Abstract
Objective
Fluid shear stress intimately regulates vasculogenesis and endothelial homeostasis. The canonical Wnt/β-catenin signaling pathways play an important role in differentiation and proliferation. In this study, we investigated whether shear stress activated Angiopoietin-2 (Ang-2) via the canonical Wnt signaling pathway with an implication in vascular endothelial repair.
Approach and Results
Oscillatory shear stress(OSS) up-regulated both TOPflash Wnt reporter activities and the expression of Ang-2 RNA and protein in human aortic endothelial cells (HAEC) accompanied by an increase in nuclear β-catenin intensity. OSS-induced Ang-2 and Axin-2 mRNA expression was down-regulated in the presence of a Wnt inhibitor, IWR-1, but was up-regulated in the presence of a Wnt agonist, LiCl. Ang-2 expression was further down-regulated in response to a Wnt signaling inhibitor, DKK-1, but was up-regulated by Wnt agonist Wnt3a. Both DKK-1 and Ang-2 siRNA inhibited endothelial cell migration and tube formation, which were rescued by human recombinant Ang-2. Both Ang-2 and Axin-2 mRNA down-regulation was recapitulated in the heat-shock inducible transgenic Tg (hsp70l:dkk1-GFP) zebrafish embryos at 72 hours post fertilization (hpf). Ang-2 morpholino injection of Tg (kdrl:GFP) fish impaired subintestinal vessel (SIV) formation at 72hpf, which was rescued by zebrafish Ang-2 mRNA (zAng-2) co-injection. Inhibition of Wnt signaling with IWR-1 also down-regulated Ang-2 and Axin-2 expression, and impaired vascular repair after tail amputation, which was rescued by zAng-2 injection.
Conclusion
Shear stress activated Ang-2 via canonical Wnt signaling in vascular endothelial cells, and Wnt-Ang-2 signaling is recapitulated in zebrafish embryos with a translational implication in vascular development and repair.
Keywords: Angiopoietin-2, Wnt signaling, endothelial repairs, human aortic endothelial cells, zebrafish, DKK-1/Dickkopfs-1, vasculogenesis
Introduction
Mechanotransduction is implicated in differentiation of embryonic stem cells to vascular endothelial cells 1–3. Hemodynamics; namely, fluid shear stress, is intimately involved in stem cell 4, 5 and mesenchymal progenitors6 differentiation to vascular endothelial cells. While the roles of angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) during vascular development have been extensively investigated, shear stress-mediated Ang-2 in mature vascular endothelium was recently reported to play a role in tubulogenesis 7 and to confer atheroprotection 8.
While Ang-1 is constitutively released by the perivascular cells, Ang-2 is released from the Weibel-Palade bodies in endothelial cells 9, 10. Ang-2 binds to endothelial specific receptor tyrosine kinase 2 (TIE-2), and acts as a negative regulator of Ang-1/TIE-2 signaling during angiogenesis 11. Earlier studies demonstrated that Ang-2 release from Weibel-Palade bodies is induced by endothelial stretch, which occurs during hypertension12. However, the mechanisms underlying reactivation of developmental genes such as Ang-2 in endothelial cells remain elusive.
Hemodynamic forces are complex regulators of endothelial homeostasis 13. Disturbed flow, including oscillatory shear stress (OSS), is a bidirectional flow associated with a net-zero forward flow that develops in the curvatures or branching points of the vasculature 14–17. OSS-induced Ang-2 promotes tubular formation and migration of cultured endothelial cells 7. While stretching isolated arterial endothelial cells further promotes the paracrine effect of Ang-2 release, Ang-1 release inhibits these effects 12. Ang-2 stimulates arteriogenesis in an C57Bl/6J mice with a ligated femoral artery 18, and confers atheroprotection in apoE-null mice. In contrast, over-expression of Ang-1 induces smooth muscle cell migration and monocyte chemotaxis 8. However, there remains a paucity of literature in shear stress-activated developmental genes, and the mechanisms underlying OSS-induced Ang-2 expression remain to be elucidated.
