Abstract
Objective
Age-related declines in physical activity are commonly observed in human and animal populations, but their physiological bases are not fully understood. We hypothesize that a lack of available energy contributes to low levels of activity in older persons.
Design
Cross-sectional analyses of relationships between physical activity level and energy availability were performed in 602 community-dwelling volunteers aged 45 to 91 yrs from the Baltimore Longitudinal Study of Aging (BLSA). Energy expenditure was measured at rest and during a maximal 400-meter walk for calculation of “available energy.” Overall and vigorous physical activity levels were assessed using standardized questionnaires. General linear regression models were used to assess the relationships between available energy and general and vigorous physical activity, and stratified analyses were used to analyze the possible differential association between available energy and physical activity across high and low (peak sustained walking VO2 <18.3 ml O2/kg/min) levels of aerobic fitness.
Results
Low available energy was associated with low levels of total physical activity (β = 64.678, p = .015) and vigorous activity (β = 9.123, p < .0001). The direct relationship between available energy and physical activity was particularly strong in persons categorized as having low aerobic fitness between available energy and physical activity with both total (β = 119.783, p = .022) and vigorous activity (β = 10.246, p = .015) and was independent of body composition and age.
Conclusions
Findings from this study support the hypothesis that available energy promotes the maintenance of physical activity in older persons. The findings also run counter to the perception that age-related declines in physical activity are primarily societally or behaviorally driven.
Keywords: Physical Activity, Energy, Aging, Aerobic Fitness
Introduction
Despite the known benefits of physical activity, in most countries rates of habitual physical activity are very low and trending dramatically downward.1–3 In older persons, low rates of physical activity are particularly deleterious, as regular activity in this group helps prevent bone loss4 and sarcopenia5 and reduces the risk of falls.6 Environmental, occupational, social, psychological, and genetic factors, together with poor self-efficacy, fear of falling and aging-adverse city planning have been identified as potential causes of age-related declines in physical activity.2,7–9 Although these and other factors certainly contribute to the volition to be physically active, there is evidence that physiological mechanisms are also at play, as evidenced by studies showing similar trends toward physical inactivity with aging in “lower” organisms such as Caenorhabditis elegans,10,11 Drosophila melanogaster,12 and other animals.13,14
Physiologic explanations of age-related declines in physical activity have been assessed in previous research, including age-related declines in maximal aerobic capacity15 and increased fatigue,9 although the specific mechanism through which these dimensions may affect daily physical activity is not fully understood. One hypothesis connecting the physiology of energy metabolism to behavioral motor activity is rooted in a paradigm recently introduced in the Baltimore Longitudinal Study of Aging (BLSA). The overarching hypothesis of this paradigm states that, “if energy becomes deficient, adaptive behaviors develop aimed at conserving energy.” Moreover, recent findings from this work support this hypothesis and suggest that older persons decrease gait speed to minimize total energy expenditure and offset declining walking efficiency.16
A similar hypothesis with respect to a possible physiologic/metabolic explanation of diminishing physical activity would contend that limited energy available for physical activity is the reason that older persons tend to have lower levels of activity. The energy available for physical activity, or “available energy” has been operationally defined as the difference between oxygen consumption during peak over-ground walking and resting oxygen consumption.17 Assessing the association between available energy and reported activity may shed light on the relationship between energy metabolism and overall activity level and offer new opportunities for physical activity intervention programs aimed at attenuating the age-related decline in physical activity. Accordingly, the primary aim of this study was to examine the relationship between available energy and overall and vigorous physical activity levels, as assessed using an interviewer-administered self-report questionnaire in 602 community-dwelling men and women aged 32 to 96 years enrolled in the BLSA.
Methods
Participants
Established in 1958 and conducted by the National Institute on Aging Intramural Research Program, the BLSA is a study of normative human aging. A general description of the sample and enrollment procedures and criteria has been previously reported.18 Briefly, the BLSA comprises a continuously enrolled cohort of volunteers who tend to be well educated, with above-average income and access to medical care. At the time of enrollment participants must pass a comprehensive health and functional screening evaluation and be free of major chronic conditions and cognitive and functional impairment. Once enrolled, participants are followed for life and undergo extensive testing every one to four years depending on age.
