Abstract
Globoid cell leukodystrophy (GLD), or Krabbe disease, is a rare and often fatal demyelinating disease caused by mutations in the galactocerebrosidase (galc) gene that result in accumulation of galactosylsphingosine (‘psychosine’). We recently reported that the extracellular matrix (ECM) protease, matrix metalloproteinase (MMP)-3, is elevated in GLD and that it regulates psychosine-induced microglial activation. Here, we examined central nervous system ECM component expression in human GLD patients and in the twitcher mouse model of GLD using immunohistochemistry. The influence of ECM proteins on primary murine microglial responses to psychosine was evaluated using ECM proteins as substrates and analyzed by quantitative real-time polymerase chain reaction (qRT-PCR), immunocytochemistry, and ELISA. Functional analysis of microglial cytotoxicity was performed on oligodendrocytes in co-culture and cell death was measured by lactose dehydrogenase assay. Tenascin-C (TnC) was expressed at higher levels in human GLD and in twitcher mice vs. controls. Microglial responses to psychosine were enhanced by TnC, as determined by an increase in ‘globoid-like’ cell formation, MMP-3 mRNA expression and higher toxicity toward oligodendrocytes in culture. These findings were consistent with a shift toward the M1 microglial phenotype in TnC grown microglia. Thus, elevated TnC expression in GLD modified microglial responses to psychosine. These data offer a novel perspective and enhance understanding of the microglial contribution to GLD pathogenesis.
Keywords: Extracellular matrix, Matrix metalloproteinase, M1 M2 phenotype, Oligodendrocyte
INTRODUCTION
Globoid cell leukodystrophy (GLD), also known as Krabbe disease, is a fatal demyelinating disease with an incidence of 1 in 100,000 in the United States (1, 2). The cause of GLD is attributed to genetic loss-of-function mutations in the lysosomal enzyme galactosylceramidase (GALC) gene (3). The natural function of GALC is to hydrolyze galactolipids, including the toxic lipid galactosylsphingosine, ‘psychosine’. Hydrolysis of psychosine is thought to prevent cellular damage (4). Thus, its mutation in GLD results in supraphysiological accumulation of psychosine (5). It is widely accepted that abnormal accumulation of psychosine drives GLD pathology through its pathophysiological effects on multiple cell types (5), but the exact mechanism of action of psychosine and the sequence of cellular involvement culminating in GLD pathology remain unclear.
The neuropathological hallmarks of GLD include severe central nervous system (CNS) demyelination, extensive astrogliosis, and the presence of large, multinucleated phagocytes called ‘globoid cells’. It has been suggested that formation of globoid cells in GLD was a secondary response to profound CNS demyelination; however, we recently determined that globoid cells are likely a primary response of microglia to psychosine (6). These data support an emerging hypothesis that psychosine induces widespread pathological changes in many cell types. Moreover, the response of microglia to psychosine provides a new model to study the unique formation and specific function(s) of globoid cells found within the CNS in GLD.
The extracellular matrix (ECM) is an intricate macromolecular network that occupies the extracellular space in tissues that provides a structural framework and homeostatic signaling environment for cells. Differential production of ECM components has been reported in many CNS diseases, including Alzheimer disease (7), stroke (8), human immunodeficiency virus encephalitis (9), and multiple sclerosis (MS) (10). One widely studied implication of altered ECM composition includes dysregulation of cellular growth, differentiation and cell fate (11–14). For example, in acutely demyelinated lesions in MS, tenascin-C (TnC) levels have been reported to be decreased relative to unlesioned or healthy tissues (15), whereas in chronically demyelinated lesions elevated production of TnC from astrocytes has been observed (15, 16). These changes in TnC levels associated with myelin lesions have led to the suggestion that TnC may negatively influence the remyelinating potential of oligodendrocyte progenitor cells in and around myelin lesions (15, 16). Increased production of fibronectin in MS has also been reported to pathologically alter glial responses. Recently Stoffels et al reported that astrocyte-derived fibronectin can prevent oligodendrocyte differentiation therein contributing to the chronicity of demyelinated lesions in MS (17). In addition, excessive fibrillar collagen production around actively demyelinating lesions in MS has been suggested to impair the expression of potentially beneficial chemokines from infiltrating monocytes (18). Thus, changes in ECM composition are closely associated with the pathology of demyelination and may represent important mediators of disease-related cellular responses in CNS diseases.
The distribution and expression of ECM constituents are tightly regulated, not only at the transcriptional and translational level, but also through extracellular proteolysis by matrix metalloproteinases (MMPs). Most ECM components are enzymatic substrates of MMPs, and degradation of ECM by MMPs can have profound effects on cellular signaling (19). We previously determined that MMP-3 (Stromelysin-1) is highly expressed in human and mouse GLD brains (6). We also determined that MMP-3 mediated psychosine-induced multinucleation of microglia with increased phagocytic activity (6). Because we have determined that psychosine induced MMP-3-dependent formation of multi-nucleated microglia, or •globoid cells•, this finding led us to hypothesize that changes in ECM may contribute to the pathophysiology of microglia in GLD. Moreover, because the precise role of globoid cells in GLD is currently unclear, we also sought to determine whether ‧globoid cells‧ had a direct effect on the survival of oligodendrocytes.
In this study, we determined that expression of TnC was specifically elevated in GLD and that it fostered the pathogenic transformation of microglia in response to psychosine by promoting their cytotoxic potential toward oligodendrocytes.
MATERIALS AND METHODS
Animals for Primary Cultures
All procedures involving animals were conducted with approval from the Institutional Animal Care and Use Committee (IACUC) at the University of Connecticut Health Center and in accordance with guidelines set forth by the National Research Council of the National Academies Guide for the Care and Use of Laboratory Animals.
Mixed Glial Cultures
Primary mixed glial cultures were established from postnatal day (P)0–3 pups of C57/BL6 wild type mice, as previously described (6, 20). Briefly, cortices were manually dissected in cold HBSS, followed by mechanical and enzymatic dissociation using a papain-based neural dissociation kit (Miltenyi Biotec, Auburn, CA), according to the manufacturer’s protocol. Dissociated cells were plated in DMEM (Gibco, Carlsbad, CA) with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), penicillin (100 U/mL, Sigma Aldrich, St Louis, MO) and streptomycin (100 mg/mL, Sigma Aldrich) in a T175 culture flask (Grenier Bio-One, Frickenhausen, Germany). After 16 hours, culture media was replaced with fresh media to remove non-adherent cells. Adhered cells were incubated in the flask at 37°C for 2 to 3 weeks until an astrocytic monolayer was established.
Primary Microglial Cultures
Pure microglial cultures were established by detaching microglia from mixed glial cultures, as previously described (6, 21). Briefly, established mixed glial cultures with 100% confluency were shaken by orbital shaker at 110 rpm at 37°C for 4 hours to detach microglia from the astrocytic monolayer. Supernatants containing detached microglia were spun at 300 X g for 10 minutes, followed by resuspension of the microglia in astrocyte-conditioned media, and then plated onto coverglass coated with either laminin (Lm) (10 µg/ml, Sigma) or TnC (1, 10 or 100 µg/ml, EMD Millipore, Billerica, MA) or into 6-well UltraLow adherence culture plates (Corning, Tewksbury, MA). Cells were acclimated for 2 to 3 days prior to experiments. Chemical inhibitors used were: GM6001 (12.5 µM, Calbiochem, Billerica, MA) and NNGH (0.1 µM, Enzo Life Sciences, Farmingdale, NY), psychosine (10 µM (Sigma), dimethylsulfoxide (0.13% •DMSO,• Sigma Aldrich).
