Abstract
Background
We hypothesized that preconditioning with a short exposure to isoflurane (ISO) would reduce neurodegeneration induced by prolonged exposure to ISO in neonatal rats, as previously shown in neuronal cell culture.
Methods
We randomly divided 7-day-old Sprague-Dawley rats into 3 groups: control, 1.5% ISO, and preconditioning (PC) + 1.5% ISO. The control group was exposed to carrier gas (30% oxygen balanced in nitrogen) for 30 min and then carrier gas again for 6 h the following day. The 1.5% ISO group was exposed to carrier gas for 30 min and then 1.5% ISO for 6 h the following day. The PC + 1.5% ISO group was preconditioned with a 30 min 1.5% ISO exposure and then exposed to 1.5% ISO for 6 h the following day. Blood and brain samples were collected 2 h after the exposures for determination of neurodegenerative biomarkers, including caspase-3, S100β, caspase-12, and an autophagy biomarker Beclin-1.
Results
Prolonged exposure to ISO significantly increased cleaved caspase-3 expression in the cerebral cortex of 7-day old rats compared to the group preconditioned with ISO and the controls using Western blot assays. However, significant differences were not detected for other markers of neuronal injury.
Conclusion
The ISO-mediated increase in cleaved caspase-3 in the postnatal day 7 rat brain is ameliorated by preconditioning with a brief anesthetic exposure, and differences were not detected in other markers of neuronal injury.
Introduction
Isoflurane (ISO) is a widely used general anesthetic for both adult and pediatric surgeries. Many studies have been performed to elucidate the harms and benefits of ISO on neurons1–9 and in the developing brain.4,10–12 Depending on the circumstances, ISO has been reported to have both neurotoxic and neuroprotective effects.
A large number of in vivo studies have shown that ISO causes apoptosis in the developing brain of various species of animals4,10,11,13–15 and that subsequent learning and memory are impaired.10,11 Furthermore, ISO has also been shown to be toxic in various cell culture models.2,4,16,17
Other studies have also shown that ISO can have neuroprotective effects. In particular, when ISO is used as a preconditioning drug, it can provide neuroprotection against various hypoxic-ischemic insults to the developing rodent brain.12,18–21 Previous in vitro work from our laboratory, and others, has shown that preconditioning with ISO has a protective effect on neuronal cell cultures subsequently exposed to ISO for a longer duration.2 However, inhibition of ISO-induced neuronal apoptosis during brain development by preconditioning has not yet been examined.
Given that ISO has been a successful preconditioning drug against subsequent anesthetic exposure for developing neurons in vitro2 and also against brain infarction induced by hypoxia and/or ischemia in vivo,12,18,20,22,23 we hypothesized that preconditioning with a short exposure to ISO would reduce neurodegeneration induced by a prolonged exposure to ISO in an animal model. We assessed the effects of 1.5% ISO exposure for 6 hours on apoptotic biomarkers in 7-day old rats, and then determined whether preconditioning with a short exposure to 1.5% ISO for 30 minutes changed the apoptotic response.
Methods
Animals
The experimental procedures and protocols used in this study were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. All efforts were made to minimize the number of animals used and their suffering. Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed with a 12-hour light-dark cycle at 22°C, with food and water provided ad libitum. Thirty-eight postnatal day 7 (P7) rats were used for the ELISA and Western blots and 11 for immunohistochemistry, with approximately equal numbers of male and female rat pups randomly assigned to each condition.
Anesthesia exposure
The groups of rats were exposed to treatments in parallel. The minimum number of rats in each group was determined by a power analysis. We anticipated a large effect size would be clinically significant and chose an effect size of 1.3, and using the desired statistical level of 0.8 and probability level of 0.05, determined a minimum sample size per group (2-tailed hypothesis) of 11 animals. P7 rats were placed in plexiglass chambers resting in a 37°C water bath to maintain a constant environmental temperature. The rat pups were exposed in these chambers to carrier gas (30% oxygen balanced in nitrogen) for 30 min and then 1.5% ISO for 6 h the following day (1.5% ISO), or preconditioned (PC) with a 30 min 1.5% ISO exposure and then exposed to 1.5% ISO for 6 h the following day (PC + 1.5% ISO). The control animals were exposed to carrier gas (30% oxygen balanced in nitrogen) for 30 min and then carrier gas again for 6 h the following day in the plexiglass chambers but not in the water bath. Exposure to ISO for 30 min alone at P7 has been shown not to be detrimental12 and thus this control group was not included. In order to maintain a steady state of anesthetic gas and to prevent accumulation of expired carbon dioxide within the chamber, we used 6 liters of total gas flow throughout the experiments. The ISO, oxygen and carbon dioxide levels in the chamber were monitored using IR absorbance (Ohmeda 5330, Datex-Ohmeda, Louisville, CO) as described in our previous studies.4,15,24 Two rats died during exposure to 1.5% ISO for 6 hrs, 1 from the ISO alone group and the other from the PC plus ISO group.