The sample for the current study consists of men and women who underwent physical function and gait speed assessments and tests of walking energy expenditure between July 2007 and March 2012 as described previously.17 Exclusion criteria for the current analysis included required use of a walking aid and the inability to complete 400 meters of peak sustained walking. Trained technicians administered all assessments following standardized protocols. The Internal Review Board of the Medstar Research Institute approved the study protocol and all participants provided written informed consent.
Physical Activity
Overall physical activity level was assessed using an interviewer-administered standardized physical activity questionnaire,19 which has relatively high construct validity in well-educated health conscious persons which characterizes most BLSA participants.20 Responses to items related to walking frequency and duration, flights of stairs climbed, and the mode, frequency, and duration of sporting or recreational activities were incorporated into a single estimate of energy expenditure through physical activity in kcal/week, as in previous studies.3,21–23
Participants reported high-intensity physical activity level through a series of questions pertaining to the number of minutes of vigorous activity performed per week, including brisk walking, and a composite value was again calculated for this category of physical activity similar to previous studies.24
Available Energy
“Available energy” is an energy construct previously operationalized17 in the BLSA and defined as the total energy available to an individual for physical activity. Available energy is calculated as the difference between the average energy expended at peak sustained walking speed and resting energy expenditure: peak sustained walking energy expenditure – resting energy expenditure (ml/kg/min), and represents the portion of aerobic capacity over and above the amount of energy required to sustain life.
Assessment of Energy Expenditure
Energy expenditure was measured using a Cosmed K4b2 portable metabolic analyzer (Cosmed, Rome, Italy) and was calculated from the volume of oxygen consumed per kilogram of body weight (VO2 ml/kg/min) during rest and walking at maximal speed for 400 meters. The Cosmed unit utilizes a rubberized facemask and turbine for gas collection. Prior to testing, the Cosmed system was warmed-up for a minimum of 20 minutes and calibrated using reference gases of known concentrations and a 3.0-L syringe for flow. Breath-by-breath measurement of VO2 data (ml/kg/min) was collected and averaged into 30-second intervals to reduce variability.
Resting Energy Expenditure
Resting energy expenditure, represented by resting metabolic rate (RMR) was assessed using indirect calorimetry first thing in the morning, in a fasted, rested state. Participants breathed into a mask attached to the Cosmed K4b2 portable metabolic analyzer for 16 minutes. To calculate average RMR, values obtained during the first 5 minutes were omitted to allow the participant to acclimate to the face mask and the testing environment and the subsequent minutes were averaged to arrive at a single value for RMR (ml/kg/min).
Peak Sustained Walking Energy Expenditure
Peak sustained walking energy expenditure was assessed during the 400m portion of the long distance corridor walk (LDCW), a validated measure of cardiorespiratory fitness in older adults.25,26 The participant was instructed to walk “as fast as possible, at a pace you can sustain for 400 meters.” The hallway course measured 20 meters, marked by cones at both ends. Standardized encouragement was given with each lap along with the number of laps remaining. Split times for each lap and total time to walk 400m was recorded. The Cosmed remained on the participant for 2 minutes after the completion of the test to ensure adequate breath collection.
To calculate peak sustained walking energy expenditure per kilogram of body weight (peak sustained walking VO2 ml/kg/min), readings from the first 1.5 minutes of the 400m walk were discarded to allow the participant to adjust to the workload and the remaining readings were averaged to arrive at single measure of the average energy expended (ml/kg/min) during 400 meters of peak sustained over-ground walking. This measure of over-ground energy expenditure is more consistent with the energy required for higher intensity tasks related to daily living than treadmill-based measures of cardiorespiratory fitness.27
Weight (SR Scales 725L) and height were measured according to standard protocols and body mass index (BMI) was calculated as weight (kg) over height squared (m2). Body composition was assessed using dual-energy X-ray absorptiometry scan, providing information on total body fat mass and fat-free mass.
