Summary
How do people cope with setbacks and persist with their goals? We examine how perceiving control over setbacks alters neural processing in ways that increase persistence through adversity. For example, a student might retake a class if initial failure was due to controllable factors (e.g., studying) but give up if failure was uncontrollable (e.g., unfair exam questions). Participants persisted more when they perceived control over setbacks, and when they experienced increased negative affect to setbacks. Consistent with previous observations involving negative outcomes, ventral striatum and ventromedial prefrontal (VMPFC) activity was decreased in response to setbacks. Critically, these structures represented distinct neural mechanisms for persistence through adversity. Ventral striatum signal change to controllable setbacks correlated with greater persistence, whereas VMPFC signal change to uncontrollable setbacks mediated the relationship between increased negative affect and persistence. Taken together, the findings highlight how people process setbacks and adapt their behavior for future goal pursuit.
Introduction
Success is often determined by persistence, that is, the continuance of a course of action despite setbacks. A failing grade in a required class, for example, can be a setback for a student completing a degree. Potential success depends on whether the student responds to the setback by persisting (i.e., retaking the failed class) or by giving up (i.e., switching to a less preferred, possibly easier degree). The belief that a person has control over the setback is one factor that encourages persistence (Andrews and Debus, 1978). For instance, a student who believes that the failing grade was due to an incorrect studying strategy may be more likely to persist and retake the class than a student who attributes the failing grade to unfair exam questions. In both cases, the setback yields the same consequence – a negative outcome and inherent negative affect – but the context in which the outcome is perceived, controllable or uncontrollable, may differentially influence behavior. Therefore, a fruitful avenue for understanding how people respond to setbacks is to examine how the perception of control influences affective and neural responses to setbacks and their relation to persistence behavior.
The perception of control is likely to influence strategies that people use to cope with the negative affect and daily life disruptions caused by negative outcomes. For example, problem-focused strategies that focus on changing behavior to avoid future negative outcomes are appropriate when individuals perceive control over such outcomes (Folkman and Lazarus, 1988; LeDoux and Gorman, 2001; Troy et al., 2013). These strategies can increase persistence after setbacks by focusing on how to avoid an outcome while persisting with a goal (e.g., change studying behavior to avoid a failing grade). Neural signals in the striatum may be important in problem-focused coping strategies as these signals underlie outcome-based behavioral changes (LeDoux and Gorman, 2001; Delgado et al., 2009; Lewis et al., 2013). Specifically, striatum signals can represent prediction errors, which can devalue a current behavior in favor of an alternative by indicating that an outcome was worse than expected (Li et al., 2011; Schönberg et al., 2007; Sutton and Barto, 1998). Striatum signals coinciding with negative outcomes may occur as decreases below a neutral outcome baseline (Breiter et al., 2001; Delgado et al., 2000; Tricomi and Fiez, 2008), and can influence behavioral responses (e.g., to avoid a controllable negative outcome; Darvas et al., 2011; Schönberg et al., 2007). It is unclear, however, how these signals relate to persistence after a setback (e.g., retaking a failed class after changing studying behavior).
When setbacks are perceived to be uncontrollable, an alternative strategy may be to employ an emotion-focused coping strategy aimed at interpreting negative affect in an advantageous manner (Folkman and Lazarus, 1988; Gross, 1998; Troy et al., 2013). This type of strategy might involve reframing the negative outcome to focus on less negative (or more positive) consequences (Gross, 1998). For example, a student who believed a failed exam was due to unfair questions may focus on the possibility that the exam will be better in the future, and thus persist by retaking the class. Prior research identifies various cortical regions in cognitively reframing negative affective information (Wager et al., 2008), but ventromedial prefrontal cortex (VMPFC) activity is of particular importance because it is also reported to coincide with incurred negative outcomes such as monetary loss or physical pain (Clark et al., 2009; Schiller and Delgado, 2010; Sokol-Hessner et al., 2013). VMPFC signals decrease for monetary losses (Clark et al., 2009; Sokol-Hessner et al., 2013), and are also modulated by cognitive regulation strategies, for example, to focus on something calming (Schiller and Delgado, 2010; Sokol-Hessner et al., 2013). Thus, VMPFC responses may be part of emotion-focused coping with negative affect generated by uncontrollable setbacks, but how VMPFC responses relate to persistence behavior is not yet known.
