Abstract
Although recipients of donor lungs from smokers have worse clinical outcomes, the underlying mechanisms are unknown. We tested the association between donor smoking and the degree of pulmonary edema (as estimated by lung weight), the rate of alveolar fluid clearance (measured by airspace instillation of 5% albumin) and biomarkers of lung epithelial injury and inflammation (bronchoalveolar lavage surfactant protein-D and IL-8) in ex vivo lungs recovered from 298 organ donors. The extent of pulmonary edema was higher in current smokers (n=127) compared to non-smokers (median 408g, IQR 364-500 vs. 385g, IQR 340 - 460, p=0.009). Oxygenation at study enrollment was worse in current smokers versus non-smokers (median PaO2/FiO2 214 mmHg, IQR 126-323 vs. 266 mmHg, IQR 154-370, p=0.02). Current smokers with the highest exposure (≥20 pack-years) had significantly lower rates of alveolar fluid clearance, suggesting that the effects of cigarette smoke on alveolar epithelial fluid transport function may be dose related. BAL IL-8 was significantly higher in smokers while surfactant protein-D was lower. These findings indicate that chronic exposure to cigarette smoke has important effects on inflammation, gas exchange, lung epithelial function and lung fluid balance in the organ donor that could influence lung function in the lung transplant recipient.
INTRODUCTION
Donor smoking has been associated with both short and long term adverse effects in the lung transplant recipient (1). Smoking may have a variety of harmful effects on the lung that could contribute to lung dysfunction after lung transplantation. In experimental studies, exposure to tobacco smoke causes lung epithelial and endothelial injury, facilitates leukocyte activation, and induces accumulation of neutrophils in the pulmonary circulation (2-8). These observations may help to explain the recent observation that both active and passive cigarette smoke exposure are associated with development of acute respiratory distress syndrome (ARDS) in patients with severe trauma (9). However, the mechanisms by which donor smoking leads to adverse outcomes in the lung transplant recipient have not been studied.
Better understanding of the mechanisms by which donor smoking leads to adverse outcomes in the lung transplant recipient could help to guide therapeutic interventions to improve outcomes in recipients of lungs from smokers. Based on the potential deleterious effects of cigarette smoking on the lung epithelium, we hypothesized that donor smoking would be associated with lower rates of alveolar epithelial fluid clearance, more pulmonary edema, and changes in bronchoalveolar lavage (BAL) lung injury biomarkers consistent with lung epithelial injury in the ex vivo lung. To test this hypothesis, we studied human lungs that were procured from 298 brain dead organ donors whose lungs were not utilized for transplantation. Some of the results of these studies have been previously reported in the form of an abstract (10).
METHODS
Donors
The study population was derived from brain dead organ donors who were managed by the California Transplant Donor Network (CTDN) from April 2006 to April 2011. Donors were eligible for inclusion in the current study if they were evaluated for inclusion in the Beta-agonists for Oxygenation in Lung Donors (BOLD) study of albuterol vs. placebo (11), and the next-of-kin authorized lung recovery for research (n = 661). As part of the BOLD study, if the lungs were not used for transplantation and a qualified surgeon was available, the intact lungs were recovered by standard protocol at the time of organ procurement for physiologic and BAL evaluation (n = 302). Among these 302 donors, smoking history was available from 298 donors and these donors form the study population for the current study. Clinical data was obtained from the CTDN medical record and included demographics, smoking and alcohol history and donor oxygenation as measured by the arterial to inspired oxygen fraction (PaO2/FiO2) at the time that donor care was assumed by the CTDN. Donors were managed during the study with a standard ventilator protocol (volume assist control, 10 cc/kg predicted body weight, PEEP 5 cmH2O). The smoking and alcohol history for each donor was obtained by the CTDN in a face-to-face interview with the donor’s closest relative and included quantity and duration of use of tobacco products and alcoholic beverages.
