Abstract
This is a narrative review of new ideas and concepts related to differences between men and women in their risk of developing dementia or Alzheimer's disease (AD). We introduce the concept of dimorphic neurology and the distinction between sex and gender. We then provide three examples of risk factors related to sex and gender from the literature. Apolipoprotein E genotype is equally common in men and women but has a stronger effect in women. Apolipoprotein E genotype is a biological factor that cannot be modified but interacts with sex or gender related factors that can be modified. Low education has a similar harmful effect in men and women but has been historically more common in women. Education is a social factor related to gender that can be modified. Finally, bilateral oophorectomy is a factor restricted to women. Bilateral oophorectomy is a surgical practice related to sex that can be modified. Consideration of risk and protective factors in men and women separately may accelerate etiologic research for neurological diseases in general, and for dementia and AD in particular. Similarly, future preventive interventions for dementia should be tailored to men and women separately.
Keywords: Dementia, sex, gender, APOE genotype, education, oophorectomy
1. Introduction
1.1. Importance of dimorphic neurology
We have observed two important conceptual trends in the last 20 years that will contribute to our future understanding of the risk of developing dementia or Alzheimer's disease (AD). First, there is increasing attention to differences between men and women in the causes, manifestations, response to treatments, and outcomes of neurological diseases (dimorphic neurology) [1-5]. This attention to dimorphic medicine has historically been stronger in fields like cancer, cardiovascular diseases, and endocrine diseases [1, 6, 7]. However, there is now a growing awareness of differences in brain structure and function between men and women throughout the entire life course (early childhood development, adult life, and aging) [2, 3, 8, 9]. Second, there is increasing recognition of the distinction between sex and gender. Sex is biology: chromosomal, hormonal, or reproductive differences between men and women [1, 4, 5]. By contrast, gender refers to psychological, social, political, and cultural differences between men and women [4, 5, 10]. These two conceptual trends are likely to transform our approach to identifying risk factors for dementia or AD.
1.2. Dementia in men versus women
Dementia is one of the most common diseases related to aging, and its impact on society is growing with time because of the rapid aging of populations worldwide [11, 12]. It remains unclear whether women have a higher risk than men to develop dementia or AD at a given age [12, 13]. Several European studies have suggested that women have a higher incidence rate of dementia or AD than men. However, studies in the United States have not shown a difference, or the difference has varied with age [12]. Regardless of this difference in risk (in incidence rates) across continents, all studies consistently showed that more women than men have AD at any given age, possibly because women survive longer [11, 14, 15]. This higher number of women affected may not be true for other types of dementia such as vascular dementia or Lewy body dementia.
1.3. Sex versus gender
It is important to distinguish sex and gender for the understanding of risk and protective mechanisms of disease. The US Institute of Medicine clarified the difference between sex and gender in a 2010 report: “Sex” refers to the classification of living things as male or female according to their reproductive organs and functions assigned by chromosomal complement, and “gender” refers to a person's self-representation as male or female or to how that person is responded to by social institutions on the basis of that presentation [5]. Thus, sex refers to biological characteristics of men and women, such as chromosomal differences (e.g., XX vs. YY chromosomes), hormonal differences (e.g., effects of estrogen or testosterone), or reproductive differences (e.g., pregnancy or menopause) [1, 4, 5].
Limited attention has been given to the sex chromosomes in relation to the etiology of diseases in general and of dementia or AD in particular [16]. Women have two copies of chromosome X, one of maternal origin and one of paternal origin. The X-chromosome carries approximately 1,600 genes (approximately 155 million base pairs), including genes encoding the androgen receptor and several proteins involved with mitochondrial function, adipose tissue distribution, apoptosis, and response to hypoxia [16, 17]. To avoid a genetic overdose, most of the genes encoded on one of the two X-chromosomes are inactivated in female cells [17-19].
We are now discovering that women are not only complex mosaics of cells with paternal X or maternal X chromosome expressed, but that this mosaic pattern varies from organ to organ (e.g., liver vs. retina vs. brain) and within organs (e.g., hippocampus vs thalamus vs cerebral cortex). Of particular interest to brain functioning, the mosaic pattern of X-chromosome inactivation may vary on a spatial scale from neighboring cells to the left versus the right side of the brain. For example, the right and left hippocampi of a mouse brain (and probably of a woman's brain) may have different amounts and patterns of paternal and maternal X-chromosome inactivation [20, 21]. Therefore, patterns of X-chromosomes inactivation may give a new perspective on the concept of laterality of brain functions in women compared with men. This mosaic pattern of X-chromosome inactivation varies from woman to woman. In addition, the mosaic pattern has been shown to change over the lifespan of female mice [22], and could, conceivably, change also in women.