The current study examines the neural mechanisms underlying our responses to setbacks and their relation to persistence behavior. While undergoing functional magnetic resonance imaging (fMRI), participants played a game designed to measure persistence with a goal after a setback. We manipulated the perceived controllability of the setback (controllable or uncontrollable) as well as the potential value of persisting with a goal in comparison to alternatives (high or low alternative value). In the game, participants chose a goal to pursue (depicted as a path) and encountered setbacks. After every setback, participants had to decide whether to persist with their previously chosen path or pursue a different path (Figure 1). Controllable setbacks could be avoided by pressing the correct button (learned by trial and error). Uncontrollable setbacks could be avoided by a random determination of the computer. Although both controllable and uncontrollable setbacks were determined by chance, and in fact equivalent, we hypothesized that participants would persist more after controllable than uncontrollable setbacks (Andrews and Debus, 1978). Furthermore, we hypothesized that striatum responses to controllable setbacks and VMPFC responses to uncontrollable setbacks would relate to persistence behavior, consistent with roles for these regions in alternative ways of coping with negative outcomes (Delgado et al., 2009; LeDoux and Gorman, 2001; Lewis et al., 2013; Schiller and Delgado, 2010; Sokol-Hessner et al., 2013).
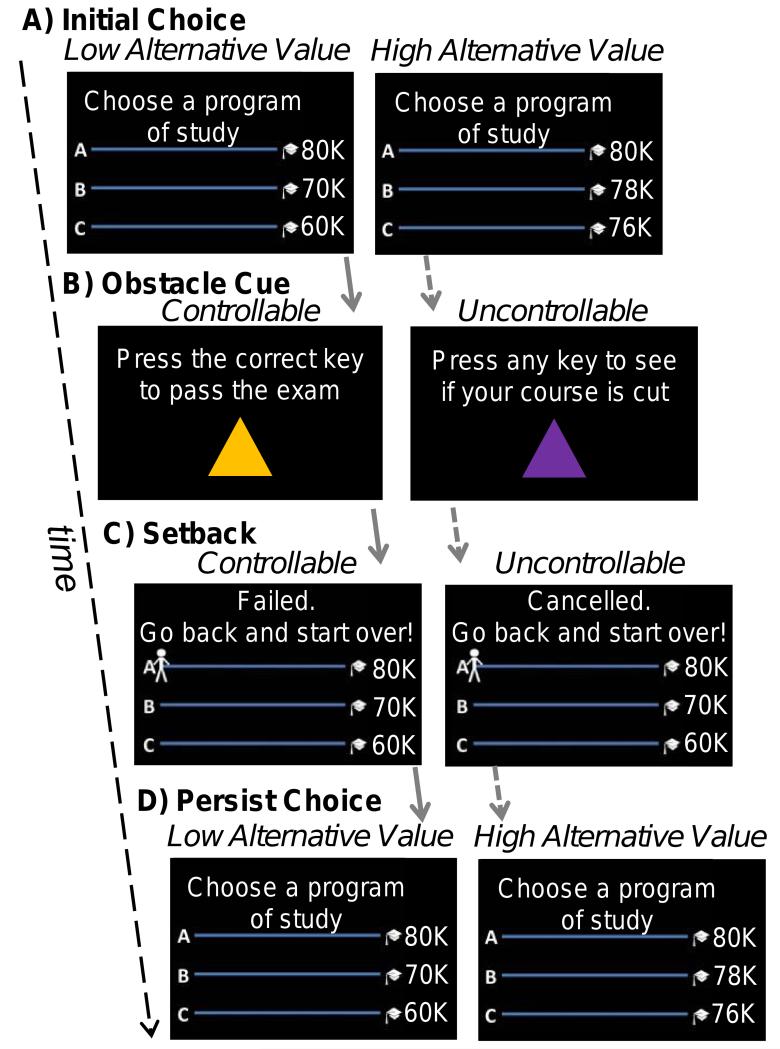
Figure 1.
Persistence after setbacks (PAS) task. A) Initial path choice (2s), B) Respond to an obstacle cue (2s), C) Experience a setback (2s), and D) Choose to persist (or not) on the previously chosen path (2s). Inter stimulus intervals (2/4/6s) occurred between each event.
Results
Perceived control over negative outcomes influences persistence after setbacks
Participants persisted more often following controllable than uncontrollable setbacks, even though both occurred with the same frequency. A 2 (setback controllability: controllable or uncontrollable) × 2 (alternative value: high or low) ANOVA showed persistence was influenced by a main effect of setback controllability (F(1,29) = 20.69, p<.001). Participants persisted more after controllable than uncontrollable setbacks in the low (t(29) = 3.68, p = .001) and high (t(29) = 3.95, p < .001) alternative value conditions (see Tables S1, S2). Persistence was not significantly influenced by the value of alternatives (main effect and interaction Fs < 1). In these results persistence is measured by choices to continue on the same path after a setback. Using an alternative measure that includes choices to switch to a higher value path, the results remain the same (see Supplemental Experimental Procedures).