Canonical Wnt/β-catenin signaling pathway regulates development, cell proliferation and migration19. In this study, we investigated whether shear stress activated Ang-2 via canonical Wnt signaling pathway. Both endothelial Ang-2 expression and Wnt TOPflash reporter activity were up-regulated in response to OSS. While Wnt agonist, Wnt3a, promoted Ang-2 mRNA expression, Dkk-1 treatment or Ang-2 siRNA inhibited endothelial cell migration and tube formation. Wnt-Ang-2 signaling was further recapitulated in the zebrafish embryos, in which mRNA of Angiopoietin 2b homolog was down-regulated in heat-shock inducible DKK-1 transgenic Tg (hsp70l:Dkk1-GFP) fish (For the zebrafish related studies, zebrafish Angiopoietin 2b homolog is denoted as Ang-2). Ang-2 morpholino micro-injection further impaired development of subintestinal vessels (SIV) at 72 hours post fertilization (hpf). Thus, we provide new insights into shear stress-activated Wnt-Ang-2 signaling with a translational implication in vascular development and repair.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement
Results
Oscillatory shear stress activated Ang-2 expression via Wnt signaling
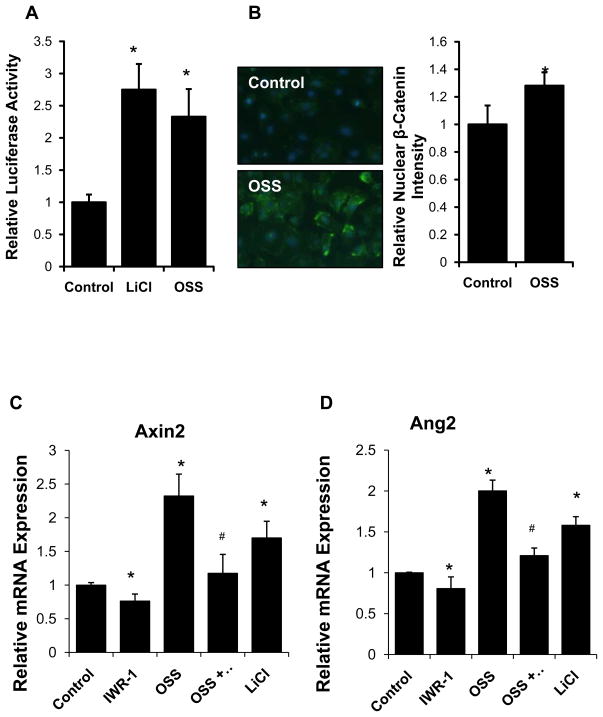
In a dynamic flow system20, oscillatory shear stress (OSS) up-regulated Wnt signaling activity in HAEC. TOPflash reporter assay demonstrated a 2.3-fold-increase in Wnt signaling activity in response to OSS, and a 2.8-fold increase in response to LiCl, a positive control (p <0.05, n=3) (Fig. 1A). In parallel, OSS increased nuclear β-catenin content by 1.33-fold compared to static condition (p < 0.05, n=4) (Fig. 1B). Wnt signaling inhibitor Ionomycin inhibited nuclear β-catenin translocation (Supplemental Fig V). Furthermore, OSS up-regulated Axin-2 mRNA, a well-known Wnt target gene, by 2.3-fold (p < 0.05, n=4), which was attenuated by a Wnt inhibitor, IWR-1 (Fig. 1C). OSS also up-regulated Ang-2 mRNA expression by 2-fold (p <0.05, n=4), which was attenuated by IWR-1 (Fig. 1D). OSS further up-regulated Ang-2 mRNA to a greater extent than did pulsatile shear stress (PSS), and OSS also up-regulated Ang-2 protein expression (p < 0.05, n=4) (Figs. 1E and 1F). Thus, OSS induced Ang-2 expression via cannonical Wnt signaling in HAEC.7.
Fig. 1. Oscillatory shear stress (OSS) promoted Ang-2 expression via Wnt signaling.
(A) Topflash reporter assay revealed that OSS for 8 hours significantly activated Wnt signaling. LiCl, a wnt-signaling inducer, was used as positive control. (Control=1.00±0.06; LiCl=2.78±0.42; OSS=2.35±0.46, *p < 0.05 vs. control, n=4). (B) OSS induced a 1.3-fold increase in nuclear β-catenin fluorescence in canonical Wnt signaling pathway. (*p < 0.05, n=4). (C) OSS up-regulated a well-recognized Wnt target gene, Axin-2, which was attenuated in the presence of IWR-1, a Wnt inhibitor (normalized to GAPDH: control=1.00±0.038; IWR-1=0.76±0.10; LiCl=1.70±0.25; OSS=2.32±0.32; OSS+IWR-1=1.18.±0.28, *p < 0.05 vs. control; #p < 0.05 vs. OSS, n=4). IWR down-regulated, but LiCl up-regulated Axin-2 expression (*p < 0.05 vs. control, n=4). (D) OSS further up-regulated Ang-2 mRNA expression, which was also attenuated in the presence of IWR-1 (normalized to GAPDH: control=1.00±0.01; IWR-1=0.81±0.14; LiCl=1.58.±0.10; OSS=2.00±0.13; OSS+IWR-1=1.21±0.09, *p < 0.05 vs. control; #p < 0.05 vs. OSS, n=4). IWR down-regulated, but LiCl up-regulated Ang-2 expression (*p < 0.05 vs. control, n=4). (E) Pulsatile shear stress (PSS) up-regulated Ang-2 mRNA expression by 1.21.±0.10-fold (*p < 0.05 vs. control, n=4), while OSS up-regulated Ang-2 expression by 2.08±0.12-fold (normalized to GAPDH: *p < 0.05 vs. control, n=4). (F) In corollary, both OSS (24 hr) and LiCl significantly up-regulated Ang-2 protein expression as quantified by densitometry (normalized to β-tubulin: *p < 0.05 vs. control, n=4).
Ang-2 is a Wnt target gene for endothelial repair
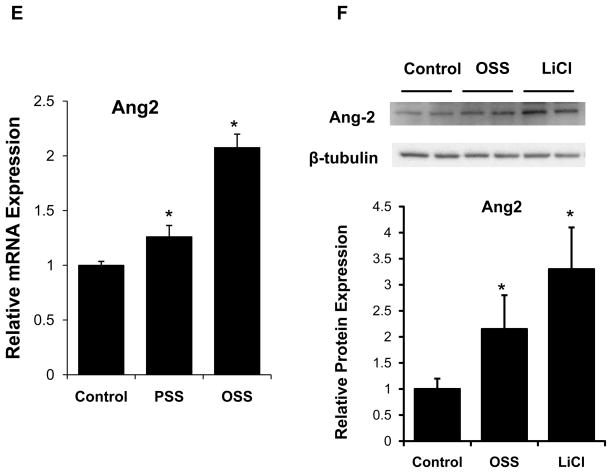
Ang-2 knock-down with siRNA (siAng-2) significantly reduced both Ang-2 mRNA and protein expression (Figs. 2A and 2B). Transfecting HAEC with siAng-2 impaired tube formation at 8 hours (Fig. 2C), and cell migration at both 4 and 8 hours (Fig. 2D). siAng-2 studies were further validated with a second set of independently designed Ang-2 siRNA sequences (Fig. 2A–2D).
Fig. 2. Knock-down of Angiopoeitin-2 retarded HAEC migration and tube formation.