Primary Oligodendrocyte Cultures
Pure oligodendrocytes cultures were established from postnatal (P)0–3 rat pups, as described previously (22), with modifications. Briefly, brains from rat pups were dissected out and meninges were removed. After removal of the cerebellum, the brain tissue was mechanically dissociated by mincing by scissors and passing through fire-polished Pasteur pipet. The single cell suspension was then plated in T75 flask in maintenance medium composed of DMEM/F12 (Gibco), 10% fetal bovine serum (FBS) (Atlanta Biologicals), penicillin (100 U/mL, Sigma Aldrich), and streptomycin (100 mg/mL, Sigma Aldrich). Cells were incubated at 37° C for 3 weeks or until monolayer was formed. Cultures were shaken for 30 minutes (37°C at 55 rpm) to remove microglia from the monolayer. Oligodendrocyte progenitor cells were removed by shaking in an orbital shaker at 225 rpm at 37°C for 16 hours. Medium containing detached cells were collected and centrifuged at 300 X g for 10 minutes. Cells were resuspended in maintenance media and plated 3 to 4 h at 37°C to eliminate microglia by differential adhesion. Non-adhering cells were collected and plated on poly-ornithine (0.1% Sigma Aldrich)-coated coverglass, incubated for 1 to 2 hours at 37°C, and media was replaced with oligodendrocytes differentiation media, composed of Neurobasal media (Gibco), L-glutamine (1X, Life Technologies, Grand Island, NY), B-27 supplement (1X, Life Technologies), and Triiodothyronine (T3, 10 ng/mL, Sigma Aldrich).
Co-cultures of Microglia and Oligodendrocytes
Primary microglial cultures in 6-well UltraLow adherence culture plates and oligodendrocyte cultures on poly-ornithine coated coverslips were established as described above. To induce globoid cells in microglia, psychosine, psychosine +TnC, or vehicle treatments were applied for 7 consecutive days; then, treated microglia were collected by gentle pipetting to detach loosely adhered cells at the bottom of wells. Collected cells were spun (300 X g for 10 minutes), and cells were carefully resuspended in oligodendrocyte differentiation media (see above). The microglia in single cell suspension were counted by hemacytometer and seeded at 0.5–1 × 105 cells/mL onto oligodendrocyte cultures, which resulted in approximately 1:1–2 microglia/oligodendrocyte ratio. Co-cultures were incubated for 3 d and media were collected for cytotoxicity assay and the cells were fixed for immunocytochemistry.
Primary Neuronal Cultures
Primary cortical or hippocampal neuronal cultures were established from rat pups at P1 (23). Briefly, brains from rat neonates were dissected out and meninges were stripped off. Cortices and hippocampi were isolated in HBSS, followed by incubation for 15 minutes at 37°C in trypsin (0.25%). Tissues settled at the bottom of conical tubes were washed with HBSS 3 times; this was followed by tituration with a fire-polished Pasteur pipette 10 times in plating media (Neurobasal media with 10% FBS). Single cell suspensions were passed through a 70-µm filter and then plated on poly-L-lysine coated plates. Media was replaced with maintenance media A (Neurobasal media, 2% B-27, 2% FBS, 0.5 mM L-glutamine, 25 µM L-glutamic acid, 1X antibiotic/antimycotic solution) 3 hours after plating. Half of the media in the cultures was then replaced with media B (Neurobasal media, 2% B-27, 0.5 mM L-glutamine, 1X antibiotic/antimycotic solution) every 3 to 5 days.
Human Brain Tissues
Brain specimens from GLD and age-matched control patients (1–2 years old) were obtained from the National Institute of Child Health and Human Development (NICHD) Brain and Tissue Bank for Developmental Disorders (Baltimore, MD). Brain tissues were paraffin-embedded, cut into 15-µm-thick sections, and then processed for immunohistochemistry (IHC).
Mouse Brain Tissues
Twitcher mice were bred and housed at the Sanford-Burnham Institute (La Jolla, CA), where the brain specimens from twitcher mice and age-matched littermate control mice were collected. Mice were deeply anesthetized in accordance with IACUC approved protocols and were transcardially perfused with phosphate buffer saline (PBS) followed by 4% paraformaldehyde (PFA). Brain tissues were collected at the age of P0, 10, 20, 31, 40, as previously described (24).
IHC
Collected brain and spinal cord tissues were paraffin-embedded, and 15-µm sagittal sections were made, as previously described (25, 26). Tissue sections were subjected to antigen retrieval using 95°C citric acid buffer (0.01 M, pH 6.0), followed by blocking non-specific binding site by 5% normal goat serum ([NGS]; Life Technologies) in PBS-Tween for 1 hour at room temperature. Sections were then incubated with primary antibodies against TnC (N-19, Santa Cruz Biotechnology, Dallas, TX), Ln [α-2 (6D580), Santa Cruz], Vitronectin [10/65/75 (H-202), Santa Cruz], Fibronectin (C-20, Santa Cruz), Iba-1 (019–19741, Wako, Richmond, VA) and/or CD16/32 (553142, BD PharMingen) followed by PBS wash and 1-hour incubation with Alexa 594- or 488- conjugated secondary antibodies (Life Technologies) against appropriate species from which primary antibodies were raised. To identify nuclei, tissues were counter-stained with 4‧,6-diamidino-2-phenylindole ([DAPI], Life Technologies) and then coverslipped using Fluoromount-G (SouthernBiotech, Birmingham, AL). Immunoreactivity was visualized and then analyzed using a computer-assisted image analysis system and software (Eclipse, Empix Imaging) on an Olympus IX71 microscope. For each experimental treatment, 2 to 4 different specimens were analyzed and experiments were also repeated in triplicate. Data presented are from a representative experiment unless otherwise indicated.
Thioflavin-S Staining
Following labeling of TnC using primary and secondary antibodies in IHC (see IHC section), tissues were then incubated with 0.1% Thioflavin-S (Sigma Aldrich) in PBS:EtOH (1:1, v:v) for 5 minutes at room temperature. Tissues were then washed with 80% EtOH several times, rehydrated with PBS, and then analyzed.
Quantitative Real-time Polymerase Chain Reaction
Total RNA was isolated from cultured cells as described previously (6) and converted into complementary DNA (cDNA) via reverse transcription (iScript cDNA synthesis kit, BioRad, Hercules, MA), according to the manufacturer’s protocol. Synthesized cDNA samples were amplified for qRT-PCR using primer pairs specific to mmp-3 and tenascin-c mRNA (Integrated DNA Technologies, Coralville, IA), as described previously (27), and SsoFast EvaGreen Supermix (BioRad) according to the manufacturer’s protocol. Target cDNA were amplified and analyzed by a Mastercycler ep realplex (Eppendorf, Hauppauge, NY). Primers for GAPDH were used to assess general expression level of housekeeping gene among samples. Relative expression of target RNA was calculated using the comparative cycle threshold analysis (ΔΔCT), as previously described (6).
Immunocytochemistry
Cells plated and grown on Lm-coated or poly-ornithine-coated glass coverslips were fixed using 4% PFA and processed for immunocytochemistry (ICC) using antibodies against Iba-1 (Wako), CD11b (eBioscience, San Diego, CA), Olig2 (EMD Millipore), myelin basic protein ([MBP]; aa82-87, EMD Millipore), CD206 (C-20, Santa Cruz), and major histocompatibility class II ([MHCII]; Y-Ae, Santa Cruz), following blocking by 5% NGS (Life Technologies), as previously described (26). Immunoreactivity was visualized using fluorescently conjugated secondary antisera against the species of the primary antibodies (Alexa Fluor 488 or 594, Life Technologies). Positive immunostaining were viewed and analyzed using computer-assisted image analysis software on an Olympus IX71 microscope. DAPI staining was used to label nuclei in order to determine cellular densities per field and multinucleated cell analyses, as previously described (6). Four to 6 visual fields were randomly chosen per coverslip and number of cells with single and/or multiple immunoreactivities were manually counted using ImageJ software (NIH) and also normalized to total DAPI-positive nuclei per field.
Lactose Dehydrogenase Assay
Media from cell cultures were collected at the end of the treatment time course. The level of lactose dehydrogenase (LDH) in collected media was measured using a commercial LDH assay kit, as per manufacture’s protocol (BioVision, Milpitas, CA).
Enzyme-linked Immunosorbent Assay (ELISA)
Media were collected from cell cultures at the end of experiments. The level of tumor necrosis factor (TNF) released into media was analyzed using a commercially available TNF ELISA DuoSet kit per manufacturer’s instructions (R&D Systems, Minneapolis, MN).