Statistical Analysis
Unadjusted relationships between age, physical activity, and energy expenditure variables were represented by LOWESS scatterplots and analyzed using multiple linear regression models. Adjusted models included as covariates sex and two established, independent contributors to physical activity level: fat mass and fat-free mass.28–30 No significant interactions were found between age and sex, age and available energy, and sex and available energy, and therefore these interactions were not included in the final models presented.
To address the potential differential association across fitness levels between energy availability and physical activity, fitness level-stratified analyses were performed. The cutpoint delineating “higher fit” from “low fit” strata was a peak sustained walking energy value of 18.3 ml/kg/min, as previously designated in this cohort31 and other research indicating this is the minimal level adequate for independent function.32–34 All analyses were performed using Stata MP, version 12 (Statacorp, College Station, TX) and p-values of <.05 were considered significant.
Results
Participant characteristics are presented in Table 1. The mean age was 68.0 (± 10.8) years, approximately half (51.8%) of the participants were men, and average BMI fell within the overweight category (27.3 (± 4.3) kg/m2). Only 5.1% of participants were current smokers or quit within the past ten years. As a descriptive measure of functional capacity, usual gait speed—which was assessed in a separate 6-meter test that is part of the BLSA physical performance battery16—ranged from 0.5 m/s to 1.9 m/s and averaged 1.2 (± 0.2) m/s. These values match the distribution of usual walking speed in the older population already reported in other studies.8,9,28
Table 1. Participant Characteristics.
| Entire Sample Mean Values (n = 602) | Higher Fit Group Mean Values (n = 261) | Low Fit Group Mean Values (n = 341) | P for Difference (Higher vs. Low Fit Group) | |
|---|---|---|---|---|
|
| ||||
| Characteristics | ||||
| Age (years), mean (SD) | 68.1 (10.8) | 64.7 (10.1) | 70.6 (10.7) | <0.001a |
| Male sex, No. (%) | 290 (51.83) | 151 (57.85) | 161 (47.21) | <0.01a |
| Body Mass Index (kg/m2), mean (SD) | 27.28 (4.73) | 26.22 (4.16) | 28.09 (4.98) | <0.001a |
| Usual Gait Speed (m/s), mean (SD) | 1.16 (0.23) | 1.26 (.21) | 1.08 (.21) | <0.001a |
| Resting Metabolic Rate (ml/kg/min), mean (SD) | 2.63 (0.67) | 2.80 (.68) | 2.50 (.63) | <0.001a |
| Peak Sustained Energy Expenditure (ml/kg/min), mean (SD) | 17.81 (4.63) | 21.99 (3.23) | 14.61 (2.49) | <0.001a |
| Available Energy (ml/kg/min), mean (SD) | 15.18 (4.49) | 19.19 (3.23) | 12.11 (2.44) | <0.001a |
| Total Physical Activity Level (kcal/week) (SD) | 2178.18 (2659.55) | 2703.49 (2871.57) | 1776.11 (2413.14) | <0.001a |
| Vigorous Activity Level (min/week) (SD) | 167.95 (216.81) | 225.87 (235.69) | 123.62 (189.90) | <0.001a |
Values are means ± SD or n (%).
Statistically significantly difference between Higher and Low Fitness Groups at the 0.05 level.
