Abstract
Purpose
16α-[18F]-fluoro-17β-estradiol positron emission tomography (FES-PET) quantifies estrogen receptor (ER) expression in tumors and may provide diagnostic benefit.
Procedures
Women with newly diagnosed metastatic breast cancer (MBC) from an ER-positive primary tumor were imaged before starting endocrine therapy. FES uptake was evaluated qualitatively and quantitatively, and associated with response and with ER expression.
Results
Nineteen patients underwent FES imaging. Fifteen had a biopsy of a metastasis and 15 were evaluable for response. Five patients had quantitatively low FES uptake, six had at least one site of qualitatively FES-negative disease. All patients with an ER-negative biopsy had both low uptake and at least one site of FES-negative disease. Of response-evaluable patients, 2/2 with low FES standard uptake value tumors had progressive disease within 6 months, as did 2/3 with qualitatively FES-negative tumors.
Conclusions
Low/absent FES uptake correlates with lack of ER expression. FES-positron emission tomography can help identify patients with endocrine resistant disease and safely measures ER in MBC.
Keywords: FES-PET, FDG-PET, Metastatic breast cancer, ER expression, Endocrine therapy, Response
Introduction
Over 200,000 new cases of invasive breast cancer were diagnosed in 2011 [1]. Improving and targeting treatment of metastatic breast cancer (MBC) has been shown to improve survival by approximately 30 % [2]. Given the efficacy and low morbidity of endocrine therapy for estrogen-receptor-expressing (ER+) tumors and the lack of efficacy of endocrine therapy in ER-negative (ER-) tumors, assay for ER expression in histologic tumor samples is critical to select treatment for MBC [3–5]. While ER expression is readily determined in biopsy material from primary breast tumors, biopsy of metastatic sites is more technically challenging and may be associated with higher morbidity. Moreover, ER expression in MBC may be heterogeneous since ER expression at the primary site or one metastatic site does not predict similar expression at all metastatic sites. Because it is neither practical nor feasible to biopsy every metastasis, therapy selection in patients with MBC is challenging. A predictive assay to assess ER expression at all sites of disease simultaneously is an urgent unmet need to select patients who are most likely to benefit from endocrine therapy.
The positron-emitting radiopharmaceutical 16α-[18F]-fluoro-17β-estradiol (FES) is a radiolabeled estrogen that has been tested as a positron emission tomography (PET) imaging agent to non-invasively measure regional estrogen receptor (ER) binding in tumors [6–11]. Prior studies have shown that the level of FES uptake correlates with the results of in vitro assay for ER expression both by radioligand binding [12] and immunohistochemistry (IHC) [13]. Prior studies have also shown that low or absent FES uptake [FES standard uptake value (SUV) ≤ 1.5] is predictive of poor response to endocrine therapy in heterogeneous groups of patients including patients undergoing initial therapy with tamoxifen [14] and in patients undergoing salvage aromatase inhibitor (AI) therapy [9].
This clinical phase II, NCI investigational new drug (IND)-enabling study's primary objective was to investigate the utility of FES-PET to predict overall response to first-line endocrine therapy in patients who had not received prior therapy for MBC. Secondary objectives included comparing FES-PET to tissue IHC and to further document the safety of FES. This study differed from the prior work [9, 15] in that it was prospective and included only patients with newly diagnosed MBC with no prior endocrine therapy in the metastatic setting.
We predicted that the use of FES-PET to measure ER expression would provide useful information to better predict response to hormonal therapy, to understand resistance to endocrine therapy, and to potentially help design multi-center therapeutic trials for better treatment outcomes.
Materials and Methods
Patients
Study participants were recruited from the University of Washington Medical Center and the Seattle Cancer Care Alliance. Eligible patients were non-pregnant pre- and post-menopausal women with a pathologically confirmed ER+ invasive primary breast cancer with clinical, radiographic, and/or pathologic evidence of stage IV disease. Biopsy of a metastatic disease site was requested, but not required for study participation. Entry criteria included a requirement for planned first-line endocrine therapy with one of the following: tamoxifen (±ovarian suppression in premenopausal women), AI±fulvestrant (with ovarian suppression in pre-menopausal patients) or fulvestrant alone. Prior adjuvant endocrine therapy was permitted with a required 6-month washout period. Alternatively, patients who had received >2 years of adjuvant endocrine therapy at the time of first recurrence and planned to change to alternate endocrine therapy with the metastatic diagnosis were also eligible. Additionally, a washout period of 6–8 weeks for prior adjuvant tamoxifen treatment was required prior to FES-PET imaging.
Patients with prior treatment for stage IV disease were not eligible. Patients with metastasis solely confined to the liver were excluded because normal hepatic uptake of the radiotracer and metabolites obscures scan interpretation. Patients with HER2/neu-positive tumors planning to undergo HER-2 directed therapy (trastuzumab or lapatinib) were not eligible for the study.
Initially, measurable disease by response evaluation criteria in solid tumors (RECIST) criteria (version 1.0) was required, but the protocol was amended in 2009 to include patients with non-RECIST measurable disease whose disease burden and response to therapy could be monitored by other means, including CA 27.29, CEA, circulating tumor cells, 2-deoxy-2-[18F]fluoro-D-glucose (FDG)-PET, [18F]-fluoride PET, or bone scans. This was an observational study; FES-PET results were not used to direct therapy. All patients provided informed consent and the protocol was approved by the Investigational Review Board. Use of [18 F]FES was carried out under an NCI-held IND (IND #79005).
