Abstract
Obsessive‐compulsive disorder (OCD) is an often severely disabling illness with onset generally in childhood or adolescence. Little is known, however, regarding the pattern of brain resting state activity in OCD early in the course of illness. We therefore examined differences in brain resting state activity in patients with pediatric OCD compared with healthy volunteers and their clinical correlates. Twenty‐three pediatric OCD patients and 23 healthy volunteers (age range 9–17), matched for sex, age, handedness, and IQ completed a resting state functional magnetic resonance imaging exam at 3T. Patients completed the Children's Yale Brown Obsessive Scale. Data were decomposed into 36 functional networks using spatial group independent component analysis (ICA) and logistic regression was used to identify the components that yielded maximum group separation. Using ICA we identified three components that maximally separated the groups: a middle frontal/dorsal anterior cingulate network, an anterior/posterior cingulate network, and a visual network yielding an overall group classification of 76.1% (sensitivity = 78.3% and specificity = 73.9%). Independent component expression scores were significantly higher in patients compared with healthy volunteers in the middle frontal/dorsal anterior cingulate and the anterior/posterior cingulate networks, but lower in patients within the visual network. Higher expression scores in the anterior/posterior cingulate network correlated with greater severity of compulsions among patients. These findings implicate resting state fMRI abnormalities within the cingulate cortex and related control regions in the pathogenesis and phenomenology of OCD early in the course of the disorder and prior to extensive pharmacologic intervention. Hum Brain Mapp 35:5306–5315, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: fMRI, functional connectivity, child, adolescent, cingulate cortex, neurobiology
INTRODUCTION
Obsessive‐compulsive disorder (OCD) is a disabling condition that is often associated with cognitive deficits [Burdick et al., 2008] and affects approximately 1–3% of the general population [Karno et al., 1988; Nestadt et al., 2000]. The disorder frequently develops during the formative childhood years, with as many as 75% of cases classified as early onset and tending to develop between the ages of 9 and 14 [Taylor, 2011]. Early age of onset in OCD has been shown to be reliably distinguished from late onset OCD, as early onset cases tend to be associated with a greater prevalence of the disorder in first degree relatives, more severe symptoms, a higher frequency in males, and a higher incidence of comorbid tics [Taylor, 2011]. Although such evidence suggests that early and late onset OCD may have different etiologies, the majority of neuroimaging research to date in OCD has been conducted in adult cohorts, in which age of onset may be mixed. Given evidence that OCD may be a neurodevelopmental disorder [Huyser et al., 2009], more pediatric studies are necessary to better elucidate aberrant neurodevelopmental processes during childhood and adolescence that may lead to pathological changes in adult tissue. Moreover, a focus on pediatric OCD may be more likely to limit the potentially confounding effects of illness duration and extensive pharmacologic exposure on study findings that is often observed in studies of adult patients.
Aberrations within frontal‐striatal‐thalamic‐cortical loops have been the most robust findings in the pathophysiology of OCD, including abnormalities involving the anterior cingulate cortex, orbitofrontal cortex, thalamus, and basal ganglia [Harrison et al., 2009; Insel, 1992; Maia et al., 2008]. Structural and functional abnormalities of the gray matter nodes comprising these circuits have also been repeatedly implicated, although the precise locations of the neuroanatomical abnormalities as well as the direction of findings have been inconsistent overall. The results of two recent meta‐analyses, however, both implicated volume reductions of the anterior cingulate gray matter in children and adults with OCD. One found less gray matter in the bilateral anterior cingulate/dorsal medial frontal gyri and more gray matter in the bilateral lenticular nuclei [Radua and Mataix‐Cols, 2009]; and another found less gray matter in the left anterior cingulate and bilateral orbitofrontal cortex, and more gray matter in the bilateral thalami [Rotge et al., 2009].
More recent studies have examined resting state functional magnetic resonance imaging activity in OCD, most often assessing connectivity in adult patients, using seed based region‐of‐interest analyses [Harrison et al., 2009; Jang et al., 2010; Li et al., 2012; Sakai et al., 2011; Stern et al., 2012]. These studies implicate abnormalities of fronto‐striatal‐thalamic regions, though direct comparisons across studies are difficult to make given variations in seed placement. Yang et al. [2010] conducted a study of resting state activity in psychotropic medication naive adults with OCD using the method of regional homogeneity [Yang et al, 2010]. They reported that compared with controls, patients showed higher regional homogeneity in the left anterior cingulate cortex and lower regional homogeneity in the left inferior temporal gyrus, which they interpreted as reflecting dysfunction of the action monitoring system in OCD. Hou et al. [2012] examined the amplitude of spontaneous low frequency fluctuation (ALFF), a measure thought to reflect the intensity of regional spontaneous brain activity, in adult OCD patients [Hou et al., 2012]. They found that compared with controls, patients demonstrated increased ALFF in the anterior cingulate cortex and bilateral orbitofrontal cortex, as well as decreased ALFF in the bilateral cerebellum and parietal cortex.
To date, two studies have investigated resting state activity in pediatric OCD. Fitzgerald et al. [2010] examined functional connectivity in children and adolescents with OCD both during a performance monitoring task and at rest [Fitzgerald et al., 2010]. They used regions of hyperactivation identified during the performance monitoring task as seeds for the resting state connectivity analyses. Placing the seeds in the posterior and ventral medial frontal cortex (MFC), they found that patients demonstrated less dorsal anterior cingulate cortex to right anterior operculum connectivity, as well as less ventral MFC to posterior cingulate connectivity during rest. Fitzgerald et al. [2011] examined functional connectivity during the resting state using a region‐of‐interest analysis, with seeds in the striatum and thalamus, in a cross‐sectional sample of OCD patients at child, adolescent, and adult stages of development [Fitzgerald et al., 2011]. They reported that OCD in children, compared with age‐matched controls, was associated with reduced connectivity of dorsal striatum and medial dorsal thalamus to rostral and dorsal anterior cingulate cortex, respectively.
