed into ODY545 with selection on SCD–His.
2.4. In vitro, microsomal lysophospholipid acyltransferase (LPLAT) assays
Microsomes from ODY545 transformed with pRS423GP/C.a. LPT1 or pRS423GP/– (vector only) were isolated as described previously [31]. LPLAT activity was measured by A) the incorporation of [1 – 14C]oleoyl CoA into the respective PL or B) the incorporation of [14C]palmitoyl lysoPC into PC. A) 50 μM [14C]oleoyl CoA (20,000 dpm/nmol), 50 μM lysoPL, 10 μg microsomal protein, and 100 mM Tris–HCl, pH 7.4 in a final volume of 100 μl were incubated at 37 °C for 4 min. and stopped by adding chloroform:methanol (2:1). Lipids were extracted, resolved using a TLC buffer system of 75:45:3:1 chloroform:methanol:acetic acid:water, and products quantified by scintillation counting. SigmaPlot software was used for nonlinear regression and curve fit analysis of kinetic data. Hill plots were used to calculate apparent Km and Vmax. B) 50 μM [14C]palmitoyl lysoPC (20,000 dpm/nmol), 4–120 μM of acyl-CoA, 10 μg of microsomal protein, and 100 mM Tris–HCl, pH 7.4 in a final volume of 100 μl were incubated at 37 °C for 4 min for α linolenoyl-CoA (18:3), linoleoyl-CoA (18:2), oleoyl-CoA(18:1) and 15 min for stearoyl-CoA (18:0). The products were analyzed as in (A) except the TLC buffer system was 57:33:7:3 chloroform:methanol: acetic acid:water.
2.5. Quantitative real time PCR
SN152 and lpt1Δ/lpt1Δ C. albicans were cultured in YPD into logarithmic phase. RNA was isolated using the hot phenol method. ThermoScript reverse transcriptase (Invitrogen) mediated oligo dT primed cDNA synthesis from 3 μg of RNA. Real-time PCR, using CFX Connect cycler (BioRad), included iTaq Universal mix (BioRad), 0.4 μM primers specific for C.a. SLC1 or ACT1, and serial dilutions of cDNA. Melting curves were uniform and efficiencies were similar (1% difference). Fold difference was calculated using the 2−ΔΔCt method. Statistical significance, compared to a fold difference of 1, was determined using a one sample, one tailed t-test.
2.6. Anti-fungal sensitivity assay
Growth in the presence of antifungal drugs was assayed as described previously [32]. Briefly, freshly plated C. albicans cultures were grown over night, diluted to %T530 = 75 – 80% in 0.9% (w/v) PBS, diluted 1:1000 in RPMI-1640 media, and added to 96-well plates containing a range of concentrations of amphoteracin B (0.1–1.0 μg/ml), fluconazole (0.1–1.0 μg/ml), or terbinafine (1–32 μg/ml). After 48 h of growth at 37 °C, cell density, as Abs530, was measured using a microplate reader. Minimal inhibitory concentration (MIC) was the drug dose required to decrease cell density by 80% compared to mock-treated controls.
2.7. In vivo phospholipid remodeling assay
Overnight, C. albicans cultures in YPD were diluted to approximately 5 × 105 cells/ml in 250 ml of fresh YPD and grown 1 h. 40 ml of the culture was transferred to a 250 ml flask and cultured for 4 h. To the remaining 210 ml of culture, Tween-40 (polysorbitan palmitate) was added to 0.2% (v/v) for a 4-hour pulse. Following two rinses in 0.5% (v/v) tergitol and water, YPD-cultured cells and 40 ml of YPDT-cultured cells (0 h chase) were frozen. The remaining YPDT cultured cells were rinsed, suspended in 170 ml YPD, split into 4 × 40 ml cultures and cultured (i.e. chased) for 15, 30, 60, and 120 min. Cells were rinsed and frozen.
Lipids were extracted as described previously [31]. Thawed cells were treated with 75 μl of 3 mg/ml lyticase, 0.02% (w/v) cyanide, 10% (v/v) glycerol for 15 min at 37 °C. 300 μl of isopropanol, 4 ml of hexane:isopropanol (2:1), and 4 ml 2 M KCl:methanol (4:1) were added sequentially with mixing. After centrifugation, the upper layer was removed and evaporated under N2 gas. Lipids were suspended in 2 ml chloroform, dried under N2 gas, and frozen prior to ESI-MS2 analysis. As described previously [2], cell lipids were analyzed using ESI-MS2 at the Kansas Lipidomics Research Center.
2.8. Statistical analysis of phospholipid compositions determined by ESI-MS2
Remodeling assay data involved a multivariate response with seven response variables (i.e. seven PL species based on number of double bonds). These represent proportions (p) of a whole and sum to 1. Such compositional data precluded the use of standard multivariate techniques such as MANOVA to the raw data. Previously, Aitchison [33] developed the concept of a log ratio transformation to allow multivariate techniques to be applied. The univariate analog of the log ratio transformation is the logit function transformation for a ratio p, 0 < p < 1, logit(p) = ln(p/(1 – p)). Logit transformation allowed the two-sample t-test to be employed as the transformed data are on the real line. For response vector (p1, p2, …, pD) with the sum of the variables being 1, the log ratio transformation leads to the transformed response vector (b1, b2, …, bd), d = D – 1 and where bi = Log(pi/pD). This transformed variable lies in unrestricted Euclidian space of dimension d. Accordingly, the data was transformed and MANOVA linear modeling was performed to compare the normal and lpt1Δ/lpt1Δ strains with respect to whether or not there was interaction between time and strain (i.e. whether the regression lines for the two strains vs time were parallel or not). Univariate tests on logits were also applied to better understand for what variables the strains differed over time. Statistical processing was performed using Minitab and SAS packages.
3. Results
3.1. Identification of a S.c. LPT1 homolog in the C. albicans genome
Given the effectiveness of antifungal drugs at targeting lipid synthesis and membrane lipids in C. albicans, we sought to characterize reactions in phospholipid (PL) metabolism as potential targets for novel antifungal drugs. The first reaction chosen was lysophospholipid acyltransferase (LPLAT) based on its putative role in phospholipid remodeling. Using the S. cerevisiae Lpt1p, an established LPLAT [10–15], as the query in a BLASTP [34] search of the C. albicans genome identified only one distinct homolog, orf 19.1881. This gene, hereafter referred to as C.a. LPT1, predicts a 642 amino acid protein which shares 42% identity with S.c. Lpt1p over the entire peptide. Both genes belong to the membrane bound O-acyltransferase (MBOAT) family [35].