PET Imaging Protocols
FDG-PET Imaging
FDG-PET was used clinically to identify sites of active disease. All patients followed the standard clinical protocol for an FDG-PET scan oneither a GE ADVANCE PET or one of two GE DSTE PET/computed tomography (CT) (GE Medical Systems, Waukesha, WI) machines. All scanners are routinely cross-calibrated with a quantitative imaging phantom measurement. Six patients were imaged on the GE ADVANCE scanner and 13 were imaged on a GEDSTE PET/CT with an average of 3 days between the FDG and FES-PET scan (range -2–19 days). Briefly, all patients fasted for at least 4 h before FDG-PET scanning and were either non-diabetic or had a pre-scan serum glucose level less than 150 mg/dl. A dose of 260–370 MBq (7–10 mCi) of FDG was used. Patients rested for 60 min after injection in a supine position prior to imaging. Each study was performed in two-dimensional (2D) and included 5 15-cm axial field-of-view (FOV) images. For attenuation correction, on the GE ADVANCE, each FOV included a 3-min attenuation scan. On GE DSTE PET/CT, a low-dose CT (120 kV, 10 mA) over the entire torso was performed. As in past studies, all emission images were performed in 2D on both machines with identical emission images of 7 min/FOV.
FES-PET Imaging
16α-[18F]-fluoro-17β-estradiol (FES) was prepared locally by the PET Radiochemistry Group at the University of Washington using established methods [16–18]. Specific activity averaged 2,465 Ci/mmol at injection (range 427-6254 Ci/mmol), radiochemical purity was always over 99 % and at no point was the total mass of FES in the injectate more than 2.8 μg. All tracer characteristics were well within the established ranges from prior studies at our center [9, 10, 13]. Imaging procedures were conducted as previously described [9, 19, 20] on the GE ADVANCE PET scanner. For patients studied on the GE DSTE PET/CT, the imaging procedure was the same with the exception of a low dose (120 kV, 10 mA) CT scan instead of a 25-min attenuation scan. Briefly, an intravenous line was established for the FES injection. Fourteen patients had their FES scan on the GE ADVANCE, and five were imaged on the GE DSTE PET/CT. The FES was administered by intravenous injection of 20 ml solution of 0.07 mCi/kg, not to exceed 6 mCi. The injection was infused over 2 min followed by a saline flush. In order to maintain the parameters of previous studies, imaging included a 60-min dynamic scan over a single apparent field-of-view (AFOV) at the time of injection. The location for the dynamic scan was determined using prior imaging (including CT and FDG-PET) by the principal investigator. The dynamic scan was followed by a 5AFOV torso survey with the same timing for attenuation or low-dose CT as in the FDG studies but with 5-min emission scans per FOV. After the completion of these scans the patient's vital signs were measured to monitor for any acute side effects of drug administration. Each patient was contacted within 24 h of the scan to assess any other side effects or toxicity.
FDG Image Analysis
FDG images were processed as for a typical clinical scan, corrected for attenuation, tracer radioactive decay, and normalized to the injected dose and body weight. This results in regional SUVs: SUV = A/(ID/BW) where A is the tissue tracer uptake in microcuries per gram (μCi/g), ID is the injected dose in millicuries (mCi), and BW is the body weight in kilograms (kg).
The FDG-PET served to help identify active sites of breast cancer and was matched to the FES-PET scan to help determine sites on the FES-PET where estradiol binding would be expected. The FDG SUVmax values for each lesion were used to calculate the FES/FDG index, as described below.
FES Qualitative Analysis
Qualitative (visual) assessment of uptake in lesion sites was performed for all studies in both the dynamic and torso survey scans. Active sites of disease visualized by clinical FDG-PET scan or CT scan were matched to the FES scan using a rigid co-registration/fusion module (PMOD Technologies, Zurich, Switzerland). Lesions identified by FDG-PET were corroborated on at least one other imaging modality—either the accompanying CT or other imaging study such as bone scan or MRI. Each site of disease was assessed as positive or negative for FES uptake. The qualitative assessment was summarized at the patient level as presence/absence of site(s) qualitatively negative for FES uptake. Three experienced imagers performed the qualitative analysis for all 19 patients to evaluate reader concordance. Readers had simultaneous access to both FES and FDG images and FDG clinical reports and were blinded to clinical outcomes.
FES Quantitative Assessment
For quantitative assessment of FES uptake, lesions that were identified and localized using the FDG-PET results were matched with the lesions on the FES-PET images as described in the “FES Qualitative Analysis” section above. In this cohort of untreated patients with MBC, no lesions were visualized by FES that were not assessable by FDG. Using PMOD (PMOD Technologies) [9], ROIs of approximately 1.5-cm diameter were drawn on three adjacent planes with the highest lesion FES uptake to determine maximal FES uptake for each disease site. Up to three lesions were quantified on the summed dynamic scan (single AFOV), and all lesions outside of the liver recorded on the clinical FDG scan were quantified on the static torso survey (5AFOV). Of note, because only sites with 1.5 cm or greater diameter were included in the analysis, partial volume correction was not used. FES uptake was measured using SUV on 30-60 min summed images of the dynamic scan and also for the 5AFOV torso survey. Average and maximum SUV (SUVmean and SUVmax) were recorded for each lesion.
The primary quantitative FES uptake measure was derived from the single FOV dynamic scan, as for prior reported studies [9]. Additional analysis was done to potentially refine measurement of FES uptake as a quantitative biomarker:
Inclusion of lesions beyond the single AFOV of the dynamic scan, by examining the entire torso survey [21, 22].
Log-transformation of SUVs prior to averaging to limit the influence of extreme measurements [23].
Weighting the FES uptake by metabolic aggressiveness as measured by FDG-PET SUV, using the FES/FDG uptake ratio, with the added benefit of controlling for partial volume effects [24]. This approach also weights the FES uptake by metabolic aggressiveness as measured by FDG-PET SUV [24].
The following patient-level quantitative summary measures were used to examine associations with clinical response:
Average of FES SUVmean of up to three lesions with the highest FES SUVmean in the dynamic scan. An average SUV of 1.5 was previously proposed as the cutpoint distinguishing low and high FES uptake [9]
Geometric mean (exponentiated average of log-transformed FES SUVmean) of the three lesions with the highest FDG SUVmax in the torso scan [see items (a) and (b) above], with three most metabolically active lesions selected [20].