Measurements
In the 298 donors included in the study, lungs were recovered without perfusion and inflated with room air to full inflation although the pressure and volume were not measured. Lungs were then transported to our laboratory at University of California San Francisco on ice. All lungs were subjected to the same standard evaluation, but for technical reasons, some measurements could not be made in every lung. The number of lungs included in each analysis is indicated below. Upon arrival in the laboratory, intact lungs were weighed for estimation of the extent of pulmonary edema (n = 570 lungs) and average lung weight was calculated for each donor. If only one lung was available from a donor because the other lung was transplanted or surgical issues prevented recovery (n = 16), then the weight of that lung was used as the average lung weight for that donor. We chose to estimate the extent of pulmonary edema using lung weight because this is the standard method used by pathologists at autopsy; in a small subset of donors early in the study, we also measured the lung wet-to-dry weight ratio in tissue samples from anterior and posterior aspects of each lobe. However, these measurements were highly variable, likely reflecting heterogeneous edema accumulation in the deceased donor lung, whereas the total lung weight correlated well with radiographic assessment of the degree of pulmonary edema (12). After the lungs were weighed, a BAL was done in a segment of a single upper lobe (n = 204). The other upper lobe (n = 242) was reperfused using previously published methods (13-16). Briefly, the lobe was suspended from a mass transducer to monitor lung weight and a pulmonary artery (PA) catheter was inserted to monitor PA pressure. The lung was rewarmed to 37°C by reperfusing with a solution of Dulbecco’s modified Eagle’s medium with low glucose containing 5% bovine serum albumin using a peristaltic pump at an output of 0.3 L/min to maintain a mean PA pressure of approximately 10 mm Hg. The pulmonary veins were not cannulated, and venous drainage was passive (13). Perfusate was continuously recirculated from a drainage reservoir. The lung was inflated with continuous positive airway pressure of 10 cm H2O with 95% O2, 5% CO2. AFC was measured by airspace instillation of a 5% albumin solution as previously described (14, 15). IL-8 (R&D Systems, Minneapolis, MN) and surfactant protein D (SP-D, Yamasa Corporation, Tokyo Japan) were measured in duplicate in BAL fluid by enzyme linked immunosorbent assay. Remaining lobes were used for unrelated studies. Laboratory staff carrying out all measures were blinded to donor smoking status.
Statistical Analysis
Normally distributed variables are expressed as mean ± SD and compared between groups using Student’s T-test. Non-normally distributed variables are expressed as median (intraquartile range, IQR) and compared between groups using Mann-Whitney U Test or Kruskal Wallis test. Categorical variables were compared by Fisher’s Exact Test. Correlation between continuous variables was assessed using Spearman’s rank test. A p-value ≤ 0.05 was considered statistically significant.
RESULTS
Donors
Donor characteristics are summarized in Table 1. Donors whose lungs were recovered for physiologic analysis were similar demographically to other donors in the study whose lungs were not used for transplantation (data not shown). Smoking was common in the donors included in the study: 43% of donors were current smokers and 58% of donors were ever smokers. Among current smokers, the average number of pack years was 19 ± 21. Among ever smokers, the average number of pack years was 18 ± 20. Current smokers were significantly younger and more likely to use alcohol than non-current smokers (Table 1). Similar trends were observed for ever versus never smokers.
Table 1.
Clinical characteristics of 298 donors by current and ever smoking status
| Characteristic | Current Smoker | Not Current Smoker | P Value | Ever Smoker | Never Smoker | P Value |
|---|---|---|---|---|---|---|
| N = 127 | N = 171 | N = 172 | N = 126 | |||
|
| ||||||
| Age | 44 ± 13 | 48 ± 15 | 0.011 | 47 ± 14 | 46 ± 15 | 0.067 |
|
| ||||||
| Male | 83 (65%) | 101 (59%) | 0.28 | 114 (66%) | 70 (56%) | 0.070 |
|
| ||||||
| Caucasian | 86 (68%) | 107 (63%) | 0.26 | 116 (67%) | 77 (61%) | 0.29 |
|
| ||||||
| Cause of Brain Death | ||||||
| Head trauma | 43 (34%) | 48 (28%) | 0.16 | 46 (27%) | 45 (36%) | 0.21 |
| CVA/bleed | 52 (41%) | 86 (51%) | 85 (50%) | 53 (42%) | ||
| Anoxia/other | 32 (25%) | 36 (21%) | 40 (23%) | 28 (22%) | ||
|
| ||||||
| Chronic Alcohol Use | 54 (57%) | 46 (34%) | 0.001 | 70 (56%) | 30 (29%) | < 0.001 |
|
| ||||||
| History of Lung Disease (other than childhood asthma) |
10 (8%) | 18 (11%) | 0.55 | 14 (8%) | 14 (11%) | 0.43 |
Pulmonary edema in the recovered lung
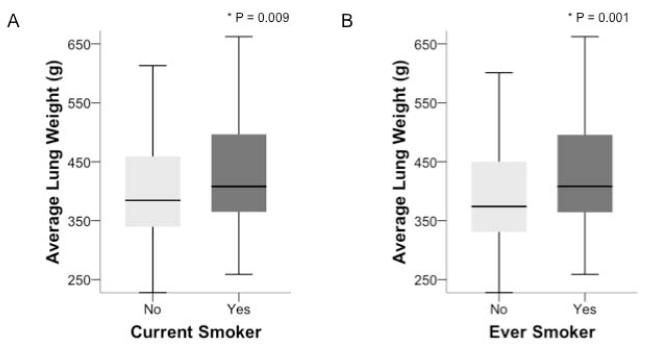
The degree of pulmonary edema was estimated by measuring the weight of the recovered lung(s) for each donor (12). Compared to current non-smokers, current smokers had more pulmonary edema as evidenced by significantly higher lung weights (median 408 g, IQR 364-500 vs. 385 g, IQR 340 - 460, p = 0.009), (Figure 1A). Similarly, compared to never smokers, ever smokers had more pulmonary edema as evidenced by significantly higher lung weights (median 408 g, IQR 364 - 496 vs. 374 g, IQR 331 - 452, p = 0.001) (Figure 1B). These differences persisted when adjusted for donor height. Compared to current non-smokers, current smokers had higher lung weight/height (median lung weight/cm height 2.4 g/cm, IQR 2.1 – 2.8 vs. 2.3 g/cm, IQR 2.0 vs 2.7, p = 0.045). Compared to never smokers, ever smokers had higher lung weight/height (median lung weight/cm height 2.4 g/cm, IQR 2.1 – 2.7 vs. 2.2 g/cm, IQR 2.0 vs 2.6, p = 0.018).
