Abstract
Gastric pacing is used to modulate normal or abnormal gastric slow-wave activity for therapeutic purposes. New protocols are required that are optimized for motility outcomes and energy efficiency. A computational tissue model was developed, incorporating smooth muscle and interstitial cell of Cajal layers, to enable predictive simulations of slow-wave entrainment efficacy under different pacing frequencies. Concurrent experimental validation was performed via high-resolution entrainment mapping in a porcine model (bipolar pacing protocol: 2 mA amplitude; 400 ms pulse-width; 17-s period; midcorpus). Entrained gastric slow-wave activity was found to be anisotropic (circular direction: 8.51 mm s−1; longitudinal: 4.58 mm s−1), and the simulation velocities were specified accordingly. Simulated and experimental slow-wave activities demonstrated satisfactory agreement, showing similar propagation patterns and frequencies (3.5–3.6 cycles per minute), and comparable zones of entrainment (ZOEs; 64 cm2). The area of ZOE achieved was found to depend on the phase interactions between the native and entrained activities. This model allows the predictions of phase interactions between native and entrained activities, and will be useful for determining optimal frequencies for gastric pacing, including multichannel pacing studies. The model provides a framework for the development of more sophisticated predictive gastric pacing simulations in future.
Index Terms: Computational simulation, gastric electrical stimulation (GES), gastric pacing, gastrointestinal modeling, pacemaker potential, slow wave
I. INTRODUCTION
In the normal stomach, there is an omnipresent gastric electrical activity (GEA) that propagates in the antegrade direction toward the gastric antrum [1]. GEA is a collective term for the spontaneous rhythmic electrical activity, known as the pacemaker potential that occurs within interstitial cells of Cajal (ICCs), and the subsequent depolarization, known as slow waves, of the adjoining smooth muscle (SM) cells [2]. The pacemaker potential initiates the peristaltic activity of the stomach, by depolarizing the membrane potential of the SM cells in a rhythmic coordination fashion, leading to calcium influx and an increased probability of contractions [3].
Gastric electrical stimulation (GES) is a therapeutic strategy that attempts to modulate GI electrophysiology to improve motility and symptoms in gastroparesis and other dysmotility syndromes [26], [27]. There are two types of GES. The first is high-frequency (also called “short pulse”) GES, which is thought to work primarily via neurostimulation. The second type is long-pulse GES (also called “gastric pacing”), which aims to directly manipulate GEA [26]. Gastric pacing has also been proposed as a treatment for obesity, whereby the direction of the GEA is reversed, leading to gastric distension and satiety [4]. While GES has shown significant promise in animal studies and uncontrolled clinical trials, and there have been commercial applications, these approaches to treatment are still largely experimental. This is because there is a lack of agreement as to which of the many possible stimulation protocols is appropriate or optimal, in terms of stimulus location, amplitude, width, and frequency. It is necessary to define the protocols that are effective, and most energy efficient, if GES is to develop as a therapeutic strategy and be implemented in an implantable device [4]. To date, evaluating the effects of the different combinations of parameters has only been conducted on trial-and-error basis in animal models, which is tedious and inefficient [6]. New methods of computational analysis are required to assist experimental studies and this is now considered a research priority [7].
Computational simulations have been proposed as a powerful tool to augment the assessment of GES protocols, achieving much greater efficiency than can be achieved in animal models alone [7]. In earlier work, the relaxation oscillator approach was used to simulate the propagation of normal GEA (referred to as the gastric electrical control activity in the original papers [31], [32]). However, due to the lack of understanding regarding the electrophysiological role of the ICC at the time, the relaxation oscillator models were simplistic, as most relaxation oscillator models did not take into account the multiple electrical active cell types in GEA. More recently, Mintchev and Bowes have employed a conical dipole model of GEA to calculate the effects of GES, focusing in particular on very high frequency stimulations that target neuromuscular pathways [8]. Overall, there are currently no existing mathematical models based on sound physiological principles that are appropriate for assessing GES protocols of other frequencies. By contrast, in the field of cardiac electrophysiology, a number of biophysically based cell models have been developed and successfully employed in simulations to assist basic applied studies [9].
In this study, a novel platform for the modeling of GEA has been developed, which is the potential to be used in the assessment of GES protocols. This model is based on the concept of “multiscale” modeling, which provides an integrated description of the electrophysiological events from the cellular level to the tissue level. Multiscale modeling has become a standard method in cardiac modeling, but has yet to be widely applied in gastrointestinal modeling.
The description of cellular level events (pacemaker potentials and slow waves) in the multiscale approach is achieved through the use of cell models. The Aliev model is one of the most prominent cell models that has previously been used to represent the GEA in 3-D anatomical models of the stomach [10]–[12]. However, the Aliev cell model also lacks a biophysical basis, without which it is unable to reproduce effects under physiologically relevant parameters, and therefore, has limited capacity for predictive modeling. Detailed descriptions of the ion channels and intracellular activities of gastric SM cells have recently led to the development of a biophysically based SM cell model [13]. This is significant because the biophysically based SM cell model allows individual ion currents to be evaluated quantitatively under the effects of physical parameters, such as temperature, ion concentration, and voltage. In this study, we present the development of this model and simulated results of normal GEA and the effects of GES in an area covered by experimental electrode array platform, together with a small validation study.