HAEC were transfected with 50 nM scrambled siRNA (Scr), or in-house designed or independently designed Ang-2 siRNA (siAng2-1 and siAng2-2, respectively) for 48 hours. The cells were used for Ang-2 mRNA expression, Matrigel assay for tube formation, and scratch assay for cell migration. (A) Transfection with siAng-2 significantly reduced Ang-2 mRNA expression (*p < 0.05, n=4), and (B) protein expression by more than 50%. (C) HAEC tube formation was inhibited at 8 hour after siAng-2 transfection. (D) HAEC monolayers were scratched by using pipette tips and cultured in the presence or Scr, siAng2-1 and siAng2-2 (50 nM). siAng-2 also inhibited HAEC migration. Bar graphs quantified cell migrations in terms of percentage after scratching at 4 hr (*p < 0.05, n=4) and 8 hr (*p < 0.05, n=4). Both the migration studies and Matrigel assays were representative of four independent experiments with reproducible findings.
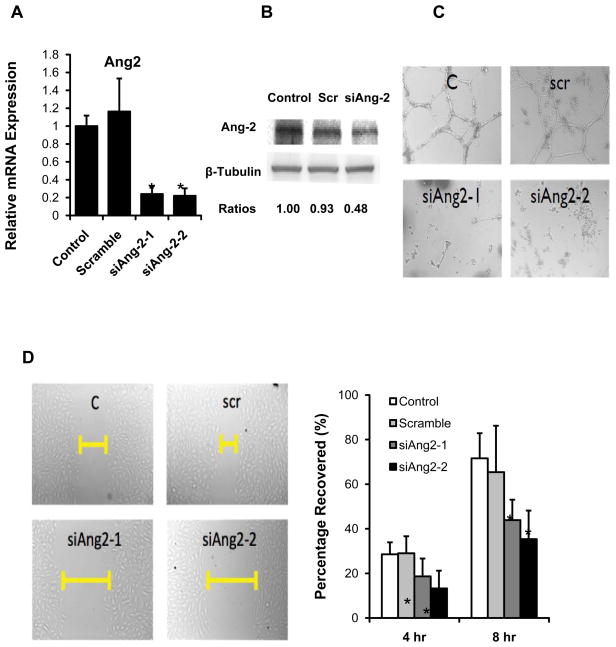
To assess Ang-2 as one of the Wnt target genes, we demonstrated that human recombinant DKK-1 treatment down-regulated Ang-2 mRNA expression in a dose- and time-dependent manner (normalized to GAPDH, p < 0.05 vs. Control, n=3) (Fig. 3A), whereas recombinant Wnt3a treatment up-regulated Ang-2 in a dose-dependent manner (p < 0.05 vs. control, n=3) (Fig. 3B). DKK-1 treatment also impaired endothelial migration (Fig. 3C) and tube formation at 8 hours (Fig. 3D), which were rescued by recombinant Ang-2 treatment (Figs. 3C and 3D). The down-regulation of Ang-2 by DKK-1 was not due to apoptosis since DKK-1 treatment had no effect on cell viability at our time points (Supplemental Fig II). Ionomycin treatment similarly reduced endothelial cell migration and tube formation (Supplemental Fig VI). Taken together, Ang-2 is a Wnt target gene, with an implication in endothelial repair.
Fig. 3. Wnt signaling mediated HAEC migration and tube formation is Ang-2-dependent.
(A) HAEC monolayers were treated with 0.1 and 0.5 μg/mL of DKK-1 for 3 hours and 6 hours, respectively. Quantitative RT-PCR revealed down-regulation of Ang-2 mRNA expression in the presence of DKK-1 in a dose- and time-dependent manner (normalized to GAPDH: *p < 0.05 vs. Control n=3). (B) Ang-2 mRNA expression was up-regulated in response to treatment with human recombinant Wnt3a for 3 hours (normalized to GAPDH: *p < 0.05 vs. control. n=3). (C) HAEC monolayers were scratched by using pipette tips and cultured in the presence or absence of 0.5 μg/mL of human recombinant DKK-1. DKK-1 inhibited cell migration, which was rescued by recombinant Ang-2 treatment (0.5 μg/mL). Bar graphs quantified cell migrations in terms of percentage after scratching at 4 hr and 8 hr (*p < 0.05, n=4). (D) HAEC were cultured in the Matrigel in the presence or absence of 0.5 μg/mL of DKK-1. After 8 hours, tube formation was inhibited in the presence of DKK-1 (0.5 μg/mL), which was rescued by Ang-2 treatment (0.5 μg/mL). Both the migration studies and Matrigel assays were representative of four independent experiments with reproducible findings.