Average of square root-transformed FES/FDG SUVmax ratio of the three lesions with the lowest FES/FDG ratio in the torso scan [see item (c) above]
Study Treatment and Clinical Monitoring
Patients were expected to start endocrine therapy within 2 weeks of the FES scan. Response assessment was evaluated at 3 and 6 months with the 6-month time point serving as the endpoint in the absence of earlier progression. For patients with at least one site of evaluable disease, size-based response criteria were used to assess response, as per RECIST 1.1 [25]. Per RECIST 1.1, results from serial FDG-PET scans were considered to supplement response classification, specifically for progressive disease (PD) if new lesions detected by FDG PET were also seen on the CT portion of PET/CT, or other imaging modality. For patients without disease valuable by RECIST 1.1, largely patients with bone-dominant MBC (n=4), serial FDG PET was used to determine response based on prior studies that have shown serial FDG-PET accurately and effectively monitors response of bone dominant breast cancer and predicts time to progression [26, 27] in patients without clear measurable disease. Similar to prior published experience for both FDG PET and tumor markers [26, 28-30], for this study, a decline of 30 % or more was considered as response for FDG SUV and/or tumor markers and an increase of 20 % or more was considered to be PD. To use FDG-PET as an indicator of response, the scans were performed at our center, followed NCI guidelines for quantitative FDG studies and were performed either on the same scanner or an identical scanner that had undergone cross-calibration using the protocol describing in the “FDG-PET Imaging” section above.
Biopsy tissue from a metastatic site was used to examine biologic correlates of FES uptake and factors known to be predictive of endocrine response. Assays and scoring for ER, progesterone receptor (PgR), and HER2 were performed per standard IHC protocols [31-35]. All tissue analysis was performed at the Fred Hutchinson Breast Cancer Research Lab under the direction of a study co-investigator. When tissue was missing or not analyzable, biopsy assay results from the patient's clinical record were reported.
Statistical Methods
FES uptake was compared to clinical benefit (PD versus other outcomes at 6 months). Associations between patient-level summaries of FES uptake and clinical benefit were assessed using the mid-P adjustment to Fisher's exact test [36]. Associations among continuous measures were evaluated using Spearman correlation coefficients and inspection of scatterplots. The primary objective of the study was to assess patient-level measures of FES uptake as predictors of overall response. As listed above, we examined a quantitative summary from the dynamic scan (as used in prior studies), as well as qualitative assessment (selected for ease of implementation) and additional quantitative summaries from the torso sweep.
Results
Patient Characteristics
Nineteen women were enrolled in this study between November 2008 and January 2011 and underwent FES-PET imaging. Patient characteristics and outcomes are summarized in Table 1. Five patients had de novo stage IV disease, and the 14 others were newly diagnosed as stage IV after having an ER+ primary breast cancer 3 months to 29 years earlier. Fifteen patients successfully underwent biopsy of metastases. In patients with recurrent disease 3/11 (27 %) had ER- biopsy results. In four patients with de novo metastatic disease, available primary and metastatic site biopsies concordantly expressed ER.
Table 1. Patient characteristics.
| Patients (n=19) | N (%) or median (min-max) |
|---|---|
| Age, years | 62 (38-77) |
| Menopausal status | |
| Pre-menopausal | 2 (11 %) |
| Post-menopausal | 17 (89 %) |
| Ethnicity | |
| Not Hispanic or Latino | 19 (100 %) |
| Race | |
| White | 16 (84 %) |
| Black or African American | 2 (11 %) |
| American Indian or Alaska Native | 1 (5 %) |
| Histology (primary diagnosis) | |
| Invasive ductal | 15 (79 %) |
| Invasive lobular | 1 (5 %) |
| Mixed ductal and lobular | 2 (11 %) |
| Unknown | 1 (5 %) |
| De novo stage IV | |
| Yes | 5 (26 %) |
| No | 14 (74 %) |
| ER (primary diagnosis) | |
| Positive | 18 (95 %) |
| Weakly positive | 1 (5 %) |
| PgR (primary diagnosis) | |
| Positive | 5 (26 %) |
| Weakly positive | 8 (43 %) |
| Negative | 5 (26 %) |
| Unknown | 1 (5 %) |
| HER2neu (primary diagnosis) | |
| Positive | 1 (5 %) |
| Negative | 15 (79 %) |
| Unknown | 3 (16 %) |
| Prior adjuvant chemotherapy | |
| Yes | 12 (63 %) |
| No | 7 (37 %) |
| Prior adjuvant radiation | |
| Yes | 10 (53 %) |
| No | 8 (42 %) |
| Unknown | 1 (5 %) |
| Prior adjuvant endocrine therapy | |
| Aromatase inhibitor (AI) | 6 (31 %) |
| AI and tamoxifen | 2 (11 %) |
| Tamoxifen | 2 (11 %) |
| None | 9 (47 %) |
| Number of lesions (FES PET dynamic FOV) | |
| 1 | 2 (11 %) |
| 2-3 | 11 (57 %) |
| 4-5 | 6 (32 %) |
| Number of lesions (FES PET sweep) | |
| 1 | 1 (5 %) |
| 2-4 | 5 (26 %) |
| 5-7 | 6 (32 %) |
| 8-12 | 7 (37 %) |
| Study treatment | 4(21 %) |
| Tamoxifen | 3 (16 %) |
| Anastrozole | 1 (5 %) |
| Exemestane + fulvestrant | 4 (21 %) |
| Letrozole | 4 (21 %) |
| Letrozole + fulvestrant | 1 (5 %) |
| Fulvestrant | 2 (11 %) |
| Did not undergo endocrine treatment on study | |
| Biopsy Results (n=15) | |
| ER (metastatic lesion) | |
| Positive | 10 (67 %) |
| Weakly positive | 2 (13 %) |
| Negative | 3 (20 %) |
| PgR (metastatic lesion) | |
| Positive | 6 (40 %) |
| Weakly positive | 4 (27 %) |
| Negative | 4 (27 %) |
| Unknown | 1 (6 %) |
| HER2neu (metastatic lesion) | |
| Positive | 3 (20 %) |
| Negative | 8 (53 %) |
| Unknown | 4 (27 %) |
| Clinical response | |
| Partial response | 4 (21 %) |
| Stable disease | 45 (216 %) |
| Radiographic progressive disease | 76 (372 %) |
| No response assessmentc | 4 (21 %) |
One patient also underwent ovarian suppression
When Allred scores were available, scoring was 0 “negative,” 2–6 “weakly positive,” 7–8 “positive.” When Allred scores were not available, clinical report scores of 1+ were “weakly positive”
Includes two patients who did not undergo endocrine monotherapy, one who had complications from a non-breast cancer-related surgery after 2 days of anastrozole, and one who changed therapy (from tamoxifen) when a successful metastatic biopsy revealed ER- disease
Safety
Since this was an NCI investigative new drug (IND)-enabling study, we report here on the safety of FES. During this study, there were no adverse events related to FES administration or FES imaging. There were two serious events that occurred during the follow-up period unrelated to FES imaging. One patient developed pneumonia and another had complications from a non-breast cancer-related surgery.
