Abstract
The generation of pigs with genetic modifications has significantly advanced the field of xenotransplantation. New genetically-engineered pigs were produced on an α1,3-galactosyltransferase gene-knockout background with ubiquitous expression of human CD46 (GTKO/CD46 pigs), with islet beta cell-specific expression of human tissue factor pathway inhibitor (hTFPI) and/or human CD39 and/or porcine CTLA4-lg. Isolated islets from pigs with 3, 4, or 5 genetic modifications were transplanted intraportally into streptozotocin-diabetic, immunosuppressed cynomolgus monkeys (n=5). Immunosuppression was based on anti-CD154mAb costimulation blockade. Monitoring included features of early islet destruction, glycemia, exogenous insulin requirement, and histopathology of the islets at necropsy. Using these modified pig islets, there was evidence of reduced islet destruction in the first hours after transplantation, compared with two series of historical controls that received identical therapy but were transplanted with islets from pigs with either no or only one genetic modification. Despite encouraging effects on early islet loss, these multi-transgenic islet grafts did not demonstrate consistency in regard to long-term success, with only 2 of 5 demonstrating function beyond 5 months.
INTRODUCTION
Xenotransplantation (xenoTx) of porcine islets is poised to become a therapeutic alternative to pancreas and islet allotransplantation (alloTx) for patients with Type 1 diabetes (1-5). A significant advance has been the ability to produce pigs with specific genetic modifications (6-13), which may prove useful in overcoming the metabolic and immunological barriers between species, and ultimately contribute to reduce the islet mass as well as the intensity of immunosuppressive therapy necessary to sustain islet graft survival.
As also seen in the human islet alloTx setting, intraportal infusion of pig islets in monkeys results in an immediate loss of islets, along with the release of insulin and C-peptide, making it more difficult to achieve and maintain normoglycemia (14) Some of the mechanisms involved in early islet loss have been characterized as the instant blood-mediated inflammatory reaction (IBMIR) (15-17).
Previously, our group achieved insulin-independence in diabetic, immunosuppressed cynomolgus monkeys following the Tx of islets from pigs expressing a single human complement-regulatory protein (hCD46) (18). However, while hCD46 was associated with successful engraftment when compared with wild-type pig islets, a reduction of early islet loss was not observed, suggesting that other modulatory transgenes would be beneficial to islet survival.
Several studies have indicated that tissue factor, complement and coagulation activation, antibody binding and inflammation contribute to primary non-function (15,19-21). To reduce such effects, new genetically-engineered (GE) pigs have been generated on a background of α1,3-galactosyltransferase gene-knockout and ubiquitous expression of hCD46 (GTKO/hCD46 pigs). Specific transgenes were selected to target relevant mechanisms. Human tissue factor pathway inhibitor (hTFPI) was aimed at inhibition of coagulation and inflammation associated with IBMIR (22,23). Human CD39, through its ATPase activity, has been shown to decrease platelet activation and prevent clotting in transgenic mouse models (24,25). In addition, the porcine CTLA4-Ig transgene was incorporated with the goal of inhibiting the cellular immune response (26,27).
Upon reaching sufficient adult size and age, the pigs’ pancreases were harvested for isolation of the islets, which were infused into diabetic, immunosuppressed cynomolgus monkeys. We report the effects of these novel GE pig islets on early islet loss and Tx outcome in the first 5 experiments. Despite significant limitations, this study provides insights into the use of GE pigs in islet xenoTx.
MATERIALS AND METHODS
Sources of animals
Six female pigs aged 16-36mo, weighing 350-400lbs (159-181kg) (Revivicor, Blacksburg, VA) were sources of islets (Table 1). The production of these GE pigs and their glucose metabolism are detailed in Wijkstrom, et al (28) (Supplementary Methods).
Table 1.
Characteristics of islet-source pigs
| Pig # | Genetic Manipulation | Pig Weight (kg) | Islet Yield (IEQ) | Islet Yield Per Gram Pancreas (IEQ/gram) | Viability (%) | Purity (%) | Stimulation Index High/low g- high + theoph/ low g |
|---|---|---|---|---|---|---|---|
| P388-03 | 4-GE* | 159 | 338,083 | 1,356 | 91 | 85 | 2.0 – 8.1 |
| P384-02 | 4-GE* | 159 | 399,083 | 2,237 | 94 | 75 | 2.3 – 6.3 |
| P388-2 | 5-GE* | 159 | 331,846 | 1,869 | 94 | 75 | 3.1 – 5.3 |
| P462-04 | 3-GE* | 159 | 325,420 | 1,574 | 95** | 80** | 2.6 – 6.9** |
| P474-07 | 3-GE* | 159 | 300,750 | 1,012 | 95** | 80** | 2.6 – 6.9** |
| P388-01 | 5-GE* | 182 | 149,606 | 1,202 | 90 | 75 | 2.2 – 10.8 |
g = glucose
theoph = theophylline
3GE = GTKO/CD46/CD39
4GE = GTKO/CD46/TFPI/CTL4-Ig
5GE = GTKO/CD46/TFPI/CTL4-Ig/CD39
islets from P462-04 and P474-07 were pooled and the combined prep was tested
***islets from all four of the 4-GE and 5-GE pigs are clones of each other with respect to the 4-GE background. The 4-GE cell line 548A.3 was used to clone P384-02 and P388-03. This same 4-GE cell line was subsequently transfected with the ins-CD39 vector, to generate the two 5-GE cloned animals, P388-01 and P388-2. While P388-03 was part of the cell pool transfected with ins-CD39, genotype analysis showed that this animal was a “no-take” with respect to integration of the CD39 transgene, and thus only 4-GE.
Five male cynomolgus monkeys (Macaca fascicularis, Three Springs Scientific, Perkasie, PA, and Alpha Genesis, Yemassee, SC) aged 2.5-4.0years, weighing 2.5-4.1kg, were islet recipients (Table 2). One monkey received islets from two GTKO/CD46/hCD39 (3-GE) cloned pigs (P462-04, P474-07).
Table 2.
Islet mass transplanted (IEQ/kg), number of recipient CD3+T cells on day of transplantation, mean mycophenolate mofetil (MMF) trough levels after transplantation, and graft survival.
| Recipient Monkey # | Monkey Weight at Time of Tx (kg) | Pig Islet Donor # | Islet Mass Transplanted (IEQ/kg) | # CD3+T Cells on Day of Tx (cells/uL) | Mean (+/−SEM) MMF Trough Levels (μg/mL) | Graft Survival (days) |
|---|---|---|---|---|---|---|
| M2-11 | 3.3 | P388-03 | 100,000 | 491 | 0.59±0.15 | 0 |
| M3-11 | 2.8 | P384-02 | 100,000 | 340 | 1.25± 0.17 | 365 |
| M1-11 | 4.1 | P388-2 | 75,000 | 441 | 2.78±0.53 | 160 |
| M14-12 | 2.5 | {P462-04 {P474-07 |
100,000 | 183 | 1.63±0.40 | 5 |
| M12-12 | 2.8 | P388-01 | 50,000 | 91 | 1.86±0.47 | 3 |
All procedures that impacted the care of animals were in compliance with guidelines in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 86-23, revised 2011), and approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Pig islet isolation and islet quality assurance
Pig pancreases were excised as non-survival procedures, as described (29,30). With a warm ischemia time of <5min, the cold pancreas was transported within 60min to the laboratory for immediate islet isolation, purification, and culture. Islets were counted as islet equivalents (IEQ) (29). CIzyme™ Collagenase MA and BP Protease were used (VitaCyte, Indianapolis, IN) following the manufacturer's guidelines.