Figure 1.
Pulmonary edema as measured by lung weight is higher in current smokers (n = 121) compared to current non-smokers (n = 167) (Panel A) and in ever smokers (n = 124) compared to never smokers (n = 164) (Panel B). Data shown in boxplot format (horizontal bar represents the median, boxes encompass the 25th to 75th percentile and error bars encompass the 10th to 90th percentile), groups compared by Mann Whitney U test.
Donor gas exchange
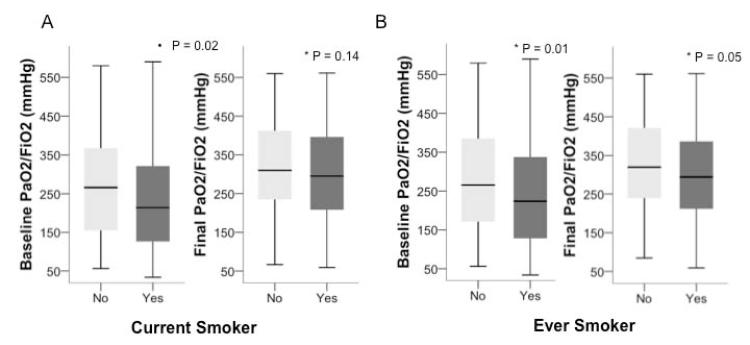
To determine whether the increased lung weight observed in lungs recovered from smokers was associated with lung dysfunction prior to lung recovery, we compared the PaO2/FiO2 ratio at study enrollment and prior to lung recovery between current and non-current smokers as well as ever and never smokers. Non-current smokers had significantly better enrollment oxygenation than current smokers (median PaO2/FiO2 266 mmHg, [IQR 154-370] vs 214 [126 – 323], p = 0.02) and a trend towards better oxygenation prior to lung recovery (median PaO2/FiO2 310 mmHg, [IQR 235-415] vs 298 [210 – 401], p = 0.14) (Figure 2A and B). Likewise, never smokers had significantly better oxygenation than ever smokers both at baseline (median PaO2/FiO2 266 mmHg, [IQR 169-385] vs 224 [128 – 342], p = 0.01) and immediately prior to lung recovery (median PaO2/FiO2 320 mmHg, [IQR 239-421] vs 296 [213 – 394], p = 0.05) (Figure 2C and D).
Figure 2.
Oxygenation was worse in current smokers (n = 120) compared to non current smokers (n = 163) (Panel A) and ever smokers (n = 122) compared to never smokers (n = 161) (Panel B) as measured by the PaO2/FiO2 ratio at enrollment (baseline) and prior to organ procurement (final). Data shown in boxplot format (horizontal bar represents the median, boxes encompass the 25th to 75th percentile and error bars encompass the 10th to 90th percentile), groups compared by Mann Whitney U test.