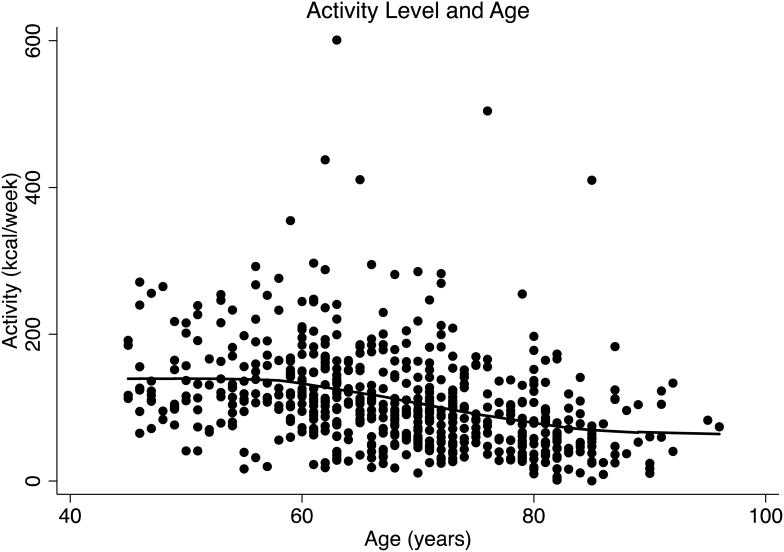
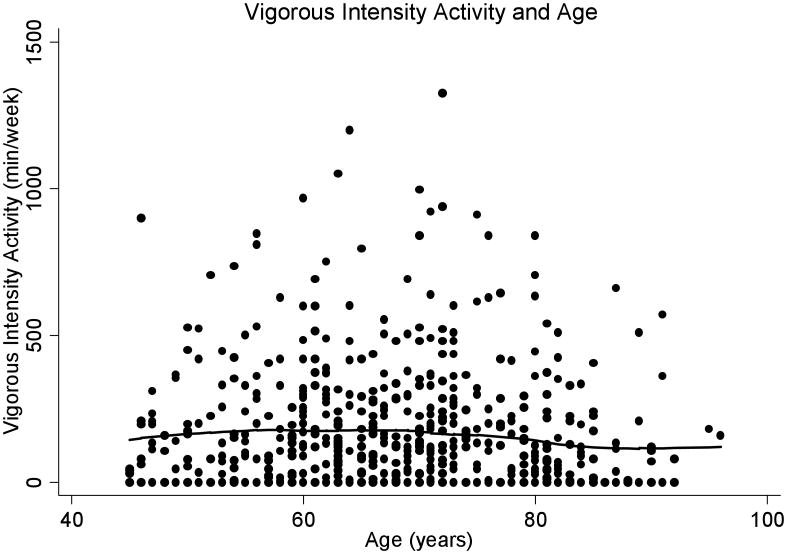
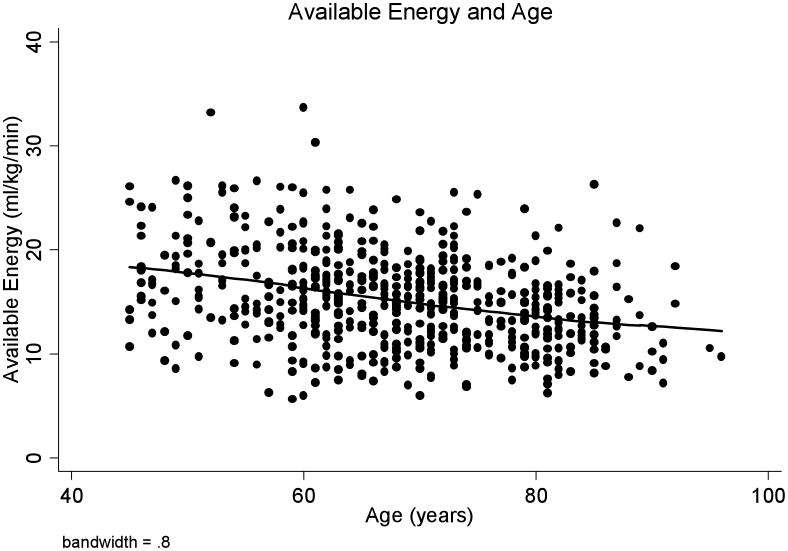
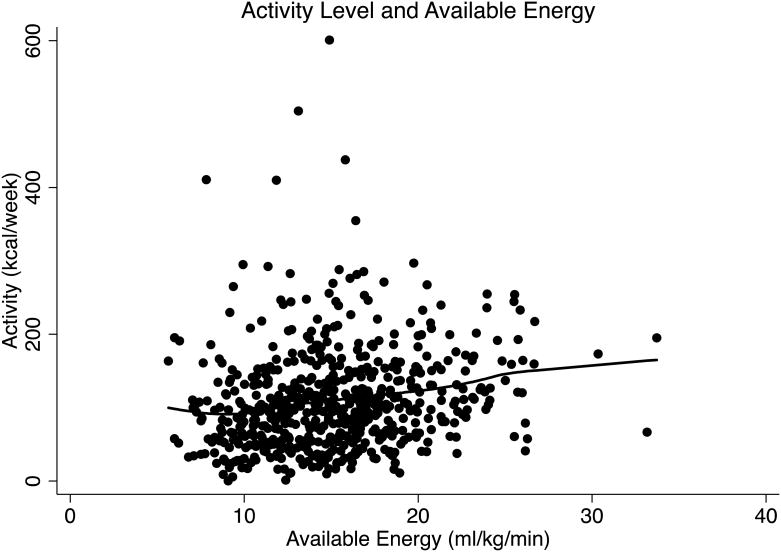
Total physical activity was progressively lower with age (β = -23.5, p = .02), especially after age 65 (β = -41.0, p=.02) (Figure 1A). In contrast, there was no significant age-related difference in reported vigorous physical activity (β = -2.37, p = .13) (Figure 1B). Available energy averaged 15.2 (± 4.2) ml/kg/min, and was lower with increasing age (β = -.12, p < .001) (Figure 2). Higher available energy was associated with higher total (β = 125.7, p < .01) (Figure 3A) and vigorous physical activity (β = 14.9, p < .001) (Figure 3B). Given these results, a mediation analysis was performed to assess if reductions in available energy substantially explain the decline in both total and vigorous physical activity. When available energy was entered into a model with age as the predictor and physical activity as the outcome, the significant relationship between age and total physical activity was reduced