Viability was determined by double fluorescent calcein-AM/propidium iodide staining, a method validated for human islets (31) (Supplementary Methods). Islet preparations were stained with dithizone and the percent of dithizone-positive aggregates (at least 50) over whole tissue was used to express purity (32). For qualitative analysis, islets were subjected to dynamic secretagogue challenges (21) (Supplementary Methods). Table 1 summarizes islet graft characteristics.
Induction of diabetes and baseline metabolic studies in recipient monkeys
To facilitate blood withdrawal and i.v. drug administration, catheters were inserted into the carotid artery and jugular vein and connected through a tether and jacket system to the exterior of the animal cage (33). The vascular lines were removed 5-6 weeks later (approximately 2 weeks after islet Tx) to minimize the risk of infection. Additional blood draws were obtained from the femoral vein, after ketamine sedation (Ketaset, Fort Dodge, IA, 10mg/kg).
Two to three weeks before islet Tx, diabetes was induced by i.v. streptozotocin (Zanosar, Sicor Pharmaceuticals, Irvine, CA; 125mg/kg, never exceeding 1,500 mg/m2 to avoid nephrotoxicity). Blood glucose levels were measured using Precision Xtra (Becton Dickinson, Franklin Lakes, NJ). Monkeys were considered diabetic if the following conditions were met: (i) hyperglycemia (>350mg/dL) on at least two occasions, (ii) baseline primate C-peptide reduced by >75% (34), (iii) no increase in C-peptide after intravenous glucose tolerance test (IVGTT) and arginine stimulation test (AST) (35), and (iv) need for exogenous insulin to prevent ketoacidosis. All monkeys met these criteria. Monkey C-peptide was measured by radioimmunoassay (RIA, Millipore, Billerica, MA) using anti-human antibodies, and concentrations were confirmed with Immulite System (UPMC Presbyterian Hospital Laboratory). Serum porcine C-peptide was measured by radioimmunoassay (RIA, Millipore) using antibodies that do not cross-react with monkey C-peptide. The successful induction of diabetes was confirmed by histological immunostaining of pancreatic tissue at necropsy.
Islet transplantation and peri-transplant prophylaxis
All islet preparations were cultured overnight prior to Tx, with the exception of islets from pig P462-04 that were cultured for one week and mixed with a second islet batch (P474-07) cultured overnight (Table 2). Culture was in CMRL-1066 (Life Technologies, Carlsbad, CA) supplemented with 10% heat-inactivated porcine serum, 100units/mL penicillin, 0.1mg/mL streptomycin, and 2mmol/L L-glutamine (all from Life Technologies) at 24°C in 5% CO2. Prior to Tx, islets were resuspended in fresh 20mL CMRL-1066 medium with the addition of low molecular weight dextran sulfate (4.5mg/kg of recipient, Sigma-Aldrich, St. Louis, MO) (36). The insulin content of the transplant medium was negligible (<0.5U).
The islets were infused intraportally by gravity over 5-10min. Immediately before islet infusion, anti-inflammatory and anticoagulant treatment was administered (Table 3). Activated clotting time (ACT) was monitored (I-Stat, Abbott, Princeton, NJ) and anticoagulants discontinued if the ACT >190sec (37). Postoperative treatment consisted of prophylactic cefazolin (10mg/kg i.m. x2 daily) and buprenorphine (0.03mg/kg i.m. x2 daily) for 3 days. Monkeys were allowed to eat on the evening of surgery.
Table 3.
Treatment aimed at reducing the IBMIR, immunosuppressive regimen, and supportive therapy
| Treatment | Dose and administration | Route | ||
|---|---|---|---|---|
| Prostacyclin (Flolan; GlaxoSmithKline, Philadelphia, PA) | 20 ng/kg/min | Day of Tx | 30min prior to Tx, 3h infusion | i.v. |
| Methylprednisolone (SoluMedrol; Pfizer, New York, NY) | 10 mg/kg | Day of Tx | bolus | i.v. |
| Dextran sulfate | 5 mg/kg | Day of Tx | bolus | i.v. |
| 2 mg/kg/h | Day of Tx | 6h infusion. | i.v. | |
| Target: ACT 150-190s | discontinued if ACT>190s | |||
| Antithymocyte globulin (ATG, Thymogolbulin; Genzyme, Cambridge, MA) | 25 mg/5-25 mg | Day −3,−1 | over 7h | i.v. |
| Target: <500 CD3+ cells/uL | ||||
| Mycophenolate mofetil (MMF, Cellcept, Roche, Nutley, NJ) | 100 mg/kg/day | From day −7 | bolus | p.o. |
| Target trough level: 3-5 μg/mL | ||||
| Anti-CD154 mAb (NIH NHP Reagent Resource, Boston, MA) | 25 mg/kg | Day −1, 0, 3, 7, 11, and 15 and every other week subsequently | bolus | i.v. |
| Aspirin | 81 mg/day | From day-7 to day+7 | bolus | p.o. |
| Ganciclovir (Cytovene, Roche, Welwyn Garden City, UK) | 5 mg/kg/day | From day of Tx until removal of vascular lines | bolus | i.v. |
| Valganciclovir (Valcyte, Genentech, San Francisco, CA) | 15 mg/kg x2 daily | After line removal | bolus | p.o |
| Famotidine (APP Pharmaceuticals, Schaumburg, IL, and Baxter Healthcare, Deerfield, IL) | 0.25 mg/kg/day | From day of Tx until removal of vascular lines | bolus | i.v. |
| 1 mg/kg/day | After line removal | bolus | p.o. | |
ACT = activated clotting time Tx = transplantation
Immunosuppressive and supportive therapy (Table 3)
Prostacyclin, methylprednisolone, dextran sulfate, and aspirin were administered for their anticoagulant and/or anti-inflammatory effects; antithymocyte globulin (ATG) for induction and mycophenolate mofetil (MMF) and anti-CD154mAb for maintenance immunosuppression. Ganciclovir and valganciclovir were administered to prevent cytomegalovirus reactivation, and famotidine to prevent peptic ulceration.