Alveolar fluid clearance in the recovered lung
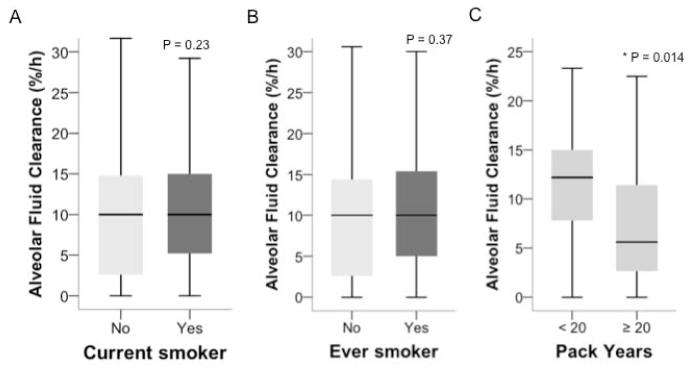
Impaired alveolar epithelial fluid clearance is an important mechanism that affects net lung fluid balance and can lead to accumulation of pulmonary edema in the airspaces of the lung (17). Overall there was no difference in the mean rates of alveolar fluid clearance between current and non-current smokers (Figure 3A) or between ever and never smokers (Figure 3B) and the rate of alveolar fluid clearance was not associated with lung weight. However, among current smokers with the highest cigarette smoke exposure (≥20 pack years, n = 35), the median rate of alveolar fluid clearance was less than half that of subjects with less than 20 pack years (n = 48) (median 5.6%/h [2.6 – 12.5] vs. 12.2 %/h [IQR 7.8 – 15.0], p = 0.014) suggesting that the effects of cigarette smoke on alveolar epithelial fluid transport function may be dose related (Figure 3C). Similar findings were observed comparing ever smokers with ≥ 20 pack years (n = 40) to those with < 20 pack years (n = 64) (median 5.3%/h [2.5 – 10.3] vs. 10.8 %/h [IQR 5.5 – 15.0], p = 0.009). In further support of a dose response, the number of pack years of smoking was modestly but significantly inversely correlated with the rate of alveolar fluid clearance among current smokers (rho = −0.28, p = 0.018) and among ever smokers (rho = −0.22, p = 0.037).
Figure 3.
The rate of alveolar fluid clearance did not differ between current smokers (n = 102) and current non-smokers (n = 135) (Panel A) or between ever smokers (n = 129) and never smokers (n = 95) (Panel B). Among current smokers, donors with ≥ 20 pack years of smoking (n = 35) had significantly slower rates of alveolar fluid clearance than donors with < 20 pack years (n = 48) (Panel C). Data shown in boxplot format (horizontal bar represents the median, boxes encompass the 25th to 75th percentile and error bars encompass the 10th to 90th percentile), groups compared by Mann Whitney U test.
Biomarkers of inflammation and lung epithelial injury
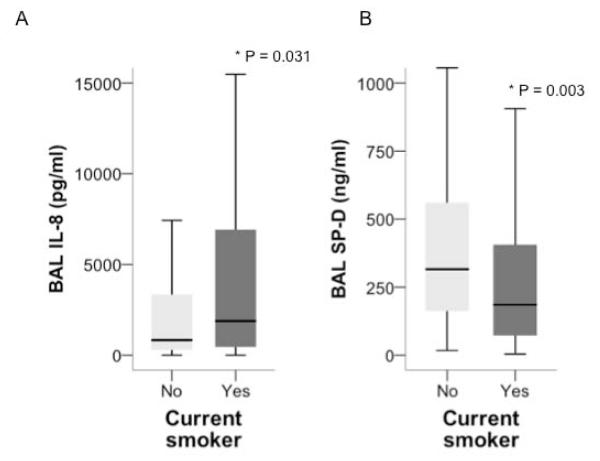
Biomarkers of inflammation and lung epithelial injury were measured in the BAL in a subset of 204 donors. Current smokers had significantly higher levels of the proinflammatory chemokine IL-8 in the BAL and significantly lower levels of the alveolar epithelial type II cell product surfactant protein D (Figure 4).
Figure 4.
Current smokers had higher levels of BAL IL-8 (Panel A) and lower levels of Surfactant Protein D (Panel B). Data shown in boxplot format (horizontal bar represents the median, boxes encompass the 25th to 75th percentile and error bars encompass the 10th to 90th percentile), groups compared by Mann Whitney U test.
Potential confounding by alcohol use
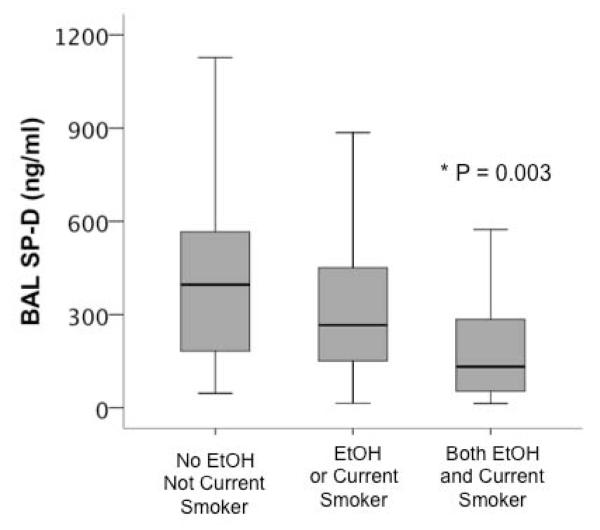
To determine whether any of the findings related to cigarette use could be confounded by heavier alcohol use in smokers compared to non-smokers, we compared physiologic parameters between donors with and without a history of chronic alcohol use. There was no difference in lung weight, donor oxygenation or rates of alveolar fluid clearance between donors with and without a history of chronic alcohol use (data not shown). Biomarkers in the BAL were also compared. BAL IL-8 levels were not different between donors with and without a history of chronic alcohol use. However, SP-D levels were significantly lower in chronic alcohol users compared to non-users (median 175 ng/ml [IQR 69 – 345] vs. 352 [168 – 565], p < 0.001). Donors who had a history of both alcohol use and current smoking had the lowest levels of BAL SP-D (p = 0.003 by Kruskal-Wallis test) (Figure 5).