Inhibition of Wnt signaling down-regulated Ang-2 expression in Zebrafish embryos
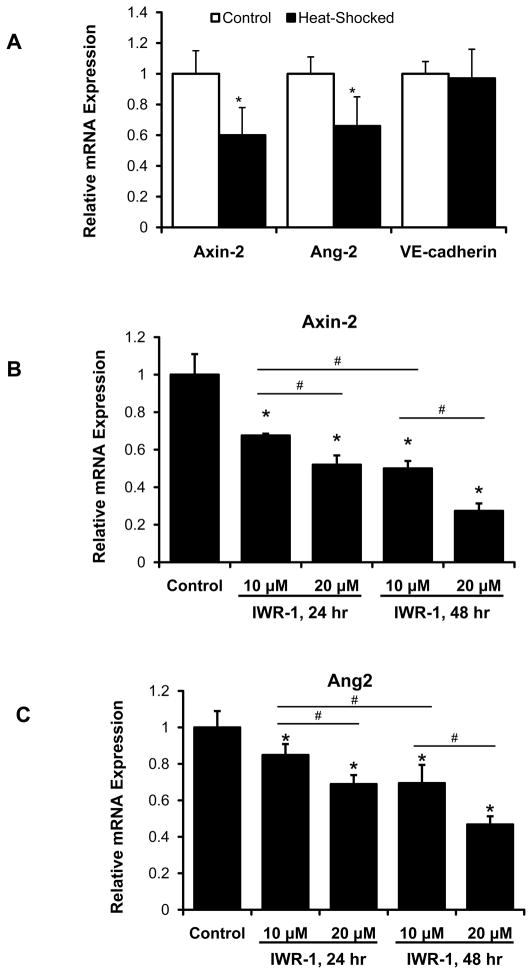
To recapitulate Ang-2 as a Wnt target gene in zebrafish embryos, we used transgenic Tg (hsp70l:Dkk1-GFP) lines. (For the zebrafish related studies, Angiopoietin 2b homolog is denoted as Ang-2). Heat-shock induction of DKK-1-GFP resulted in down-regulation of both Axin-2 and Ang-2 mRNA expression while VE-cadherin expression remained unchanged (Fig. 4A); whereas heat shock of wild-type fish did not have any effect on Axin-2 or Ang-2 expression (Supplementary Fig. III). To further validate Ang-2 as a Wnt target gene, we used IWR-1, a small molecule Wnt inhibitor, to interrogate Axin-2 and Ang-2 mRNA expression. Both genes were down-regulated in dose- and duration-dependent manners at 72 hpf (Fig. 4B and 4C). These findings corroborated Ang-2 as a Wnt target gene in the zebrafish embryos.
Fig. 4. Treatment of Tg (hsp70:DKK-1-GFP) zebrafish embryos with IWR-1 recapitulated Ang-2 as a Wnt target gene.
(A) Tg (hsp70l:Dkk1-GFP) embryos were heat-shocked at 48 hpf at 37°C for 1 hour. Axin-2, a well-recognized Wnt target gene, was used as a reference control. In Tg (hsp70l:Dkk1-GFP) embryos, both Axin-2 and Ang-2 mRNA expressions were down-regulated in response to heat-shock induction of DKK-1 (*p < 0.05 vs. control, n=4). DKK-1 did not significantly change the expression of VE-Cadherin suggesting DKK-1-induced down-regulation of Axin-2 and Ang-2 was not due to potential vascular toxicity by heat-shock induction of DKK-1. (B,C) IWR-1 also down-regulated both Axin-2 and Ang-2 mRNA in a dose-dependent manner in the Tg (kdrl:GFP) fish at 72hpf. Ang-2 expression was down-regulated to a greater extent over 48 hr treatment (starting at 24 hpf) compared to 24 hr treatment (starting at 48 hpf). (*p < 0.05 vs. control; #p < 0.05 for pair-wise comparison, n=4).
Ang-2 Morpholinos (MO) impaired vascular development in Zebrafish embryos
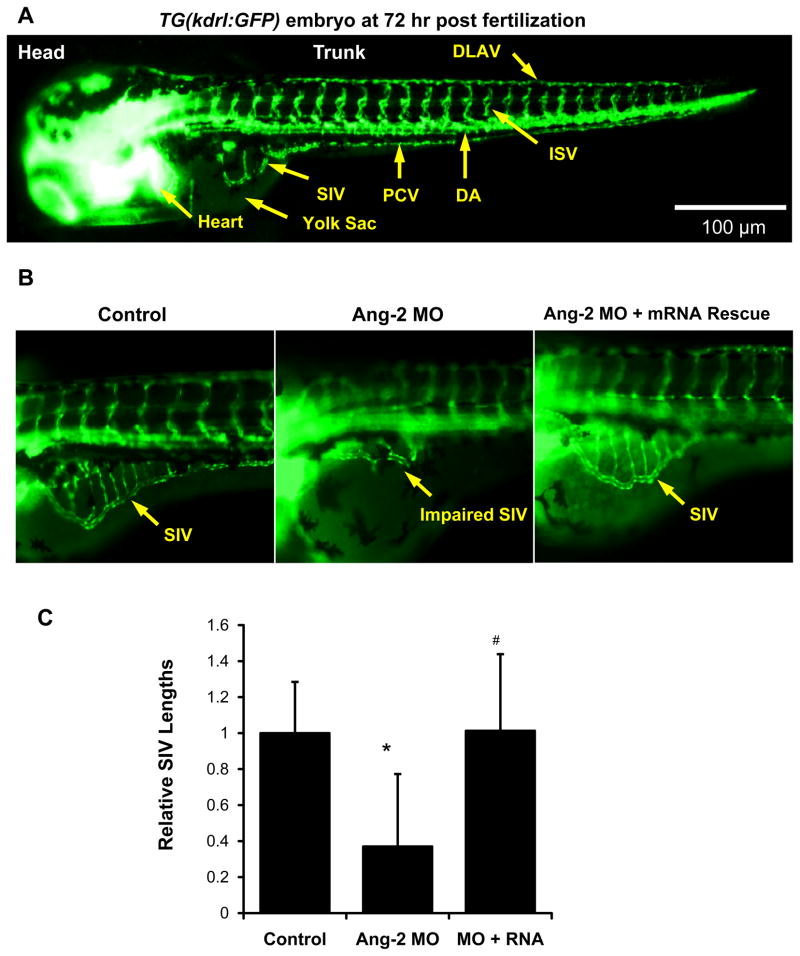
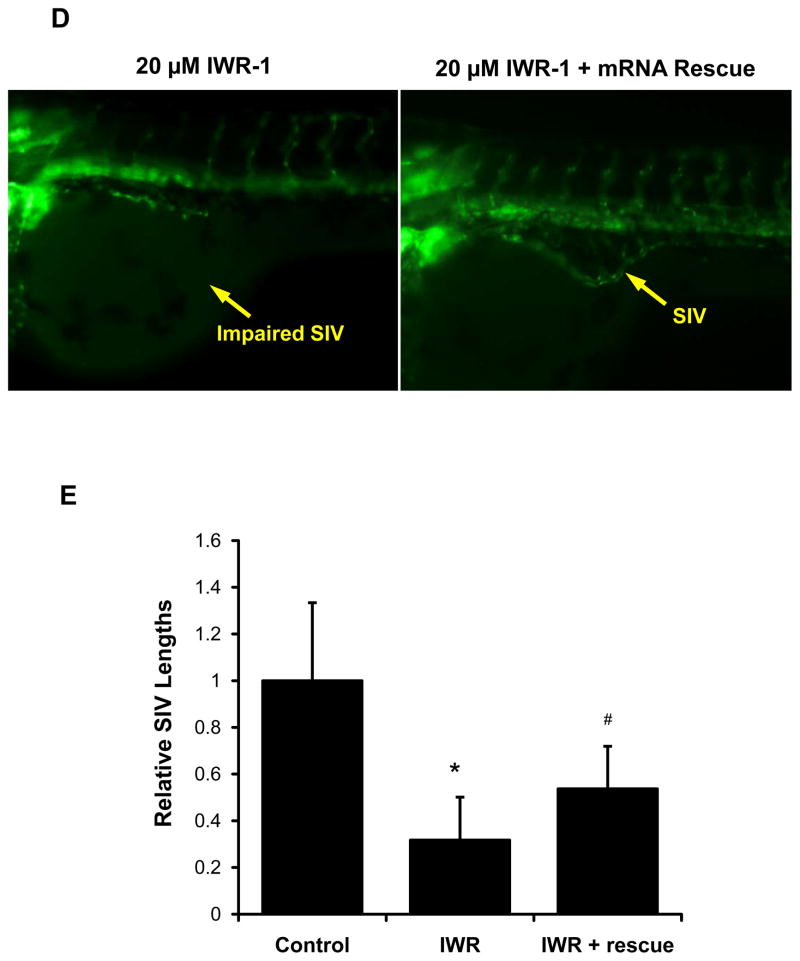
To further elucidate whether Ang-2 was implicated in subintestinal vessel (SIV) development, we used transgenic Tg (kdrl:GFP) zebrafish embryos (Fig. 5A). Micro-injection of 0.5 μM Ang-2 ATG MO or splicing MO to the 2-cell stage embryos impaired SIV development at 72 hpf (Fig. 5B, Supplemental Fig. IV). Co-injection of zebrafish Ang-2 (zAng-2) mRNA restored SIV formation (Fig. 5B). Quantitatively, SIV length