Imaging Results
Imaging examples from two patients are provided (Fig. 1). Both patients had lymph node metastases from ER+ breast cancer. Patient A (15-002) whose axillary mass biopsy demonstrated ER positivity had high FES uptake at each site identified on FDG-PET. Patient B (3-001) whose T10 vertebral lesion tumor biopsy was ER- had nodal metastases clearly visualized on FDG-PET, but not appreciated by FES-PET.
Fig. 1.
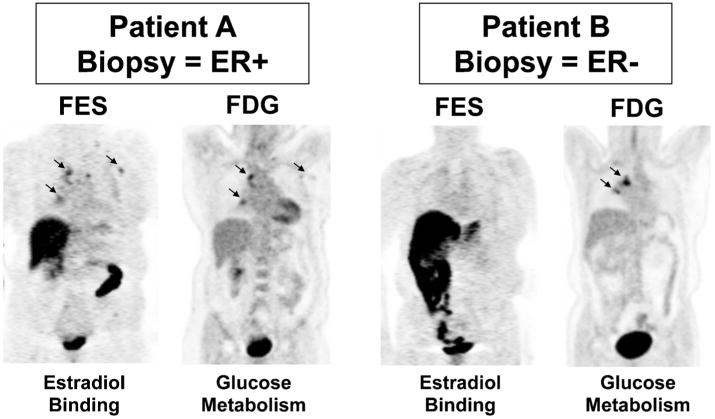
Imaging examples from two patients who underwent both FES and FDG scans prior to therapy. Left panel: Patient A (15-002) had mediastinal lesions appreciated by both FES and FDG. Right panel: Patient B (3-001) also had mediastinal disease clearly seen by FDG-PET, not visible on FES-PET. The core biopsy of a metastatic axillary lesion from Patient A showed ER+ breast cancer, while the needle biopsy of a vertebral lesion from Patient B showed ER- breast cancer.
Evaluating summed images from the dynamic acquisition, 56 lesions were identified with a median FES SUVmean of 2.2 (range 0.4–7.7). Using an average SUVmean ≤ 1.5 for lesions in the dynamic FOV as a cutpoint, 5/19 patients had disease with low FES uptake (26 %, 95 % CI 12 %–49 %).
For the alternative analysis of the torso scan, one hundred twenty-four tumors were identified by both FES and FDG scans (Table 2). The median FES SUVmean for the 124 lesions measured in the torso survey was 2.0 (range 0.0–8.3). Qualitative assessment of the FES-PET torso survey revealed 6/19 patients (32 %, 95 % CI 15 %–54 %) with at least one site that was considered FES-negative. For the six patients (32 lesions) with at least one qualitative site of FES-negative disease, the median torso SUVmean was 0.7 (0.0–2.4). There were no discordant results among the three reviewers evaluating qualitative results.
Table 2. Lesion level and patient level summaries of FES PET and FDG PET measures.
| N | N (%) or median (min–max) | |
|---|---|---|
| Lesion level | ||
| Tumor site | 124 | |
| Breast | 5 (4 %) | |
| Soft tissue/nodes | 52 (42 %) | |
| Spine | 32 (26 %) | |
| Other bone | 35 (28 %) | |
| FES PET SUVmean (dynamic FOV) | 56 | 2.2 (0.4,7.7) |
| FES PET SUVmean | 124 | 2.0 (0.0,8.3) |
| FDG PET SUVmax | 124 | 4.6 (1.3,17.3) |
| Ratio (FES PET SUVmax/FDG PET SUVmax) | 124 | 0.7 (0.1,3.6) |
| Patient level | ||
| Any FES qualitatively negative sites | 19 | |
| No | 13 (68 %) | |
| Yes | 6 (32 %) | |
| Average FES PET SUVmean (dynamic FOV) | 19 | 2.0 (0.6, 5.9) |
| Geometric mean of SUVmean (three highest FDGmax-sweep) | 19 | 2.2 (0.4, 6.5) |
| Average of sqrt(FES/FDG ratio) (three highest FDGmax-sweep) | 19 | 0.9 (0.3, 1.5) |
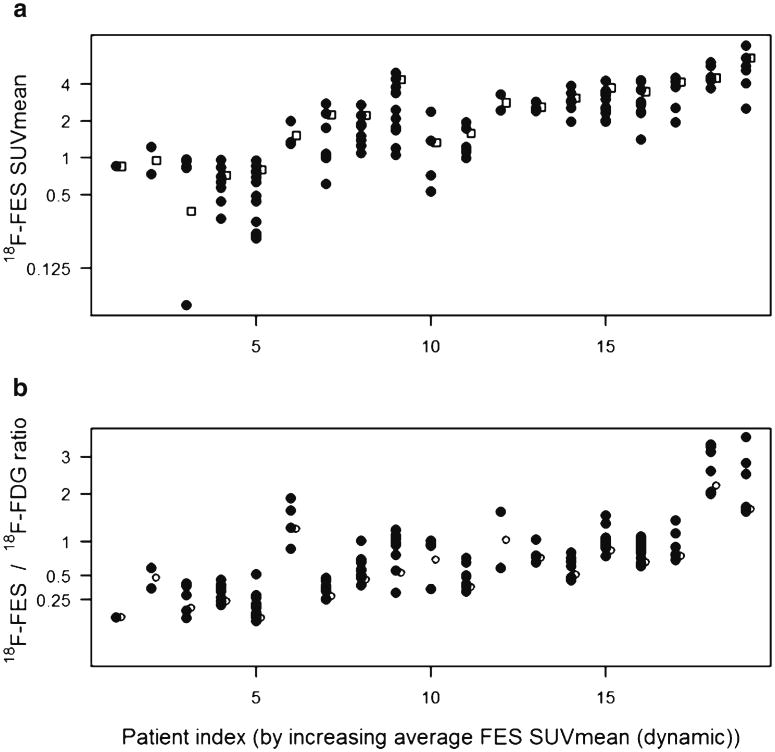
The median FDG SUVmax was 4.6 (range 1.3–17.3). Fig. 2a shows FES SUVmean for all lesions in 19 patients. Also displayed is the average for up to three lesions with highest FDG SUVmax (hollow squares). The FES/FDG ratio had a median value of 0.7 (range 0.1–3.6). Fig. 2b shows the FES/FDG ratio for all lesions in the torso scans, as well as the average for up to three lesions with lowest FES/FDG ratio (hollow circles). In some cases the FES/FDG ratio appeared to be more homogeneous within-patient than the FES SUV (patient index 7-010,16-012) but in other cases the FES/FDG ratio was discrepant between lesions compared to FES SUV (patient index 12-020).