Figure 5.
Donors who were both current smokers and current alcohol users had the lowest levels of Surfactant Protein D in the BAL (p = 0.003 for significant difference between groups by Kruskal Wallis test). Data shown in boxplot format (horizontal bar represents the median, boxes encompass the 25th to 75th percentile and error bars encompass the 10th to 90th percentile.
DISCUSSION
Several recent studies have associated cigarette smoking in the organ donor with adverse outcomes in the lung transplant recipient. For example, in a prospective cohort study of 1255 lung transplant recipients by the Lung Transplant Outcomes Group, donor smoking was an independent risk factor for primary graft dysfunction after lung transplantation (18). In another study of 1295 lung transplant recipients in the UK Transplant registry, those who received lungs from donors who were smokers had significantly lower three-year survival (19). The current study was designed to investigate potential mechanisms for these reported relationships between donor smoking and short and long-term adverse outcomes in lung transplant recipients.
In lungs recovered from 298 donors, we found that current or ever smokers had significantly higher recovered lung weights, suggestive of increased pulmonary edema. This finding was associated with poorer donor oxygenation during the donor management period. To determine whether the increases in pulmonary edema as estimated by lung weight were due to lung epithelial dysfunction, we measured the rate of alveolar epithelial fluid clearance in recovered lungs. Although there were no significant differences in mean rates of net alveolar fluid clearance between smokers and non-smokers, donors with the highest cigarette smoke exposure (≥ 20 pack years) had slower rates of alveolar fluid clearance, suggesting that detrimental effects of cigarette smoke on alveolar epithelial fluid transport function may be dose related. Although we have previously reported that alveolar fluid clearance rates are impaired in lung transplant recipients with primary graft dysfunction (20), this is the first study, to our knowledge, to systematically measure the rate of alveolar fluid clearance in a large number of donor lungs. Since intact alveolar fluid clearance mechanisms are critical to the resolution of both acute lung injury (21) and primary graft dysfunction (20), the finding of an inverse association between pack years of smoking and rates of alveolar fluid clearance suggests one potential mechanism to explain the reported association between donor smoking and primary graft dysfunction in lung transplant recipients (18).
Levels of SP-D, a biomarker of alveolar epithelial type II injury, were lower in the BAL in current or ever smokers. Decreased levels of SP-D in the pulmonary edema fluid have been previously reported as a marker of lung epithelial injury in the acute respiratory distress syndrome (22). In addition, in one study of 110 healthy volunteers, BAL SP-D levels were lower in smokers compared to non-smokers (23). Interestingly, the median SP-D levels in the BAL were substantially higher (~600 ng/ml in non-smokers) in that study compared to the levels that we report in donor lungs. Although these differences could be due to different immunoassay and bronchoalveolar lavage methods, another potential explanation is that lower levels in the BAL reflect lung epithelial injury even in non-smoking donors perhaps due to mechanical ventilation, critical illness or the underlying insult leading to brain death.
Levels of the proinflammatory chemokine IL-8, a chemokine that is produced abundantly by activated lung epithelium (24), and induced in the lung epithelium by cigarette smoke (25) were increased in the BAL from smokers. This finding is in contrast to several prior reports of BAL IL-8 levels in healthy volunteers that found no significant differences (26-28) perhaps due to the small numbers of patients enrolled (n = 18 – 39). Kuschner and colleagues (29) did report higher BAL IL-8 levels in smokers (n = 16) compared to non-smokers (n = 14). Of note, in all prior studies where actual IL-8 levels are available, the levels in the BAL were substantially lower than levels measured in the current study with median levels in the 30 pg/ml range compared to medians of 834 pg/ml in non-current smokers and 1888 pg/ml in current smokers in this study. Although the higher IL-8 levels in the current study could be due to methodologic differences in immunoassays and BAL, the high levels are comparable to the levels measured in normal volunteers after LPS challenge (28) and may reflect brain death-related (30) or ventilator-induced lung inflammation (31) in the critically ill donor population. There was no difference in the mean time from brain death to organ procurement between current smokers and current non-smokers (data not shown), indicating that differences in IL-8 were not due to the timing of organ procurement with relation to the early pro-inflammatory and late immunosuppressive effects of brain death. When taken together, the findings of higher IL-8 and lower SP-D in the BAL of smokers suggest that donor smoking is associated with significant lung epithelial dysfunction and release of proinflammatory mediators that could contribute both to pre-transplant lung dysfunction as manifested by pulmonary edema and to post-transplant lung dysfunction including primary graft dysfunction and long term graft and recipient survival. Furthermore, the high BAL IL-8 levels in smokers may have contributed to the lack of efficacy of albuterol in the parent BOLD trial of albuterol vs. placebo (11) since we have recently reported that IL-8 can impair beta-adrenergic agonist stimulated upregulation of AFC (32).