Increased negative affect relates to greater persistence after setbacks
After the scan, participants rated how they felt (valence and intensity) when they received controllable or uncontrollable setbacks, and ratings were tested for a correlation with persistence behavior. Greater negative affect experienced with setbacks correlated with increased levels of behavioral persistence. Specifically, greater negative valence and intensity ratings experienced with controllable setbacks correlated with greater persistence after controllable setbacks (low alternative value condition; valence: r = .42, p = .02; intensity: r = .34, p = .06; positive numbers indicate greater negative valence and intensity). The same was true for uncontrollable setbacks: greater negative affect correlated with greater persistence (valence: r = .35, p = .06; intensity: r = .40, p=.03). Affect ratings did not significantly differ for controllable (valence mean = .90, s.d. = .55; intensity mean = 2.40, s.d. = .81) compared to uncontrollable setbacks (valence mean = 1.00, s.d. = .79, t(29) = .57, p = .57; intensity mean = 2.70, s.d. = 1.21, t(29) = −1.39, p = .17).
Controllable and uncontrollable setbacks elicit distinguishable neural responses
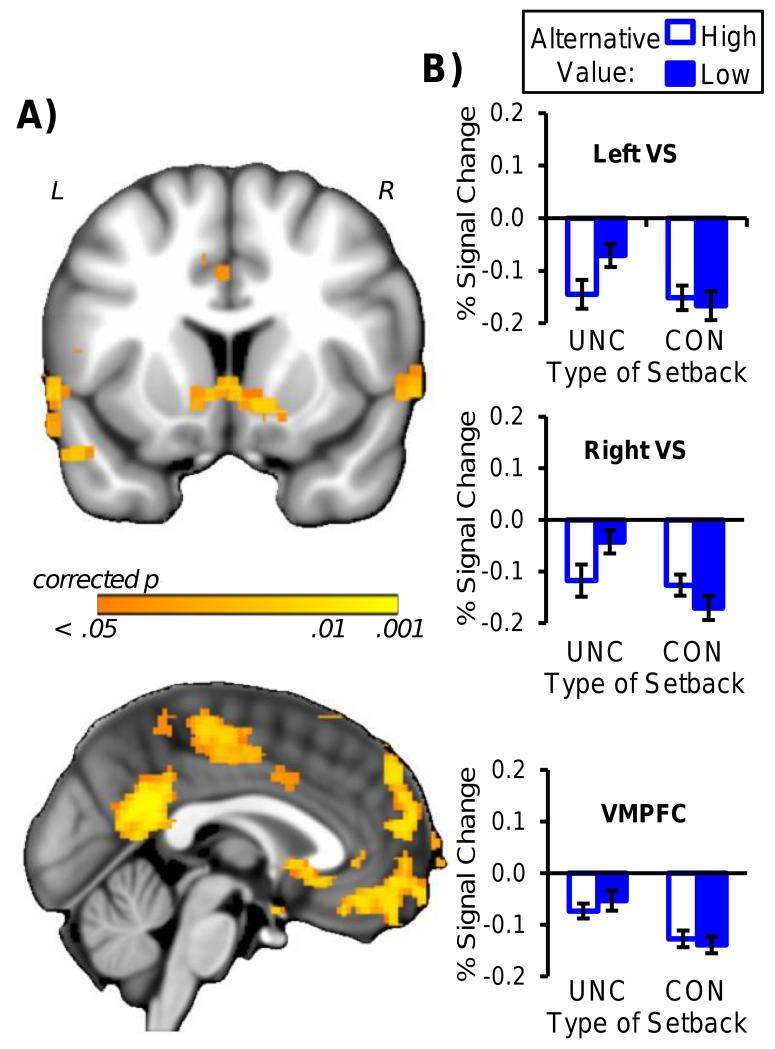
The effect of perceived control on neural responses to setbacks was assessed by a 2 (setback controllability: controllable or uncontrollable) × 2 (alternative value: high or low) ANOVA conducted on BOLD signals associated with the setback phase of trials (Figure 1C). The main effect of setback controllability influenced activity in ventral striatum and VMPFC, in addition to other regions in prefrontal, parietal, and temporal cortex (Table S3). No regions exhibited responses to setbacks that were significantly influenced by the manipulation of alternative value or by its interaction with setback controllability. Consistent with prior reports of decreases in neural activity elicited by negative outcomes (Breiter et al., 2001; Clark et al., 2009; Sokol-Hessner et al., 2013; Tricomi and Fiez, 2008), parameter estimates extracted from activation peaks in ventral striatum (x,y,z peaks: left = −6, 14, −6, right = 12, 10, −8) and VMPFC (left: −10, 44, −6) revealed that activity decreased below baseline to a greater degree for controllable compared to uncontrollable setbacks (Figure 2).
Figure 2.
Neural regions modulated by setback controllability. A) Regions displaying greater activity for controllable compared to uncontrollable setbacks (p < .05, corrected (TFCE), top image at y = 10, bottom image at x = −2). B) Ventral striatum and VMPFC signal change (SEM). L=left, R=right, UNC=Uncontrollable, CON=Controllable.