Fig. 2.
Alternative analysis methods for FES uptake. Panel a shows SUVmean for all lesions in the torso survey (black circles). Also displayed is the average for up to three lesions with highest FDG SUVmax (hollow squares). Panel b shows the FES/FDG ratio for all lesions in the torso survey, as well as the average for up to three lesions. For consistency, the horizontal axis for both panels is arranged by increasing average FES SUVmean (from the dynamic FOV). The different patient-level summaries follow different rank orders. For example, patient 6-018 (index #6 on the horizontal axis) had lesions with low FDG uptake, so the FES/FDG ratio was high relative to FES uptake.
Five of the 19 patients had average FES SUVmean (dynamic FOV) ≤1.5, and 6/19 had at least one site that was considered FES-negative in qualitative analysis of the torso survey. Qualitative image interpretation and the quantitative summary agreed for 18/19 patients, with one patient having ER+ disease by the dynamic scan, but sites of ER-metastases outside the dynamic FOV (Table 3). For the six patients with at least one qualitative site of FES-negative disease, the median torso SUVmean was 0.7 (range 0.0–2.4) for 32 lesions. Listings of study data (patient characteristics, imaging results, response to therapy) for the 19 participants are included in an online appendix (Appendix Table 1).
Table 3. Qualitative and quantitative summary measures to classify disease burden as ER+ or ER- by FES, with response.
| N (%) | Response summary | |
|---|---|---|
| Average SUVmean (dynamic) > 1.5 | 13 (69 %) | 4 PR |
| AND no qualitatively negative sites on FES PET | 3 SD | |
| 5 PD | ||
| 1 not evaluable for responsea | ||
| Average SUVmean (dynamic) ≤ 1.5 | 5 (26 %) | 2 PD |
| AND qualitatively negative sites detected on FES PET | 3 not evaluable for responseb | |
| Average SUVmean (dynamic) > 1.5 | 1 (5 %) | 1 SD (index #10-015) |
| AND qualitatively negative sites detected on FES PET |
Four patients withdrew from the study due to unrelated adverse events (n=2) or an unexpected biopsy results (n=2)
Tissue Biopsy, Prognostic Marker Expression, and FES Uptake
All primary tumors were ER+ and the majority were also PgR + and HER2neu- (Table 1). Assays from biopsy of metastasis tissue were available for lesions from 15 of the 19 patients. Of the metastasis biopsies, 12/15 (80 %) were ER+ and 10/14 (71 %) were PgR+ (one case not evaluated). HER2neu was measurable in 11 lesions, and eight (73 %) were negative.
FES uptake correlated with tissue assay of ERs. All three patients with ER- metastasis biopsies harbored qualitative FES-negative sites and had average dynamic scan FES SUVs of 0.7, 0.8, and 1.0. Three other patients with a confirmed ER+ tissue biopsy had at least one qualitatively FES-negative un-biopsied site. A tabular listing of biopsy and scan results for all patients is presented in the online appendix (Appendix Table 2).
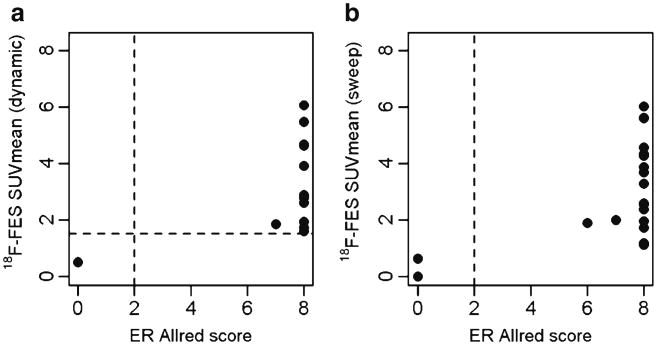
Fig. 3 shows associations between FES SUVmean for the dynamic FOV (Fig. 3a) and the torso sweep (Fig. 3b) of biopsied lesions, with Allred ER expression scores for those lesions. The appendix also includes a similar figure showing FES uptake for all lesions (not only the biopsied lesion), by Allred ER score for the biopsied lesion. For all lesions in the dynamic FOV (Fig. 3a) and most lesions in the sweep (Fig. 3b), biopsy and imaging are correlated and unambiguously classify lesions as ER+ or ER-.