Statistics
Data are presented as mean ± SE. Statistical significance was tested by one-way analysis of variance (ANOVA) with Tukey’s post hoc analysis, or two-way ANOVA with Bonferroni’s post hoc test, using Prism software (GraphPad, San Diego, CA). For all comparisons, p < 0.05 was considered statistically significant.
RESULTS
TnC Expression Is Elevated in the GLD Brain
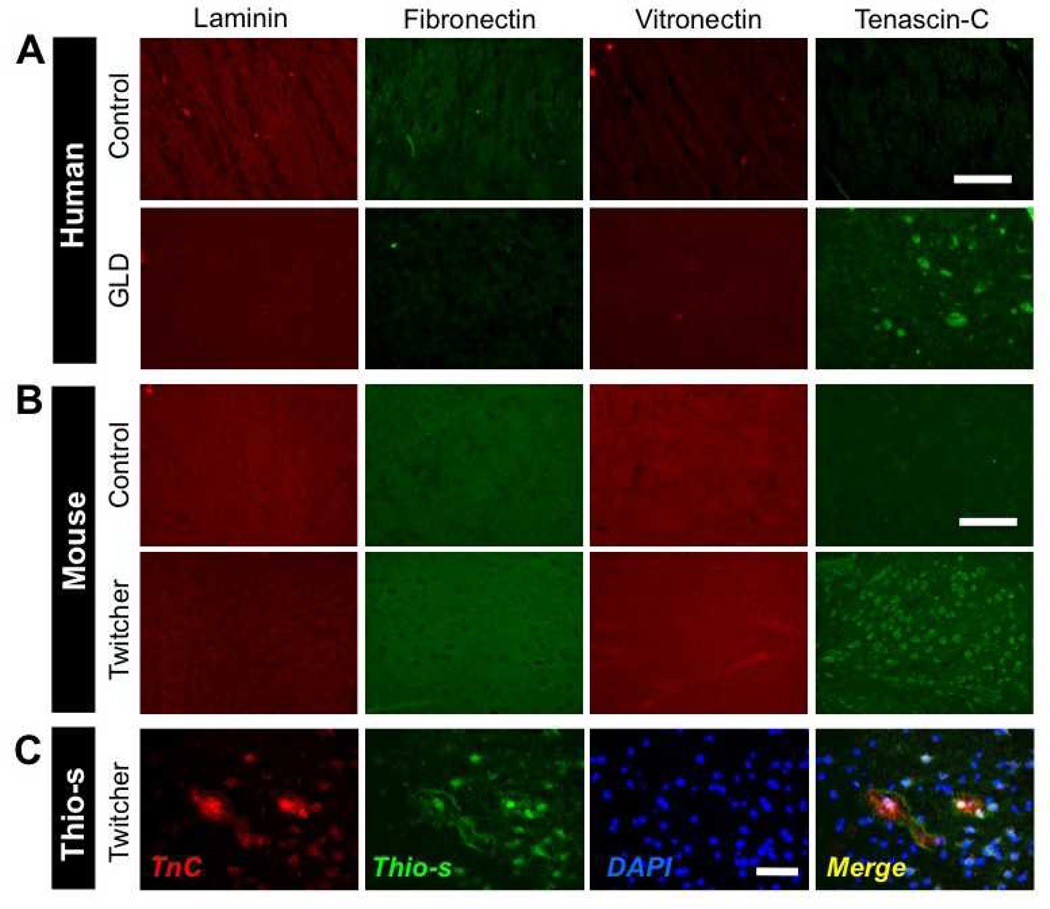
The emerging recognition of the significance of ECM molecules on glial cell functions led us to consider whether differential expression of ECM molecules occurred in GLD. To explore this, we performed IHC for 4 prominent ECM molecules: Lm, fibronectin, vitronectin, and TnC on brain tissue sections from human brain from GLD cases and age-matched control subjects. Examination of the expression of the ECM components Lm, fibronectin, and vitronectin revealed no significant differences between GLD and control brains in terms of their pattern of expression or intensity of immunostaining (Fig. 1A). However, there were TnC-positive deposits in the brains of GLD patients but not in age-matched control subjects (Fig. 1A). Next, we examined whether TnC was a generalized feature of GLD brain pathology across species. TnC immunoreactivity was analyzed in brain tissues from twitcher mice, an authentic mouse model of GLD (28). Similar to the human GLD cases and control brains, there were no significant differences in staining for Lm, fibronectin, or vitronectin between the 2 genotypes but TnC-positive deposits were more frequently observed in twitcher brains than in age-matched controls (Fig. 1B). Interestingly, the high TnC immunoreactivity in twitcher mouse was frequently observed in plaque-like patterns, which resembled the staining observed in human GLD. This pattern also exhibited similarities to β-amyloid deposits observed in Alzheimer disease; and, in fact, TnC-positive plaques were frequently colocalized with Thioflavin-S, a marker of amyloid fibrils ([29), Fig. 1C). Together, these data indicate that the intensity and pattern of TnC immunoreactivity are altered in the CNS of GLD-affected humans and mice.
Figure 1.
Aberrant pattern of tenascin-C (TnC) expression in globoid cell leukodystrophy (GLD). (A) Immunohistochemical (IHC) analysis of the extracellular matrix (ECM) proteins laminin, fibronectin, vitronectin, and TnC in the midbrain of human infantile GLD patients (bottom row) and age-matched control human subjects (top row). (B) IHC analysis of ECM proteins in the midbrain of the P30 twitcher mouse model of GLD (bottom row), and age-matched littermate wild type control mice (top row). Note the intense punctate pattern of TnC immunoreactivity in human and murine GLD tissues vs. that in control subjects. (C) TnC immunoreactivity colocalizes with Thioflavin-S positive staining in the hippocampus of P30 twitcher mouse brain as visualized by IHC for TnC and Thioflavin-S staining. Nuclei are stained with DAPI (blue). Two to 4 specimens were analyzed. Scale bar: A, 250 µm; B, 150 µm; C, 100 µm.
TnC Enhances Glial Responses to Psychosine In Vitro
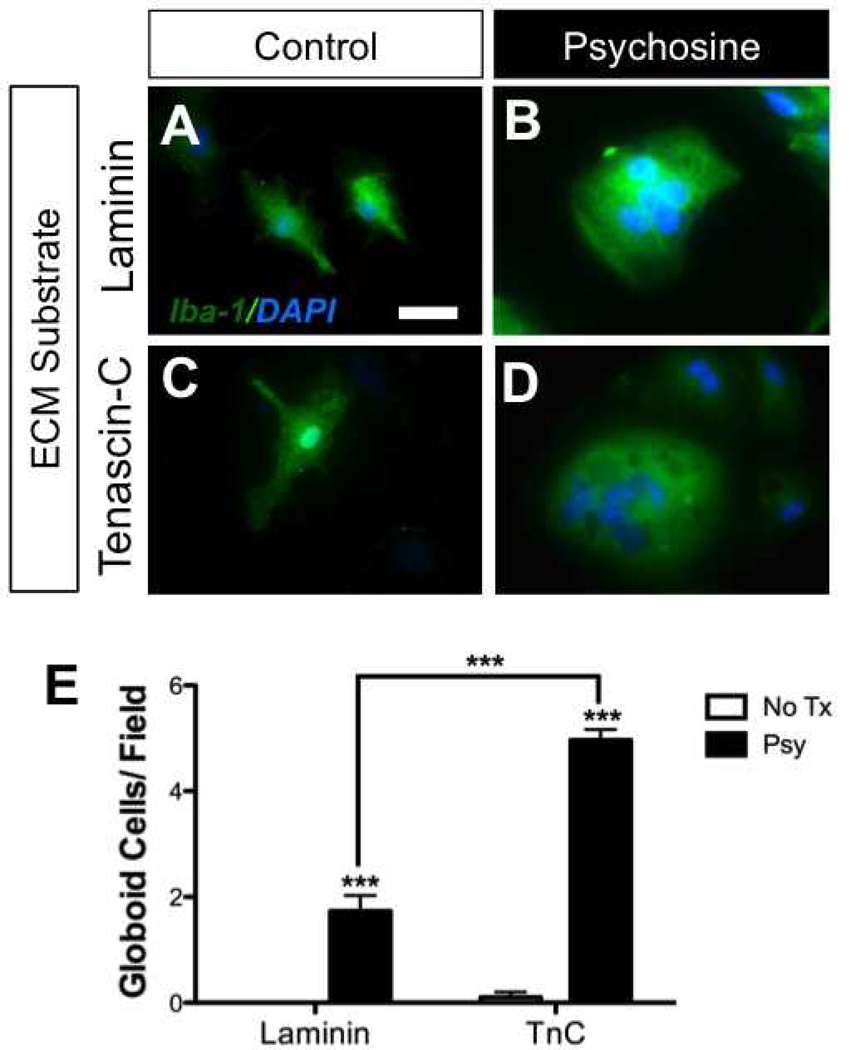
ECM proteins have the potential to potently modulate glial functions. Based on the high expression of TnC in GLD brains (and previously reported effects of TnC on glia [30–32]), we next examined whether TnC influenced microglial responses to psychosine. We previously reported that psychosine induced globoid cell formation in glial cultures (6). Using this in vitro model system, we prepared primary mixed glial cultures that were grown on either TnC or Lm (Fig. 2). Cultures were then exposed to psychosine (10 µM) for 7 consecutive days. Immunocytochemical analyses determined that globoid cells formed in response to psychosine when grown on either ECM substrate (Fig. 2A–D). However, in glial cultures grown on TnC, there were significantly more psychosine-induced globoid cells formed than in glial cultures grown on Lm (Fig. 2E). These data suggest that TnC enhanced globoid cell formation in response to psychosine in vitro.