In the current study, we examined resting state functional connectivity in a child and adolescent cohort of OCD patients and well‐matched healthy volunteers using group independent component analysis (ICA). A potential advantage of using group ICA is that it is inherently data driven and accounts for interactions among multiple brain regions, thus avoiding dependence on seed region selection. Based on basal ganglia‐thalamocortical circuits [Alexander et al., 1990; Alexander et al., 1986; Cummings, 1993] considered relevant to OCD pathophysiology and prior work [Gruner et al., 2012; Harrison et al., 2009; Szeszko et al., 2004, 2008; Jang et al., 2010; Li et al., 2012; Sakai et al., 2011; Stern et al., 2012; Zhang et al., 2011], we hypothesized that patients would exhibit resting state abnormalities within regions comprising cortical‐striatal‐thalamic‐cortical loops compared with healthy volunteers including the orbital frontal cortex and anterior cingulate. We further predicted that a greater magnitude of abnormalities would be associated with worse symptom severity among patients.
METHODS
Participants
Twenty three (12M/11F) children and adolescents with a DSM‐IV diagnosis of OCD and 23 (13M/10F) controls participated in this study. Patients and controls were individually matched for age, handedness, and IQ. All participants were between the ages of 9 and 17. The mean age of patients was 14.3 (SD = 2.1) and the mean age of healthy volunteers was 14.2 (SD = 2.2). Diagnoses were based on the Schedule for Affective Disorders and Schizophrenia for School‐Age‐Children, Present and Lifetime Version (K‐SADS‐PL) [Kaufman et al., 1997]. All children and adolescents with OCD were comprehensively assessed and diagnosed by a licensed psychologist (PG) experienced in the assessment of OCD. Among patients, comorbid diagnoses included major depressive disorder, social anxiety disorder, panic disorder, and attention deficit hyperactivity disorder (ADHD). Nine patients were psychotropic drug‐naive at the time of the scan, two were free of treatment with psychotropic drugs for at least 30 days prior to the scan, and the remaining patients were being treated with selective serotonin reuptake inhibitors (n = 12). All healthy controls were assessed using the K‐SADS‐PL and were determined to be free of any current or past psychiatric disorder. Exclusion criteria for all participants included: (1) MRI contraindications; (2) significant medical illness (3) prior psychosurgery; (4) DSM‐IV diagnosis of Tourette syndrome, schizophrenia, schizoaffective disorder, delusional disorder, brief reactive psychosis, bipolar disorder, substance use disorder, or mood disorder with psychotic features; (5) DSM‐IV mental retardation; and (6) pregnancy. All procedures were approved by the Institutional Review Board and written informed consent was obtained from one parent or legal guardian for each participant. Written assent was obtained for all participants.
Clinical Assessments
All pediatric patients with OCD were interviewed using the Children's Yale‐Brown Obsessive‐Compulsive Scale (CY‐BOCS) [Scahill et al., 1997]. All participants also completed the Multidimensional Anxiety Scale for Children (MASC) to evaluate general anxiety symptoms and severity. Handedness was assessed using the Edinburgh Handedness Inventory. Intellectual ability was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI).
Functional Magnetic Resonance Imaging
All participants received a structural MR imaging exam on a GE Signa HDx 3T system using an 8 channel head coil (TR = 7.5 ms, TE = 3 ms, slice thickness= 1 mm, matrix = 256 x 256, FOV = 240 mm). Resting state fMRI was 5 min in duration. 150 echo‐planner imaging (EPI) volumes were acquired with the following parameters: TR = 2,000 ms, TE = 30 ms, matrix = 64 x 64, FOV = 240 mm, slice thickness = 3 mm, 40 continuous axial oblique slices (one voxel = 3.75 x 3.75 x 3 mm). During the resting state exam all subjects were instructed to keep their eyes closed, not to sleep, and to “not think of anything in particular.” We investigated the potential effects of motion on functional connectivity given recent concerns in the literature [e.g. Power et al., 2012] by examining both relative and absolute motion displacement during the resting state fMRI exam.
Image Processing and Statistical Analysis
Using the brain extraction tool (BET) in the FMRIB software library [Smith, 2002], non‐brain tissue was removed from the images. Standard preprocessing was carried out using FSL including removal of the first four volumes, motion correction, spatial smoothing with kernel FWHM of 8 mm, registration to the anatomical image, and intensity normalization. Data were decomposed into 36 functional networks using spatial group independent component analysis (ICA) using GIFT software [Calhoun et al., 2001]. The number of components (or sources) in our dataset was determined empirically by using minimum description length criterion, which is a standard information theoretic method, which makes a decision based on the complexity or information content of the dataset [Calhoun et al., 2001; Rissanen, 1983].
