Abstract
The increased abundance and activity of cathelicidin and kallikrein 5 (KLK5), a predominant trypsin-like serine protease (TLSP) in the stratum corneum, have been implicated in the pathogenesis of rosacea, a disorder treated by the use of low-dose doxycycline. Here we hypothesized that doxycycline can inhibit activation of tryptic KLKs through an indirect mechanism by inhibition of matrix metalloproteinases (MMPs) in keratinocytes. The capacity of doxycycline to directly inhibit enzyme activity was measured in surface collections of human facial skin and extracts of cultured keratinocytes by fluorescence polarization assay against fluorogenic substrates specific for MMPs or TLSPs. Doxycycline did inhibit MMP activity but did not directly inhibit serine protease activity against a fluorogenic substrate specific for TLSPs. However, when doxycycline or other MMP inhibitors were added to live keratinocytes during the production of tryptic KLKs, this treatment indirectly resulted in decreased TLSP activity. Furthermore, doxycycline under these conditions inhibited the generation of the cathelicidin peptide LL-37 from its precursor protein hCAP18, a process dependent on KLK activity. These results demonstrate that doxycycline can prevent cathelicidin activation, and suggest a previously unknown mechanism of action for doxycycline through inhibiting generation of active cathelicidin peptides.
INTRODUCTION
Cathelicidin is one of the most well-characterized antimicrobial peptides (AMPs) found in the human skin. Because of its direct antimicrobial action against a wide range of bacteria (Larrick et al., 1995b), the release of these peptides is responsible in part for innate defense against infectious pathogens. Human cathelicidin is produced as an inactive precursor protein with a mass of 18 kDa, and is named hCAP18 (Larrick et al., 1995a). Posttranscriptional processing cleaves the C-terminal cathelin domain from hCAP18 and produces active AMPs such as a peptide composed of 37 amino acids beginning with two leucines, named LL-37. Recently, our group showed that stratum corneum trypsin-like serine proteinases (TLSPs) of the kallikrein (KLK)-related peptidase family participate in the proteolytic activation of hCAP18 to LL-37 in the skin (Yamasaki et al., 2006, 2007). KLKs are present in virtually all tissues and fluids of the body at varying levels of expression. KLK5, originally denoted as stratum corneum tryptic enzyme, is the most predominant TLSP in the stratum corneum of human skin (Brattsand and Egelrud, 1999). KLK5 is highly expressed in the stratum granulosum, and is then transported by lamellar granules and accumulates in the stratum corneum (Ishida-Yamamoto et al., 2005). KLK14 is also a TLSP found in human stratum corneum (Stefansson et al., 2006), which may contribute to proteolytic activation of hCAP18 to LL-37 in addition to KLK5. KLKs are released as inactive preproenzymes with a signal peptide removed upon secretion and profragment removed during activation (Kantyka et al., 2011). Activation entails either autoactivation and/or activation by other KLKs in a cascade-like manner, or removal of the profragment by enzymes such as matrix metalloproteinases (MMPs) or other endopeptidases (Ryu et al., 2002; Lundwall and Brattsand, 2008; Sotiropoulou et al., 2009). For example, pro-KLK4 undergoes proteolytic activation by MMP-3 and MMP-20 (Ryu et al., 2002; Beaufort et al., 2010; Chun et al., 2010). Pro-KLK7 is processed by another epidermal metalloproteinase, meprin β, before activation of pro-KLK7 by tryptic enzymes (Ohler et al., 2010). Thus, we have hypothesized that MMPs may stimulate KLK activity and thus indirectly catalyze proteolytic activation of hCAP18 to LL-37.
The cathelicidin peptide LL-37 not only has the capacity to kill microbes but can also modify host immunity and growth responses by promoting leukocyte chemotaxis (De et al., 2000), angiogenesis (Koczulla et al., 2003), and altering expression of extracellular matrix components (Wolf et al., 2001; Park et al., 2009). Recent clinical observations indicate that abnormal production of LL-37 in the skin is implicated in a wide variety of inflammatory skin diseases including psoriasis (Lande et al., 2007) and atopic dermatitis (Ong et al., 2002). In addition, abnormal expression of KLK5 was observed in rosacea skin, causing aberrant proteolytic activation of hCAP18 to the long peptide form of cathelicidin (LL-37) that is not found in large amounts on normal noninflamed skin (Yamasaki et al., 2007). KLK5 itself triggers a proinflammatory microenvironment independently of external stimuli by inducing cytokine overexpression and defective stratum corneum adhesion (Briot et al., 2009; Voegeli et al., 2009). It also affects dermal matrix and vascular remodeling via the digestion of corneodesmosin, desmocollin 1, desmoglein 1 (Caubet et al., 2004; Jiang et al., 2011), collagens type I, II, III, and IV, fibronectin, and laminin (Michael et al., 2005). Therefore, blocking the activity of tryptic KLKs may have a therapeutic capacity to relieve inflammation on two levels.
Doxycycline has been reported to nonselectively inhibit MMPs by binding to zinc and calcium atoms and causing conformational changes and loss of enzymatic activity (Garcia et al., 2005). In addition to this direct inhibitory activity, doxycycline has been reported to suppress gene expression of MMPs in human corneal epithelial cells (Li et al., 2004), human umbilical vein endothelial cells (Hanemaaijer et al., 1998), and skin keratinocytes (Uitto et al., 1994). Doxycycline has also been shown to have efficacy in the systemic treatment of rosacea at subantimicrobial doses (40 mg day−1), a dose below the conventional level (100–200 mg day−1) used in antimicrobial action (Korting and Schollmann, 2009a). These observations suggest that doxycycline may exert its therapeutic effects in rosacea by a mechanism that does not include alteration of microbial abundance. However, the precise mechanism of action of doxycycline in rosacea treatment is still unknown.
In this study, we investigated whether the ability of doxycycline to inhibit MMPs could influence the activity of tryptic KLKs and the activation of cathelicidin in human keratinocytes. Our findings offer a hypothesis that may explain the therapeutic potential of doxycycline, and could have broad implications for the treatment of disease states where overexpression of LL-37 has a role in inflammation.
