Abstract
In the past years, the relationship between the endocannabinoid system (ECS) and other hormonal and neuromodulatory systems has been intensively studied. G protein-coupled receptors (GPCRs) can stimulate endocannabinoid (eCB) production via activation of Gq/11 proteins and, in some cases, Gs proteins. In this review, we summarize the pathways through which GPCR activation can trigger eCB release, as well as the best known examples of this process throughout the body tissues. Angiotensin II-induced activation of AT1 receptors, similar to other Gq/11-coupled receptors, can lead to the formation of 2-arachido-noylglycerol (2-AG), an important eCB. The importance of eCB formation in angiotensin II action is supported by the finding that the hypertensive effect of angiotensin II, injected directly into the hypothalamic paraventricular nucleus of anaesthetized rats, can be abolished by AM251, an inverse agonist of CB1 cannabinoid receptors (CB1Rs). We conclude that activation of the ECS should be considered as a general consequence of the stimulation of Gq/11-coupled receptors, and may mediate some of the physiological effects of GPCRs.
Keywords: Endocannabinoid, CB1, GPCR, Angiotensin II
1. Introduction
The endocannabinoid system (ECS) is a complex endogenous modulatory system that affects a variety of physiological functions. In the central nervous system (CNS), it has a role in such important processes as learning, thinking, emotional functions, regulation of food intake or pain sensation (Freund et al., 2003; Kano et al., 2009). In the periphery, its activity can be attributed, among others, to cardiovascular, immune, metabolic or reproductive functions. Moreover, its involvement in different pathophysiological processes of the above systems has also been documented (Pacher et al., 2006). It is therefore not surprising that in the past years the relationship of the ECS with other hormonal and neuromodulatory systems has been intensively studied. Thus, it has been established that one of the key stimuli of ECS is the activation of G protein-coupled receptors (GPCRs), which, through Gq/11 or Gs protein signaling, can trigger the release of endocannabinoid (eCB) molecules from the cells, leading to cannabinoid receptor activation (Pertwee et al., 2010; Turu et al., 2009). This scenario was first described in CNS synapses, where its presence, contributing to ‘retrograde endocannabinoid signaling’, has turned out to be fundamental (Hashimotodani et al., 2007). However, as outlined in this review, several data point to the presence of this mechanism in peripheral systems, suggesting a more general distribution of the phenomenon.
In this review we summarize the signaling pathways through which the activation of a GPCR can lead to the formation of eCBs and subsequent transactivation of cannabinoid receptors. We also cite some prominent examples from different systems in which such effects have been shown, to demonstrate that this kind of interaction between receptor systems can be considered as a quite general, although not obligate, component of Gq/11 protein signaling.
2. Biosynthesis of the major endocannabinoids
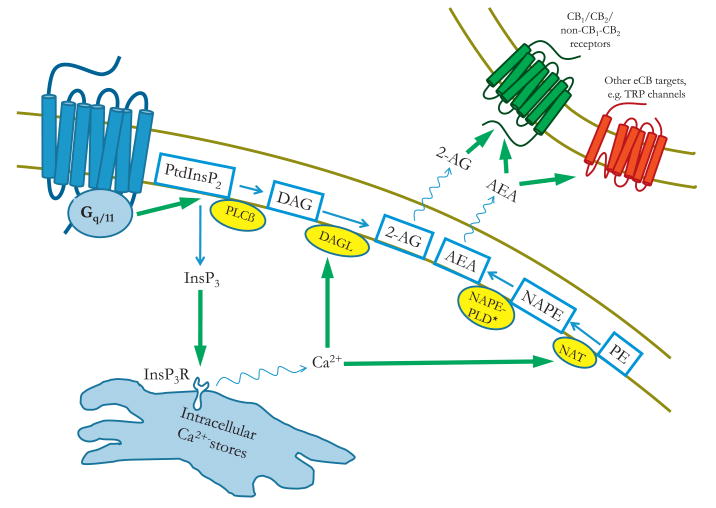
The two major eCBs are 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamide (AEA), also known as anandamide. Since their discovery as eCBs, various enzymes and metabolic pathways have been described to be involved in their biosynthesis (for reviews, see (Basavarajappa, 2007; Bisogno, 2008). Although these pathways probably do not contribute equally to eCB formation under physiological conditions, a clear picture regarding their relative physiological role in the various tissues is currently lacking. Here we describe only the pathways that are generally considered to constitute the main route of eCB production (Fig. 1).
Fig. 1.
Signaling mechanisms by which the activation of a Gq/11-coupled receptor enhances endocannabinoid production. Activation of a Gq/11-coupled receptor leads to the activation of phospholipase Cß enzyme, which cleaves PtdInsP2 in the plasma membrane to release InsP3 and DAG. DAG can serve as a substrate for the DAGL enzyme, which produces 2-AG in the cell membrane. On the other hand, InsP3 can trigger the release of Ca2+ from intracellular stores through InsP3R activation. The elevated cytoplasmic Ca2+ level can activate DAGL, further potentiating 2-AG production, as well as the NAT enzyme, leading to the production of NAPE, which can serve as a substrate for NAPE-PLD to yield AEA. * Indicates alternative pathways for anandamide production (see text for details). The released eCBs, after leaving the cell, can activate cannabinoid receptors or other eCB target molecules in a paracrine way. Abbreviations: PtdInsP2, phosphatidylinositol 4,5-bisphosphate; PLCß, phospholipase Cß; DAG, diacylglycerol; DAGL, diacylglycerol lipase; 2-AG, 2-arachidonoyl-glycerol; AEA, N-arachidonoyl-ethanolamine (anandamide); InsP3, inositol 1,4,5-trisphosphate; InsP3R, InsP3 receptor; NAPE, N-arachidonoyl-phosphatidylethanolamine; NAPE-PLD, NAPE-specific phospholipase D; NAT, N-acyltransferase; PE, phosphatidylethanolamine; TRP, transient receptor potential.