II. METHODS
A. Mathematical Model
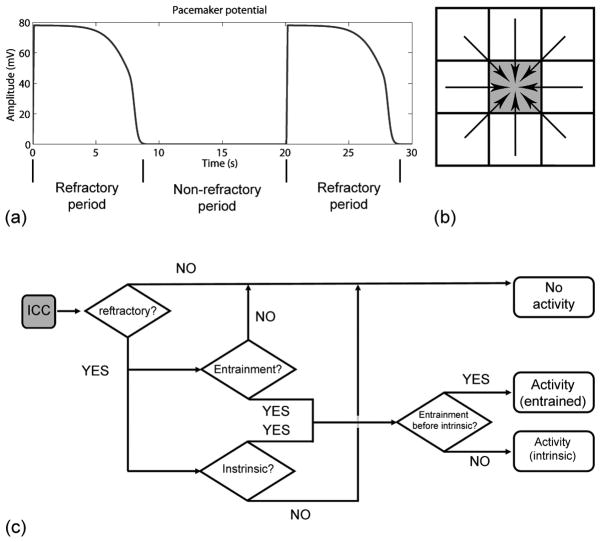
A computational model of gastric electrical activity was developed by modeling the electrical events of the ICC and SM layers as two interconnected tissue domains. Multiple experiments have now shown that ICCs act as pacemakers and mediators of slow-wave propagation through the entrainment of pacemaker potentials [14], [15]. Furthermore, SM layers cannot actively propagate slow waves without the presence of ICCs [3]. In previous simulations of GEA, multiple ICC and SM layers have been incorporated [11], [12]. In this study, two interconnected layers were incorporated to represent the averaged effects of all electrically active layers measured from the serosal surface. While this approach does not allow microscopic details of GEA to be investigated, it presents a computationally efficient way of reliably comparing simulated data to experimental measurements. There is also presently insufficient anatomical knowledge of the gastric microstructure to facilitate accurate 3-D modeling. Each layer was represented as a 2-D continuum tissue, into which mathematical descriptions of the ICCs and SM cells were embedded. The solution of the ICC layer was the pacemaker current used to depolarize the SM layer. While biophysically based cell models of isolated ICCs have recently been developed [16], [17], the underlying cell-to-cell entrainment mechanisms have yet to be successfully modeled. For this reason, a cellular automata algorithm was used in this study to simulate entrainment of single cell behavior in the ICC layer. The algorithm employed (outlined in Fig. 1) involved assigning a set of conditions for which an ICC continuum cell was to generate a pacemaker potential, such that the overall frequency of pacemaker potentials would be entrained to the continuum cell containing the highest intrinsic frequency in the ICC layer.
Fig. 1.
(a) Refractory period is defined as the period during which the pacemaker potential occurs and nonrefractory period is defined as the period during which the rest potential occurs. An ICC continuum cell in a nonrefractory state (b; gray square) is capable of either generating a new pacemaker potential due its own intrinsic frequency, or being entrained by one of the eight neighboring continuum cells, according to the flowchart of the cellular automata algorithm outlined in (c).
1) ICC Layer
Once a continuum cell in the ICC layer entered the refractory period in accordance with the automata algorithm, a predefined ICC membrane potential trace was used to represent the ICC electrical activity [see Fig. 1(a)]. The predefined trace was a fitted curve of the membrane potential of a single excitation of an isolated ICC that was experimentally obtained from guinea pigs [13]. This trace has been previously shown to adequately depolarize the SM cell model [13]. Each ICC continuum cell was assigned an intrinsic frequency at which it produced pacemaker potentials. Based on previous experimental recordings of decoupled canine gastric slow-wave frequencies [18], a gradient of the intrinsic frequency was set linearly ranging from 3.65 to 2 cycles per minute (cpm) from the proximal to the distal end of the tissue model. A new pacemaker potential could only be invoked in each ICC cell during the nonrefractory period [see Fig. 1(b)]. Conversely, during the refractory period, the ICC continuum cell was insensitive to the depolarizing currents from another ICC continuum cell. A time counter was assigned to each ICC continuum cell for recording the time since the onset of a pacemaker potential. The counter was reset to 0 s each time that a continuum cell produced a pacemaker potential. Once an ICC continuum cell entered the nonrefractory period (i.e., counter > refractory period), the automata algorithm determined whether any of the eight surrounding continuum cells produced a pacemaker potential that could entrain the resting ICC continuum cell [see Fig. 1(b)]. The automata algorithm also checked whether entrainment occurred in advance of a pacemaker potential arising due to the intrinsic frequency in the resting ICC continuum cell [see Fig. 1(c)]. The counter of the resting ICC was then assigned a “delay time” before the next pacemaker event occurred. The time delay (n) was calculated using the following equation:
| (1) |
where d and θ are the distance and angle between the resting and depolarizing ICC, respectively, and Vcirc and Vlong are the conduction velocities in the circular and longitudinal directions, respectively. The assigned slow-wave conduction velocity in the circular direction (Vcirc = 8.51 mm s−1) and in the longitudinal direction (Vlong = 4.58 mm s−1), were based on preliminary analysis of the normal slow-wave recordings in this study. Equation (1) was used in conjunction with the algorithm outlined in Fig. 1(c) to assign the timing of the entrained pacemaker activity in the ICC layer.