Determination of plasma S100β
Two hours after the completion of the anesthetic treatment, P7 rats from the control, 1.5% ISO and PC+1.5%ISO groups were deeply anesthetized with 2–3% ISO. Blood (0.1 ml) was collected from the left ventricle and centrifuged to separate the plasma. We measured levels of S100β, a neuronal injury marker, using Sangtec 100 ELISA kits (DiaSorinInc, Stillwater, MN) following the manufacturer’s protocol and as we described previously.25 Briefly, 50 µl of plasma from each rat was placed in each well of a 96-well-plate and mixed with 150 µl of tracer from the kit, and incubated for 2 hours. Afterwards, 3,3’,5,5’tetramethylbenzidine substrate and stop solution were added to each well. The optical density was read at 450 nm. The sensitivity was determined by plotting the standard curve and then measuring concentrations of the samples from the standard curve.
Western Blot Assays
Western blots were performed as we described previously.15,24 Two hours after the ISO exposure, after the mice were anesthetized and blood samples collected from the heart (see above), the mice were perfused with ice-cold saline through the heart and the parietal cortex dissected, frozen in liquid nitrogen and stored at −80. At the time of the assay, the brain tissue from the P7 rat cortical tissue was thawed and homogenized and the total protein concentrations were quantified. The proteins were then separated by 12% gel electrophoresis and were transferred to a nitrocellulose membrane. The blots were incubated with an antibody against cleaved caspase-3 (Cell Signaling #9664), caspase-12 (Cell Signaling #2202), or Beclin-1 (Cell Signaling #3495). The density was measured by Quantity One software (BIO-RAD version 4.5.0) and GS-800 Densitometer (BIO-RAD, Hercules, CA) and the data are expressed as the percent of control of the means from 1 blot per animal per group.
Immunohistochemistry
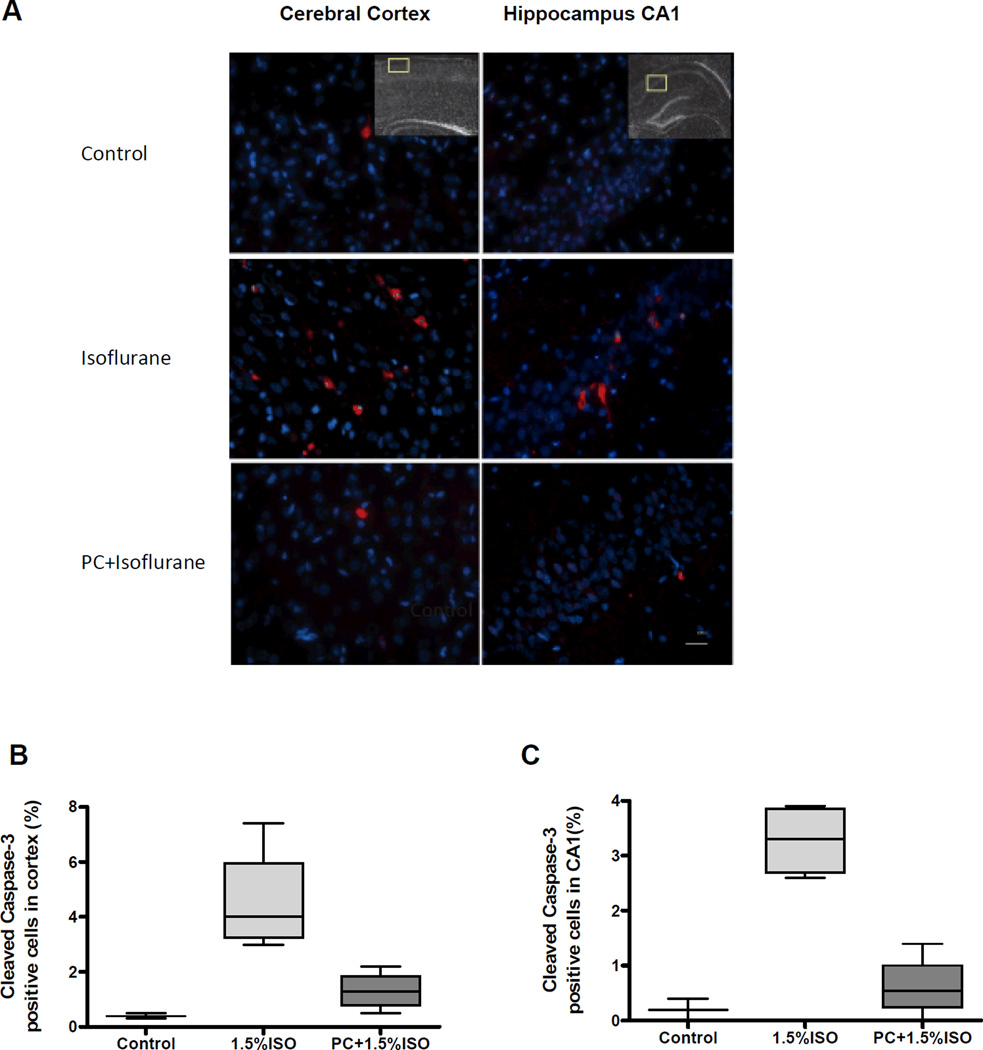
Immunohistochemical localization of caspase-3 was performed in a separate group of P7 rats, as previously described.15 Briefly, 2 hours after the ISO exposure, P7 pups were deeply anesthetized with ISO and transcardially perfused with ice cold saline before the brains were removed, fixed with 4% paraformaldehyde, cryprototected in 30% sucrose, frozen in isopentane and stored at −80°C. Coronal cryosections (10µm) were incubated in 3% hydrogen peroxide, 10% normal goat serum and cleaved caspase-3 antibody (1:400; Cell Signaling Technology, #9664) overnight at room temperature. The next day, the sections were incubated with Alexa Fluor® 594 goat anti-rabbit IgG and coverslipped using ProLong® Gold Antifade Reagent containing the nuclear stain, DAPI (Invitrogen). Quantitative imaging was conducted on an Olympus IX70 microscope equipped with a Cooke SensiCam camera (Applied Scientific Instrumentation, Eugene, OR) and IP lab 4.0 software (Biovision Technologies, Exton, PA). Caspase-positive and total number of cells were counted in the CA1 region of the hippocampus and the adjacent parietal cortex at 20× magnification. The brain sampled and analyzed in parietal cortex was the same region used in the Western blot from the opposite brain hemisphere. The mean number of cells was calculated from 3 sections per animal and the data expressed as the percentage of caspase-3 positive cells in each region.