RESULTS
Doxycycline inhibits MMP activity in human skin and cultured keratinocytes
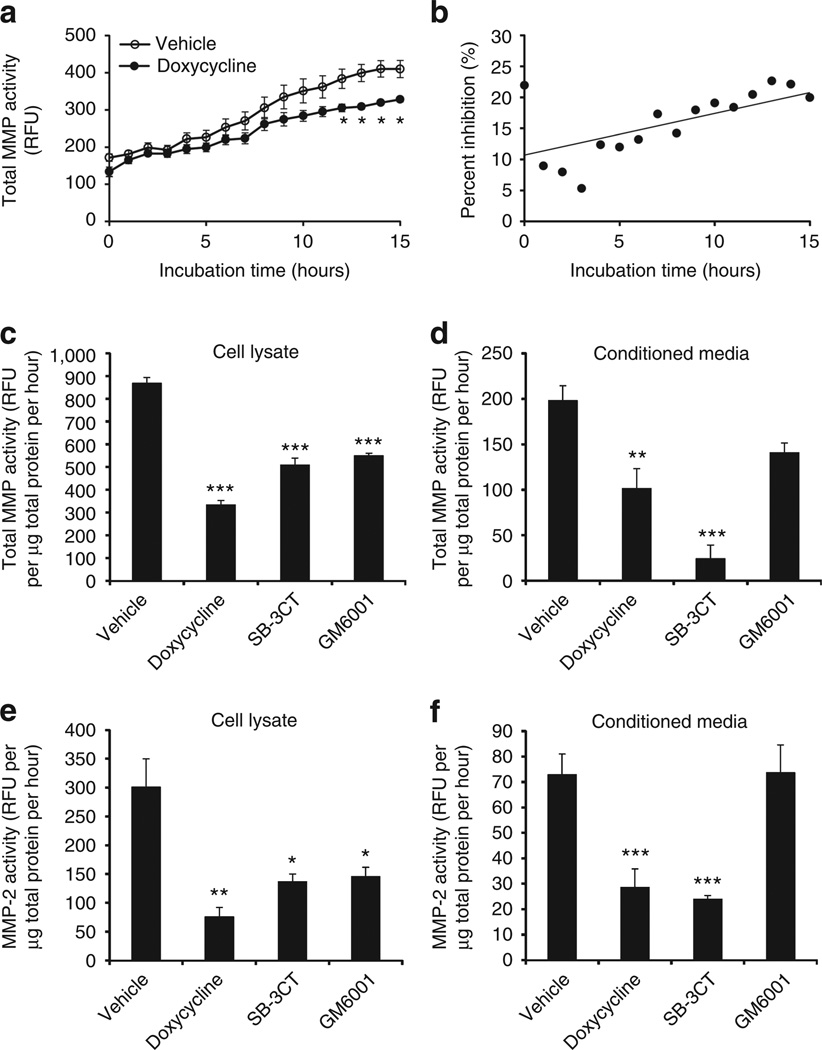
Prior observations that doxycycline nonselectively inhibits MMP activity led us to investigate the ability of doxycycline to attenuate the activity of cutaneous MMPs. Surface material from normal facial skin of healthy volunteers was collected by tape stripping, and enzymes were extracted. Cumulative activity of MMPs was measured by fluorescence polarization assay using an MMP-specific substrate. Enzymatic activity collected by tape strip catalyzed increased processing of the MMP substrate, and this was detected by increased fluorescent emission over time. Compared with skin surface protein extract treated with vehicle, total enzyme activity was significantly decreased when facial tape-strip proteins were treated with doxycycline at a concentration of 225 µm (Figure 1a). As expected for a kinetic assay, the percentage of total inhibition of MMP product by doxycycline, calculated every hour, grew linearly throughout the time period to >25% inhibition at 24 hours (Figure 1b). These observations made in a cell-free system of protein extracted from human facial skin suggested that doxycycline had a direct inhibitory effect on human cutaneous MMP activity.
Figure 1. Doxycycline inhibits human cutaneous and normal human epidermal keratinocyte (NHEK) matrix metalloproteinase (MMP) activity in vitro.
(a) Tape-strip samples were obtained from normal human facial skin, and total protein was extracted and normalized to a final protein concentration of 115 µg ml−1. MMP activity was measured in relative fluorescence units (RFU) using fluorogenic substrate (5 µm) sensitive to most MMP enzymes (MMP-1, MMP-3, MMP-7, MMP-8, MMP-9, MMP-12, MMP-13, MMP-14, MMP-16, and MMP-20). Total-MMP activity of enzymatic extract treated with doxycycline (225 µm) was decreased throughout incubation as compared with vehicle control. *P<0.05, **P<0.01 by Student’s t-test, versus vehicle. (b) Doxycycline inhibition of total MMP activity, expressed as percent inhibition (%) at every hour, increased linearly throughout. (c) Incubating NHEK cell lysate with doxycycline (225 µm) for 18 hours significantly attenuated total MMP activity, showing direct inhibitory action. SB3CT (5 µm), a selective inhibitor of MMP-2 and MMP-9, and GM6001 (25 µm), a nonselective MMP inhibitor, were used as positive controls and showed similar effect. Vehicle control was 0.1% (v/v) DMSO. (d) Total MMP activity in NHEK-conditioned media (for secreted MMPs) was measured with fluorogenic substrate in the presence of calcium and zinc. All three inhibitors suppressed total MMP activity. (e) Cell lysate was assayed for MMP-2 activity in the presence of zinc ion required for its enzymatic activity as described in the Materials and Methods. (f) Conditioned medium was assayed for MMP-2 activity as in e. *P<0.05, **P<0.01, ***P<0.005 by Student’s t-test, versus vehicle or control.
To determine whether the observed inhibitory effect of doxycycline on extracted human facial skin samples could be also measured in cultured keratinocytes, MMP activity was determined after doxycycline addition to lysates of normal human epidermal keratinocytes (NHEKs), or addition to culture media. Both total-MMP and MMP-2 activities in cell lysates (demonstrating cellular MMPs) and conditioned media (demonstrating secreted MMPs) were significantly suppressed (Figure 1c–f). At the concentrations used here, the degree of inhibition of MMP activity in the cell-associated fraction was similar between doxycycline (half-maximal inhibitory concentration (IC50)=15 µm) and known inhibitors of MMPs, SB-3CT (IC50=185–2,900 nm), a selective inhibitor of MMP-2 and MMP-9, and GM6001 (IC50=0.4 nm), a nonselective MMP inhibitor.