was reduced by 65% in response to ATG-MO injection, which was rescued by zAng-2 mRNA injection (p < .01, n = 20) (Fig. 5C). Furthermore, Wnt inhibitor IWR-1 impaired SIV formation, which was partially rescued by zAng-2 co-injection at 72 hpf (Figs. 5D and 5E).. A similar effect was observed with Ionomycin treatment (Supplemental Fig VII). Thus, Ang-2 is implicated in SIV development, recapitulating endothelial tube formation (Fig. 2).
Fig. 5. Ang-2 morphant injection impaired subintestinal vein (SIV) formation in Zebrafish embryos.
(A) Vasculature of transgenic zebrafish Tg (kdrl:GFP) at 72hpf reveals subintestinal vessel (SIV), intersegmental vessel (ISV), dorsal longitudinal vein (DLAV), dorsal aorta (DA), and posterior cardinal vein (PCV). (B) Embryos injected with the control MO developed normal SIV at 72 hpf. Ang-2 ATG-MO injection (0.5 μM) impairs SIV formation. Co-injection of zAng-2 mRNA (25ng) with Ang-2 MO rescued SIV formation. (C) Quantification of SIV length was performed and there was a significant difference between control and injection with morpholino (*p < .001, n = 20). zAng-2 mRNA injection rescued the SIV formation (#p < .001, n = 20). (D) Treatment with Wnt signaling inhibitor IWR-1 impaired SIV formation. Injection with zAng-2 mRNA (25ng) was able to partially rescue SIV formation. (E) Quantification of SIV length showed significant reduction after IWR-1 treatment (*p < .001, n = 20), while injection with zAng-2 mRNA significantly increased SIV length (#p < .001, n = 20).
IWR-1 impaired vascular repair
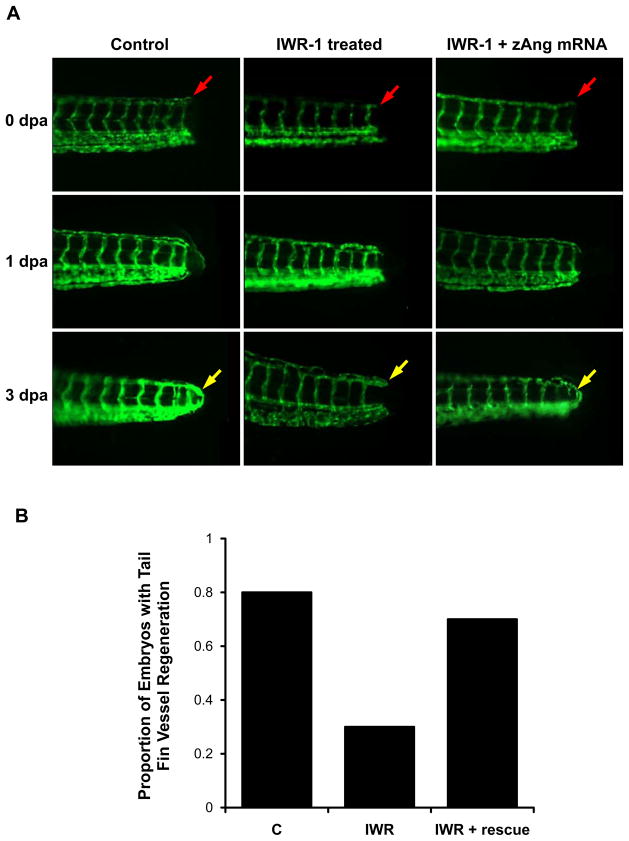
We further assessed whether Wnt signaling was implicated in endothelial repair in the Tg (kdrl:GFP) zebrafish embryos at 72 hpf. Tail amputation was performed approximately 100 μm from the tip (Fig. 6A). In the control group, vascular repair led to a closed loop between dorsal longitudinal anastomotic vessels (DLAV) and dorsal aortas (DA) at 3 days post amputation (dpa) (Fig. 6A). Treatment with 10μM IWR-1 inhibited vascular endothelial repair at 3 dpa (Fig. 6A). Tail amputation performed at 72 hpf to the fish injected with zAng-2 mRNA at 2-cell stage and treated with 10μM IWR-1 exhibited tail repair at 3 dpa (Fig. 6A). Both the control and zAng-2 injection groups exhibited a significantly higher rate of regeneration as compared to IWR-1 treatment alone (p < .05, n = 20) (Fig. 6B) These findings support the implication of Wnt-Ang-2 signaling in vascular repair.