Statistical analysis
All data were analyzed using the Mann-Whitney U test to determine between-group differences and exact p-values using STATA statistical software. Differences were considered statistically significant at p<0.01.
Results
Preconditioning significantly reduces ISO’s apoptotic effect
This study tested the effects of PC, with a short exposure to ISO before a prolonged exposure to ISO, on apoptotic neurodegeneration in postnatal rats. The level of apoptosis was evaluated by determining cleaved caspase-3 levels in the cerebral cortex using Western blot immunoassays (Fig. 1A). The cleaved caspase-3 levels, expressed as percent of control, in the P7 rats exposed to ISO alone were significantly higher than the PC group (P=0.0009) or the controls (P=0.0004) (Fig. 1B) (Tables 1–2). In addition, the animals that were PC with ISO were also significantly different than controls (P=0.0007) (Fig. 1B) (Tables 1–2). When the P7 cortex and hippocampus were quantitatively analyzed for the immunohistochemical localization of caspase-3 (Fig. 2), significant differences were not detected in the number of caspase-3 positive-cells in the ISO-exposed group compared to controls or the PC group in the cortex or the hippocampus (Fig. 2 B, C) (Tables 1–2). These data support the claim that PC, with a short exposure to ISO, before a prolonged ISO exposure, significantly reduces the ISO-mediated apoptotic neurodegeneration in the developing rat brain using Western blot assays.
Figure 1. Preconditioning inhibits the increase of cleaved caspase-3 induced by isoflurane.
A) Representative Western blot of cleaved caspase-3 in the cerebral cortex after 6 h exposure to isoflurane (1.5%ISO) or preconditioning (PC) with 1.5% isoflurane before the same 6 h isoflurane exposure (PC+1.5%ISO) at postnatal day 7. B) Quantitative analysis of the Western blots of caspase-3, normalized to β-actin as percent of control, showed that prolonged exposure to 1.5% isoflurane (1.5%ISO) significantly increased cleaved caspase-3 compared to controls (P=0.0004), while PC (PC+1.5% ISO) significantly ameliorated the increase compared to 1.5% ISO (P=0.0009). The PC+1.5% ISO group was also significantly different from controls (P=0.0007). N=16 for all groups, **P<0.001. Data are presented as box plots of the means with whiskers (min to max).
Table 1.
P values (95% confidence intervals) of the Western blot and immunohistochemical analyses
| Western blots | Control vs Isoflurane |
Con vs Preconditioning + Isoflurane |
Isoflurane vs Preconditioning + Isoflurane |
|---|---|---|---|
| Caspase-3 | 0.0004 (−1310 to −428.7) | 0.0007 (−777.8 to 103.8) | 0.0009 (91.69 to 973.2) |
| S100β | 0.19 (−147.4 to 7.044) | 0.49 (−101.0 to 53.43) | 0.34 (−23.46 to 116.2) |
| Caspase-12 | 0.96 (−79.41 to 48.46) | 0.27 (−44.11 to 83.76) | 0.17 (−28.64 to 99.24) |
| Beclin-1 | 0.71 (−59.91 to 44.18) | 0.57 (−38.61 to 63.09) | 0.53 (−30.75 to 70.96) |
| Immunohistochemistry | |||
| Caspase-3 Cortex | 0.034 (−7.155 to −1.245) | 0.049 (−3.880 to 2.030) | 0.021 (0.539 to 6.011) |
| Caspase-3 Hippocampus | 0.034 (−4.346 to −1.804) | 0.21 (−1.696 to 0.846) | 0.021 (1.474 to 3.826) |
Table 2.