Serine protease inhibitors decrease TLSP activity in the stratum corneum of human skin in vitro
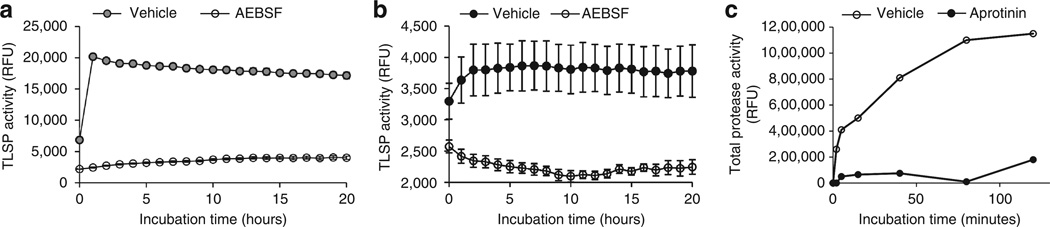
We next verified that serine protease inhibitors could directly attenuate the enzymatic activity in the stratum corneum of healthy skin using a similar fluorescence polarization assay and a TLSP-specific fluorogenic substrate. Because stratum corneum TLSPs, such as tryptic KLKs, are implicated in the direct activation of human cathelicidin precursor protein hCAP18 to mature peptide LL-37 on the skin surface (Yamasaki et al., 2006), we used Boc-Phe-Ser-Arg-AMC, a fluorogenic substrate specific for TLSPs, to establish this assay. As shown in Figure 2a, addition of purified tissue KLK rapidly cleaved the substrate to generate maximal fluorogenic product, and the activity of tissue-purified KLK was significantly inhibited for up to 20 hours by AEBSF (4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride), an irreversible serine protease inhibitor. This effect was also demonstrable in the proteins extracted from tape-strip samples collected from the facial skin of healthy volunteers. Protein extract from facial tape strips demonstrating TLSP activity reached a peak at 2 hours and could be suppressed in the presence of AEBSF (Figure 2b). In a similar manner, aprotinin, a competitive serine protease inhibitor, was also assessed for its ability to diminish TLSP activity in normal facial skin protein extract. Aprotinin strongly attenuated cutaneous total protease activity as compared with vehicle (Figure 2c), suggesting a predominance of serine proteases on the human skin surface. These data suggest that much of the total enzymatic activity detected by tape stripping human facial skin is serine protease in nature.
Figure 2. Serine protease inhibitors inhibit cutaneous trypsin-like serine protease (TLSP) activity in vitro.
(a) Tissue-purified kallikrein from porcine pancreas (50 µg ml−1) was incubated with and without 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF; 250 µm), an irreversible serine protease inhibitor. AEBSF directly inhibited TLSP activity as compared with vehicle control as measured using a fluorogenic TLSP-specific substrate (2.5 mm) to assay TLSP activity (relative fluorescence units (RFU)). (b) Tape-strip samples from normal facial skin were obtained, and total enzymes were extracted. Enzymatic extract was incubated with and without AEBSF to gauge the serine protease inhibitor’s ability to attenuate cutaneous TLSP activity (RFU). Compared with vehicle control, AEBSF inhibited cutaneous TLSP activity throughout 20 hours of incubation. (c) Tape-strip samples from normal facial skin were measured with EnzChek Protease Assay Kit (Invitrogen) in the absence of inhibitor (vehicle) and in the presence of aprotinin (50 µg ml−1), a competitive inhibitor for serine proteases. Aprotinin strongly attenuated cutaneous total protease activity.
Doxycycline directly inhibits MMP activity, but indirectly inhibits TLSP activity
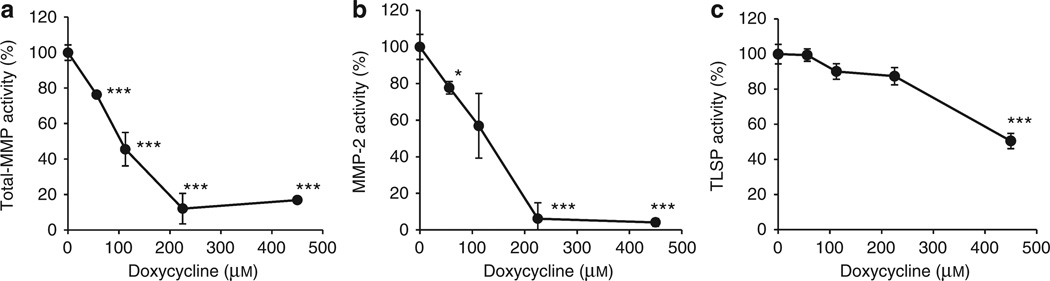
Having established sensitive direct assays of human skin MMP and TLSP activity, we next sought to examine the inhibitory effects of doxycycline on these enzymes in human keratinocytes. The activities of these enzymes were first measured directly in lysates of NHEKs where the final proteolytic activation of tryptic KLKs could proceed before the addition of inhibitors. Final enzyme activity was determined in the presence of various concentrations of doxycycline (0, 56.25, 112.5, 225, and 450 µm) added to the NHEK lysate. The ability of doxycycline to diminish cellular enzyme activity was reflected in the change in fluorescence emitted at each doxycycline concentration compared with baseline (0 µm doxycycline). Similar to results from facial skin tape strips, total-MMP and MMP-2 activities were directly inhibited in NHEK lysate by doxycycline in a dose-dependent manner (Figure 3a and b), with 90% inhibition occurring at a doxycycline concentration of 225 µm. In contrast, TLSP enzymatic activity was not significantly inhibited by doxycycline until exposed to the highest dose of doxycycline (475 µm), and even then abundant activity remained compared with control (Figure 3c). Therefore, we used 225 µM of doxycycline for the following experiments as a dose that effectively directly inhibited MMP activity but not TLSP activity.
Figure 3. Doxycycline directly inhibits keratinocyte matrix metalloproteinase (MMP) but not trypsin-like serine protease (TLSP) activity.
To examine the concentration at which doxycycline directly inhibits MMP and TLSP in normal human epidermal keratinocytes (NHEKs), MMP and TLSP activities in NHEK lysates were determined in the presence of various concentrations of doxycycline (0, 56.25, 112.5, 225, and 450 µm). Cell lysate from NHEKs was measured for baseline catalysis of enzyme-specific substrates and then compared with catalysis in the presence of differing concentrations of doxycycline. Relative enzymatic activity is represented as percentage of no doxycycline control (100%). (a, b) Total-MMP catalysis is directly inhibited in a dose-dependent manner by direct addition of doxycycline to NHEK extracts. (c) In contrast to the inhibition of MMP activity, maximum inhibition of TLSP activity by doxycycline did not exceed 50% and was not significant at a concentration of doxycycline that was effective at inhibition of MMP activity (225 µm). *P<0.05, ***P<0.005 by Student’s t-test, versus vehicle or control.