Fig. 6. Wnt-Ang-2 signaling and vascular endothelial repair.
(A) The tails of transgenic Tg (kdrl:GFP) zebrafish embryos were amputated at 72 hpf. At 0 day post amputation (dpa), the red arrow pointed to the site of injury. At 1 dpa, initiation of endothelial repairs was present. At 3 dpa, complete tail repair was observed, as indicated by the yellow arrow. IWR-1 treatment (10 μM) inhibited tail repair at 3 dpa. zAng-2 mRNA injection restored tail repair in IWR-1-treated fishes at 3 dpa. (B) Quantification of tail repair. These experiments were repeated for n = 20 in each treatment group, and each fish was assessed for the presence of tail repair at 3 dpa. The proportion of fish exhibiting tail repair in each group showed a significant difference between the control and IWR-1 treatment conditions (*p < 0.01, n = 20) and between IWR-1 treatment with and without zAng-2 mRNA injection (# p < 0.05, n = 20).
Discussion
In this study, we recapitulate a shear stress-activated Wnt-Ang-2 signaling pathway using the developmental zebrafish model. In our dynamic flow system, canonical Wnt signaling was implicated in OSS-induced Ang-2 expression 7, which influenced vascular endothelial cell migration and tube formation. In the zebrafish embryos, the mechano-reactivated Wnt-Ang-2 signaling was implicated in both subintestinal vessel development and tail repair. Thus, shear stress-reactivated Wnt target genes (Supplemental Table I), in this case, Ang-2 confers therapeutic potential in restoring endothelial repair.
The Wnt/β-catenin signaling pathway plays an important role in both development and tissue repair 21, 22,23,24,25. Several molecules negatively regulate canonical Wnt signaling, including Dickkopfs (DKK-1), the secreted frizzled-related proteins (sFRP-1, sFRP-2, sFRP-3, and sFRP-4), and the Wnt inhibitory factor (Wif-1) 26, 27 as well as small molecules such as IWR-1. Treatment with DKK-1 and siAng-2 inhibited endothelial cell migration and tube formation. In corollary, Ionomycin, a Calcium ionophore, is well-recognized to down-regulate β-catenin/Tcf signaling in Wnt pathway 28. In the colon cancer cells, Ionomycin disrupted β-catenin and TCF binding, nuclear translocation of β-catenin, and suppression of TCF complexes binding to its specific DNA-binding sites29. We also demonstrated that Ionomycin attenuated nuclear translocation of β-catenin, resulting in: 1) down-regulation of both Ang-2 mRNA and protein expression (Supplemental Figure V), 2) inhibition of tube formation, 3) endothelial migration, 4) proliferation (Supplemental Figure VI), and 5) inhibition of SIV development in the zebrafish model (Supplemental Figure VII). In this context, the complementary use of Wnt signaling inhibitors; namely, DKK-1, IWR-1 or siAng-2 knockdown, with recombinant or zebrafish Ang-2 mRNA corroborated reactivation of Wnt-Ang-2 signaling in vascular endothelial repair.
Using the Angiogenesis PCR SuperArray (PAHS-024), we identified a host of Wnt/β-catenin target genes. Ang-2 was one of the shear stress-responsive angiogenic factors (data not shown),. In response to low shear stress (1 dyne/cm2), VEGF-dependent induction of Ang-2/Tie-2 system is implicated in endothelial homeostasis, proliferation and differentiation; in response to high shear stress (30 dyne/cm2), FOXO1-dependent down-regulation of Ang-2 occurs 30, 31. We demonstrate that OSS up-regulated Ang-2 mRNA to a greater extent than did PSS, and OSS-regulated Ang-2 protein expression by 2.2-fold (Figs. 1E and 1F). Furthermore, OSS activated Ang-2 expression via Wnt signaling both in mature endothelial cells and in a developmental zebrafish model. Ang-2 is a secreted glycoprotein that is expressed by endothelial cells and vascular progenitor cells, and the release of Ang-2 from activated endothelial cells antagonizes the binding of Ang-1 to the Tie-2 receptor, thus sensitizing the endothelial cells to pro-angiogenic and/or pro-inflammatory stimuli 11. Ang-2 promotes endothelial chemotaxis and tube formation by inhibiting Ang-1-mediated phosphorylation of Tie-2 32. Over-expression of Ang-2 can impart an anti-angiogenic effect as an Ang-1/Tie-2 inhibitor by disrupting embryonic blood vessel formation, resulting in a phenotype similar to that of Tie-2 knockout 33. Ang-2 is further implicated in regulating Wnt target Survivin expression to mitigate oxidized LDL-induced apoptosis in human aortic endothelial cells 34. Elevated Ang-2 levels promote tumor progression 35, and are associated with obesity 36. Endothelial-specific Ang-2 over-expression further promotes vascular permeability and hypotension during septic shock, whereas inhibition of the Ang-2/Tie-2 interaction attenuates lipopolysaccharide-induced hypotension and reduces mortality rate37. Nevertheless, the precise mechanism whereby OSS modulates Ang-2 expression in maintaining endothelial homeostasis and in promoting vascular repair warrants further investigation.
