Abstract
The paradigm that resident macrophages in steady-state tissues are derived from embryonic precursors has never been investigated in the intestine, which contains the largest pool of macrophages. Using fate mapping models and monocytopenic mice, together with bone marrow chimeric and parabiotic models, we show that embryonic precursors seeded the intestinal mucosa and demonstrated extensive in situ proliferation in the neonatal period. However these cells did not persist in adult intestine. Instead, they were replaced around the time of weaning by the CCR2-dependent influx of Ly6Chi monocytes that differentiated locally into mature, anti-inflammatory macrophages. This process was driven largely by the microbiota and had to be continued throughout adult life to maintain a normal intestinal macrophage pool.
Introduction
Macrophages are found in every tissue of the body, where they act as a first line of defense against pathogenic insult, but also contribute to the maintenance of tissue homeostasis and wound repair1. Traditionally macrophages were thought to be part of a linear mononuclear phagocyte system (MPS) and assumed to derive exclusively from blood monocytes2. However other more recent work has challenged this concept, generating a paradigm that all macrophage in steady-state tissues are derived from embryonic progenitors in the yolk sac (YS) and/or fetal liver (FL). Thereafter, it has been proposed that these macrophage populations are maintained by self renewal in situ, with little or no contribution from circulating blood monocytes3-8. However despite the presence of a large population of functionally specialized macrophages in the intestine9,10, these concepts have not been addressed in this tissue.
There is considerable evidence that monocytes can give rise to intestinal macrophages during inflammation in both man and mice11,12. Initial studies also showed that ‘inflammatory’ Ly6Chi monocytes could replenish intestinal macrophages after diphtheria toxin (DT)-mediated intense depletion of myeloid cells in the CD11c-DTR mouse13,14. We recently extended these findings by showing that Ly6Chi monocytes were present in the colonic mucosa of unmanipulated mice and could enter the intestine after transfer into unmanipulated mice. There they appeared to differentiate locally into mature F4/80hi CX3CR1hi MHCII+ macrophages through a series of intermediaries, eventually acquiring the typical properties of gut-resident macrophages, through a process sometimes referred to as the “monocyte waterfall”15. The resulting cells showed constitutive production of interleukin 10 (IL-10), phagocytic activity and resistance to stimulation via Toll-like receptors (TLRs)15,16. However these studies did not address the possibility that YS and/or FL precursors might seed the intestinal mucosa and persist into adulthood alongside Ly6Chi monocyte-derived macrophages. Furthermore, we could not identify at what stage of development the monocyte dependent replenishment of intestinal macrophages began, and the developmental, environmental and signaling requirements for monocyte recruitment to the intestine remain unexplored.
Here we have used a combination of immunophenotyping, lineage tracing and parabiosis to explore the origin of the intestinal macrophage compartment from birth until adulthood in mice. We show that although YS- and FL-derived macrophages were present in the neonatal intestine, these cells failed to persist into adulthood and instead were diluted out by monocyte-derived macrophages that began to arrive around the time of weaning in a process that is highly dependent on CCR2 and commensal microbiota. These findings substantially increase our understanding of the regulatory processes that control how intestinal macrophage play critical roles in maintaining epithelial integrity and local homeostasis in this highly dynamic microenvironment10.
Results
Origins of intestinal macrophages early in life
To explore the origins of intestinal macrophages, we first developed methods for identifying these cells in the neonatal intestine, by adapting a rigorous gating strategy we had developed in adult mice15,16. Live (7-AAD−) CD45+ cells could be identified readily in enzymatic digests of colon from newborn mice and as expected, the numbers of live leukocytes rose progressively into adulthood, beginning after the first week of life (Supplementary Fig. 1a). After excluding SiglecF+ eosinophils, Ly6G+ neutrophils and F4/80−CD11chi dendritic cells (DCs), a clear population of F4/80+CD11b+ cells could be found at E19.5, increasing progressively in numbers up to adulthood (Fig. 1a,b and Supplementary Fig. 1b). In both adult and newborn colon, we confirmed their identity as macrophages on the basis of their abundant expression of the macrophage-specific markers CD64 (FcγR1) and CX3CR1 (Fig. 1c), as well as substantial amounts of surface CD11c and mRNA for the hemoglobin-haptoglobin scavenger receptor CD163 (Supplementary Fig. 1c,d). Interestingly, macrophages in neonatal colon expressed less MerTK than those found in adult colon or F4/80hi CD11blo Kupffer cells in the liver (Supplementary Fig. 1e).
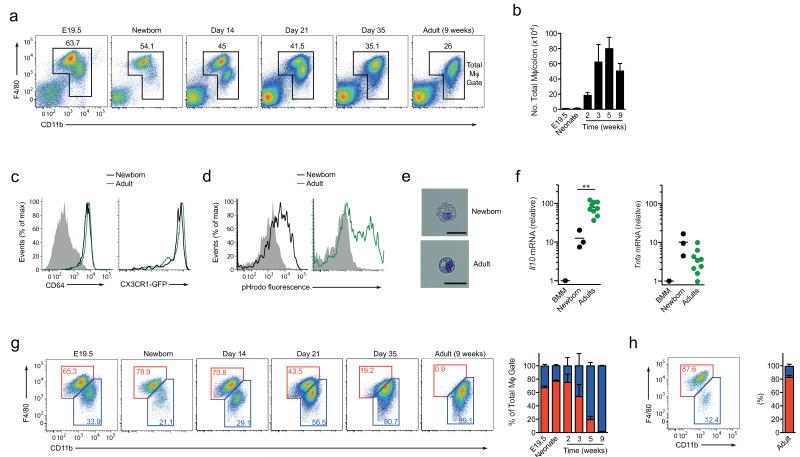
Figure 1. F4/80 and CD11b expression identifies macrophage subsets in neonatal and adult colon.
(a) F4/80 and CD11b expression by live CD45+SiglecF−Ly6G−CD11clo colonic LP cells from neonatal (day 2), day 14, day 21, day 35 and 9 week old CX3CR1+/gfp mice. (b) Absolute numbers of total F4/80+ CD11b+ cells per colon in day 2, day 14, day 21, day 35 and 9 week old CX3CR1+/gfp mice. (c) CD64 and CX3CR1-GFP expression by F4/80+ CD11b+ colonic LP cells from newborn (day 2; black line) and 8 week old (green line) CX3CR1+/gfp mice. Shaded histogram represents isotype control. (d) Phagocytic activity of F4/80+ CD11b+ colonic LP cells from newborn (day 2) or adult CX3CR1+/gfp mice measured by the uptake of pHrodo Escherichia coli bioparticles for 30-60 mins at 37°C or 4°C as a control (shaded histograms). (e) Morphological appearance of F4/80+ CD11b+ colonic mφ from newborn and adult mice. Scale bar 25μm. (f) qRT-PCR of mRNA for IL10 and TNFα by F4/80hi LP mφ from newborn (day 1) or adult mice, and BM-derived mφ (BMM). Results are mean expression relative to cyclophilin A (CPA) + 1SD obtained using the 2−dΔC(t), with BMM set to 1. **P<0.01 (Student’s t-test). (g, h) Representative plots and mean frequencies of F4/80hi CD11blo (red) and F4/80lo CD11b+ (blue) subsets of total colonic LP mφ from newborn (day 2), day 14, day 21, day 35 and 9 week old CX3CR1+/gfp mice (g) or from 8 week old wild-type liver (h). Data are from one of 3 (a, b, g, h), or 2 independent experiments with two mice (c), of two independent experiments with 3 mice per group (d), or are representative images from two independent experiments (e). Results in b, g, h are means ± 1 SD of 6 (neonate timepoint) or 4 (day 14, 21 and 35, and 9 week old timepoints) mice per group. Data from E19.5 embryos are from two experiments each using cells pooled from 8 mice. For qRT-PCR analysis (f), data represent 3 (neonate) or 9 (adult) biological replicates using RNA pooled from 1-2 adults or 5 neonates.