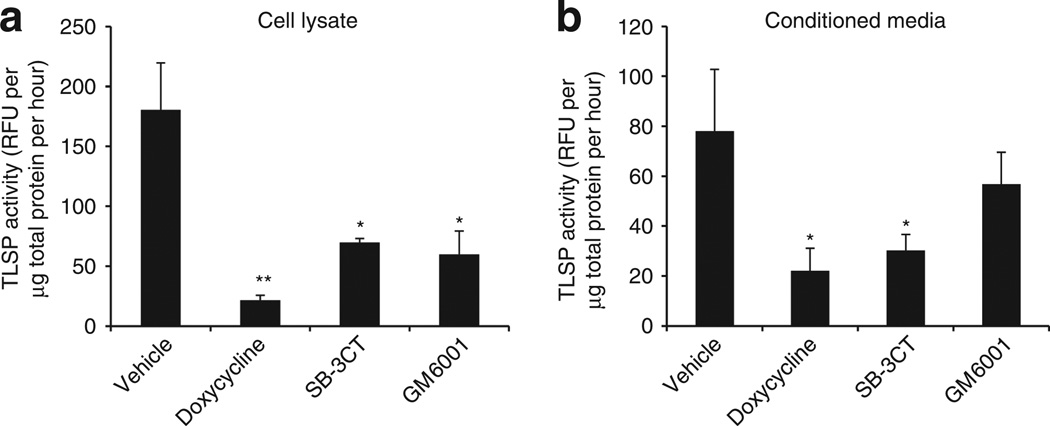
Live NHEKs in culture were then exposed to doxycycline to test the effect of this drug, or other MMP inhibitors, on the generation of nascent TLSP activity from live cells. Under these conditions, a concentration of doxycycline that had no direct inhibitory effect on NHEK TLSP activity (225 µm) significantly attenuated both cellular (Figure 4a) and secreted (Figure 4b) TLSP activity generated by living cells. Incubating keratinocytes with specific MMP inhibitors SB-3CT and GM6001 also resulted in lower TLSP activity. Thus, although there was minimal effect on TLSP activity when doxycycline was added directly to cells under conditions in which KLKs were already activated, addition to live cells resulted in decreased serine protease activity. These results suggested that doxycycline indirectly suppressed tryptic KLK activity in keratinocytes by blocking the action of MMPs.
Figure 4. Doxycycline inhibits cellular and secreted trypsin-like serine protease (TLSP) activity of live keratinocytes.
(a) To examine the activity of doxycycline on TLSP activity produced by live normal human epidermal keratinocytes (NHEKs), cells were cultured in media containing doxycycline (225 µm) for 18 hours. The matrix metalloproteinase (MMP) inhibitors SB3CT (5 µm) and GM6001 (25 µm) were used as positive controls for MMP inhibition. TLSP activity in cell lysate following culture with doxycycline was determined with TLSP-specific substrate (relative fluorescence units (RFU)). Doxycycline significantly attenuated cellular TLSP activity generated by live cells. Incubating cells with SB-3CT and GM6001 also significantly decreased cellular TLSP activity. (b) Secreted TLSP activity was similarly assayed in NHEK conditioned media. Doxycycline significantly decreased secreted TLSP activity. The inability of doxycycline to inhibit TLSP activity when directly added to enzyme extracts (Figure 3c) suggests that the inhibition induced in live cells is by an indirect mechanism and is related to the capacity to inhibit MMPs. *P<0.05, **P<0.01 by Student’s t-test, versus vehicle or control.
Doxycycline pretreatment to live NHEK inhibits the processing of hCAP18 to LL-37
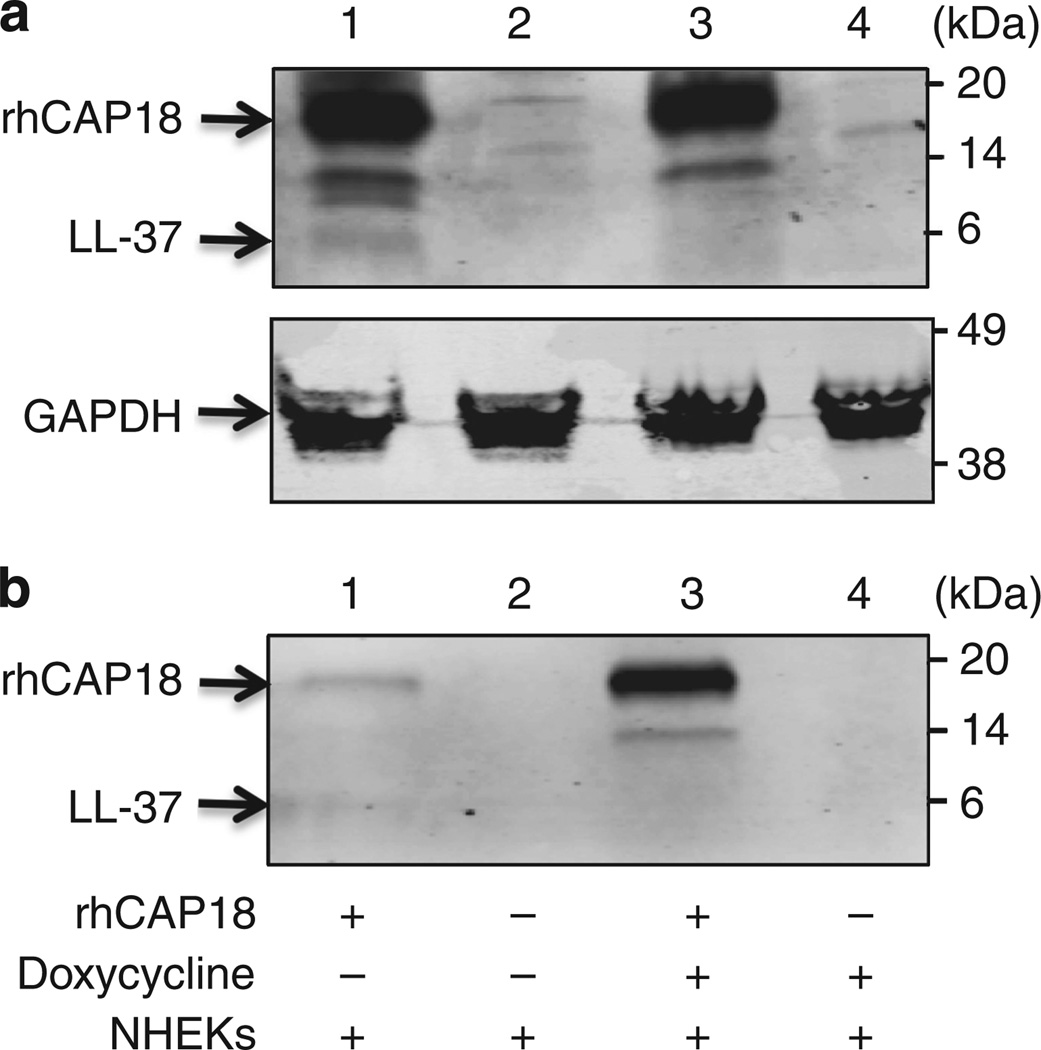
To evaluate the ability of doxycycline to inhibit processing of cathelicidin, full-length recombinant cathelicidin hCAP18 was required for functional analysis, as endogenous production of cathelicidin by NHEK is too little to detect by standard western blotting techniques. Complementary DNA encoding full-length hCAP18 was cloned into the pEcoli expression vector system. This system generated fusion proteins with an N-terminal peptide of 5 kDa containing a 6 × HN purification tag. Cultures of BL21 (DE3) bacteria transformed with pEcoli-hCAP18 were used for expression following IPTG (isopropyl β-d-1-thiogalactopyranoside) induction. A band of ~ 18 kDa, which corresponds to hCAP18, was detected and confirmed by western blot analysis using antibody against LL-37 (Figure 5).