Monitoring of early islet loss
Serum porcine C–peptide was measured 1, 2, and 24h post-Tx, and expressed as ng/mL per 10,000IEQ/Kg to take into account differences in islet mass infused. Blood glucose was measured at least every 2h during the first day and every 4h on day 1. During the first 24h post-Tx, the blood glucose target was 100-150mg/dL. If the blood glucose rose >150mg/dL, insulin was administered. If it fell <100mg/dL, dextrose was infused i.v. The total amount of dextrose (expressed as grams/24h) needed to maintain glycemia within the target range and to prevent hypoglycemia as a result of insulin leakage was calculated (17). Quantification of serum human IL-6 (Quantikine ELISA, R&D Systems, Minneapolis MN) and SC5b-9 (complement complex) Plus Enzyme Immunoassays (Quidel, San Diego, CA) was carried out.
Assessment of islet graft function
Monkeys were followed for 6-12mo or until graft failure. Graft function was defined by detectable fasting porcine C-peptide with no need for exogenous insulin to maintain blood glucose levels <200mg/dL or with exogenous insulin dose <50% of pre-Tx requirement. Fasting blood glucose levels were measured daily and C-peptide weekly.
Histopathological examination
The livers and pancreases of all recipients were examined at necropsy. Additionally, specimens from the 3-4-5 GE donor pancreases prior to islet isolation and from transgenic hCD46 pigs used as donors in our previous study (18) were examined. Tissue sections were fixed in both 10% buffered formalin and 4% paraformaldehyde. Formalin-paraffin-embedded sections were stained with hematoxylin/eosin, using standard procedures. Paraformaldehyde-fixed tissues were used for immunofluorescence analysis. Primary and secondary antibodies are listed in Table 4. Nuclear staining was done with TO-Pro-3 iodide (Molecular Probes, Eugene, OR).
Table 4.
Antibodies for Immunocytochemistry and Immunofluorescence
| Antibody | Dilution | Supplier |
|---|---|---|
| Immunocitochemistry | ||
| Rabbit anti-proinsulin | 1:100 | Scytek Laboratories (Logan, UT) |
| Rabbit anti-glucagon | 1:50 | Zymed (San Francisco, CA) |
| Immunofluorescence - Primary | ||
| Mouse anti-human CD46 | 1:100 | Thermo Fisher Scientific (Fremont, CA) |
| Mouse anti-human CD3 | 1:20 | BD Pharmingen (San Jose, CA) |
| Rabbit anti-human C4d | 1:20 | EMELCA Bio-science (Bergen op Zoom, The Netherlands) |
| Goat anti-human IgG | 1:1000 | Kirkegaard & Perry (Gaithersburg, MD) |
| Rabbit or mouse anti-insulin | 1:100 | Santa Cruz Biotechnology (Santa Cruz, CA) |
| Goat anti-glucagon | 1:50 | Santa Cruz Biotechnology |
| Rabbit anti-human TFPI | 1:10 | American Diagnostic (Hauppauge, NY) |
| Mouse anti-CD152 | 1:10 | Serotec (Raleigh, NC) |
| Secondary | ||
| Goat anti-mouse Cy3 | 1:500 | Jackson ImmunoResearch (West Grove, PA) |
| Goat anti-rabbit Cy3 | 1:500 | Jackson ImmunoResearch |
| Donkey anti-goat Cy3 | 1:500 | Jackson ImmunoResearch |
| Goat anti-rabbit Alexa 488 | 1:500 | Molecular Probes (Eugene, OR) |
| Goat anti-mouse Alexa 488 | 1:500 | Molecular Probes |
Images were captured by a Photometrics Cool SNAP digital camera (Roper Scientific, Tucson, AZ) and Nikon C1 confocal system at 40x objective lens (Nikon Instruments, Melville, NY), and analyzed by MetaMorph imaging analysis software (Molecular Devices, Downington, PA). Photographs were taken through a Nikon Eclipse E800 microscope (Nikon Instruments).
Flow cytometry for expression of transgenes in porcine cells
Cultured porcine pancreatic cells enriched in islets (50-60% islets/whole tissue) were dissociated into single-cell suspensions by gentle agitation in 0.25% trypsin-EDTA (Invitrogen, Carlsbad, CA). The suspension was filtered through a 100μm nylon cell strainer (Becton Dickinson) to remove cell clumps. Single cells (1×105 ) were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-human CD46mAb (clone MEM-258, Serotec, Raleigh, NC) or mouse IgG1k isotype control (clone MOPC-21, Becton Dickinson) or allophycocyanin (APC)-conjugated anti-human CD39mAb (clone TÜ66, or mouse IgG2bκ isotype control, clone 27-35, Becton Dickinson), and expression of hCD46 and hCD39 on cells was detected by BD™ LSR II flow cytometer (Becton Dickinson). Surface expression of hCD46 and hCD39 on peripheral blood mononuclear cells (PBMC) and primary aortic endothelial cells (PAEC) was carried out as described (38).
Flow cytometry for measurement of xenoreactive antibodies
Recipient sera drawn pre-Tx, at 1week and 1mo after islet Tx, and at the end of the study were tested for binding of xenoreactive anti-nonGal antibodies to PBMC from GTKO pigs, as described (39). Binding of lgM and lgG was assessed using relative mean fluorescence intensity (MFI), calculated as follows:-Relative MFl = (actual MFI)/(MFI of secondary antibody only, in the absence of serum). Post-Tx relative MFl was compared to pre-Tx MFI.
Statistical analyses
Continuous variables were expressed as mean±standard error (SEM), and analysis of differences was made using the Kruskal-Wallis test with Dunn's Multiple Comparison. A p value of <0.05 was considered to indicate a statistically significant difference. All analyses were performed with GraphPad Prism 4 for Windows (GraphPad Software, La Jolla, CA).
RESULTS
After streptozotocin-induced diabetes, body weight decreased in all monkeys except one, and increased in all after Tx (Supplementary Figure 1). Mean body weight at the end of the study was 123% of weight at time of Tx (SEM 6.5%). All recipients underwent islet Tx with no complications, and were restored to full diet within two days. The weight gain and minimal adverse events associated with the immunosuppressive protocol indicate their general well-being.