We next assessed if these neonatal macrophages were functionally equivalent to their adult counterparts, examining for the high phagocytic activity and constitutive production of IL-10 and tumor necrosis factor (TNF) that characterize steady state macrophages in adult intestine14,16. As expected, macrophages from adult colon excelled at taking up fluorescently labeled Escherichia coli into acidified vesicles and similar results were obtained with macrophages from 2-day-old colon (Fig. 1d). Consistent with this evidence of efficient phagocytic activity, both neonatal and adult macrophage were large and highly vacuolar in appearance (Fig.1e). Macrophages sorted from adult intestine also contained high amounts of IL-10 mRNA, together with substantial amounts of TNF mRNA compared with those in CSF-1-treated bone marrow (BM) macrophages used as controls (Fig. 1f). Macrophages from 2-day-old intestine also showed constitutive expression of mRNA for both cytokines, although the abundance of IL-10 mRNA was lower in neonatal than in adult macrophages (Fig. 1f). Together these findings show that cells with the phenotypic and functional properties of resident intestinal macrophages are already present immediately after birth.
Recent studies have proposed that the extent of F4/80 and CD11b expression can be used to identify developmentally distinct macrophage populations in multiple different tissues3. Whereas F4/80hiCD11blo are proposed to derive from embryonic precursors, those expressing low amounts of F4/80 but high amounts of CD11b appear to originate from conventional hematopoiesis in the adult3. Thus we next assessed whether F4/80 and CD11b expression could be used to define discrete macrophage populations in the colon. Clearly definable populations of F4/80hiCD11blo and F4/80loCD11bhi macrophages were seen in the colon of newborn mice, with the F4/80hiCD11blo subset being dominant at this time (Fig. 1g). Identical populations of F4/80hiCD11blo and F4/80loCD11bhi macrophages were also present in the intestine before birth, confirming their embryonic origin (Fig. 1g). Both subsets expressed substantial amounts of CD64 and CX3CR1, although their expression was somewhat higher on the F4/80hiCD11blo subset (Supplementary Fig. 1f). However the distinction between the two phenotypic subsets became progressively less clear in the intestine with age and by adulthood, there were virtually no F4/80hiCD11blo macrophages remaining in the colonic mucosa (Fig. 1a,g). In marked contrast, the monocyte/macrophage compartments found in the liver and spleen showed clear populations of F4/80hiCD11blo and F4/80loCD11bhi cells in adulthood, with the F4/80hiCD11blo remaining the dominant population at this time (Fig. 1h, Supplementary Fig. 1g and data not shown). Thus macrophages bearing the characteristic F4/80hiCD11blo signature of embryonically derived cells remain in tissues such as the liver and spleen, but appear to be lost progressively in the colonic mucosa.
Yolk sac derived macrophages do not persist in adult colon
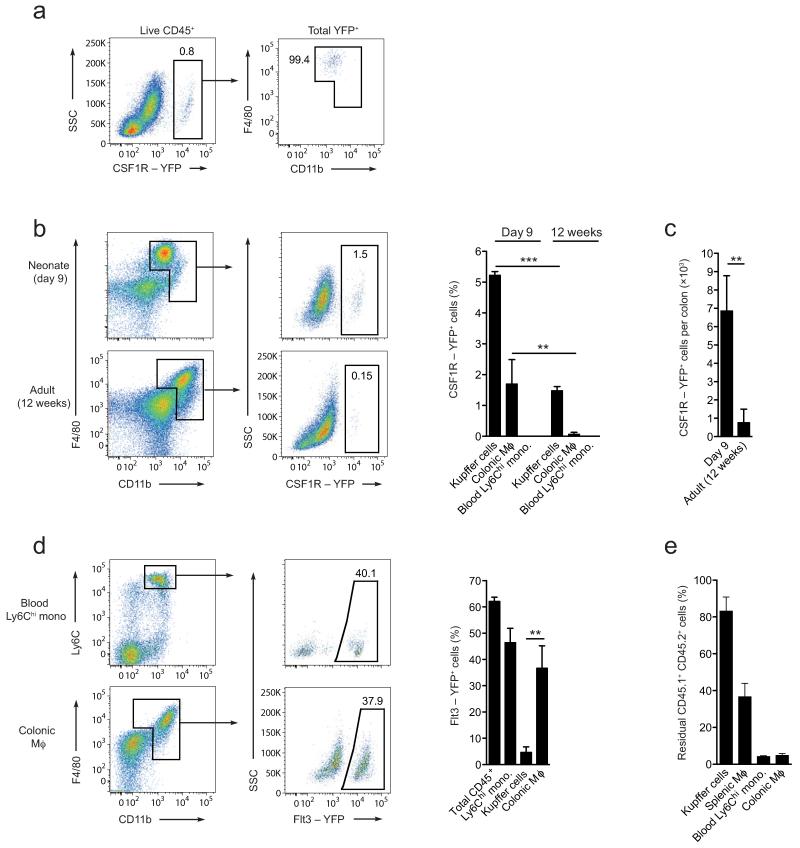
As these results suggested that adult intestinal macrophages may be developmentally distinct from those in other tissues, we examined directly whether YS progenitors contribute to the intestinal macrophage pool. To do this we used mice expressing tamoxifen-inducible Cre recombinase under the control of the Csf1r promoter17. These Csf1r-mer-iCre-mer mice were crossed with Rosa26-LSL-YFP reporter mice (referred to as Csfr1-reporter) and Cre-mediated recombination was induced with a single injection of 4-hydroxy-tamoxifen into pregnant females at E8.5. This protocol leads to irreversible expression of YFP by CSF1R+ cells in the YS and their progeny, but not by hematopoietic stem cells (HSCs) or their derivatives3. YFP+ cells were clearly present in the colonic lamina propria of 9-day-old Csf1r-reporter mice and all YFP+ cells fell within the F4/80+CD11b+ macrophage gate (Fig. 2a,b). At this age, fewer YFP+ cells were found among colonic macrophages than among F4/80hi CD11blo Kupffer cells (1.7±0.8% versus 5.2±0.1%, respectively), perhaps reflecting tissue-specific differences in the efficiency of Cre-mediated labeling3, or that monocyte-derived cells were already appearing in the colon by this time (Fig. 2b). This difference was even more marked in adult Csf1r-reporter mice, where YFP+ cells remained readily detectable in the liver, albeit at reduced numbers, whereas the colon contained very few YFP+ of the same mice (Fig. 2b). The reduction in YFP+ macrophages in adult intestine was not simply due to dilution by other cells, as their absolute number was also significantly lower in adult colon than in 9-day-old intestine (Fig. 2c). Consistent with exclusive labeling of YS-derived cells, YFP+ cells could not be detected among Ly6Chi blood monocytes at any time (Fig. 2b). Thus although YS-derived macrophages are present in the intestine early in life, they do not persist in the adult gut to any significant extent.