Figure 5. Normal human epidermal keratinocytes (NHEKs) pretreated with doxycycline display inhibited proteolytic processing of recombinant hCAP18.
Equal amounts of recombinant cathelicidin precursor (rhCAP18, 1.525 µg) were added as indicated to identical cultures of live NHEKs either untreated or pretreated overnight with doxycycline (225 µm). Cleavage of hCAP18 was monitored by western blot analysis with anti-LL-37 antibody. (a) Cathelicidin detected in whole-cell extract and (b) cathelicidin remaining in overlying NHEK culture media. Lane 1, rhCAP18 alone + NHEK; lane 2, NHEK alone; lane 3, rhCAP18 + doxycycline + NHEK; lane 4, doxycycline alone + NHEK. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown as loading control. In the absence of doxycycline (lane 1), rhCAP18 is enzymatically processed as detected by generation of a band at the expected migration of LL-37. In the presence of doxycycline (lane 3), LL-37 is not seen in the cell-associated fraction, and a greater amount of rhCAP18 remains in the supernatant.
To evaluate the ability of doxycycline to attenuate live NHEK processing of full-length recombinant hCAP18, we differentiated NHEK in high calcium conditions known to upregulate expression of KLK5 (Morizane et al., 2010). Identical amounts of rhCAP18 were added to all cultures. Western blot analysis for LL-37 demonstrated that under normal conditions a portion of recombinant hCAP18 added was cleaved to a size consistent with LL-37 (Figure 5a, lane 1). If NHEKs were pretreated with doxycycline (225 µm) and then incubated with an identical amount of added recombinant hCAP18, proteolytic processing was diminished as evidenced by the disappearance of the LL-37 band (Figure 5a, compare lane 1 with lane 3). Western blot analysis for LL-37 was also performed on equal fractions of culture media overlying NHEKs to compare with the results seen with the cell fraction. Recombinant hCAP18 added to the media of untreated cells became associated with the cell fraction (Figure 5a, lane 1), resulting in a decreased amount of rhCAP18 present in the conditioned media (Figure 5b, lane 1). However, when pretreated with doxycycline, hCAP18 persisted in the media (Figure 5b, lane 3), and was similar in final processed size to that detected in the cell fraction (Figure 5a, lane 3). Taken together, these results show that doxycycline added to live NHEKs altered the proteolytic cleavage of hCAP18 and association with keratinocytes.
DISCUSSION
As a physical and chemical barrier, the skin is reliant on proteases to control differentiation and turnover of keratinocytes, process structural components, and activate AMPs. The latter function combats bacterial challenges and provides fast and first-line protection against microorganisms. Endogenous and exogenous proteases such as KLKs, MMPs, matriptases, caspases, and proteases derived from microorganisms are in dynamic balance with protease inhibitors such as SERPINs, LEKTI, elafin, and cystatins to regulate desquamation of the stratum corneum (Egelrud, 2000) and activation of AMPs (Yamasaki et al., 2006; Eissa et al., 2011). The mechanisms that regulate this network of proteases in the epidermis are an important area of investigation not only to understand their normal physiology and interplay, which allows for epidermal homeostasis, but also because changes in the proteolytic balance in the skin can result in inflammation, ichthyosis, and other skin disease states such as Netherton syndrome (Meyer-Hoffert, 2009; Sales et al., 2010).
Dysregulation of AMPs, such as cathelicidin that is activated by proteases, has an important role in the pathogenesis of psoriasis, atopic dermatitis, and rosacea. Whereas skin in atopic dermatitis demonstrates decreased cathelicidin expression and increased susceptibility to super-infection, overabundant and abnormal production of cathelicidin in rosacea and psoriasis is implicated in the cutaneous inflammation seen in these disease states because of the immune-modifying ability of LL-37 (Schauber et al., 2008). In this study, to better understand the role of LL-37 in rosacea, we examined the influence of a drug commonly used in rosacea therapy, our hypothesis being that a drug that ameliorates the inflammation of rosacea may work through normalization of cathelicidin processing. We identified both doxycycline and serine protease inhibitors as capable of inhibiting key proteolytic enzymes in the cascade that activates cathelicidin to the inflammatory peptide LL-37. In human skin tape-strip samples, and both keratinocyte lysates and media, doxycycline inhibited MMP activity but did not attenuate TLSP activity at a concentration that suppressed 90% of the MMP activity. However, when doxycycline or MMP-specific inhibitors were added to live keratinocytes in culture, this resulted in a decrease in TLSP activity. As these do not directly suppress the activity of KLK5 or other serine proteases that contribute to the TLSP activity measured, we conclude that the capacity of doxycycline to function as a MMP inhibitor indirectly resulted in a decrease in TLSP activity.
The significance of the capacity of doxycycline to inhibit TLSP action was seen upon assay of cathelicidin enzymatic processing by keratinocytes. The use of recombinant hCAP18 protein revealed that doxycycline decreased the proteolytic processing of hCAP18 to LL-37 (Figure 5). As this step is dependent on the activity of stratum corneum tryptic KLKs, but because doxycycline had little effect on directly inhibiting these serine proteases at the concentrations tested, we conclude that doxycycline functioned indirectly by preventing tryptic KLK activation by MMPs. This is an important step, as when mammalian cathelicidin is produced as an 18-kDa precursor proprotein, hCAP18, it is biologically inactive until it is cleaved into active LL-37 or other active peptides. Thus, the chain of proteolytic activation implicates MMPs as indirect mediators of cathelicidin function.