Subject spatial maps and temporal dynamics were estimated using the dual‐regression approach. For each component, we computed expression scores by taking the dot product of the mean group and the subject's spatial map, allowing us to explain the individual level of expression for the particular map. The expression of a given network pattern in an individual subject was quantified using a voxel‐based topographic profile rating (TPR) algorithm as in our prior work [Spetsieris, 2007, 2009; Ma et al., 2007]. A subject score for a network pattern k was calculated by , where is a set of group spatial independent component maps with k = 1,2, .,C is the number of desired components, is the transpose of corresponding spatial map k for subject j. This matrix product is summed across the entire brain [Spetsieris et al., 2007]. In advantage of using the dot product is not having to use arbitrary thresholds. Regions of higher expression have a higher relative weight and regions of lower expression have a lower relative weight. The subject expression scores for each independent component per subject were fed into a forward‐selection multivariate logistic regression and utilized to identify those components that together best discriminated patients from healthy volunteers. In addition, we used leave‐one‐out cross validation to determine the level of accuracy for which we could predict group membership using those components that best discriminated the groups.
Pearson Product correlations (P < 0.05; two‐tailed) were used to investigate the clinical correlates of abnormal resting state fMRI activity in both approaches using R (v. 2.15.2) and SPSS.
RESULTS
There were no significant group differences in age, sex, handedness, or full scale IQ (P > 0.05). For patients, the mean total score on the CY‐BOCS was 26.67 (SD = 4.48), the mean CY‐BOCS Obsessions score was 13.09 (SD = 2.92) and the mean CY‐BOCS Compulsions score was 13.78 (SD = 2.28), indicating severe symptoms overall in the OCD group. Scores on the MASC did not differ significantly between groups (P > 0.05). There were no significant (Ps > 0.05) differences between patients and healthy volunteers in either relative or absolute movement displacement measures during the resting state fMRI exam. The relative displacement was 0.076 ± 0.067 mm in the control group and 0.118 ± 0.094 mm (mean ± SD) in the patient group. The absolute displacement was 0.26 ± 0.38 mm in the control group and 0.45 ± 0.38 mm (mean ± SD) in the patient group.
Logistic regression indicated that a combination of three independent components yielded maximum separation between groups (χ 2 = 16.12; df = 3, P = 0.001), including a middle frontal/dorsal anterior cingulate network, a visual network, and an anterior/posterior cingulate network. These independent components are illustrated in Figures 1, 2, 3 and the regions comprising these networks are provided in Table 1. Expression scores were higher in patients than controls in the middle frontal/dorsal anterior cingulate and anterior/posterior cingulate networks. In contrast, expression scores were lower in patients than controls in the visual network. The effect sizes (odds ratios) and the 95% confidence intervals corresponding to the three independent components were: middle frontal/dorsal anterior cingulate network: 1.70 (1.13–2.56), P = 0.01; visual network: 0.70 (0.49–0.99), P = 0.045; and anterior/posterior cingulate network 1.71 (1.12–2.60), P = 0.013. In the final model, R 2 = 0.394 with 76.1% (60.9–86.9%) accuracy. Sensitivity was 78.3% (55.8–91.7%) and specificity was 73.9% (55.8–88.9%). With a combination of these three independent components we were able to predict group membership with accuracy of 80.1% using leave‐one‐out cross validation. Investigation of the clinical correlates of the three expression scores that significantly differentiated the groups indicated that higher scores among patients in the anterior/posterior cingulate network correlated significantly with greater severity of compulsions on the CY‐BOCS (r = 0.43; df = 21, P = 0.039; Fig. 4).
Figure 1.
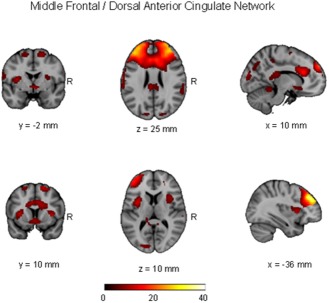
Spatial maps representing middle frontal/ dorsal anterior cingulate network for the entire 46 participants (pediatric OCD = 23 and healthy control = 23). Spatial maps are plotted as t‐statistics, thresholded at t > 3.5, and are displayed at the most informative slices.
Figure 2.
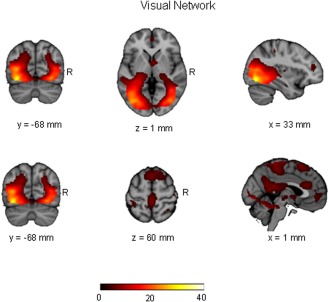
Spatial maps representing visual network for the entire 46 participants (pediatric OCD = 23 and healthy control = 23). Spatial maps are plotted as t‐statistics, thresholded at t > 3.5, and are displayed at the most informative slices.
Figure 3.
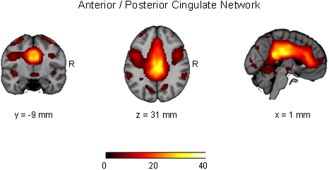
Spatial maps representing the anterior/ posterior cingulate network for the entire 46 participants (pediatric OCD = 23 and healthy control = 23). Spatial maps are plotted as t‐statistics and thresholded at t > 3.5, and are displayed at the most informative slices.
Table 1.