Figure 2. The colonic macrophage compartment is dependent on conventional hematopoiesis.
(a) YFP expression by total live CD45+ colonic leukocytes (left panel) from 9 day old progeny of Csf1r-mer-iCre-mer Rosa26-LSL-YFP mice injected with tamoxifen at E8.5 to pulse label CSF1R+ YS macrophages (left panel); expression of F4/80 and CD11b by total YFP+ cells (right panel). (b) Representative YFP expression by F4/80+ CD11b+ colonic LP cells from 9 day or 12 week old progeny of Csf1r-mer-iCre-mer Rosa26-LSL-YFP mice treated as in a. Bar chart shows the mean frequency of YFP+ cells amongst F4/80hi CD11blo liver Kupffer cells, total F4/80+ CD11b+ colonic LP cells and amongst Ly6Chi blood monocytes from 9 day or 12 week old progeny of Csf1r-mer-iCre-mer Rosa26-LSL-YFP mice treated as above. **P<0.01, ***P<0.0001 (Student’s t-test). (c) Absolute number of eYFP-expressing F4/80+ CD11b+ colonic LP cells from 9 day or 12 week old adult progeny of Csf1r-mer-iCre-mer Rosa26-LSL-YFP mice. (d) Representative eYFP expression (upper panels) and mean eYFP expression (lower panel) by total CD45+ blood leukocytes, Ly6Chi blood monocytes, F4/80hi CD11blo liver KC and colonic F4/80+ CD11b+ cells from 8 week old progeny of Flt3-Cre Rosa26-LSL-YFP mice. **P<0.005 (Student’s t-test). (e) Proportions of CD45.1+CD45.2+ residual host cells amongst Ly6Chi blood monocytes, total F4/80+ CD11b+ colonic LP cells, F4/80hi CD11blo liver Kupffer cells and F4/80hi CD11blo splenic macrophages of WT:Ccr2−/− mixed BM chimeric mice 8 weeks after reconstitution. a, b, c: Data are means + 1 SD and are pooled from two independent experiments with 3 or 4 mice per group (12 week old), or from one experiment with 3 mice (9 day old). d, e: Data are means + 1 SD and are from one of two independent experiments with 3 mice.
Colon macrophages derive from conventional hematopoiesis
Given the apparent paucity of YS-derived macrophages in the adult intestine, we next used Flt3-Cre Rosa26-LSL-YFP reporter mice (referred to as Flt3-reporter mice) to assess the contribution of conventional hematopoiesis to the intestinal macrophage pool. As the cytokine receptor Flt3 is expressed by all pluripotent hematopoietic progenitors in the BM, the progeny of these cells become permanently YFP+ in Flt3-reporter mice3. 50-60% of total leukocytes and of Ly6Chi monocytes in the blood of adult Flt3-reporter mice were YFP+ and similar proportions of YFP+ cells were also seen among macrophages in the adult colon (Fig. 2d). This evidence that intestinal macrophages are derived from conventional hematopoiesis was supported further by the fact that virtually all colonic macrophages were eliminated by whole body irradiation and were replaced by BM-derived progenitors in BM chimeras (Fig. 2e). In stark contrast, very few F4/80hi CD11blo Kupffer cells (4.6±2.1%) were labeled in Flt3-reporter mice (Fig. 2d), and large proportions of Kupffer cells and splenic macrophages remained of host origin in radiation chimeras, consistent with previous reports that these cells are radio-resistant18 (Fig. 2e). Taken together, these results suggest that although YS-derived cells seed the intestinal lamina propria early in development, they are replaced by the progeny of conventional hematopoiesis in adulthood.
Ly6Chi monocytes drive colonic macrophage accumulation
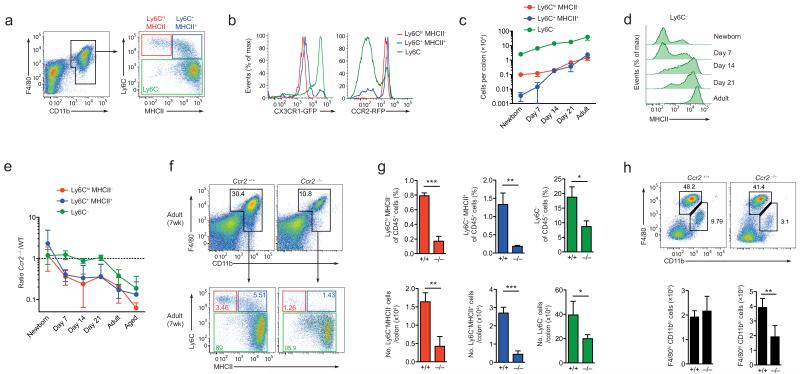
We reasoned that this switch from embryonic to hematopoietic origin might occur at the time when we observed the most intense increase in the number of macrophages, 2-3 weeks after birth. To test this hypothesis, we carried out a comprehensive analysis of the macrophage compartment from birth to adulthood. Confirming our previous findings15,16, we could identify 3 subsets of F4/80+CD11b+ cells based on their expression of Ly6C and MHCII in the colon of adult wild-type mice (Fig. 3a). Colonic Ly6Chi MHCII− cells were phenotypically similar to classical blood monocytes19, expressing intermediate amounts of CX3CR1 and high amounts of CCR2 (Fig. 3b), whereas the Ly6C+MHCII+ cells showed loss of CCR2 expression and upregulation of CX3CR1; this finding is consistent with these cells being an intermediary stage between Ly6Chi monocytes and resident macrophages15,16 (Fig. 3b). The majority of F4/80+CD11b+ cells was Ly6C− and had the CX3CR1hi CCR2− phenotype of mature macrophages (Fig. 3b). Whereas the numbers of mature Ly6C− macrophages increased progressively with age, there were very few Ly6ChiMHCII− cells in the intestine of wild-type mice at the time of birth and their numbers remained very low until after 14 days of life (Fig. 3c). Notably, the Ly6C+MHCII+ cells were essentially absent from the colonic mucosa until around three weeks of life, suggesting that differentiation of classical monocytes through the ‘monocyte waterfall’ does not occur until 2-3 weeks of life. Furthermore, the appearance of Ly6C+MHCII+ intermediaries was paralleled by a progressive acquisition of MHCII expression by mature Ly6C− macrophages (Fig. 3d).
Figure 3. Maturation and CCR2-dependence of intestinal macrophage development.