3.2. Determining if C.a. LPT1 is a functional homolog
To determine if C.a. LPT1 is a functional homolog of S.c. LPT1, we expressed C.a. LPT1 in a S.c. lpt1Δ strain and assayed for restoration of LPLAT activity. Such expression required site directed mutagenesis to change four CUG codons at positions 13, 123, 152, and 230 in the C.a. LPT1 open reading frame to UCG codons. This addresses the difference in the genetic code between C. albicans (CUG: serine) and S. cerevisiae (CUG: leucine). In vitro, microsomal LPLAT assays in S.c. lpt1Δ strains transformed with the C.a. LPT1 expression vector showed robust esterification of all lysoPL species provided (Fig. 1). Even though three lysoPA acyltransferases (LPAATs) besides LPT1 exist in the S. cerevisiae genome [16,17,36], C.a. LPT1 expression still conferred a 20-fold increase in LPAAT activity. These data indicate that C.a. Lpt1p may participate in both de novo PL synthesis and PL remodeling.
Fig. 1.
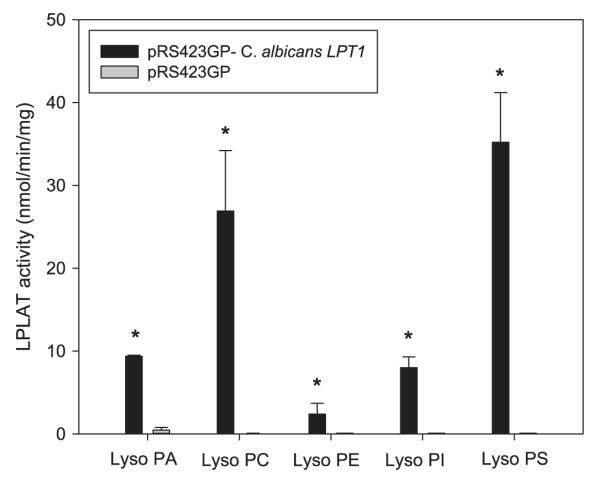
In vitro, microsomal LPLAT assays testing for C.a. LPT1 complementing S.c. lpt1Δ. LPLAT activity was measured in microsomes from S.c. lpt1Δ strains transformed with pRS423GP/C.a. LPT1 (4 CUG–UCG) or pRS423GP/– (vector only) 10 μg microsomes were incubated with 50 μM [14C]oleoyl CoA (20,000 dpm/nmol), 50 μM of lysoPA, lysoPC, lysoPE, lysoPI or lysoPS, and 100 mM Tris–HCl, pH 7.4 in 100 μl for 4 min. at 37 °C. After stopping the reactions, lipids were extracted, resolved using TLC, and quantified by scintillation counting. Data represent means ± standard deviation (n = 3) *p < 0.01.
3.3. Acyl-CoA substrate specificity of C.a. Lpt1p
Establishing the acyl-CoA specificity for C.a. Lpt1p is critical for understanding the physiological role of this enzyme in cellular PL metabolism. Given that the majority of PL species in eukaryotes from animals to S. cerevisiae contain unsaturated acyl-chains in the sn-2 position [37,38], we hypothesized that C.a. Lpt1p preferentially utilizes unsaturated acyl-CoA substrates. Due to the low background of lysoPC esterification in Fig. 1, this hypothesis was tested using the same microsomes as above. In vitro, LPLAT assays were performed using lysoPC and concentration series of four acyl-CoA substrates of identical length but with decreasing numbers of double bonds: α linolenoyl CoA (18:3Δ9,12,15), linoleoyl-CoA (18:2Δ9,12), oleoyl-CoA (18:1Δ9), and stearoyl-CoA (18:0) (Fig. 2). All occur naturally in C. albicans. Curve fitting of the plots identified a sigmoidal relationship between acyl-CoA concentration and LPLAT activity, suggesting cooperativity. Accordingly, Hill plots were used to determine the apparent Km and Vmax values (Table 1). Based on the ratio of Vmax/Km, the substrate preference was α linolenoyl-CoA > oleoyl-CoA > linoleoyl-CoA > stearoyl-CoA. While the Km values were similar, the Vmax values differentiated the use of the acyl-CoA species. Parallel assays with the substrate arachidonyl-CoA (20:4Δ5,8,11,14) showed no activity (data not shown).
Fig. 2.
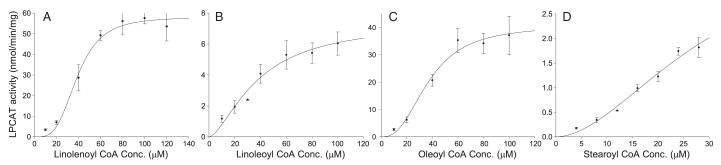
In vitro, microsomal LPLAT assays testing for C.a. LPT1 acyl-CoA specificity. LPLAT activity was measured in microsomes from S.c. lpt1Δ strains transformed with pRS423GP/C.a. LPT1 (4 CUG–UCG) or pRS423GP/– (vector only). 10 μg of microsomes was incubated with 50 μM [14C]palmitoyl lysophosphatidylcholine, 4–120 μM of acyl-CoA, and 100 mM Tris–HCl, pH 7.4 in 100 μl for 4 min. (15 min. for stearoyl-CoA) at 37 °C. (A) α linolenoyl CoA (18:3), (B) linoleoyl CoA (18:2), (C) oleoyl CoA( 18:1) and (D) stearoyl CoA (18:0). Activity measured in microsomes from vector-only strains was considered background and subtracted. Data represent means ± standard error (n ≥ 3).
Table 1.