The use of transgenic zebrafish model recapitulated shear stress-reactivated Wnt-Ang-2 signaling pathway. Zebrafish Ang-2 orthologs have been recognized to play an important role in zebrafish vascular development, particularly for intersegmental vessel (ISV) sprouting and subintestinal vessel (SIV) formation prior to 72 hpf 38. ISV sprouting occurs between 24 hpf to 72 hpf, and SIV formation originate from the duct of Cuvier between 48 to 72 hpf 39. Both ISV and SIV are anatomic milestones for monitoring disrupted angiogenesis 40. Analogous to the in vitro model of vascular repairs, we demonstrate Ang-2 knock-down with morpholinos resulted in impaired SIV formation in Tg (kdrl:GFP) fish (Fig. 5). Furthermore, we demonstrate that inhibition of Wnt-signaling pathway disrupted vascular repair in response to tail amputation (Fig. 6). Taken together, these findings provide new mechanotransduction insights underlying the reactivation of Wnt target genes with a therapeutic implication for vascular development and repair.
Supplementary Material
Significance.
The canonical Wnt/β-catenin signaling pathways play an important role in differentiation and proliferation. In this study,, we investigated whether the Wnt signaling pathway was implicated in shear stress-activated Ang-2. Both Ang-2 expression and Wnt TOPflash reporter activity were up-regulated in response to oscillatory shear stress (OSS). While Wnt agonist Wnt3a promoted Ang-2 mRNA expression, Dkk-1 treatment or Ang-2 siRNA inhibited endothelial cell migration and tube formation. Wnt-Ang-2 signaling was further elucidated in the zebrafish embryos, in which Ang-2 mRNA was down-regulated in heat-shock inducible DKK-1 transgenic Tg(hsp70l:Dkk1-GFP) fish. Ang-2 morpholino micro-injection further impaired development of subintestinal vessels (SIV) and blood flow in at 72 hours post fertilization (hpf). Thus, we provide new insights in shear stress-activated Wnt-Ang-2 signaling with a translational implication in vascular repair.
Acknowledgments
We are grateful to Dr. Roel Nusse at Stanford University School of Medicine for providing us with the plasmid to produce TOPFlash reporter lentiviruses. We are also thankful to Dr. Sara Childs at the University of Calgary for providing us with the zebrafish Ang-2 cDNA (in plasmid pDONR221).
Sources of Funding
These studies were supported by the National Institutes of Health R01HL-083015 (T.K.H.), R01HD069305 (N.C.C., T.K.H.), R01HL111437 (T.K.H., N.C.C.), and R01HL096121(C.L.L.).
Non-standard Abbreviations and Acronyms
- HAEC
human aortic endothelial cells
- Ang-2
Angiopoietin-2
- SIV
Subintestinal vessel
- MO
Morpholino
Footnotes
Disclosures
None
References
- 1.Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. American Journal of Physiology-Heart and Circulatory Physiology. 2005;288:H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 2.Obi S, Yamamoto K, Shimizu N, Kumagaya S, Masumura T, Sokabe T, Asahara T, Ando J. Fluid shear stress induces arterial differentiation of endothelial progenitor cells. Journal of Applied Physiology. 2009;106:203–211. doi: 10.1152/japplphysiol.00197.2008. [DOI] [PubMed] [Google Scholar]
- 3.Toh Y-C, Voldman J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. The FASEB Journal. 2011;25:1208–1217. doi: 10.1096/fj.10-168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915–1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 5.Huang H, Nakayama Y, Qin K, Yamamoto K, Ando J, Yamashita JK, Itoh H, Kanda K, Yaku H, Okamoto YN, emoto Y. Differentiation from embryonic stem cells to vascular wall cells under in vitro pulsatile flow loading. J Artif Organs. 2005;8:110–118. doi: 10.1007/s10047-005-0291-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 7.Tressel SL, Huang RP, Tomsen N, Jo H. Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2–dependent mechanism. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:2150–2156. doi: 10.1161/ATVBAHA.107.150920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, Fujisawa T, Niu XL, Ahmad S, Al-Ani B, Chudasama K, Abbas A, Potluri R, Bhandari V, Findley CM, Lam GKW, Huang J, Hewett PW, Cudmore M, Kontos CD. Angiopoietin-2 confers atheroprotection in apoe−/− mice by inhibiting ldl oxidation via nitric oxide. Circulation research. 2009;104:1333–1336. doi: 10.1161/CIRCRESAHA.109.196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed A, Fujisawa T. Multiple roles of angiopoietins in atherogenesis. Current Opinion in Lipidology. 2011;22:380–385. doi: 10.1097/MOL.0b013e32834b26b3. [DOI] [PubMed] [Google Scholar]
- 10.Thomas M, Augustin HG. The role of the angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 11.Felcht M, Luck R, Schering A, Seidel P, et al. Angiopoietin-2 differentially regulates angiogenesis through tie2 and integrin signaling. The Journal of Clinical Investigation. 2012;122:1991. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korff T, Ernst E, Nobiling R, Feldner A, Reiss Y, Plate KH, Fiedler U, Augustin HG, Hecker M. Angiopoietin-1 mediates inhibition of hypertension-induced release of angiopoietin-2 from endothelial cells. Cardiovascular research. 2012;94:510–518. doi: 10.1093/cvr/cvs124. [DOI] [PubMed] [Google Scholar]
- 13.Resnick N, Gimbrone M. Hemodynamic forces are complex regulators of endothelial gene expression. The FASEB Journal. 1995;9:874–882. doi: 10.1096/fasebj.9.10.7615157. [DOI] [PubMed] [Google Scholar]