Figure 2.
Tenascin-C (TnC) promotes globoid cell formation. (A-D) Primary glial cell cultures (mixture of astrocytes and microglia) grown either on laminin (A, B) or TnC (C, D), were treated with either vehicle (A, C) or psychosine (B, D) for 7 consecutive days. Cells were fixed and immunostained for the microglial marker, Iba-1 (green) and counter-stained with DAPI (blue; nuclei). (E) Seven to 9 visual fields generally containing 50 to 70 total DAPI-positive nuclei under 20x magnification were randomly chosen and numbers of multinucleated cells were counted. Psychosine treatment induced multinucleation of Iba-1-positive cells regardless of ECM substrate although glia grown on TnC produced significantly more •globoid-like• cells when compared to laminin-grown cultures. Scale bar: A-D, 20 µm. Two-way ANOVA; ***p < 0.0001 with n = 3/condition. Data are shown as mean ± SE. No Tx, no treatment; Psy, psychosine.
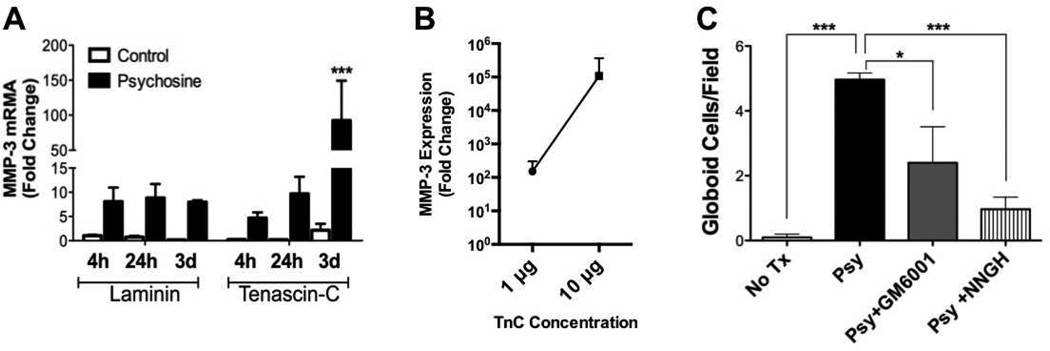
Because we had previously determined that induction of globoid cells by psychosine required MMP-3 (6), we examined MMP-3 expression. To determine whether TnC modified psychosine-induced MMP-3 expression by glia, we grew mixed glial cultures on either TnC or Lm, and treated with psychosine for 4 or 24 hours, or 3 days, and quantified MMP-3 mRNA expression by qRT-PCR. At earlier time points there was no significant difference in psychosine-induced MMP-3 expression on either substrate but MMP-3 mRNA expression by psychosine-treated glia on TnC reached a 92-fold change by day 3 of psychosine treatment vs. controls (Fig. 3A). To determine whether the concentration of TnC was a factor determining the magnitude of MMP-3 expression, we plated mixed glial cultures onto varying concentrations (1, 10 or 100 µg) of TnC substrate and then treated these cultures with psychosine for 72 hours, the time-point of maximal MMP-3 expression on 1 µg TnC (Fig. 3A). On 100 µg TnC, cultures exhibited highly variable adherence and survival, which precluded these cultures from further analysis. However, analysis of MMP-3 mRNA expression in the adherent cultures treated with psychosine grown on either 1 or 10 µg concentrations of TnC revealed a strong correlation between the concentration of TnC and MMP-3 (Fig. 3B). These data suggest that microglia grown on TnC exhibit a more robust transcriptional response to psychosine than on Lm, and that MMP-3 expression in response to psychosine is strongly influenced by TnC in a concentration-dependent manner.
Figure 3.
Matrix metalloproteinase-3 (MMP-3) mediates globoid cell formation in response to psychosine. (A) Primary glial cell cultures, which consisted of astrocytes and microglia exclusively, grown on either laminin- or tenascin-C (TnC)-coated plates, were challenged with psychosine for 4 hours, 24 hours, or 3 days. Total RNA were isolated, reverse-transcribed into cDNA, and MMP-3 mRNA expression was analyzed by qRT-PCR. Data represent mean ± SE of fold change normalized to laminin-treated 4-hour samples. (B) Analysis of TnC concentration dependent effect on MMP-3 expression following 3 days of psychosine treatment. (C) Primary glial cultures grown on TnC-coated coverslips were treated with either psychosine (Psy; black bar), psychosine with the pan-MMP inhibitor, GM6001 (12.5 µM; gray bar) or the MMP-3 specific inhibitor, NNGH (0.1 µM; striped bar), for consecutive 7 days. Cells were fixed and immunostained for microglial marker, Iba-1 and DAPI for nuclei. Four to 6 visual fields under 20x magnification were randomly chosen and globoid cells were counted. Numbers of globoid cells were significantly decreased when GM6001 or NNGH was applied in culture in conjunction with psychosine treatment (Two-way ANOVA; interaction: **p < 0.0099; treatment: ***p < 0.0002). Data represent mean ± SE. N = 3/condition.
We next tested whether the enhanced formation of globoid cells grown on TnC was also mediated by MMP-3. TnC-grown glial cultures were challenged with psychosine in combination with a pan-MMP inhibitor (GM6001), MMP-3-specific inhibitor (NNGH), or vehicle (DMSO) for 7 consecutive days. Immunocytochemical analysis determined that treatment with either GM6001 or NNGH significantly attenuated the psychosine-induced globoid cell formation of TnC-grown microglial cultures (Fig. 3C). These data confirmed that psychosine-induced globoid cell formation was mediated by MMP-3, but also revealed that this process was enhanced by recapitulating the ECM environment of the GLD brain, i.e. TnC.