C.a. Lpt1 kinetic parameters. Kinetic data from Fig. 2 were used to calculate apparent Km and Vmax.
| Acyl-CoA species | Km |
Vmax |
Vmax/Km |
|---|---|---|---|
| μM | nmol/min/mg | ml min−1 mg−1 | |
| α Linolenoyl-CoA (18:3) | 38.8 | 58.2 | 1.50 |
| Linoleoyl-CoA (18:2) | 38.3 | 7.4 | 0.19 |
| Oleoyl-CoA (18:1) | 36.6 | 39.4 | 1.08 |
| Stearoyl-CoA (18:0) | 29.4 | 4.0 | 0.14 |
3.4. Generation of LPT1 homozygous deletion strains and the affect on LPLAT activity
To further address the physiological role of C.a. Lpt1p, we generated deletion-mutant strains with one or both LPT1 alleles replaced with autotrophic marker genes. PCR was used to generate DNA strands that targeted replacement of the LPT1 alleles through homologous recombination upon transformation into a normal (SN152) C. albicans strain (Fig. 3A). Two heterozygous, LPT1-deletion strains, OMY5 and OMY10 were initially generated using the LEU2 autotrophic gene. These were subsequently used in parallel to generate homozygous LPT1-deletion strains, OMY15 and 20, by replacing the remaining LPT1 allele with the HIS1 gene (Fig. 3B). All subsequent phenotypic analysis of the lpt1Δ/lpt1Δ genotype involved these independently generated strains to address the possibility of spurious mutations.
Fig. 3.

Generation of homozygous, C.a. LPT1 deletion strains OMY15, OMY20. C. albicans strain SN152 (29) was transformed with a PCR-generated construct containing C.a. LEU2 flanked by ~60 nucleotides of C.a. LPT1 sequence (A). Transformants were selected on SC–Leu media and genotyped by PCR using oligo pairs lp1, lp2 or lp1, le2 (B). Subsequently, LPT1/lpt1Δ::LEU2 strains were transformed with a similar PCR-generated construct containing C.a. HIS1 with PCR genotyping using a lp1, hi2 oligo pair.
Removal of LPT1 from the genome allowed examining the contribution of Lpt1p to cellular LPLAT activity. We hypothesized that C.a. Lpt1p mediates the majority of cellular LPLAT activity and has broad lysoPL specificity based on the precedent of S.c. Lpt1p [10]. Providing an array of lysoPL substrates (Fig. 4) showed that the esterification of lysoPC, lysoPE, and lysoPS was significantly reduced upon deletion of LPT1. LysoPI esterification, already at low levels in the normal strains, was unaffected. LysoPA esterification was reduced but not to statistical significance. The C. albicans genome contains one distinct homolog, orf19.250, of the S.c. SLC1 gene. It is possible that this homolog mediates enhanced LPAAT activity in the absence of LPT1. In fact, quantitative real time PCR showed orf19.250 RNA to be 20% more abundant (fold difference = 1.21 ± 0.14, p < 0.05) in lpt1Δ/lpt1Δ compared to SN152. This apparent up-regulation may mask a significant reduction in LPAAT activity upon C.a. LPT1 deletion.
Fig. 4.
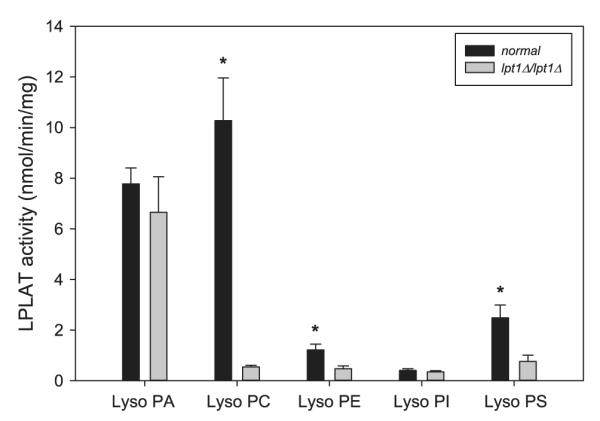
Lysophospholipid substrate specificity of C.a. Lpt1. LPLAT activity was determined in microsomes from SN152 (black bars) and lpt1Δ/lpt1Δ (gray bars) C. albicans strains using [14C]oleoyl-CoA and lysoPA, lysoPC, lysoPE, lysoPI or lysoPS as acyl acceptors as described in Fig. 1. Data represent means ± standard error (n = 7) *p < 0.05.
Why the relative pattern of lysoPL utilization differs between C.a. LPT1 expression in S.c. lpt1Δ (Fig. 1) and the C.a. LPT1 expression in normal C. albicans (Fig. 4) is not clear. Particularly striking is the difference in lysoPS esterification relative to lysoPC in the two environments. Also interesting is that normal C. albicans microsomes esterified lysoPC significantly faster than all other lysoPL species, even lysoPA. This in contrast to normal S. cerevisiae microsomes which esterify lysoPA and lysoPC at similar rates [10].
3.5. Effect of LPT1 deletion on growth rate and sensitivity to antifungals
Limiting the ability of cells to esterify lysoPLs should limit PL remodeling and potentially affect the maintenance of membrane composition. In such cells, the rate of growth may be slowed. To address this possibility, normal (SN152) and lpt1Δ/lpt1Δ C. albicans strains were cultured in liquid YPD media at two temperatures, 37 and 30 °C. Cell density was monitored using spectrophotometry. While deletion of LPT1 seems to reduce the growth rate during the log phase in YPD at 37 °C (Fig. 5), the difference in log phase doubling times, 1.19 h for normal and 1.25 h for lpt1Δ/lpt1Δ, stopped just short of statistical significance. Similar analysis at 30 °C showed a significant (p < 0.05) difference between log phase doubling times of normal (1.14 h) and lpt1Δ/lpt1Δ (1.24 h) C. albicans (data not shown). Therefore, Lpt1p function is necessary for optimal growth in the conditions tested.
Fig. 5.
