Abstract
Loss of endothelial barrier function is implicated in the etiology of metastasis, atherosclerosis, sepsis and many other diseases. Studies suggest that sphingosine-1-phosphate (S1P), particularly HDL-bound S1P (HDL–S1P) is essential for endothelial barrier homeostasis and that HDL–S1P may be protective against loss of endothelial barrier function in disease. This review summarizes evidence providing mechanistic insights into how S1P maintains endothelial barrier function, highlighting the recent findings that implicate the major S1P carrier, HDL, in the maintenance of the persistent S1P-signaling needed to maintain endothelial barrier function. We review the mechanisms proposed for HDL maintenance of persistent S1P-signaling, the evidence supporting these mechanisms and the remaining fundamental questions.
Keywords: sphingosine-1-phosphate, endothelial barrier function, HDL, S1P
1. Introduction
S1P is a lysosphingolipid found at highest levels in the blood, particularly in HDL and in red blood cells [2]. S1P is critical for cardiovascular development [4-6], for the regulation of hemodynamics and vascular permeability [9-11] and for the differentiation, trafficking and functions of hematopoietic cells in immunity [13-17]. The effects of S1P are largely attributable to S1P-binding and signaling through the S1P (EDG) family of G protein coupled receptors (i.e., S1P receptors S1PR1-5), though several intracellular receptors are identified [18-22]. The diversity of S1P effects likely reflects differences both in the coupling of S1P receptors to G proteins and downstream signaling pathways and in the expression of S1P receptor family members in tissues [23]. Of relevance to our discussion here is that S1PR1, S1PR2 and S1PR3 are expressed in endothelial cells and together regulate endothelial cell functions in vascular development [12, 24-26], in vascular tone [10, 27] and in vascular permeability [9, 28-30].
Vascular leak of fluid from plasma to interstitial space (i.e., edema) increases in ischemia and inflammation. At this time, no therapeutics sufficiently address the loss of endothelial barrier function, the underlying issue causing vascular leak in cancer [31], atherosclerosis [32, 33], lung injury [34], kidney injury [39], sepsis [40] and other diseases. The current lack of endothelial barrier-promoting therapeutics reflects the historic lack of knowledge of the mechanisms promoting endothelial barrier function.
Recent evidence suggests a central role for S1P-signaling in the maintenance of endothelial barrier function. First, it was demonstrated that the secreted ligands angiopoietin-1 [41-43] and S1P [1, 44, 45] are sufficient to promote endothelial barrier function. Later, angiopoietin-1-promotion of endothelial barrier function was found to be dependent on transactivation of signaling of the endothelial barrier-promoting S1P receptor, S1PR1 [46]. Further evidence demonstrated that S1P signaling through S1PR1 is essential for endothelial barrier function, as is the carrier of S1P on HDL, apolipoprotein M (APOM). For example, mice genetically lacking the sphingosine kinases (both SphK1 and SphK2), S1pr1 or ApoM all display increased vascular leak related to decreased endothelial barrier function [9, 48, 49]. Likewise, antagonism of S1PR1 increases vascular leak in mice [30, 50]. Together, this evidence points to a central role for the SPHK/HDL–S1P/S1PR1-signaling pathway in the maintenance of endothelial barrier function. We therefore review evidence for how S1P maintains endothelial barrier function through the S1PR1-signaling pathway in the sections below. We also highlight recent evidence for the role of plasma S1P carriers in endothelial barrier-promoting S1P-signaling.
1.1 S1P promotes endothelial cell spreading
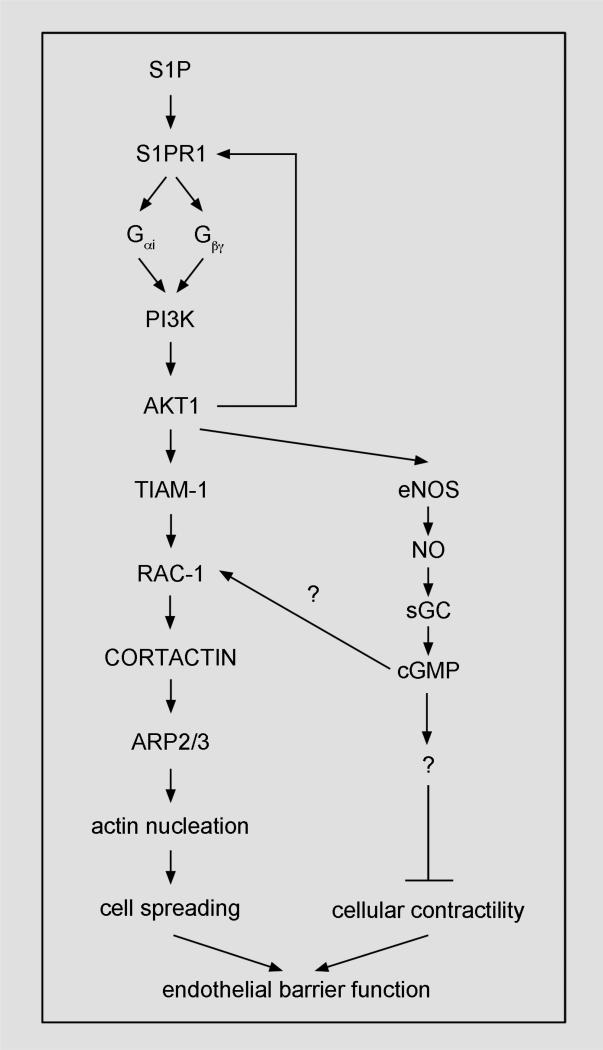
In response to S1P, endothelial barrier function increases partly through filling intercellular gaps by spreading of endothelial cells, and partly thereafter through the stabilization of endothelial cell–cell junctions [9, 51, 52]. Mechanistically, most evidence shows that S1P induces the spreading of endothelial cells through actin polymerization downstream of the S1PR1/PI3K pathway (Fig. 1). We will therefore focus our discussion on the S1PR1/PI3K pathway, though some evidence suggests that S1PR3 can promote PI3K-dependent endothelial barrier function similar to S1PR1 [29] and that S1PR2 can inhibit endothelial barrier function by increasing RHOA-dependent cellular contractility [47, 53, 54].
Figure 1. S1P/S1PR1-signaling.
In response to S1P, the S1PR1 signaling pathway may increase endothelial barrier function by stimulating actin-dependent outward spreading of endothelial cells and suppressing cellular contractility. Arrows, induction; bar-headed lines, inhibition.
To induce actin polymerization-dependent spreading of endothelial cells, S1P first binds the S1PR1 receptor, which recruits G proteins [55-57] to form the active, heterotrimeric complex (i.e., the G protein-coupled receptor; GPCR). We infer in part from mechanistic studies of endothelial cell responses to S1P and in part from other literature on PI3K-signaling that binding of Gβγ subunit or RAS GTPase—two mediators of S1P responses [58-60]—activates PI3K [61, 62]. As a result, membrane PIP3 generated by PI3K activity recruits AKT-1 to the membrane. There, AKT-1 is phosphorylated and is thereby activated [63]. Following activation, AKT-1 activates Rac-1 and eNOS, which may act in concert to induce actin-dependent outward spreading of endothelial cells that increases endothelial barrier function in response to S1P.
To activate Rac-1, AKT-1 activity recruits the guanine exchange factor for Rac-1, TIAM1, to the cell periphery [1, 35, 64]. AKT-1 and TIAM1 bind to S1PR1, and together may form a signaling complex [35, 36]. Following that binding, Rac-1 activity recruits cortactin to the ARP2/3-containing actin-nucleation complex, which consequently starts the polymerization of actin filaments at the cell periphery that distend the membrane and thereby spread endothelial cells [65-70].
PI3K activity and Rac-1 activity are reciprocal. In addition to PI3K activation of Rac-1, Rac-1 activates PI3K to increase endothelial barrier function in response to S1P [64]. Thus, PI3K and Rac-1 form a positive feedback loop that promotes outward spreading of endothelial cells to fill gaps and thereby increases endothelial barrier function in response to S1P.
Increasing evidence suggests that S1P, in addition to promoting actin-dependent endothelial cell spreading, may otherwise promote endothelial cell spreading by suppressing cellular contractility. Evidence in support of this includes that S1P activates RAC-1, which suppresses RHOA-dependent stress fiber formation and transforms cells to an epithelial phenotype [71]. In most studies using endothelial cells, S1P activation of RAC-1 predominates over S1PR2-dependent RHOA-activation [1, 3, 35, 45, 69, 72, 73], though studies using aged endothelial cells and vessels find the reverse [27, 53, 74-77].
Further evidence supporting that S1P suppresses endothelial cell contractility is that S1P maintenance of endothelial barrier function involves nitric oxide, an inhibitor of myosin activity [78-82]. To increase nitric oxide production in response to S1P, active AKT-1 phosphorylates serine 1177 in eNOS [58, 83-89]. In our recent study, inhibition of eNOS abolished maintenance of endothelial barrier function in response to HDL–S1P [90]. Likewise, inhibition of the nitric oxide target, soluble guanylate cyclase (SGC), abolished maintenance of endothelial barrier function in response to HDL–S1P. More work is needed to identify the specific cGMP-dependent mediators of HDL–S1P-induced endothelial barrier function. Of possible relevance in this regard, cGMP both can inhibit nonmuscle myosin activity [82] and can promote RAC-1 activity [91]. We speculate that, S1P through induction of nitric oxide, cGMP and RAC-1 activity, is able to induce the coordinated endothelial barrier-promoting conditions of actin-dependent spreading and of suppression of myosin-mediated cellular contractility.