- 14.Hwang J, Michael H, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates nadph oxidase subunit expression: Implication for native ldl oxidation. Circulation research. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conway DE, Lee S, Eskin SG, Shah AK, Jo H, McIntire LV. Endothelial metallothionein expression and intracellular free zinc levels are regulated by shear stress. American Journal of Physiology-Cell Physiology. 2010;299:C1461–C1467. doi: 10.1152/ajpcell.00570.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang J, Saha A, Boo YC, Sorescu GP, McNally JS, Holland SM, Dikalov S, Giddens DP, Griendling KK, Harrison DG. Oscillatory shear stress stimulates endothelial production of from p47phox-dependent nad (p) h oxidases, leading to monocyte adhesion. Journal of Biological Chemistry. 2003;278:47291–47298. doi: 10.1074/jbc.M305150200. [DOI] [PubMed] [Google Scholar]
- 17.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. Journal of the American College of Cardiology. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 18.Tressel SL, Kim H, Ni C-W, Chang K, Velasquez-Castano JC, Taylor WR, Yoon Y-s, Jo H. Angiopoietin-2 stimulates blood flow recovery after femoral artery occlusion by inducing inflammation and arteriogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1989–1995. doi: 10.1161/ATVBAHA.108.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logan CY, Nusse R. The wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 20.Jen N, Yu F, Lee J, Wasmund S, Dai X, Chen C, Chawareeyawong P, Yang Y, Li R, Hamdan MH. Atrial fibrillation pacing decreases intravascular shear stress in a new zealand white rabbit model: Implications in endothelial function. Biomechanics and Modeling in Mechanobiology. 2012:1–11. doi: 10.1007/s10237-012-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin G, Slack JM. Requirement for wnt and fgf signaling in xenopus tadpole tail regeneration. Developmental biology. 2008;316:323–335. doi: 10.1016/j.ydbio.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan C-W, Wei S, Hao W, Kilgore J, Williams NS. Small molecule–mediated disruption of wnt-dependent signaling in tissue regeneration and cancer. Nature chemical biology. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vijayaragavan K, Bhatia M. Early cardiac development: A wnt beat away. Proc Natl Acad Sci U S A. 2007;104:9549–9550. doi: 10.1073/pnas.0703731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galli LM, Barnes T, Cheng T, Acosta L, Anglade A, Willert K, Nusse R, Burrus LW. Differential inhibition of wnt-3a by sfrp-1, sfrp-2, and sfrp-3. Dev Dyn. 2006;235:681–690. doi: 10.1002/dvdy.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. Dkk1, a negative regulator of wnt signaling, is a target of the beta-catenin/tcf pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- 28.Park CH, Hahm ER, Lee JH, Jung KC, Rhee HS, Yang CH. Ionomycin downregulates β-catenin/tcf signaling in colon cancer cell line. Carcinogenesis. 2005;26:1929–1933. doi: 10.1093/carcin/bgi145. [DOI] [PubMed] [Google Scholar]
- 29.Park CH, Hahm ER, Lee JH, Jung KC, Rhee HS, Yang CH. Ionomycin downregulates beta-catenin/tcf signaling in colon cancer cell line. Carcinogenesis. 2005;26:1929–1933. doi: 10.1093/carcin/bgi145. [DOI] [PubMed] [Google Scholar]
- 30.Goettsch W, Gryczka C, Korff T, Ernst E, Goettsch C, Seebach J, Schnittler HJ, Augustin HG, Morawietz H. Flow-dependent regulation of angiopoietin-2. Journal of cellular physiology. 2008;214:491–503. doi: 10.1002/jcp.21229. [DOI] [PubMed] [Google Scholar]
- 31.Chlench S, Mecha Disassa N, Hohberg M, Hoffmann C, Pohlkamp T, Beyer G, Bongrazio M, Da Silva-Azevedo L, Baum O, Pries AR. Regulation of foxo-1 and the angiopoietin-2/tie2 system by shear stress. FEBS letters. 2007;581:673–680. doi: 10.1016/j.febslet.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-fes and c-fyn. J Cell Sci. 2002;115:175–183. doi: 10.1242/jcs.115.1.175. [DOI] [PubMed] [Google Scholar]
- 33.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 34.Li R, Mittelstein D, Fang K, Beebe T, Quigley K, Berliner J, Hsiai TK. Angiopoeitin-2 modulates survivin expression in oxldl-induced endothelial cell apoptosis. Biochemical and Biophysical Research Communications. 2011;417:619–622. doi: 10.1016/j.bbrc.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: Possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. [PubMed] [Google Scholar]
- 36.Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. The Journal of Clinical Investigation. 2013;123:3436. doi: 10.1172/JCI66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kubota Y, Oike Y, Satoh S, Tabata Y, Niikura Y, Morisada T, Akao M, Urano T, Ito Y, Miyamoto T. Cooperative interaction of angiopoietin-like proteins 1 and 2 in zebrafish vascular development. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13502–13507. doi: 10.1073/pnas.0501902102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: An atlas of embryonic and early larval development. Developmental biology. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- 40.Zheng P-P, Severijnen L-A, Willemsen R, Kros JM. Circulation status of subintestinal vessels is a sensitive parameter for monitoring suboptimal systemic circulation in experimental zebrafish embryos. Cell Cycle. 2009;8:3782–3783. doi: 10.4161/cc.8.22.10000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.