Decreases in the ventral striatum signal to controllable setbacks are associated with greater persistence
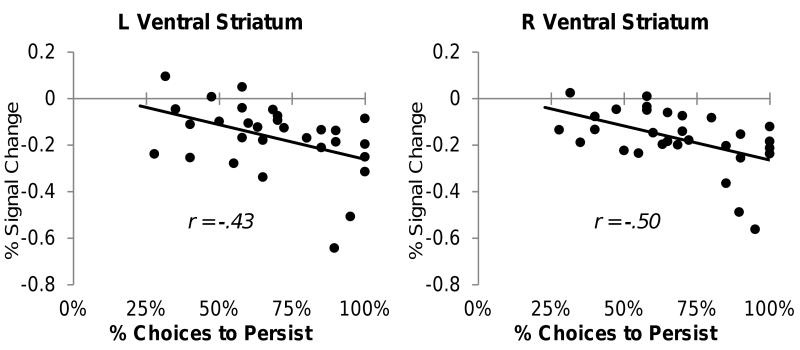
At the level of individual participants, decreases in ventral striatum activity for controllable setbacks were associated with greater persistence after controllable setbacks. In the low alternative value condition, larger bilateral ventral striatum signal decreases for controllable setbacks related to greater persistence (left: r = −.43, p = .017, right: r = −.50, p = .005, Figure 3; the relationships were similar if averaging across the alternative value conditions, left: r = −.44, p = .016, right: r = −.35, p = .06). In other words, individuals who exhibited more pronounced decreases in activation in response to controllable setbacks were those who exhibited more behavioral persistence. Notably, this relationship was only observed during controllable setbacks, as ventral striatum responses for uncontrollable setbacks showed no significant relationship to behavioral persistence (left: r = −.12, p = .54; right: r = .24, p = .21). Furthermore, the ventral striatum relation to persistence in the controllable setback condition was apparent even when controlling for the relation between negative affect and persistence (partial correlation left ventral striatum: r = −.54, p = .003; right: r = −.52, p = .005). Ventral striatum signal change to controllable setbacks was not significantly correlated with affective valence (left: r = .03, p = .90; right: r = −.10, p = .61) or intensity ratings (left: r = .16, p = .40; right: r = −.04, p = .83) of controllable setbacks.
Figure 3.
Individual percent signal change in ventral striatum in relation to behavioral persistence (controllable, low alternative value condition).
VMPFC signal change to uncontrollable setbacks mediates the relationship between negative affect and persistence
VMPFC responses to uncontrollable setbacks exhibited significant relationships with persistence behavior as well as with setback-related affect. VMPFC percent signal change positively correlated with behavioral persistence in the uncontrollable setback condition (low alternative value condition: r = .49, p = .006). Notably, this effect was observed only during uncontrollable setbacks, as VMPFC percent signal change to controllable setbacks did not significantly correlate with persistence (low alternative value condition: r = −.26, p = .17). Given that inverse correlations between VMPFC and subcortical activity have proven to be predictive of negative emotion processing (Kim et al., 2011; Pezawas et al., 2005), we further examined condition specific functional connectivity of VMPFC with the ventral striatum ROIs. Although VMPFC activity during uncontrollable setbacks (low alternative condition) was negatively related to right ventral striatum activity (t(29) = 2.63, p < .05), the strength of VMPFC connectivity with ventral striatum was not significantly related to persistence (Figure S1).
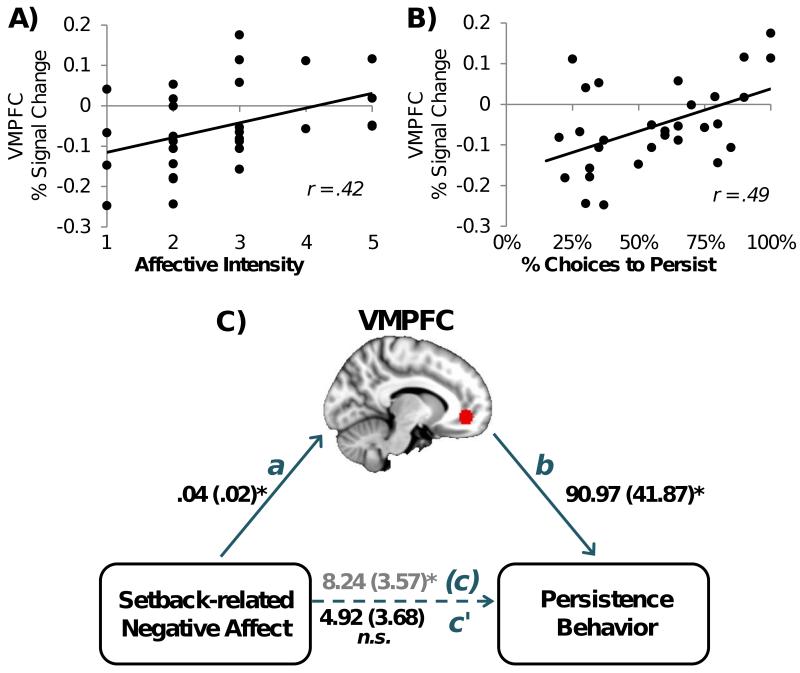
The significant relationship between VMPFC and persistence prompted us to examine the relationships between VMPFC signal change and uncontrollable setback-related affect. Increased VMPFC percent signal change to uncontrollable setbacks in the low alternative value condition was positively correlated with uncontrollable setback-related negative affective valence (r = .37, p = .04) and intensity ratings (r = .42, p = .02).