Porcine C-peptide release, blood glucose levels, and dextrose administration in the first 24h post-transplantation
Porcine C-peptide release
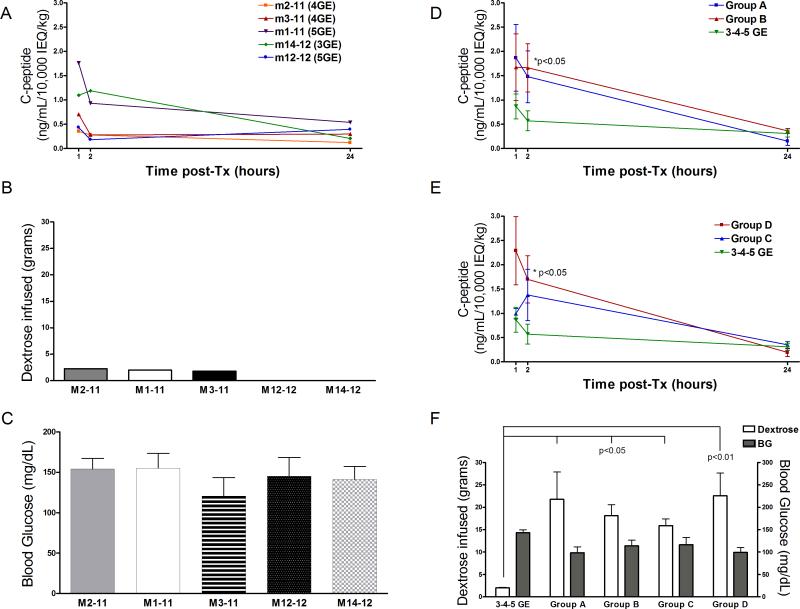
To monitor early islet cell loss, porcine C-peptide was measured in recipient serum at 1, 2, and 24h, as calculated from the end of islet infusion (Figure 1). In panel A, C-peptide is shown individually in recipients of 3-4-5 GE pig islets. These levels are also shown as a mean of all 3-4-5 GE recipients and compared with those of two historical groups comprised of monkey recipients of wild-type (Group A) or hCD46 transgenic (Group B) islets subjected to the same treatment (islet mass and immunosuppression) (18). Porcine C-peptide levels at 1h and 2h were significantly lower in the recipients of 3-4-5 GE islets than those of historical Groups A and B (p<0.05 vs Group B at 2h) (Figure 1D) and historical monkeys from Groups A and B that (i) became normoglycemic after successful islet Tx and remained so for >3mo (Group C) or (ii) did not achieve stable normoglycemia (Group D) (p<0.05 vs Group D at 2h, Figure 1E). In both comparisons, data were normalized for 10,000IEQ/Kg. Insulin content of the islets was not significantly different between the groups (data not shown).
Figure 1.
Panels A, B, C: Individual data for the 5 monkey recipients of 3-4-5 GE islets. (A) Porcine C-peptide levels measured 1, 2, and 24h after islet infusion. (B) Amount of dextrose infused in the first 24h post-Tx and (C) mean blood glucose during the same time period.
Panels D, E, F: comparison between 3-4-5 GE recipients historical groups of monkey (D) Porcine C-peptide levels (mean of all recipients for each group) measured 1, 2, and 24h after islet infusion of 3-4-5 GE recipients and historical Groups A and B (monkeys that received the same islet mass and the same immunosuppressive and peri-Tx treatment but received islets from wild-type pigs (Group A, n=5) or hCD46 pig islets (Group B, n=5); or had (E) successful outcomes, i.e., normoglycemia >3mo (Group C, n=4) or unsuccessful, i.e., did not achieve normoglycemia after islet Tx (Group D, n=6). (F) Dextrose infused and mean blood glucose in the first 24h post-Tx for all groups (3-4-5 GE, Group A, Group B, Group C, and Group D). For monkeys of Group B (that received two transplants, C-peptide levels are those of the first transplant.
Blood glucose levels and dextrose administration
Exogenous insulin treatment was suspended immediately prior to islet infusion, and only one monkey from the current study and one from historical Group B (and D) were given insulin within the first 24h post-Tx to reduce the blood glucose to ≤150mg/dL. The monkey in our present study that required insulin within the first 24h received no dextrose. Figure 1B shows the amount of dextrose administered during the first 24h post-Tx in the 3-4-5 GE islet recipients. Two recipients in the present study required no dextrose (M12-12, and M14-12). Mean of 3-4-5 GE recipient dextrose infused (Figure 1F) was substantially lower than in Groups A, B, and C (all p<0.05) and Group D (p<0.01).
Individual blood glucose levels for 3-4-5 GE recipients and mean blood glucose levels during the first 24h post-Tx are shown in Figure 1C and F, respectively. Only 1 of 5 monkeys in the present study had a blood glucose ≤50mg/dL at one time-point, whereas 7 out of 10 monkeys from Groups A and B (or C and D) combined had a total of 18 blood glucose readings ≤50mg/dL in the same period.
Additional serum analysis
No difference was found in split soluble C5b-9 (soluble complement complex) in the serum pre-Tx, 1, and 2h post-Tx between the 3-4-5 GE recipients and historical groups. However, serum IL-6 levels were significantly lower in 3-4-5 GE recipients compared to Groups A and B (2.27±1.48pg/mL vs 14.33±2.95pg/mL and 11.75±3.72pg/mL, mean±SEM, p<0.05 3-4-5 GE vs Groups A and B) 1h post-Tx. Pre-Tx ACT levels (mean±SEM) were 164±11s vs 150±10s and 178±13s in 3-4-5 GE, Group A, and Group B recipient sera, respectively; 2hr post-Tx levels were 134±27s vs 189±45s and 162±36s, respectively (differences not statistically significant).
Metabolic outcome of islet transplantation
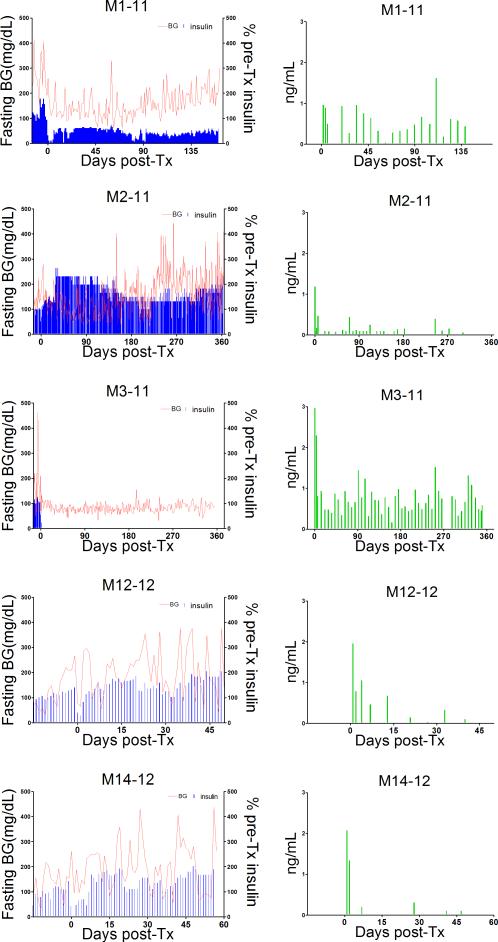
Despite evidence indicating a reduced early islet loss, the long-term effect of the GE pig islets on porcine C-peptide levels, blood glucose, and exogenous insulin requirement was inconsistent (Figure 2).
Figure 2.
Left panels show blood glucose levels (red line) and daily exogenous insulin (as percent of pre-Tx dosages) in all monkey recipients of pig islets from the time of streptozotocin administration. Right panels show porcine C-peptide levels over the same time-periods.