Chronic alcohol ingestion has also been associated with adverse effects on the lung (33) including reduced antioxidant capacity (34), propensity to develop acute lung injury (35, 36), and lung epithelial dysfunction including alterations in alveolar epithelial barrier properties (37), ion transport and fluid clearance. Chronic alcohol consumption was common in the donor population studied and was significantly more common in current or ever smokers. This finding raised the concern that alcohol use might be confounding the association between smoking and increased pulmonary edema, decreased oxygenation, and decreased SP-D and increased IL-8 in the BAL. When chronic alcohol users were compared to non-users, there were no significant differences in lung weight, oxygenation, alveolar fluid clearance rates or BAL IL-8 levels. However, BAL SP-D levels were lower in alcohol users consistent with more severe epithelial injury in this group; the lowest BAL SP-D levels were observed in donors who both smoked and drank alcohol, suggesting a possible additive effect of cigarette smoke exposure and alcohol use on the lung epithelium. These findings are important, in that studies of the impact of cigarette smoking on lung function do not typically take into account possible confounding by alcohol use. In addition, alcohol use can be difficult to quantify without the use of standardized validated questionnaires (38, 39). Future prospective studies of the impact of cigarette smoking on donor and lung transplant recipient outcomes should aim to collect quantitative measures of alcohol use.
One question that arises from the current study is whether the findings support a limitation on the use of lungs from donors with cigarette smoke exposure. A detailed analysis of outcomes in the UK Registry study suggested that limiting the use of lungs from smokers would lead to increased death on the waiting list for lung transplantation that would not be offset by improved survival in lung transplant recipients (19). Our study focused on the effect of donor smoking on lung epithelial function in order to understand mechanisms of disease, and by necessity did not include clinical outcomes in transplant recipients; therefore, drawing conclusions related to clinical practice would be premature. Rather than limit the use of lungs from donors that smoked, one potential benefit of the current study is to provide targets for potential therapeutic interventions that might be used to improved outcomes in recipients who received lungs from donors that smoked. The current findings suggest that therapies that target the lung epithelium and/or the pro-inflammatory response might be helpful.
This study has several strengths. First, to our knowledge, it is one of the only studies to date to quantify the effects of long term cigarette smoke exposure on physiologic and biochemical indices of lung epithelial injury in the explanted human lung. Close to 300 explanted human lungs were studied, providing a robust sample size for analysis. Second, the study includes predominantly young and otherwise healthy organ donors without chronic lung disease, making it likely that the observed changes are related to cigarette smoke exposure and less likely that they are due to advanced cigarette smoke-related lung disease. Indeed, only a small minority of the donors studied had any history of chronic lung disease. Finally, the experimental model which includes measurement of rates of alveolar fluid clearance in the isolated perfused human lung is a novel feature of this study that has not previously been applied to such a large number of human lung explants.
This study also has some limitations. First, both smoking history and alcohol history were obtained from the donor social history. The donor social history is usually obtained by the organ procurement organization from the closest available relative of the brain dead organ donor and may not be completely accurate. In addition, quantitative exposure estimates, particularly for alcohol, but also for duration and number of cigarettes smoked are likely to be inaccurate. Moreover, exposure to secondhand cigarette smoke, which might also be harmful, is not captured in the social history. In future studies, measurement of biomarkers of cigarette smoke exposure in organ donors such as serum cotinine or urine NNAL (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol) (40) could provide better quantification of cigarette smoke exposure. A second limitation is that all the lungs that were studied were deemed to be not suitable for transplantation. It was not possible in this study to obtain samples of lungs that were utilized for transplantation, and thus it is not possible to determine if any of the observed changes in the lungs that were recovered for this study might have had an impact on lung function if transplanted. A third limitation is that the measure used to assess pulmonary edema, total lung weight, may not be as quantitative as gravimetric methods. However, in initial studies in the BOLD cohort, we observed that the lung wet-to-dry weight ratio was highly variable and inconsistent, likely reflecting heterogeneity in distribution of excess lung water, particularly between dependent and non-dependent lung regions. By contrast, total lung weight was highly correlated with the extent of radiographic infiltrates as scored on the anterior-posterior chest radiograph (12). For this reason, it is likely that the total lung weight is actually more accurate as a global index of pulmonary edema than the lung wet-to-dry weight ratio. In addition, these lungs have very little intravascular blood volume so this means that the wet weight measurement should primarily reflect extravascular lung water. A final limitation is that certain variables in the study could not be controlled; for example, the lungs were not flushed at the time of resection, and retained blood volume may have been variable. Likewise, the cold ischemic time prior to reperfusion for measurement of alveolar fluid clearance was also variable.