As a next step to explore the relationship between setback-related affect, neural responses, and persistence behavior, we then tested whether VMPFC signal change to uncontrollable setbacks mediated the relationship between uncontrollable setback-related affect and persistence behavior. As described above, negative affect correlated with persistence behavior, and VMPFC signal change to uncontrollable setbacks correlated with negative affect (Figure 4A) as well as persistence behavior (Figure 4B). Thus, we tested a mediation model in which increased negative affect indirectly relates to persistence by way of its relation to VMPFC signal change (Preacher and Hayes, 2004). Using uncontrollable setback-related affective intensity ratings as an indicator of negative affect in this mediation model, negative affective intensity predicted persistence (total effect: B = 8.24, t(27) = 2.31, p = .03, Figure 4C path c), but the relation was no longer significant when controlling for VMPFC percent signal change (direct effect: B = 4.92, t(27) = 1.33, p = .19, Figure 4C path c’). Furthermore, the path from negative affective intensity, through VMPFC percent signal change, to behavioral persistence was significant (indirect effect: B = 3.45, bias corrected 95% confidence interval (CI) = .03 to 9.99; Figure 4C, paths a and b), indicating that VMPFC percent signal change mediated the relationship between negative affective intensity and behavioral persistence.
Figure 4.
VMPFC mediates the relationship between setback-related negative affect and persistence. A) and B) Individual VMPFC signal change for uncontrollable setbacks (low alternative value) in relation to A) negative affective intensity and B) persistence. C) Path a: effect of negative affective intensity on VMPFC; path b: effect of VMPFC on persistence, controlling for negative affective intensity; path c: total effect of negative affective intensity on persistence; and path c’: direct effect of negative affective intensity on persistence, controlling for VMPFC. Path coefficients are unstandardized, to indicate change expected (in units of the outcome) for a 1 unit change in the predictor (i.e., 1 point on the affect scale or 1% VMPFC signal change). * indicates p < .05.
Results were similar with negative valence ratings as the measure of negative affect (total effect: B = 10.95, t(27) = 1.96, p = .06; direct effect: B = 6.04, t(27) = 1.08, p = .29; indirect effect: B = 5.19, bias corrected 95% CI = .04 to 16.07). We also tested the alternative model that setback-related affect might mediate the relationship between VMPFC signal change and persistence behavior. However, VMPFC percent signal change remained a significant predictor of persistence in this model and the indirect effect was not significant (with valence as mediator: VMPFC direct effect B = 97.12, t(27) = 2.34, p = .03, indirect effect: B = 14.29, bias corrected 95% CI = −12.48 to 57.49; with intensity as mediator: VMPFC direct effect B = 90.67, t(27) = 2.17, p = .04, indirect effect: B = 21.93, bias corrected 95% CI = −5.43 to 67.14).
Discussion
How do we persist when faced with a setback? The present study investigated the mechanisms through which we cope with negative affect inherent in a setback to promote persistence. Ventral striatum responses related to increased persistence behavior after controllable setbacks whereas VMPFC responses related to persistence after uncontrollable setbacks. Critically, VMPFC responses mediated the relationship between negative affect and persistence after uncontrollable setbacks. The findings suggest different mechanisms through which people can cope with negative outcomes and adapt their behavior in situations where setbacks are a necessary obstacle on the route to success.