(a) Ly6C and MHCII expression by colonic F4/80+CD11b+ cells from adult CX3CR1+/gfp mice. (b) CX3CR1 and CCR2 expression by Ly6ChiMHCII−, Ly6ChiMHCII+ and Ly6C− subsets from CX3CR1+/gfp and CCR2+/rfp mice respectively. (c) Absolute numbers of colonic Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets from newborn (day 0-1), day 7, day 14, day 21 and 7-8 week old CX3CR1+/gfp mice. (d) MHCII expression by F4/80+CD11b+Ly6C− colonic LP cells from newborn (day 0-1), day 7, day 14, day 21 and 7-8 week old CX3CR1+/gfp mice. (e) Numbers of Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets in colon of newborn (day 0-1), day 7, day 14, day 21, 7 week old and in 9-12 month old adult Ccr2−/− mice, shown as ratios to the equivalent populations in age-matched Ccr2+/+ mice. (f) Representative F4/80 and CD11b expression by live CD45+SiglecF−Ly6G−CD11clo colonic LP cells and Ly6C and MHCII expression by total F4/80+CD11b+ cells from 7 week old Ccr2+/+ or Ccr2−/− mice. (g) Mean proportions and absolute numbers of Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets in colon of 7 week old Ccr2+/+ or Ccr2−/− mice. *P<0.05, **P<0.01, ***P<0.001 (Student’s t-test). (h) Representative F4/80 and CD11b expression by live CD45+SiglecF−Ly6G−CD11clo liver cells from 8 week old Ccr2+/+ or Ccr2−/− mice. Bar charts show the absolute numbers of F4/80hiCD11blo and F4/80loCD11b+ cells from livers of 8wk old Ccr2+/+ or Ccr2−/− mice. **P<0.01 (Student’s t-test). Data are representative of 3 independent experiments (a, f, g, h and Cx3cr1+/gfp mice in b), or from one experiment (Ccr2+/rfp mice in b), or are pooled from two independent time course experiments (c, d, e). Aged mice were examined in one experiment. Results in c, e, g, h are means ± 1 SD of 6-8 (c, e) or 3-4 mice (g, h) per group.
To assess the contribution of classical blood monocytes at this time, we examined Ccr2−/− mice in whom Ly6Chi monocyte egress from the BM is defective20. Although all Ly6C- and MHCII-defined populations of macrophages were present in normal numbers in newborn Ccr2−/− mice, progressively severe deficiencies in Ly6ChiMHCII− and Ly6C+MHCII+ cells relative to wild-type mice of the same ages appeared after 7 days of age (Fig. 3e and Supplementary Fig. 2a,b). By adulthood, these cells were essentially absent from Ccr2−/− colon (Fig. 3e-g). In contrast, the numbers of mature Ly6C− macrophages in Ccr2−/− colon remained equivalent to those in wild-type mice until 7 weeks of age, when there was a 50% deficit in their numbers (Fig. 3e-g). Notably, this defect developed further with age, with 9-12-month-old Ccr2−/− mice showing a much more severe defect in the numbers of mature Ly6C− macrophages compared with their younger counterparts (Fig. 3e and Supplementary Fig. 2c). The absence of CCR2 did not affect other tissue-resident macrophage populations, with the numbers of F4/80hiCD11blo Kupffer cells in the liver being normal in adult Ccr2−/− mice, consistent with them being embryonically derived (Fig. 3h). Conversely, Ccr2−/− livers had significantly reduced numbers of F4/80lo cells, which are presumptively derived from classical monocytes.
Interestingly, despite their normal numbers in 3-week-old Ccr2−/− colon, a proportion of the mature Ly6C− macrophages seen in these mice remained MHCII−, when all Ly6C− cells had become MHCII+ in wild-type colon (Fig. 3d and Supplementary Fig. 2a,c). A small population of MHCIIlo Ly6C− macrophages also remained present in adult Ccr2−/− colon (Fig. 3f,g). Together these data suggest that the adult colonic macrophage pool first becomes established around the time of weaning at 2-3 weeks of age and that this is associated with CCR2-dependent accumulation of Ly6Chi monocytes and their subsequent differentiation into MHCII-expressing macrophages.
Macrophages proliferate in neonatal, but not adult colon
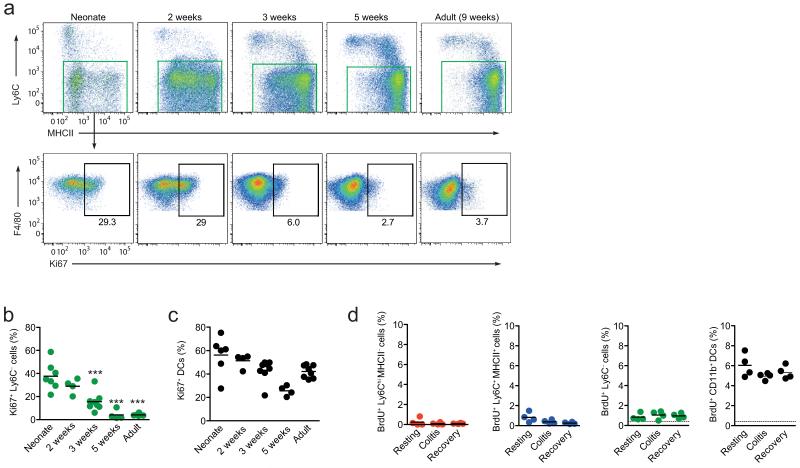
Given that the abundance of mature Ly6C− macrophages remained normal in Ccr2−/− mice until adulthood, despite the severe reduction in classical blood monocytes, we next tested the hypothesis that there may be in situ proliferation of the embryonically derived macrophages which were clearly present at birth. To examine this we assessed the expression of the Ki67 antigen, which is expressed exclusively by cells in active cell cycle. At 2 days of age, around 30% of mature Ly6C− macrophages (MHCII+ and MHCII−) from the colon showed high Ki67 expression and similar results were seen with macrophages from 2 week old mice, before the numbers of Ki67+ macrophages fell significantly at 3 weeks (Fig. 4a,b). By 5 weeks of age, the frequency of Ki67+ macrophages had decreased further, roughly around the limit of detection, and this remained the case for macrophages from older adults (Fig. 4a,b). There were no differences between the MHCII+ and MHCII− subsets of Ly6C− mature macrophage in terms of Ki67 expression, indicating that this phenotypic distinction does not correlate with an ability to self-renew (Supplementary Fig. 3). Similarly, those F4/80hi CD11blo macrophages that could be identified discretely in adult colon did not differ in Ki67 expression, as would be expected if proliferative capacity was restricted to any remaining embryonically derived cells (Supplementary Fig. 3). The changing pattern of Ki67 expression by colonic macrophages was not a property of all myeloid cells in the mucosa during development, as intestinal eosinophils failed to express Ki67 at any time point (Supplementary Fig. 3), while mucosal DC displayed high expression of Ki67 at all times from birth until adulthood, consistent with the known ability of mature tissue DCs to divide in situ21 (Fig. 4c). It is well known that certain macrophage populations display enhanced proliferative capacity following acute inflammation in an attempt to replenish their numbers4,22,23. However we found no evidence for increased cell division by any of the populations of monocytes/macrophages defined on expression of Ly6C/MHCII during acute colitis induced by oral administration of dextran sodium sulfate (DSS), or in the period of tissue repair after removal of DSS (Fig. 4d). Thus resident intestinal macrophages proliferate in situ in the first few weeks of life, but this diminishes in parallel with the arrival of BM-derived monocytes around 3 weeks of age. Although the adult macrophages retain some capacity to divide locally, this does not appear to account for the maintenance of this population in the steady state, or during their response to inflammation.
Figure 4. Proliferative activity of intestinal macrophages in situ.