The production of 2-AG is mainly regulated by the sn-1-diacyl-glycerol-lipase (DAGL) α and β isoenzymes, which can release 2-AG by hydrolyzing diacylglycerol (DAG) at the sn-1 position. DAG, in turn, can be produced in the cell membrane, either from phosphoinositides or from phosphatic acid (PA), requiring the enzymatic hydrolysis by phospholipase C (PLC) or PA-phosphohydrolase enzymes, respectively (Bisogno, 2008) (Fig. 1).
The source of anandamide is principally the N-arachidonoyl-phosphatidylethanolamine (NAPE) molecule (Fig. 1). NAPE is generally produced by an enzymatic transfer reaction catalyzed by a Ca2+-dependent N-acyl transferase (NAT), which transfers an arachidonoyl group from the sn-1 position of membrane phospholipids to the N-position of phosphatidylethanolamide (PE) (Bisogno, 2008). More recently, Ca2+-independent isoforms of the NAT enzyme have also been identified (Jin et al., 2007). This reaction seems to be the rate limiting step in anandamide biosynthesis (Okamoto et al., 2007). NAPE can be directly converted into anandamide by a NAPE-specific phospholipase D (NAPE-PLD) (Okamoto et al., 2004), and this was originally considered as the most important route for anandamide formation. However, the existence of alternative biosynthetic pathways has been documented (Liu et al., 2006; Simon and Cravatt, 2006; Sun et al., 2004), and studies with enzyme-knockout animals suggest that these pathways are able to substitute for one another in vivo (Leung et al., 2006; Simon and Cravatt, 2010).
The overall importance of the eCB biosynthetic pathways is further underlined by the fact that all of the involved enzymes and substrates are almost ubiquitously present throughout the body tissues (Basavarajappa, 2007).
3. Involvement of Gq/11-coupled receptor signaling in endocannabinoid release
The role of Gq/11 protein-mediated signaling in eCB release was implicated already in the first models of retrograde cannabinoid signaling in the CNS (Ohno-Shosaku et al., 2002; Wilson and Nicoll, 2002). According to this classical model, presynaptically released glutamate neurotransmitter can activate both ionotropic (e.g. NMDA-type) and metabotropic (mGluR) glutamate receptors on the postsynaptic neuronal membrane. This leads, besides the formation of excitatory postsynaptic potentials (EPSPs), to eCB production in the postsynaptic cell, mainly via two mechanisms: calcium signal generation, which can activate enzymes involved in eCB formation, and metabolism of DAG to 2-AG (Fig. 1).
Calcium signals are generated in the postsynaptic cells, either upon depolarization resulting from ionotropic receptor activation (via voltage-gated Ca2+-channels) or by the activation of a Gq/11 protein-coupled mGluR (via IP3 formation and Ca2+-release from intracellular stores). This Ca2+ signal can mediate eCB formation, since the key enzymes of 2-AG and anandamide biosynthesis (i.e. DAGL and NAT, respectively) are activated by Ca2+. The released eCBs can then leave the postsynaptic cell and activate presynaptically localized CB1 cannabinoid receptors (CB1Rs) in a retrograde manner.
On the other hand, activation of a Gq/11-coupled (i.e. group (1) postsynaptic mGluR also leads to the activation of the phospholipase Cß enzyme, which cleaves phosphatidylinositol 4,5-bisphosphate (PtdInsP2) in the plasma membrane to form DAG and inositol 1,4,5-trisphosphate (InsP3). The produced DAG can serve as a substrate for 2-AG production.
The above model of retrograde cannabinoid signaling has been extensively studied since its first description, and is still considered to be one of the most important functions of the ECS in the CNS in various physiological and pathophysiological processes. In terms of molecular pharmacology, however, a key element of this model is a receptor transactivation mechanism by which the activation of a G protein-coupled receptor can lead to the formation of eCB ligands, which then cause paracrine activation of their receptors. More importantly, this mechanism does not have to be specific to a certain type of Gq/11-coupled receptor (e.g. mGluR), nor to the CNS, as the molecular components of the transactivation process (i.e. various Gq/11-coupled receptors, signaling molecules and eCB biosynthetic enzymes as well as cannabinoid receptors) are known to be present in most of the tissues throughout the body. This led to the conclusion that the activation of the ECS through Gq/11-coupled receptors is a general physiological phenomenon (Turu et al., 2009). In other words, eCB release most likely constitutes a general intercellular signaling mechanism that contributes to the physiological effects of Gq/11-coupled receptors. In the next part of this paper, characteristic examples of such phenomena as well as the physiological roles of these processes will be discussed.
4. Endocannabinoid release upon Gq/11 protein activation in the CNS
4.1. Group 1 metabotropic glutamate receptors
As indicated above, the first example of the phenomenon that a Gq/11-coupled receptor can trigger the release of eCBs was described in the glutamatergic synapses of the central nervous system. The involvement of retrogradely acting eCBs in the presynaptic inhibition following postsynaptic depolarization (a phenomenon termed depolarization-induced suppression of inhibition or excitation, DSI or DSE, respectively) was reported in 2001 by several research groups (Kreitzer and Regehr, 2001; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). In the same year, however, the role of group 1 mGluR activation in retrograde eCB signaling was also demonstrated. Maejima and co-workers described that the activation of postsynaptic mGlu1 receptors in the cerebellum inhibited the activity of presynaptic excitatory climbing fibers by a mechanism, which required G protein activation and presynaptic CB1 receptors, but was on its own independent of intracellular calcium (Maejima et al., 2001). Another study demonstrated the same signaling mechanism in the pyramidal cells of the hippocampal cortex, where the enhancement of DSI upon mGlu1 receptor activation could be blocked by CB1R inverse agonist and was absent in CB1R-deficient mice (Varma et al., 2001). These results soon led to the integrative model that depolarization and group 1 mGluR activation on the postsynaptic cell may act in a parallel, cooperative manner to enhance eCB release, thus modifying synaptic transmission (Ohno-Shosaku et al., 2002; Wilson and Nicoll, 2002). Since then, eCB release upon group 1 mGluR activation has been described in many other brain areas, revealing a wide-spread and physiologically important signaling mechanism in the CNS (Kano et al., 2009).