2) SM Layer
The membrane potentials of the SM layer were modeled based on a passive conduction continuum monodomain in which a biophysically based and validated SM cell model was embedded [13]. The monodomain description has been used to quantify the effects of cardiac electrophysiology for many years, but has only been recently transferred to study the gastric slow waves [13]. The passive conduction of slow waves in the SM layer was modeled using a monodomain model
| (2) |
Here, Am denotes the cell surface to volume ratio, Cm denotes the SM cell membrane capacitance, Vm denotes the SM cell membrane potential, Iion is the sum of the ionic currents, IICC denotes the current from the ICC layer, and σ denotes the conductivity tensor of the SM tissue. The values of all the parameters except for σ were as defined by the authors of the SM cell model. The passive conduction of slow waves within the SM layer, influenced by the σ, does not contribute appreciably to the active slow-wave propagation based on the experiments conducted by Sanders et al. on slow-wave decay in canine colon tissues devoid of ICC [24]. The σ value was set to 0.005 mS mm−1 to represent the passive current conduction within the modeled SM tissues. The second derivatives of the SM membrane potential were scaled to represent the serosal slow waves, based on a previous study on the relationship between extracellular potential and second derivative of membrane potential in neurons [25]. The amplitudes of the extracellular slow waves were scaled to match the relative size of the experimental recording.
B. Animal Preparation
Experimental validation studies were conducted in a porcine model and ethical approval for porcine experiments was obtained from the local institutional committee (The University of Auckland Animal Ethics Committee). The International Guiding Principles for Biomedical Research Involving Animals were followed. Recordings were performed in two female weaner crossbreed pigs (weight range: 35.0–37.4 kg). The recording setup and methodology has been described in a previous study [19].
1) Stimulation Protocol
The positions of six printed-circuit-board (PCB) electrode array (8×24 array; interelectrode distance 7.62 mm) on the anterior porcine gastric corpus is shown in Fig. 2(a). PCB orientation and serosal contact was maintained with gentle overlying pressure using warm saline-soaked gauze. Two 23 g hollow-bore stainless steel pacing electrodes were inserted 11 mm below the fundal line, as shown in Fig. 2. The electrodes were 8 mm apart, providing an average tissue resistance of 16.5 kΩ. The pacing wires leading out of the wound were connected via an electrical isolator to a DS8000 multichannel stimulator(World Precision Instruments, Sarasota, FL). The wound edges were approximated, and a 15-min period of stabilization was allowed prior to a 10-min recording period of baseline gastric slow waves. Electrical stimulation [see Fig. 2(b)] was then administrated for 20 min. The position of the PCBs and pacing leads was not disturbed during the recordings. Slow waves were filtered, characterized, and activation maps were constructed according to our described methods [19]. The acquisition device was a Biosemi Active Two System (The Netherlands). The common mode sense (reference) electrode was placed on the body surface of the lower abdomen, 5 cm below the incision for all recordings. The right-leg drive electrode was placed on the lower hind right leg.
Fig. 2.
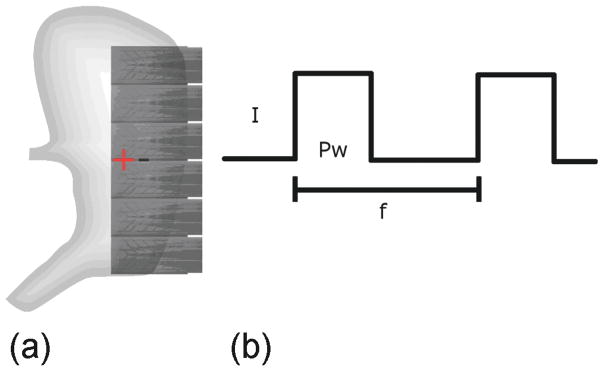
(a) Placement of the PCB electrodes. The “+” and “−” represent the location of the positive and negative leads of the stimulator, respectively. (b) Bipolar stimulus was used. The amplitude (I) for all stimulation trials was 2 mA and the pulse width (Pw) was 400 ms. This protocol was selected based on protocols that have previously been shown to successfully entrain gastric slow waves [20]. The frequency (f) of stimulation was 3.5 cpm (one pulse per 17 s).
2) Signal Processing
The raw data were saved and filtered post-recording using a second-order Bessel low-pass filter of 4 Hz. The following definitions were used to accurately quantify the effects of gastric pacing over the 2-D-mapped tissue area. The area of tissue that was successfully entrained by gastric pacing was termed the “zone of entrainment” (ZOE), whereas the area where the native slow-wave activity continued to occur was termed the “zone-of-native” activity (ZON). The ZOE was distinguished from the ZON by the direction of the slow-wave propagation. The entrained displacement in the retrograde direction from the location of stimulus site to the midpoint of the isochronal band where the ZOE meets the ZOE was measured in millimeters. Similarly, the entrained displacement in the proximal direction was also measured in millimeters. In the simulation, ZON and the entrained displacements could be measured by tracking the source of entertainment to distinguish whether a continuum in the SM domain was activated by the native pacemaker or by the stimulus.