Infusion of 4-GE pig islets (GTKO/CD46/TFPI/CTLA4lg) in M3-11 achieved normoglycemia within 2 days and maintained it for the entire period of follow-up (12mo). The porcine C-peptide response to AST was measured at 1, 4, and 9mo post-Tx and was positive at each time-point with a basal porcine C-peptide of 0.81ng/mL and a stimulated level of 1.45ng/mL 9mo after Tx (not shown). M1-11 showed partial graft function characterized by substantial reduction of exogenous insulin for 24 weeks with no insulin requirement on 6 days (days 4, 16, 80, 82, 90, 93), and detectable porcine C-peptide levels. At 14 weeks, response to arginine remained positive, with a basal porcine C-peptide of 0.36ng/mL and a stimulated level of 0.48ng/mL (not shown).
In the 3 remaining monkeys, the islet graft failed to achieve metabolic control. In M12-12 (recipient of an islet mass of only 50,000 IEQ/kg), porcine C-peptide was detected for approximately 2 weeks. In the other two, low C-peptide levels were detected intermittently for 4 weeks in one case (M14-12) and for 35 weeks in the other (M2-11) (Figure 2). In these 3 recipients, circulating porcine C-peptide was not associated with metabolic improvement. Prior to euthanasia, porcine C-peptide fell below the assay's detection limit of 0.1ng/mL on at least two occasions in each recipient. We suggest that C-peptide release may have been associated with ongoing islet graft destruction.
Histopathology of liver and pancreas of monkey recipients
At the end of the study, the pancreases of all 5 monkeys were stained with anti-insulin and anti-glucagon antibodies, and, as expected, showed no endogenous beta cells (Supplementary Figure 2).
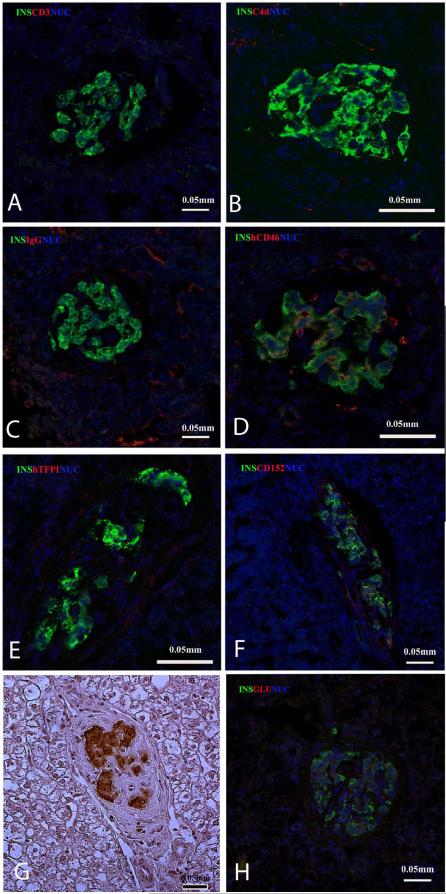
In the livers of the 3 monkeys that demonstrated islet graft failure, a definitive histopathological diagnosis could not be made because the islets were most likely destroyed before necropsy (which was some weeks after graft failure). In M3-11 (12mo follow-up), however, immunostaining for insulin in the liver at necropsy indicated viable islets (Figure 3A-G). Immunostaining with anti-insulin antibodies in association with anti-CD3 (T lymphocytes), C4d (as a marker of complement activation), and IgG was used to determine possible cell infiltration and complement and antibody deposition. No CD3+ cellular infiltrate, and no C4d or IgG was found around the islets.
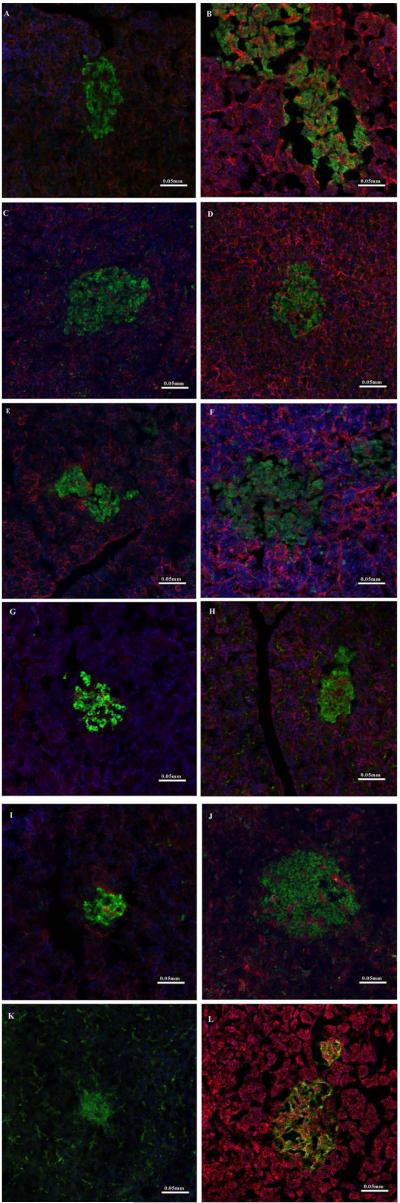
Figure 3.
Immunofluorescence of M3-11 liver at necropsy one year after islet Tx (A-F). Staining for insulin (green) and nuclei (blue) with co-staining with antibodies (in red) significant for rejection (CD3 for T lymphocytes in panel A, C4d for complement activation in panel B, and IgG in panel C). There was no evidence of rejection. Immunostaining for human transgenes (hCD46 in D, hTFPI in E, and CD152 as a marker for CTLA4-Ig in F) shows transgene persistence except for hTFPI. (G) Immunostaining for insulin in the liver from M3-11 one year after Tx is also confirmed by immunocytochemistry in H.
Immunostaining of the liver of M1-11 6mo after islet Tx shows well-preserved islet beta cells (green = insulin; red = glucagon; blue = nuclei).
Figure 3 also shows immunostaining with anti-insulin antibodies (to identify the islets) in association with anti-hCD46, hTFPI, and CD152 (a marker for CTLA4-Ig) antibodies to assess the expression of the transgenes in the liver of M3-11 one year after Tx. hCD46 remained weakly detectable within the islets, CD152 was co-expressed with insulin in the islets, but staining for hTFPI was not detectable. Figure 3H shows insulin-positive cells in the liver of M1-11 6mo after Tx.