4.2. Gq/11-coupled muscarinic acetylcholine receptors
Soon after the first descriptions of mGluR-mediated eCB release in the CNS, another subfamily of metabotropic receptors, namely the muscarinic acetylcholine receptors (mAChRs), were shown to modulate synaptic transmission partly through the release of eCBs. In the hippocampus, cholinergic agonists enhance depolarization-induced suppression of inhibition (DSI) in a way which requires postsynaptic G proteins, and is sensitive to inhibition of CB1R (Kim et al., 2002). The involvement of Gq/11-coupled mAChRs (M1 and M3) in this mechanism was also described (Fukudome et al., 2004; Ohno-Shosaku et al., 2003). This led to the conclusion that postsynaptically localized Gq/11-coupled mAChRs can, in cooperation with group 1 mGluRs, modulate synaptic transmission through retrograde eCB signaling in the hippocampal cortex. Since then, the effect ofmAChR activation on eCB release has been demonstrated in other CNS structures, which further underlines its physiological importance (Lau and Vaughan, 2008; Narushima et al., 2007).
4.3. Other Gq/11-coupled receptors in the CNS
The studies described above represent characteristic examples of how Gq/11-coupled receptors in the CNS can utilize eCBs to exert their effects on the synaptic transmission and related physiological functions. However, there are several other data with different Gq/11-coupled receptors indicating that a similar mechanism is generally present in various parts of the brain. We mention these studies briefly to demonstrate that eCB release upon Gq/11 activation can be considered as a generally occurring signaling event in the CNS.
The neuropeptide cholecystokinin (CCK) has been shown to regulate the GABAergic input of hippocampal pyramidal cells through an eCB-dependent mechanism (Foldy et al., 2007). The inhibition of GABA release from CCK+ basket cells required postsynaptic CCK2-receptor activation with subsequent Gq/11 protein-dependent eCB release and retrograde cannabinoid signaling (Lee and Soltesz, 2011). The interplay between CCK and the ECS has also been demonstrated in behavioral studies (Chhatwal et al., 2009; Kurrikoff et al., 2008).
Activation of serotonin 5HT2A and 5HT2C receptors led to 2-AG production in cultured NIH3T3 cells with a phospholipase C-dependent mechanism (Bockaert et al., 2006; Parrish and Nichols, 2006). In the inferior olive, activation of postsynaptic 5HT2 receptors caused the suppression of glutamatergic excitatory inputs, and this effect could be blocked by the CB1R inverse agonist AM251 (Best and Regehr, 2008). Furthermore, serotonergic negative feedback and functionality of 5-HT1 and 5-HT2A/C receptors were impaired in CB1R-deficient mice (Aso et al., 2009; Mato et al., 2007). The role of ECS–serotonin interaction in the regulation of trait anxiety has been shown in human genetic studies (Lazary et al., 2009) and a similar relationship is the main candidate also for the CB1R antagonist rimonabant-induced anxiety (Lazary et al., 2011), further suggesting the involvement of the ECS in physiological serotonin signaling.
In the hypothalamic supraoptic nucleus (SON), endothelin depresses glutamatergic transmission via ETA receptor-mediated retrograde eCB signaling (Zampronio et al., 2010). Oxytocin, released from the magnocellular neurons of SON, was shown to activate autoreceptors to generate eCBs, which then retrogradely modify the synaptic inputs of these neurons (Hirasawa et al., 2004). Ghrelin, a brain-gut peptide, was shown to exert its orexigenic effect through the activation of the cannabinoid system in the parvocellular neurons of the paraventricular nucleus. Ghrelin-induced inhibition of the excitatory inputs on these neurons, as well as its stimulatory effect on AMP-kinase activity and food intake required an intact cannabinoid signaling pathway in this hypothalamic area of mice (Kola et al., 2008; Tucci et al., 2004). In the dorsal raphe nucleus, the wake-promoting neuropeptide orexin-B inhibited the glutamatergic input presynaptically, but via a postsynaptic, G protein-dependent mechanism, and this pathway involved postsynaptic PLC and DAGL activity as well as presynaptic CB1 receptors (Haj-Dahmane and Shen, 2005). In a very recent report, activation of the PAR1 receptor with thrombin or PAR1-specific peptide agonist led to the suppression of inhibitory transmission in cultured hippocampal neurons, with the involvement of postsynaptic DAGL and presynaptic CB1 receptors, independently of mGluR activation (Hashimotodani et al., 2011).
In studies published by the same research group, centrally administered vasopressin, corticotropin-releasing factor (CRF) and bombesin all elevated the central adrenomedullary outflow and thus plasma cathecholamine levels through the activation of PLC and DAGL and subsequent generation of 2-AG. However, the effects exerted by 2-AG itself were most likely inhibitory, with the involvement of central CB1 receptors, and the stimulatory effects on sympathetic outflow were caused by the arachidonic acid derivatives generated after 2-AG degradation (Shimizu et al., 2004, 2005, 2010, 2011; Shimizu and Yokotani, 2008; Yokotani et al., 2001).
Interestingly, it has also been shown that a fast, i.e. non-genomic, action of corticosteroids in the hypothalamic paraventricular nucleus was the suppression of glutamatergic synaptic inputs with the involvement of eCBs, through a putative, G protein-coupled membrane corticosteroid receptor (Di et al., 2003, 2005).
5. Angiotensin II and endocannabinoids
We have previously demonstrated that stimulation of AT1 angiotensin receptors (AT1Rs) with angiotensin II (Ang II) in heterologous expression systems can lead to stimulation of cannabinoid receptors (Turu et al., 2007), because activation of AT1R, similar to other Gq/11-coupled receptors, can lead to 2-AG release and paracrine transactivation of CB1R (Turu et al., 2009). In order to demonstrate the physiological relevance of this finding, we have investigated the role of CB1Rs in the hypertensive effect of centrally administered Ang II.