Likely related to S1P activation of the endothelial barrier-promoting S1PR1-signaling pathway is that S1P triggers the subcellular translocation of several components of this signaling pathway. After S1P stimulation, S1PR1, PI3K, AKT-1 and eNOS are enriched in lipid raft-containing fractions of endothelial cells (Table 1). Also, TIAM1, RAC-1, cortactin and filamentous actin are increased at endothelial cell junctions after S1P stimulation (Table 1). Given their functional relationships in the S1PR1 pathway, the synchrony and colocalization of these subcellular translocations in response to S1P likely reflects the binding and signaling of S1PR1 pathway components. Some subcellular translocation events in response to S1P are explainable based on affinity to active signaling molecules (e.g., the recruitment of G proteins and AKT-1 to active S1PR1, the recruitment of phosphorylated cortactin to the ARP2/3 complex, etc.). The trafficking mechanism(s) mediating the translocation of other S1PR1 pathway components (e.g., the recruitment of S1PR1 to lipid rafts in response to S1P, the recruitment of cortactin to the membrane in response to RAC-1 activation), however, are unclear and need further study.
Table 1.
Translocation of proteins important for endothelial barrier function to endothelial cell–cell junctions and to lipid rafts in response to S1P.
| Junction proteins | Location after S1P stimulation | |
| VE-cadherin | junctions | [1] |
| Claudin-5 | junctions | [3] |
| α/β/γ-catenin | junctions | [1, 7, 8] |
| ZO-1 | junctions | [3, 12] |
| JAM | junctions | [3, 12] |
| PECAM | junctions | [3] |
| α-actinin-1/4 | rafts | [35] |
| S1PR1/PI3K/AKT pathway | Location after S1P stimulation | |
| S1PR1 | rafts, endocytosis | [35-37] |
| PI3K | rafts | [9, 12, 25, 35, 38] |
| AKT-1 | rafts | [35] |
| eNOS | rafts | [35, 36] |
| TIAM1 | junctions | [1] |
| RAC-1 | junctions | [1, 9, 25, 38, 47] |
| Cortactin | junctions | [3] |
| Actin | junctions | [1, 9, 10, 54] |
1.2 S1P promotes formation of endothelial cell–cell junctions
After promoting actin-dependent endothelial cell spreading, S1P sustains the endothelial barrier by stabilizing endothelial cell–cell junctions [52]. The basis for S1P stabilization of endothelial cell–cell adhesion and endothelial barrier function is increased homotypic binding of VE-cadherin and of claudin-5 in endothelial cell–cell junctions [92, 93]. Evidence shows that S1P induces the translocation of VE-cadherin, claudin-5 and several other junction proteins to the cell periphery (Table 1; [1, 3, 35, 52, 94-96]).
Several pieces of evidence imply that the actin-dependent endothelial cell spreading in response to S1P and the stabilization of endothelial cell–cell junctions in response to S1P are related and are interdependent. First, actin-dependent endothelial cell spreading leads to new endothelial cell–cell contacts, which, in turn, can lead to new junction formation [52]. Second, both the junction protein translocation in response to S1P and the actin dynamics in response to S1P require the activities of RAC-1 and RHOA [1]. Endothelial barrier-mediating junction proteins like VE-cadherin are bound to the cortical actin cytoskeleton by catenin and actinin proteins [97]. Furthermore, the trafficking of VE-cadherin is actin-dependent [98]. Third, the formation of VE-cadherin complexes stimulates actin dynamics within the cell periphery [99, 100]. Based on evidence for the interdependence of actin-dependent endothelial cell spreading and stabilization of endothelial cell–cell junctions in response to S1P, the concept of a “stabilizing loop” for strengthening the endothelial barrier was proposed by Spindler et al. [101] and we subscribe to this concept.
2. The roles of plasma S1P carriers in plasma S1P homeostasis and related endothelial barrier-promoting S1P-signaling
Most cells can synthesize S1P, but two pieces of evidence suggest that tight regulation of plasma S1P levels is needed to maintain vascular functions related to S1P-signaling. First, S1P is more concentrated in plasma (200-900 nM) and in lymph (~90 nM) than in tissues (<10 nM) [102]. Accordingly, the S1pr1 receptor is enriched on the apical surface of endothelial cells adjacent to the high concentrations of S1P in plasma [103]. Second, the gradient of S1P concentration in plasma>lymph>tissue is essential for maintenance of endothelial barrier function [9] and other S1P-dependent processes such as leukocyte trafficking [104, 105].
Mechanistically, the maintenance of the plasma>lymph>tissue S1P concentration gradient involves at least three modes of constant S1P flux: 1) S1P generation by SPHK activity [4], 2) S1P degradation by S1P lyase and S1P phosphatases [106-109] and 3) cellular uptake, storage and release of S1P [110-112]. The evidence for each of these modes of S1P flux is already reviewed in detail [102, 113-119]. Below, we review evidence that suggests roles for S1P carriers in S1P stability in vivo, in the release of S1P stored in red blood cells and in influencing the duration of S1P-signaling responses in endothelial cells. As growing evidence suggests that modulating S1P levels could have therapeutic benefit, determining the relative activities of each carrier in S1P flux and in S1P-signaling deserves more study.
2.1 The role of carriers in S1P stability
Recent evidence suggests a role for plasma S1P carriers in S1P stability in vivo. In plasma, S1P is bound to both serum albumin and HDL, though only HDL–S1P is sufficient for maintenance of endothelial barrier function [49]. Also, most plasma S1P is bound to HDL [2]. Recent findings suggest that the basis for the ‘preference’ of S1P for HDL in plasma, rather than the more abundant carrier, serum albumin, may be greater stability of the S1P bound to HDL. In support of this, S1P clearance from culture medium is lower in HepG2 cells overexpressing APOM than in control HepG2 cells [120]. Similarly, increased stability of S1P is reported in the presence of elevated APOM in vivo [121]. Also corroborating this is that plasma S1P is increased in mice overexpressing APOM and decreased in mice lacking ApoM [49]. Thus, S1P bound to HDL may be better protected from degradation than S1P bound to serum albumin.
The basis for the role of HDL in S1P stability in vivo remains unclear, but findings from two studies point to a role for APOM in the “remodeling” of HDL particles, whereby the stability of HDL [38], and, inferably, the stability of S1P bound to HDL, is regulated through the transfer and the metabolism of lipids on HDL by LCAT, PLTP, CETP and lipases [38]. More specifically, LCAT activity facilitates the maturation of nascent HDL (i.e., pre-β-HDL, β-HDL, pre-α-HDL and α-HDL, in that order [38]) whereas PLTP, CETP and hepatic lipase counteract this maturation, deconstructing HDL through either catabolism of lipids in HDL or transfer of lipids from HDL to triglyceride-rich lipoproteins.
While some findings of the two studies using mice overexpressing human APOM are similar (e.g., increases in plasma levels of cholesterol and phospholipids and an altered composition of HDL), these studies differ notably in their conclusions about the mechanism of APOM influence on HDL remodeling. The conclusion of the first study is that APOM promotes PLTP-dependent HDL remodeling [122], an important factor for HDL stability in vivo [123], whereas the conclusion of the second study is that APOM increases LCAT function in HDL [121], another important factor to HDL stability in vivo [124]. The conclusion of the first study is supported by two key observations. First, the activity of Pltp measured in vitro in plasma samples corresponds to the level of APOM function while hepatic lipase activity and Lcat activity in plasma were unaffected by either overexpression of APOM or deletion of ApoM. Second, a type of HDL remodeling that can reflect either PLTP activity [125] or LCAT activity [126, 127]— the conversion of α-HDL to pre- β-HDL (i.e., in plasma in vitro at 37 °C)—is increased in mice overexpressing APOM, whereas another type of HDL remodeling that corresponds to Lcat activity [127]—the conversion of α-HDL to pre-βa-HDL (i.e., in plasma in vitro at 37 °C) —is unaffected in plasma from mice overexpressing APOM. Accordingly, Christoffersen et al. conclude that APOM promotes PLTP-dependent HDL remodeling. Considering that the catabolism of HDL is increased 2-3-fold in mice lacking Pltp whereas the production of HDL was unaffected [123] the conclusion of the first study suggests that the mechanism of HDL stabilization of S1P in vivo involves APOM-dependent PLTP activity. Corroborating this suggestion, a recent study reports that S1P is reduced in plasma and in HDL from mice lacking Pltp [128]. Thus, APOM may augment HDL stability—augmenting the stability of S1P bound to HDL, in turn—by somehow augmenting PLTP activity.
The conclusion of the second study is that that APOM increases LCAT function in HDL [121]. This conclusion is supported by three key observations. First, the level of Lcat protein in HDL is increased in mice overexpressing APOM. Second, the level of esterified cholesterol (i.e., the product of Lcat activity) in HDL is increased in mice overexpressing APOM. Third, consistent with the role of LCAT in HDL maturation [38], HDL size is increased in mice overexpressing APOM. Accordingly, Liu et al. conclude that increased plasma S1P and altered HDL structure observed in mice overexpressing APOM relates to increased LCAT activity in HDL. Similarly, another study showed reduced plasma S1P in patients having LCAT mutations [129]. Thus, APOM may augment HDL size and stability—augmenting the stability of S1P bound to HDL, in turn—by somehow recruiting LCAT to HDL.
Thus, these studies are expressly contentious regarding the role of APOM in LCAT activity. This contention likely reflects differences in experimental design. For example, the pattern of APOM expression is likely a major variable: Liu et al. use a hepatocyte-specific promoter to drive APOM expression, whereas the APOM transgene in the mouse used by Christoffersen et al. included the APOM promoter. Additionally, the studies examine vastly different indicators of HDL remodeling: Christoffersen et al. focused their analysis of HDL remodeling on relevant activity assays in plasma specimens, whereas Liu et al. focused on the relevant protein and lipid composition of HDL. The role of APOM in HDL remodeling by LCAT might be reconciled through a study of S1P stability in mice or patients having loss of function in LCAT. Further investigation of the mechanisms underlying APOM functions in the enhancement of PLTP activity and in the recruitment of LCAT to HDL is also needed.