Fig. 3.
Association between quantitative FES uptake measures and ER expression in biopsy tissue. a The relationship between FES SUVmean (dynamic scan) mean and Allred scored levels of ER expression, for 13 lesions in 11 patients. The vertical dotted lines represent the division of a positive or negative Allred score. The horizontal dotted line depicts the previously established cutpoint of 1.5. Imaging and tissue assay classifications agree in all cases. b The relationship between FES SUVmean (torso scan) and ER expression, for 20 lesions in 13 patients. Imaging and tissue assay classifications appear to agree, although two lesions have low SUVmean scores in contrast to the Allred score of 8 for the biopsied material. Both discrepancies were from a single patient's axillary nodes with SUVmean of 1.1–1.2, in contrast to biopsy results (Allred 8 from a core biopsy, 11-019).
Response
Response was evaluated in 15 patients (Table 3). The four non-evaluable patients either had adverse events unrelated to FES imaging (n=2; pneumonia and surgical complications) or opted to receive chemotherapy due to an ER- metastatic lesion biopsy (n=2; one patient received the ER- result before initiating endocrine therapy, and the other discontinued endocrine therapy when the biopsy results were available.) Only one patient continued on endocrine therapy following an ER- metastatic biopsy, and she was found to have PD when assessed at 3 months.
Of three response-evaluable patients harboring qualitatively negative sites of disease by FES-PET, two had PD and the other had SD at 6 months. Evaluating qualitatively negative FES sites as a predictor of progressive disease, the one-sided mid-p value (mid-p adjustment to Fisher's exact test) was 0.26.
Although 5 of 19 patients had average FES SUVmean in the dynamic FOV ≤1.5, only two of these patients remained on study and were available for response assessment; both had PD. Five of 14 patients with average FES SUVmean 91.5 had PD: the one-sided mid-p value for FES SUVmean ≤1.5 as a predictor of PD was 0.10.
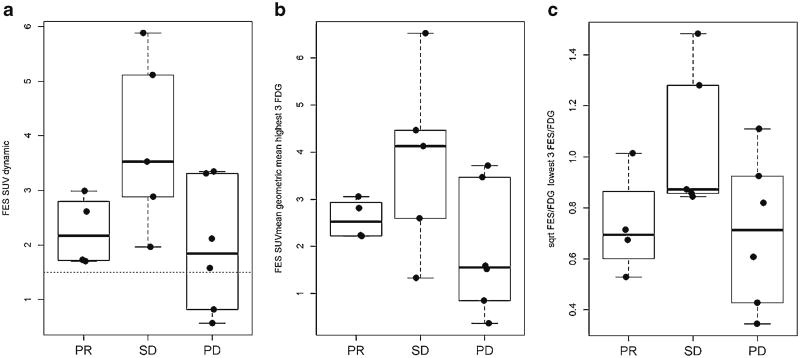
Fig. 4 shows associations between patient-level quantitative measures of FES uptake and clinical response. The patients with lowest average FES SUV (Fig. 4a) had PD. Fig. 4b shows patient-level summaries including lesions outside the dynamic FOV; most patients' relative positions are similar, except for one patient (10-015) with stable disease. The average FES/FDG SUVmax ratio (Fig. 4c) did not predict which patients experienced PD on endocrine therapy.
Fig. 4.
Association between three patient-level quantitative measures of FES uptake and clinical response. a shows the distribution of response compared to FES SUVmean (dynamic scan). b The response distribution of FES SUVmean, for the three lesions in the torso sweep with highest FDG SUVmax. c The response distribution compared to the average FES/FDG ratio, for the three lesions in the torso sweep with lowest FES/FDG ratio.
Discussion
Our goal of this IND-enabling phase II clinical trial was to test the ability of FES-PET to predict response in patients with newly diagnosed MBC undergoing first-line endocrine treatment. Because patients are not routinely subjected to multiple biopsies, we report on the ability of FES-PET to quantify ER function over the whole body burden of tumor without biopsy. Tumor ER expression is used to guide therapy, and patients could be spared futile therapy if the ER status of their tumors is optimally evaluated prior to starting treatment.
The study population was clinically less heterogeneous than our previous series [9, 20] in that all patients' breast cancer was newly diagnosed as stage IV. Our previous study using FES-PET in 47 patients with MBC treated with endocrine therapies for any line of therapy showed FES-PET correlation of tumor SUV and endocrine therapy response. Similar to the results seen in the current report, FES uptake and response were significantly associated; 0/15 patients with initial FES SUV ≤1.5 had clinical benefit from endocrine therapy, compared to 11/32 (34 %) with FES SUV ≥1.5 (p<.01). Of the 47 patients, 15 (33 %) had average dynamic FES SUVs ≤1.5 [9] as compared with this study (5/19, 26 %). The rate of low tumoral FES uptake and the association with response were similar for patients receiving endocrine therapy as nth-line or first-line treatment for MBC.
Other investigators have correlated the tumor ER status and response to systemic therapy with FES imaging data. Mortimer et al. [15] suggested that the functional assessment of hormone sensitivity by PET imaging identified patients with ER+ disease whose tumors are likely to be hormone refractory and predicted tumor response to tamoxifen therapy in ER+ MBC [14]. In this study, only two of five patients with low FES SUV were evaluated for response to endocrine therapy; however, two of the others went off study because the ER- biopsy raised the concern that ER-directed therapy would be ineffective. Our findings suggest that low FES SUV predicts a lack of response to ER-directed therapy, and that FES can help determine patients for whom endocrine therapy is inappropriate.
We and other investigators have also previously shown that FES uptake correlates well with breast cancer tumor ER status [12, 13] and that FES uptake is not affected by exogenous estradiol administration or other hormonal status variables [11]. This current study further validates the correlation between FES uptake and ER expression. In addition, both qualitative and quantitative measure of FES uptake strongly predicted resistance to endocrine therapy when FES uptake was low or absent. Our series identified some within-patient heterogeneity in FES uptake in some tumors. Three patients with low average FES and/or one of more sites of qualitatively negative disease had an ER+ metastasis biopsy result, (Appendix Tables 1, 2). This illustrates the potential sampling error associated with metastasis biopsy and the advantage of imaging: the ability to evaluate ER levels in tumors throughout the whole body.