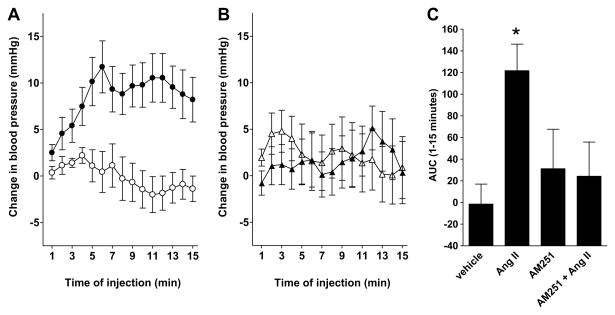
Ang II was microinfused directly into the hypothalamic paraventricular nucleus (PVN) of male Wistar rats (weighing 484.1 ± 10 g; mean ± SE). As shown in Fig. 2, infusion of Ang II in the PVN caused elevation of the blood pressure, which is a well-known effect of centrally administered Ang II (Bains and Ferguson, 1995; Miyakubo et al., 2002; Zhang et al., 2002). Our data show that this change could be abolished by co-administration of AM251, an inverse agonist of CB1R (Fig. 2). AM251 alone caused modest, immediate but short-term increase in blood pressure. These results suggest that the endocannabinoids have dual role in blood pressure regulation in the hypothalamus, and the mechanism by which Ang II can cause hypertension involves the activation of CB1Rs. Although it is likely that, similar to other systems, Gq/11-mediated eCB release can mediate this central effect of Ang II, cross inhibition of heterodimerized GPCRs may also serve as an alternative explanation for these results (Hudson et al., 2010; Szidonya et al., 2008). In fact, a recent study has demonstrated that heterodimerization of CB1Rs and AT1Rs can occur, and affect the function of these receptors (Rozenfeld et al., 2011). It has also been proposed that the effect of Ang II in the PVN is mediated by reactive oxygen species (Chen and Pan, 2007; Li and Pan, 2005). However, in many cases reactive oxygen species serve as a signal amplifier (Hunyady and Catt, 2006), and Ang II-induced eCB release can also mediate the disinhibition of the GABAergic tone, which mediates the effects of AT1R activation in the PVN. Additional studies are required to elucidate the exact role of CB1Rs in the mechanism of action of Ang II in the hypothalamus.
Fig. 2.
CB1R plays a role in the hypertensive effects of angiotensin II in the rat paraventricular nucleus. AM251 (150 ng/rat, Biozol) and angiotensin II (500 ng/rat, Sigma) and/or vehicle were administered through a cannula inserted into a previously implanted guide cannula into the paraventricular nucleus of the hypothalamus (PVN) in a single 10 min microinfusion in urethane anaesthetized male Wistar rats. Mean arterial pressure (MAP) was measured from the left femoral artery. Surgery, localization of the PVN and verification of the site of injection were performed as described previously (Bagdy, 1996; Bagdy and Makara, 1994; To and Bagdy, 1999). (A) Grouped data showing the changes from baseline in the MAP of animals treated by vehicle (open circles) or Ang II (closed circles). (B) Grouped data showing the changes from baseline in the MAP of animals treated by AM251 (open triangles) or Ang II + AM251 (closed triangles). (C) Area under the curve (AUC) was determined for MAP changes over a 15-min period from the beginning of the treatment. Statistical analysis (two-way ANOVA followed by Tukey's post hoc test) showed significant differences between AUC values of AngII- and AngII + AM251-treated animals (*p < 0.05 compared to vehicle treatment). All data are mean ± SEM, n = 3. All animal experiments were carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the National Institutes of Health “Principles of Laboratory Animal Care” (NIH Publications No. 85-23, revised 1985), as well as specific national laws (the Hungarian Governmental Regulations on animal studies, December 31, 1998). Permission was obtained from the local ethical committees.
6. Endocannabinoid release upon Gq/11 protein activation in peripheral tissues
More and more data are becoming available about the presence and function of the ECS in peripheral tissues, including the cardiovascular, gastrointestinal and immune systems as well as the liver, adipose tissue, peripheral nerves and reproductive organs (Pacher et al., 2006). Discussing the functions of the ECS in these systems in its full complexity is beyond the scope of this review. Therefore, we focus here on examples, where eCB production and subsequent cannabinoid receptor-mediated effects upon activation of a Gq/11-coupled receptor, analogously to the central nervous system, have been demonstrated in peripheral tissues.
The ECS has long been known to have a role in the regulation of vascular tone under physiological and pathophysiological conditions. Its effect proved to be largely vasodilatory in different systems, involving various effector mechanisms, the complexity of which is still not fully understood (for reviews, see (Pacher et al., 2005; Randall et al., 2002). Soon after their discovery, eCBs were suggested to represent the enigmatic endothelium-derived hyperpolarizing factor (EDHF). Two early studies showed that the endothelium-dependent vasodilatory effects of bradykinin and/or carbachol can be antagonized by the CB1R inverse agonist SR141716A in the rat mesenteric artery. In both studies, application of the calcium ionophore A23187 also caused endothelium-dependent vasodilation, which was inhibited by SR141716A (Randall et al., 1996; White and Hiley, 1997). In the next few years, the presence of CB1 receptors on vascular smooth muscle (Gebremedhin et al., 1999) and endothelial cells (Liu et al., 2000), as well as on sympathetic nerve endings innervating the vessels (Ishac et al., 1996) was also documented. Given that these receptors (i.e. B1 and B2 bradykinin receptors and M1 or M3 muscarinic acetylcholine receptors) are able to activate Gq/11 proteins and generate an intracellular calcium signal, these data indicate that a part of the mechanism through which they induce vasodilation could be the production of eCBs in endothelial cells and the transactivation of cannabinoid receptors. Indeed, elevated levels of 2-AG after carbachol stimulus have been reported in the rat aorta (Mechoulam et al., 1998).