2.2 The role of carriers in S1P release from red blood cells
Some evidence suggests a role for S1P carriers in the release of S1P stored in blood cells, an activity that may be important for plasma S1P homeostasis. Early studies showed that HDL and serum albumin promote the release of S1P from isolated platelets [130-132], which could suggest that carrier is required for the solubility of released S1P. A later study showed that plasma S1P levels more than doubled after co-incubation of plasma with red blood cells in vitro [8], demonstrating not only that red blood cells are the chief store of S1P in blood but also that S1P transfers readily from red blood cells to plasma. Although isolated red blood cells produce little or no S1P [8, 133], a collectively significant role for red blood cells in the production of plasma S1P is possible given they are the major cell type in blood. But this notion is controversial: while multiple studies find plasma S1P is associated with red blood cell numbers [8, 134-137], some studies find no such association [138-140]. Nevertheless, based on these demonstrations many hold that red blood cells produce, release and take up S1P to buffer against fluctuations in plasma S1P and thereby maintain plasma S1P homeostasis. Considering that plasma S1P levels are tightly regulated but that so little is known about the factors regulating plasma S1P levels and flux, the disparate findings of these studies are unsurprising and likely reflect differences in methods (e.g., indicators of red blood cell numbers, strategies to modulate red blood cell numbers) and models (e.g., mice, patient groups). The replication of these experiments using consistent methods and a uniform model might both clarify the role of red blood cells in plasma S1P homeostasis and identify the idiosyncratic factors of these studies that regulate S1P release from red blood cells.
The need to study the relative importance of S1P release from red blood cells in plasma S1P homeostasis is emphasized by the connection between plasma S1P homeostasis and endothelial barrier homeostasis [9]. However the lack of knowledge of the underlying mechanism of S1P release from red blood cells hampers such a study. Currently, two studies provide insights into the mechanism of S1P release from red blood cells. The first study shows that S1P release from red blood cells is dependent on ATP and is sensitive to glyburide [141], which is consistent with the involvement of the transporter ABCA1 in S1P export from red blood cells. The notion of ABCA1 activity promoting plasma S1P homeostasis, however, remains controversial. In this regard, contrary findings show that while patients having ABCA1 mutations have reduced plasma S1P [129], mice lacking Abca1 do not have significant reductions in plasma S1P [142]. Thus, more work is needed to clarify the mechanism of S1P export from red blood cells and the implications of this mechanism for plasma homeostasis and endothelial barrier homeostasis.
The second study providing insight into the mechanism of S1P release from red blood cells shows that both serum albumin and HDL speed S1P release from red blood cells in vitro [135]. Moreover, S1P released to red blood cell-conditioned media in response to addition of serum albumin can reduce the permeability of isolated microvessels [143], demonstrating that carrier-dependent S1P release from red blood cells can increase endothelial barrier function. Considering the evidence that carriers trigger S1P release from red blood cells, it is possible that the correlation between plasma S1P levels and APOM function [49, 129] not only relates to the influence of APOM on S1P stability (i.e., through effects of APOM on HDL remodeling) but also relates to the role of APOM in S1P release from red blood cells. Mechanistically, Bode et al. suggested that the affinity of the carriers for S1P could be the primary basis for S1P release from red blood cells [i.e., the binding affinity of S1P for its carriers extracts S1P from red blood cells; [135]]. Indirectly supporting this mechanism is that an S1P antibody can mimic the effects of carriers on S1P release from red blood cells [135], but this suggestion has not been tested using carriers having disrupted S1P-binding sites (e.g., HDL from Apom null mice). Further study of the mechanistic basis for carrier-dependent S1P release from red blood cells might reveal ways to augment plasma S1P.
2.3 The role of S1P carriers in influencing the duration of S1P-signaling responses in endothelial cells
Evidence suggests that S1P carriers variably influence the duration of S1P-signaling responses in endothelial cells. In early studies, it was shown that HDL–S1P promotes longer duration of eNOS activation and longer duration of maintenance of endothelial barrier function than albumin–S1P [144, 145], but these studies did not compare the effects of equivalent doses of albumin–S1P and HDL–S1P. Since, two studies provide further evidence that HDL–S1P and albumin–S1P differ in their activity. In the first recent study, Christoffersen and colleagues showed that mice lacking ApoM have undetectable HDL–S1P and have increased basal vascular leak [49]. By inference, the remaining plasma albumin–S1P in mice lacking ApoM (54% of wildtype plasma S1P levels) was insufficient to sustain endothelial barrier function, suggesting that HDL–S1P has greater endothelial barrier function-sustaining activity than albumin–S1P.
In the second recent study, we showed that HDL–S1P indeed sustains endothelial barrier function and AKT/eNOS activation for a longer duration than albumin–S1P in cultured endothelial cells when HDL–S1P and albumin–S1P are used at equivalent doses [90]. In full accordance with the inference that HDL–S1P is essential while albumin–S1P is non-essential for endothelial barrier function in vivo, the presence of albumin–S1P did not impact the sustained endothelial barrier response to HDL–S1P in vitro [90]. Yet, as much as 45% of plasma S1P is bound to serum albumin [2, 146] and the addition of albumin–S1P increases endothelial barrier function rapidly and transiently in isolated endothelial cells [1, 44, 45, 90]. These results raise questions as to the relative importance of serum albumin-bound S1P (albumin–S1P) to endothelial barrier function, particularly in light of the results from the other recent study showing that mice lacking HDL–S1P display increased vascular leak in their lungs at baseline despite the presence of levels of albumin–S1P sufficient to elicit endothelial barrier-promoting responses [100-400 nM; [49]] Taken together, this evidence suggests that the role of albumin–S1P in endothelial barrier function is secondary to that of HDL–S1P. The role of albumin–S1P in endothelial barrier function might be clarified by monitoring vascular leak after modulating serum albumin levels or otherwise disrupting the binding of S1P to albumin.
The longer duration of endothelial responses to HDL–S1P than to albumin–S1P carries many implications for experimental and therapeutic applications of S1P. But the basis for the longer duration of responses to HDL–S1P than albumin–S1P is not understood. We have considered several possibilities for mechanisms:
2.3.1 Differences in carrier clearance
Clearance rates of serum albumin and HDL differ in vivo [147, 148] and differences in clearance of extracellular HDL–S1P and albumin–S1P might variably limit S1P responses. Though recent findings suggest that albumin–S1P turnover is greater than HDL–S1P in vivo [120], we found no significant difference in the clearance of albumin–S1P and HDL–S1P from culture medium by endothelial cells [90]. Thus, the longer duration of endothelial responses to HDL–S1P than to albumin–S1P seen in isolated endothelial cells are not explained by differences in clearance of extracellular HDL–S1P and albumin–S1P.
2.3.2 Differences in carrier recruitment to the cell surface
HDL binding to HDL receptors recruits HDL to the plasma membrane [149, 150]. Others therefore proposed that HDL recruitment to the plasma membrane may speed S1P transfer from HDL to S1P receptors [10]. In support of this idea is that two HDL receptors—endothelial lipase and scavenger receptor-BI—mediate eNOS activation in response to HDL–S1P in endothelial cells [144, 151]. Evidence is needed to prove that these HDL receptors are indeed in proximity to S1P receptors and that they speed responses to HDL–S1P relative to albumin–S1P. To the contrary, multiple studies have examined whether the speed of acute signaling and acute endothelial barrier responses differ for HDL–S1P and albumin–S1P, but no differences were reported [49, 90, 144, 145].
2.3.3 Differences in carrier binding and release of S1P
Many hold that HDL and serum albumin bind and sequester plasma S1P and that the plasma carriers of S1P thereby prevent S1P receptor activation and maintain the apical surface localization of S1PR1. N. Murata and colleagues inferred this theory based on the finding that S1P-signaling responses are reduced when measured in the presence of serum [2] and that HDL and serum albumin bind S1P [2, 130]. Murata et al. point out that this theory is only one possibility, however, leaving open the possibility that, rather than by the sequestration of S1P by carriers, some unrecognized serum component might otherwise inhibit S1P-signaling. Notwithstanding that alternative possibility, this theory can explain the seeming paradox that is raised by evidence that: 1) S1pr1 (mouse) is present on the apical endothelial surface in vivo [103], 2) S1P is higher in plasma [2] than the Kd for the binding of S1P to S1P receptors [152-160] and 3) S1P triggers internalization of S1PR1 [37]. If differences in carrier affinity for S1P exist, then carriers could variably sequester S1P and limit S1P responses.
Two pieces of evidence are contrary to the theory that plasma carriers prevent S1P receptor activation by plasma S1P. First, rather than preventing S1P receptor activation or variably activating endothelial cell responses, albumin–S1P and HDL–S1P activate both endothelial barrier function and AKT/eNOS-signaling to similar degrees [49, 145]. This was the case even at concentrations near the kD values determined for albumin–S1P binding to S1PR1 ([8.1 nM), [152, 153]] and the EC50 for albumin–S1P activation of eNOS ([30 nM), [86]], suggesting that both albumin–S1P and HDL–S1P are potent, even at concentrations far below their physiological levels in plasma. Second, rather than finding that S1pr1 in endothelial cells is largely inactive, R. Proia and colleagues demonstrated using a novel genetic reporter for S1pr1 activity that S1pr1 is signaling in many endothelial cells in vivo in mice [161]. Considering the evidence that is to the contrary, we suggest the theory that the plasma carriers of S1P prevent S1P receptor activation by plasma S1P has insufficient supporting evidence and that variability in the sequestration of S1P by the carriers is not likely a factor in the longer duration of endothelial responses to HDL–S1P than to albumin–S1P. To test whether there is any basis for this theory, the relative binding affinities of S1P for serum albumin and APOM, as well as the rates for association and disassociation of HDL–S1P and albumin–S1P in the presence of S1P receptors, should be determined in like assays.
2.3.4 Inequitable S1P transfer between carriers
S1P transfer between albumin and HDL has been demonstrated [135]. Theoretically, S1P transfer to HDL may limit albumin–S1P responses, or vice versa. Accordingly, if S1P transfer limits responses, the presence of excess albumin or albumin–S1P might blunt or augment, respectively, the endothelial cell responsiveness to HDL–S1P. Yet, the sustained endothelial barrier response to HDL–S1P was affected by neither albumin–S1P nor S1P-free albumin [90]. While we do not question that S1P can transfer between carriers, our findings do not support the theory that S1P transfer to HDL limits albumin–S1P responses or vice versa.