Correct mistakes but stay the course: Ventral striatum response to behavior-correcting controllable setbacks correlates with persistence
When we perceive a setback as controllable, we believe that taking a different action might have avoided the negative outcome. For example, when a student believes that a failed class was due to poor studying, the student can change behavior to avoid failure the next time around. Students who cope with failures in this way may be more likely to persist after setbacks (Andrews and Debus, 1978). In the current study, a neural region previously associated with devaluing a behavior based on a negative outcome displayed activity correlated with persistence after controllable setbacks. Similar to prior findings (Delgado et al., 2000; Schönberg et al., 2007; Tricomi and Fiez, 2008), ventral striatal activity decreased for negative outcomes. This signal decrease below baseline is consistent with a negative prediction error signal in the striatum that can drive learning to avoid aversive outcomes (Darvas et al., 2011; Delgado et al., 2008; Schönberg et al., 2007; Tricomi and Fiez, 2008). Notably, the magnitude of ventral striatal signal decrease correlated with behavioral persistence, suggesting that neural signals underlying behavior correction may be part of a process for persisting after controllable setbacks (i.e., problem-focused coping; Folkman and Lazarus, 1988; LeDoux and Gorman, 2001). An alternative interpretation is that ventral striatum indicates the likelihood of success or expected value of the current path. However, it was decreases in ventral striatum responses that related to valuing the current path (i.e., persisting), rather than signal increases which have been more typically associated to likelihood of success or expected value (Abler et al., 2006; Delgado, 2007; Yacubian et al., 2006). The precise role of ventral striatal signals in persistence might be better understood with further research to examine the influence of expected value on setback-related signals, or whether expected value signals might occur at other time-points (e.g., when making a decision after a negative outcome).
VMPFC signal change to setbacks link affect to persistence behavior
Whether an affective response to a negative outcome promotes one behavior (e.g., persistence) over another (e.g., avoidance) depends on an individual’s appraisal of the negative outcome information (Weiner, 1985). One way of coping with a negative affective response to an uncontrollable setback is to reappraise the initially negative affective information in a manner that reduces threatening aspects (Folkman and Lazarus, 1988; Gross, 1998). Importantly, negative affect is not exclusively related with avoidance behavior and can predict approach behaviors. For example, frustration and anger are associated with increased reward seeking (Carver, 2004) and optimistic assessments of risk and uncertainty (Lerner and Keltner, 2001). Also, when negative affective information is non-threatening (e.g., to self-esteem) and likely to change (e.g., turn out better next time), then people show increased motivation and persistence (Dweck, 1986; Di Paula and Campbell, 2002; Weiner, 1985). In the current study, participants with greater negative affective responses to uncontrollable setbacks exhibited greater persistence. VMPFC setback-related signals accounted for the relationship between negative affect and persistence, suggesting a possible role for VMPFC in forming adaptive behavioral responses based on negative affective information.
This finding is consistent with a view that VMPFC plays a role in flexibly reappraising affective information as well as formulating a behavioral response based on affective information (Beer et al., 2006; Schiller and Delgado, 2010; Roy et al., 2012). For example, VMPFC is involved in modulating behavior when multiple interpretations of an outcome might be valid, as when cue-outcome relationships are extinguished, reversed, or reappraised (O’Doherty et al., 2003; Schiller and Delgado, 2010; Wager et al., 2008). Furthermore, animal research shows that VMPFC activation during an uncontrollable stressor improves subsequent learning and reduces exaggerated fear behavior (Amat et al., 2005). Importantly, VMPFC is not necessarily involved in reducing an affective response (Buhle et al., 2013), rather forming an appropriate response based on affective information. Uncontrollable setbacks, and inherent negative affect, were unavoidable in the current task. However, those subjects who were able to form an adaptive behavioral response (persistence) by engaging VMPFC activity were able to be most successful in the task.
The findings suggest that setback-related negative affect relates to persistence indirectly by way of VMPFC responses, rather than the alternative possibility that VMPFC modulates negative affect, which then influences behavior. This relationship between VMPFC and negative affect differs from other research suggesting that VMPFC reduces negative affective responses during extinction, reversal, or reappraisal of negative cues (Schiller and Delgado, 2010; Wager et al., 2008). Importantly, reduction of negative affect is not integral to perform the current task as is the case in extinction, reversal, or reappraisal paradigms. In fact, setback-related negative affect was always appropriate in the current task because setbacks always impeded performance. Furthermore, negative affect can increase motivation and persistence when it is interpreted as non-threatening and likely to change (Dweck, 1986; Weiner, 1985). Indeed, success in our experiment required that participants interpret uncontrollable setbacks as non-threatening and likely to change. Thus, performance demands may explain why VMPFC activity might reduce negative affect in cases where affect reduction is the goal, but may show a different relationship with negative affect when the goal is to interpret negative affect in an advantageous way rather than reduce it. The role of VMPFC in affect regulation and persistence might be better understood by further research manipulating affect reappraisal goals.