Transgene expression on donor tissue
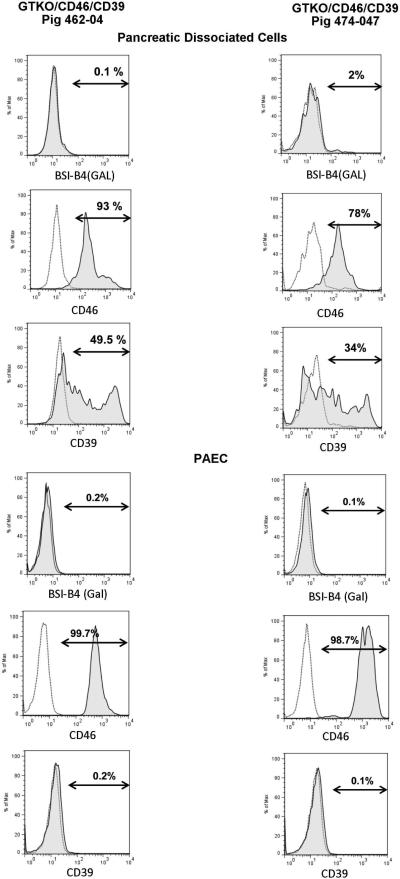
Transgene expression was confirmed by immunofluorescence when pigs were generated (28). Flow cytometry on two randomly-selected dissociated islet preparations (3-GE pig donor tissues) showed 93% and 78% hCD46-positive cells, and 49.5% and 34% hCD39-positive cells, respectively. PAEC were 99.7 and 98.7% hCD46- positive, whereas hCD39 expression was minimal (Figure 4).
Figure 4.
A single-cell suspension of pancreatic islets (A, B, C, G, H, and I) and PAEC (D, E, F, J, K, and L) from pigs P462-04 and P474-07 were stained with FITC-conjugated anti-Gal antibodies, anti-hCD46 and anti-hCD39 and subjected to flow cytometry. Expression of hCD39 was restricted to islets only (not in PAEC) since under control of the islet-specific rIns-2 promoter.
We also reviewed hCD46 immunostaining in sections of the pig pancreases before islet isolation. Figure 5 shows hCD46 expression in donor pig pancreatic tissue from the present study (A,C,E,G, and I) compared to pig pancreatic tissue of hCD46 donors used in our previously published study (B,D,F,H, and J) (18). The number of hCD46-positive cells varies between pigs and within groups.
Figure 5.
Immunostaining for insulin (green) and hCD46 (red) of pancreatic sections from the GE pig donors in the present study (A, C, E, G, I) and from hCD46 transgenic pigs used previously (B, D, F, H, J) (18). Tissues shown in A, C, E, G, I are respectively from donors for M2-11, M12-12, M1-11, M14-12, and M3-11. B, D, F, H tissues are homozygous for hCD46 while J shows tissue from a pig heterozygous for hCD46. Wild-type pig (K) and human (L) pancreases are used as negative and positive controls, respectively. Note that hCD46 transgene expression is not limited to beta cells. There is some variation in hCD46 expression, particularly in the pancreases from pigs in the present study (A, C, E, G, I). Nuclei are stained in blue.
Xenoreactive antibody titers
There was no increase in xenoreactive anti-nonGal IgM and IgG antibodies in any recipient after Tx, indicating no sensitization to pig antigens (not shown).
DISCUSSION
Islets from genetically-unmodified pigs can normalize blood glucose levels in diabetic monkeys (40,41), but intensive immunosuppressive therapy is required to protect the islets from rejection. An additional hurdle is the islet graft loss that occurs immediately after intravascular islet infusion, which involves activation of coagulation, complement and inflammatory mediators, events that have been characterized as IBMIR (42-44). The advent of GE pigs renders donor tissue more compatible to the recipients (1,6,7,18,45). hCD46 transgenic porcine islets transplanted intraportally demonstrated graft function for >1 year, despite a less intensive immunosuppressive regimen (18). However, the hCD46 pig islets were not associated with a substantial reduction of early islet destruction (18).
One potential solution was to engineer donor pig islets to express additional molecules that protect against destruction. A limited number of GE pigs, with various combinations of transgenes (Table 1) on a GTKO/hCD46 background were made available for this purpose, and therefore we could not test each individual transgene in this first series of transplants. The additional transgenes were designed to be expressed only on the pancreatic beta cells under regulation of the insulin promoter (28). Transgenic expression of hTFPI or hCD39 has been shown to provide anti-thrombotic and anti-inflammatory effects beneficial to islets in vitro and in vivo (22,23,25). Moreover, expression of CTLA4-Ig on the beta cells was aimed to provide local co-stimulation blockade (46).
In the first hours after islet Tx, when significant porcine C-peptide release indicates islet breakage (47), porcine C-peptide release was lower in recipients of 3-4-5 GE islets than in historical groups of wild-type and hCD46 islet recipients. This, along with the absence of any significant fall in blood glucose and the need for minimal dextrose infusion to prevent hypoglycemia, was interpreted as a sign of reduced islet lysis compared to historical studies. Additionally, serum IL-6 levels 1h post-Tx were significantly lower in 3-4-5 GE recipients compared to historical groups. Reduced islet lysis was, therefore, considered to be associated with the expression of one or more of the coagulation-regulatory transgenes, even though there was no clinical evidence of coagulation impairment (no bleeding, no increased ACT).
We do not know the contributions of the individual transgenes. However, preliminary in vitro observations, in which pig neonatal islet-like clusters transgenic for hCD39 were exposed to human blood, indicate that hCD39 did not affect coagulation, complement activation, or cell damage, nor prevented IgG and IgM deposition, whereas mouse islets transgenic for hTFPI exposed in vitro to human blood delayed coagulation. Nevertheless, none of the recipients of hTFPI and hCD39 islets showed impaired coagulation during early post-Tx or follow-up.
Despite this encouraging result, the 3-4-5 GE islet transplants did not show the improved long-term function that we had expected, particularly in view of the decreased early islet loss. Although we are unable to fully explain this outcome, several factors may have contributed: (i) The limitations of GE pig availability did not allow selection of donors of optimum size and age (such as retired breeders), which typically provide more robust islets (29,48,49). (ii) For the same availability reasons, we could not carry out a second transplant with genetically-identical islets, as was done in two of the recipients in the previous study (18). (iii) Due to the discontinuance of Liberase PI (Roche, Indianapolis, IN), used in our historical work, in the present study we used VitaCyte, a widely utilized enzyme blend. Although these differences could potentially affect the outcome of the transplant, we believe that the surplus islet mass transplanted should have compensated for some qualitative variability associated with these factors.
The blood levels of oral MMF were frequently lower than planned (Table 2). However, this is unlikely to have accounted for what may be considered primary graft failure and the levels were not significantly higher in the monkey (M3-11) in which prolonged graft function (1 year) was documented. Additionally, no histopathological features of rejection were found in M3-11 or M1-11, suggesting adequate immunosuppression. A unique feature of this study was the employment of islets with specific transgene expression. Questions can be raised as to whether (i) the transgenes were sufficiently expressed, (ii) the proteins (encoded by the transgenes) exerted the expected biological function, and (iii) transgenic expression of hTFPI, hCD39, and CTLA4Ig under the insulin promoter possibly interfered with the normal physiology of the beta cells. Most of these aspects require additional studies, which have to date not been possible due to the unavailability of further pigs. Nevertheless, a few observations can be made.