2.3.5 HDL effects on cholesterol-enriched lipid raft microdomains
HDL mediates cholesterol uptake (i.e., reverse cholesterol transport) from cells [162], which may involve the S1P carrier on HDL, ApoM [163]. Because S1PR1-signaling requires cholesterol-enriched lipid raft microdomains [35, 164], HDL-mediated cholesterol uptake might be expected to limit S1PR1-signaling. To the contrary, S1PR1-signaling is more persistent in the presence of HDL–S1P than albumin–S1P [90]. Accordingly, we conclude that the cholesterol uptake activity of HDL is not likely promoting persistent S1PR1-signaling in response to HDL–S1P.
Most evidence shows that HDL mediates cholesterol uptake from endothelial cells, but some findings raise the possibility that HDL might also transfer cholesterol to endothelial cells. Specifically, isolated endothelial cells take up labeled HDL [165], which likely involves scavenger receptor-BI-mediated endocytosis [166, 167]. While cholesterol transfer from HDL to endothelial cells has not, to our knowledge, been explicitly demonstrated, such a mechanism might expand cholesterol-enriched lipid raft microdomains and thereby augment the signaling of S1PR1.
2.3.6 Carrier influence on S1PR1 desensitization
After S1P stimulation, S1P-signaling is attenuated by endocytosis and proteolysis of S1P receptors [37, 168]. Differences in S1PR1 internalization and proteolysis in response to HDL–S1P and albumin–S1P could therefore variably limit S1P responses. More studies are needed to determine if there are such differences. Both HDL–S1P and albumin–S1P cause internalization of S1PR1 by 15 minutes. Loss of S1PR1 six hours after HDL–S1P and albumin–S1P stimulation is abolished by proteasomal inhibition [90]. The continuous observation, however, of S1PR1 endocytosis using live cell imaging might reveal carrier-specific differences in S1PR1 trafficking.
2.3.7 Carrier influence on S1PR1 recycling
The persistence of GPCR signaling responses to agonism are governed partly by the efficiency of ‘desensitization’ through receptor internalization and proteolysis and partly by the efficiency of ‘re-sensitization’ through the recycling of receptors to the cell surface [169, 170]. To examine the possibility that the longer duration of endothelial responses to HDL–S1P than to albumin–S1P relate to increased S1PR1 recycling in response to HDL–S1P, we examined S1PR1 protein dynamics after HDL–S1P and albumin–S1P treatment. Consistent with the possibility that HDL–S1P promotes greater S1PR1 recycling than albumin–S1P is that, following HDL–S1P treatment, S1PR1 protein increases in total lysate, in fractions of surface protein and in fractions containing lipid rafts, the site of actively signaling S1PR1 [35, 86]. Furthermore, inhibition of GPCR recycling using monensin abolishes endothelial barrier function sustained in response to HDL–S1P; monensin shortens the HDL–S1P response to a duration similar to that of albumin–S1P. Accordingly, we reason that HDL–S1P promotes S1PR1 recycling and thereby stabilizes S1PR1 levels on the cell surface to prolong S1PR1 signaling versus albumin–S1P. Indeed, HDL–S1P elicits a longer duration of activation of both AKT and eNOS than did albumin–S1P. Moreover, the sustained endothelial barrier function response to HDL–S1P was abolished by HDL–S1P washout or by inhibition of the S1PR1/PI3K/AKT/eNOS/SGC pathway. These findings together imply that greater S1PR1 recycling to the cell surface in response to HDL–S1P lengthens endothelial responses to HDL–S1P versus albumin–S1P. This implication might be confirmed using photoconvertible tags to trace recycled pools of tagged S1PR1 in live cells after HDL– S1P versus albumin–S1P.
2.4 S1PR1 recycling
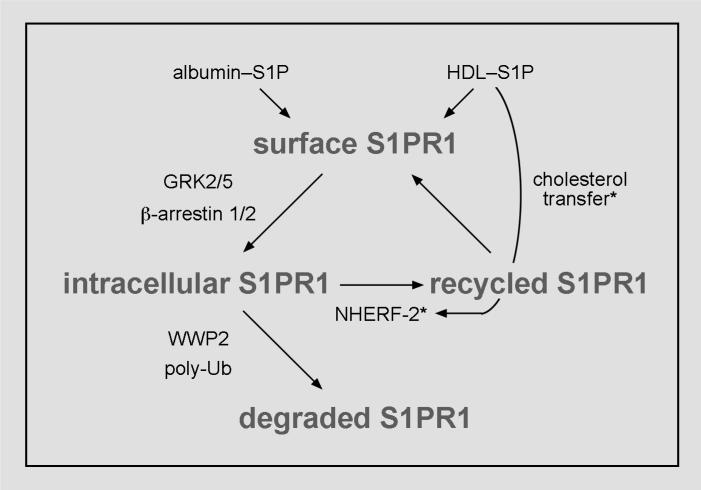
Evidence indicating that endothelial barrier maintenance in response to HDL–S1P involves increased S1PR1 recycling to the cell surface highlights the need for more mechanistic studies of S1PR1 recycling. But little is known about S1P receptor recycling to the cell surface. So far, N-glycosylation of S1PR1 in the N-terminal extracellular domain [171, 172], protein kinase C activity [173] and HDL [90] are implicated in S1PR1 recycling, but their respective roles in the mechanism of recycling of S1PR1 are unclear. The role of these factors in endocytic sorting of S1PR1 for recycling to the cell surface should therefore be examined. We speculate that HDL and S1PR1 traffic together in endocytic vesicles and that once there, HDL recruits factors that target S1PR1 to recycling endosomes (Fig. 2) through a mechanism that may involve cholesterol transfer from HDL to the endosomal membrane.
Figure 2. Hypothetical mechanism for greater S1PR1 recycling in response to HDL–S1P.
Following activation of S1PR1 by albumin–S1P or HDL–S1P, S1PR1 is targeted for internalization and degradation by the proteasome. We speculate that HDL recruits factors such as NHERF-2 to the S1PR1 complex, thereby targeting S1PR1 to recycling endosomes. HDL might induce recruitment of such factors in S1PR1 recycling through its lipid transport activity. To contrast the mechanistic insights supported by experimental evidence with our speculations, we identify the components of our hypothetical mechanism by asterisks.
Based on the consensus of recycling mechanisms determined for other GPCRs, the binding of S1PR1 to PDZ domain-containing proteins is likely important for sorting of S1PR1 to recycling endosomes [170]. S1PR1 itself contains neither a consensus PDZ domain nor a PDZ-binding site, but S1PR1 binds both to TRIP6, a PDZ domain-binding protein [174] and to AKT-1, which contains a PDZ domain [36]. Further suggesting a role for TRIP6 in S1PR1 recycling is that TRIP6 complexes with the PDZ domain-containing protein NHERF-2, an established mediator of sorting of GPCRs to recycling endosomes [170]. Thus, it is inferable that S1PR1 recycling to the cell surface occurs through a known paradigm for GPCR recycling, but this hypothesis needs testing.
3. Implications and future directions
Despite growing evidence that S1P promotes endothelial barrier function, studies have not addressed what the role is for endothelial S1P-signaling in endothelial barrier dysfunction during pathological processes such as wound healing, heart failure, metastasis, microbial infections and atherosclerosis. Whether the loss of endothelial barrier function in disease relates to perturbations in SPHK/HDL–S1P/S1PR1-signaling needs more research. In this regard, we recently reported that S1P levels in an HDL-containing fraction of plasma are reduced in patients having ischemic heart disease [175]. Similarly, others showed that vessel sites susceptible to atherosclerosis have disrupted expression patterns of the endothelial barrier-promoting receptor, S1PR1, and lower levels of active eNOS [48] while the endothelial barrier-inhibiting receptor, S1PR2, is increased in vessels after aging, diabetes and heart failure [27, 53, 74-77]. Furthermore, eNOS activity is decreased in atherosclerosis and also is decreased coincident with multiple risk factors for atherosclerosis: dyslipidemia, hypertension, diabetes, smoking [176-180]. Such decreases in S1P, S1PR1 and eNOS and increases in S1PR2 in disease might diminish endothelial barrier-promoting responses to S1P. Findings from two recent studies suggest that S1P-signaling is also disrupted in two settings where microbial infections evoke systemic inflammatory responses leading to endothelial barrier dysfunction. The first study demonstrates that S1PR1 agonism increases survival in influenza virus-infected by suppressing the secretion of inflammatory cytokines by endothelial cells [181]. The second study demonstrates that APOM is decreased in patients having sepsis [182]. These findings suggest that the replacement of S1P or otherwise augmenting S1PR1-signaling may have therapeutic value.
Highlighting the potential therapeutic value of promoting endothelial barrier function using S1P is that S1P infusion can decrease vascular leak and associated inflammatory injury in dogs after acute lung injury [40]. Thus, an important implication of recent evidence showing that HDL–S1P activates persistent S1PR1-signaling is that S1P delivery on HDL might confer greater therapeutic benefit in diseases related to loss of endothelial barrier function. Along similar lines, analytical tools for measuring HDL–S1P/S1PR1-signaling (e.g., plasma HDL–S1P levels) might be of value for diagnosis and management of diseases related to loss of endothelial barrier function. These implications emphasize the need for further study of the mechanisms whereby HDL augments the stability of S1P in vivo, the release of S1P stored in red blood cells to plasma and the duration of S1P-signaling responses in endothelial cells.