In contrast to sex, gender includes both a subjective component of self-representation (or sexual identity) and societal components related to the social, cultural, and legal contexts in which women live. For example, a woman may rate herself higher or lower on a masculinity vs. femininity personality scale [23]. However, her right to drive a car, vote for political elections, or own property will depend on the legal system of the country in which the woman lives in a given point in history (e.g., Sweden vs. Saudi Arabia). The personal aspects of gender (e.g., psychology, personality, or behavior) are linked with the social and political aspects (e.g., legal system, religious practices, or local traditions), and it is sometimes difficult to determine to which extent the self-representation of gender is the determinant or the consequence of cultural, political, or religious norms. Thus, sex and gender are tightly related and interdependent; however, they are not the same. Each variable should be studied independently [7, 23].
Gender-related factors have also varied over history. For example, women in the United States were not allowed to vote until the passage of the Nineteenth Amendment to the United States Constitution in 1920 (Women's Suffrage). Similarly, women in the United States have been less likely than men to smoke cigarettes during most of the 20th century. The gap in smoking behavior is now narrowing [12, 24].
2. Methods
This is a narrative review of new ideas and concepts that are developing regarding the etiology of dementia in men and women. Unfortunately, because different studies used different diagnostic categories, the data available specifically refer to AD in some studies and to dementia as a syndrome in other studies. More recently, the definition of dementia has expanded to include pre-clinical stages, such as mild cognitive impairment [25-27]. As a result, we are forced to use narrower or broader definitions of the disease being described depending on the study being quoted (cognitive decline, mild cognitive impairment, dementia, AD, other types of dementia, etc.).
We did not attempt to summarize studies or data from existing clinical or epidemiological studies in a systematic way. In particular, we did not conduct an exhaustive literature search to identify all studies related to sex, gender, and dementia. We selected the papers to discuss by judgment and based on our personal experience and our interpretation of the findings. Similarly, we did not use statistical testing or concepts of statistical significance. More conventional reviews of the literature have been published by us [12] and by others [13-15]. Thus, this review is intended to provide an alternative point of view.
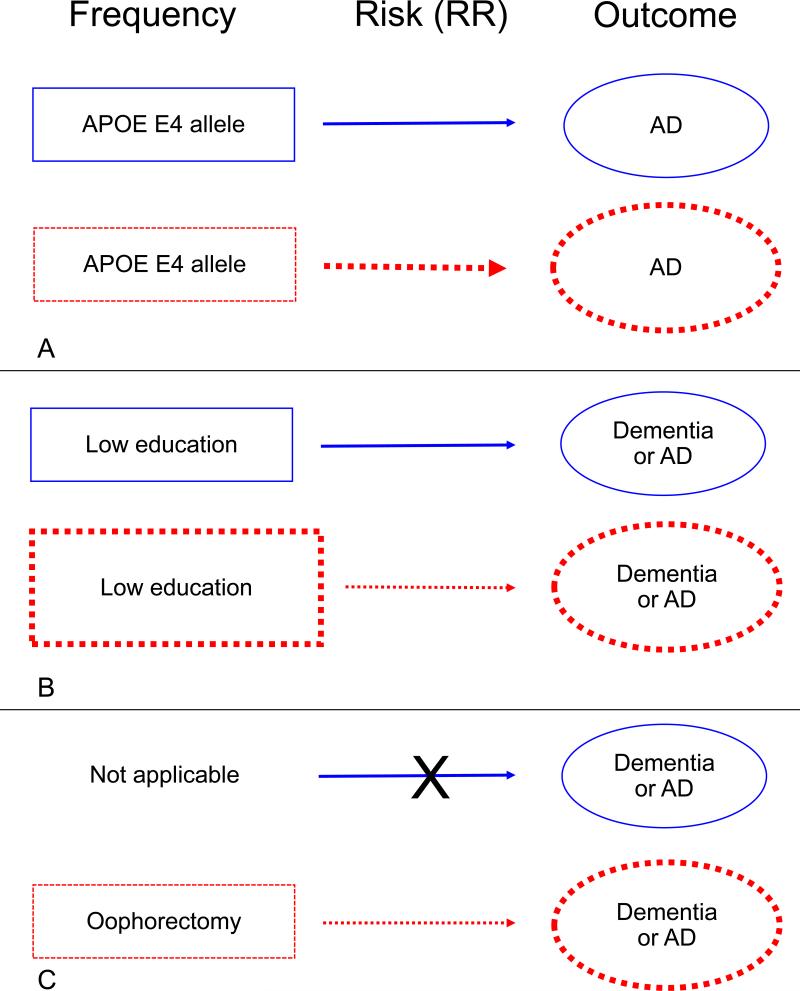
We considered three scenarios of sex and gender differences in disease risk: 1) Risk factors that are equally common in men and women but have a stronger effect in one sex or gender group (e.g., APOE genotype). 2) Risk factors that have a similar effect in men and women but are more common in one sex or gender group because they are gender related (e.g., education). 3) Risk factors restricted to one sex (e.g., oophorectomy). In this review, we provide a conceptual narrative of the current evidence for each example. Figure 1 provides a schematic representation of the three examples.