TnC Modifies Microglial Phenotype in Response to Psychosine
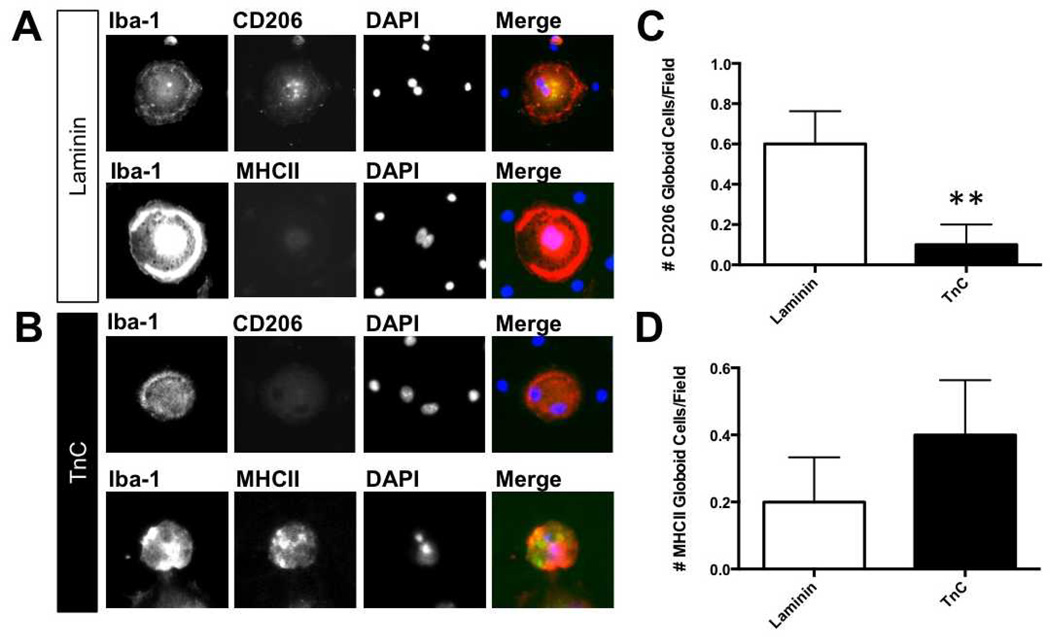
Globoid cells originate from microglia (6). Our previous study had determined that psychosine induced phagocytic activity of microglia, but we had not fully characterized the phenotype of these cells. Because microglia can have detrimental or beneficial effects, based on their phenotypes, known as M1 and M2, respectively (33, 34), we next determined the phenotype of both mononuclear and multinucleated microglia following treatment with psychosine or psychosine and TnC. Microglial phenotypes were determined by ICC using expression of MHCII or CD206 on Iba-1-positive microglia as indicators of the classic M1 and M2 phenotypes, respectively. We analyzed the phenotype of the multinucleated microglia (globoid cells) induced by psychosine when grown on either in the presence or absence of TnC (Fig. 4A, B). The number of MHCII-positive globoid cells in psychosine-treated microglia grown on TnC was slightly higher than those in microglia grown without TnC, albeit without a statistically significant difference (Fig. 4D). Conversely, the number of CD206-positive ‧globoid cells‧ from psychosine-treated microglia was significantly greater than psychosine-treated microglia exposed to TnC (Fig. 4C). These data provide support for a distinct M1-microglial phenotype of microglia in response to psychosine when grown on TnC, whereas microglia without TnC tended to exhibit an M2 phenotype. Similar results were observed among mononucleated Iba-1-positive microglia in co-culture (Supplemental Fig. 1). These data suggest that the presence of TnC during psychosine exposure switches the phenotype from a potentially beneficial (M2) to a more harmful (M1) phenotype.
Figure 4.
Tenascin-C (TnC) changes M1/M2 phenotype in psychosine-treated microglia/globoid cells. (A, B) Isolated microglial cultures were grown on either laminin (Lm) (A) or TnC (B) and treated with psychosine for 7 days. Expression of M1 and M2 markers (major histocompatibility complex II [MHCII], CD206, respectively) was identified by immunocytochemistry. Representative images show that with microglia plated on Lm predominantly express CD206 but not MHC II (A), while cells grown on TnC predominantly express MHC II, but not CD206 (B). (C, D) Data represent percent of marker-positive cell staining of total cell number/field (n = 5–8 fields per treatment) (mean ± SE), **, p < 0.01. Scale bar: 20 µm.
TnC Determines the Cytotoxicity of Globoid Cells for Oligodendrocytes
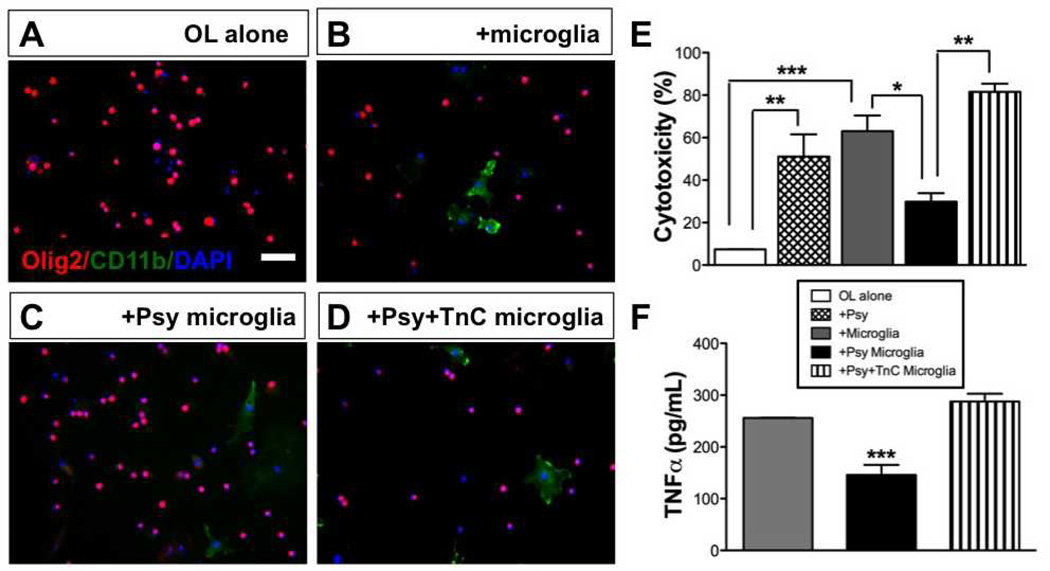
We previously reported that when microglia were treated with psychosine in vitro, they transform into large, highly-phagocytic multinucleated cells (6). Also, globoid cells in the brains of human GLD patients, twitcher mice, and other animal models of GALC deficiency have often found close to myelin lesion sites and to contain tubular inclusions and myelin debris (35). Thus, we hypothesized that globoid cells phagocytize myelin and possibly accelerate disease process(es) of GLD by promoting demyelination and oligodendrocyte death. To test this, isolated microglia were grown on low adherence plates either with or without TnC and then treated with psychosine (10 µM) or vehicle. Microglia were then collected and co-cultured with primary oligodendrocyte cultures. The impact of microglia on oligodendrocytes in culture was then assessed by ICC for Olig2, an oligodendrocyte marker (Fig. 5A), and the amount of cell death in cultures was evaluated using an LDH assay. Psychosine-treated microglia exhibited less cytotoxicity to oligodendrocytes than observed in control-treated microglia (Fig. 5B, C). In contrast, psychosine-treated microglia grown on TnC evoked a 2.9-fold increase over psychosine-treated microglia without TnC (5D), in terms of cytotoxicity to oligodendrocytes, or when compared with control treated microglia (Fig. 5E).
Figure 5.
Globoid cells grown on tenascin-C (TnC) are toxic to oligodendrocytes. Isolated microglia treated with psychosine (+Psy), or grown on TnC and treated with psychosine (+Psy+TnC), or vehicle for 7 days were then co-cultured with oligodendrocytes for 4 subsequent days. Oligodendrocyte cultures were about 50% myelin basic protein (MBP)-positive at the time of co-culture. (A-D) Representative photomicrographs of immunocytochemistry for Olig2 (red) and CD11b (green) counterstained with DAPI (nuclei; blue) in cultures of oligodendrocytes (A), oligodendrocytes co-cultured with microglia (B), oligodendrocytes cultured with psychosine-treated microglia that were grown on a laminin substrate (C), or oligodendrocytes cultured with psychosine-treated microglia that were grown on a TnC substrate (D). Scale bar = 100 µm. (E) Analysis of cell death under each condition was measured by lactose dehydrogenase assay. Cytotoxicity is expressed as percent of full kill control (0.1% sterile Triton X-100). Psychosine-induced globoid cells without TnC (black bar) had minimal toxicity in co-culture to oligodendrocytes vs. untreated microglia (gray bar), whereas TnC-grown psychosine-treated microglia exhibited significant toxicity (striped bar). (F) Level of tumor necrosis factor (TNFα) released from isolated microglia treated with psychosine, TnC grown microglia treated with psychosine, or vehicle control (7 days) was measured by ELISA. Note that psychosine-treated microglia (black bar) exhibited the least amount of TNFα release vs. untreated or TnC-exposed psychosine-treated microglia (gray, striped bar, respectively). One-way ANOVA, p < 0.001; where, *p < 0.05; **p < 0.01; ***p < 0.001. N = 3/condition.
