Abstract
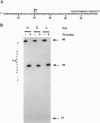
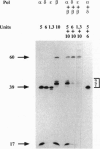
We have examined the capacity of calf thymus DNA polymerases alpha, beta, delta, and epsilon to perform in vitro translesion synthesis on a substrate containing a single d(GpG)-cisplatin adduct placed on codon 13 of the human HRAS gene. We found that DNA synthesis catalyzed by DNA polymerases alpha, delta, and epsilon was blocked at the base preceding the lesion. Addition of proliferating cell nuclear antigen to DNA polymerase delta and replication protein A to DNA polymerase alpha did not restore their capacity to elongate past the adduct. On the other hand, DNA polymerase beta efficiently bypassed the cisplatin adduct. Furthermore, we observed that DNA polymerase beta was the only polymerase capable of primer extension of a 3'-OH located opposite the base preceding the lesion. Likewise, DNA polymerase beta was able to elongate the arrested replication products of the other three DNA polymerases, thus showing its capacity to successfully compete with polymerases alpha, delta, and epsilon in the stalled replication complex. Our data suggest (i) a possible mechanism enabling DNA polymerase beta to bypass a d(GpG)-cisplatin adduct in vitro and (ii) a role for this enzyme in processing DNA damage in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belguise-Valladier P., Maki H., Sekiguchi M., Fuchs R. P. Effect of single DNA lesions on in vitro replication with DNA polymerase III holoenzyme. Comparison with other polymerases. J Mol Biol. 1994 Feb 11;236(1):151–164. doi: 10.1006/jmbi.1994.1125. [DOI] [PubMed] [Google Scholar]
- Bradley L. J., Yarema K. J., Lippard S. J., Essigmann J. M. Mutagenicity and genotoxicity of the major DNA adduct of the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1993 Jan 26;32(3):982–988. doi: 10.1021/bi00054a031. [DOI] [PubMed] [Google Scholar]
- Burnouf D., Duane M., Fuchs R. P. Spectrum of cisplatin-induced mutations in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3758–3762. doi: 10.1073/pnas.84.11.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M. P., Levine A. S., Dixon K. HeLa cell single-stranded DNA-binding protein increases the accuracy of DNA synthesis by DNA polymerase alpha in vitro. Mutat Res. 1992 Jun;274(1):29–43. doi: 10.1016/0921-8777(92)90041-z. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Low molecular weight deoxyribonucleic acid polymerase from calf thymus chromatin. I. Preparation of homogeneous enzyme. J Biol Chem. 1973 Jun 10;248(11):3789–3795. [PubMed] [Google Scholar]
- Chin A. J., Sanders S. P., Sherman F., Lang P., Norwood W. I., Castaneda A. R. Accuracy of subcostal two-dimensional echocardiography in prospective diagnosis of total anomalous pulmonary venous connection. Am Heart J. 1987 May;113(5):1153–1159. doi: 10.1016/0002-8703(87)90928-8. [DOI] [PubMed] [Google Scholar]
- Comess K. M., Burstyn J. N., Essigmann J. M., Lippard S. J. Replication inhibition and translesion synthesis on templates containing site-specifically placed cis-diamminedichloroplatinum(II) DNA adducts. Biochemistry. 1992 Apr 28;31(16):3975–3990. doi: 10.1021/bi00131a013. [DOI] [PubMed] [Google Scholar]
- Davies J. F., 2nd, Almassy R. J., Hostomska Z., Ferre R. A., Hostomsky Z. 2.3 A crystal structure of the catalytic domain of DNA polymerase beta. Cell. 1994 Mar 25;76(6):1123–1133. doi: 10.1016/0092-8674(94)90388-3. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Zmudzka B., Hollander M. C., Wilson S. H. Induction of beta-polymerase mRNA by DNA-damaging agents in Chinese hamster ovary cells. Mol Cell Biol. 1989 Feb;9(2):851–853. doi: 10.1128/mcb.9.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgaki A., Hübscher U. DNA unwinding by replication protein A is a property of the 70 kDa subunit and is facilitated by phosphorylation of the 32 kDa subunit. Nucleic Acids Res. 1993 Aug 11;21(16):3659–3665. doi: 10.1093/nar/21.16.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. S., Fry M., Ji J., Williams K. J., Loeb L. A. Codons 12 and 13 of H-ras protooncogene interrupt the progression of DNA synthesis catalyzed by DNA polymerase alpha. Cancer Res. 1993 Jun 15;53(12):2895–2900. [PubMed] [Google Scholar]
- Huang L., Turchi J. J., Wahl A. F., Bambara R. A. Effects of the anticancer drug cis-diamminedichloroplatinum(II) on the activities of calf thymus DNA polymerase epsilon. Biochemistry. 1993 Jan 26;32(3):841–848. doi: 10.1021/bi00054a015. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Spadari S. DNA replication and chemotherapy. Physiol Rev. 1994 Apr;74(2):259–304. doi: 10.1152/physrev.1994.74.2.259. [DOI] [PubMed] [Google Scholar]