Regions comprising independent components
| Independent component | Anatomical regions identified | Key MNI coordinates |
|---|---|---|
| Middle frontal/ dorsal anterior cingulate network | Bilateral dorsal anterior cingulate | (−2, 26, 24) |
| Bilateral dorsal and ventral lateral prefrontal cortex (DLPFC) (VLPFC) | (−36, 42, 28) and (40, 42, 28) | |
| Bilateral precuneus | (56, −42, 46) and (−56, −44, 40) | |
| Bilateral posterior cingulate | (0, −32, 18) | |
| Bilateral planum temporale | (56, −34, 16) and (−62, −32, 18) | |
| Bilateral anterior insula | (−32, 14, 2) and (36, 12, 6) | |
| Bilateral anterior cerebellum | (6, −46, −10) | |
| Visual network | Bilateral occipital cortex including occipital fusiform gyrus | (−44, −76, −4) and (38, −72, −4) |
| Supplementary motor cortex (SMA) | (2, 22, 60) | |
| Bilateral paracentral lobule | (0, −36, 66) | |
| Bilateral posterior cingulate gyrus | (0, −46, 14) | |
| Bilateral ventral anterior cingulate | (2, 30, 22) | |
| Bilateral ventral medial prefrontal cortex (VMPC) | (2,56, 2) | |
| Anterior/posterior cingulate network | Bilateral cingulate gyrus | (0, −6, 30) |
| Bilateral precentral gyrus | (−32, −14, 64) and (36, −14, 64) | |
| DLPFC | (−48, 12, 34) and (44, 10, 32) | |
| Bilateral planum temporale | (−40, −32, 12) and (42, −26, 12) |
Positively correlating regions comprising each of the three independent components/networks that separated patients from healthy volunteers. Key MNI coordinates are provided for reference.
Figure 4.
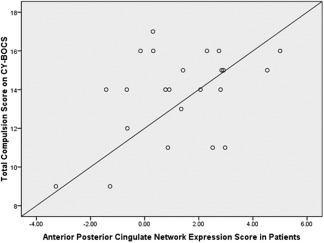
Relationship of patient expression scores within the anterior/posterior cingulate network to CYBOCS compulsions scores (r = 0.43; df = 21, P = 0.039). Expression scores for the anterior/posterior cingulate network are relative values. CY‐BOCS = Children's Yale‐Brown Obsessive Compulsive Score.
DISCUSSION
To our knowledge this study represents the first application of independent component analysis (ICA) to resting state functional magnetic resonance imaging data in pediatric OCD. Logistic regression of independent components acquired from the analysis of resting state functional magnetic resonance imaging data in child and adolescent OCD patients and age‐ and sex‐matched healthy volunteers revealed three components that accounted for 39% of the variance (i.e., R2) in activity that differentiated these groups from each other. Moreover, using leave one out cross‐validation we were able to predict group status with accuracy of 80.1% from these three independent components. Expression scores were significantly higher in two independent components comprising the cingulate in patients compared with healthy volunteers: a middle frontal/dorsal anterior cingulate and an anterior/posterior cingulate network. Moreover, higher expression scores within the anterior/posterior cingulate network were associated with greater severity of compulsions. These findings indicate that pediatric and adolescent OCD may be characterized by aberrant functional connectivity of the cingulate. Possible advantages of our approach include the use of a pediatric population, which allowed us to examine activation patterns closer to illness onset and with less exposure to psychotropic medications and the use of independent components analysis, which is primarily a data driven approach that accounts for interactions among multiple brain regions. Moreover, an additional advantage of ICA is that the components were derived from both patients and healthy volunteers, and thus, OCD specific patterns can be potentially captured as either increases or decreases.
Our findings converge with the results of prior resting state functional magnetic resonance imaging studies that have implicated anterior cingulate abnormalities in OCD. Yang et al. [2010] showed that medication naive adults with OCD demonstrated higher regional homogeneity of the left anterior cingulate [Yang et al., 2010] and Hou et al. [2012] found that adults with OCD had an increased amplitude of low frequency fluctuation in the anterior cingulate [Hou et al., 2012]. Moreover, although the only two other studies that examined resting state activity in children with OCD were region‐of‐interest analyses designed to examine functional connectivity in the medial frontal cortex and the striatum and thalamus, both demonstrated abnormal functional connectivity of the anterior cingulate to other regions compared with matched controls. Fitzgerald et al. [2010] reported decreased dorsal anterior cingulate functional connectivity to the right anterior operculum [Fitzgerald et al., 2010] and Fitzgerald et al. [2011] reported reduced functional connectivity of the rostral anterior cingulate to the dorsal striatum and reduced functional connectivity of the dorsal anterior cingulate to the medial dorsal thalamus [Fitzgerald et al., 2011]. It should be acknowledged, however, that it is difficult to directly compare the current study with these prior studies, given the different analytic approaches employed.
The finding of greater expression scores in two networks using ICA that encompass the cingulate is consistent with the growing body of literature implicating cingulate dysfunction in the neurobiology of OCD. For example, gray matter structural abnormalities in this region have been consistently demonstrated in both children and adults [Radua and Mataix‐Cols, 2009; Rotge et al., 2009]. In addition, functional neuroimaging studies have consistently reported greater brain activity in the cingulate in adult patients at rest [Swedo et al., 1989] and during symptom provocation [Adler et al., 2000; Breiter and Rauch, 1996; Rauch et al., 1994]; and during executive functioning tasks in both pediatric [Huyser et al., 2010] and adult samples [van den Heuvel et al., 2005]. Finally, abnormalities have also been demonstrated in the associated white matter of the cingulum bundle in both children [Gruner et al, 2013, Zarei et al., 2011] and adults [Cannistraro et al., 2007; Garibotto et al., 2010; Szeszko et al., 2005] with OCD.