We sought to validate the previously defined cutoff of FES SUV ≤1.5 to identify patients who would be highly unlikely to benefit from endocrine therapy [9]. This cutoff was based on the average uptake for the three lesions with greatest uptake in the dynamic FES AFOV. When comparing qualitative and quantitative evaluations of the tumors using our previously defined cutoff, it is interesting to note that in all 13 patients with no qualitatively negative sites, the average SUV was >1.5 in the dynamic scan. In five of the six patients with at least one qualitatively negative site the average SUV was ≤1.5. While the average FES uptake for up to three lesions is likely a good patient-level summary for patients with localized disease [20], subsequent development of FES-PET measures has suggested alternative image data parameterizations, especially in patients with metastases in multiple fields-of-view. Further investigation with a larger number of patients is needed in order to characterize qualitative vs. quantitative mismatch or to adjust the SUV cutoff value of 1.5. We note that the qualitative interpretation of FES-PET images is quite robust, with a high degree of concordance between the three readers in this study.
We also averaged values for the three lesions with highest FDG uptake and/or lowest FES/FDG ratios to identify tumors that would be unlikely to respond to endocrine therapy. Successful targeting of an indolent FES-avid lesion would not be successful therapy if an aggressive lesion remained that was not responsive to endocrine therapy.
Serum Estradiol was measured in 14 patients, and all but two of those patients had levels ≤10 pg/ml with the highest value being 82 pg/ml. As expected, we found no correlation between measured serum estradiol levels and tumor FES uptake. Further, there was no correlation between other serum hormone status values and response (data not shown). Serum SHBG was measured in 15 patients with an average of 29 nmol/l (range 11–59 nmol/l), within established normal range. As in prior studies, this study result suggests that physiologic levels of circulating estrogens do not interfere with the ability of FES to measure regional ER binding [11].
There were several limitations to this study, primarily the small number of patients available. We were unable to select the location of the metastasis biopsy or to perform multiple biopsies, so a direct comparison between tumor FES uptake and ER status biopsy results for all lesions was not possible. Additionally, although all patients had planned endocrine therapy at the time of FES imaging, only 15/19 had evaluable response due to complications unrelated to the imaging procedure preventing completion of treatment, or to individual patient and physician choices to forego endocrine therapy in the case of biopsy result showing a lack of ER expression.
With the sample size of an extended case series rather than a definitive confirmatory cohort, we were unable to compare the performance of alternate versions of quantitative biomarkers of FES uptake as predictors of response to endocrine therapy. Further refinement of uptake measures (such as lean body mass adjustment [11] and cutpoint selection) will be explored in a future pooled analysis. Finally, due to hepatic clearance, FES PET cannot be used to assess liver lesions. Some clinicians may think that patients with any liver lesions should eschew endocrine therapy for chemotherapy, making FES PET assessment perhaps less important for patients with any liver tumors. However, one of the four patients in our series with liver disease appeared to show clinical benefit from endocrine therapy (with stable disease for 8 months on letrozole and fulvestrant). Two of the other three patients with liver disease experienced PD within 6 months of starting endocine therapy, and the third (5-011) was hospitalized soon after undergoing FES PET and did not undergo endocrine therapy as had been planned.
Conclusions and Future Directions
This NCI-sponsored, IND-enabling phase II study supports the use of FES-PET to measure ER expression in newly diagnosed MBC. Study results corroborate that very low FES uptake predicts a lack of response to endocrine therapy, an ER- tumor tissue biopsy status, or both. Future analyses will examine the optimal quantitative or qualitative threshold to indicate disease as ER+ or ER- by imaging, as well as the use of additional tissue markers. FES is also confirmed as a safe imaging agent. Larger multi-center clinical trials to further validate the use of FES-PET in routine practice are warranted.
Supplementary Material
Acknowledgments
Support. The authors would like to thank Eunice How, the radiochemistry staff, nuclear medicine technologists, and physicists in the UW Department of Radiology as well as the staff of the Fred Hutchinson Breast Cancer Research Center Cancer Biology lab. This study was sponsored by the NCI Phase I and II Cancer Imaging Program (Contract #N01-CM-37008, Protocol 8052) and was supported by the UPCI Biostatistics Shared Resource (P30CA047904).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11307-013-0699-7) contains supplementary material, which is available to authorized users.