In contrast, some studies carried out using other species or tissues showed that bradykinin- or acetylcholine-mediated vasodilatation in these other tissues either shows SR141716A-sensitivity only at high agonist concentrations or is not sensitive to SR141716A at all (Chataigneau et al., 1998; Fulton and Quilley, 1998; Niederhoffer and Szabo, 1999). Therefore this paracrine effect, and the involvement of eCB formation in the vasodilatory actions of bradykinin and acetylcholine, seems to be tissue-dependent.
In rat cerebral arteries, application of the thromboxane A2-mimetic compound U-46619 led to a significant increase in the 2-AG-and AEA-contents of these vessels. Moreover, the vasoconstrictor effect of U-46619 was more potent in the presence of CB1R inverse agonist, whereas compounds interfering with 2-AG degradation reduced the effect of U-46619, suggesting a physiological feedback mechanism exerted by the ECS in response to TP receptor stimulation (Hillard et al., 2007; Rademacher et al., 2005).
In addition to the above mentioned examples, only a few other cases of such transactivation procedures have been described in peripheral tissues. Although activation of the ECS by Gq/11-coupled receptors is a well-established phenomenon in the CNS, relatively few studies have addressed this question in peripheral organs. Moreover, in many cases it is difficult to identify a well-defined cannabinoid transactivation mechanism in the periphery, because the tissue environment is often diverse, with less tightly organized structures, compared e.g. to CNS synapses. For example, the release of 2-AG upon platelet activating factor (PAF) stimulus was reported in 2001 in platelets and macrophage cells, and the involvement of the Gq/11-coupled PAF receptor and phospholipase C was also demonstrated (Berdyshev et al., 2001). This may theoretically have important effects on immune and endothelial functions. However, a circumscribed mechanism through which this would lead to cannabinoid transactivation, and a picture about the relative physiological role of the phenomenon are still lacking.
7. Involvement of G proteins other than Gq/11, in endocannabinoid release
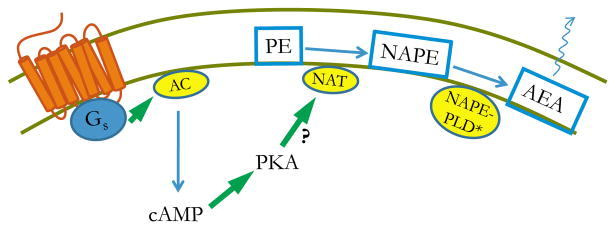
So far we have focused on the effects of Gq/11-coupled GPCRs on eCB production. However, activation of other G proteins can also stimulate the release of eCBs. To our knowledge, no data have been hitherto presented indicating that activation of the Gi/o- or G12/13-subtypes could stimulate eCB release. However, some results have suggested that Gs protein activation, through subsequent elevation of cAMP levels and protein kinase A (PKA) activation, could possibly participate in GPCR-induced eCB formation (Fig. 3).
Fig. 3.
The putative mechanism by which the activation of a Gs-coupled receptor may enhance endocannabinoid production. Activation of a Gs-coupled receptor leads to the activation of the adenylyl cyclase enzyme, which produces cAMP. cAMP can activate PKA, which, through the phosphorylation of an as yet unidentified target, may enhance the production of eCBs, principally that of anandamide. As the rate-limiting step in AEA-biosynthesis is catalyzed by NAT, it would seem reasonable that PKA exerts its potentiating effect on NAT. However, no direct evidence demonstrating this effect is available at the moment. Abbreviations: cAMP, cyclic AMP; PKA, protein kinase A; AC, adenylyl cyclase; AEA, N-arachidonoyl-ethanolamine (anandamide); NAPE, N-arachidonoyl-phosphatidylethanolamine; NAPE-PLD, NAPE-specific phospholipase D; NAT, N-acyltransferase; PE, phosphatidylethanolamine. * Indicates alternative pathways for anandamide production (see text for details).
In 1996, Cadas et al. showed that in rat cortical neurons the ionomycin-induced (i.e. calcium-dependent) biosynthesis of the anandamide precursor molecule NAPE is enhanced by the simultaneous activation of PKA, either directly by forskolin, or through activation of Gs-coupled receptors by stimulating neurons with VIP. However, application of forskolin or VIP alone (i.e. without elevating intracellular calcium with ionomycin) did not result in elevated NAPE-levels. This suggested a possible role of Gs protein-coupled receptors in potentiating the calcium-dependent formation of eCBs (Cadas et al., 1996).
Since then, this question has not been in the main focus of eCB research, and therefore we do not have much data on whether this kind of effect can have a role in the different physiological systems. However, some studies provide important additions to this question. In 2006, Malcher-Lopes et al. reported that the rapid, non-genomic effect of glucocorticoids in the PVN, which requires a putative metabotropic glucocorticoid receptor and is mediated by eCB release, can be inhibited by blocking the activity of Gs proteins or PKA. Both 2-AG and anandamide were involved in this effect (Malcher-Lopes et al., 2006). Whether or not this process requires intracellular calcium at any level was not addressed in this study, but the results suggest that Gs protein activation and subsequent activation of PKA can increase eCB levels, thus modulating retrograde eCB signaling.
In another study, eCB levels in HEK293 cell cultures were increased upon forskolin stimulus. A similar effect was observed in dorsal root ganglia preparations in the case of AEA, however, without a significant increase in 2-AG levels. The increase in the AEA levels caused by forskolin was not significant, if intracellular calcium was buffered with BAPTA (Vellani et al., 2008).
The above results provide interesting information regarding the capability of Gs-coupled receptors to enhance eCB release. However, to gain a clearer view it will be important to identify the enzymatic targets (e.g. eCB biosynthetic or degrading enzymes) through which PKA can exert its effect, and to clarify whether this signaling pathway can stimulate eCB biosynthesis on its own, or has only a modifying effect on the ‘canonical’, Gq/11- and/or calcium-mediated eCB release.
8. Further aspects of the interplay between GPCRs and the ECS
As demonstrated in the previous parts of our review, modulation of the endocannabinoid system upon GPCR activation can be considered as a quite general physiological phenomenon. However, this model cannot be extended to any system without limitations, and one should be careful when evaluating results concerning the GPCR-mediated activation of the ECS.