Figure 1.
Schematic representation of three examples of sex and gender differences related to the risk of dementia or Alzheimer's disease (AD). Men are represented by blue boxes, arrows, and ovals and women by red boxes, arrows and ovals. In all three examples, women experienced a higher risk of dementia or AD attributable to the specific risk factors (bigger red oval). Panel A: APOE E4 allele is equally frequent in men and women (equal boxes) but has a stronger effect in women (thicker red arrow). Panel B: low education has the same effect on the risk of dementia or AD in both men and women (equal strength of the blue and red arrow). However, low education has been historically more common in women than men in many countries (bigger red box in women). Panel C: oophorectomy increases the risk of dementia or AD in women but is not applicable to men. RR = relative risk; APOE = apolipoprotein E: AD = Alzheimer's disease.
3. Results
3.1. APOE genotype and Alzheimer's disease
Traditional genetic studies that examined the association between single-nucleotide polymorphisms (SNPs) and AD, have normally considered sex as an adjustment variable. For example, case-control studies of individual SNPs, or their extension into genome-wide association studies of thousands of SNPs, have matched cases and controls by sex to avoid confounding. These analyses have not emphasized the role of sex as an effect modifier (interaction effect) because many studies did not have adequate power to split the sample and analyze the association in men and women separately [6, 12].
As we reported in greater details elsewhere [12], the E4 allele of the apolipoprotein E gene (APOE) is the strongest known susceptibility variant for AD [28, 29]. There are three major isoforms of the ApoE protein (ApoE2, ApoE3, and ApoE4) that are encoded by three alleles of the APOE gene (E2, E3, and E4). Carriers of one E4 allele are three to four times more likely to develop AD than non-carriers. Carriers of the E4 allele also have an earlier age at onset of AD that can be visualized in cumulative incidence curves. Carriers of two E4 alleles have an even higher risk of AD than carriers of one allele (trend by genetic dose). The majority of studies, and a large meta-analysis published by Farrer et al., in 1997, showed higher age-specific odds ratios of AD in women compared with men both for carriers of one E4 allele and for carriers of two E4 alleles. Interestingly, the women to men differences were greater for carriers of one E4 allele than for carriers of two E4 alleles. The effect of the E4 allele was reduced after age 85 years in both men and women [28].
Among E4 allele carriers, women showed greater hippocampal atrophy, more changes in the default mode connectivity, more cortical atrophy, and worse memory performance compared with men [30-32]. In addition, a large autopsy study showed a higher burden of amyloid plaques and neurofibrillary tangles in the brain of women than of men who were carriers of an E4 allele [33]. Finally, a recent study suggested that the greater risk of AD in women compared with men who carry one APOE E4 allele may be mediated by tau pathology [34].
The stronger effect of the APOE E4 allele in women compared with men offers an excellent example of a completely biological factor (a genetic variant) interacting with other biological factors (e.g., hormones produced by the ovaries or other genes hosted on chromosomes X or Y) or with gender-related factors (e.g., education, physical activity, behavioral preferences, type of occupation). We will first describe possible interactions between APOE E4 and sex mediated by hormonal mechanisms.