to non-significance (β = -11.4, p = .28) while available energy was still strongly and significantly related to total physical activity (β = -89.5, p < 0.001). Similarly, a nearly significant relationship between age and vigorous physical activity was attenuated (β = .22, p = .79) by available energy, with a significant relationship between available energy and vigorous physical activity (β = 12.7, p < 0.001) prevailing.
Figure 1.
A: Physical Activity (kcal/week) Level and Age
A LOWESS fit curve illustrates the curvilinear relationship between physical activity level (kcal/wk) and age. This figure illustrates an age-related decline in total physical activity that accelerates after age 65.
B: Vigorous Physical Activity (min/week) and Age
A LOWESS fit curve displays no significant age-related change in vigorous physical activity.
Figure 2.
Available Energy (ml/kg/min) and Age
Available energy, which represents the difference between peak sustained walking energy expenditure and RMR, also declines with age.
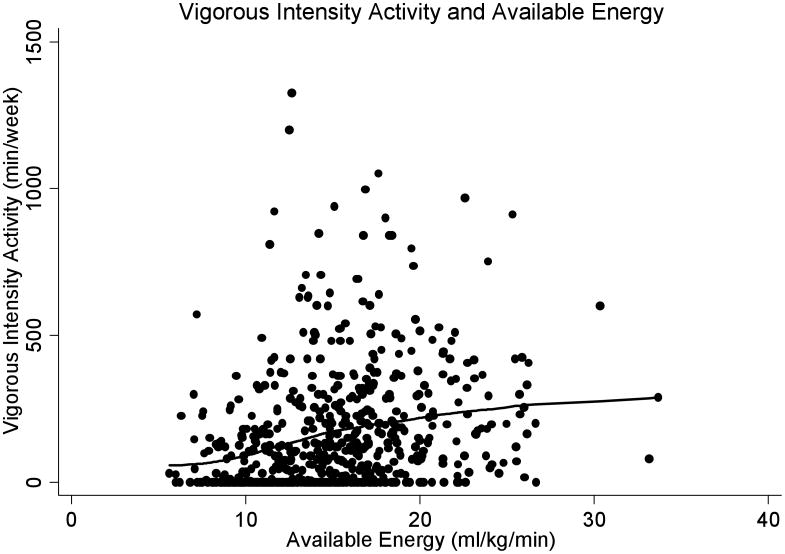
Figure 3.