(a) Expression of Ki67 by F4/80+ CD11b+ Ly6C− colonic mφ from early neonate (day 3), day 14, day 21, day 35 and 9-12 week old adult CX3CR1+/gfp mice. (b, c) Proportions of Ki67+ F4/80+ CD11b+ Ly6C− mφ (b) and Ki67+ CD11chi F4/80− DC (c) in colon of mice of different ages. The results are of 4-7 individual mice pooled from 2 independent experiments. Each dot represents an individual mouse. ***P<0.0001 (One way ANOVA followed by Bonferroni’s multiple comparison test). (d) Proportion of BrdU+ cells amongst CD64+ Ly6ChiMHCII−, Ly6C+MHCII+, Ly6C− cells and CD64− CD11b+ DC from the colons of resting mice, mice administered 2% DSS for 3 days (colitis) and mice given DSS for 3 days followed by 3 days of normal water (recovery). Mice were administered BrdU 3 hours prior to sacrifice and the dotted line represents the limit of detection as shown by BrdU staining in a non-DNase treated control sample. Data are pooled from two independent experiments with 4-7 seven mice at each time point (a, b, c), or are from one of two independent experiments with 4 mice per group (d). Each circle represents an individual mouse.
Monocytes maintain macrophages in steady state colon
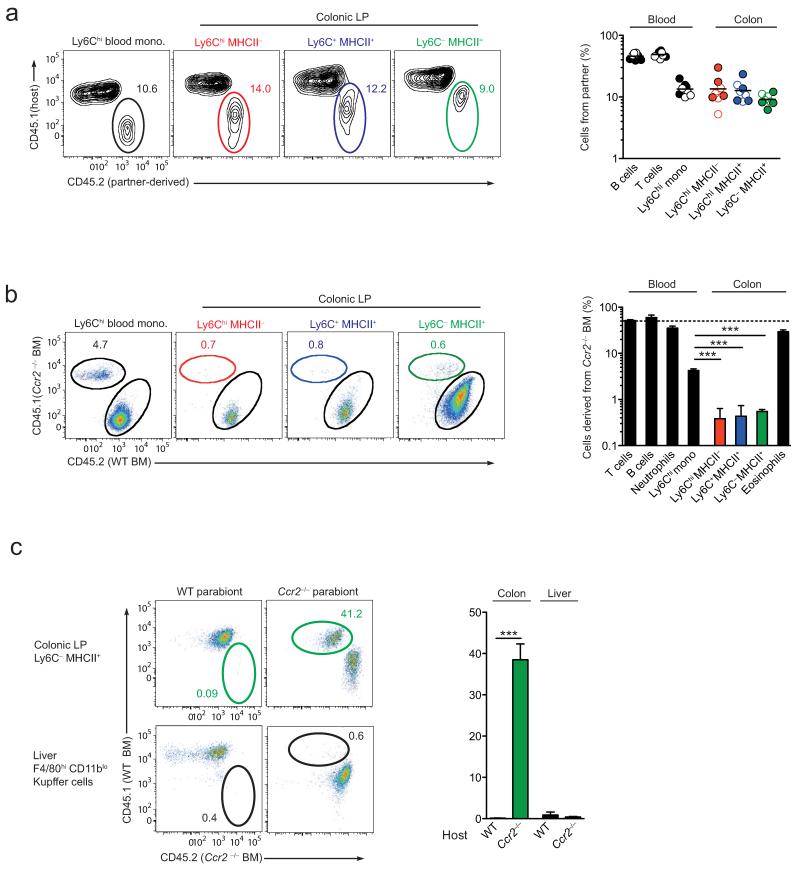
Thus far, our results indicate that the macrophage pool in adult intestine is derived from classical hematopoiesis, rather from self-renewing YS-derived precursors and that this process needs to be continued throughout adult life to maintain normal numbers of macrophages. To study this in more detail, we used a parabiotic approach, in which blood monocytes were able to exchange between adult hosts. Congenic wild-type CD45.1+ and CD45.2+ mice were joined surgically and the presence of non-host cells was assessed in each parabiont 10 weeks later. Confirming that there had been efficient exchange between the circulations at this time, B and T lymphocytes in the bloodstream showed an approximately 50:50 mix of host:donor origin, regardless of which parabiont was examined (Fig. 5a). As reported previously7,24, the degree of chimerism of myeloid cells was considerably lower than that of lymphocytes, with Ly6Chi blood monocytes showing ~12% chimerism in either partner (Fig. 5a). Similar frequencies of chimerism were found among all the populations of Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− MHCII+ macrophages in the colon, with ~10% deriving from the opposite parabiont in each case (Fig. 5a).
Figure 5. Colonic macrophages derive from classical Ly6Chi monocytes.
(a) Representative chimerism of Ly6Chi blood monocytes and Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets of colonic F4/80+ CD11b+ cells from the CD45.1+ partner of wild-type:wild-type parabionts 10 weeks after joining. Scatter plot shows the proportions of non-host derived cells amongst B and T lymphocytes and Ly6Chi monocytes in blood, and colonic Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets from individual CD45.1+ (open circles) and CD45.2+ (filled circles) parabionts. (b) Representative dot plots of the chimerism of Ly6Chi blood monocytes and of the colonic Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets from wild-type (CD45.2+):Ccr2−/− (CD45.1+) mixed BM chimeric mice. Bar chart shows the mean proportions of cells derived from Ccr2−/− CD45.1+ BM amongst CD3+ T cells, CD19+ B cells, Ly6Chi monocytes and Ly6G+ neutrophils in blood, and amongst the colonic Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets, together with F4/80+ CD11b+ MHCII− SSChi eosinophils in the colon of WT (CD45.1+):Ccr2−/− (CD45.2+) mixed BM chimeric mice. ***P<0.0001 (Student’s t-test). (c) Representative non-host chimerism amongst Ly6C− colonic mφ and F4/80hi CD11blo liver Kupffer cells from the WT partner (left plots) and the Ccr2−/− partner (right plots) of WT-Ccr2−/− parabiotic mice 10 weeks after establishment of parabiosis. Bar chart shows the mean proportion of non-host chimerism amongst Ly6C− colonic mφ and F4/80hi CD11blo liver Kupffer cells in the WT and Ccr2−/− partner of WT-Ccr2−/− parabiotic mice. ***P<0.0001 (Student’s t-test). Data are from one experiment (a), or from one of two independent experiments (b), or are pooled from three experiments (c). Results are the means ± 1 SD of 3 (b) or 4-6 mice per group (c).
We next examined the role of CCR2 in the replenishment of the adult macrophage pool, first by analyzing mixed BM chimeric mice that had been reconstituted with a 50:50 mice of wild-type and Ccr2−/− BM. As expected, there was a marked bias towards reconstitution of Ly6Chi monocytes in blood from wild-type BM in these mice (Fig. 5b). In parallel, macrophages in the colon were derived almost exclusively from wild-type BM in these animals. Indeed, even fewer intestinal macrophages were derived from Ccr2−/− BM compared with circulating Ly6Chi monocytes in the same animals (0.5% versus ~4%, Fig. 5b), suggesting that CCR2 is required for entry to the colonic mucosa, in addition to its role in BM egress. In contrast, eosinophils of Ccr2−/− origin accumulated normally in the intestine of chimeric mice, indicating successful engraftment of both types of BM (Fig. 5b). These results did not reflect potential disruption of intestinal homeostasis by irradiation, as similar findings were obtained in wild-type:Ccr2−/− parabiotic mice (wild-type). Under these conditions, wild-type-derived macrophages were readily detected in the colon of the Ccr2−/− parabionts, whereas Ccr2−/− derived macrophages could not be found in the colon of their wild-type partner (Fig. 5c). The same pattern of chimerism was not seen when F4/80hi CD11blo Kupffer cells in the liver of these parabionts were examined, where no exchange between either partner could be seen, consistent with their independence from circulating monocytes (Fig. 5c). Together these results confirm that the adult intestinal macrophage pool requires constant replenishment by CCR2-dependent recruitment of Ly6Chi monocytes.