It has been postulated that the estrogen produced by the ovaries in a woman before the onset of menopause has an important neuroprotective effect on the brain [35, 36]. The stronger effect of the APOE E4 allele on the risk of dementia in women may be mediated by estrogen. Indeed, it has been hypothesized that the apolipoprotein E (apoE: the protein coded by the APOE gene) may be a critical factor in the neuroprotective actions of estrogen [37, 38]. There is increasing evidence from both in vivo (mice) and in vitro (cell cultures) studies that estrogen may modulate the apoE protein and its receptor, namely, the low density lipoprotein receptor-related protein [38, 39]. Laboratory studies have shown that: 1) Nerve regeneration was severely delayed in APOE-gene knockout mice as compared to wild-type littermates [38, 40]. 2) Estrogen treatment given to ovariectomized mice resulted in a significant increase in levels of the apoE protein and of the low density lipoprotein receptor-related protein in the olfactory bulb and other brain areas [38, 41]. 3) Estrogen treatment increased apoE protein and also increased neurite outgrowth in cortical and olfactory neuronal cultures [38, 42]. 4) Finally, estrogen treatment had no effect on neurite outgrowth in cultures deprived of apoE protein or in cultures with the apoE4 protein (abnormal gene product) [38, 42]. In summary, these studies suggest that the apoE protein is a critical intermediary for the beneficial effects of estrogen on neuronal protection and repair [38, 40-42]. The hypothesis that the neuroprotective effects of estrogen may be modified by the APOE genotype is supported also by some epidemiologic studies in women. [43-46].
Another line of reasoning for the differences between men and women focuses on possible interactions between APOE genotype and more conventional risk or protective factors for dementia or AD. APOE E4 genotype may interact synergistically with alcohol intake, cigarette smoking, physical inactivity, and high intake of saturated fat with the diet [14, 15]. These interactions may explain the increased risk of dementia and AD in APOE carriers in general. These interactions may also explain the differential effects of APOE genotype in men and women because men and women differ in their exposure to cigarette smoking, alcohol drinking, dietary preferences, and willingness to engage in physical activity. It remains unclear whether these behavioral factors are completely gender-related or whether they are partly biologically driven (sex related). It has also been suggested that higher education may reduce the harmful effects of APOE E4. Indeed, women who carried an APOE E4 allele had reduced risk of developing dementia if they obtained a higher level of education early in life [47].
3.2. Education and dementia
Lower education is recognized as one of the most established risk factors for dementia and AD. Some studies suggested that the effect of lower education may be even stronger than the effect of the APOE E4 genotype. For example, in the risk score to predict dementia proposed by Kivipelto et al., in 2006 using Finnish data, the scores given to lower education strata were four for 0 – 6 years and three for 7 – 9 years of education (compared with people with 10 or more years of education). The scores given to age were five for people older than 53 years and three for people 47 – 53 years old (compared with people younger than 47 years). For comparison, the score for APOE genotype was only two [48]. Interestingly, in the same model, being a man received a score of one, suggesting that sex and gender may have an important effect on the risk of dementia even after accounting for the possible mediation effects of several known risk factors (education, systolic blood pressure, body-mass index, total cholesterol, physical activity, and APOE genotype).
It remains unknown how education may prevent dementia and AD, and current data suggest that the impact of education on the risk of dementia or AD is similar in men and women (Figure 1) [49-53]. It may be strategic to consider education within a broader concept of intellectual enrichment that includes other protective activities or behaviors. For example, it has been shown that subjects who are involved in mentally stimulating activities at work (e.g., occupations requiring complex interactions with data and people) may reduce their initial risk related to lower education [54]. Therefore, education, primary occupation in earlier life, and cognitively stimulating leisure activities in midlife or later life have been combined into the concept of lifetime intellectual enrichment. It has been hypothesized that lifetime intellectual enrichment may provide an important brain reserve mechanism to delay the onset of cognitive decline and dementia [52, 53].
Education in earlier life (through schooling or formal training), mental stimulation as part of a job, and stimulating leisure activities later in life are three examples of factors that are primarily gender-related and historically contingent. In some countries, men had historically more access to advanced education than women; this pattern has now reversed. For example, at the most recent US Census, the educational attainment in women was higher than in men [55]. Similarly, cognitively demanding jobs used to be restricted to men (e.g. directing public or private institutions, serving in high ranking political roles, holding high academic ranks, etc.); the pattern is changing in some countries [12].