In conclusion, chronic exposure to cigarette smoke results in more pulmonary edema (as estimated by lung weight) and worse oxygenation in the potential organ donor. Mechanistically, these findings may be explained in part by more alveolar inflammation (elevated IL-8) and a dysregulated alveolar epithelium (impaired alveolar epithelial fluid clearance and reduced levels of SP-D), findings that may be exacerbated by chronic alcohol use. These abnormalities in lung fluid balance, gas exchange, and alveolar epithelial function could be important determinants of the risk of acute and chronic lung dysfunction following lung transplantation in donor lungs exposed to cigarette smoke.
Acknowledgments
We would like to acknowledge the dedication and hard work of all the transplant coordinators, advanced practice coordinators, surgical coordinators and support staff at the California Transplant Donor Network as well as members of Dr. Matthay’s laboratory including Jason Abbott who contributed to this study. This study was supported by National Institutes of Health HL081332, HL110969, HL51854 and HL51856.
ABBREVIATIONS
- AFC
alveolar fluid clearance
- ARDS
acute respiratory distress syndrome
- BAL
broncho-alveolar lavage
- BOLD
beta-agonists for oxygenation in lung donors
- CTDN
California Transplant Donor Network
- Il-8
interleukin-8
- IQR
intra-quartile range
- NNAL
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol
- PA
pulmonary artery
- SP-D
surfactant protein-D
Footnotes
Disclosure The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Corbett C, Armstrong MJ, Neuberger J. Tobacco smoking and solid organ transplantation. Transplantation. 2012;94:979–987. doi: 10.1097/TP.0b013e318263ad5b. [DOI] [PubMed] [Google Scholar]
- 2.Jones JG, Minty BD, Lawler P, Hulands G, Crawley JCW, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1:66–68. doi: 10.1016/s0140-6736(80)90493-6. [DOI] [PubMed] [Google Scholar]
- 3.Mason GR, Uszler JM, Effros RM, Reid E. Rapidly reversible alterations of pulmonary epithelial permeability induced by smoking. Chest. 1983;83:6–11. doi: 10.1378/chest.83.1.6. [DOI] [PubMed] [Google Scholar]
- 4.Li XY, Rahman I, Donaldson K, MacNee W. Mechanisms of cigarette smoke induced increased airspace permeability. Thorax. 1996;51:465–471. doi: 10.1136/thx.51.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blann AD, McCollum CN. Adverse influence of cigarette smoking on the endothelium. Thromb Haemost. 1993;70:707–711. [PubMed] [Google Scholar]
- 6.Fernandez JA, Gruber A, Heeb MJ, Griffin JH. Protein C pathway impairment in nonsymptomatic cigarette smokers. Blood Cells Mol Dis. 2002;29:73–82. doi: 10.1006/bcmd.2002.0542. [DOI] [PubMed] [Google Scholar]
- 7.MacNee W, Wiggs B, Belzberg AS, Hogg JC. The effect of cigarette smoking on neutrophil kinetics in human lungs. N Engl J Med. 1989;321:924–928. doi: 10.1056/NEJM198910053211402. [DOI] [PubMed] [Google Scholar]
- 8.Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, et al. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest. 1997;99:2358–2364. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, et al. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. American journal of respiratory and critical care medicine. 2011;183(12):1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ware LB, Lee JW, Landeck M, Wickersham N, Matthay MA, Calfee CS. Pulmonary edema and biomarkers of inflammation and lung epithelial dysfunction are associated with donor smoking in the ex vivo donor lung. J Heart Lung Transplant. 2013;32:S152. doi: 10.1111/ajt.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware LB, Landeck M, Koyama T, Zhao Z, Singer J, Kern R, et al. A randomized trial of the effects of nebulized albuterol on pulmonary edema in brain-dead organ donors. Am J Transpl. 2014;14:621–628. doi: 10.1111/ajt.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ware LB, Neyrinck A, O’Neal HR, Lee JW, Landeck M, Johnson E, et al. Comparison of chest radiograph scoring to lung weight as a quantitative index of pulmonary edema in organ donors. Clin Transplant. 2012:65–71. doi: 10.1111/j.1399-0012.2011.01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briot R, Frank JA, Uchida T, Lee JW, Calfee CS, Matthay MA. Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest. 2009;135(2):269–275. doi: 10.1378/chest.08-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. Coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. American journal of physiology Lung cellular and molecular physiology. 2007;293(1):L52–59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAuley DF, Curley GF, Hamid UI, Laffey JG, Abbott J, McKenna DH, et al. Clinical Grade Allogeneic Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. American journal of physiology Lung cellular and molecular physiology. 2014 doi: 10.1152/ajplung.00358.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 18.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. American journal of respiratory and critical care medicine. 2013;187(5):527–534. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonser RS, Taylor R, Collett D, Thomas HL, Dark JH, Neuberger J. Effect of donor smoking on survival after lung transplantation: a cohort study of a prospective registry. Lancet. 2012;380(9843):747–755. doi: 10.1016/S0140-6736(12)60160-3. [DOI] [PubMed] [Google Scholar]