Conflict of interest. None
References
- 1.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics. CA Cancer J Clin. 2011;61(6):409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Chia SK, Speers CH, D'Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110(5):973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 3.Carlson RW. Sequencing of endocrine therapies in breast cancer—integration of recent data. Breast Cancer Res Treat. 2002;75(Suppl 1):S27–S32. doi: 10.1023/a:1020361700012. discussion S33–S25. [DOI] [PubMed] [Google Scholar]
- 4.Major MA. Clinical trials update: medical management of advanced breast cancer. Cancer Nurs. 2003;26(6 Suppl):10S–15S. [PubMed] [Google Scholar]
- 5.Carlson RW, Allred DC, Anderson BO, et al. Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(7):821–829. doi: 10.6004/jnccn.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dehdashti F, Mortimer JE, Siegel BA, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36(10):1766–1774. [PubMed] [Google Scholar]
- 7.Linden HM, Ramsey S, Anderson N. An ounce of prevention or a pound of cure? Investing to improve breast cancer outcomes for African American women. J Clin Oncol. 2004;22(13):2517–2518. doi: 10.1200/JCO.2004.04.902. [DOI] [PubMed] [Google Scholar]
- 8.Linden HM, Link JM, Stekhova S, et al. Serial 18F-fluoroestradiol positron emission tomography (FES PET) measures estrogen receptor binding during endocrine therapy. Breast Cancer Res Treat. 2005;94(Suppl 1):S1–S287. [Google Scholar]
- 9.Linden HM, Stekhova SA, Link JM, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24(18):2793–2799. doi: 10.1200/JCO.2005.04.3810. [DOI] [PubMed] [Google Scholar]
- 10.Mankoff DA, Peterson LM, Tewson TJ, et al. [18F]fluoroestradiol radiation dosimetry in human PET studies. J Nucl Med. 2001;42(4):679–684. [PubMed] [Google Scholar]
- 11.Peterson LM, Kurland BF, Link JM, et al. Factors influencing the uptake of 18F-fluoroestradiol in patients with estrogen receptor positive breast cancer. Nucl Med Biol. 2011;38(7):969–978. doi: 10.1016/j.nucmedbio.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mintun MA, Welch MJ, Siegel BA, et al. Breast cancer: PET imaging of estrogen receptors. Radiology. 1988;169(1):45–48. doi: 10.1148/radiology.169.1.3262228. [DOI] [PubMed] [Google Scholar]
- 13.Peterson LM, Mankoff DA, Lawton T, et al. Quantitative imaging of estrogen receptor expression in breast cancer with PET and 18F-fluoroestradiol. J Nucl Med. 2008;49(3):367–374. doi: 10.2967/jnumed.107.047506. [DOI] [PubMed] [Google Scholar]
- 14.Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19(11):2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 15.Mortimer JE, Dehdashti F, Siegel BA, et al. Positron emission tomography with 2-[18F]Fluoro-2-deoxy-D-glucose and 16alpha-[18F]fluoro-17beta-estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res. 1996;2(6):933–939. [PubMed] [Google Scholar]
- 16.Lim JL, Zheng L, Berridge MS, Tewson TJ. The use of 3-methoxymethyl-16 beta, 17 beta-epiestriol-O-cyclic sulfone as the precursor in the synthesis of F-18 16 alpha-fluoroestradiol. Nucl Med Biol. 1996;23(7):911–915. doi: 10.1016/s0969-8051(96)00126-6. [DOI] [PubMed] [Google Scholar]
- 17.Romer J, Fuchtner F, Steinbach J, Johannsen B. Automated production of 16alpha-[18F]fluoroestradiol for breast cancer imaging. Nucl Med Biol. 1999;26(4):473–479. doi: 10.1016/s0969-8051(98)00098-5. [DOI] [PubMed] [Google Scholar]
- 18.Romer J, Fuchtner F, Steinbach J, Kasch H. Automated synthesis of 16alpha-[18F]fluoroestradiol-3,17beta-disulphamate. Appl Radiat Isot. 2001;55(5):631–639. doi: 10.1016/s0969-8043(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 19.Mankoff DA, Tewson TJ, Eary JF. Analysis of blood clearance and labeled metabolites for the estrogen receptor tracer [F-18]-16 alpha-fluoroestradiol (FES) Nucl Med Biol. 1997;24(4):341–348. doi: 10.1016/s0969-8051(97)00002-4. [DOI] [PubMed] [Google Scholar]
- 20.Kurland BF, Peterson LM, Lee JH, et al. Between-patient and within-patient (site-to-site) variability in estrogen receptor binding, measured in vivo by 18F-fluoroestradiol PET. J Nucl Med. 2011;52(10):1541–1549. doi: 10.2967/jnumed.111.091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehdashti F, Mortimer JE, Trinkaus K, et al. PET-based estradiol challenge as a predictive biomarker of response to endocrine therapy in women with estrogen-receptor-positive breast cancer. Breast Cancer Res Treat. 2009;113(3):509–517. doi: 10.1007/s10549-008-9953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Kruchten M, Glaudemans AW, de Vries EF, et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 2012;53(2):182–190. doi: 10.2967/jnumed.111.092734. [DOI] [PubMed] [Google Scholar]
- 23.Thie JA, Hubner KF, Smith GT. The diagnostic utility of the lognormal behavior of PET standardized uptake values in tumors. J Nucl Med. 2000;41(10):1664–1672. [PubMed] [Google Scholar]
- 24.Mankoff DA. Molecular imaging to select cancer therapy and evaluate treatment response. Q J Nucl Med Mol Imaging. 2009;53(2):181–192. [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Stafford SE, Gralow JR, Schubert EK, et al. Use of serial FDG PET to measure the response of bone-dominant breast cancer to therapy. Acad Radiol. 2002;9(8):913–921. doi: 10.1016/s1076-6332(03)80461-0. [DOI] [PubMed] [Google Scholar]
- 27.Specht JM, Tam SL, Kurland BF, et al. Serial 2-[18F] fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP) Breast Cancer Res Treat. 2007;105(1):87–94. doi: 10.1007/s10549-006-9435-1. [DOI] [PubMed] [Google Scholar]
- 28.Gennari A, Donati S, Salvadori B, et al. Role of 2-[18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) in the early assessment of response to chemotherapy in metastatic breast cancer patients. Clin Breast Cancer. 2000;1(2):156–161. doi: 10.3816/cbc.2000.n.014. discussion 162–153. [DOI] [PubMed] [Google Scholar]
- 29.Tateishi U, Gamez C, Dawood S, et al. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247(1):189–196. doi: 10.1148/radiol.2471070567. [DOI] [PubMed] [Google Scholar]
- 30.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter PL, El-Bastawissi AY, Mandelson MT, et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91(23):2020–2028. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 32.Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–2542. doi: 10.1002/cncr.20279. [DOI] [PubMed] [Google Scholar]
- 33.Porter PL, Malone KE, Heagerty PJ, et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3(2):222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 34.Yaziji H, Goldstein LC, Barry TS, et al. HER-2 testing in breast cancer using parallel tissue-based methods. JAMA. 2004;291(16):1972–1977. doi: 10.1001/jama.291.16.1972. [DOI] [PubMed] [Google Scholar]
- 35.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 36.Lancaster H. Significance tests in discrete distributions. J Am Stat Assoc. 1961;56:226–234. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.