The usefulness of current S1P mimetics such as Fingolimod for promoting endothelial barrier function may be limited due to their strong immunosuppressive effects and their effects leading to the degradation of S1PR1 [50, 183-185]. Indeed, the side effects noted after Fingolimod treatment—pulmonary edema, pulmonary infection and retinal leak [50]—are consistent with the effects of Fingolimod in disrupting endothelial barrier function after long-term dosage in mice [184]. Targeting S1P synthesis and catabolism also results in both immune and vascular defects and therefore does not present an obvious advantage over Fingolimod. Alternate approaches to promoting endothelial barrier function using S1P meriting more investigation include raising HDL–S1P by promoting favorable HDL remodeling (e.g., by increasing PLTP or LCAT activity), by transfusion of blood or blood products and by the infusion of HDL–S1P mimetics. The prospect of HDL–S1P mimetics seems promising following the resolution of structures for both APOM [49] and S1PR1 [186]. Augmenting the activity of HDL– S1P specifically might have greater selectivity for endothelium than the immune system— promoting endothelial barrier function without causing immunosuppression.
HIGHLIGHTS.
HDL-bound S1P promotes endothelial barrier function.
HDL can influence the mobility, stability and endothelial signaling of plasma S1P.
Mechanisms explaining the influence of HDL on S1P levels and function are examined.
Recommendations on how to resolve these mechanisms are made.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grants HL109829, HL094883 and HL080404 (K. M. A.). B.A.W. was supported by National Institutes of Health Training Grant HL007260 and by a fellowship from the American Heart Association (Grant 10PRE3910006).
Abbreviations
- S1P
sphingosine-1-phosphate
- HDL–S1P
HDL-bound S1P
- albumin–S1P
albumin-bound S1P
- AKT
protein kinase B
- SPHK
sphingosine kinase
- PI3K
phosphatidylinositol 3-kinase
- S1PR1-3
S1P receptor 1-3
- RAC-1
ras-related C3 botulinum toxin substrate 1
- SGC
soluble guanylate cyclase
- TIAM1
T-cell lymphoma invasion and metastasis 1
- APOM
apolipoprotein M
- TRIP6
TRIP6 thyroid hormone receptor interactor 6
- NHERF-2
Na+/H+ exchanger regulatory factor-2
- ARP
actin-related protein
- GPCR
G protein-coupled receptor
- RHOA
ras homolog family member A
- VE-cadherin
vascular endothelial cadherin/cadherin 5
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 2.Murata N, Sato K, Kon J, Tomura H, Yanagita M, Kuwabara A, Ui M, Okajima F. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352(Pt 3):809–815. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, Wang E, Lee MJ. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem. 2006;281:29190–29200. doi: 10.1074/jbc.M604310200. [DOI] [PubMed] [Google Scholar]
- 4.Mizugishi K, Li C, Olivera A, Bielawski J, Bielawska A, Deng CX, Proia RL. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest. 2007;117:2993–3006. doi: 10.1172/JCI30674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulsen RR, McClaskey CM, Rivkees SA, Wendler CC. The Sphingosine-1-phospate receptor 1 mediates S1P action during cardiac development. BMC developmental biology. 2011;11:37. doi: 10.1186/1471-213X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Means CK, Brown JH. Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc Res. 2009;82:193–200. doi: 10.1093/cvr/cvp086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–1209. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 9.Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest. 2009;119:1871–1879. doi: 10.1172/JCI38575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, Schafers M, Fobker M, Zidek W, Assmann G, Chun J, Levkau B. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J Clin Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 12.Inoki I, Takuwa N, Sugimoto N, Yoshioka K, Takata S, Kaneko S, Takuwa Y. Negative regulation of endothelial morphogenesis and angiogenesis by S1P2 receptor. Biochem Biophys Res Commun. 2006;346:293–300. doi: 10.1016/j.bbrc.2006.05.119. [DOI] [PubMed] [Google Scholar]
- 13.Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, Jacques Y, Baratin M, Tomasello E, Vivier E. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nature immunology. 2007;8:1337–1344. doi: 10.1038/ni1523. [DOI] [PubMed] [Google Scholar]
- 14.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P4) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. FASEB J. 2005;19:1731–1733. doi: 10.1096/fj.05-3730fje. [DOI] [PubMed] [Google Scholar]
- 16.Du W, Takuwa N, Yoshioka K, Okamoto Y, Gonda K, Sugihara K, Fukamizu A, Asano M, Takuwa Y. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010;70:772–781. doi: 10.1158/0008-5472.CAN-09-2722. [DOI] [PubMed] [Google Scholar]
- 17.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 18.Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Futerman AH. Characterization of Ceramide Synthase 2. Journal of Biological Chemistry. 2008;283:5677–5684. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- 19.Xu SZ, Muraki K, Zeng F, Li J, Sukumar P, Shah S, Dedman AM, Flemming PK, McHugh D, Naylor J, Cheong A, Bateson AN, Munsch CM, Porter KE, Beech DJ. A sphingosine-1-phosphate-activated calcium channel controlling vascular smooth muscle cell motility. Circ Res. 2006;98:1381–1389. doi: 10.1161/01.RES.0000225284.36490.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strub GM, Paillard M, Liang J, Gomez L, Allegood JC, Hait NC, Maceyka M, Price MM, Chen Q, Simpson DC, Kordula T, Milstien S, Lesnefsky EJ, Spiegel S. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 2011;25:600–612. doi: 10.1096/fj.10-167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. Sphingosine 1-Phosphate Receptor Signaling. Annual Review of Biochemistry. 2009;78:743–768. doi: 10.1146/annurev.biochem.78.072407.103733. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Watanabe T, Sato K, Kon J, Tomura H, Tamama K, Kuwabara A, Kanda T, Kobayashi I, Ohta H, Ui M, Okajima F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem J. 2000;348(Pt 1):71–76. [PMC free article] [PubMed] [Google Scholar]
- 25.Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- 26.Paik JH, Chae S, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J Biol Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- 27.Hoefer J, Azam MA, Kroetsch JT, Leong-Poi H, Momen MA, Voigtlaender-Bolz J, Scherer EQ, Meissner A, Bolz SS, Husain M. Sphingosine-1-phosphate-dependent activation of p38 MAPK maintains elevated peripheral resistance in heart failure through increased myogenic vasoconstriction. Circ Res. 2010;107:923–933. doi: 10.1161/CIRCRESAHA.110.226464. [DOI] [PubMed] [Google Scholar]
- 28.Sammani S, Moreno-Vinasco L, Mirzapoiazova T, Singleton PA, Chiang ET, Evenoski CL, Wang T, Mathew B, Husain A, Moitra J, Sun X, Nunez L, Jacobson JR, Dudek SM, Natarajan V, Garcia JG. Differential effects of sphingosine 1-phosphate receptors on airway and vascular barrier function in the murine lung. Am J Respir Cell Mol Biol. 2010;43:394–402. doi: 10.1165/rcmb.2009-0223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singleton PA, Moreno-Vinasco L, Sammani S, Wanderling SL, Moss J, Garcia JG. Attenuation of vascular permeability by methylnaltrexone: role of mOP-R and S1P3 transactivation. Am J Respir Cell Mol Biol. 2007;37:222–231. doi: 10.1165/rcmb.2006-0327OC. [DOI] [PubMed] [Google Scholar]
- 30.Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, Cahalan MD, Wong CH, Rosen H. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- 31.Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGill HC, Jr., Geer JC, Holman RL. Sites of vascular vulnerability in dogs demonstrated by Evans blue. AMA Arch Pathol. 1957;64:303–311. [PubMed] [Google Scholar]
- 33.Sun C, Wu MH, Yuan SY. Nonmuscle Myosin Light-Chain Kinase Deficiency Attenuates Atherosclerosis in Apolipoprotein E-Deficient Mice via Reduced Endothelial Barrier Dysfunction and Monocyte Migration. Circulation. 2011;124:48–57. doi: 10.1161/CIRCULATIONAHA.110.988915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 35.Singleton PA, Dudek SM, Chiang ET, Garcia JGN. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and Œ±-actinin. The FASEB Journal. 2005;19:1646–1656. doi: 10.1096/fj.05-3928com. [DOI] [PubMed] [Google Scholar]
- 36.Lee MJ, Thangada S, Paik JH, Sapkota GP, Ancellin N, Chae SS, Wu M, Morales-Ruiz M, Sessa WC, Alessi DR, Hla T. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8:693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 37.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis GF, Rader DJ. New Insights Into the Regulation of HDL Metabolism and Reverse Cholesterol Transport. Circulation Research. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 39.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of Clinical Investigation. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am J Respir Crit Care Med. 2004;170:987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- 41.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 42.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 43.Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, Honda Y, Wiegand SJ, Yancopoulos GD, Nishikawa S-I. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. The Journal of Clinical Investigation. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Stankovic M, Bonder CS, Hahn CN, Parsons M, Pitson SM, Xia P, Proia RL, Vadas MA, Gamble JR. Basal and angiopoietin-1-mediated endothelial permeability is regulated by sphingosine kinase-1. Blood. 2008;111:3489–3497. doi: 10.1182/blood-2007-05-092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- 48.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, Skoura A, Kinzel B, Brinkmann V, Rafii S, Evans T, Hla T. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Developmental cell. 2012;23:600–610. doi: 10.1016/j.devcel.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, Dahlback B. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc Natl Acad Sci U S A. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oo ML, Chang S-H, Thangada S, Wu M-T, Rezaul K, Blaho V, Hwang S-I, Han DK, Hla T. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. The Journal of Clinical Investigation. 2011;0:0–0. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arce FT, Whitlock JL, Birukova AA, Birukov KG, Arnsdorf MF, Lal R, Garcia JGN, Dudek SM. Regulation of the Micromechanical Properties of Pulmonary Endothelium by S1P and Thrombin: Role of Cortactin. Biophysical journal. 2008;95:886–894. doi: 10.1529/biophysj.107.127167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu M, Waters CL, Hu C, Wysolmerski RB, Vincent PA, Minnear FL. Sphingosine 1-phosphate rapidly increases endothelial barrier function independently of VE-cadherin but requires cell spreading and Rho kinase. Am J Physiol Cell Physiol. 2007;293:C1309–1318. doi: 10.1152/ajpcell.00014.2007. [DOI] [PubMed] [Google Scholar]