Means ± standard errors of the Western blot and immunohistochemical analyses
| Western blots | Control vs Isoflurane |
Con vs Preconditioning + Isoflurane |
Isoflurane vs Preconditioning + Isoflurane |
|---|---|---|---|
| Caspase-3 | 100 ± 36.03 | 1106 ± 219.7 | 489.9 ± 116.3 |
| S100β | 100 ± 6.26 | 170 ± 30.03 | 123.8 ± 9.97 |
| Caspase-12 | 100 ± 13.78 | 115.5 ± 22.06 | 80.17 ± 15.79 |
| Beclin-1 | 100 ± 16.63 | 107 ± 15.40 | 87.76 ± 10.55 |
| Immunohistochemistry | |||
| Caspase-3 Cortex | 0.4 ± 0.058 | 4.600 ± 0.984 | 1.325 ± 0.357 |
| Caspase-3 Hippocampus | 0.2 ± 0.116 | 3.275 ± 0.335 | 0.625 ± 0.290 |
Figure 2. Isoflurane preconditioning significantly inhibits neuronal apoptosis induced by prolonged exposure to isoflurane in the cerebral cortex and hippocampus.
A) Representative images of immunostaining for cleaved caspase-3 in the postnatal day 7 (P7) rat cerebral cortex (left panels) and hippocampus (right panels) of controls (top panels), after isoflurane exposure (middle panels), and preconditioning (PC) with a brief anesthetic exposure before the isoflurane exposure (PC + Isoflurane) (bottom panels). Apoptotic cells are stained for caspase-3 (red) and total cells are stained with DAPI (blue). The insets indicate the areas sampled in the cortex and hippocampus for quantitation. Scale bar, 25 µm. B) Quantitative analysis of the percentage of cleaved caspase-3 positive cells in the cerebral cortex and C) the hippocampal CA 1 region. No significant differences were found in the number of caspase-3 positive cells in the cortex in the isoflurane (P=0.034) or PC + isoflurane (0.049) groups compared to controls or between the isoflurane and PC groups (P=0.021), or in the hippocampus (P=0.034, P=0.21, P=0.021 respectively). Data represent the mean of 3 adjacent brain sections per animal, n=4 animals for both experimental groups and n=3 for controls. Data are presented as box plots of the means with whiskers (min to max).
The level of neurodegeneration was further studied by determining the plasma levels of S100β, a marker of neuronal injury, in P7 rats exposed to 1.5% ISO for 6 h, with and without ISO PC (Fig. 3). The ELISA assay did not reveal significant differences in plasma S100β levels between these groups (Tables 1–2).
Figure 3. Effects of isoflurane preconditioning on S100β in plasma.
No significant differences were found in plasma S100β levels with ELISA assays from postnatal day 7 (P7) rats after exposure to 1.5% isoflurane (1.5%ISO) for 6 hours with (P=0.34) or without preconditioning (PC) (P=0.19) or with PC (PC+1.5%ISO) compared to controls (P=0.049). Controls, n=9; 1.5%ISO, n=13; PC+1.5%ISO, n=13. Data are presented as box plots of the means with whiskers (min to max).
The effect of PC on the apoptotic pathway was also examined by caspase-12 activation in the developing brain. Western blot analysis of caspase-12 levels in the P7 cerebral cortex (Fig. 4A) after either a 6h exposure to 1.5% ISO or PC with ISO before the prolonged exposure, were not significantly different from controls (Fig. 4B) (Tables 1–2).
Figure 4. Effects of isoflurane on cleaved caspase-12 in the cerebral cortex.
A) Representative Western blot of cleaved caspase-12 in the cerebral cortex after 6 h exposure to isoflurane (1.5%ISO) or preconditioning (PC) with 1.5% isoflurane before the same 6 h isoflurane exposure (PC+1.5%ISO). B) Quantitation of caspase-12 levels, normalized to β-actin as percent of control, showed no significant differences in the levels of cleaved caspase-12 in the postnatal day 7 (P7) brain between groups exposed to1.5% isoflurane for 6 hours compared to controls (P=0.96) or compared with isoflurane PC (P=0.17) or the PC group compared to controls (P=0.27). Controls, n=9; 1.5%ISO, n=9; PC+1.5%ISO, n=9. Data are presented as box plots of the means with whiskers (min to max).
Effect of ISO treatment on Autophagy
Autophagy, after anesthetic exposures, with and without PC, was examined in the P7 developing brain by measuring Beclin-1 levels. Western blot analysis (Fig. 5A) showed that a 6 h ISO exposure did not significantly reduce Beclin-1 levels (Fig. 5B) (Tables 1–2).
Figure 5. Effects of isoflurane exposure on Beclin-1 in the cerebral cortex.