III. RESULTS
A. Simulation and Validation of Normal Slow-Wave Propagation
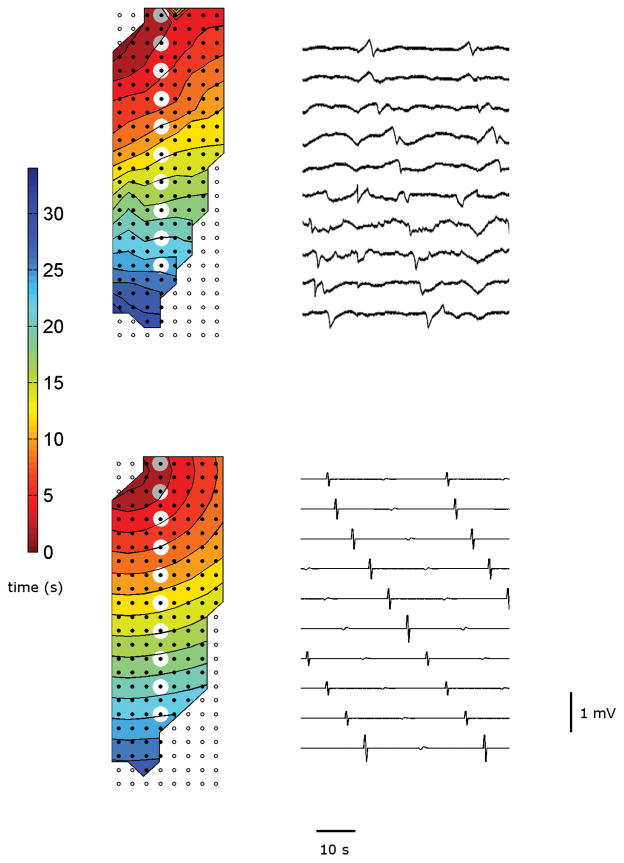
Slow-wave propagation maps and gastric electrograms from the simulated and experimental studies are compared in Fig. 3. Overall, the simulated slow-wave activity produced good agreement with the recording of normal slow waves, in terms of frequency and propagation velocity. The frequency of recorded normal slow waves was 3.62±0.07 and 3.56±0.03 cpm (p-value = 0.52) over ten consecutive waves in the porcine trials. The overall frequency of the simulated slow waves was 3.60 cpm, which overrode the underlying gradient of intrinsic frequencies across the tissue model, demonstrating that successful entrainment to the dominant intrinsic frequency was achieved. The propagation velocity in the longitudinal direction was 5.05±0.04 and 5.48±0.50 mm s−1 (p-value=0.4659); the propagation velocity in the circular direction was 8.31±0.18 and 8.71±0.17 mm s−1 (p-value=0.1470). The simulated slow waves propagated at the designated velocity of 4.58 mm s−1 in the antegrade direction and 8.51 mm s−1 in the circular direction. The sample of the selected channels of experimentally recorded slow waves [see Fig. 3(a)] showed a more gradual upstroke phase than the simulated slow waves [see Fig. 3(b)]. Both activation plots of normal porcine slow wave events (experimental and simulated) demonstrated that slow waves propagated in the antegrade direction, and in the case of the experimental recording, that they originated from the porcine gastric fundus and propagated in the organoaxial direction towards the gastric antrum.
Fig. 3.
(a) Activation map of normal porcine gastric slow waves recorded via high-resolution flexible electrode platform. (b) Simulated results. The selected channels, highlighted in gray circles, show the electrograms of those channels over a 30 s period.