The dimorphic effects of education, occupation, and leisure activities on the risk of dementia and AD should be further investigated and may be leveraged in developing preventive interventions [52, 53]. The dramatic changes in social and cultural attitudes and norms about gender that are occurring in many western countries in recent decades may modify our projections on the future burden of dementia on society [56].
3.3. Oophorectomy and dementia
Oophorectomy and other gynecological surgeries are examples of factors restricted to one sex because of anatomical differences (Figure 1). The neuroprotective effect of estrogen may be lost in women who experience premature (before age 40 years), or early (between age 40 and 45 years) menopause either naturally or because of medical or surgical interventions (more commonly, bilateral oophorectomy) [36, 57]. In 2007, the Mayo Clinic Cohort Study of Oophorectomy and Aging showed that women who underwent bilateral oophorectomy before the onset of menopause experienced a long-term increased risk of cognitive impairment or dementia [35, 36, 57-59]. The risk increased with younger age at oophorectomy, did not vary by indication for the oophorectomy, and was eliminated by estrogen therapy initiated after the surgery and continued up to age 50 years or longer. In most of the women, the bilateral oophorectomy was performed at the time of a hysterectomy. The Mayo Clinic study also suggested that unilateral oophorectomy, with or without concurrent hysterectomy, is associated with increased risk of cognitive decline or dementia [57-59].
The findings from the Mayo Clinic study for both unilateral and bilateral oophorectomy were first replicated three years later, in 2010, by a Danish nationwide study [59, 60]. The findings for unilateral oophorectomy were subsequently replicated by a 2011 Chinese study [61]. However, some studies did not confirm the associations, as discussed by Bove et al [62].
In 2014, Bove et al reported the results of a cohort study on the association between surgical menopause and cognitive decline and AD pathology [62]. Earlier age at surgical menopause was associated with faster decline in global cognition, and specifically in episodic memory and semantic memory. Earlier age at surgical menopause was also associated with increased AD neuropathology, in particular neuritic plaques. Estrogen therapy that was initiated within 5 years of the surgery and that was continued for at least 10 years was associated with a slower decline in global cognition. None of these associations were observed for women who underwent natural menopause. Strengths of the study included the long duration of follow-up, the detailed assessment of cognitive functions, and the large number of autopsies. Weaknesses of the study included the lack of information needed to separate women who underwent different gynecological surgeries that may result in surgical menopause, the lag time between the time of surgery and the enrollment in the study, and the use of self-reported information about gynecological surgeries [35, 36].
The consistent findings from the Mayo Clinic, the Danish, and the Bove et. al., studies suggest that bilateral oophorectomy is a risk factor for cognitive decline and dementia. It has been suggested that bilateral oophorectomy causes an abrupt decline in the levels of circulating estrogen, and that this decline may trigger a chain of causality leading to degenerative and vascular lesions in the brain. These brain lesions may manifest as cognitive impairment or dementia several decades after the oophorectomy. The role of other ovarian hormones (e.g., progesterone) and of other etiologic mechanism (e.g., disruption of the hypothalamus-pituitary-ovarian axis) remains uncertain [35, 36]. Similarly, the effects of hysterectomy on the remaining two ovaries, or the effects of removing one ovary on the single remaining ovary are unknown [57-61].
If the major mechanism linking bilateral oophorectomy with cognitive impairment or dementia is estrogen deprivation, we must postulate that estrogen is neuroprotective in women before the age of natural menopause [36, 57]. This hypothesis may appear to be in contrast with the findings from the clinical trials conducted by the Women's Health Initiative Memory Study (WHIMS) that showed an increased risk of cognitive impairment and dementia in women randomized to receive either estrogen alone or estrogen plus a progestin at age 65-79 years [63, 64]. However, these findings are only apparently conflicting because they refer to two distinct periods in women's life [63, 64]. The effects of estrogen on the brain are different in women younger than age 50 years compared with women with age 65-79 years (timing hypothesis). A full discussion of the timing hypothesis for the effects of estrogen on the brain has been reported elsewhere. [36, 57]
Hysterectomy, unilateral oophorectomy, and bilateral oophorectomy are examples of sex specific conditions restricted to women. Similar sex specific conditions have been investigated less frequently in men. For example, it remains unclear whether men who are treated for prostate hypertrophy or prostate cancer have an increased risk of dementia.