A) Representative Western blot of Beclin-1 levels in the cerebral cortex after a 6 h exposure to isoflurane (1.5%ISO) or preconditioning (PC) with 1.5% isoflurane before the same 6 h isoflurane exposure (PC+1.5%ISO). B) Quantitative analysis of Beclin-1, normalized to β-actin as percent of control, showed that 1.5% isoflurane for 6 hours, with (P=0.53) or without isoflurane PC (P=0.71), or PC compared to controls (P=0.57) did not significantly affect the levels of Beclin-1 in the cerebral cortex at postnatal day 7 (P7). Controls, n=10; 1.5%ISO, n=10; PC+1.5%ISO, n=11. Data are presented as box plots of the means with whiskers (min to max).
Discussion
This study provides new evidence that PC with ISO, before a long ISO exposure, significantly decreases ISO-mediated apoptosis in the developing brain. This finding is based on a significant reduction in the caspase-3 levels using Western blot assays. However, significant differences were not detected in caspase-3 levels in the cerebral cortex and hippocampus after either ISO exposure or PC. Other markers of neuronal injury, S100β, caspase-12 and Beclin-1 were not significantly affected by either the prolonged ISO exposure or PC. While we have been able to exclude a large effect of these markers, it is possible that we could not detect significant effects due to our small sample size (e.g., Figures 2 and 3).
Caspase-3 is one of the final mediators of the apoptotic pathway and is a well-established biomarker of apoptosis. The most important aspect of our results is that ISO PC decreased caspase-3 levels induced by a prolonged ISO exposure in the developing brain. In a similar study, Shu et al26 showed that xenon pretreatment before a combined ISO/nitrous oxide exposure decreased apoptosis, while nitrous oxide PC had no effect. Furthermore, we have previously shown sevoflurane PC can also inhibit neuronal cell death induced by prolonged exposure to ISO.2 Thus, more studies are necessary to determine the mechanism for the protective effect of PC so that novel approaches can be developed to mimic this effect.
Anesthetics have been shown to be both neuroprotective and neurotoxic in the developing brain and thus concentrations and durations must be considered in pediatric anesthetic practice. Our previous study suggested that prolonged exposure to sevoflurane can induce neuronal damage in vitro.27 The IV anesthetic, propofol, has also been shown to be both neurotoxic28,29 and neuroprotective against brain damage induced by ischemia and other stress factors.30,31 Therefore, it is important to investigate the dose and time responses of anesthetic-induced effects in the developing brain to use their neuroprotective features but minimize their neurotoxic effects.
S100β, a dimeric cytosolic calcium binding protein released by glial cells, is a biomarker of blood-brain barrier dysfunction32 and overall brain distress.33 It has been studied clinically as a biomarker for traumatic brain injury and hypoxic-ischemic brain injury.34,35 We have previously studied S100β in the developing fetal rat brain and found that an in utero exposure to 3% ISO for 1 hour resulted in higher levels of S100β in the plasma of fetal rats when compared to controls.25 Furthermore, we showed an increase in plasma S100β after exposure to a subclinical concentration of ISO in neonatal mice.15 Though the current study did not show a significant difference in S100 β, S100β may be a useful biomarker to detect anesthetic-mediated damage in the developing brain as indicated above, although further studies are needed to investigate its role in pediatric patients.
Caspase-12, part of the apoptotic pathway, is activated by disruption of the calcium homeostasis in the endoplasmic reticulum (ER).36 Anesthetics have been shown to cause calcium dysregulation in the ER via multiple mechanisms.37 In immature hippocampal neurons, ISO exposure was shown to enhance gamma-aminobutyric acid-induced intracellular calcium increase, which was blocked by dantrolene, indicating that ISO exposure causes ryanodine receptor-dependent calcium release from the ER,17 which is consistent with our previous studies in different types of neurons.5 Other studies also suggest that ISO exposure during brain development causes increased activation of inositol triphosphate receptors (InsP3R) resulting in increased calcium release from the ER leading to cell damage and neurodegeneration.3,4,27,38 Furthermore, caspase-12 positive neurons in the hippocampus were significantly increased in fetal rats exposed to 1.3% ISO for 4 hours.39 Caspase-12 has also been shown to indirectly activate caspase-3 in the neuronal apoptotic pathway.40 Although a previous study suggested that ISO-induced neuroapoptosis during brain development in rodents involved both intrinsic and extrinsic pathways,41 it is not clear whether the caspase-12-dependent pathway is also involved. In this study, we did not find significant caspase-12 activation after the ISO exposures, possibly because our ISO concentration may not have been high enough to cause ER stress and caspase-12-dependent neuroapoptosis. Further dose-dependent and exposure duration studies are needed to clarify this question.
Beclin-1, a protein required for autophagosome formation, is an important regulator and biomarker of autophagy activity.42–44 Interestingly, autophagy may have both beneficial and harmful effects on the brain, depending on the experimental conditions. Autophagy appears to be essential to both ischemic and hyperbaric oxygen PC before cerebral ischemia.45 Furthermore, reducing Beclin-1 levels has been shown to exacerbate neurodegeneration in Alzheimer disease models, while overexpression of Beclin-1 can prevent neuronal cell death.46 Autophagy activity can be regulated by InsP3R activity, while ISO has been shown to activate InsP3R and cause cell apoptosis by overactivation of InsP3R.47,48 In addition, autophagy activity may be an upstream regulator of apoptosis, and excessive autophagy may lead to cell death by apoptosis. The effects of InsP3R activity on autophagy depend on the level of InsP3R activation, which then determines whether the effect will be protective or toxic. While this study did not find an effect on Beclin-1 expression at P7 after exposure to 1 concentration of ISO, further studies are needed to investigate the effects of general anesthetics on cell autophagy, and therefore neuroprotection or neurotoxicity, especially in the developing brain.