- 53.Lee JF, Gordon S, Estrada R, Wang L, Siow DL, Wattenberg BW, Lominadze D, Lee MJ. Balance of S1P1 and S1P2 signaling regulates peripheral microvascular permeability in rat cremaster muscle vasculature. Am J Physiol Heart Circ Physiol. 2009;296:H33–42. doi: 10.1152/ajpheart.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang G, Yang L, Kim GS, Ryan K, Lu S, O'Donnell RK, Spokes K, Shapiro N, Aird WC, Kluk MJ, Yano K, Sanchez T. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood. 2013 doi: 10.1182/blood-2012-11-467191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee M-J, Evans M, Hla T. The Inducible G Protein-coupled Receptor edg-1 Signals via the G/Mitogen-activated Protein Kinase Pathway. Journal of Biological Chemistry. 1996;271:11272–11279. doi: 10.1074/jbc.271.19.11272. [DOI] [PubMed] [Google Scholar]
- 56.Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol. 2004;286:C1130–1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- 57.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 58.Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase beta. Evidence for divergence and convergence of receptor-regulated endothelial nitric-oxide synthase signaling pathways. J Biol Chem. 2001;276:36281–36288. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- 59.Usui S, Sugimoto N, Takuwa N, Sakagami S, Takata S, Kaneko S, Takuwa Y. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J Biol Chem. 2004;279:12300–12311. doi: 10.1074/jbc.M305025200. [DOI] [PubMed] [Google Scholar]
- 60.Miura S, Tanigawa H, Matsuo Y, Fujino M, Kawamura A, Saku K. Ras/Raf1-dependent signal in sphingosine-1-phosphate-induced tube formation in human coronary artery endothelial cells. Biochem Biophys Res Commun. 2003;306:924–929. doi: 10.1016/s0006-291x(03)01065-9. [DOI] [PubMed] [Google Scholar]
- 61.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nature reviews. Molecular cell biology. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 62.Roztocil E, Nicholl SM, Davies MG. Mechanisms of sphingosine-1-phosphate-induced akt-dependent smooth muscle cell migration. Surgery. 2009;145:34–41. doi: 10.1016/j.surg.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PRJ, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein Kinase B Kinases That Mediate Phosphatidylinositol 3,4,5-Trisphosphate-Dependent Activation of Protein Kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–3216. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 65.Uruno T, Liu J, Zhang P, Fan Y, Egile C, Li R, Mueller SC, Zhan X. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat Cell Biol. 2001;3:259–266. doi: 10.1038/35060051. [DOI] [PubMed] [Google Scholar]
- 66.Vouret-Craviari V, Bourcier C, Boulter E, van Obberghen-Schilling E. Distinct signals via Rho GTPases and Src drive shape changes by thrombin and sphingosine-1-phosphate in endothelial cells. Journal of cell science. 2002;115:2475–2484. doi: 10.1242/jcs.115.12.2475. [DOI] [PubMed] [Google Scholar]
- 67.Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JG. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- 68.Li Y, Uruno T, Haudenschild C, Dudek SM, Garcia JG, Zhan X. Interaction of cortactin and Arp2/3 complex is required for sphingosine-1-phosphate-induced endothelial cell remodeling. Exp Cell Res. 2004;298:107–121. doi: 10.1016/j.yexcr.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 69.Lee JF, Ozaki H, Zhan X, Wang E, Hla T, Lee MJ. Sphingosine-1-phosphate signaling regulates lamellipodia localization of cortactin complexes in endothelial cells. Histochem Cell Biol. 2006;126:297–304. doi: 10.1007/s00418-006-0143-z. [DOI] [PubMed] [Google Scholar]
- 70.Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine-1-phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res. 2010;88:344–351. doi: 10.1093/cvr/cvq184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tauseef M, Kini V, Knezevic N, Brannan M, Ramchandaran R, Fyrst H, Saba J, Vogel SM, Malik AB, Mehta D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ Res. 2008;103:1164–1172. doi: 10.1161/01.RES.0000338501.84810.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adyshev DM, Moldobaeva NK, Elangovan VR, Garcia JG, Dudek SM. Differential involvement of ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cell Signal. 2011;23:2086–2096. doi: 10.1016/j.cellsig.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Estrada R, Zeng Q, Lu H, Sarojini H, Lee JF, Mathis SP, Sanchez T, Wang E, Kontos CD, Lin CY, Hla T, Haribabu B, Lee MJ. Up-regulating sphingosine 1-phosphate receptor-2 signaling impairs chemotactic, wound-healing, and morphogenetic responses in senescent endothelial cells. J Biol Chem. 2008;283:30363–30375. doi: 10.1074/jbc.M804392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hemmings DG, Xu Y, Davidge ST. Sphingosine 1-phosphate-induced vasoconstriction is elevated in mesenteric resistance arteries from aged female rats. Br J Pharmacol. 2004;143:276–284. doi: 10.1038/sj.bjp.0705752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bautista-Perez R, Arellano A, Franco M, Osorio H, Coronel I. Sphingosine-1-phosphate induced vasoconstriction is increased in the isolated perfused kidneys of diabetic rats. Diabetes research and clinical practice. 2011;94:e8–11. doi: 10.1016/j.diabres.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Yang J, Noyan-Ashraf MH, Meissner A, Voigtlaender-Bolz J, Kroetsch JT, Foltz W, Jaffray D, Kapoor A, Momen A, Heximer SP, Zhang H, van Eede M, Henkelman RM, Matthews SG, Lidington D, Husain M, Bolz SS. Proximal cerebral arteries develop myogenic responsiveness in heart failure via tumor necrosis factor-alpha-dependent activation of sphingosine-1-phosphate signaling. Circulation. 2012;126:196–206. doi: 10.1161/CIRCULATIONAHA.111.039644. [DOI] [PubMed] [Google Scholar]
- 78.Peng XQ, Damarla M, Skirball J, Nonas S, Wang XY, Han EJ, Hasan EJ, Cao X, Boueiz A, Damico R, Tuder RM, Sciuto AM, Anderson DR, Garcia JG, Kass DA, Hassoun PM, Zhang JT. Protective role of PI3-kinase/Akt/eNOS signaling in mechanical stress through inhibition of p38 mitogen-activated protein kinase in mouse lung. Acta Pharmacol Sin. 31:175–183. doi: 10.1038/aps.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 80.Filep JG, Foldes-Filep E, Sirois P. Nitric oxide modulates vascular permeability in the rat coronary circulation. Br J Pharmacol. 1993;108:323–326. doi: 10.1111/j.1476-5381.1993.tb12803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murad F. Ferid Murad - Nobel Lecture: Discovery of Some of the Biological Effects of Nitric Oxide and Its Role in Cell Signaling. 1998. [DOI] [PubMed]
- 82.Gunduz D, Thom J, Hussain I, Lopez D, Hartel FV, Erdogan A, Grebe M, Sedding D, Piper HM, Tillmanns H, Noll T, Aslam M. Insulin stabilizes microvascular endothelial barrier function via phosphatidylinositol 3-kinase/Akt-mediated Rac1 activation. Arterioscler Thromb Vasc Biol. 2010;30:1237–1245. doi: 10.1161/ATVBAHA.110.203901. [DOI] [PubMed] [Google Scholar]
- 83.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PR, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845–848. doi: 10.1016/s0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 84.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 86.Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. eNOS activation by sphingosine 1-phosphate and the role of caveolin-1 in sphingolipid signal transduction. J Biol Chem. 2000;275:32363–32370. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- 87.Igarashi J, Bernier SG, Michel T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J Biol Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- 88.Kwon YG, Min JK, Kim KM, Lee DJ, Billiar TR, Kim YM. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J Biol Chem. 2001;276:10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- 89.Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- 90.Wilkerson BA, Grass GD, Wing SB, Argraves WS, Argraves KM. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J Biol Chem. 2012;287:44645–44653. doi: 10.1074/jbc.M112.423426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baumer Y, Burger S, Curry FE, Golenhofen N, Drenckhahn D, Waschke J. Differential role of Rho GTPases in endothelial barrier regulation dependent on endothelial cell origin. Histochem Cell Biol. 2008;129:179–191. doi: 10.1007/s00418-007-0358-7. [DOI] [PubMed] [Google Scholar]
- 92.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 93.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krump-Konvalinkova V, Yasuda S, Rubic T, Makarova N, Mages J, Erl W, Vosseler C, Kirkpatrick CJ, Tigyi G, Siess W. Stable knock-down of the sphingosine 1-phosphate receptor S1P1 influences multiple functions of human endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:546–552. doi: 10.1161/01.ATV.0000154360.36106.d9. [DOI] [PubMed] [Google Scholar]