4. Conclusions
At this point in the history of research on the etiology of dementia or AD, we need new concepts, new theories, and new points of view rather than simply additional data. An impressive number of individual papers, monographs, books, literature reviews, and meta-analyses on the etiology of dementia or AD have been written [12]. It may be time to take some distance from the existing literature and see whether there are new lines of investigation to be explored.
We hope that this review will stimulate other groups of investigators to further explore the impact of sex and gender related factors on cognitive aging [12, 35, 36, 57, 65]. We also hope that this review will prompt new funding from the National Institutes of Health and other funding agencies worldwide for studies exploring risk and protective factors for cognitive decline or dementia that are related to sex, hormonal differences, and gender factors in men and women. Consideration of risk and protective factors in men and women separately may accelerate etiologic research in neurological diseases in general, and for dementia and AD in particular [2, 3, 12, 35, 65].
Acknowledgements
We would like to thank Ms. Carol J. Greenlee for her assistance in typing and formatting the manuscript.
Funding Information
The authors receive funding from several NIH institutes (AG034676; AG006786; AG037526; AG044170).
ABBREVIATIONS
- AD
Alzheimer's disease
- SNP
single nucleotide polymorphism
- APOE
apolipoprotein E (gene)
- apoE
apolioprotein E (protein)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
- Walter A. Rocca - first author: he drafted the manuscript.
- Michelle M. Mielke - conducted an extensive literature review and reviewed the manuscript from an epidemiological perspective.
- Prashanthi Vemuri - reviewed the manuscript from a brain imaging perspective.
- Virginia M. Miller - reviewed the manuscript as an expert of sex and gender issues in medicine.
Competing Interests
The authors declare no conflict of interest.
Provenance and Peer Reviewed
Commissioned and externally peer reviewed.
References
- 1.Exploring the Biological Contributions to Human Health: Does Sex Matter? The National Academies Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- 2.Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A. Sex and gender differences in Alzheimer's disease: recommendations for future research. Journal of Women's Health. 2012;21:1018–23. doi: 10.1089/jwh.2012.3789. [DOI] [PubMed] [Google Scholar]
- 3.Cahill L. A half-truth is a whole lie: on the necessity of investigating sex influences on the brain. Endocrinology. 2012;153:2541–3. doi: 10.1210/en.2011-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods NF, Tsui AO. Editorial: Epidemiologic approaches to women's health. Epidemiol Rev. 2014;36:1–4. doi: 10.1093/epirev/mxt013. [DOI] [PubMed] [Google Scholar]
- 5.Women's Health Research: Progress . Pitfalls, and Promise. The National Academies Press; 2010. [PubMed] [Google Scholar]
- 6.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–22. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller VM. Why are sex and gender important to basic physiology and translational and individualized medicine? Am J Physiol Heart Circ Physiol. 2014;306:H781–8. doi: 10.1152/ajpheart.00994.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nugent BM, Tobet SA, Lara HE, Lucion AB, Wilson ME, Recabarren SE, et al. Hormonal programming across the lifespan. Horm Metab Res. 2012;44:577–86. doi: 10.1055/s-0032-1312593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014;111:823–8. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N. Epidemiology and the Peoples Health Theory and Context. Oxford University Press, Inc.; New York: 2011. [Google Scholar]
- 11.Alzheimer's Association Alzheimer's Disease Facts and Figures. Alzheimer's & Dement.10. 2014 doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998;55:809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 14.Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L. Dementia prevention: current epidemiological evidence and future perspective. Alzheimers Res Ther. 2012;4:6. doi: 10.1186/alzrt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomon A, Kivipelto M, Soininen H. Prevention of Alzheimer's disease: moving backward through the lifespan. J Alzheimers Dis. 2013;33(Suppl 1):S465–9. doi: 10.3233/JAD-2012-129021. [DOI] [PubMed] [Google Scholar]