While we have not yet discovered the mechanism by which ISO PC prevents ISO-induced apoptosis, other groups have studied ISO PC before cerebral ischemia and have linked several mechanisms to this process. One study found that ISO PC caused a decrease in glutamate receptor activation,49 and another found that it decreased protein aggregation.50 Changes in the expression of various genes have also been discovered, but the significance of these genetic changes has not been fully determined.51–53 Furthermore, ISO may provide PC neuroprotection by causing a moderate increase of cytosolic calcium concentrations via adequate activation of the InsP3R calcium channel.54,55 While we recognize that ISO PC for brain ischemia is not the same as for a subsequent anesthetic exposure, it is possible that there may be similarities in the cascade of events and consequences. One of the limitations of the current study is that we only investigated a few of the potential mechanisms underlying the dual effects of neuroprotection and neurotoxicity caused by ISO. Future experiments could continue to address the many other mechanisms that are likely involved in this process.
The long-term behavioral adaptations to the effects of pre-adolescent drug exposures are more permanent compared to the same exposures later in life,56 with the peak period of anesthetic-induced apoptosis occurring during synaptogenesis.57 This vulnerable period during rat development is between approximately postnatal days 2 and 14 and between post-conception day 153 to postnatal 288 days in the human, based on a species prediction model recently developed to correlate the timing of neural events between species.58 Translating exact developmental milestones between rats and humans is complicated. Postnatal brain maturation in the rat encompasses many developmental events, such as neurogenesis, neuronal migration, synaptogenesis and apoptosis, the extent of which varies greatly depending on the brain region of interest.59,60
The present study tested the hypothesis that PC with ISO can prevent apoptosis caused by a prolonged exposure to ISO, and our results with caspase-3, but not other markers of neuronal injury, support this hypothesis and indicate that in vivo ISO PC is neuroprotective, while a prolonged exposure to ISO is neurotoxic during early postnatal brain development. It is important to determine the optimal concentration and duration range for anesthetic exposures during postnatal brain development, which has implications for our pediatric patients.
Acknowledgments
The authors would like to thank Rebecca Speck, PhD, MPH, Department of Anesthesiology and Critical Care, University of Pennsylvania, for advice and assistance with the statistical analyses.
Funding: Supported by National Institute of General Medicine (NIGMS), NIH (GM-073224, GM084979, GM084979-02S1 to H.W.), Bethesda, Maryland, United States, March of Dimes Birth Defects Foundation Research Grant (#12-FY08-167 to H.W.), White Plains, New York, United States, Research Fund at the Department of Anesthesiology and Critical Care, University of Pennsylvania (to H.W.), Philadelphia, Pennsylvania, United States.
Footnotes
The authors declare no conflicts of interest.
This report was previously presented, in part, at the Society for Neuroscience, 2010. San Diego, CA. Program # 157.6.
DISCLOSURES:
Name: Jun Peng, MD
Contribution: This author helped design the study, conduct the study, and analyze the data.
Attestation: Jun Peng has seen the original study data, reviewed the analysis of the data and approved the final manuscript.
Name: Julie K. Drobish, MD
Contribution: This author helped with the writing of the manuscript.
Attestation: Julie K. Drobish has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Ge Liang, MD
Contribution: This author helped design the study, conduct the study and analyze the data.
Attestation: Ge Liang has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Zhen Wu, MD
Contribution: This author helped conduct the study and analyze the data.
Attestation: Zhen Wu has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Chunxia Liu, MD
Contribution: This author helped conduct the study and analyze the data.
Attestation: Chunxia Liu has seen the original study data, reviewed the analysis of the data and approved the final manuscript
Name: Donald J. Joseph, PhD
Contribution: This author helped conduct the study and analyze the data.
Attestation: Donald J. Joseph reviewed the analysis of the data and approved the final manuscript.
Name: Hossam Abdou, BS
Contribution: This author helped with the writing of the manuscript.
Attestation: Hossam Abdou reviewed the analysis of the data and approved the final manuscript.
Name: Maryellen F. Eckenhoff, PhD
Contribution: This author helped with the writing of the manuscript.
Attestation: Maryellen F. Eckenhoff reviewed the analysis of the data and approved the final manuscript.
Name: Huafeng Wei, MD, PhD
Contribution: This author helped design the study, analyze the data and write the manuscript.
Attestation: Huafeng Wei has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
Contributor Information
Jun Peng, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Julie K. Drobish, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Ge Liang, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Zhen Wu, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Chunxia Liu, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Donald J. Joseph, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Hossam Abdou, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Maryellen F. Eckenhoff, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
Huafeng Wei, Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania.