B. Gastric Pacing at 3.5 cpm
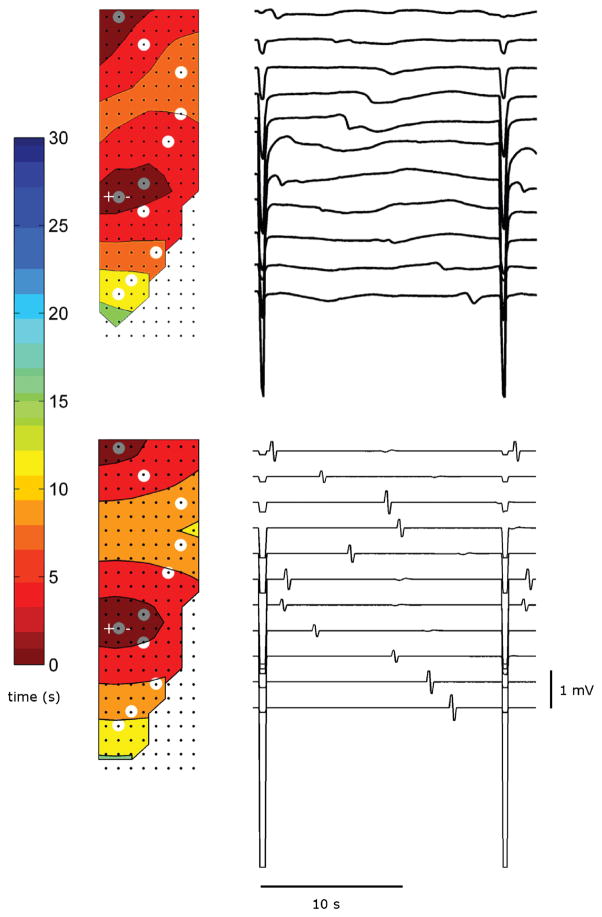
Slow-wave activation maps and gastric electrograms for the simulated and experimental studies, under gastric pacing at 3.53 cpm (period 17 s), are shown in Fig. 4. The pacing frequency of 3.53 cpm was chosen for the purposes of model validation, because pacing at a similar frequency to the native activity allowed a detailed assessment of the interaction between the native and entrained activities in both the experiment and simulation. Slow-wave entrainment was consistently and reliably achieved in the experimental study, and the frequency of the invoked events was similar to that of the native baseline frequency of 3.6 cpm. The stimulus effectively produced a secondary pacemaker of slow-wave activity in addition to the native pacemaker. The electrograms shown in Fig. 4(a) demonstrate that the entrained slow-wave events propagated simultaneously in both the antegrade and retrograde directions from the point of stimulus.
Fig. 4.
(a) Activation map of paced porcine gastric slow waves recorded via high-resolution flexible electrode platform. The location of pacing needles are marked by “+” and “−”. (b) Simulated results. The selected channels, which are highlighted in gray circles, show the electrograms of those channels over a 20 s period.
To simulate the effects of stimulation protocol in the tissue model, a virtual stimulus was placed at a location corresponding to the site of the experimental stimulus, as shown in Fig. 2. The intrinsic frequency of the ICC paced by the stimulus was set to the stimulation frequency (3.53 cpm). The frequency of the normal slow waves remained at 3.60 cpm. Both the intrinsic and paced activity began at t = 0 s. The origin of the secondary pacemaker corresponded to the location of the pacing needles, which was within 11 mm distal to the fundal line in both experimental recording and simulation. Both experiment and simulation achieved an overall entrainment frequency of 3.53 cpm. The simulation demonstrated that the displacement of entrainment in the retrograde direction was 53 mm from the point of stimulation, and 77 mm in the antegrade direction from the point of stimulation [see Fig. 4(b)]. The experimental data also demonstrated similar results in term of entrainment displacements. Over ten consecutive entrained events in the trial demonstrated in Fig. 4(b), the entrained displacement in the retrograde direction was 55±3 mm, and 71±1 mm in antegrade direction (to the extent of the mapped boundary), both measured relative to the location of pacing needles.
Over multiple consecutive waves during pacing at 3.53 cpm, the experimental data demonstrated a ZOE of 64±10 cm2. The high degree of variability in the ZOE was due to the variable phase interaction between the native and entrained activities. During some events, the majority of the mapped area could be entrained, whereas in other events a native event was found to be propagating distally, clashing with the entrained event, and limiting propagation in the retrograde direction, at a variable distance down the porcine stomach. An additional theoretical study was therefore conducted to define the relationship of the phase interaction between the native and entrained slow-wave activities on the ZON and ZOE achieved during pacing in the computational model. The onset of stimulation was delayed from 0 to 17 s in 1 s increments relative to the onset of the native slow waves, and the results are presented in Fig. 5. As the delay time between the onsets of stimuli relative to the native slow-wave activity lengthened, the stable ZOE of the stimulus decreased. The highest ZOE was 78%, which translates to approximately 64 cm2 after taking into consideration the 39 inactive electrodes in the ZOE. (These electrodes were inactive because the related areas of the PCBs lay off the edge of the greater curvature; refer Fig. 2.)
Fig. 5.
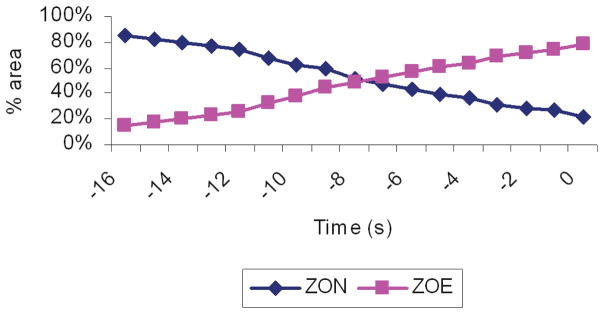
ZOE is the proportion of total mapped area containing entrained slow waves following the stimulation protocol. ZON is the proportion of total mapped area containing normal slow waves. The onset of the stimulus was incrementally delayed relative to onset of the native activity, with the values being the number of seconds of the onset of stimulus behind the native activity. The ZOE of the stimulus and native activity interchanged depending on the length of the delay.