A: Physical Activity (kcal/week) and Available Energy (ml/kg/min)
A LOWESS curve fit displays a significant increase in total physical activity (kcal/week) with increasing available energy, indicating that the more available energy an individual has, the more active they are.
B: Vigorous Physical Activity (min/week) and Available Energy
Similarly, a LOWESS curve fit displays a significant increase in vigorous physical activity (kcal/week) with increasing available energy, indicating that the more available energy an individual has, the more vigorously active they are.
Additional analyses using general linear models indicated direct relationships between lean mass and both total and vigorous physical activity (β = 0.076, p = .02, β = 0.004, p = .03, respectively) (Table 2). These models also found a negative relationship between fat mass and total and vigorous physical activity (β = -0.007, p = .01, β = -0.003, p = .001). No significant association between age and either measure of physical activity were found in the adjusted models; however, low available energy was negatively associated with levels of total physical activity (β = 64.678, p = .015) and vigorous activity (β = 9.123, p < .0001) in older persons, independent of body composition and age (Table 2).
Table 2. Association Between Age, Energy Availability and Physical Activity Level.
| Dependent Variable: Total Energy Expenditure (kcal/week) | Coefficient | P-value | Dependent Variable: Vigorous Activity Level (min/week) | Coefficient | P-value |
|---|---|---|---|---|---|
| Independent Variables: | |||||
| Age (years) | -7.159 | .535 | -.399 | .675 | |
| Male sex | 60.759 | .885 | -9.102 | .791 | |
| Lean mass (kg) | .076 | .021a | .004 | .029a | |
| Fat mass (kg) | -.007 | .012a | -.003 | .001a | |
| Intercept | -1798.984 | .217 | -26.940 | .822 | |
| Available energy (ml/kg/min) | 64.678 | .015a | 9.123 | <.0001a |
Statistically significant at the 0.05 level.
Finally, stratified analyses were used to analyze the possible differential association between available energy and physical activity across levels of aerobic fitness. In this sample, 341 (56.6%) individuals were below the 18.3 ml/kg/min threshold. This low fit group had a greater percentage of females and tended to have a higher BMI and lower usual gait speed, RMR, available energy and total and vigorous activity levels (Table 1). In the more fit group, lean mass was associated with total physical activity (β = 0.086, p = 0.01); however, available energy was not associated with either measure of physical activity in this higher fit group (Table 3). Analyses of the low fit (or debilitated) group (peak sustained walking energy expenditure < 18.3 ml/kg/min) generally indicated similar trends in terms of the association of lean mass with both total physical activity (β = .074, p = .026) and vigorous activity (β = .004, p = .061), and the association of fat mass with both total physical activity (β = -.009, p = .509) and vigorous activity (β = -.002, p = .033). However, in contrast to the higher fit group, in this low fit group there was a significant positive relationship between available energy and physical activity with both total (β = 119.783, p = .022) and vigorous activity (β = 10.246, p = .015) (Table 3).