We next measured TNF production in these co-cultures by ELISA to determine whether psychosine modulated microglial production of TNF. Microglia treated with psychosine exhibited a measurable reduction in TNF production (Fig. 5B). Overall, psychosine-treated microglia had significantly less cytotoxicity on oligodendrocytes than non-treated microglia in co-culture, whereas psychosine-treated microglia grown on TnC displayed significantly greater toxicity to co-cultured oligodendrocytes and higher TNF. These data suggest that psychosine can promote a potentially pathogenic phenotype of microglia in the presence of TnC. These data also suggest that psychosine reduced the intrinsic toxicity of microglia to oligodendrocytes, but that the local production of TnC in GLD overcomes and promotes this cytotoxicity, potentially through modulating TNF production from microglia.
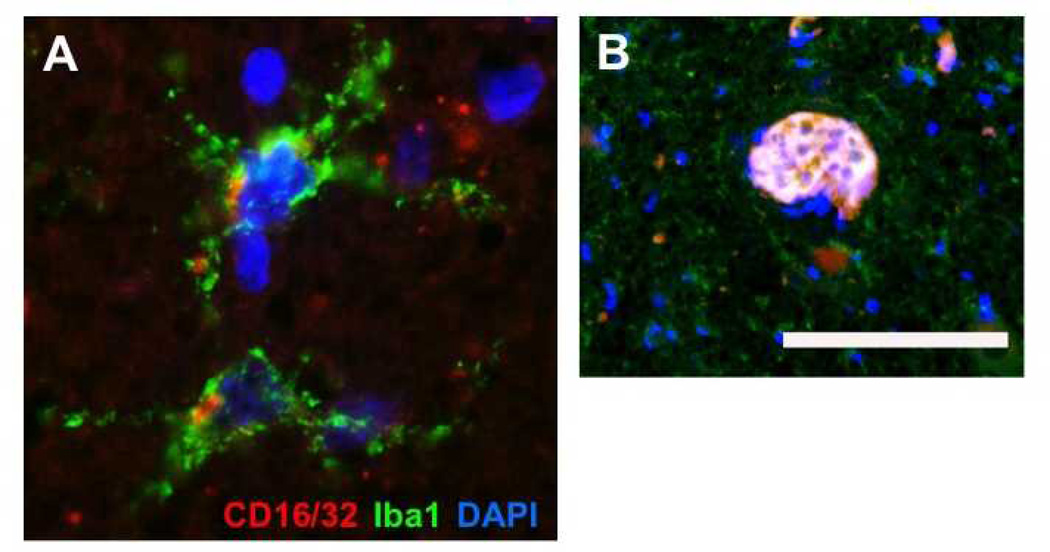
Detection of M1 Polarized Microglia and Globoid Cells in Human GLD
Recent studies have determined that the polarization of microglia and macrophages can dramatically impact the potential for recovery and repair in models of white matter injury (33, 36). Our in vitro data indicated that the presence of TnC in GLD may polarize the phenotype of microglia toward a classically activated oligotoxic •M1• phenotype. To evaluate the phenotype of microglia in GLD, we examined whether Iba-1-positive microglia also co-expressed CD16/32, an M1 activation marker, in human GLD brain tissue (Fig. 6). Microglia were abundant in the brain stem and exhibited highly activated states, as evident in their morphology; with short, thickened processes or amoeboid forms. Immunohistochemical detection of CD16/32 also revealed colocalization with clusters of Iba-1-positive microglia (Fig. 6A). Expression of the CD16/32 M1 phenotype marker was most pronounced on multinucleated globoid cells within regions of abundant Iba-1-positive cells in presumptive, degenerating white matter tracts (Fig. 6B). Together, these data indicate the presence of M1 microglia in GLD and suggest a potential role for microglial polarization in GLD pathology and white matter injury.
Figure 6.
Identification of M1 polarized microglia and Globoid Cells in globoid cell leukodystrophy (GLD). (A) Immunohistochemistry performed on brainstem tissues of an infantile GLD case identified multiple microglial cells expressing both Iba-1 and the M1 polarization marker CD16/32. (B) Within a white matter lesion with evidence of intense microgliosis, a multi-nucleated globoid cell was also identified that was immunopositive of both Iba-1 and CD16/32. Scale bar in B = 29 µm for panel A and 166 µm for panel B.
DISCUSSION
In this study, we determined that TnC production was enhanced in twitcher mouse and human GLD brain samples. Microglial responses to psychosine were enhanced by TnC, as measured by both increased MMP-3 expression and globoid cell formation in vitro. These data suggest that localized accumulation of TnC may have the potential to modify microglial responses to psychosine. The impact of this TnC modulation was revealed by our investigation on the cytotoxicity of globoid cells toward oligodendrocytes. We determined that globoid cells plated on TnC evoked a higher level of cell death in co-culture with oligodendrocytes than globoid cells without TnC. Strikingly, naïve microglia also showed significantly greater toxicity to oligodendrocytes than psychosine-treated microglia, which reduced the native toxicity of microglia toward oligodendrocytes. Together, these data suggest that by recapitulating the ECM microenvironment of GLD, we influenced the cellular phenotype of microglia; this may be an important consideration for modeling disease-like responses in cell culture systems. Moreover, the findings support a more prominent and primary function for microglia and globoid cells as putative direct inducers of myelin pathology in GLD.
Alterations of the ECM have gained increasing interest for their relationship and contribution to myelin pathologies. In this study, we determined that TnC was differentially expressed in GLD. TnC has many known functions in modulating cellular growth and maturation, and also the repulsion of cells via local detachment (37). We determined that microglia grown on TnC elicited a more robust increase in psychosine-induced MMP-3 expression and enhanced •globoid-like• cell formation. In previous studies on traumatic brain injury and Alzheimer disease, TnC expression has been closely associated with both astrocytes and microglia (30, 32, 38–40). The cellular source of TnC remains an open but important question related to GLD pathology. We speculate that if TnC is not transcriptionally regulated by psychosine then inflammatory factors, such as TNF and/or interleukin-1 (IL-1) (41, 42), which are greatly upregulated in GLD brains (43), may modify the ECM in GLD.
We previously reported that psychosine transforms microglia into a multinucleated phenotype with high phagocytic activity (6). Here, we explored the potential effect of psychosine-induced globoid cells on oligodendrocytes. Interestingly, psychosine-induced globoid cells exhibited a predominant M2 phenotype with reduced cytotoxicity to oligodendrocytes when compared to untreated microglia. This suggests that globoid cells may either be benign or beneficial in GLD. The effect of TnC on the microglial response to psychosine-induced globoid cells (and this effect the oligodendrocyte co-culture system) were dramatic with a shift toward an oligotoxic M1 phenotype. Our data also indicated that psychosine modulation of microglial function correlated with and may be mediated by TNF. We hypothesize that TnC accumulation in GLD contributes to white matter pathology via altering the microglia response to psychosine induce demyelination. This provides a new prospective on the role of globoid cells and the extracellular environment in GLD. The mechanism(s) by which the combination of TnC and psychosine promote microglial toxicity to oligodendrocytes is presently unclear. Previous studies in other disease models have shown that altered ECM, associated with neuropathology, can modify production of growth factors such as ciliary neurotrophic factor, fibroblast growth factor, epidermal growth factor, and tumor growth factor (30, 32, 38–40). Future studies would be warranted to understand how TnC-modified microglia, in association with aberrant MMP-3 expression, contribute to the inflammatory environment of the CNS in GLD.