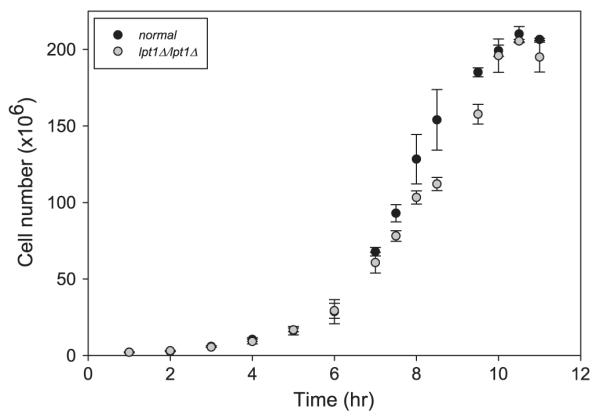
Growth rate of normal and lpt1Δ/lpt1Δ C. albicans. Stationary cultures of C. albicans strains SN152, OMY15, and OMY20 were diluted to 1 × 106 cells/ml in 25 ml of YPD and shaken at 37 °C. Cell density was calculated from Abs600. Data represent means ± standard deviation (n ≥ 3).
Two targets for clinically utilized antifungal drugs are sterols in the plasma membrane (e.g. amphoteracin B) and sterol synthesis (e.g. fluconazole) [27]. Since both sterols and PLs influence membrane dynamics [39], it is conceivable that altered PL compositions will affect the necessary levels for sterols in membranes and therefore impact sensitivity to these drugs. To test this hypothesis, the sensitivity of normal and lpt1Δ/lpt1Δ strains to amphoteracin B, fluconazole, and terbinafine (an inhibitor of sterol synthesis later in the pathway than fluconazole) was assayed using the broth microdilution method [32]. Drug concentrations between the standard two-fold dilutions were included so to detect subtle differences in sensitivity. Even so, the average minimum inhibitory concentration (MIC) for the three drugs was, respectively, 0.9, 0.5, and 28 μg/ml regardless of genotype. Under the growth conditions utilized, limiting lysoPL esterification does not affect sensitivity to the antifungals tested.
3.6. In vivo phospholipid remodeling measured by pulse/chase and mass spectrometry
If C.a. Lpt1p has a physiological role in PL remodeling then the homozygous deletion strains should have a diminished capacity for this process when assayed in vivo. Accordingly, a novel PL remodeling assay was developed using a Tween-40 (polysorbitan palmitate) pulse–chase format (Fig. 6). We hypothesized that providing a fatty acid species that is saturated and shorter (16 carbons vs. 18 carbons) than the majority of acyl chains in C. albicans PLs [4,28] would steer cellular PL synthesis toward atypical composition. Chasing in media lacking supplemental fatty acids would allow remodeling of PLs back toward typical composition. Slowed remodeling was expected to result in prolonged retention of saturated acyl chains in PLs. Electrospray ionization tandem mass spectroscopy (ESI-MS2) was chosen for maximal resolution of PL species. To allow manageable statistical analysis of changes in the complex array of cellular PLs, we initially grouped PLs based solely on the number of double bonds in the acyl chains.
Fig. 6.
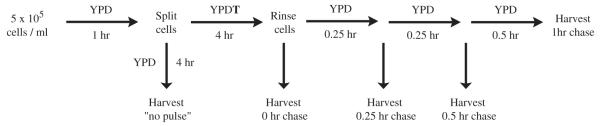
Schematic of the in vivo, pulse/chase, phospholipid remodeling assay design.
Normal C. albicans were first analyzed to validate the assay. The abundance of PLs containing zero or one double bond markedly increased when palmitate was provided in the media (Fig. 7; light gray vs. black bars). This occurred at the expense of PLs with two or more double bonds. Six is the maximum number of double bonds in a C. albicans PL given that linolenoyl-CoA (18:3) is the most unsaturated species this yeast produces. After 1 h in the chase media, these values returned to those for cells grown in media without fatty acids (Fig. 7; dark gray vs. black bars). Given the distinct ability for Lpt1 to esterify lysoPC, remodeling in PC specifically was also analyzed (supplemental Fig. 1). Aside from a distribution of acyl chains skewed toward having more double bonds, PC species changes after the pulse and at the end of the chase were similar to those observed when all PLs were analyzed together.
Fig. 7.
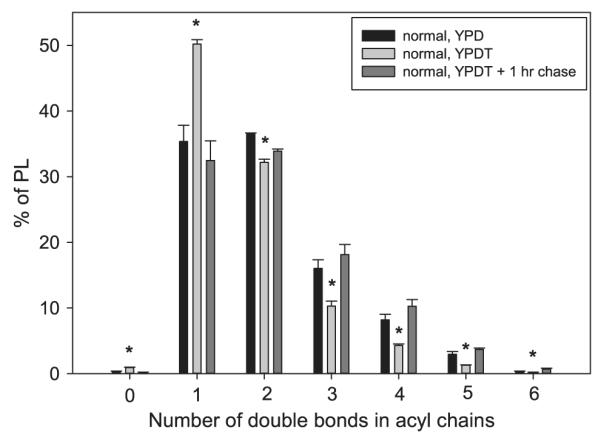
Phospholipid composition at the three extremes of the pulse–chase assay. Dense cultures of normal strain SN152 were diluted to 5 × 105 cells/ml in YPD and assayed for PL remodeling as described in Fig. 6 and Materials and methods. To show the efficacy of the pulse and the chase, the YPD (no pulse; black bars), YPDT — 0 h chase (pulse; light gray), and YPDT — 1 h chase (pulse and longest chase; dark gray) data are shown. Lipids were extracted from the yeast and PL composition determined by ESI-MS2. PL species were grouped based on the number of double bonds in the acyl chains, regardless of head group, and expressed as percent of all PL. Data represent means ± standard deviation (n = 3). Asterisks indicate significant statistical difference between the YPD and YPDT — 0 h chase (p < 0.05).