References
- 1.Liang G, Wang QJ, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei HF. A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg. 2008;106:492–500. doi: 10.1213/ane.0b013e3181605b71. [DOI] [PubMed] [Google Scholar]
- 2.Wei H, Liang G, Yang H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett. 2007;425:59–62. doi: 10.1016/j.neulet.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Q, Liang G, Yang H, Wang S, Eckenhoff MF, Wei H. The common inhaled anesthetic isoflurane increases aggregation of huntingtin and alters calcium homeostasis in a cell model of Huntington's disease. Toxicol Appl Pharmacol. 2011;250:291–298. doi: 10.1016/j.taap.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Liang G, Chen Q, Joseph DJ, Meng Q, Eckenhoff RG, Eckenhoff MF, Wei H. Anesthetic-induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J Pharmacol Exp Ther. 2010;333:14–22. doi: 10.1124/jpet.109.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Xie ZC, Dong YL, Maeda U, Moir RD, Xia WM, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–1254. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culley DJ, Boyd JD, Palanisamy A, Xie Z, Kojima K, Vacanti CA, Tanzi RE, Crosby G. Isoflurane decreases self-renewal capacity of rat cultured neural stem cells. Anesthesiology. 2011;115:754–763. doi: 10.1097/ALN.0b013e318223b78b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng S, Zuo Z. Isoflurane preconditioning reduces purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Z, Wang Y, Huang Y. Isoflurane preconditioning protects human neuroblastoma SH-SY5Y cells against in vitro simulated ischemia-reperfusion through the activation of extracellular signal-regulated kinases pathway. Eur J Pharmacol. 2006;542:84–91. doi: 10.1016/j.ejphar.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Ma D, Williamson P, Januszewski A, Nogaro MC, Hossain M, Ong LP, Shu Y, Franks NP, Maze M. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–753. doi: 10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 11.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–703. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Palanisamy A, Baxter MG, Keel PK, Xie Z, Crosby G, Culley DJ. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114:521–528. doi: 10.1097/ALN.0b013e318209aa71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–1334. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–825. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao YL, Xiang Q, Shi QY, Li SY, Tan L, Wang JT, Jin XG, Luo AL. GABAergic excitotoxicity injury of the immature hippocampal pyramidal neurons' exposure to isoflurane. Anesth Analg. 2011;113:1152–1160. doi: 10.1213/ANE.0b013e318230b3fd. [DOI] [PubMed] [Google Scholar]
- 18.Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane provides long-term protection against focal cerebral ischemia in the rat. Anesthesiology. 2007;106:92–99. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- 19.McAuliffe JJ, Joseph B, Vorhees CV. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia-ischemia. Anesth Analg. 2007;104:1066–1177. doi: 10.1213/01.ane.0000260321.62377.74. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAuliffe JJ, Loepke AW, Miles L, Joseph B, Hughes E, Vorhees CV. Desflurane, isoflurane, and sevoflurane provide limited neuroprotection against neonatal hypoxia-ischemia in a delayed preconditioning paradigm. Anesthesiology. 2009;111:533–546. doi: 10.1097/ALN.0b013e3181b060d3. [DOI] [PubMed] [Google Scholar]
- 22.Michenfelder JD, Sundt TM, Fode N, Sharbrough FW. Isoflurane when compared to enflurane and halothane decreases the frequency of cerebral ischemia during carotid endarterectomy. Anesthesiology. 1987;67:336–340. doi: 10.1097/00000542-198709000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41:1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Liang G, Wang S, Meng Q, Wang Q, Wei H. Effect of fetal exposure to isoflurane on postnatal memory and learning in rats. Neuropharmacology. 2007;53:942–950. doi: 10.1016/j.neuropharm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Peretich K, Zhao Y, Liang G, Meng Q, Wei H. Anesthesia-induced neurodegeneration in fetal rat brains. Pediatr Res. 2009;66:435–440. doi: 10.1203/PDR.0b013e3181b3381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu Y, Patel SM, Pac-Soo C, Fidalgo AR, Wan Y, Maze M, Ma D. Xenon pretreatment attenuates anesthetic-induced apoptosis in the developing brain in comparison with nitrous oxide and hypoxia. Anesthesiology. 2010;113:360–368. doi: 10.1097/ALN.0b013e3181d960d7. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei HF. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–250. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cattano D, Young C, Straiko MM, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–1714. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- 29.Tu S, Wang X, Yang F, Chen B, Wu S, He W, Yuan X, Zhang H, Chen P, Wei G. Propofol induces neuronal apoptosis in infant rat brain under hypoxic conditions. Brain Res Bull. 2011;86:29–35. doi: 10.1016/j.brainresbull.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Cai J, Hu Y, Li W, Li L, Li S, Zhang M, Li Q. The neuroprotective effect of propofol against brain ischemia mediated by the glutamatergic signaling pathway in rats. Neurochem Res. 2011;36:1724–1731. doi: 10.1007/s11064-011-0487-1. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi M, Furuya H, Patel PM. Neuroprotective effects of anesthetic agents. J Anesth. 2005;19:150–156. doi: 10.1007/s00540-005-0305-5. [DOI] [PubMed] [Google Scholar]