One possible mechanism by which TnC alters the phenotype and response of microglia to psychosine is through differential expression of their ECM receptors. Several integrins, including αv, β1, and β2 families (44), are thought to be TnC receptors. TnC binds to αvβ3, αvβ6, α9β1, and α8β1; these are distinct from the integrin receptors for Lm, which include α1β1 and α6β1 (44). Thus, the presence of TnC may stimulate microglia to express more TnC receptors, from which downstream signaling may result in functionally different outcomes. Microglia express αv and β1 integrins (45), and while altered integrin expression on microglia may result from psychosine levels in GLD, other cytokines that are also produced in the CNS during GLD may also contribute to microglial activation (43). In particular, the cytokines transforming growth factor β, TNF, and IL-1β, which are known to be elevated in GLD, have also been reported to induce a diversity of α and β integrin expression on microglia (45). Moreover, the ECM composition itself can induce changes in microglial integrin expression (45). Based on our findings in this report, further analysis of integrin expression on microglia and their role in mediating the cellular responses to psychosine in the presence of TnC would be expected to provide additional mechanistic insights into the pathological activation of microglia in GLD.
It is important to point out that TnC is a putative substrate of MMP-3. The concomitant increase of MMP-3 expression in GLD with the significant modulation of microglial response to psychosine on TnC suggests several potentially important associations between TnC and MMP-3. For example, we have determined that TnC dramatically increased mmp-3 mRNA expression in glia (Fig. 2), and previous work by others has investigated enzymatically targeted TnC and several cleaved TnC products (46, 47). Although the binding capacities or the signaling potentials of cleaved TnC fragments, it is possible that fragmented TnC may bind to integrins differently from full-length TnC or they may modulate signaling that results in microglial phenotypic changes. Future studies would be required to elucidate the involvement of integrins and possible TnC proteolysis on cellular phenotype changes induced by TnC on microglia in response to psychosine.
Globoid cells in situ frequently contain tubular inclusions and myelin debris (35, 48), but their function in GLD is unclear. Our in vitro analyses and modeling indicate that the local presence of TnC dramatically limits any potential beneficial function of globoid cells in GLD. Consistent with our in vitro data, we also identified M1 polarized microglia and globoid cells within brain tissues from human GLD specimens. We report that globoid cells behave differently depending on their local extracellular environment. Thus, GLD pathophysiology is likely a much more dynamic process than previously recognized. Indeed, cord stem cell and bone marrow transplantation, while yielding encouraging but limited effectiveness, may be influenced by altered ECM constituents in GLD. In particular, TnC, may be a limiting factor for current therapeutic strategies, and because it has diverse functions on cellular growth, maturation and repulsion of cells (37), it may also impact the activation phenotype of transplanted cells. At MS lesions, increased TnC may have a beneficial role to promote cell growth but it is also possible that prolonged TnC elevations may also prevent the migration of progenitor cells necessary for mediating repair (49, 50). TnC deposits in twitcher brain exhibited a unique pattern of plaque-like clustering in multiple brain regions and some TnC-positive clusters were positive for Thioflavin-S, an amyloid marker (51). Additionally, neuronal source would be consistent with the colocalization of TnC foci that also stained for Thioflavin-S in the twitcher mouse brain. These findings are also in agreement with our recent report on the development of intraneuronal inclusions containing α-synuclein in GLD (51). Further analysis of GLD pathology is needed to improve our understanding of the cellular source of TnC in GLD and to characterize the composition of extracellular plaques identified by TnC staining patterns in this disease.
In summary, we have defined a major role for globoid cells in the pathophysiology of GLD.
The importance of the ECM microenvironment in GLD was also highlighted by the enhanced transformation of microglia in response to psychosine when in the presence of TnC. Further detailed analyses on the temporal and spatial functions of TnC expression in the CNS would be required to elucidate the stage and full significance of TnC to GLD pathology. Future consideration of whether this process could be adopted to modify pathogenic microglial functions and how this understanding may be therapeutically applied in GLD may be warranted.
Supplementary Material
ACKNOWLEDGMENT
We would like to thank Rodney Ritzel for technical assistance.
This study was sponsored by grants from the National Institutes of Health (NIH; to SJC; NS078392), Health Center Research Advisory Council (SJC), CT Innovations (to SJC; 12-SCA-UCHC-06), Kim Family Fund (KIC), NIH (to ERB; RNS065808A), the Morton Cure Paralysis Foundation (to ERB), Legacy of Angels Foundation (to ERB), and the Board of Trustees at the University of Illinois (to ERB). Human tissue was obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, contract HHSN2752009000TIC, Ref. No. N01-HD-9-0011.
REFERENCES
- 1.Duffner PK, Jalal K, Carter RL. The Hunter’s Hope Krabbe family database. Pediatr Neurol. 2009;40:13–18. doi: 10.1016/j.pediatrneurol.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Wenger DA, Rafi MA, Luzi P, et al. Krabbe disease: genetic aspects and progress toward therapy. Mol Genet Metab. 2000;70:1–9. doi: 10.1006/mgme.2000.2990. [DOI] [PubMed] [Google Scholar]
- 3.Zlotogora J, Chakraborty S, Knowlton RG, et al. Krabbe disease locus mapped to chromosome 14 by genetic linkage. Am J Hum Genet. 1990;47:37–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Svennerholm L, Vanier MT, Mansson JE. Krabbe disease: a galactosylsphingosine (psychosine) lipidosis. J Lipid Res. 1980;21:53–64. [PubMed] [Google Scholar]
- 5.Miyatake T, Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem Biophys Res Commun. 1972;48:539–43. doi: 10.1016/0006-291x(72)90381-6. [DOI] [PubMed] [Google Scholar]
- 6.Ijichi K, Brown GD, Moore CS, et al. MMP-3 mediates psychosine-induced globoid cell formation: implications for leukodystrophy pathology. Glia. 2013;61:765–777. doi: 10.1002/glia.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morawski M, Bruckner G, Jager C, et al. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer’s disease neuropathology. Brain Pathol. 2012;22:547–561. doi: 10.1111/j.1750-3639.2011.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji K, Tsirka SE. Inflammation modulates expression of laminin in the central nervous system following ischemic injury. J Neuroinflammation. 2012;9:159. doi: 10.1186/1742-2094-9-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belichenko PV, Miklossy J, Celio MR. HIV-I induced destruction of neocortical extracellular matrix components in AIDS victims. Neurobiol Dis. 1997;4:301–310. doi: 10.1006/nbdi.1997.0143. [DOI] [PubMed] [Google Scholar]
- 10.Bonneh-Barkay D, Wiley CA. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009;19:573–585. doi: 10.1111/j.1750-3639.2008.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetz AK, Scheffler B, Chen HX, et al. Temporally restricted substrate interactions direct fate and specification of neural precursors derived from embryonic stem cells. Proc Natl Acad Sci U S A. 2006;103:11063–11068. doi: 10.1073/pnas.0510926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eyermann C, Czaplinski K, Colognato H. Dystroglycan promotes filopodial formation and process branching in differentiating oligodendroglia. J Neurochem. 2012;120:928–947. doi: 10.1111/j.1471-4159.2011.07600.x. [DOI] [PubMed] [Google Scholar]