Before our study, little was known about the mechanism of action for submicrobial doses of doxycycline, currently the only systemic approved therapy for rosacea (Del Rosso et al., 2007), or why the inhibition of MMPs by tetracycline derivatives such as doxycycline leads to clinical improvement in skin diseases such as acne and rosacea (Monk et al., 2011). More than 19 MMPs are expressed in normal human skin (Quan et al., 2009), and expression of MMP-2 and MMP-9 is increased in rosacea skin (Jang et al., 2011). Prior work has shown that, in rosacea, proinflammatory cytokines trigger the release of MMPs, especially MMP-1, -3, and -9, and lead to the degradation of extracellular matrix components (Kahari and Saarialho-Kere, 1997) and inflammatory damage in the form of papulopustular lesions (Golub et al., 1998; Kang et al., 2005). Furthermore, the observations that tetracycline derivatives are able to inhibit the activity of various MMPs (Korting and Schollmann, 2009b) and suppress MMP expression in human corneal epithelial cells, endothelial cells, and skin keratinocytes are well documented. Until now, however, no one has correlated the role of MMP in LL-37 activation with the anti-inflammatory and therapeutic effect of doxycycline. We found that through the suppression of cutaneous and keratinocyte MMPs, and indirect inhibition of tryptic KLKs, doxycycline can block the LL-37 proteolytic pathway and potentially normalize LL-37 in vitro.
The correlation between in vivo doxycycline levels and the effective concentration required to suppress MMP enzymatic activity in vitro remains unclear. Our results confirm previous studies that have shown that doxycycline is not a very potent inhibitor of MMPs in comparison with other inhibitors. According to our results, the IC50 of doxycycline for total MMPs in the NHEKs is ~ 100µm. The inhibition of collagenase purified from culture media of human gingival fibroblasts (MMP-1) was reported to require even higher doxycycline levels (IC50=280 µm; Golub et al., 1995). The oral administration of subantimicrobial-dose doxycycline (40 mg day−1) results in a steady-state serum level at the range of 400–600 ng ml−1 (Skidmore et al., 2003). These concentrations appear to be too little to suppress MMP function. However, doxycycline is known to show organ-specific tissue affinity that is presumably related to its lipophilicity (Belli et al., 1968; Cars and Ryan, 1988). Thus, the local concentration of doxycycline on the skin may be much higher. This could be particularly evident at the air–liquid interface of the surface of the skin where evaporation and subsequent concentration will occur. Under these conditions it is conceivable that the concentration of doxycycline could approach the measured IC50. Furthermore, as our assays on skin tape-strip samples have shown, this superficial compartment is a site of abundant TLSP activity. Thus, both enzyme and substrate are localized in a unique environment when soluble concentrations are unclear. Additional work is necessary to conclude that the action of doxycycline to inhibit MMP and TLSP observed here is relevant to the in vivo situation.
Another implication of the present findings is in revealing that inhibition of serine protease activity may have therapeutic effect in rosacea. This observation suggests that the use of such inhibitors could be an entirely new class of treatment to add to the rosacea therapeutic arsenal. Prior work from our group was the first to identify that increased serine protease activity and cathelicidin promote skin inflammation in rosacea (Yamasaki et al., 2007). Serine protease inhibitors have long been used clinically to treat excessive postoperative bleeding secondary to their ability to inhibit both plasmin, the enzyme responsible for fibrinolysis (McNicol and Douglas, 1964), and KLK, which leads to inhibition of factor XIIa and the intrinsic pathway of the coagulation cascade. This report represents the first recommendation for serine protease inhibitors as a treatment modality for rosacea secondary to our observation that they can directly control the processing of LL-37 from hCAP18 by tryptic KLKs, such as KLK5. These findings reveal a role, which to our knowledge is previously unreported, for serine protease inhibitors in epidermal homeostasis, and suggest that they could be used to target a specific molecular cause of the inflammation of rosacea.
MATERIALS AND METHODS
Reagents
The following reagents were purchased from Enzo Life Sciences (Plymouth Meeting, PA): Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate for total-MMP enzymes and GM6001. Boc-Phe-Ser-Arg-AMC fluorogenic substrate for TLSPs was purchased from Bachem Bioscience (King of Prussia, PA). Tissue-purified KLK from porcine pancreas, SB-3CT, AEBSF, and aprotinin were purchased from Sigma-Aldrich (St Louis, MO). D-Squame standard sampling discs were purchased from CuDerm Corporation (Dallas, TX). Micro BCA Protein Assay Kit was purchased from Thermo Fisher Scientific (Chicago, IL).
Tape stripping and protein extraction
This study was approved by the Human Research Protection Program at the University of California, San Diego. Tape strips were obtained from normal facial skin by applying and removing a single D-Squame standard sampling disc at the same site 10 times serially. Proteins were extracted from sampling discs by placing in BeadBeater tubes with 1 g of 1.0mm Zirconia/Silica beads and 500 µl fluorescence assay buffer. Tubes were homogenized for 3 minutes with a BeadBeater homogenizer, and then centrifuged for 2 minutes at 14,000 r.p.m. Protein extracts were kept on ice. Micro BCA was performed on the protein extracts according to the manufacturer’s instructions to determine protein concentration.
Protease activity fluorescence polarization assays
TLSP activity was assayed by extracting proteins with buffer A composed of 0.1mm Tris-HCl (pH 8.0) and 200mm NaCl. Activity was determined by adding TLSP-specific substrate (2.5mm) to protein extract, incubating for 1 hour at 37 °C, and subsequently measuring relative fluorescence units at fluorescence excitation wavelength 354nm and emission wavelength 422 nm.
Total-MMP activities were determined in 0.1 m Tris-HCl (pH 7.5) containing 150mm NaCl, 0.05% (v/v) Brij35, and 0.1% (w/v) PEG6000. For the determination of total MMP or MMP-2 activity, both 10mm CaCl2 and 10mm ZnCl2 or 0.1mm ZnCl2 were added, respectively, based on their dependency on metal ions as cofactors. MMP-2 and total-MMP activity was determined by adding total-MMP substrate (5 µm) to protein extracts, incubating for 1 hour at 37 °C, and subsequently measuring relative fluorescence units at fluorescence excitation wavelength 328nm and emission wavelength 400 nm. Data were normalized by protein amounts.
Total protease activities were determined with the EnzChek Protease Assay Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instruction.