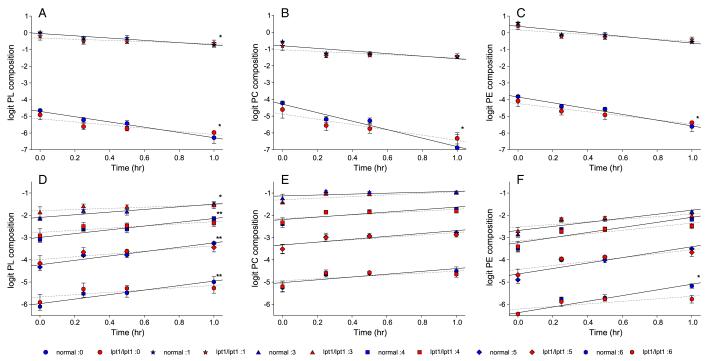
The rate of PL remodeling was established by monitoring the change in composition over the time of the chase. Since percent composition data are constrained between 0 and 1 (or 0–100%), the data were logit transformed [33]. Larger percent compositions yield larger logit values. Initially, a multiple analysis of variance (MANOVA) was performed to determined if during the chase, the changes observed in all PL species, grouped by number of double bonds, differed significantly between normal and lpt1Δ/lpt1Δ strains. The calculated p-value was 0.055. Subsequently, univariate regression analysis analyzed one PL group at a time. As predicted from Fig. 7, the slopes of the lines during the chase were negative for species with zero and one double bond (Fig. 8A) and positive for species with three or more double bonds (Fig. 8D). PL species with two double bonds were not included since those amounts changed very little. The rate of decrease of PLs containing zero and one double bond was significantly (p < 0.05) less in lp1Δ/lpt1Δ compared to normal yeast (Fig. 8A). Additionally, the corresponding rate of increase in PLs with three double bonds was significantly flattened upon deletion of LPT1 (Fig. 8D). Remodeling of PLs with four, five, and six double bonds was also significantly slowed if the thresh hold for significance was raised to p < 0.10. This data supports C.a. Lpt1 having a physiological role in PL remodeling under the conditions utilized.
Fig. 8.
Phospholipid remodeling during the chase in normal and lpt1Δ/lpt1Δ strains. Cultures of normal strain SN152 and lpt1Δ/lpt1Δ strains (OMY15, 20) were assayed for PL remodeling as described in Figs.6, 7 and Materials and methods. Lipids were extracted and PL composition determined by ESI-MS2. PL species were grouped based on the number of double bonds in the acyl chains. Percent compositions were transformed to logit values (logit = ln (p/(1 – p)). Number of double bonds (symbol): 0 (circle), 1 (star), 3 (triangle), 4 (square), 5 (diamond), and 6 (hexagon). Blue symbols are SN152 and red symbols are OMY 15, 20 data, representing means ± standard deviation (n = 3). Regression analysis was performed to compare the slopes of normal (solid lines) vs. lpt1Δ/lpt1Δ data (dashed lines) *p < 0.05; **p < 0.10. (A–C) PL, PC, and PE species, respectively, decreasing during the chase. (D–F) PL, PC, and PE species, respectively, increasing during the chase.
Due to the robust esterification of lysoPC observed in Figs. 1 and 4, the change in PC composition during the chase was also examined. The calculated MANOVA p-value was 0.16. However, remodeling of PC with two saturated acyl chains was significantly slowed (Fig. 8B). The differences between normal and lpt1Δ/lpt1Δ for the other PC species were not significant (Fig. 8B, E). MANOVA regarding PE species changes during the chase identified a significant difference (p = 0.02) between normal and lpt1Δ/lpt1Δ. Univariate regression found that remodeling of PE with zero or six double bonds was significantly slowed (Fig. 8C and F). Analysis of PS, PI, and PA species showed no significant compositional differences between normal and lpt1Δ/lpt1Δ during the chase (data not shown).
4. Discussion
Esterifying lysoPLs is part of de novo PL and triglyceride synthesis, PL remodeling, scavenging lysoPLs produced by intracellular phospholipases, and utilizing lysoPLs produced by extracellular phospholipases and imported [18]. Here, we biochemically characterized the first broad-substrate LPLAT in C. albicans. C.a. LPT1 belongs to the membrane bound o-acyltransferase (MBOAT) gene family [35] which has five members in the S. cerevisiae genome: LPT1, ARE1, ARE2, GUP1, and GUP2. Higher eukaryotes such as mice have 11 members [40]. C.a. Lpt1p has seven [41], eight [42], or 13 [43] predicted transmembrane domains, making purification of active enzyme challenging. Therefore, a gene-activity relationship was established using expression in a low-background system and deletion of the gene with subsequent LPLAT activity measurement. Results from both types of experiments support that C.a. Lpt1p has a major role in the lysoPL esterification that is likely part of PL remodeling and a smaller role in lysoPA esterification that is part of PL synthesis.
Regarding the preference of acyl-CoA species, C.a. Lpt1p showed a similarity with S.c. Lpt1p by using monounsaturated acyl-CoA (e.g. 18:1Δ9) at a much higher Vmax than the saturated acyl-CoA [10,15]. However, the presence of a second double bond at the Δ12 position in linoleoyl-CoA greatly reduced the Vmax of C.a. Lpt1p. In contrast, S.c. Lpt1p used 18:2Δ9,12 equally well as oleoyl-CoA [21]. Including a third double bond at Δ15 returned C.a. Lpt1p activity to levels similar to the monounsaturated acyl-CoA. The importance of the number and positions of double bonds in 18 carbon acyl-CoA substrates on LPLAT activity has also observed with mouse and human LPCAT3. From single substrate concentration assays, mouse LPCAT3 has acyl-CoA preference of 18:2 ⪢ 18:3 > 18:1. Mouse LPCAT3 preferably utilized arachidonyl-CoA over any 18 carbon acyl-CoA [44], while C.a. Lpt1p showed no activity with arachidonyl-CoA. Substrate concentration series showed human LPCAT3 to have a distinctly lower apparent Km and higher Vmax for 18:1 acyl-CoA than 18:3 [45]. 18:2 was not measured. For C.a. Lpt1p, acyl-CoA species with one to three double bonds bind with similar affinity but may have different orientations in the active site, leading to different turn over rates.
The ability for remodeling to introduce acyl chains of a different structure into the sn-2 position than introduced during de novo synthesis suggests the corresponding enzymes have different substrate specificity. However, all LPAATs in S. cerevisiae, Slc1 [46], Ict1p [16], Loa1p [19] and Lpt1 [10], none of which are homologous to each other, show a preference for unsaturated over saturated acyl-CoA substrates in vitro. Whether this is also true in C. albicans awaits kinetic characterization of additional acyltransferases. BLASTP analysis of the C. albicans genome identifies one SLC1 homolog, one LOA1 homolog, and four S.c. ICT1 homologs, suggesting the presence of other LPAATs in the C. albicans genome. The physiological need for this apparent redundancy remains to be determined.