The anterior cingulate is known to be heterogeneous and there is evidence that it may consist of several cytoarchitecturally and functionally distinct regions [Bush et al., 2000; Devinsky et al., 1995]. The region surrounding the genu contains afferent connections from the amygdala [Vogt and Pandya, 1987] and has been linked to affective processing [Whalen et al., 1998]. This rostral‐ventral “affective division” has generally been considered to be distinct from a dorsal “cognitive division” that is activated during tasks tapping error‐monitoring and response inhibition to nonemotional stimuli [Bush et al., 2000, 1998; Devinsky et al., 1995]. However, new evidence suggests that a portion of the dorsal anterior cingulate, the anterior subdivision known as the anterior midcingulate cortex, may constitute a control center that integrates information, including information about negative affect and pain, and determines optimal course of action in the face of uncertainty or competition among alternatives [Shackman et al., 2011] This portion of the dorsal anterior cingulate cortex may thus be better thought of as a “hub” where information regarding reinforcing elements, both cognitive and affective in nature, are processed to execute goal‐directed behavior. It is therefore noteworthy that resting state abnormalities among patients in the middle frontal/dorsal anterior network in the current study were observed within the dorsal anterior cingulate and that functions subserved by this region have been shown to be disrupted in OCD. This is true both for those functions traditionally associated with the dorsal ‘cognitive' division, such as error monitioring and response inhibition to non‐emotional stimuli [e.g. Menzies et al., 2007], as well as general deficits in executing goal oriented behavior that have recently been demonstrated in patients with OCD [e.g. Gillan et al., 2011, 2014].
Moreover, it may be noteworthy that both independent components, which comprised the dorsal division of the AC, also encompassed the dorsolateral prefrontal cortex (DLPFC). The anterior midcingulate cortex is known to be reciprocally connected with the DLPFC, another region implicated in cognitive control and the maintenance of goals [Shackman et al., 2011]. Specifically, the DLPFC is associated with cognitive set‐shifting, planning and working memory [Schultz et al., 1999]; and has typically been implicated in perseverative, disinhibited behavior [Cummings and Mega, 2003]. Also, structural abnormalities of the DLPFC have been reported in adults with OCD [Christian et al., 2008] and neurobiological abnormalities of this region have been reported in children with OCD [Russell et al., 2003]. The DLPFC acts together with the basal ganglia and thalamus via frontostriatal circuits [Friedlander and Desrocher, 2006]; and it is likely that the DLPFC, the anterior cingulate cortex, and the orbitofrontal cortex are all involved in the processes of inhibiting and terminating inappropriate responses and selecting and monitoring preferred behavior [Evans et al., 2004].
In the current study, the middle frontal/dorsal anterior cingulate and anterior/posterior cingulate networks also included critical cognitive control centers of the brain such as the dorsolateral prefrontal cortex, precuneus, posterior cingulate, anterior insula, and anterior cerebellum. In this regard, our findings are similar to those reported by Zhang et al. who demonstrated greater functional connectivity in regions involved in cognitive control, including the cingulate, precuneus, and cerebellum in patients with OCD [Zhang et al., 2011]. It is also particularly noteworthy that both these networks encompassed the anterior insula, as recent research indicates that the dorsal anterior cingulate and bilateral anterior insula are integral for higher order control processes [Dosenbach et al., 2006]. Moreover, the dorsal anterior cingulate and anterior insula are important nodes within the salience network, a network thought to signal the presence of events found to be personally salient to the individual, assist with conflict‐monitoring and facilitate behavioral responses to these stimuli [Menon and Lucina, 2010; Seeley et al., 2007]. Increased functional connectivity among regions comprising the salience network in patients with OCD may therefore be related to the greater attribution of importance and/or danger that these patients place on stimuli within their environment and the associated compulsive behaviors that they engage in to reduce these threats [Stern et al., 2011]. The finding in our study that higher expression scores within the anterior/posterior cingulate network were associated with severity of compulsive behavior among patients strongly supports this possibility.
Control processes are generally employed when habitual responses are inadequate to support goal oriented behavior [Shackman et al., 2011], such as situations in which there is uncertainty, when actions are associated with risks, or when contingencies change. In such situations, the dorsal anterior cingulate, particularly the anterior midcingulate cortex, is thought to synthesize information from multiple sources and signal other brain regions to provide control operations as needed. As such, it is feasible that abnormal functional connectivity of the cingulate to other control regions may contribute to problems terminating dysfunctional compulsive behavior and engaging goal oriented control. Alternatively, aberrant functional connectivity of the cingulate may develop as a compensatory mechanism for compulsive behavioral problems that are difficult to terminate.
In the ICA we also found decreased expression within a visual network in patients that included regions of the bilateral occipital cortex along with the supplementary motor cortex. Although this network was not predicted a priori and the visual system has been less well investigated in OCD, some studies support a role for the occipital cortex in OCD. Menzies et al. [2008] revealed consistent activation abnormalities in OCD in the middle occipital cortices and other regions outside of orbitofrontal striatal regions, suggesting that more distributed large scale brain regions including the occipital cortex may be involved in OCD pathology [Menzies et al., 2008]. In addition, two studies have revealed less grey matter in regions within the occipital cortices in adult OCD patients [Szeszko et al., 2008; Togao et al., 2010] and structural abnormalities of the splenium have also been identified suggesting abnormal physical connectivity in posterior brain regions including the occipital lobes [Park et al., 2011]. Moreover, the supplementary motor cortex is implicated in aspects of cognitive control such as conflict monitoring [Garavan et al. 2003; Hester et al. 2004]; and recent functional imaging studies have reported abnormalities of the supplementary motor area and pre‐supplementary motor area in adult patients with OCD [Ciesielski et al., 2012; Woon et al., 2012]. The supplementary motor area has been shown to be an effective target area for repetitive transcranial magnetic stimulation in the treatment of OCD [Jaafari et al., 2012; Kumar and Chadda, 2011]. While the supplementary motor area is generally thought to be hyperactive in OCD patients, the current study demonstrated decreased expression of this region within a visual network. It is important to note, however, that hyperactivity does not necessarily equal hyper‐synchronicity.