Cell culture conditions
NHEKs were grown in serum-free EpiLife cell culture media (Cascade Biologics, Portland, OR) containing 0.06mm CaCl2 and 1 × EpiLife defined growth supplement at 37 °C under standard tissue culture conditions. The cultures were maintained for up to four passages in this media. Cell differentiation was induced by the addition of high calcium (2mm) for 72 hours after reaching 100% confluence.
Expression of recombinant hCAP18 in E. coli
A PCR product encoding a full length of hCAP18 was generated by using complementary DNA obtained from NHEKs stimulated with vitamin D as a template, the forward PCR primer (5′-TAAGGCCTCTGTCGACCAGGTCCTCAGCTACAAGGAAGC-3′) and the reverse PCR primer (5′-CAGAATTCGCAAGCTTCTAGGACTCTGTCCTGGGTACAAG-3′). The amplified DNA products were inserted into the In-Fusion Ready pEcoli-Nterm 6 ×HN vector (Clontech Laboratories, Mountain View, CA) and transformed into competent cells (E. coli and BL21 (DE3); Invitrogen). IPTG (1mm) was used to induce protein synthesis. The expressed protein was purified with a TALON Express Purification Kit (Clontech Laboratories). The amount of recombinant protein was determined by BCA assay (Thermo Scientific, Rockport, IL).
SDS-PAGE and western blotting
The expression of hCAP18 protein was confirmed by SDS-PAGE followed by western blot analysis. Gels (4–20%) were run and transferred onto polyvinylidene difluoride membranes (Thermo Scientific). Membranes were blocked with Odyssey infrared imaging system blocking buffer (LI-COR, Lincoln, NE) and then probed overnight at 4 °C with rabbit anti-LL-37 (1:1,000) primary Ab. Membranes were washed and incubated with goat anti-rabbit IRDye 680 secondary Ab (LI-COR) for 1 hour at room temperature. Membranes were washed, and fluorescence was detected using the Odyssey infrared imaging system (LI-COR). Overexpressed protein was observed at the expected size and showed immunoreactivity against anti-LL-37 IgG. To verify equal loading of cell lysates, the membrane was washed with Newblot polyvinylidene difluoride stripping buffer (LI-COR) and incubated with anti-glyceraldehyde-3-phosphate dehydrogenase mAb (1:10,000; Fitzgerald, Acton, MA), followed by anti-mouse IRDye800 secondary Ab (LI-COR).
Processing of full-length hCAP18 to LL-37
NHEKs (100% confluent) differentiated with 72 hours of 2mm calcium conditions were pretreated with doxycycline (100 µg ml−1) overnight. Full-length hCAP18 recombinant protein (1.525 µg) was incubated with doxycycline-treated NHEKs at 37 °C for 48 hours. Conditioned medium was collected and lyophilized. The sample was subsequently boiled in Laemmli sample buffer and run by SDS-PAGE, followed by western blot analysis with anti-LL-37 antibody. NHEKs were lysed with −80 °C freeze/thaw cycle, boiled in Laemmli sample buffer, and run by SDS-PAGE followed by western blot analysis with anti-LL37 IgG.
Statistical analysis
Experiments were performed at least three times, and the data are presented as means±SE. To determine statistical significance between groups, comparisons were made using two-tailed t-tests. For all statistical tests, a P-value of <0.05 was accepted for statistical significance.
ACKNOWLEDGMENTS
This work was supported in part by an investigator-initiated grant from Galderma and the NIH grants R01 AR052728 and R01 AI052453. KNK was supported by a Howard Hughes Medical Institute Research Training Fellowship.
Abbreviations
- AMP
antimicrobial peptide
- IC50
half-maximal inhibitory concentration
- KLK
kallikrein
- MMP
matrix metalloproteinase
- NHEK
normal human epidermal keratinocyte
- TLSP
trypsin-like serine protease
Footnotes
CONFLICT OF INTEREST
RLG is a co-inventor of technology held by UCSD that applies inhibition of serine protease activity as a therapy for rosacea. The other authors state no conflict of interest.
REFERENCES
- Beaufort N, Plaza K, Utzschneider D, et al. Interdependence of kallikrein-related peptidases in proteolytic networks. Biol Chem. 2010;391:581–587. doi: 10.1515/BC.2010.055. [DOI] [PubMed] [Google Scholar]
- Belli G, Ciaffi G, Ricci P. Blood and tissue levels of 2 antibiotics of the tetracycline group orally administered to man. Antibiotica. 1968;6:109–115. [PubMed] [Google Scholar]
- Brattsand M, Egelrud T. Purification, molecular cloning, and expression of a human stratum corneum trypsin-like serine protease with possible function in desquamation. J Biol Chem. 1999;274:30033–30040. doi: 10.1074/jbc.274.42.30033. [DOI] [PubMed] [Google Scholar]
- Briot A, Deraison C, Lacroix M, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cars O, Ryan DM. Concentrations of doxycycline in muscle tissue and muscle tissue fluid. Scand J Infect Dis Suppl. 1988;53:18–21. [PubMed] [Google Scholar]
- Caubet C, Jonca N, Brattsand M, et al. Degradation of corneodesmosome proteins by two serine proteases of the kallikrein family, SCTE/KLK5/hK5 and SCCE/KLK7/hK7. J Invest Dermatol. 2004;122:1235–1244. doi: 10.1111/j.0022-202X.2004.22512.x. [DOI] [PubMed] [Google Scholar]
- Chun YH, Yamakoshi Y, Yamakoshi F, et al. Cleavage site specificity of MMP-20 for secretory-stage ameloblastin. J Dent Res. 2010;89:785–790. doi: 10.1177/0022034510366903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791–802. doi: 10.1016/j.jaad.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Egelrud T. Desquamation in the stratum corneum. Acta Derm Venereol Suppl (Stockh) 2000;208:44–45. doi: 10.1080/000155500750012513. [DOI] [PubMed] [Google Scholar]
- Eissa A, Amodeal V, Smith CR, et al. Kallikrein-related peptidase-8 (KLK8) is an active serine protease in human epidermis and sweat and is involved in a skin barrier proteolytic cascade. J Biol Chem. 2011;286:687–706. doi: 10.1074/jbc.M110.125310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RA, Pantazatos DP, Gessner CR, et al. Molecular interactions between matrilysin and the matrix metalloproteinase inhibitor doxycycline investigated by deuterium exchange mass spectrometry. Mol Pharmacol. 2005;67:1128–1136. doi: 10.1124/mol.104.006346. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, et al. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Golub LM, Sorsa T, Lee HM, et al. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol. 1995;22:100–109. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R, Visser H, Koolwijk P, et al. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res. 1998;12:114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Deraison C, Bonnart C, et al. LEKTI is localized in lamellar granules, separated from KLK5 and KLK7, and is secreted in the extracellular spaces of the superficial stratum granulosum. J Invest Dermatol. 2005;124:360–366. doi: 10.1111/j.0022-202X.2004.23583.x. [DOI] [PubMed] [Google Scholar]