The microbiota regulates the colonic macrophage pool
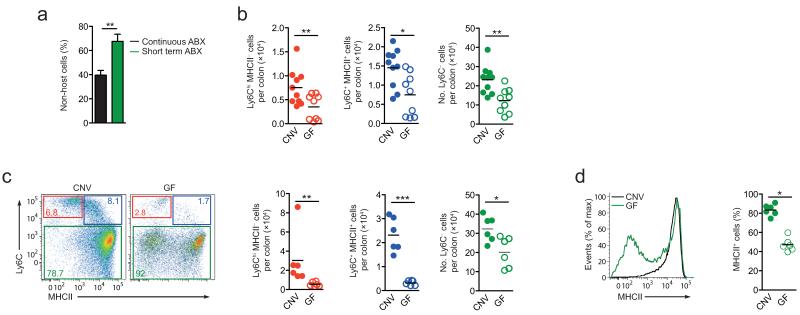
As this need for continuous replenishment by monocytes marks the intestine out from other tissues, we reasoned that this may be driven by local signals, such as the commensal microbiota. To test this hypothesis, we first administered broad-spectrum antibiotics (Abx) to conventionally reared wild-type mice for two weeks. This protocol has been described to alter the composition and burden of commensal bacteria in the intestine25. In our hands, it resulted in small, but significant reductions in the numbers of the Ly6ChiMHCII− and Ly6C+MHCII+ monocyte-like subsets in the colon, but not in the number of Ly6C−MHCII+ mature macrophages (Supplementary Fig. 4). We next depleted the microbiota under conditions in which CCR2-dependent recruitment of monocytes could be assessed by parabiosis. Wild-type:Ccr2−/− parabionts received antibiotics for either the first 4 weeks after surgery, or for the entire period before the degree of non-host chimerism was assessed in the Ccr2−/− parabionts at 8 weeks. We found a higher degree of chimerism in parabionts that had only received antibiotics for the first 4 weeks after surgery compared with those receiving continuous antibiotics (~40% versus ~60%; Fig. 6a).
Figure 6. Influence of the commensal microbiota on the development of colonic macrophages.
(a) Non-host chimerism of CD64+/F4/80+ Ly6C− colonic mφ in Ccr2−/− partners of WT-Ccr2−/− parabiotic mice given broad spectrum antibiotics for the first 4 weeks after surgery (Abx. 4wks), compared with the group of mice shown in Fig. 5c that received antibiotics for the entire 8 weeks of the study (continuous Abx). **P<0.01 (Student’s t-test). (b) Absolute numbers of the Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets of F4/80+ CD11b+ cells per colon of adult conventionally housed (CNV) and germ free (GF) mice. *P<0.05, **P<0.005 (Mann Whitney). (c) Representative dot plots of Ly6C and MHCII expression by F4/80+ CD11b+ colonic LP cells from 3 week old Rag1−/− CNV and GF mice. Scatter plots show the absolute numbers of the Ly6ChiMHCII−, Ly6C+MHCII+ and Ly6C− subsets of F4/80+ CD11b+ cells per colon of individual 3 week old CNV and GF mice. *P<0.05, ***P<0.0001 (Mann Whitney). (d) Representative expression of MHCII by F4/80+ CD11b+ Ly6C− colonic LP cells from 3 week old mice and mean proportion of F4/80+ CD11b+ Ly6C− MHCII+ cells amongst total F4/80+ CD11b+ colonic LP cells. *P<0.05 (Student’s t-test). Data are means + 1 SD of results pooled from two independent experiments using 3 (a) or 10 mice per group (b), or are from one of two independent experiments with 6 mice per group (c, d).
As the accumulation of colonic macrophages between 2-3 weeks of age coincides with acquisition of commensal bacteria, we addressed the hypothesis that commensal bacteria-derived signals may be essential for the burst of monocyte-derived colonic macrophages. Analysis of the colonic macrophage compartment in germ-free mice revealed strikingly fewer of all the monocyte-macrophage subsets in the colon of adult germ-free mice compared with conventionally housed controls (Fig. 6b). Importantly, at 3 weeks of age, there were very few Ly6ChiMHCII− and Ly6C+MHCII+ cells in the germ-free colon compared with conventionally housed mice (Fig. 6c). This was paralleled by a decrease in the numbers of mature Ly6C− macrophages (Fig. 6c) and significantly fewer of these expressed MHCII than among Ly6C− macrophages in wild-type colon (~40% vs ~80%, Fig. 6d). Together these data support the idea that the defect in macrophage numbers seen in germ-free conditions might reflect impaired recruitment of the monocytes that normally begin to replenish the intestinal macrophage population around weaning. Consistent with this we found no difference in the abundance of Ly6C− macrophages in the colonic mucosa of 7-day-old germ-free mice compared with conventionally housed mice (data not shown). Together these experiments indicate that homeostasis of resident intestinal macrophage requires microbiota and CCR2-dependent recruitment of classical monocytes.
Discussion
The concept of the MPS in which blood monocytes replenish tissue macrophages has been called into question recently by a new paradigm proposing that tissue macrophages arise from embryonic precursors and are independent of blood monocytes3,4,6. Here we show that the intestinal mucosa is an exception to this idea. Although seeded early in life by embryonic precursors, the colonic macrophage compartment becomes entirely dependent on constant replenishment by classical Ly6Chi blood monocytes.
Macrophages could be identified readily in newborn and late fetal intestine, where they comprised a mixture of the F4/80hi CD11blo and F4/80lo CD11b+ cells proposed to be derived from the YS and fetal liver respectively3,5. Csf1r and Flt3 driven fate mapping confirmed the presence of YS-derived macrophages in the neonatal intestine, but they were virtually absent from the adult mucosa, where they were replaced by the progeny of conventional hematopoiesis. This contrasts with the liver, lung, spleen, kidney, epidermis and brain, where macrophages retain their embryonic signature into adulthood3,4,26. Although there was also an apparent age-related decrease in the frequency of labeled liver Kupffer cells in the Csf1r-reporter mouse, the vast majority of these remained independent of conventional hematopoiesis. Notably, there were already fewer labeled colonic macrophages in of day 9 Csf1r-reporter mice compared with amongst Kupffer cells, perhaps indicating that conventional hematopoietic precursors had already entered the intestine by this time.