There were several limitations to the current study that should be acknowledged. Our sample included both prepubescent children and adolescents. In addition, the cross‐sectional design of the study precluded the investigation of how abnormalities in functional connectivity may change with age or treatment history and when these abnormalities develop. Longitudinal studies that follow children over time as symptoms develop are necessary to provide critical information regarding changes in resting state patterns. Given that our sample included both medicated and psychotropic drug‐naive children we could not draw definitive conclusions regarding the effects of psychotropic medications on these findings. Moreover, the lack of an additional cohort to replicate the findings from the logistic regression and leave one out cross validation approaches should be considered additional study limitations.
In summary, we provide evidence for resting state fMRI abnormalities within the cingulate cortex and related control centers of the brain in pediatric OCD using independent components analysis.
ACKNOWLEDGMENTS
The authors thank the children and parents who participated in this study.
Dr. Gruner, Dr. Vo, Dr. Argyelan, Dr. Ikuta, Dr. Degnan, Dr. Peters, Dr. John, Dr. Ulug, and Dr. Szeszko declare that, except for income received from their primary employers, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. Dr. Malhotra has received compensation from Eli Lilly, Schering‐Plough/Merck, Sunovion Pharmaceuticals Inc., Genomind, and Shire.
REFERENCES
- Adler CM, McDonough‐Ryan P, Sax KW, Holland SK, Arndt S, Strakowski SM (2000): fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res 34:317–324. [DOI] [PubMed] [Google Scholar]
- Alexander GE (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR (1990): Basal‐ganglia‐thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85:119–146. [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X‐N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombs S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL (1996): Functional MRI and the study of OCD: From symptom provocation to cognitive‐behavioral probes of cortico‐striatal systems and the amygdala. Neuroimage 4:S127–S138. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Robinson DG, Malhotra AK, Szeszko PR (2008): Neurocognitive profile analysis in obsessive‐compulsive disorder. J Int Neuropsychol Soc 14:640–645. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL (1998): The counting Stroop: an interference task specialized for functional neuroimaging—Validation study with functional MRI. Hum Brain Mapp 6:270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson G, Pekar J (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistraro PA, Makris N, Howard JD, Wedig MM, Hodge SM, Wilhelm S, Kennedy DN, Rauch SL (2007): A diffusion tensor imaging study of white matter in obsessive‐compulsive disorder. Depress Anxiety 24:440–446. [DOI] [PubMed] [Google Scholar]
- Christian C, Lencz T, Robinson D, Burdick K, Ashtari M, Malhotra AK, Betensky JD, Szeszko PR (2008): Gray matter structural alterations in obsessive‐compulsive disorder: Relationship to neuropsychological functions. Psychiatry Res Neuroimaging 164:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesielski KT, Rauch SL, Ahlfors SP, Vangel ME, Wilhelm S, Rosen BR, Hamalainen MS (2012): Role of medial cortical networks for anticipatory processing in obsessive‐compulsive disorder. Hum Brain Mapp 33:2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi L, Harrison BJ, Pujol J, Harding IH, Fornito A, Pantelis C, Yucel M (2012): Functional alterations of large‐scale brain networks related to cognitive control in obsessive‐compulsive disorder. Hum Brain Mapp 33:1089–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega MS (2003): Neuropsychiatry and Behavioral Neuroscience. New York: Oxford University Press. [Google Scholar]
- Cummings JL (1993): Frontal‐subcortical circuits and human behavior. Arch Neurol 50:873–880. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behavior. Brain 118:279–306. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006): A core system for the implementation of tasks. Neuron 50:799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DW, Lewis MD, Iobst E (2004): The role of the orbitofrontal cortex in normally developing compulsive‐like behaviors and obsessive‐compulsive disorder. Brain Cogn 55:220–234. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson‐Muth KC, Maynor MR, Welsh RC, Hanna GL, Taylor SF (2010): Altered function and connectivity of the medial frontal cortex in pediatric obsessive‐compulsive disorder. Biol Psychiatry 68:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Stern ER, Angstadt M, Hanna GL, Abelson JL, Taylor SF (2011): Developmental alterations of frontal‐striatal‐thalamic connectivity in obsessive‐compulsive disorder. J Am Acad Child Adolesc Psychiatry 50:938–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander L, Desrocher M (2006): Neuroimaging studies of obsessive‐compulsive disorder in adults and children. Clin Psychol Rev 26:32–49. [DOI] [PubMed] [Google Scholar]
- Garavan, H , Ross, T.J , Kaufman, J , Stein, EA (2003): A midline dissociation between error‐processing and response‐conflict monitoring. Neuroimage 20:1132–1139. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Scifo P, Gorini A, Alonso CR, Brambati S, Bellodi L, Perani D (2010): Disorganization of anatomical connectivity in obsessive compulsive disorder: A multi‐parameter diffusion tensor imaging study in a subpopulation of patients. Neurobiol Dis 37:468–476. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG (2000): Action‐monitoring dysfunction in obsessive‐compulsive disorder. Psychol Sci 11:1–6. [DOI] [PubMed] [Google Scholar]