- Jang YH, Sim JH, Kang HY, et al. Immunohistochemical expression of matrix metalloproteinases in the granulomatous rosacea compared with the non-granulomatous rosacea. J Eur Acad Dermatol Venereol. 2011;25:544–548. doi: 10.1111/j.1468-3083.2010.03825.x. [DOI] [PubMed] [Google Scholar]
- Jiang R, Shi Z, Johnson JJ, et al. Kallikrein-5 promotes cleavage of desmoglein-1 and loss of cell-cell cohesion in oral squamous cell carcinoma. J Biol Chem. 2011;286:9127–9135. doi: 10.1074/jbc.M110.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahari VM, Saarialho-Kere U. Matrix metalloproteinases in skin. Exp Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–1699. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantyka T, Fischer J, Wu Z, et al. Inhibition of kallikrein-related peptidases by the serine protease inhibitor of Kazal-type 6. Peptides. 2011;32:1187–1192. doi: 10.1016/j.peptides.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korting HC, Schollmann C. Current topical and systemic approaches to treatment of rosacea. J Eur Acad Dermatol Venereol. 2009a;23:876–882. doi: 10.1111/j.1468-3083.2009.03167.x. [DOI] [PubMed] [Google Scholar]
- Korting HC, Schollmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009b;22:287–294. doi: 10.1159/000235550. [DOI] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Larrick JW, Hirata M, Balint RF, et al. Human CAP18: a novel antimicrobial lipopolysaccharide-binding protein. Infect Immun. 1995a;63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick JW, Hirata M, Zhong J, et al. Anti-microbial activity of human CAP18 peptides. Immunotechnology. 1995b;1:65–72. doi: 10.1016/1380-2933(95)00006-2. [DOI] [PubMed] [Google Scholar]
- Li DQ, Chen Z, Song XJ, et al. Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:4302–4311. doi: 10.1167/iovs.04-0299. [DOI] [PubMed] [Google Scholar]
- Lundwall A, Brattsand M. Kallikrein-related peptidases. Cell Mol Life Sci. 2008;65:2019–2038. doi: 10.1007/s00018-008-8024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicol GP, Douglas AS. Epsilon-aminocaproic acid and other inhibitors of fibrinolysis. Br Med Bull. 1964;20:233–239. doi: 10.1093/oxfordjournals.bmb.a070338. [DOI] [PubMed] [Google Scholar]
- Meyer-Hoffert U. Reddish, scaly, and itchy: how proteases and their inhibitors contribute to inflammatory skin diseases. Arch Immunol Ther Exp (Warsz) 2009;57:345–354. doi: 10.1007/s00005-009-0045-6. [DOI] [PubMed] [Google Scholar]
- Michael IP, Sotiropoulou G, Pampalakis G, et al. Biochemical and enzymatic characterization of human kallikrein 5 (hK5), a novel serine protease potentially involved in cancer progression. J Biol Chem. 2005;280:14628–14635. doi: 10.1074/jbc.M408132200. [DOI] [PubMed] [Google Scholar]
- Monk E, Shalita A, Siegel DM. Clinical applications of non-antimicrobial tetracyclines in dermatology. Pharmacol Res. 2011;63:130–145. doi: 10.1016/j.phrs.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Morizane S, Yamasaki K, Kabigting FD, et al. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010;130:1297–1306. doi: 10.1038/jid.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohler A, Debela M, Wagner S, et al. Analyzing the protease web in skin: meprin metalloproteases are activated specifically by KLK4, 5 and 8 vice versa leading to processing of proKLK7 thereby triggering its activation. Biol Chem. 2010;391:455–460. doi: 10.1515/BC.2010.023. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843–850. doi: 10.1038/jid.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, Qin Z, Xia W, et al. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu O, Hu JC, Yamakoshi Y, et al. Porcine kallikrein-4 activation, glycosylation, activity, and expression in prokaryotic and eukaryotic hosts. Eur J Oral Sci. 2002;110:358–365. doi: 10.1034/j.1600-0722.2002.21349.x. [DOI] [PubMed] [Google Scholar]
- Sales KU, Masedunskas A, Bey AL, et al. Matriptase initiates epidermal prokallikrein activation and disease onset in a mouse model of Netherton syndrome. Nat Genet. 2010;42:676–683. doi: 10.1038/ng.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Ruzicka T, Rupec RA. Cathelicidin LL-37. A central factor in the pathogenesis of inflammatory dermatoses? Hautarzt. 2008;59:72–74. doi: 10.1007/s00105-007-1457-z. [DOI] [PubMed] [Google Scholar]
- Skidmore R, Kovach R, Walkder C, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol. 2003;139:459–464. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G, Pampalakis G, Diamandis EP. Functional roles of human kallikrein-related peptidases. J Biol Chem. 2009;284:32989–32994. doi: 10.1074/jbc.R109.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson K, Brattsand M, Ny A, et al. Kallikrein-related peptidase 14 may be a major contributor to trypsin-like proteolytic activity in human stratum corneum. Biol Chem. 2006;387:761–768. doi: 10.1515/BC.2006.095. [DOI] [PubMed] [Google Scholar]
- Uitto VJ, Firth JD, Nip L, et al. Doxycycline and chemically modified tetracyclines inhibit gelatinase A (MMP-2) gene expression in human skin keratinocytes. Ann NY Acad Sci. 1994;732:140–151. doi: 10.1111/j.1749-6632.1994.tb24731.x. [DOI] [PubMed] [Google Scholar]
- Voegeli R, Rawlings AV, Breternitz M, et al. Increased stratum corneum serine protease activity in acute eczematous atopic skin. Br J Dermatol. 2009;161:70–77. doi: 10.1111/j.1365-2133.2009.09142.x. [DOI] [PubMed] [Google Scholar]
- Wolf WC, Evans DM, Chao L, et al. A synthetic tissue kallikrein inhibitor suppresses cancer cell invasiveness. Am J Pathol. 2001;159:1797–1805. doi: 10.1016/S0002-9440(10)63026-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Schauber J, Coda A, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]