- 32.Cata JP, Abdelmalak B, Farag E. Neurological biomarkers in the perioperative period. Br J Anaesth. 2011;107:844–858. doi: 10.1093/bja/aer338. [DOI] [PubMed] [Google Scholar]
- 33.Michetti F, Gazzolo D. S100B protein in biological fluids: a tool for perinatal medicine. Clin Chem. 2002;48:2097–2104. [PubMed] [Google Scholar]
- 34.Bloomfield SM, McKinney J, Smith L, Brisman J. Reliability of S100B in predicting severity of central nervous system injury. Neurocritical Care. 2007;6:121–138. doi: 10.1007/s12028-007-0008-x. [DOI] [PubMed] [Google Scholar]
- 35.Tavarez MM, Atabaki SM, Teach SJ. Acute evaluation of pediatric patients with minor traumatic brain injury. Curr Opin Pediatr. 2012;24:307–313. doi: 10.1097/MOP.0b013e3283531ce6. [DOI] [PubMed] [Google Scholar]
- 36.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 37.Wei H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth Analg. 2011;113:972–974. doi: 10.1213/ANE.0b013e3182323261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei HF, Liang G, Yang H, Wang QJ, Hawkins B, Madesh M, Wang SP, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 39.Kong F, Xu L, He D, Zhang X, Lu H. Effects of gestational isoflurane exposure on postnatal memory and learning in rats. Eur J Pharmacol. 2011;670:168–174. doi: 10.1016/j.ejphar.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 40.Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett. 2004;357:127–130. doi: 10.1016/j.neulet.2003.12.080. [DOI] [PubMed] [Google Scholar]
- 41.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 42.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicencio JM, Ortiz C, Criollo A, Jones AW, Kepp O, Galluzzi L, Joza N, Vitale I, Morselli E, Tailler M, Castedo M, Maiuri MC, Molgo J, Szabadkai G, Lavandero S, Kroemer G. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 2009;16:1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 44.Maiuri MC, Criollo A, Kroemer G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010;29:515–516. doi: 10.1038/emboj.2009.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei K, Wang P, Miao CY. A double-edged sword with therapeutic potential: an updated role of autophagy in ischemic cerebral injury. CNS Neurosci Ther. 2012;18:879–886. doi: 10.1111/cns.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaeger PA, Wyss-Coray T. Beclin 1 complex in autophagy and Alzheimer disease. Arch Neurol. 2010;67:1181–1184. doi: 10.1001/archneurol.2010.258. [DOI] [PubMed] [Google Scholar]
- 47.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 49.Zheng S, Zuo Z. Isoflurane preconditioning decreases glutamate receptor overactivation-induced Purkinje neuronal injury in rat cerebellar slices. Brain Res. 2005;1054:143–151. doi: 10.1016/j.brainres.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 50.Zhang HP, Yuan LB, Zhao RN, Tong L, Ma R, Dong HL, Xiong L. Isoflurane preconditioning induces neuroprotection by attenuating ubiquitin-conjugated protein aggregation in a mouse model of transient global cerebral ischemia. Anesth Analg. 2010;111:506–514. doi: 10.1213/ANE.0b013e3181e45519. [DOI] [PubMed] [Google Scholar]
- 51.Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27:1108–1128. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–970. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- 53.Zhu W, Wang L, Zhang L, Palmateer JM, Libal NL, Hurn PD, Herson PS, Murphy SJ. Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience. 2010;169:758–769. doi: 10.1016/j.neuroscience.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bickler PE, Fahlman CS. The inhaled anesthetic, isoflurane, enhances Ca2+-dependent survival signaling in cortical neurons and modulates MAP kinases, apoptosis proteins and transcription factors during hypoxia. Anesth Analg. 2006;103:419–429. doi: 10.1213/01.ane.0000223671.49376.b2. [DOI] [PubMed] [Google Scholar]
- 55.Gray JJ, Bickler PE, Fahlman CS, Zhan X, Schuyler JA. Isoflurane neuroprotection in hypoxic hippocampal slice cultures involves increases in intracellular Ca2+ and mitogen-activated protein kinases. Anesthesiology. 2005;102:606–615. doi: 10.1097/00000542-200503000-00020. [DOI] [PubMed] [Google Scholar]
- 56.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Slikker W., Jr Strategies and experimental models for evaluating anesthetics: effects on the developing nervous system. Anesth Analg. 2008;106:1643–1658. doi: 10.1213/ane.ob013e3181732c01. [DOI] [PubMed] [Google Scholar]
- 58.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pressler R, Auvin S. Comparison of Brain Maturation among Species: An Example in Translational Research Suggesting the Possible Use of Bumetanide in Newborn. Front Neurol. 2013;4:36. doi: 10.3389/fneur.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]