- 20.Ware LB, Golden JA, Finkbeiner WE, Matthay MA. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. American journal of respiratory and critical care medicine. 1999;159:980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 21.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 22.Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Critical care medicine. 2003;31:20–27. doi: 10.1097/00003246-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 23.More JM, Voelker DR, Silveira LJ, Edwards MG, Chan ED, Bowler RP. Smoking reduces surfactant protein D and phospholipids in patients with and without chronic obstructive pulmonary disease. BMC pulmonary medicine. 2010;10:53. doi: 10.1186/1471-2466-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP, 3rd, Toews GB, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model of cytokine networks in the lung. J Clin Invest. 1990;86:1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. American journal of respiratory and critical care medicine. 1997;155(5):1770–1776. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- 26.McCrea KA, Ensor JE, Nall K, Bleecker ER, Hasday JD. Altered cytokine regulation in the lungs of cigarette smokers. American journal of respiratory and critical care medicine. 1994;150(3):696–703. doi: 10.1164/ajrccm.150.3.8087340. [DOI] [PubMed] [Google Scholar]
- 27.Burnham EL, Kovacs EJ, Davis CS. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. American journal of physiology Lung cellular and molecular physiology. 2013;304(12):L873–882. doi: 10.1152/ajplung.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wesselius LJ, Nelson ME, Bailey K, O’Brien-Ladner AR. Rapid lung cytokine accumulation and neutrophil recruitment after lipopolysaccharide inhalation by cigarette smokers and nonsmokers. The Journal of laboratory and clinical medicine. 1997;129(1):106–114. doi: 10.1016/s0022-2143(97)90167-0. [DOI] [PubMed] [Google Scholar]
- 29.Kuschner WG, D’Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking-related inflammatory responses in healthy adults. The European respiratory journal. 1996;9(10):1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 30.Stangl M, Zerkaulen T, Theodorakis J, Illner W, Schneeberger H, Land W, et al. Influence of brain death on cytokine release in organ donors and renal transplants. Transplantation proceedings. 2001;33(1-2):1284–1285. doi: 10.1016/s0041-1345(00)02479-9. [DOI] [PubMed] [Google Scholar]
- 31.Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- 32.Roux J, McNicholas CM, Carles M, Goolaerts A, Houseman BT, Dickinson DA, et al. IL-8 inhibits cAMP-stimulated alveolar epithelial fluid transport via a GRK2/PI3K-dependent mechanism. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:1096–1106. doi: 10.1096/fj.12-219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boe DM, Vandivier RW, Burnham EL, Moss M. Alcohol abuse and pulmonary disease. Journal of leukocyte biology. 2009;86(5):1097–1104. doi: 10.1189/jlb.0209087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss M, Guidot DM, Wong-Lambertina M, Teh Hoor T, Perez R, Brown LAS. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. American journal of respiratory and critical care medicine. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 35.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, et al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Critical care medicine. 2003;31(3):869–877. doi: 10.1097/01.CCM.0000055389.64497.11. [DOI] [PubMed] [Google Scholar]
- 36.Thakur L, Kojicic M, Thakur SJ, Pieper MS, Kashyap R, Trillo-Alvarez CA, et al. Alcohol consumption and development of acute respiratory distress syndrome: a population-based study. International journal of environmental research and public health. 2009;6(9):2426–2435. doi: 10.3390/ijerph6092426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoseph BP, Breed E, Overgaard CE, Ward CJ, Liang Z, Wagener ME, et al. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PloS one. 2013;8(5):e62792. doi: 10.1371/journal.pone.0062792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donovan DM, Dunn CW, Rivara FP, Jurkovich GJ, Ries RR, Gentilello LM. Comparison of trauma center patient self-reports and proxy reports on the Alcohol Use Identification Test (AUDIT) The Journal of trauma. 2004;56(4):873–882. doi: 10.1097/01.ta.0000086650.27490.4b. [DOI] [PubMed] [Google Scholar]
- 39.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) Journal of studies on alcohol. 1975;36(1):117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh SJ, Ware LB, Eisner MD, Yu L, Jacob P, 3rd, Havel C, et al. Biomarkers increase detection of active smoking and secondhand smoke exposure in critically ill patients. Critical care medicine. 2011;39(1):40–45. doi: 10.1097/CCM.0b013e3181fa4196. [DOI] [PMC free article] [PubMed] [Google Scholar]