IV. Discussion
This study has presented a multiscale gastric tissue model, containing a biophysically based SM cell model and a cellular automata model of ICCs. We have successfully employed this model to simulate normal slow-wave propagation, and the effects of a typical gastric pacing protocol. Simulated normal slow waves were successfully shown to originate from the pacemaker region and the results showed propagation in the antegrade direction at the designated velocities. The slow waves in the SM layer accurately reflected the pacemaker potentials in the underlying ICC network. Importantly, the simulation results were in good agreement with the experimental data from a small porcine validation study, in terms of the velocities and directions of slow-wave propagation, and in terms of the slow-wave entrainment pattern and area entrained following a single gastric pacing protocol.
This model is a significant advance over existing models of GEA, which have not yet been biophysically based, and which lack the same degree of predictive potential [11]. In this study, the simulations of GES and experimental results do suggest that the new model can form a sound initial platform for predictive modeling for evaluating gastric pacing protocols. In particular, the model successfully reproduced the correct sequence of events and demonstrated successful slow-wave entrainment (see Fig. 3). The ICC layer was entrained before the secondary depolarization of the SM layer, the stimulated region successfully acted as a secondary pacemaker, and retrograde slow waves propagated throughout the SM layer. The stimulated region was shown to entrain a similar portion of the ICC and SM layers, as occurred during the experimental GES study, due to the phase interaction between the normal and entrained activities. The stimulated region was shown to entrain a similar portion of the ICC and SM layers to that achieved in the experimental GES study. During stimulation, the region entrained by the stimulus is, therefore, electrically decoupled from the normal region. Interestingly, both the simulated and experimental results of this study (see Fig. 4) show that the distance of entrainment mapped in the retrograde direction is shorter than the distance of entrainment mapped in the antegrade direction (53 mm versus 77 mm). It should be noted that the distance entrained in the antegrade direction is underestimated by entrainment mapping, because the slow waves would be expected to continue to propagate beyond the mapped area, until the pylorus [21], [23]. The difficulty in achieving effective entrainment in the retrograde direction has been noted in a previous study, in which the authors believed that the “resistance” was lower for antegrade entrainment than for retrograde entrainment [22]. This study suggests an alternative plausible explanation, that the efficacy of entrainment in the retrograde direction could be strongly dependent on the phase interaction between the native and entrained activities in the proximal stomach. Traditionally, gastric pacing has been employed at frequencies somewhat higher than the native activity. This is in part because if stimulation was performed at a similar frequency to the native activity, while recording with few sparsely placed electrodes, then it would be very difficult for the investigator to determine whether entrainment was being achieved or not. With high-resolution entrainment mapping, as shown in this study, the precise spatiotemporal effects of GES on GEA can be determined at any stimulation frequency. When attempting to treat obesity using gastric pacing, researchers have placed the pacing leads into the distal stomach, in order to disrupt normal antegrade slow-wave activity, and therefore, motility [4]. Pilot studies have suggested that it may be possible to employ this type of “retrograde pacing” without inducing intolerable symptoms [22]. This study demonstrates that if this methodology is likely to be successful in achieving significant entrainment in the retrograde direction, then the pacing protocols must take into consideration the native slow-wave frequency and the slow-wave refractory period. The model presented here is ideally suited to this research task, offering a medium to screen inefficient stimulation protocols by predicting whether effective entrainment is likely to be achieved in experiments.
There is a need to improve the energy efficiency of gastric pacing to allow implantable devices, and multichannel pacing has recently been promoted as a possible means to achieve this goal [28]. However, protocol development for multichannel pacing is a complex task, because a vast range of possible parameter combinations (principally pulse-width, amplitude, frequency, and ON–OFF timing) must be considered and evaluated for each individual channel, yet coordinated across all channels [30]. To date, trial and error has been the dominant strategy for evaluating the efficacy of the various GES protocols, requiring laborious animal-model testing [6]. Computational models to improve research efficiency in multichannel pacing are, therefore, an identified research priority [4]. In this model, the location of the pacemaker region, the number of pacemakers, and the frequency of each pacemaker can be readily adjusted. The ZOEs achieved by each individual channel can, therefore, be predicted under different pacing frequencies, presenting a useful initial tool for the computational screening of effective multichannel protocols.
Ultimately, the clinical translation of gastric pacing for dysmotility syndromes will require demonstrated improvements in motility and symptoms. To date, only modest clinical improvements have been demonstrated following pacing in gastroparetic patients, partly because motility also involves a complex interaction of neural, hormonal, and paracrine factors, besides GEA [7]. In future, computational simulations and high-resolution entrainment mapping may assist in addressing the challenge of improving motility via GES. For example, simulations on biophysically based cell models may help to predict the effects of GES on the membrane potential and calcium influx into SMCs [7], [34]. High-resolution mapping has also recently revealed that gastric dysrhythmias may involve complex focal and reentrant behaviors, similar to those occurring in the heart [23], and the effects of pacing on these complex activities now awaits to be defined via entrainment mapping.