- 16.Wise AL, Gyi L, Manolio TA. eXclusion: toward integrating the X chromosome in genome-wide association analyses. Am J Hum Genet. 2013;92:643–7. doi: 10.1016/j.ajhg.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spatz A, Borg C, Feunteun J. X-chromosome genetics and human cancer. Nat Rev Cancer. 2004;4:617–29. doi: 10.1038/nrc1413. [DOI] [PubMed] [Google Scholar]
- 18.Brown CJ, Ballabio A, Rupert JL, Lafreniere RG, Grompe M, Tonlorenzi R, et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349:38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- 19.Ross MT, Grafham DV, Coffey AJ, Scherer S, McLay K, Muzny D, et al. The DNA sequence of the human X chromosome. Nature. 2005;434:325–37. doi: 10.1038/nature03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu H, Luo J, Yu H, Rattner A, Mo A, Wang Y, et al. Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron. 2014;81:103–19. doi: 10.1016/j.neuron.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zimmer C. Seeing X Chromosomes in a New Light. The New York Times; New York: 2014. p. D1. [Google Scholar]
- 22.Berletch JB, Yang F, Disteche CM. Escape from X inactivation in mice and humans. Genome Biol. 2010;11:213. doi: 10.1186/gb-2010-11-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ristvedt SL. The evolution of gender. JAMA Psychiatry. 2014;71:13–4. doi: 10.1001/jamapsychiatry.2013.3199. [DOI] [PubMed] [Google Scholar]
- 24.American Lung Association Research and Program Services, Epidemiology and Statistics Unit. [April 7, 2014];Trends in tobacco use. 2011 PDF available from http://www.lung.org/finding-cures/our-research/trend-reports/Tobacco-Trend-Report.pdf.
- 25.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364:2227–34. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 26.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. [PubMed] [Google Scholar]
- 29.Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21:1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Paajanen T, Westman E, Wahlund LO, Simmons A, Tunnard C, et al. Effect of APOE epsilon4 allele on cortical thicknesses and volumes: the AddNeuroMed study. J Alzheimers Dis. 2010;21:947–66. doi: 10.3233/JAD-2010-100201. [DOI] [PubMed] [Google Scholar]
- 31.Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, et al. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:8254–62. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleisher A, Grundman M, Jack CR, Jr., Petersen RC, Taylor C, Kim HT, et al. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62:953–7. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- 33.Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24–8. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- 34.Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer's disease. Ann Neurol. 2014 doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocca WA, Henderson VW. Is there a link between gynecologic surgeries and Alzheimer disease? Neurology. 2014;82:196–7. doi: 10.1212/WNL.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 36.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen, and dementia: A 2014 update. Mol Cell Endocrinol. 2014 doi: 10.1016/j.mce.2014.01.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAsey ME, Cady C, Jackson LM, Li M, Randall S, Nathan BP, et al. Time course of response to estradiol replacement in ovariectomized mice: brain apolipoprotein E and synaptophysin transiently increase and glial fibrillary acidic protein is suppressed. Exp Neurol. 2006;197:197–205. doi: 10.1016/j.expneurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 38.Struble RG, Cady C, Nathan BP, McAsey M. Apolipoprotein E may be a critical factor in hormone therapy neuroprotection. Front Biosci. 2008;13:5387–405. doi: 10.2741/3088. [DOI] [PubMed] [Google Scholar]
- 39.Cheng X, McAsey ME, Li M, Randall S, Cady C, Nathan BP, et al. Estradiol replacement increases the low-density lipoprotein receptor related protein (LRP) in the mouse brain. Neurosci Lett. 2007;417:50–4. doi: 10.1016/j.neulet.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer's disease. J Neurosci. 1998;18:3180–5. doi: 10.1523/JNEUROSCI.18-09-03180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horsburgh K, Macrae IM, Carswell H. Estrogen is neuroprotective via an apolipoprotein E-dependent mechanism in a mouse model of global ischemia. J Cereb Blood Flow Metab. 2002;22:1189–95. doi: 10.1097/01.wcb.0000037991.07114.4e. [DOI] [PubMed] [Google Scholar]
- 42.Nathan BP, Barsukova AG, Shen F, McAsey M, Struble RG. Estrogen facilitates neurite extension via apolipoprotein E in cultured adult mouse cortical neurons. Endocrinology. 2004;145:3065–73. doi: 10.1210/en.2003-1707. [DOI] [PubMed] [Google Scholar]