First, the fact that activation of a GPCR leads to elevated eCB levels in a tissue does not necessarily mean that this will result in cannabinoid receptor transactivation. The relatively rapid metabolic degradation of eCBs resulting in inactive products or their conversion into other biologically active compounds may have a great impact on the observed effects (Vandevoorde and Lambert, 2007). Moreover, since it is known that non-CB1/CB2 GPCRs or transient receptor potential (TRP) channels can also serve as eCB targets, their presence may thus also be responsible for some of the results obtained (De Petrocellis and Di Marzo, 2010).
Second, eCB molecules, once produced by a cell, may not only act intercellularly, i.e. as a paracrine messenger, but can also have autocrine mediatory functions through cannabinoid receptors. In the CNS, Bacci and co-workers reported the phenomenon of slow self-inhibition, by which repetitive firing of low-threshold spiking (LTS) interneurons enhances the production of 2-AG in these cells, followed by the autocrine activation of CB1Rs, which mediate the long-lasting hyperpolarization of the same cell (Bacci et al., 2004; Marinelli et al., 2008). Although this question has not been addressed directly in peripheral systems, it is likely that eCBs can exert autocrine effects also in these tissues, and transactivation of CB1R by other GPCRs may also have such physiological aspects. It is, however, hard to differentiate between paracrine and autocrine effects when both receptors are present in the same cell type, as is the case in many peripheral tissues. Moreover, heterodimerization of CB1R with other GPCRs has been demonstrated in many cases (Hudson et al., 2010; Rozenfeld et al., 2011; Ward et al., 2011), which makes this scenario even more complex. Nevertheless, the concept of eCBs as autocrine messengers should be taken into account when studying the G protein-mediated regulation of eCB release.
Third, an interesting issue concerning GPCR-mediated eCB release is the concept of the existence of 2-AG pools in cell membranes, described very recently (Alger and Kim, 2011). According to this concept, basal synthesis of 2-AG might not be always coupled to its immediate release, and this leads to the formation of basal, non-signaling 2-AG pools in the cell membranes. Furthermore, neuronal activation might not only stimulate on-demand 2-AG synthesis (followed by prompt eCB release), but, in an as yet unidentified way, also triggers the release of 2-AG from these preformed pools. This can have important consequences e.g. on the duration and kinetics of distinct eCB-mediated signaling events, and might also be taken into account when studying GPCR-evoked eCB release, especially by short-term application of pharmacological inhibitors (e.g. those of PLC or DAGL), because release from a preformed pool can transitionally mask the inhibitory effects exerted on the ‘on-demand’ synthetic pathway. However, more direct investigations are needed to demonstrate the existence of such pools and the mechanisms by which GPCR activation or intracellular Ca2+ could stimulate eCB release from these pools, simultaneously with (or even independently of) on-demand eCB synthesis.
Finally, even if such transactivation is unequivocally demonstrated in a given system, differences between body regions or species may still exist (as seen in the case of vascular preparations). The amount and activity of the key enzymes of the process is known to vary with tissue, and the biological regulation of the participating receptors (e.g. receptor numbers, receptor sensitization or desensitization) can also modify the responses.
9. Conclusions
In this review we have summarized the best-known examples of how G protein-coupled receptor signaling can lead to activation of the ECS. The classical model of this transactivation was described in 2001 in CNS synapses, but we extend this scenario by suggesting that eCB formation and subsequent paracrine transactivation of cannabinoid receptors form a general part of Gq/11-mediated signaling in the most diverse tissues of the body. We have paid attention in this review to underline the physiological relevance of this process in the different systems, at least where we have enough data to draw such conclusions, to show that this phenomenon is widespread and physiologically meaningful. We have also discussed some further important aspects of the GPCR-ECS interplay, which contribute to the complexity of this issue and should be kept in mind when studying these regulatory phenomena.
Nevertheless, it would be interesting to see whether the many GPCRs that are theoretically capable of stimulating eCB release (i.e. couple to Gq/11 proteins), but have as yet not been implicated in this model of cannabinoid transactivation, may utilize this type of receptor cross-talk to exert their physiological effects. This is especially true for peripheral systems, e.g. the immune system or the tissues principally involved in metabolic regulation, where the interplay between the ECS and other hormones and modulators is currently less understood.
Acknowledgments
The help provided by Jolán Józan in the drawing of the figures is greatly appreciated. This work was supported in part by Grants from the Hungarian Science Foundation (OTKA NK-072661), the Hungarian Ministry of Public Health (ETT 337/2009, ETT 318/2009), TAMOP-4.2.1.B-09/1/KMR-2010-0001 and EU, LSHM-CT-2004-503474.