Despite this apparent redundancy, Lpt1p seems to have a distinct physiological role given the increase, albeit modest, in doubling times in C.a. lpt1Δ/lpt1Δ strains. Slowed growth in C. albicans due to disrupted plasma membrane composition and organization occurs upon deletion of SUR7 which encodes for a putative membrane organizing protein. Diminished endocytosis and inappropriate cell wall construction was observed [47]. Doubling times were also increased in C. albicans strains harboring deletions of GUP1, another member of the MBOAT gene family. Gup1p has been implicated in GPI anchor remodeling and its deletion alters ergosterol distribution among membranes and hyphal morphogenesis [48]. Hyphal morphogenesis and direct virulence analysis of lpt1Δ/lpt1Δ strains were not reported here since those experiments are part of a separate study examining the affects of a series of gene-deletions on PL metabolism. Altering membrane PL composition may mirror the affect that altering sterol composition has on lateral diffusion of the C. albicans drug resistance protein-1 (Cdr1p), in artificial membranes [49].
Previous ESI-MS2 analysis of S. cerevisiae lpt1Δ strains found little to no affect on cellular PL composition [12]. These yeast were grown into exponential phase in YP + 2% galactose media. Based on this precedent, we sought a more dynamic assay for detecting altered PL metabolism in C.a. lpt1Δ/lpt1Δ strains. One such in vivo, pulse–chase assay has been developed previously in S. cerevisiae [50] using a custom synthesized, short-chain PC species, (methyl-13C)3-diC8PC. After a five minute pulse, the abundance of (methyl-13C)3-8:0–16:1-PC and (methyl-13C)3-8:0–16:0-PC, intermediates of remodeling at the sn-2 position, were found to decrease fairly linearly for 60 min. This matches our finding of steady recovery of PL composition during a 60-minute chase. While that study did not analyze remodeling in a. lpt1Δ strain, it did generate a S. cerevisiae pem1Δpem2Δale1Δ strain. Pem1p and Pem2p (aka Cho2p, Opi3p) mediate the methylation of PE to for PC so that their deletion causes choline auxotrophy. When diC8PC was the sole choline source, pem1Δpem2Δale1Δ yeast showed reduced cell density at stationary phase compared to pem1Δpem2Δ strains. Therefore, S.c. Ale1p (Lpt1p) was implicated in the utilization of the exogenous, short-chain PC and by extension, implicated in PL remodeling.
Described here is a novel pulse–chase assay using an exogenously provided, physiological fatty acid, palmitate. ESI-MS2 analysis showed that deleting LPT1 from the C. albicans genome significantly slowed the remodeling of PL with two saturated acyl chains. Such PLs are normally in low abundance, allowing their relative cellular composition to increase three-fold during the pulse. This large fold-change allowed the greatest relative recovery during the chase and accordingly, the greatest sensitivity. However, the importance of Lpt1 was also evident in the remodeling of major constituents of cell membranes, namely, PLs containing one and three double bonds. Flux through PLs with two double bonds may have differed with genotype but this species is at the fulcrum of PL remodeling under these conditions, lying between PLs with few and many double bonds.
PL species were also analyzed by head-group. Based on the preference of C.a. Lpt1 for lysoPC displayed in in vitro assays, slowed PC remodeling was expected to be evident in lpt1Δ/lpt1Δ strains. However, only the effect on the disaturated species was observed. While it is tempting to note that PC contains relatively few saturated acyl chains so that its composition was less perturbed than other PL species during the pulse, lessening the possible change during the chase, PC composition changed on a similar scale to all PL during the pulse and chase. Reduced PC remodeling in lpt1Δ/lpt1Δ strains during the chase may be masked by on-going de novo synthesis and continued cell proliferation. This same explanation pertains to the modest affect deleting LPT1 had on PE remodeling. The absence of a PS remodeling phenotype, even though lysoPS was shown to be a substrate for C.a. Lpt1p, may be due to the limited abundance of lysoPS in cells. Our ESI-MS2 analysis only detected lysoPC and lysoPE species. In summary, our data support that C.a. Lpt1 is a broad-substrate LPLAT with a physiological role in PL composition.
Supplementary Material
Acknowledgements
We thank Ruth Welti and Mary Roth for the mass spectrometry analysis performed at the Kansas Lipidomics Research Center (KLRC). Instrument acquisition and method development at KLRC were supported by NSF grants MCB 0455318, MCB 0920663, DBI 0521587, DBI 1228622, Kansas INBRE (NIH Grant P20 RR16475 from the INBRE program of the National Center for Research Resources), NSF EPSCoR grant EPS-0236913, Kansas Technology Enterprise Corporation, and Kansas State University. We thank Susan Noble for providing strain SN152, Tom Edlind for the C.a. HIS1 plasmid, and undergraduates David Dadiomov, Amada Ismail, Ibrahim Haidar-Ahmad, Walaa Tout and Lama Wehbi for their assistance. A.A. was supported by an Undergraduate Fellowship sponsored by the University of Michigan — Dearborn Office of Research and Sponsored Programs. Funding was also provided as a seed grant from the same office to P.O.
Abbreviations
- CoA
coenzyme A
- ESI-MS2
electrospray ionization tandem mass spectrometry
- ER
endoplasmic reticulum
- LPAAT
lysophosphatidic acid acyltransferase
- LPLAT
lysophospholipid acyltransferase
- PA
phosphatidic acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- PL
phospholipid
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbalip.2013.12.015.