Our parabiosis studies supported a direct precursor-product relationship between Ly6Chi monocytes and colonic macrophages. Although Ly6Chi monocytes have been shown to replenish intestinal CX3CR1+ F4/80+ cells by other investigators13,27, this was following intense depletion of resident cells. Here we have extended our previous findings that adoptively transferred Ly6Chi monocytes enter the steady state colonic mucosa16, by showing that the monocyte replenishment process becomes essential for maintaining the colonic macrophage pool. This was also evidenced by the severe deficit in colonic macrophages found in older Ccr2−/− mice. In contrast, most other tissue macrophages show poor exchange during parabiosis4,7,28,29 and are unaffected by CCR2-deficiency26,30,31. Interestingly however, skin dermal macrophages have been shown to be partly dependent on CCR2 and to exchange during parabiosis, suggesting that some of these cells may also require replenishment by Ly6Chi monocytes28.
We cannot exclude the possibility that small numbers of embryonically derived macrophages may remain present in the intestine throughout adult life. Indeed, as the fate mapping systems used to identify the progeny of YS derived precursors show relatively low efficiencies of recombination, they probably underestimate the true size of these populations32. The presence of colonic macrophages in adult Ccr2−/− mice could also argue for the persistence of macrophages from primitive sources, although it must be noted that Ccr2−/− mice do have circulating Ly6Chi monocytes, albeit ~90% fewer than wild-type mice. Nevertheless, the numbers of YS-derived macrophages in the intestine were ≤ 1000 labeled cells/intestine, suggesting that such cells may not make a substantial contribution to the adult compartment.
During the neonatal period, colonic macrophages showed high levels proliferation, similar to that seen with other macrophages early in life5,22,30. However, following the onset of monocyte accumulation, the proliferative capacity of colonic macrophage diminished, supporting previous studies showing a low turnover rate of mature intestinal macrophages in situ16,33. Adult colonic macrophages also failed to show a capacity to self renew in response to acute inflammation, or during its repair phase, situations where embryonically-derived macrophages proliferate in other organs4,22,23,30,34. Thus the mechanism maintaining the colonic macrophage compartment reflects a unique switch from local proliferation to replenishment by Ly6Chi blood monocytes.
Because the most dramatic changes in macrophage number and monocyte recruitment occurred during the third week of life, we considered that this could reflect establishment of the microbiota. Previous studies have reached discrepant conclusions on the abundance of colonic macrophages in germ-free mice14,35,36 and we found that although these mice had a normal intestinal macrophage compartment at birth, they had markedly reduced monocyte recruitment at 3 weeks of age. As a result, adult germ-free mice had significantly fewer of all the subsets within the so-called monocyte “waterfall”16,37. This effect was partially recapitulated by administering broad spectrum antibiotics to conventionally housed adult mice, although this had no effect on mature macrophages. This might reflect the short duration of antibiotic regime in comparison with the half life of mature cells, which we estimate at ~4-6 weeks on the basis our studies of parabiotic and Ccr2−/− mice. Antibiotic treatment also reduced the recruitment of non-host monocytes into the intestine of parabiotic partners and taken together, these results demonstrate definitively that the microbiota influences the kinetics of macrophage replenishment in the colonic mucosa. Notably, this contrasts with the recruitment of Ly6Chi monocytes to other barrier surfaces such as the skin dermis28,29.
Nevertheless it is intriguing to note that all stages of the monocyte-macrophage waterfall could be detected in adult germ-free colon, as well as in conventional small intestine where the microbial burden is much lower. That the microbiota is not the only factor involved in the homeostasis of intestinal macrophages is supported by our findings that although macrophages in the neonatal and adult intestine have distinct origins, they shared many of the features that make of intestinal macrophages unique. Both expressed high levels of CX3CR1 and scavenger receptors, were avidly phagocytic and produced IL-10 and TNF constitutively, but were hyporesponsive to TLR stimulation (data not shown). Thus intestinal macrophages may have similar roles in the presence or absence of the microbiota, irrespective of the stage of host development (Supplementary Fig.5) Likely functions of this kind could include removal of dying epithelial cells and tissue remodeling, highly appropriate to such a rapidly dividing organ. Indeed, apoptotic debris from epithelial cells has been observed in LP macrophage38,39.
Our results indicate that the ontogeny of resident tissue macrophages is more complex than can be explained only by the paradigms of the MPS or embryonic origin. Rather, the relative contributions of these sources depends on the tissue, with different anatomical and microbiological factors demanding distinct forms of adaptation. The intestine is at one end of the spectrum, as its continual exposure to environmental stimuli warrants constant replenishment by highly plastic blood monocytes (Supplementary Fig.5). Conversely, the physiological demands of tissue remodeling may dominate over responsiveness to external agents in the brain, epidermis and alveolar space, and are best served by a stable population of embryonically derived macrophages throughout life. Tissues like the liver, dermis, heart and spleen appear to exhibit a mixed pattern of origin, allowing stability and flexibility to be combined. Importantly, all tissues can readily recruit classical monocytes when infection or tissue damage demand a rapid and flexible reaction, mirroring the position that occurs on a daily basis in the intestine.
Methods
Mice
Wild-type C57BL/6 (B6) (Harlan Olac), Cx3cr1+/gfp40 and Ccr2-/-41) mice were maintained under specific pathogen-free conditions at the Central Research Facility at the University of Glasgow, UK. Ccr2+/rfp mice19 were maintained at the Division of Neuroimmunology, Roslin Institute, University of Edinburgh. Csf1r-mer-Cre-mer17, Flt3-Cre42 and Rosa26-LSL-YFP mice have been described previously3 and were maintained at the Centre for Molecular and Cellular Biology of Inflammation, King’s College London, UK. GF mice were maintained in the University of Pennsylvania Gnotobiotic Mouse Facility. GF mice were confirmed to be free of all culturable bacteria prior to and at the culmination of all experiments. Conventionally housed counterparts were bred and maintained at the Perelman School of Medicine, University of Pennsylvania, USA. All mice had been backcrossed for at least nine generations on to the B6 background and were used between embryonic day 19.5 and 12 weeks, unless stated otherwise. For determining the stage of development, vaginal plug formation was taken as 0.5 day post-coitum (d.p.c.). All experiments were carried out according to UK Home Office regulations or in agreement with University of Pennsylvania Institutional Animal Care and Use Committee-approved protocols.
Generation of parabiotic mice
CD45.1+ WT mice were sutured to either CD45.2+ WT or Ccr2−/− mice and left for 8-10 weeks before blood and organs were assessed for the level of non-host chimerism. All mice were 9 weeks old at the time of surgery and kept under ibuprofen treatment started few days before the surgery. Mice received either Bactrim for the entire period after surgery, or an antibiotic cocktail containing ampicillin (1 g/l), neomycin (1 g/l), metrodinazole (1 g/l) and vancomycin (0.5 g/l) for only the first 4 weeks following surgery, before returning to normal drinking water. Antibiotics were replaced weekly.
Generation of bone marrow chimeric mice
7-8-week-old CD45.1+CD45.2+ WT mice were lethally irradiated with 2 doses of 5 Gy, 2 h apart and then reconstituted immediately with 5 × 106 BM cells from CD45.1+ WT and CD45.2+ Ccr2−/− mice at a 50:50 ratio by intravenous infusion. Mice were left for 8 weeks before blood and organs were assessed for chimerism.