- 95.Sun X, Shikata Y, Wang L, Ohmori K, Watanabe N, Wada J, Shikata K, Birukov KG, Makino H, Jacobson JR, Dudek SM, Garcia JG. Enhanced interaction between focal adhesion and adherens junction proteins: involvement in sphingosine 1-phosphate-induced endothelial barrier enhancement. Microvasc Res. 2009;77:304–313. doi: 10.1016/j.mvr.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singer II, Tian M, Wickham LA, Lin J, Matheravidathu SS, Forrest MJ, Mandala S, Quackenbush EJ. Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol. 2005;175:7151–7161. doi: 10.4049/jimmunol.175.11.7151. [DOI] [PubMed] [Google Scholar]
- 97.Dejana E. Endothelial cell-cell junctions: happy together. Nature reviews. Molecular cell biology. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 98.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 99.Mierke CT. Cancer cells regulate biomechanical properties of human microvascular endothelial cells. J Biol Chem. 2011;286:40025–40037. doi: 10.1074/jbc.M111.256172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stroka KM, Aranda-Espinoza H. Effects of Morphology vs. Cell-Cell Interactions on Endothelial Cell Stiffness. Cellular and molecular bioengineering. 2011;4:9–27. doi: 10.1007/s12195-010-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 102.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akiyama T, Sadahira Y, Matsubara K, Mori M, Igarashi Y. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 in vascular and lymphatic endothelial cells. J Mol Histol. 2008;39:527–533. doi: 10.1007/s10735-008-9193-y. [DOI] [PubMed] [Google Scholar]
- 104.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 105.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279:15396–15401. doi: 10.1074/jbc.M314291200. [DOI] [PubMed] [Google Scholar]
- 106.Mao C, Wadleigh M, Jenkins GM, Hannun YA, Obeid LM. Identification and characterization of Saccharomyces cerevisiae dihydrosphingosine-1-phosphate phosphatase. J Biol Chem. 1997;272:28690–28694. doi: 10.1074/jbc.272.45.28690. [DOI] [PubMed] [Google Scholar]
- 107.Le Stunff H, Peterson C, Thornton R, Milstien S, Mandala SM, Spiegel S. Characterization of murine sphingosine-1-phosphate phosphohydrolase. J Biol Chem. 2002;277:8920–8927. doi: 10.1074/jbc.M109968200. [DOI] [PubMed] [Google Scholar]
- 108.Long J, Darroch P, Wan KF, Kong KC, Ktistakis N, Pyne NJ, Pyne S. Regulation of cell survival by lipid phosphate phosphatases involves the modulation of intracellular phosphatidic acid and sphingosine 1-phosphate pools. Biochem J. 2005;391:25–32. doi: 10.1042/BJ20050342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Siow DL, Anderson CD, Berdyshev EV, Skobeleva A, Natarajan V, Pitson SM, Wattenberg BW. Sphingosine kinase localization in the control of sphingolipid metabolism. Advances in enzyme regulation. 2011;51:229–244. doi: 10.1016/j.advenzreg.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mendoza A, Breart B, Ramos-Perez WD, Pitt LA, Gobert M, Sunkara M, Lafaille JJ, Morris AJ, Schwab SR. The transporter spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell reports. 2012;2:1104–1110. doi: 10.1016/j.celrep.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, Uemura A, Kiyonari H, Abe T, Fukamizu A, Hirashima M, Sawa H, Aoki J, Ishii M, Mochizuki N. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cellular Signalling. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 114.Orr Gandy KA, Obeid LM. Targeting the sphingosine kinase/sphingosine 1-phosphate pathway in disease: Review of sphingosine kinase inhibitors. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2013;1831:157–166. doi: 10.1016/j.bbalip.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Karliner JS. Sphingosine kinase and sphingosine 1-phosphate in the heart: A decade of progress. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2012 doi: 10.1016/j.bbalip.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12:688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Neubauer HA, Pitson SM. Roles, regulation and inhibitors of sphingosine kinase 2. FEBS Journal. 2013;280:5317–5336. doi: 10.1111/febs.12314. [DOI] [PubMed] [Google Scholar]
- 118.Saba JD, Hla T. Point-Counterpoint of Sphingosine 1-Phosphate Metabolism. Circulation Research. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 119.Nishi T, Kobayashi N, Hisano Y, Kawahara A, Yamaguchi A. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. doi: 10.1016/j.bbalip.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 120.Kurano M, Tsukamoto K, Ohkawa R, Hara M, Iino J, Kageyama Y, Ikeda H, Yatomi Y. Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis. 2013;229:102–109. doi: 10.1016/j.atherosclerosis.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 121.Liu M, Seo J, Allegood J, Bi X, Zhu X, Boudyguina E, Gebre AK, Avni D, Shah D, Sorci-Thomas MG, Thomas MJ, Shelness GS, Spiegel S, Parks JS. Hepatic ApoM overexpression stimulates formation of larger, ApoM/S1P-enriched plasma HDL. Journal of Biological Chemistry. 2013 doi: 10.1074/jbc.M113.499913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Christoffersen C, Jauhiainen M, Moser M, Porse B, Ehnholm C, Boesl M, Dahlback B, Nielsen LB. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J Biol Chem. 2008;283:1839–1847. doi: 10.1074/jbc.M704576200. [DOI] [PubMed] [Google Scholar]
- 123.Qin S, Kawano K, Bruce C, Lin M, Bisgaier C, Tall AR, Jiang X.-c. Phospholipid transfer protein gene knock-out mice have low high density lipoprotein levels, due to hypercatabolism, and accumulate apoA-IV-rich lamellar lipoproteins. Journal of Lipid Research. 2000;41:269–276. [PubMed] [Google Scholar]
- 124.Rousset X, Vaisman B, Auerbach B, Krause BR, Homan R, Stonik J, Csako G, Shamburek R, Remaley AT. Effect of Recombinant Human Lecithin Cholesterol Acyltransferase Infusion on Lipoprotein Metabolism in Mice. Journal of Pharmacology and Experimental Therapeutics. 2010;335:140–148. doi: 10.1124/jpet.110.169540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Korhonen A, Jauhiainen M, Ehnholm C, Kovanen PT, Ala-Korpela M. Remodeling of HDL by Phospholipid Transfer Protein: Demonstration of Particle Fusion by1H NMR Spectroscopy. Biochemical and Biophysical Research Communications. 1998;249:910–916. doi: 10.1006/bbrc.1998.9162. [DOI] [PubMed] [Google Scholar]
- 126.Ishida BY, Albee D, Paigen B. Interconversion of prebeta-migrating lipoproteins containing apolipoprotein A-I and HDL. Journal of Lipid Research. 1990;31:227–236. [PubMed] [Google Scholar]
- 127.Holvoet P, De Geest B, Van Linthout S, Lox M, Danloy S, Raes K, Collen D. The Arg123-Tyr166 Central Domain of Human ApoAI Is Critical for Lecithin:Cholesterol Acyltransferase–Induced Hyperalphalipoproteinemia and HDL Remodeling in Transgenic Mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:459–466. doi: 10.1161/01.atv.20.2.459. [DOI] [PubMed] [Google Scholar]
- 128.Yu Y, Guo S, Feng Y, Feng L, Cui Y, Song G, Luo T, Zhang K, Wang Y, Jiang XC, Qin S. Phospholipid transfer protein deficiency decreases the content of S1P in HDL via the loss of its transfer capability. Lipids. 2014;49:183–190. doi: 10.1007/s11745-013-3850-y. [DOI] [PubMed] [Google Scholar]
- 129.Karuna R, Park R, Othman A, Holleboom AG, Motazacker MM, Sutter I, Kuivenhoven JA, Rohrer L, Matile H, Hornemann T, Stoffel M, Rentsch KM, von Eckardstein A. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 2011;219:855–863. doi: 10.1016/j.atherosclerosis.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 130.Yatomi Y, Ohmori T, Rile G, Kazama F, Okamoto H, Sano T, Satoh K, Kume S, Tigyi G, Igarashi Y, Ozaki Y. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- 131.Kobayashi N, Nishi T, Hirata T, Kihara A, Sano T, Igarashi Y, Yamaguchi A. Sphingosine 1-phosphate is released from the cytosol of rat platelets in a carrier-mediated manner. J Lipid Res. 2006;47:614–621. doi: 10.1194/jlr.M500468-JLR200. [DOI] [PubMed] [Google Scholar]
- 132.Aoki S, Yatomi Y, Ohta M, Osada M, Kazama F, Satoh K, Nakahara K, Ozaki Y. Sphingosine 1-phosphate-related metabolism in the blood vessel. J Biochem. 2005;138:47–55. doi: 10.1093/jb/mvi100. [DOI] [PubMed] [Google Scholar]
- 133.Ito K, Anada Y, Tani M, Ikeda M, Sano T, Kihara A, Igarashi Y. Lack of sphingosine 1-phosphate-degrading enzymes in erythrocytes. Biochem Biophys Res Commun. 2007;357:212–217. doi: 10.1016/j.bbrc.2007.03.123. [DOI] [PubMed] [Google Scholar]
- 134.Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, Osima N, Yokota H, Ikeda H, Yatomi Y. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Annals of clinical biochemistry. 2008;45:356–363. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- 135.Bode C, Sensken SC, Peest U, Beutel G, Thol F, Levkau B, Li Z, Bittman R, Huang T, Tolle M, van der Giet M, Graler MH. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem. 2010;109:1232–1243. doi: 10.1002/jcb.22507. [DOI] [PubMed] [Google Scholar]
- 136.Ikeda H, Ohkawa R, Watanabe N, Nakamura K, Kume Y, Nakagawa H, Yoshida H, Okubo S, Yokota H, Tomiya T, Inoue Y, Nishikawa T, Ohtomo N, Tanoue Y, Koike K, Yatomi Y. Plasma concentration of bioactive lipid mediator sphingosine 1-phosphate is reduced in patients with chronic hepatitis C. Clinica chimica acta; international journal of clinical chemistry. 2010;411:765–770. doi: 10.1016/j.cca.2010.02.063. [DOI] [PubMed] [Google Scholar]