- Gillan CM, Papameyer MA, Morein‐Zamir S, Sahakian, BJ , Fineberg NA, Robbins T, de Wit, S (2011): Disruption in the balance between goal‐directed behavior and habit learning in obsessive‐compulsive disorder. Am J Psychiatry 168:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillan CM, Morein‐Zamir S, Urcelay GP, Sule A, Voon V, Apergis‐Schoute AM, Fineberg NA, Sahakian BJ, Robbins TW (2014): Enhanced avoidance habits in obsessive‐compulsive disorder. Biol Psychiatry 75:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner P, Vo A, Ikuta T, Mahon K, Peters BD, Malhotra AK, Ulug AM, Szeszko PR (2012): White matter abnormalities in pediatric obsessive‐compulsive disorder. Neuropsychopharmacology 37:2730–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Soriano‐Mas C, Pujol J, Ortiz H, Lopez‐Sola M, Hernandez‐Ribas R, Deus J, Alonso P, Yucel M, Pantelis C, Menchon JM, Cardoner N (2009): Altered corticostriatal functional connectivity in obsessive‐compulsive disorder. Arch Gen Psychiatry 66:1189–1200. [DOI] [PubMed] [Google Scholar]
- Hester, R , Fassbender, C , Garavan H (2004). Individual differences in error processing: A review and reanalysis of three event‐related fMRI studies using the GO/NOGO task. Cereb Cortex 14:986–994. [DOI] [PubMed] [Google Scholar]
- Hou J, Wu W, Lin Y, Wang J, Zhou D, Guo J, Gu S, He M, Ahmed S, Hu J, Qu W, Li H (2012): Localization of cerebral functional deficits in patients with obsessive‐compulsive disorder: A resting‐state fMRI study. J Affect Disord 138:313–321. [DOI] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, de Haan E, Boer F (2009): Paediatric obsessive‐compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev 33:818–830. [DOI] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, Wolters LH, de Haan E, Boer F (2010): Functional magnetic resonance imaging during planning before and after cognitive‐behavioral therapy in pediatric obsessive‐compulsive disorder. J Am Acad Child Adolesc Psychiatry 49:1238–1248. [DOI] [PubMed] [Google Scholar]
- Insel TR (1992): Toward a neuroanatomy of obsessive‐compulsive disorder. Arch Gen Psychiatry 49:739–744. [DOI] [PubMed] [Google Scholar]
- Jaafari N, Rachid F, Rotge JY, Polosan M, El‐Hagen W, Belin D (2012): Safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of obsessive‐compulsive disorder: a review. World J Biol Psychiatry 13:164–177. [DOI] [PubMed] [Google Scholar]
- Jang JH, Kim JH, Jung WH, Choi JS, Jung MH, Lee JM, Choi CH, Kang DH, Kwon JS (2010): Functional connectivity in fronto‐subcortical circuitry during the resting state in obsessive‐compulsive disorder. Neurosci Lett 474:158–162. [DOI] [PubMed] [Google Scholar]
- Karno M, Golding JM, Sorenson SB, Burnam MA (1998): The epidemiology of obsessive‐compulsive disorder in five US communities. Arch Gen Psychiatry 45:1094–1099. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997): Schedule for affective disorders and schizophrenia for school‐age children‐present and lifetime version (K‐SADS‐PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- Kumar N, Chadda RK (2011): Augmentation effect of repetitive transcranial magnetic stimulation over the supplementary motor cortex in treatment refractory patients with obsessive compulsive disorder. Indian J Psychiatry 53:340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Li S, Dong Z, Luo J, Han H, Xiong H, Guo Z, Li Z (2012): Altered resting state functional connectivity patterns of the anterior prefrontal cortex in obsessive‐compulsive disorder. Neuroreport 23:681–686. [DOI] [PubMed] [Google Scholar]
- Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D (2007): Abnormal metabolic network activity in Parkinson's disease: Test‐retest reproducibility. J Cereb Blood Flow Metab 27:597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia TV, Cooney RE, Peterson BS (2008): The neural bases of obsessive‐compulsive disorder in children and adults. Dev Psychopathol 20:1251–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Lucina QU (2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Archard S, Chamberlain SR, Fineberg N, Chen C, del Campo N, Sahakian BJ, Robbins TW, Bullmore E (2007): Neurocognitive endophenotypes of obsessive‐compulsive disorder. Brain 130:3223–3236. [DOI] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET (2008): Integrating evidence from neuroimaging and neuropsychological studies of obsessive‐compulsive disorder: the orbitofrontal‐striatal model revisited. Neurosci Biobehav Rev 32:525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestadt G, Samuels J, Riddle M, Bienvenu OJ III, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn‐Saric R (2000): A family study of obsessive‐compulsive disorder. Arch Gen Psychiatry 57:358–363. [DOI] [PubMed] [Google Scholar]
- Park HY, Park JS, Kim SH, Jang JH, Jung WH, Choi JS, Kang DH, Lee JM, Kwon JS (2011): Midsagittal structural differences and sexual dimorphism of the corpus callosum in obsessive‐compulsive disorder. Psychiatry Res 192:147–153. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J, Mataix‐Cols D (2009): Voxel‐wise meta‐analysis of grey matter changes in obsessive‐compulsive disorder. Br J Psychiatry 195:393–402. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ (1994): Regional cerebral blood flow measured during symptom provocation in obsessive‐compulsive disorder using oxygen 15‐labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 51:62–70. [DOI] [PubMed] [Google Scholar]