- Jenkins T. M., Saxena J. K., Kumar A., Wilson S. H., Ackerman E. J. DNA polymerase beta and DNA synthesis in Xenopus oocytes and in a nuclear extract. Science. 1992 Oct 16;258(5081):475–478. doi: 10.1126/science.1411545. [DOI] [PubMed] [Google Scholar]
- Kamiya H., Murata N., Murata T., Iwai S., Matsukage A., Masutani C., Hanaoka F., Ohtsuka E. Cyclobutane thymine dimers in a ras proto-oncogene hot spot activate the gene by point mutation. Nucleic Acids Res. 1993 May 25;21(10):2355–2361. doi: 10.1093/nar/21.10.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Loeb L. A. Depurination-induced infidelity of deoxyribonucleic acid synthesis with purified deoxyribonucleic acid replication proteins in vitro. Biochemistry. 1983 May 10;22(10):2378–2384. doi: 10.1021/bi00279a012. [DOI] [PubMed] [Google Scholar]
- Loehrer P. J., Einhorn L. H. Drugs five years later. Cisplatin. Ann Intern Med. 1984 May;100(5):704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- Mamenta E. L., Poma E. E., Kaufmann W. K., Delmastro D. A., Grady H. L., Chaney S. G. Enhanced replicative bypass of platinum-DNA adducts in cisplatin-resistant human ovarian carcinoma cell lines. Cancer Res. 1994 Jul 1;54(13):3500–3505. [PubMed] [Google Scholar]
- Morrison A., Araki H., Clark A. B., Hamatake R. K., Sugino A. A third essential DNA polymerase in S. cerevisiae. Cell. 1990 Sep 21;62(6):1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- O'Day C. L., Burgers P. M., Taylor J. S. PCNA-induced DNA synthesis past cis-syn and trans-syn-I thymine dimers by calf thymus DNA polymerase delta in vitro. Nucleic Acids Res. 1992 Oct 25;20(20):5403–5406. doi: 10.1093/nar/20.20.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier H., Sawaya M. R., Kumar A., Wilson S. H., Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994 Jun 24;264(5167):1891–1903. [PubMed] [Google Scholar]
- Perrino F. W., Loeb L. A. Differential extension of 3' mispairs is a major contribution to the high fidelity of calf thymus DNA polymerase-alpha. J Biol Chem. 1989 Feb 15;264(5):2898–2905. [PubMed] [Google Scholar]
- Pillaire M. J., Margot A., Villani G., Sarasin A., Defais M., Gentil A. Mutagenesis in monkey cells of a vector containing a single d(GPG) cis-diamminedichloroplatinum(II) adduct placed on codon 13 of the human H-ras proto-oncogene. Nucleic Acids Res. 1994 Jul 11;22(13):2519–2524. doi: 10.1093/nar/22.13.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillaire M. J., Villani G., Hoffmann J. S., Mazard A. M., Defais M. Characterization and localization of cis-diamminedichloro-platinum(II) adducts on a purified oligonucleotide containing the codons 12 and 13 of H-ras proto-oncogene. Nucleic Acids Res. 1992 Dec 25;20(24):6473–6479. doi: 10.1093/nar/20.24.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya M. R., Pelletier H., Kumar A., Wilson S. H., Kraut J. Crystal structure of rat DNA polymerase beta: evidence for a common polymerase mechanism. Science. 1994 Jun 24;264(5167):1930–1935. doi: 10.1126/science.7516581. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Izuta S., Yoshida S. DNA polymerase alpha overcomes an error-prone pause site in the presence of replication protein-A. J Biol Chem. 1994 Apr 8;269(14):10225–10228. [PubMed] [Google Scholar]
- Sweasy J. B., Loeb L. A. Mammalian DNA polymerase beta can substitute for DNA polymerase I during DNA replication in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1407–1410. [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Tornaletti S., Pfeifer G. P. Slow repair of pyrimidine dimers at p53 mutation hotspots in skin cancer. Science. 1994 Mar 11;263(5152):1436–1438. doi: 10.1126/science.8128225. [DOI] [PubMed] [Google Scholar]
- Villani G., Cazaux C., Pillaire M. J., Boehmer P. Effects of a single intrastrand d(GpG) platinum adduct on the strand separating activity of the Escherichia coli proteins RecB and RecA. FEBS Lett. 1993 Oct 25;333(1-2):89–95. doi: 10.1016/0014-5793(93)80380-d. [DOI] [PubMed] [Google Scholar]
- Weiser T., Gassmann M., Thömmes P., Ferrari E., Hafkemeyer P., Hübscher U. Biochemical and functional comparison of DNA polymerases alpha, delta, and epsilon from calf thymus. J Biol Chem. 1991 Jun 5;266(16):10420–10428. [PubMed] [Google Scholar]
- Wold M. S., Weinberg D. H., Virshup D. M., Li J. J., Kelly T. J. Identification of cellular proteins required for simian virus 40 DNA replication. J Biol Chem. 1989 Feb 15;264(5):2801–2809. [PubMed] [Google Scholar]