- 13.Lei WL, Xing SG, Deng CY, et al. Laminin/beta1 integrin signal triggers axon formation by promoting microtubule assembly and stabilization. Cell Res. 2012;22:954–972. doi: 10.1038/cr.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lathia JD, Patton B, Eckley DM, et al. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol. 2007;505:630–643. doi: 10.1002/cne.21520. [DOI] [PubMed] [Google Scholar]
- 15.Gutowski NJ, Newcombe J, Cuzner ML. Tenascin-R and C in multiple sclerosis lesions: relevance to extracellular matrix remodelling. Neuropathol Appl Neurobiol. 1999;25:207–214. doi: 10.1046/j.1365-2990.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 16.Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 17.Stoffels JM, de Jonge JC, Stancic M, et al. Fibronectin aggregation in multiple sclerosis lesions impairs remyelination. Brain. 2013;136:116–131. doi: 10.1093/brain/aws313. [DOI] [PubMed] [Google Scholar]
- 18.Mohan H, Krumbholz M, Sharma R, et al. Extracellular matrix in multiple sclerosis lesions: Fibrillar collagens, biglycan and decorin are upregulated and associated with infiltrating immune cells. Brain Pathol. 2010;20:966–975. doi: 10.1111/j.1750-3639.2010.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim Biophys Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Crocker SJ, Frausto RF, Whitton JL, et al. A novel method to establish microglia-free astrocyte cultures: comparison of matrix metalloproteinase expression profiles in pure cultures of astrocytes and microglia. Glia. 2008;56:1187–1198. doi: 10.1002/glia.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welser-Alves JV, Crocker SJ, Milner R. A dual role for microglia in promoting tissue inhibitor of metalloproteinase (TIMP) expression in glial cells in response to neuroinflammatory stimuli. J Neuroinflammation. 2011;8:61. doi: 10.1186/1742-2094-8-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan J, Munro TP, Barbarese E, et al. A molecular mechanism for mRNA trafficking in neuronal dendrites. J Neurosci. 2003;23:8859–8866. doi: 10.1523/JNEUROSCI.23-26-08859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor RM, Lee JP, Palacino JJ, et al. Intrinsic resistance of neural stem cells to toxic metabolites may make them well suited for cell non-autonomous disorders: evidence from a mouse model of Krabbe leukodystrophy. J Neurochem. 2006;97:1585–1599. doi: 10.1111/j.1471-4159.2006.03986.x. [DOI] [PubMed] [Google Scholar]
- 25.Crocker SJ, Whitmire JK, Frausto RF, et al. Persistent macrophage/microglial activation and myelin disruption after experimental autoimmune encephalomyelitis in tissue inhibitor of metalloproteinase-1-deficient mice. Am J Pathol. 2006;169:2104–2116. doi: 10.2353/ajpath.2006.060626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore CS, Milner R, Nishiyama A, et al. Astrocytic tissue inhibitor of metalloproteinase-1 (TIMP-1) promotes oligodendrocyte differentiation and enhances CNS myelination. J Neurosci. 2011;31:6247–6254. doi: 10.1523/JNEUROSCI.5474-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YW, Boyartchuk V, Lewis BC. Differential roles of insulin-like growth factor receptor- and insulin receptor-mediated signaling in the phenotypes of hepatocellular carcinoma cells. Neoplasia. 2009;11:835–845. doi: 10.1593/neo.09476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duchen LW, Eicher EM, Jacobs JM, et al. Hereditary leucodystrophy in the mouse: the new mutant twitcher. Brain. 1980;103:695–710. doi: 10.1093/brain/103.3.695. [DOI] [PubMed] [Google Scholar]
- 29.Kelenyi G. Thioflavin S fluorescent and Congo red anisotropic stainings in the histologic demonstration of amyloid. Acta Neuropathol. 1967;7:336–348. doi: 10.1007/BF00688089. [DOI] [PubMed] [Google Scholar]
- 30.Nash B, Thomson CE, Linington C, et al. Functional duality of astrocytes in myelination. J Neurosci. 2011;31:13028–13038. doi: 10.1523/JNEUROSCI.1449-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Battisti WP, Wang J, Bozek K, et al. Macrophages, microglia, and astrocytes are rapidly activated after crush injury of the goldfish optic nerve: a light and electron microscopic analysis. J Comp Neurol. 1995;354:306–320. doi: 10.1002/cne.903540211. [DOI] [PubMed] [Google Scholar]
- 32.Smith GM, Hale JH. Macrophage/Microglia regulation of astrocytic tenascin: synergistic action of transforming growth factor-beta and basic fibroblast growth factor. J Neurosci. 1997;17:9624–9633. doi: 10.1523/JNEUROSCI.17-24-09624.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miron VE, Boyd A, Zhao JW, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chhor V, Le Charpentier T, Lebon S, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figols J, Zimmer C, Warzok R, et al. Immuno-lectin histochemistry and ultrastructure in two cases of globoid cell leukodystrophy (Krabbe’s disease) Clin Neuropathol. 1992;11:312–317. [PubMed] [Google Scholar]
- 36.Kigerl KA, Gensel JC, Ankeny DP, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 38.Dobbertin A, Czvitkovich S, Theocharidis U, et al. Analysis of combinatorial variability reveals selective accumulation of the fibronectin type III domains B and D of tenascin-C in injured brain. Exp Neurol. 2010;225:60–73. doi: 10.1016/j.expneurol.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Wiese S, Karus M, Faissner A. Astrocytes as a source for extracellular matrix molecules and cytokines. Front Pharmacol. 2012;3:120. doi: 10.3389/fphar.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie K, Liu Y, Hao W, et al. Tenascin-C deficiency ameliorates Alzheimer’s disease-related pathology in mice. Neurobiol Aging. 2013;34:2389–2398. doi: 10.1016/j.neurobiolaging.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Nakoshi Y, Hasegawa M, Sudo A, et al. Regulation of tenascin-C expression by tumor necrosis factor-alpha in cultured human osteoarthritis chondrocytes. J Rheumatology. 2008;35:147–152. [PubMed] [Google Scholar]
- 42.Noda N, Minoura H, Nishiura R, et al. Expression of tenascin-C in stromal cells of the murine uterus during early pregnancy: induction by interleukin-1 alpha, prostaglandin E(2), and prostaglandin F(2 alpha) Biol Reprod. 2000;63:1713–1720. doi: 10.1095/biolreprod63.6.1713. [DOI] [PubMed] [Google Scholar]
- 43.Kondo Y, Adams JM, Vanier MT, et al. Macrophages counteract demyelination in a mouse model of globoid cell leukodystrophy. J Neurosci. 2011;31:3610–3624. doi: 10.1523/JNEUROSCI.6344-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milner R, Campbell IL. The integrin family of cell adhesion molecules has multiple functions within the CNS. J Neurosci Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- 45.Milner R, Campbell IL. The extracellular matrix and cytokines regulate microglial integrin expression and activation. J Immunol. 2003;170:3850–3858. doi: 10.4049/jimmunol.170.7.3850. [DOI] [PubMed] [Google Scholar]
- 46.Siri A, Knauper V, Veirana N, et al. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem. 1995;270:8650–8654. doi: 10.1074/jbc.270.15.8650. [DOI] [PubMed] [Google Scholar]
- 47.Imai K, Kusakabe M, Sakakura T, et al. Susceptibility of tenascin to degradation by matrix metalloproteinases and serine proteinases. Febs Letters. 1994;352:216–218. doi: 10.1016/0014-5793(94)00960-0. [DOI] [PubMed] [Google Scholar]
- 48.Jesionek-Kupnicka D, Majchrowska A, Krawczyk J, et al. Krabbe disease: an ultrastructural study of globoid cells and reactive astrocytes at the brain and optic nerves. Folia Neuropathol. 1997;35:155–162. [PubMed] [Google Scholar]
- 49.Frost E, Kiernan BW, Faissner A, et al. Regulation of oligodendrocyte precursor migration by extracellular matrix: evidence for substrate-specific inhibition of migration by tenascin-C. Dev Neurosci. 1996;18:266–273. doi: 10.1159/000111416. [DOI] [PubMed] [Google Scholar]
- 50.Garcion E, Faissner A, ffrench-Constant C. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development. 2001;128:2485–2496. doi: 10.1242/dev.128.13.2485. [DOI] [PubMed] [Google Scholar]
- 51.Smith BR, Santos MB, Marshall MS, et al. Neuronal inclusions of alpha-synuclein contribute to the pathogenesis of Krabbe disease. J Pathol. 2014;232:509–521. doi: 10.1002/path.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.