Conclusions
Different manners of coping with setbacks may be related to two distinct neural mechanisms for persistence with adversity: signals in ventral striatum and VMPFC that coincide with negative outcome processing during setbacks. Ventral striatum activity may signal that a behavioral correction is needed to persist, whereas VMPFC activity may be part of a process of appraising negative feelings to inform the best behavioral response. These mechanisms were explored in a situation where persistence was the best response to an outcome, but further work is needed to know whether these mechanisms underlie persistence in other situations, or other more long-term forms of persistence (e.g., choosing to persist with career goal despite failing multiple academic classes over several years). Nevertheless, these findings may provide another perspective for further research to understand important achievement-related individual differences, such as differences between students who believe they can improve after a setback and those who do not (i.e., incremental and fixed mindsets; Dweck, 1986). The findings may also contribute to future understanding of important problems, such as high dropout rates among certain groups of students (Lord et al., 2009), behavioral patterns that may contribute to depression (Maier and Seligman, 1976), or the manner in which substance dependent individuals cope with negative life events (Sinha, 2007). Further research exploring neural mechanisms underlying different manners of coping may be useful to understand why individuals in difficult circumstances may fail to persist even when persistence would be clearly beneficial.
Experimental Procedures
Participants
31 right-handed participants from the Rutgers University community underwent functional Magnetic Resonance Imaging (fMRI) while performing a Persistence After Setbacks (PAS) task for monetary compensation. One participant was excluded from analysis due to head movement during (>3mm between fMRI volumes), leaving 30 participants (18 female, mean age 23.4, s.d. = 4.9, range 18-38). All participants provided informed consent and the study was approved by the institutional review board of Rutgers University.
PAS task
Before entering the scanner, participants received instructions for the task and played two practice rounds. The task involved many rounds of an “academic degree decision game” and each round was a chance to earn points. Total points determined the size of their monetary performance bonus ($0 to $10). Participants made an initial path choice between three paths, each with a distinct point value at the end (Figure 1A; low alternative value: 80/70/60 points; high alternative value: 80/78/76). Participants next encountered a controllable or uncontrollable obstacle (Figure 1B). For controllable obstacles (midterm exams), participants were instructed to press the correct button (from 4 possible) to pass the exam, which could only be learned by trial and error each round (Delgado et al., 2009). The participant moved forward with a correct response but an incorrect response (failed exam) sent the participant to the beginning of the path (controllable setback; Figure 1C). For uncontrollable obstacles (course cancellations), participants pressed any button to see if their course was randomly selected to be cancelled. The participant moved forward if the course was not cancelled but a cancelled course sent the participant to the beginning of the path (uncontrollable setback; Figure 1C).
Critically, after a controllable (failed exam) or uncontrollable setback (cancelled course) participants decided to persist with their previously chosen path (scored as persistence), choose a lower value path, or choose a higher value path (Figure 1D). The behavioral measure was the percent of choices to persist (an alternative measure included choices for a higher value path, see Supplemental Experimental Procedures). Persistence is thus defined as the continuance of a course of action despite difficulty. This operationalization is consistent with a lay understanding of persistence as well as prior research on persistence (Andrews and Debus, 1978; Di Paula and Campbell, 2002). Persistence was computed for each condition of the 2 (controllability) × 2 (high/low alternative value) design. The alternative value manipulation guarded against the possibility that participants would fail to persist with a path simply to explore the environment. That is, when one path was clearly better than another (low alternative value), exploration was more costly than when one path was only slightly better (high alternative value). Thus, behavior in the low alternative value condition would be most indicative of individual tendencies to persist after setbacks, rather than tendencies to explore the environment. Participants were explicitly instructed that path difficulty was not necessarily related to point value. A round included either controllable or uncontrollable setbacks, but not both, and the path point values remained the same for the round. A round ended when the player reached the end of a path (three steps), or time ran out (after a pseudo-randomly determined number of events). To facilitate participants achieving goals, they were occasionally presented with a third cue signaling a “class meeting,” which allowed participants to move forward. Participants received 20 setbacks in each condition (see Supplemental Information for complete description). The distribution of setbacks in each condition was predetermined to ensure that every participant had the same amount of trials and chances to persist. A post-experimental probe showed that no participants suspected that the setbacks were predetermined. After completing the task and exiting the scanner, participants rated their affective responses (valence and intensity) to each type of setback.