References
- [1].Spector AA, Yorek MA. Membrane lipid composition and cellular function. J. Lipid Res. 1985;26:1015–1035. [PubMed] [Google Scholar]
- [2].Lattif AA, Mukherjee PK, Chandra J, Roth MR, Welti R, Rouabhia M, Ghannoum MA. Lipidomics of Candida albicans biofilms reveals phase-dependent production of phospholipid molecular classes and role for lipid rafts in biofilm formation. Microbiology. 2011;157:3232–3242. doi: 10.1099/mic.0.051086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mahmoudabadi AZ, Drucker DB. Comparison of polar lipids from yeast and mycelial forms of Canadida albicans and Candida dubliniensis. Mycoses. 2006;49:18–22. doi: 10.1111/j.1439-0507.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- [4].Krishnamurthy S, Plain A, Albert J, Prasad T, Prasad R, Ernst JF. Dosage-dependent functions of fatty acid desaturase Ole1p in growth and morphogenesis of Candida albicans. Microbiology. 2004;150:1991–2003. doi: 10.1099/mic.0.27029-0. [DOI] [PubMed] [Google Scholar]
- [5].Xu D, Sillaots S, Davison J, Hu W, Jiang B, Kauffman S, Martel N, Ocampo P, Oh C, Trosok S, Veillette K, Wang H, Yang M, Zhang L, Becker J, Martin CE, Roemer T. Chemical genetic profiling and characterization of small-molecule compounds that affect the biosynthesis of unsaturated fatty acids in Candida albicans. J. Biol. Chem. 2009;284:19754–19764. doi: 10.1074/jbc.M109.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pineau L, Bonifait L, Berjeaud J-M, Alimardani-Theuil P, Berges T, Ferreira T. A lipid-mediated quality control process in the Golgi apparatus in yeast. Mol. Biol. Cell. 2008;19:807–821. doi: 10.1091/mbc.E07-06-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ariyama H, Kono N, Matsuda S, Inoue T, Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J. Biol. Chem. 2010;285:22027–22035. doi: 10.1074/jbc.M110.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. U. S. A. 2013;110:4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Athenstaedt K, Daum G. Biosynthesis of phosphatidic acid in lipid particles and endoplasmic reticulum of Saccharomyces cerevisiae. J. Bacteriol. 1997;179:7611–7616. doi: 10.1128/jb.179.24.7611-7616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jain S, Stanford N, Bhagwat N, Seiler B, Costanzo M, Boone C, Oelkers P. Identification of a novel lysophospholipid acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:30562–30569. doi: 10.1074/jbc.M706326200. [DOI] [PubMed] [Google Scholar]
- [11].Chen Q, Kazachkov M, Zheng Z, Zou J. The yeast acylglycerol acyltransferase LCA1 is a key component of lands cycle for phosphatidylcholine turnover. FEBS Lett. 2007;581:5511–5516. doi: 10.1016/j.febslet.2007.10.061. [DOI] [PubMed] [Google Scholar]
- [12].Benghezal M, Roubaty C, Veepuri V, Knudsen J, Conzelmann A. SLC1 and SLC4 encode partially redundant acyl-coenzyme A 1-acylglycerol-3-phosphate O acyltransferases of budding yeast. J. Biol. Chem. 2007;282:30845–30855. doi: 10.1074/jbc.M702719200. [DOI] [PubMed] [Google Scholar]
- [13].Tamaki H, Shimada A, Ito Y, Ohya M, Takase J, Miyashita M, Miyagawa H, Nozaki H, Nakayama R, Kumagai H. LPT1 encodes a membrane-bound O-acyltransferase involved in the acylation of lysophospholipids in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:34288–34298. doi: 10.1074/jbc.M704509200. [DOI] [PubMed] [Google Scholar]
- [14].Stahl U, Stalberg K, Stymne S, Ronne H. A family of eukaryotic lysophospholipid acyltransferases with broad specificity. FEBS Lett. 2008;582:305–309. doi: 10.1016/j.febslet.2007.12.020. [DOI] [PubMed] [Google Scholar]
- [15].Riekhof WR, Wu J, Jones JL, Voelker DR. Identification and characterization of the major lysophosphatidylethanolamine acyltransferase in Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:28344–28352. doi: 10.1074/jbc.M705256200. [DOI] [PubMed] [Google Scholar]
- [16].Ghosh AK, Ramakrishnan G, Rajasekharan R. YLR099C (ICT1) encodes a soluble acyl-CoA-dependent lysophosphatidic acid acyltransferase responsible for enhanced phospholipid synthesis on organic solvent stress in Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:9768–9775. doi: 10.1074/jbc.M708418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ayciriex S, Le Guédard M, Camougrand N, Velours G, Schoene M, Leone S, Wattelet-Boyer V, Dupuy J-W, Shevchenko A, Schmitter J-M, Lessire R, Bessoule J-J, Testet E. YPR139c/LOA1 encodes a novel lysophosphatidic acid acyltransferase associated with lipid droplets and involved in TAG homeostasis. Mol. Biol. Cell. 2012;23:233–246. doi: 10.1091/mbc.E11-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lands WEM. Stories about acyl chains. Biochim. Biophys. Acta. 2000;1483:1–14. doi: 10.1016/s1388-1981(99)00177-8. [DOI] [PubMed] [Google Scholar]
- [19].Singh A, Prasad T, Kapoor K, Mandal A, Roth M, Welti MR, Prasad R. Phospholipidome of Candida: each species of Candida has distinctive phospholipid molecular species. OMICS. 2010;14:665–677. doi: 10.1089/omi.2010.0041. [DOI] [PubMed] [Google Scholar]
- [20].Schneiter R, Brugger B, Sandhoff R, Zellnig G, Leber A, Lampl M, Athenstaedt K, Hrastnik C, Eder S, Daum G, Paltauf F, Wieland FT, Kohlwein SD. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 1999;146:741–754. doi: 10.1083/jcb.146.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Riekhof WR, Wu J, Gijon MA, Zarini S, Murphy RC, Voelker DR. Lysophosphatidylcholine metabolism in Saccharomyces cerevisiae: the role of P-type ATPases in transport and a broad specificity acyltransferase in acylation. J. Biol. Chem. 2007;282:36853–36861. doi: 10.1074/jbc.M706718200. [DOI] [PubMed] [Google Scholar]
- [22].Le Guédard M, Bessoule JJ, Boyer V, Ayciriex S, Velours G, Kulik W, Ejsing CS, Shevchenko A, Coulon D, Lessire R, Testet E. PSI1 is responsible for the stearic acid enrichment that is characteristic of phosphatidylinositol in yeast. FEBS J. 2009;276:6412–6424. doi: 10.1111/j.1742-4658.2009.07355.x. [DOI] [PubMed] [Google Scholar]