Differences in hTFPI immunostaining between islets from the same pancreas (Supplementary Figure 3) and a lack of immunoreactivity one year after successful Tx were documented.
hCD46-positive cells were quantified by flow cytometry of PBMC as well as PAEC (data not shown) from all pig donors tested and in two representative batches of dissociated islet-enriched fractions from 3-GE pigs (Figure 4). As expected, hCD46, under control of a constitutive promoter, demonstrated high level expression in both the dissociated islet fractions and PAEC. However, when analyzed by immunofluorescence, hCD46 expression in pancreatic sections and single islets was less intense. Consistent with use of the islet-specific rIns-2 promoter, expression of hCD39 was observed in dissociated islets, but not in PAEC, demonstrating effective tissue specificity for this transgene. Some differences in hCD46 immunostaining between the 3-4-5 GE donor pigs and hCD46 pigs used in our previous study were also documented. The zygosity of hCD46 may potentially affect gene expression, but this is unlikely to be associated with differences in long-term outcome since in both groups (3-4-5 GE pigs and historical hCD46 pigs) only one recipient was infused with islets heterozygous for hCD46. In the historical study, this transplant had a successful outcome. It is also difficult to determine whether variations in transgene expression are meaningful. Although all 4 pigs of the 4-GE and 5-GE genotypes were clones of each other (derived from the same 4-GE cell line), epigenetic differences resulting in modulation of certain gene expression are possible.
Additionally, interference of multiple transgenes (regulated under the insulin promoter) on beta cell function cannot be ruled out even though beta cell failure alone cannot explain the histological findings in the recipients with graft failure (as no islet cells survived), which was more indicative of rejection. Although 3-4-5 GE pigs responded to glucose challenge in vivo (28), and their isolated islets released insulin in vitro as well as non-GE pig islets, possible functional impairment and phenotype changes may still occur in beta cells in a Tx setting. If future studies prove these factors to be critical, it will become necessary to optimize the GE pigs prior to large-scale breeding.
In conclusion, our study indicates that, once we have a greater understanding of the effects of specific genetic modifications, GE pigs may provide a ready supply of islets with enhanced ability to survive early islet loss in a pre-clinical model. We are hopeful that optimization of this approach will eventually enable islet xenoTx to contribute to greater control of Type 1 diabetes in humans.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the following for their exceptional assistance: - Carmela Knoll and Catie Wong for laboratory assistance, Amber Funair and Leslie Wilson for veterinary technical support, and Bob Lacomy for flow cytometry. DJvdW is supported by a research grant from the American Society of Transplantation, and by the Derek Gray Traveling Scholarship Award of the International Pancreas & Islet Transplant Association. HH is the recipient of an NIH RO3 grant AI096296. ME is a recipient of a Joseph B. Patrick Research Fellowship in Transplantation at the Thomas E. Starzl Transplantation Institute. Work on xenotransplantation at the University of Pittsburgh has been in part supported by Department of Defense grant W81XWH-06-1-0317 (MT), JDRF grant 6-2005-1180 (MT), NIH grants #U19 AI090959-01, #U01 AI068642, and # R21 A1074844 (DKCC), and by Sponsored Research Agreements between Revivicor, Inc., Blacksburg, VA, and the University of Pittsburgh.
ABBREVIATIONS
- ACT
activated clotting time
- AST
arginine stimulation test
- GE
genetically-engineered
- GTKO
α1,3-galactosyltransferase gene-knockout
- hTFPI
tissue factor pathway inhibitor
- IBMIR
instant blood-mediated inflammatory reaction
- IEQ
islet equivalents
- IVGTT
intravenous glucose tolerance test
- Tx
transplantation
Footnotes
DECLARATION OF CONFLICT OF INTEREST
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. DA and CP are employees of Revivicor, Inc., a subsidiary of United Therapeutics. The other authors declare no conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article. Supplementary Methods
REFERENCES
- 1.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, Bottino R, et al. Clinical xenotransplantation: the next medical revolution? Lancet. 2012;379:672–683. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 2.van der Windt DJ, Bottino R, Kumar G, Wijkstrom M, Hara H, Ezzelarab M, et al. Clinical islet xenotransplantation: how close are we? Diabetes. 2012;61:3046–3055. doi: 10.2337/db12-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper DKC, Bottino R, Satyananda V, Wijkstrom M, Trucco M. Toward clinical islet xenotransplantation – are revisions to the IXA guidelines warranted? Xenotransplantation. 2013;2:68–74. doi: 10.1111/xen.12015. [DOI] [PubMed] [Google Scholar]
- 4.Dufrane D, Gianello P. Pig islet xenotransplantation in human: structural and physiological compatibility for human clinical application. Transplant Rev. 2012;26:183–188. doi: 10.1016/j.trre.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Elliott RB. Towards xenotransplantation of pig islets in the clinic. Curr Opin Organ Transplant. 2011;16:195–200. doi: 10.1097/MOT.0b013e3283449dec. [DOI] [PubMed] [Google Scholar]
- 6.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003;299:411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phelps C, Ball S, Vaught T, Vance AM, Mendicino M, Monahan JA, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–485. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 8.Phelps C, Vaught T, Ball S, Mendicino M, Walters A, Monahan JA, et al. Multi-transgenic pigs designed for xenoislet transplants [Abstract # IXA-O-7.3]. Xenotransplantation. 2009;16:374. [Google Scholar]
- 9.Yazaki S, Iwamoto M, Onishi A, Miwa Y, Hashimoto M, Oishi T, et al. Production of cloned pigs expressing human thrombomodulin in endothelial cells. Xenotransplantation. 2012;19:82–91. doi: 10.1111/j.1399-3089.2012.00696.x. [DOI] [PubMed] [Google Scholar]
- 10.Klymiuk N, van Buerck L, Bähr A, Offers M, Kessler B, Wuensch A, et al. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes. 2012;61:1527–1537. doi: 10.2337/db11-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayares D, Vaught T, Ball S, Ramsoondar J, Monahan JA, Mendicino M, et al. Islet-specific expression of TFPI, CD39, and CTLA4Ig in transgenic pigs designed for xenoislet transplantation [abstract #120]. Xenotransplantation. 2011;18:269. [Google Scholar]
- 12.Ayares D, Phelps C, Vaught T, Ball S, Mendicino M, Ramsoondar J, et al. Multi-transgenic pigs for vascularized pig organ xenografts [abstract #119]. Xenotransplantation. 2011;18:269. [Google Scholar]
- 13.Ayares D. Multi-transgenic pigs for xenotransplantation. Reprod Fertil Dev. 2012;25:320. [Google Scholar]
- 14.Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, et al. Current status of clinical islet transplantation. Transplantation. 2005;79:1289–1293. doi: 10.1097/01.tp.0000157273.60147.7c. [DOI] [PubMed] [Google Scholar]