Future research should also address two important limitations of this model to allow a more sophisticated platform for GES simulations with improved predictive capabilities for all GES parameters. First, the ICC algorithm is deterministic, and a biophysically based ICC model should ideally be incorporated. Such ICC models are now available [16], [17]; however, the mechanism of ICC entrainment must first be effectively established and modeled before they can be incorporated in this framework. Second, 2-D modeling is relatively simplistic, and incorporating a multilayered structure based on a geometrically realistic gastric microstructure would provide an improved base for predictive simulations, as has been achieved in previous cardiac work [29]. Currently, however, there is no suitable 3-D gastric microstructure to guide this aim.
In conclusion, the new computational model presented here offers a major advance over previously published multiscale gastrointestinal tissue models, and provided an effective platform for simulations of normal GEA and the effects of GES. This new tissue model does demonstrate good initial predictive value for GES simulations, and with improvements, we anticipate it will prove a useful platform for clinically relevant simulations, including evaluating and optimizing protocols for multichannel gastric pacing.
Acknowledgments
The authors thank L. Nisbet for her technical assistance, and contributions from Prof. W. Lammers and Dr. J. Egbuji.
Contributor Information
Peng Du, Email: peng.du@auckland.ac.nz, Auckland Bioengineering Institute, The University of Auckland, Auckland 1142, New Zealand.
Greg O’Grady, Email: gog@ps.gen.nz, Auckland Bioengineering Institute and the Department of Surgery, The University of Auckland, Auckland 1142, New Zealand.
J.A. Windsor, Email: j.windsor@auckland.ac.nz, Department of Surgery, The University of Auckland, Auckland 1142, New Zealand
Leo K. Cheng, Email: l.cheng@auckland.ac.nz, Auckland Bioengineering Institute, The University of Auckland, Auckland 1142, New Zealand
Andrew J. Pullan, Email: a.pullan@auckland.ac.nz, Department of Engineering Science, The University of Auckland, Auckland 1142, New Zealand
References
- 1.Kelly KA, Force RCL. Role of the gastric pacesetter potential defined by electrical pacing. Can J Physiol Pharmacol. 1972;50:1017–1019. doi: 10.1139/y72-147. [DOI] [PubMed] [Google Scholar]
- 2.Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol. 2005 Mar;288(3):C710–20. doi: 10.1152/ajpcell.00361.2004. [DOI] [PubMed] [Google Scholar]
- 3.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20:39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin J, Chen JD. Implantable gastric electrical stimulation: Ready for prime time? Gastroenterology. 2008;134:665–667. doi: 10.1053/j.gastro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 5.Song GQ, Hou X, Sun Y, Yang B, Qian W, Chen JD. Effects of retrograde gastric electrical stimulation with pulse trains on gastric emptying of solids and plasma hormones in dogs. Amer J Surg. 2007;194:122–127. doi: 10.1016/j.amjsurg.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Familoni BO, Abell TL, Nemoto D, Voeller G, Johnson B. Efficacy of electrical stimulation at frequencies higher than basal rate in canine stomach. Dig Dis Sci. 1997;42:892–897. doi: 10.1023/a:1018804128695. [DOI] [PubMed] [Google Scholar]
- 7.Cheng LK, O’Grady G, Du P, Egbuji JU, Windsor JA, Pullan AJ. Gastrointestinal system. Wiley Interdiscip Rev Syst Biol Med. 2010;2(1):65–79. doi: 10.1002/wsbm.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mintchev M, Bowes K. Computer model of gastric electrical stimulation. Ann Biomed Eng. 1997;25:726–730. doi: 10.1007/BF02684849. [DOI] [PubMed] [Google Scholar]
- 9.Pullan AJ, Cheng LK, Buist ML. Mathematically Modeling the Electrical Activity of the Heart: From Cell to Body Surface and Back. Singapore: World Scientific; 2005. [Google Scholar]
- 10.Aliev RR, Richards W. A simple nonlinear model of electrical activity in the intestine. J Theor Biol. 2000;204:21–28. doi: 10.1006/jtbi.2000.1069. [DOI] [PubMed] [Google Scholar]
- 11.Cheng LK, Komuro R, Austin TM, Buist ML, Pullan AJ. Anatomically realistic multiscale models of normal and abnormal gastrointestinal electrical activity. World J Gastroenterol. 2007;13:1378–1383. doi: 10.3748/wjg.v13.i9.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pullan A, Cheng L, Yassi R, Buist M. Modelling gastrointestinal bioelectric activity. Prog Biophys Mol Biol. 2004 Jun-Jul;85(2-3):523–50. doi: 10.1016/j.pbiomolbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Corrias A, Buist ML. A quantitative model of gastric smooth muscle cellular activation. Ann Biomed Eng. 2007;35:1595–1607. doi: 10.1007/s10439-007-9324-8. [DOI] [PubMed] [Google Scholar]
- 14.Ordog T, Baldo M, Danko R, Sanders KM. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/Wv mutant mice. Gastroenterology. 2002;123:2028–2040. doi: 10.1053/gast.2002.37056. [DOI] [PubMed] [Google Scholar]