- [23].Bosson R, Jaquenoud M, Conzelmann A. GUP1 of Saccharomyces cerevisiae encodes an O-acyltransferase involved in remodeling of the GPI anchor. Mol. Biol. Cell. 2006;17:2636–2645. doi: 10.1091/mbc.E06-02-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Testet E, Laroche-Traineau J, Noubhani A, Coulon D, Bunoust O, Camougrand N, Manon S, Lessire R, Bessoule JJ. Ypr140wp, ‘the yeast tafazzin’, displays a mitochondrial lysophosphatidylcholine (lyso-PC) acyltransferase activity related to triacylglycerol and mitochondrial lipid synthesis. Biochem. J. 2005;387:617–626. doi: 10.1042/BJ20041491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Takahashi M, Banno Y, Shikano Y, Mori S, Nozawa Y. Purification and characterization of lysophospholipase-transacylase of pathogenic fungus Candida albicans. Biochim. Biophys. Acta. 1991;1082:161–169. doi: 10.1016/0005-2760(91)90190-s. [DOI] [PubMed] [Google Scholar]
- [26].Klevay MJ, Ernst EJ, Hollanbaugh JL, Miller JG, Pfaller MA, Diekema DJ. Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn. Microbiol. Infect. Dis. 2008;60:273–277. doi: 10.1016/j.diagmicrobio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- [27].Pappas PG, Kauffman CA, Andes D, Benjamin DK, Jr., Calandra TF, Edwards JE, Jr., Filler SG, Fisher JF, Kullberg B-J, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Murayama SY, Negishi Y, Umeyama T, Kaneko A, Oura T, Niimi M, Ubukata K, Kajiwara S. Construction and functional analysis of fatty acid desaturase gene disruptants in Candida albicans. Microbiology. 2006;152:1551–1558. doi: 10.1099/mic.0.28751-0. [DOI] [PubMed] [Google Scholar]
- [29].Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. John Wiley & Sons; New York: 1998. [Google Scholar]
- [31].Oelkers P, Cromley D, Padamsee M, Billheimer JT, Sturley SL. The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J. Biol. Chem. 2002;277:8877–8881. doi: 10.1074/jbc.M111646200. [DOI] [PubMed] [Google Scholar]
- [32].CLSI . Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, third edition, CLSI document M27-A3. CLSI; Wayne, PA: 2008. [Google Scholar]
- [33].Aitchison J. The statistical analysis of compositional data, reprint ed. Blackburn Press; Caldwell, N.J: 2003. [Google Scholar]
- [34].Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for Wnt signaling. Trends Biochem. Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- [36].Nagiec MM, Wells GB, Lester RL, Dickson RC. A suppressor gene that enables Saccharomyces cerevisiae to grow without making sphingolipids encodes a protein that resembles an Escherichia coli fatty acyltransferase. J. Biol. Chem. 1993;268:22156–22163. [PubMed] [Google Scholar]
- [37].Schmitz MGJ, Renooij W. Phospholipids from rat, human, and canine gastric mucosa. Gastroenterology. 1990;99:1292–1296. doi: 10.1016/0016-5085(90)91152-v. [DOI] [PubMed] [Google Scholar]
- [38].Wagner S, Paltauf F. Generation of glycerophospholipids molecular species in the yeast Saccharomyces cerevisiae. Fatty acid pattern of phospholipid classes and selective acyl turnover at sn-1 and sn-2 positions. Yeast. 1994;10:1429–1437. doi: 10.1002/yea.320101106. [DOI] [PubMed] [Google Scholar]
- [39].Rodriguez RJ, Low C, Bottema CDK, Parks LW. Multiple functions for sterols in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1985;837:336–343. doi: 10.1016/0005-2760(85)90057-8. [DOI] [PubMed] [Google Scholar]
- [40].Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- [41].Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- [43].Pagac M, de la Mora H. Vazquez, Duperrex C, Roubaty C, Vionnet C, Conzelmann A. Topology of 1-acyl-sn-glycerol-3-phosphate acyltransferases SLC1 and ALE1 and related membrane-bound O-acyltransferases (MBOATs) of Saccharomyces cerevisiae. J. Biol. Chem. 2011;286:36438–36447. doi: 10.1074/jbc.M111.256511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hishikawa D, Shindou H, Kobayashi S, Nakanishi H, Taguchi R, Shimizu T. Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2830–2835. doi: 10.1073/pnas.0712245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jain S, Zhang X, Khandelwal PJ, Saunders AJ, Cummings BS, Oelkers P. Characterization of human lysophospholipid acyltransferase 3. J. Lipid Res. 2009;50:1563–1570. doi: 10.1194/jlr.M800398-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shui G, Guan XL, Gopalakrishnan P, Xue Y, Goh JSY, Yang H, Wenk MR. Characterization of substrate preference for Slc1p and Cst26p in Saccharomyces cerevisiae using lipidomic approaches and an LPAAT activity assay. PloS one. 2010;5:e11956. doi: 10.1371/journal.pone.0011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Alvarez FJ, Douglas LM, Rosebrock A, Konopka JB. The Sur7 protein regulates plasma membrane organization and prevents intracellular cell wall growth in Candida albicans. Mol. Biol. Cell. 2008;19:5214–5225. doi: 10.1091/mbc.E08-05-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ferreira C, Silva S, Faria-Oliveira F, Pinho E, Henriques M, Lucas C. Candida albicans virulence and drug-resistance requires the O-acyltransferase Gup1p. BMC Microbiol. 2010;10:238–251. doi: 10.1186/1471-2180-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ganguly S, Singh P, Manoharlal R, Prasad P, Chattopadhyay A. Differential dynamics of membrane proteins in yeast. Biochem. Biophys. Res. Commun. 2009;387:661–665. doi: 10.1016/j.bbrc.2009.07.054. [DOI] [PubMed] [Google Scholar]
- [50].Tanaka K, Fukuda R, Ono Y, Eguchi H, Nagasawa S, Nakatani Y, Watanabe H, Nakanishi H, Taguchi R, Ohta A. Incorporation and remodeling of extracellular phosphatidylcholine with short acyl residues in Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2008;1781:391–399. doi: 10.1016/j.bbalip.2008.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.