- 15.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–1914. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson B, Ekdahl KN, Lorsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;6:620–626. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 17.van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007;14:288–297. doi: 10.1111/j.1399-3089.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 18.van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-Term Controlled Normoglycemia in Diabetic Non-Human Primates after Transplantation with hCD46 Transgenic Porcine Islets. Am J Transplant. 2009;9:2716–2726. doi: 10.1111/j.1600-6143.2009.02850.x. [DOI] [PubMed] [Google Scholar]
- 19.van der Windt DJ, Marigliano M, He J, Votyakova TV, Echeverri GJ, Esker B, et al. Early islet damage after direct exposure of pig islets to blood: has humoral immunity been underestimated? Cell Transplant. 2012;21:1791–1802. doi: 10.3727/096368912X653011. [DOI] [PubMed] [Google Scholar]
- 20.Beuneu C, Vosters O, Ling Z, Pipeleers D, Pradier O, Goldman M, et al. N Acetylcysteine derivative inhibits procoagulant activity of human islet cells. Diabetologia. 2007;50:343–347. doi: 10.1007/s00125-006-0529-4. [DOI] [PubMed] [Google Scholar]
- 21.Johansson H, Lukinius A, Moberg L, Lundgren T, Berne C, Foss A, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54:1755–1762. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 22.Berman DM, Cabrera O, Kenyon NM, Miller J, Tam SH, Khandekar VS, et al. Interference with tissue factor prolongs intrahepatic islet allograft survival in a nonhuman primate marginal mass model. Transplantation. 2007;84:308–315. doi: 10.1097/01.tp.0000275401.80187.1e. [DOI] [PubMed] [Google Scholar]
- 23.Lin CC, Ezzelarab M, Hara H, Long C, Lin CW, Dorling A, et al. Atorvastatin or transgenic expression of TFPI inhibits coagulation initiated by anti-nonGal IgG binding to porcine aortic endothelial cell. J Thromb Haemost. 2010;8:2001–2010. doi: 10.1111/j.1538-7836.2010.03950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dwyer KM, Robson SC, Nandurkar HH, Campbell DJ, Gock H, Murray-Segal LJ, et al. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation . J Clin Invest. 2004;113:1440–1446. doi: 10.1172/JCI19560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dwyer KM, Mysore TB, Crikis S, Robson SC, Nandurkar H, Cowan PJ, et al. The transgenic expression of human CD39 on murine islets inhibits clotting of human blood. Transplantation. 2006;82:428–432. doi: 10.1097/01.tp.0000229023.38873.c0. [DOI] [PubMed] [Google Scholar]
- 26.Phelps CJ, Ball SF, Vaught TD, Vance AM, Mendicino M, Monahan JA, et al. Production and characterization of transgenic pigs expressing porcine CTLA4-Ig. Xenotransplantation. 2009;16:477–485. doi: 10.1111/j.1399-3089.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- 27.Koshika T, Phelps C, Fang J, Lee SE, Fujita M, Ayares D, et al. Relative efficiency of porcine and human cytotoxic T-lymphocyte antigen 4 immunoglobulin in inhibiting human CD4+ T-cell responses co-stimulated by porcine and human B7 molecules. Immunology. 2011;134:386–397. doi: 10.1111/j.1365-2567.2011.03496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijkstrom M, Iwase H, Ekser B, van der Windt DJ, Long C, Phelps C, et al. Glucose metabolism in pigs expressing human genes under an insulin promoter. doi: 10.1111/xen.12145. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bottino R, Balamurugan AN, Smetanka C, Bertera S, He J, Rood PP, et al. Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation. 2007;14:74–82. doi: 10.1111/j.1399-3089.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- 30.Echeverri GJ, McGrath K, Bottino R, Hara H, Dons EM, van der Windt DJ, et al. Endoscopic gastric submucosal transplantation of islets (ENDO-STI): technique and initial results in diabetic pigs. Am J Transplant. 2009;9:2485–2496. doi: 10.1111/j.1600-6143.2009.02815.x. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 32.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 2002;51:2561–2567. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- 33.Cooper DKC, Ye Y, Niekrasz M. Heart transplantation in primates. In: Cramer DV, Podesta L, Makowka L, editors. Handbook of Animal Models in Transplantation Research. CRC Press; Boca Raton: 1994. pp. p173–200. [Google Scholar]
- 34.Jonasson O, Jones CW, Bauman A, John E, Manaligod J, Tso MO. The pathophysiology of experimental insulin-deficient diabetes in the monkey. Implications for pancreatic transplantation. Ann Surg. 1985;201:27–39. [PMC free article] [PubMed] [Google Scholar]
- 35.Casu A, Bottino R, Balamurugan AN, Hara H, van der Windt DJ, Campanile N, et al. Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: implications for translation into clinical practice. Diabetologia. 2008;51:120–129. doi: 10.1007/s00125-007-0844-4. [DOI] [PubMed] [Google Scholar]
- 36.Goto M, Johansson H, Maeda A, Elgue G, Korsgren O, Nilsson B. Low molecular weight dextran sulfate prevents the instant blood-mediated inflammatory reaction induced by adult porcine islets. Transplantation. 2004;77:741–747. doi: 10.1097/01.tp.0000114872.26990.4f. [DOI] [PubMed] [Google Scholar]
- 37.Rood PP, Bottino R, Belamurugan AN, Smetanka C, Ayares D, Groth CG, et al. Reduction of early graft loss after intraportal porcine islet transplantation in monkeys. Transplantation. 2007;82:202–210. doi: 10.1097/01.tp.0000250680.36942.c6. [DOI] [PubMed] [Google Scholar]
- 38.Hara H, Long C, Lin YJ, Tai H-C, Ezzelarab M, Ayares D, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transplant Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 39.Hara H, Ezzelarab M, Rood PP, Lin YJ, Busch J, Ibrahim Z, et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation. 2006;13:357–365. doi: 10.1111/j.1399-3089.2006.00319.x. [DOI] [PubMed] [Google Scholar]
- 40.Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12:301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 41.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–306. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 42.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51:1779–1784. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 43.Lee DY. Islet surface PEGylation attenuate the instant blood-mediated inflammatory reaction in intrahepatic islet transplantation. Macromol Res. 2012;19:904–991. [Google Scholar]
- 44.Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Källen R, et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56:2008–2015. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- 45.Thompson P, Badell IR, Lowe M, Cano J, Song M, Leopardi F, et al. Islet xenotransplantation using gal-deficient neonatal donors improves engraftment and function. Am J Transplant. 2011;11:2593–602. doi: 10.1111/j.1600-6143.2011.03720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gimmi C D, Freeman J G, Gribben G, Gray G, Nadler L M. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Källen R, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–2045. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 48.Krickhahn M, Buhler C, Meyer T, Thiede A, Ulrichs K. The morphology of islets within the porcine donor pancreas determines the isolation result: successful isolation of pancreatic islets can now be achieved from young market pigs. Cell Transplant. 2002;11:827–838. [PubMed] [Google Scholar]
- 49.Ulrichs K, Heiser A. Recent Approaches to the Isolation of Adult Porcine Islets of Langerhans. In: Cooper DKC, Kemp E, Platt JL, White DJG, editors. Xenotransplantation. Springer Berlin-Heidelberg; Berlin, Germany: 1997. pp. p565–579. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.