- Rissanen J (1983): A universal prior for integers and estimation by minimum description length. Ann Statistics 11:416–431. [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B (2009): Meta‐analysis of brain volume changes in obsessive‐compulsive disorder. Biol Psychiatry 65:75–83. [DOI] [PubMed] [Google Scholar]
- Russell A, Cortese B, Lorch E, Ivey J, Banerjee P, Moore G, Rosenberg DR (2003): Localized functional neurochemical marker abnormalities in dorsolateral prefrontal cortex in pediatric obsessive‐compulsive disorder. J Child Adolesc Psychopharmacol 13:S31–S38. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Narumoto J, Nishida S, Nakamae T, Yamada K, Nishumura T, Fukui K (2012): Corticostriatal functional connectivity in non‐medicated patients with obsessive‐compulsive disorder. Eur Psychiatry 26:463–469. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin‐Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF (1997): Children's Yale‐Brown Obsessive Compulsive Scale: Reliability and validity. J Am Acad Child Adolesc Psychiatry 36:844–852. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Evans DW, Wolf M (1999): Neuropsychological models of childhood obsessive‐compulsive disorder. Child Psychiatric Clin N Am 8:513–531. [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Grecius MD (2007): Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011): The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spetsieris PG, Ma Y, Dhawan V, Eidelberg D (2009): Differential diagnosis of parkinsonian syndromes using PCA‐based functional imaging features. Neuroimage 45:1241–1252. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Ma Y, Eckert T, Dhawan V, Eidelberg D (2007): New strategies for automated differential diagnosis of degenerative brain disorders. Conf Proc IEEE Eng Med Biol Soc 3421–3425. [DOI] [PubMed] [Google Scholar]
- Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF (2012): Resting‐state functional connectivity between fronto‐parietal and default mode networks in obsessive‐compulsive disorder. PLoS One 7:e36356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Welsh RC, Fitzgerald KD, Gehring WJ, Lister JJ, Himle, JA , Abelson JL, Taylor SF (2011): Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive‐compulsive disorder. Biol Psychiatry 69:583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL (1989): Cerebral glucose metabolism in childhood‐onset obsessive‐compulsive disorder. Arch Gen Psychiatry 46:518–523. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, Ivey J, Rose M, Banerjee SP, Bhandari R, Moore GJ, Rosenberg DR (2004): Brain structural abnormalities in psychotropic drug‐naive pediatric patients with obsessive‐compulsive disorder. Am J Psychiatry 161:1049–1056. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, Lim KO (2005): White matter abnormalities in obsessive‐compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry 62:782–790. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Christian C, Macmaster F, Lencz T, Mirza Y, Taormina SP, Easter P, Rose M, Michalopoulou GA, Rosenberg DR (2008): Gray matter alterations in psychotropic drug‐naive pediatric obsessive‐compulsive disorder: an optimized voxel‐based morphometry study. Am J Psychiatry 165:1299–1307. [DOI] [PubMed] [Google Scholar]
- Taylor S (2011): Early versus late onset obsessive‐compulsive disorder: Evidence for distinct subtypes. Clin Psychol Rev 31:1083–1100. [DOI] [PubMed] [Google Scholar]
- Togao O, Yoshiura T, Nakao T, Nabeyama M, Sanematsu H, Nakagawa A, Noguchi T, Hiwatashi A, Yamashita K, Nagao E, Kanba S, Honda H (2010): Regional gray and white matter volume abnormalities in obsessive‐compulsive disorder: A voxel‐based morphometry study. Psychiatry Res 184:29–37. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS (2003): Overactive action monitoring in obsessive‐compulsive disorder: Evidence from functional magnetic resonance imaging. Psychol Sci 14:347–353. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Cath DC, van Balkom AJ, van Hartskamp J, Barkhof F, van Dyck R (2005): Frontal‐striatal dysfunction during planning in obsessive‐compulsive disorder. Arch Gen Psychiatry 62:301–309. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter SC (2002): The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav 77:477–482. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN (1987): Cingulate cortex of the rhesus monkey. II. Cortical afferents. J Comp Neurol 262:271–289. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, Rauch SL (1998): The emotional counting Stroop paradigm: A functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry 15:1219–1228. [DOI] [PubMed] [Google Scholar]
- Woon FL, Allen MD, Miller CH, Hedges DW (2012): The functional magnetic resonance imaging‐based verbal fluency test in obsessive‐compulsive disorder. Neurocase 18:424–440. [DOI] [PubMed] [Google Scholar]
- Yang T, Cheng Y, Li H, Jiang H, Luo C, Shan B, Xu L, Xu X (2010): Abnormal regional homogeneity of drug‐naive obsessive‐compulsive patients. Neuroreport 21:786–790. [DOI] [PubMed] [Google Scholar]
- Zarei M, Mataix‐Cols D, Heyman I, Hough M, Doherty J, Burge L, Winmill L, Nijhawan S, Matthews PM, James A (2011): Changes in gray matter volume and white matter microstructure in adolescents with obsessive‐compulsive disorder. Biol Psychiatry 70:1083–1090. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wang J, Yang Y, Wu Q, Li B, Chen L, Yue Q, Tang H, Yan C, Lui S, Huang X, Chan RC, Zang Y, He Y, Gong Q (2011): Abnormal small‐world architecture of top‐down control networks in obsessive‐compulsive disorder. J Psychiatry Neurosci 36:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