Pulse labeling of YS derived macrophages
To label YS derived macrophages, tamoxifen-inducible Csf1r-mer-iCre-mer transgenic mice were crossed with homozygous Rosa26-LSL-YFP reporter mice and pregnant females were given a single intraperitoneal injection of 75 g/g bodyweight 4-hydroxytamoxifen (4′OHT, Sigma) and 37.5 μg/g progesterone at E8.5 to induce Cre-mediated recombination as described previously3.
Isolation of tissue leukocytes
Lamina propria cells were obtained from fetal, neonatal and adult mouse colon by enzymatic digestion as described previously12,43. For isolation of colonic LP cells from E18.5 fetal mice, pregnant females were sacrificed by cervical dislocation, fetuses were dissected out of the uterus and washed in ice-cold phosphate-buffered saline (PBS). For the isolation of liver leukocytes, livers were removed from perfused mice, chopped finely and incubated in pre-warmed 1.25 mg/ml collagenase D (Roche), 0.85 mg/ml collagenase V (Sigma-Aldrich), 1 mg dispase (Gibco, Invitrogen), and 30 U/ml DNase (Roche Diagnostics GmbH) in RPMI-1640 for 20-30 minutes in a shaking incubator at 37°C.
Flow cytometric analysis and sorting of cells
0.5-5 × 106 cells were stained at 4°C in the dark as described previously43, using the antibodies listed in Supplementary Table 1 and analyzed using an LSR II or FACSAria I cytometer (BD Biosciences) and FlowJo software (Tree Star). Colonic macrophages were sorted as live gated CD45+ SiglecF− Ly6G− CD11cint F4/80hi. For morphological assessment, sorted cells were spun onto Polysine™ glass microscope slides (VWR International), fixed in acetone and stained using the Rapid-Romanowsky staining kit (Raymond A. Lamb Ltd).
Assessment of proliferation
For the detection of Ki67 expression, 3-4 × 106 cells were first incubated with fixable viability dye eFluor780 (eBioscience), before being incubated with anti-CD16/CD32 to block Fc receptors and then stained with the appropriate surface markers, all at 4°C. Cells were then fixed and permeabilized with Foxp3 staining buffer set overnight at 4°C (eBioscience), before staining for Ki67 (BD Bioscience) for 30 min at 20°C. In some experiments, mice were injected i.p. with 1 mg BrdU (BD Biosciences) and the incorporation of BrdU by isolated cells was assessed after 3 h using the BD BrdU Flow Kit (BD Biosciences).
Assessment of phagocytosis
1-3 × 106 cells were cultured with pHrodo Escherichia coli bioparticles (Life Technologies) according to the manufacturer’s guidelines and analyzed by flow cytometry.
Induction of colitis
Mice received 2% DSS (reagent grade; MW 36-50 kDa; MP Biomedicals), ad libitum in sterile drinking water for 3 days. To assess the recovery phase, mice were then returned to normal drinking water for 3 days.
Quantitation of gene expression by real-time reverse transcription PCR
Total RNA was extracted from FACS-purified F4/80hi cells from the colon of individual adult mice or from pooled neonatal colons using the RNeasy Micro kit (Qiagen). RNA were reverse transcribed to cDNA using the Superscript II First strand synthesis system (Invitrogen) and gene expression was assayed by quantitative reverse transcription PCR using Brilliant III Ultra Fast SYBR qPCR master mix (Agilent Technologies) on the 7500HT Fast system (Applied Biosystems) and with the primers (Integrated DNA Technologies) detailed in Supplementary Table 2. Complementary DNA samples were assayed in triplicate and gene expression levels were normalized to Cyclophilin A. The mean relative gene expression was calculated using the 2−dΔC(t) method.
Statistical analysis
Results are presented as mean ±1 s.d. and groups were compared using a Student’s t-test, Mann-Whitney test or for multiple groups, a one-way ANOVA followed by a Bonferroni post test using Prism Software (GraphPad Software).
Supplementary Material
Acknowledgements
We would like to thank D. Vaughan from the Flow Cytometry Core Facility at the University of Glasgow and B. McColl from the Roslin Institute, Edinburgh for the CCR2-reporter mice. We would also like to thank V. Cerovic for critical reading of the manuscript.
C.C.B., A.M.M. and C.L.S. were funded by The Wellcome Trust and MRC UK; A.B.-B. was funded by Conacyt (Mexico) and Tenovus Scotland; S.H. and B.M. were supported by the EE-ASI European Collaborative Research Project and the Agence National de Recherche (France).
Footnotes
Competing Interests: The authors have no competing interests to declare.
References
- 1.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat. Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Furth R, et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science (New York, N.Y. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilliams M, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J. Exp. Med. 1985;161:475–489. doi: 10.1084/jem.161.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bain CC, Mowat AM. The monocyte-macrophage axis in the intestine. Cell. Immunol. 2014 doi: 10.1016/j.cellimm.2014.03.012. doi:10.1016/j.cellimm.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm MC, et al. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995;10:387–395. doi: 10.1111/j.1440-1746.1995.tb01589.x. [DOI] [PubMed] [Google Scholar]
- 12.Platt AM, Bain CC, Bordon Y, Sester DP, Mowat AM. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J. Immunol. 2010;184:6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- 13.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamoutounour S, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 16.Bain CC, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qian B-Z, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- 19.Saederup N, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE. 2010;5:e13693. doi: 10.1371/journal.pone.0013693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 21.Ginhoux F, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 2009;206:3115–3130. doi: 10.1084/jem.20091756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davies LC, et al. A quantifiable proliferative burst of tissue macrophages restores homeostatic macrophage populations after acute inflammation. Eur. J. Immunol. 2011;41:2155–2164. doi: 10.1002/eji.201141817. [DOI] [PubMed] [Google Scholar]
- 23.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 24.Liu K, et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 25.Hill DA, et al. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol. 2010;3:148–158. doi: 10.1038/mi.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epelman S, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamoutounour S, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Jakubzick C, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chorro L, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 2009;206:3089–3100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mildner A, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 32.Epelman S, Lavine KJ, Randolph GJ. Origin and Functions of Tissue Macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smythies LE, et al. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J. Leukoc. Bio. 2006;80:492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 34.Ghigo C, et al. Multicolor fate mapping of Langerhans cell homeostasis. J. Exp. Med. 2013;210:1657–1664. doi: 10.1084/jem.20130403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueda Y, et al. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol. 2010;22:953–962. doi: 10.1093/intimm/dxq449. [DOI] [PubMed] [Google Scholar]
- 36.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J. Immunol. 2010;184:2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 37.Tamoutounour S, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur. J. Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 38.Muller AJ, et al. Salmonella gut invasion involves TTSS-2-dependent epithelial traversal, basolateral exit, and uptake by epithelium-sampling lamina propria phagocytes. Cell Host Microbe. 2012;11:19–32. doi: 10.1016/j.chom.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Nagashima R, Maeda K, Imai Y, Takahashi T. Lamina propria macrophages in the human gastrointestinal mucosa: their distribution, immunohistological phenotype, and function. J. Histochem. Cytochem. 1996;44:721–731. doi: 10.1177/44.7.8675993. [DOI] [PubMed] [Google Scholar]
- 40.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boring L, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bertrand JY, et al. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. doi: 10.1038/nature08738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bain CC, Mowat AM. CD200 receptor and macrophage function in the intestine. Immunobiology. 2012;217:643–651. doi: 10.1016/j.imbio.2011.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.