Data analysis
Functional image analysis (see Supplemental Information for acquisition and preprocessing) identified neural activity associated with setbacks, and its relation to persistence and affect. Neural activity to setbacks was estimated with a general linear model (GLM) consisting of four regressors of interest (controllable/uncontrollable setbacks received in the high/low alternative value conditions, see Supplemental Information for GLM specification). Parameter estimates (from least squares estimates) were averaged across the 4 scans. Group level random effects analysis used FMRIB Local Analysis of Mixed Effects (FLAME) approach in FSL (Woolrich et al., 2009). First, a 2 (setback controllability) × 2 (alternative value) ANOVA was specified to test for main effects and the interaction, with a cluster correction threshold applied to group level z-statistic maps (cluster defining threshold of z > 2.57, corrected cluster significance threshold of p < .05). This cluster thresholding method allows inference concerning whole clusters primarily. In a second thresholding procedure, individual peaks in striatum and VMPFC then identified using FSL’s threshold free cluster enhancement (TFCE) method (Smith and Nichols, 2009) and voxel-wise corrected p-values were computed based on FSL’s permutation-based randomize procedure with 10,000 iterations. Spheres with 8mm radius were drawn around peaks in striatum and VMPFC (including only voxels with corrected p < .05), and percent signal change estimates were extracted. These neural activity estimates were examined for correlations with the behavioral measure of persistence and with setback-related affect (valence and intensity treated as two separate measurements). In the case that a neural region was associated with affect and behavioral persistence, the region was tested as a mediator of the relationship between affect and behavioral persistence (Preacher and Hayes, 2004). Behavioral persistence and neural signal change are examined in the low alternative value conditions due to the hypothesis that behavior is less influenced by exploration, however, the results remain the same if behavior and neural signal change are averaged across low and high alternative value conditions.
Supplementary Material
Acknowledgements
We thank Meg Speer for data acquisition and helpful feedback. This research was supported by funding from the National Institute of Mental Health to M.R.D. (R01DA027764) and a National Science Foundation SBE Postdoctoral Research Fellowship to J.P.B. (1305994).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions. J.P.B. and M.R.D. designed the research and wrote the manuscript. J.P.B conducted the research and data analysis.
References
- Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probability is coded in human nucleus accumbens. Neuroimage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Andrews GR, Debus RL. Persistence and the causal perception of failure: Modifying cognitive attributions. J. Educ. Psychol. 1978;70:154–166. [Google Scholar]
- Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18:871–879. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers J, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb. Cortex. 2013:1–10. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RD. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn. Mem. 2011;18:136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Schotter A, Ozbay EY, Phelps EA. Understanding overbidding: using the neural circuitry of reward to design economic auctions. Science. 2008;321:1849–1852. doi: 10.1126/science.1158860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Front. Behav. Neurosci. 2009;3:1–9. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dweck CS. Motivational Processes Affecting Learning. Am. Psychol. 1986;41:1040–1048. [Google Scholar]
- Folkman S, Lazarus RS. Coping as a mediator of emotion. J. Pers. Soc. Psychol. 1988;54:466–475. [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. J. Pers. Soc. Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks R. a, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb. Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- Lerner JS, Keltner D. Fear, anger, and risk. J. Pers. Soc. Psychol. 2001;81:146–159. doi: 10.1037//0022-3514.81.1.146. [DOI] [PubMed] [Google Scholar]
- Lewis AH, Niznikiewicz MA, Delamater AR, Delgado MR. Avoidance-based human Pavlovian-to-instrumental transfer. Eur. J. Neurosci. 2013;38:3740–3748. doi: 10.1111/ejn.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nat. Neurosci. 2011;14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord SM, Camacho MM, Layton R. a., Long R. a., Ohland MW, Wasburn MH. Who’s persisting in engineering? A comparative analysis of female and male asian, black, hispanic, native american, and white students. J. Women Minor. Sci. Eng. 2009;15:167–190. [Google Scholar]
- Maier SF, Seligman ME. Learned helplessness: Theory and evidence. J. Exp. Psychol. Gen. 1976;105:3–46. [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paula A, Campbell JD. Self-esteem and persistence in the face of failure. J. Pers. Soc. Psychol. 2002;83:711–724. doi: 10.1037//0022-3514.83.3.711. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski B. a, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Meth., Ins., C. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönberg T, Daw ND, Joel D, O’Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. J Neurosci. 2007;27:12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr. Psychiatry Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Camerer CF, Phelps EA. Emotion regulation reduces loss aversion and decreases amygdala responses to losses. Soc. Cogn. Affect. Neurosci. 2013;8:341–350. doi: 10.1093/scan/nss002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning. MIT Press; Cambridge, MA: 1998. [Google Scholar]
- Tricomi E, Fiez JA. Feedback signals in the caudate reflect goal achievement on a declarative memory task. Neuroimage. 2008;41:1154–1167. doi: 10.1016/j.neuroimage.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy AS, Shallcross AJ, Mauss IB. A Person-by-Situation Approach to Emotion Regulation: Cognitive Reappraisal Can Either Help or Hurt, Depending on the Context. Psychol. Sci. 2013;24:2505–2514. doi: 10.1177/0956797613496434. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner B. An attributional theory of achievement motivation and emotion. Psychol. Rev. 1985;92:548–573. [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Yacubian J, Gläscher J, Schroeder K, Sommer T, Braus DF, Büchel C. Dissociable systems for gain-and loss-related value predictions and errors of prediction in the human brain. J. Neurosci. 2006;26:9530–9537. doi: 10.1523/JNEUROSCI.2915-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.