- 15.Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 16.Corrias A, Buist ML. Quantitative cellular description of gastric slow wave activity. Am J Physiol Gastrointest Liver Physiol. 2008 Apr;294(4):G989–95. doi: 10.1152/ajpgi.00528.2007. [DOI] [PubMed] [Google Scholar]
- 17.Faville RA, Pullan AJ, Sanders KM, Smith NP. A biophysically based mathematical model of unitary potential activity in interstitial cells of Cajal. Biophys J. 2008 Jul;95(1):88–104. doi: 10.1529/biophysj.107.122507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber J, Jr, Koatsu S. Pacemaker localization and electrical conduction patterns in the canine stomach. Gastroenterology. 1970;59(5):717–26. [PubMed] [Google Scholar]
- 19.Du P, O’Grady G, Egbuji JU, Lammers WJ, Budgett D, Nielsen P, Windsor JA, Cheng LK, Pullan AJ. High-resolution mapping of in vivo gastrointestinal slow wave activity using flexible printed circuit board electrodes: Methodology and validation. Ann Biomed Eng. 2009;37(4):839–846. doi: 10.1007/s10439-009-9654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bortolotti M. The “electrical way” to cure gastroparesis. Am J Gastroenterol. 2002 Aug;97(8):1874–83. doi: 10.1111/j.1572-0241.2002.05898.x. [DOI] [PubMed] [Google Scholar]
- 21.Lammers WJ, Stephen B, Adeghate E, Ponery S, Pozzan O. The slow wave does not propagate across the gastroduodenal junction in the isolated feline preparation. Neurogastroenterol Motil. 1998;10:339–349. doi: 10.1046/j.1365-2982.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 22.Yao S, Ke M, Wang Z, Xu D, Zhang Y, Chen JD. Retrograde gastric pacing reduces food intake and delays gastric emptying in humans: A potential therapy for obesity? Dig Dis Sci. 2005;50:1569–1575. doi: 10.1007/s10620-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 23.Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1200–10. doi: 10.1152/ajpgi.90581.2008. [DOI] [PubMed] [Google Scholar]
- 24.Sanders KM, Stevens R, Burke E, Ward SM. Slow waves actively propagate at submucosal surface of circular layer in canine colon. Am J Physiol. 1990 Aug;259(2 Pt 1):G258–63. doi: 10.1152/ajpgi.1990.259.2.G258. [DOI] [PubMed] [Google Scholar]
- 25.Cohen I, Miles R. Contributions of intrinsic and synaptic activities to the generation of neuronal discharges in in vitro hippocampus. J Physiol. 2000;524(2):485–502. doi: 10.1111/j.1469-7793.2000.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasler WL. Methods of gastric electrical stimulation and pacing: A review of their benefits and mechanisms of action in gastroparesis and obesity. Neurogastroenterol Motil. 2009;21(3):229–243. doi: 10.1111/j.1365-2982.2009.01277.x. [DOI] [PubMed] [Google Scholar]
- 27.O’Grady G, Egbuji JU, Du P, Cheng LK, Pullan AJ, Windsor JA. High-frequency gastric electrical stimulation for the treatment of gastroparesis: Ameta-analysis. World J Surg. 2009 Aug;33(8):1693–701. doi: 10.1007/s00268-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JD, Xu X, Zhang J, Abo M, Lin X, McCallum RW, Ross B. Efficiency and efficacy of multi-channel gastric electrical stimulation. Neurogastroenterol Motil. 2005;17(6):878–882. doi: 10.1111/j.1365-2982.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 29.Trew ML, Caldwell BJ, Sands GB, Hooks DA, Tai DC, Austin TM, LeGrice IJ, Pullan AJ, Smaill BH. Cardiac electrophysiology and tissue structure: bridging the scale gap with a joint measurement and modelling paradigm. Exp Physiol. 2006 Mar;91(2):355–70. doi: 10.1113/expphysiol.2005.031054. [DOI] [PubMed] [Google Scholar]
- 30.Lin Z, Forster J, Sarosiek I, McCallum RW. Treatment of gastroparesis with electrical stimulation. Dig Dis Sci. 2003 May;48(5):837–848. doi: 10.1023/a:1023099206939. [DOI] [PubMed] [Google Scholar]
- 31.Sarna SK, Daniel EE, Kingma YJ. Premature control potentials in the dog stomach and in the gastric computer model. Amer J Physiol. 1972;222:1518–1523. doi: 10.1152/ajplegacy.1972.222.6.1518. [DOI] [PubMed] [Google Scholar]
- 32.Sarna SK, Daniel EE, Kingma YJ. Effects of partial cuts on gastric electrical control activity and its computer model. Amer J Physiol. 1972;223:332–340. doi: 10.1152/ajplegacy.1972.223.2.332. [DOI] [PubMed] [Google Scholar]
- 33.Lammers WJ, Ver Donck L, Stephen B, Smets D, Schuurkes JA. Focal activities and re-entrant propagations as mechanisms of gastric tachyarrhythmias. Gastroenterology. 2008 Nov;135(5):1601–11. doi: 10.1053/j.gastro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Du P, Li S, O’Grady G, Cheng LK, Pullan AJ, Chen JD. Effects of electrical stimulation on isolated rodent gastric smooth muscle cells evaluated via a joint computational simulation and experimental approach. Am J Physiol Gastrointest Liver Physiol. 2009;297:G672–80. doi: 10.1152/ajpgi.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]