References
- Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci. 2011;34:304–315. doi: 10.1016/j.tins.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E, Renoir T, Mengod G, Ledent C, Hamon M, Maldonado R, Lanfumey L, Valverde O. Lack of CB1 receptor activity impairs serotonergic negative feedback. J Neurochem. 2009;109:935–944. doi: 10.1111/j.1471-4159.2009.06025.x. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bagdy G. Role of the hypothalamic paraventricular nucleus in 5-HT1A, 5-HT2A and 5-HT2C receptor-mediated oxytocin, prolactin and ACTH/corticosterone responses. Behav Brain Res. 1996;73:277–280. doi: 10.1016/0166-4328(96)00112-x. [DOI] [PubMed] [Google Scholar]
- Bagdy G, Makara GB. Hypothalamic paraventricular nucleus lesions differentially affect serotonin-1A (5-HT1A) and 5-HT2 receptor agonist-induced oxytocin, prolactin, and corticosterone responses. Endocrinology. 1994;134:1127–1131. doi: 10.1210/endo.134.3.8119151. [DOI] [PubMed] [Google Scholar]
- Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol. 1995;268:R625–R633. doi: 10.1152/ajpregu.1995.268.3.R625. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS. Critical enzymes involved in endocannabinoid metabolism. Protein Pept Lett. 2007;14:237–246. doi: 10.2174/092986607780090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdyshev EV, Schmid PC, Krebsbach RJ, Schmid HH. Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. FASEB J. 2001;15:2171–2178. doi: 10.1096/fj.01-0181com. [DOI] [PubMed] [Google Scholar]
- Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci. 2008;28:6508–6515. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T. Endogenous cannabinoids: structure and metabolism. J Neuroendocrinol. 2008;20(Suppl. 1):1–9. doi: 10.1111/j.1365-2826.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res. 2006;326:553–572. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chataigneau T, Feletou M, Thollon C, Villeneuve N, Vilaine JP, Duhault J, Vanhoutte PM. Cannabinoid CB1 receptor and endothelium-dependent hyperpolarization in guinea-pig carotid, rat mesenteric and porcine coronary arteries. Br J Pharmacol. 1998;123:968–974. doi: 10.1038/sj.bjp.0701690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Pan HL. Signaling mechanisms of angiotensin II-induced attenuation of GABAergic input to hypothalamic presympathetic neurons. J Neurophysiol. 2007;97:3279–3287. doi: 10.1152/jn.01329.2006. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Gutman AR, Maguschak KA, Bowser ME, Yang Y, Davis M, Ressler KJ. Functional interactions between endocannabinoid and CCK neurotransmitter systems may be critical for extinction learning. Neuropsychopharmacology. 2009;34:509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- Foldy C, Lee SY, Szabadics J, Neu A, Soltesz I. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10:1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Fulton D, Quilley J. Evidence against anandamide as the hyperpolarizing factor mediating the nitric oxide-independent coronary vasodilator effect of bradykinin in the rat. J Pharmacol Exp Ther. 1998;286:1146–1151. [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am J Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Ca(2+)-assisted receptordriven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol. 2007;17:360–365. doi: 10.1016/j.conb.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Yamazaki M, Sakimura K, Kano M. Neuronal protease-activated receptor 1 drives synaptic retrograde signaling mediated by the endocannabinoid 2-arachidonoylglycerol. J Neurosci. 2011;31:3104–3109. doi: 10.1523/JNEUROSCI.6000-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Ho WS, Thompson J, Gauthier KM, Wheelock CE, Huang H, Hammock BD. Inhibition of 2-arachidonoylglycerol catabolism modulates vasoconstriction of rat middle cerebral artery by the thromboxane mimetic, U-46619. Br J Pharmacol. 2007;152:691–698. doi: 10.1038/sj.bjp.0707468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson BD, Hebert TE, Kelly ME. Ligand- and heterodimer-directed signaling of the CB(1) cannabinoid receptor. Mol Pharmacol. 2010;77:1–9. doi: 10.1124/mol.109.060251. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br J Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin XH, Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J Biol Chem. 2007;282:3614–3623. doi: 10.1074/jbc.M606369200. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–10191. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kurrikoff K, Inno J, Matsui T, Vasar E. Stress-induced analgesia in mice. Evidence for interaction between endocannabinoids and cholecystokinin Eur J Neurosci. 2008;27:2147–2155. doi: 10.1111/j.1460-9568.2008.06160.x. [DOI] [PubMed] [Google Scholar]
- Lau BK, Vaughan CW. Muscarinic modulation of synaptic transmission via endocannabinoid signalling in the rat midbrain periaqueductal gray. Mol Pharmacol. 2008;74:1392–1398. doi: 10.1124/mol.108.045872. [DOI] [PubMed] [Google Scholar]
- Lazary J, Juhasz G, Hunyady L, Bagdy G. Personalized medicine can pave the way for the safe use of CB receptor antagonists. Trends Pharmacol Sci. 2011;32:270–280. doi: 10.1016/j.tips.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Lazary J, Lazary A, Gonda X, Benko A, Molnar E, Hunyady L, Juhasz G, Bagdy G. Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1118–1127. doi: 10.1002/ajmg.b.31024. [DOI] [PubMed] [Google Scholar]
- Lee SH, Soltesz I. Requirement for CB1 but not GABAB receptors in the cholecystokinin mediated inhibition of GABA release from cholecystokinin expressing basket cells. J Physiol. 2011;589:891–902. doi: 10.1113/jphysiol.2010.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricular-rostral ventrolateral medulla output neurons. J Pharmacol Exp Ther. 2005;313:1035–1045. doi: 10.1124/jpet.104.082495. [DOI] [PubMed] [Google Scholar]
- Liu J, Mirshahi F, Gao B, Khanolkar AD, Sanyal AJ, Kunos G, Makriyannis A. Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J. 2000;346(Pt. 3):835–840. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Bisogno T, Di Marzo V, Prince DA, Huguenard JR, Bacci A. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28:13532–13541. doi: 10.1523/JNEUROSCI.0847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mato S, Aso E, Castro E, Martin M, Valverde O, Maldonado R, Pazos A. CB1 knockout mice display impaired functionality of 5-HT1A and 5-HT2A/C receptors. J Neurochem. 2007;103:2111–2120. doi: 10.1111/j.1471-4159.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Fride E, Ben Shabat S, Meiri U, Horowitz M. Carbachol, an acetylcholine receptor agonist, enhances production in rat aorta of 2-arachidonoyl glycerol, a hypotensive endocannabinoid. Eur J Pharmacol. 1998;362:R1–R3. doi: 10.1016/s0014-2999(98)00777-8. [DOI] [PubMed] [Google Scholar]