- 43.Mattila KM, Axelman K, Rinne JO, Blomberg M, Lehtimaki T, Laippala P, et al. Interaction between estrogen receptor 1 and the epsilon4 allele of apolipoprotein E increases the risk of familial Alzheimer's disease in women. Neurosci Lett. 2000;282:45–8. doi: 10.1016/s0304-3940(00)00849-1. [DOI] [PubMed] [Google Scholar]
- 44.Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54:1949–54. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- 45.Burkhardt MS, Foster JK, Laws SM, Baker LD, Craft S, Gandy SE, et al. Oestrogen replacement therapy may improve memory functioning in the absence of APOE epsilon4. J Alzheimers Dis. 2004;6:221–8. doi: 10.3233/jad-2004-6302. [DOI] [PubMed] [Google Scholar]
- 46.Rippon GA, Tang MX, Lee JH, Lantigua R, Medrano M, Mayeux R. Familial Alzheimer disease in Latinos: interaction between APOE, stroke, and estrogen replacement. Neurology. 2006;66:35–40. doi: 10.1212/01.wnl.0000191300.38571.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang HX, Gustafson DR, Kivipelto M, Pedersen NL, Skoog I, Windblad B, et al. Education halves the risk of dementia due to apolipoprotein epsilon4 allele: a collaborative study from the Swedish brain power initiative. Neurobiol Aging. 2012;33:1007, e1–7. doi: 10.1016/j.neurobiolaging.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 49.Letenneur L, Gilleron V, Commenges D, Helmer C, Orgogozo JM, Dartigues JF. Are sex and educational level independent predictors of dementia and Alzheimer's disease? Incidence data from the PAQUID project. J Neurol Neurosurg Psychiatry. 1999;66:177–83. doi: 10.1136/jnnp.66.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cobb JL, Wolf PA, Au R, White R, D'Agostino RB. The effect of education on the incidence of dementia and Alzheimer's disease in the Framingham Study. Neurology. 1995;45:1707–12. doi: 10.1212/wnl.45.9.1707. [DOI] [PubMed] [Google Scholar]
- 51.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004–10. [PubMed] [Google Scholar]
- 52.Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Roberts RO, Lowe VJ, et al. Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol. 2012;72:730–8. doi: 10.1002/ana.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vemuri P, Lesnick TG, Przybelski SA, Machulda MM, Knopman DS, Mielke MM, et al. Association of lifetime intellectual enrichment with cognitive decline in the elderly. Jama Neurology. 2014 doi: 10.1001/jamaneurol.2014.963. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karp A, Andel R, Parker MG, Wang HX, Winblad B, Fratiglioni L. Mentally stimulating activities at work during midlife and dementia risk after age 75: follow-up study from the Kungsholmen Project. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2009;17:227–36. doi: 10.1097/JGP.0b013e318190b691. [DOI] [PubMed] [Google Scholar]
- 55.Ryan CL, Siebens J. Current Population Reports. US Department of Commerce, US Census Bureau; Washington, DC: 2012. Educational Attainment in the United States: 2009. Washington, D.C.: US Department of Commerce, US Census Bureau; 2012. [Google Scholar]
- 56.Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011;1379:188–98. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–83. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 59.Rocca WA, Grossardt BR, Shuster LT, Stewart EA. Hysterectomy, oophorectomy, estrogen, and the risk of dementia. Neurodegener Dis. 2012;10:175–8. doi: 10.1159/000334764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phung TK, Waltoft BL, Laursen TM, Settnes A, Kessing LV, Mortensen PB, et al. Hysterectomy, oophorectomy and risk of dementia: a nationwide historical cohort study. Dement Geriatr Cogn Disord. 2010;30:43–50. doi: 10.1159/000314681. [DOI] [PubMed] [Google Scholar]
- 61.Zhou G, Liu J, Sun F, Duan L, Yan B, Peng Q. Cognitive functioning in elderly women who underwent unilateral oophorectomy before menopause. Int J Neurosci. 2011;121:196–200. doi: 10.3109/00207454.2010.542842. [DOI] [PubMed] [Google Scholar]
- 62.Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, et al. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–9. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 64.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 65.Miller VM, Garovic VD, Kantarci K, Barnes JN, Jayachandran M, Mielke MM, et al. Sex-specific risk of cardiovascular disease and cognitive decline: pregnancy and menopause. Biol Sex Differ. 2013;4:6. doi: 10.1186/2042-6410-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]