- 137.Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, Coughlin SR. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- 138.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Selim S, Sunkara M, Salous AK, Leung SW, Berdyshev EV, Bailey A, Campbell CL, Charnigo R, Morris AJ, Smyth SS. Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin Sci (Lond) 2011;121:565–572. doi: 10.1042/CS20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nunes J, Naymark M, Sauer L, Muhammad A, Keun H, Sturge J, Stebbing J, Waxman J, Pchejetski D. Circulating sphingosine-1-phosphate and erythrocyte sphingosine kinase-1 activity as novel biomarkers for early prostate cancer detection. British journal of cancer. 2012;106:909–915. doi: 10.1038/bjc.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kobayashi N, Kobayashi N, Yamaguchi A, Nishi T. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J Biol Chem. 2009;284:21192–21200. doi: 10.1074/jbc.M109.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins Other Lipid Mediat. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Adamson RH, Clark JF, Radeva MY, Jiang Y, Kheirolomoom A, Ferrara KW, Curry FR. ALBUMIN MODULATES S1P DELIVERY FROM RED BLOOD CELLS IN PERFUSED MICROVESSELS: MECHANISM OF THE PROTEIN EFFECT. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00829.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, Murakami M, Okajima F. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem. 2006;281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 145.Argraves KM, Gazzolo PJ, Groh EM, Wilkerson BA, Matsuura BS, Twal WO, Hammad SM, Argraves WS. High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem. 2008;283:25074–25081. doi: 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sattler KJ, Elbasan S, Keul P, Elter-Schulz M, Bode C, Graler MH, Brocker-Preuss M, Budde T, Erbel R, Heusch G, Levkau B. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res Cardiol. 2010;105:821–832. doi: 10.1007/s00395-010-0112-5. [DOI] [PubMed] [Google Scholar]
- 147.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sheffield WP, Mamdani A, Hortelano G, Gataiance S, Eltringham-Smith L, Begbie ME, Leyva RA, Liaw PS, Ofosu FA. Effects of genetic fusion of factor IX to albumin on in vivo clearance in mice and rabbits. Br J Haematol. 2004;126:565–573. doi: 10.1111/j.1365-2141.2004.05106.x. [DOI] [PubMed] [Google Scholar]
- 149.Stefulj J, Panzenboeck U, Becker T, Hirschmugl B, Schweinzer C, Lang I, Marsche G, Sadjak A, Lang U, Desoye G, Wadsack C. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ Res. 2009;104:600–608. doi: 10.1161/CIRCRESAHA.108.185066. [DOI] [PubMed] [Google Scholar]
- 150.Rohrer L, Ohnsorg PM, Lehner M, Landolt F, Rinninger F, von Eckardstein A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor BI and ATP-binding cassette transporter G1. Circ Res. 2009;104:1142–1150. doi: 10.1161/CIRCRESAHA.108.190587. [DOI] [PubMed] [Google Scholar]
- 151.Tatematsu S, Francis SA, Natarajan P, Rader DJ, Saghatelian A, Brown JD, Michel T, Plutzky J. Endothelial lipase is a critical determinant of high-density lipoprotein-stimulated sphingosine 1-phosphate-dependent signaling in vascular endothelium. Arterioscler Thromb Vasc Biol. 2013;33:1788–1794. doi: 10.1161/ATVBAHA.113.301300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 153.Zondag GC, Postma FR, Etten IV, Verlaan I, Moolenaar WH. Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem J. 1998;330(Pt 2):605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Van Brocklyn JR, Tu Z, Edsall LC, Schmidt RR, Spiegel S. Sphingosine 1-phosphate-induced cell rounding and neurite retraction are mediated by the G protein-coupled receptor H218. J Biol Chem. 1999;274:4626–4632. doi: 10.1074/jbc.274.8.4626. [DOI] [PubMed] [Google Scholar]
- 155.An S, Bleu T, Huang W, Hallmark OG, Coughlin SR, Goetzl EJ. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Letters. 1997;417:279–282. doi: 10.1016/s0014-5793(97)01301-x. [DOI] [PubMed] [Google Scholar]
- 156.Sato K, Murata N, Kon J, Tomura H, Nochi H, Tamoto K, Osada M, Ohta H, Tokumitsu Y, Ui M, Okajima F. Downregulation of mRNA Expression of Edg-3, a Putative Sphingosine 1-Phosphate Receptor Coupled to Ca2+Signaling, during Differentiation of HL-60 Leukemia Cells. Biochemical and Biophysical Research Communications. 1998;253:253–256. doi: 10.1006/bbrc.1998.9745. [DOI] [PubMed] [Google Scholar]
- 157.Van Brocklyn JR, Gräler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S. Sphingosine-1-phosphate is a ligand for the G protein-coupled receptor EDG-6. Blood. 2000;95:2624–2629. [PubMed] [Google Scholar]
- 158.Yamazaki Y, Kon J, Sato K, Tomura H, Sato M, Yoneya T, Okazaki H, Okajima F, Ohta H. Edg-6 as a Putative Sphingosine 1-Phosphate Receptor Coupling to Ca2+ Signaling Pathway. Biochemical and Biophysical Research Communications. 2000;268:583–589. doi: 10.1006/bbrc.2000.2162. [DOI] [PubMed] [Google Scholar]
- 159.Im D-S, Heise CE, Ancellin N, O'Dowd BF, Shei G.-j., Heavens RP, Rigby MR, Hla T, Mandala S, McAllister G, George SR, Lynch KR. Characterization of a Novel Sphingosine 1-Phosphate Receptor, Edg-8. Journal of Biological Chemistry. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- 160.Malek RL, Toman RE, Edsall LC, Wong S, Chiu J, Letterle CA, Van Brocklyn JR, Milstien S, Spiegel S, Lee NH. Nrg-1 Belongs to the Endothelial Differentiation Gene Family of G Protein-coupled Sphingosine-1-phosphate Receptors. Journal of Biological Chemistry. 2001;276:5692–5699. doi: 10.1074/jbc.M003964200. [DOI] [PubMed] [Google Scholar]
- 161.Kono M, Tucker AE, Tran J, Bergner JB, Turner EM, Proia RL. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J Clin Invest. 2014 doi: 10.1172/JCI71194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhu X, Lee JY, Timmins JM, Brown JM, Boudyguina E, Mulya A, Gebre AK, Willingham MC, Hiltbold EM, Mishra N, Maeda N, Parks JS. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J Biol Chem. 2008;283:22930–22941. doi: 10.1074/jbc.M801408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Elsoe S, Christoffersen C, Luchoomun J, Turner S, Nielsen LB. Apolipoprotein M promotes mobilization of cellular cholesterol in vivo. Biochim Biophys Acta. 2013;1831:1287–1292. doi: 10.1016/j.bbalip.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 164.Means CK, Miyamoto S, Chun J, Brown JH. S1P1 receptor localization confers selectivity for Gi-mediated cAMP and contractile responses. J Biol Chem. 2008;283:11954–11963. doi: 10.1074/jbc.M707422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reckless JPD, Weinstein DB, Steinberg D. Lipoprotein and cholesterol metabolism in rabbit arterial endothelial cells in culture. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1978;529:475–487. doi: 10.1016/0005-2760(78)90091-7. [DOI] [PubMed] [Google Scholar]
- 166.Lim Hwee Y., Thiam Chung H., Yeo Kim P., Bisoendial R, Hii Chung S., McGrath Kristine C.Y., Tan Kar W., Heather A, Steven J, Alexander J, Angeli V. Lymphatic Vessels Are Essential for the Removal of Cholesterol from Peripheral Tissues by SR-BI-Mediated Transport of HDL. Cell metabolism. 2013;17:671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 167.Pagler TA, Rhode S, Neuhofer A, Laggner H, Strobl W, Hinterndorfer C, Volf I, Pavelka M, Eckhardt ERM, van der Westhuyzen DR, Schütz GJ, Stangl H. SR-BI-mediated High Density Lipoprotein (HDL) Endocytosis Leads to HDL Resecretion Facilitating Cholesterol Efflux. Journal of Biological Chemistry. 2006;281:11193–11204. doi: 10.1074/jbc.M510261200. [DOI] [PubMed] [Google Scholar]
- 168.Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, Lin CY, Hla T. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 169.Cotton M, Claing A. G protein-coupled receptors stimulation and the control of cell migration. Cellular Signalling. 2009;21:1045–1053. doi: 10.1016/j.cellsig.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 170.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 171.Kohno T, Wada A, Igarashi Y. N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 2002;16:983–992. doi: 10.1096/fj.01-0809com. [DOI] [PubMed] [Google Scholar]
- 172.Kohno T, Igarashi Y. Truncation of the N-terminal ectodomain has implications in the N-glycosylation and transport to the cell surface of Edg-1/S1P1 receptor. J Biochem. 2003;134:667–673. doi: 10.1093/jb/mvg191. [DOI] [PubMed] [Google Scholar]
- 173.Sensken SC, Graler MH. Down-regulation of S1P1 receptor surface expression by protein kinase C inhibition. J Biol Chem. 2010;285:6298–6307. doi: 10.1074/jbc.M109.049692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.E S, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, Yu JH, Guo H, Kihara A, Tigyi G, Lin FT. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem. 2009;284:14558–14571. doi: 10.1074/jbc.M900185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Argraves KM, Sethi AA, Gazzolo PJ, Wilkerson BA, Remaley AT, Tybjaerg-Hansen A, Nordestgaard BG, Yeatts SD, Nicholas KS, Barth JL, Argraves WS. S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 2011;10:70. doi: 10.1186/1476-511X-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ramet ME, Ramet M, Lu Q, Nickerson M, Savolainen MJ, Malzone A, Karas RH. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J Am Coll Cardiol. 2003;41:2288–2297. doi: 10.1016/s0735-1097(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 177.Zhao CX, Xu X, Cui Y, Wang P, Wei X, Yang S, Edin ML, Zeldin DC, Wang DW. Increased Endothelial Nitric-Oxide Synthase Expression Reduces Hypertension and Hyperinsulinemia in Fructose-Treated Rats. Journal of Pharmacology and Experimental Therapeutics. 2009;328:610–620. doi: 10.1124/jpet.108.143396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang XL, Sim AS, Wang MX, Murrell GAC, Trudinger B, Wang J. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Letters. 2000;471:45–50. doi: 10.1016/s0014-5793(00)01356-9. [DOI] [PubMed] [Google Scholar]
- 179.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. The Journal of Clinical Investigation. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, Peach R, Oldstone MB, Rosen H. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146:980–991. doi: 10.1016/j.cell.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kumaraswamy SB, Linder A, Akesson P, Dahlback B. Decreased plasma concentrations of apolipoprotein M in sepsis and systemic inflammatory response syndromes. Critical care (London, England) 2012;16:R60. doi: 10.1186/cc11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 184.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol. 2010;43:662–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;5:428–434. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- 186.Hanson MA, Roth CB, Jo E, Griffith MT, Scott FL, Reinhart G, Desale H, Clemons B, Cahalan SM, Schuerer SC, Sanna MG, Han GW, Kuhn P, Rosen H, Stevens RC. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]