- Miyakubo H, Hayashi Y, Tanaka J. Enhanced response of subfornical organ neurons projecting to the hypothalamic paraventricular nucleus to angiotensin II in spontaneously hypertensive rats. Auton Neurosci. 2002;95:131–136. doi: 10.1016/s1566-0702(01)00388-5. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Involvement of CB1 cannabinoid receptors in the EDHF-dependent vasorelaxation in rabbits. Br J Pharmacol. 1999;126:1383–1386. doi: 10.1038/sj.bjp.0702452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci. 2003;18:109–116. doi: 10.1046/j.1460-9568.2003.02732.x. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Wang J, Morishita J, Ueda N. Biosynthetic pathways of the endocannabinoid anandamide. Chem Biodivers. 2007;4:1842–1857. doi: 10.1002/cbdv.200790155. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. Cardiovascular pharmacology of cannabinoids. Handb Exp Pharmacol. 2005:599–625. doi: 10.1007/3-540-26573-2_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JC, Nichols DE. Serotonin 5-HT(2A) receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem. 2006;99:1164–1175. doi: 10.1111/j.1471-4159.2006.04173.x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Hansen HS, Greasley PJ, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Patel S, Ho WS, Savoie AM, Rusch NJ, Gauthier KM, Hillard CJ. U-46619 but not serotonin increases endocannabinoid content in middle cerebral artery: evidence for functional relevance. Am J Physiol Heart Circ Physiol. 2005;288:H2694–H2701. doi: 10.1152/ajpheart.00978.2004. [DOI] [PubMed] [Google Scholar]
- Randall MD, Alexander SP, Bennett T, Boyd EA, Fry JR, Gardiner SM, Kemp PA, McCulloch AI, Kendall DA. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- Randall MD, Harris D, Kendall DA, Ralevic V. Cardiovascular effects of cannabinoids. Pharmacol Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee-Ramos D, Nieto N, Devi LA. AT1R-CB(1)R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011;30:2350–2363. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Lu L, Yokotani K. Possible inhibitory roles of endogenous 2-arachidonoylglycerol during corticotropin-releasing factor-induced activation of central sympatho-adrenomedullary outflow in anesthetized rats. Eur J Pharmacol. 2010;641:54–60. doi: 10.1016/j.ejphar.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Lu L, Yokotani K. Endogenously generated 2-arachidonoylglycerol plays an inhibitory role in bombesin-induced activation of central adrenomedullary outflow in rats. Eur J Pharmacol. 2011;658:123–131. doi: 10.1016/j.ejphar.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Okada S, Yamaguchi N, Arai J, Wakiguchi H, Yokotani K. Brain phospholipase C/diacylglycerol lipase are involved in bombesin BB2 receptor-mediated activation of sympatho-adrenomedullary outflow in rats. Eur J Pharmacol. 2005;514:151–158. doi: 10.1016/j.ejphar.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Okada S, Yamaguchi-Shima N, Yokotani K. Brain phospholipase C-diacylglycerol lipase pathway is involved in vasopressin-induced release of noradrenaline and adrenaline from adrenal medulla in rats. Eur J Pharmacol. 2004;499:99–105. doi: 10.1016/j.ejphar.2004.07.087. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Yokotani K. Bidirectional roles of the brain 2-arachidonoyl-sn-glycerol in the centrally administered vasopressin-induced adrenomedullary outflow in rats. Eur J Pharmacol. 2008;582:62–69. doi: 10.1016/j.ejphar.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Characterization of mice lacking candidate N-acyl ethanolamine biosynthetic enzymes provides evidence for multiple pathways that contribute to endocannabinoid production in vivo. Mol Biosyst. 2010;6:1411–1418. doi: 10.1039/c000237b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, Kudo I, Ueda N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–756. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szidonya L, Cserzo M, Hunyady L. Dimerization and oligomerization of G-protein-coupled receptors: debated structures with established and emerging functions. J Endocrinol. 2008;196:435–453. doi: 10.1677/JOE-07-0573. [DOI] [PubMed] [Google Scholar]
- To CT, Bagdy G. Anxiogenic effect of central CCK administration is attenuated by chronic fluoxetine or ipsapirone treatment. Neuropharmacology. 1999;38:279–282. doi: 10.1016/s0028-3908(98)00176-2. [DOI] [PubMed] [Google Scholar]
- Tucci SA, Rogers EK, Korbonits M, Kirkham TC. The cannabinoid CB1 receptor antagonist SR141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol. 2004;143:520–523. doi: 10.1038/sj.bjp.0705968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turu G, Simon A, Gyombolai P, Szidonya L, Bagdy G, Lenkei Z, Hunyady L. The role of diacylglycerol lipase in constitutive and angiotensin AT1 receptor-stimulated cannabinoid CB1 receptor activity. J Biol Chem. 2007;282:7753–7757. doi: 10.1074/jbc.C600318200. [DOI] [PubMed] [Google Scholar]
- Turu G, Varnai P, Gyombolai P, Szidonya L, Offertaler L, Bagdy G, Kunos G, Hunyady L. Paracrine transactivation of the CB1 cannabinoid receptor by AT1 angiotensin and other Gq/11 protein-coupled receptors. J Biol Chem. 2009;284:16914–16921. doi: 10.1074/jbc.M109.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoorde S, Lambert DM. The multiple pathways of endocannabinoid metabolism: a zoom out. Chem Biodivers. 2007;4:1858–1881. doi: 10.1002/cbdv.200790156. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Petrosino S, De Petrocellis L, Valenti M, Prandini M, Magherini PC, McNaughton PA, Di Marzo V. Functional lipidomics. Calciumindependent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacology. 2008;55:1274–1279. doi: 10.1016/j.neuropharm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Pediani JD, Milligan G. Hetero-multimerization of the cannabinoid CB1 receptor and the orexin OX1 receptor generates a unique complex in which both protomers are regulated by orexin A. J Biol Chem. 2011 doi: 10.1074/jbc.M111.287649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Yokotani K, Murakami Y, Okada S, Hirata M. Role of brain arachidonic acid cascade on central CRF1 receptor-mediated activation of sympatho-adrenomedullary outflow in rats. Eur J Pharmacol. 2001;419:183–189. doi: 10.1016/s0014-2999(01)00987-6. [DOI] [PubMed] [Google Scholar]
- Zampronio AR, Kuzmiski JB, Florence CM, Mulligan SJ, Pittman QJ. Opposing actions of endothelin-1 on glutamatergic transmission onto vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci. 2010;30:16855–16863. doi: 10.1523/JNEUROSCI.5079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Francis J, Weiss RM, Felder RB. The renin–angiotensin– aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002;283:H423–H433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]