Table 3. Fitness-Level Stratified Analysis.
| Dependent Variable: Total Energy Expenditure (kcal/week) | Coefficient | P-value | Dependent Variable: Vigorous Activity Level (min/week) | Coefficient | P-value |
|---|---|---|---|---|---|
| Higher Fit Stratum (n = 261) | |||||
| Independent Variables: | |||||
| Age (years) | -16.087 | .429 | -.909 | .593 | |
| Male sex | -242.962 | .736 | -34.891 | .562 | |
| Lean mass (kg) | .0863 | .014a | .004 | .226 | |
| Fat mass (kg) | -.004 | .871 | -.005 | .006a | |
| Intercept | 683.658 | .217 | 178.778 | .423 | |
| Available energy (ml/kg/min) | -51.518 | .379 | 3.939 | .420 | |
| Low Fit Stratum (n = 341) | |||||
| Independent Variables: | |||||
| Age (years) | -.773 | .955 | -.097 | .930 | |
| Male sex | 197.589 | .694 | 10.40 | .798 | |
| Lean mass (kg) | .074 | .026a | .004 | .061 | |
| Fat mass (kg) | -.009 | .509 | -.002 | .033a | |
| Intercept | -2902.783 | .108 | -112.912 | .438 | |
| Available energy (ml/kg/min) | 119.783 | .022a | 10.246 | .015a | |
Statistically significant at the 0.05 level.
Discussion
In healthy, independently mobile individuals aged 45-96 there were significant positive relationships between available energy and levels of both total and vigorous physical activity, which was maintained after adjusting for age and body composition. Although the relationship between physical activity and physical fitness is well-established, with activity considered the driving factor, the analysis stratified by fitness level revealed that the relationship between available energy and physical activity was driven by the strong association in debilitated persons; i.e. those with fitness levels below the threshold of peak sustained walking energy expenditure consistent with independent living.
The results of this paper are consistent with previous findings from the BLSA indicating older individuals may employ an adaptive strategy of reducing usual gait speed to conserve limited available energy16 and indicate a similar strategy of energy conservation relating to the volume of activity (in terms of either kilocalories of total energy expenditure or minutes of vigorous activity). Both high-volume and high-intensity activities require substantial energy expenditure and, especially in individuals with compromised peak aerobic fitness that falls below critical levels, energy availability may be a critical determinant of why many individuals avoid physical exertion.2 Furthermore, physical inactivity is a core component of frailty, the stage of heightened vulnerability and diminished functional capacity observed at the end of life.35 We contend that this non-volitional reduction in activity may begin as an insidious physiological process based in low available energy.
Preservation of available energy appears to be a critical factor in maintaining sufficient physical activity levels with age and reaping the documented benefits of an active lifestyle.3,36,37 Since persons with low peak energy availability are most vulnerable/at risk, efforts should be focused first on developing low-level activities within the energetic capacity of debilitated/detrained individuals to prevent them from falling below a critical threshold. Physical activity may also assist these individuals in rising above the threshold, as suggested by previous work indicating the importance of physical activity, including resistance training, in preserving muscle mass and quality and limiting comorbidities.38,39 With available energy preserved or increased gradually through small doses of physical activity spread across the day, previously detrained individuals may then be more likely to participate in greater volume and intensity of physical activity. Specific interventions may progress toward more novel types of training such as higher-intensity interval training with its effects on peak aerobic capacity in addition to broader health benefits.40
Limitations of the current study include the cross-sectional nature of the data, which does not allow excluding a mechanism of reverse-causality (i.e. diminished physical activity may initiate losses in available energy). Additional longitudinal analyses will be possible as more follow-up data becomes available to address this issue; nevertheless the cross-sectional analysis distinguishes the low fit individuals from others in the sample. In addition, the sample used in the current study is likely more fit than the general population, as supported by a lack of significant decline in vigorous activity across the age span (Figure 1B). This feature of the current study may limit the generalizability of these findings to a more fit, active subgroup of the general population. However, even within this healthy cohort, a substantial number of individuals were classified as ‘low fit’ by established criteria. Despite their status as ‘low fit,’ all individuals in this sample met the inclusion criterion of being able to complete 400 meters of peak sustained walking, suggesting that there is sufficient physiological reserve to enable habilitation even in persons with even low levels of fitness.
The mechanisms contributing to the worldwide downward trends of physical activity are multifactorial and interrelated. Findings from this study underscore the potential importance of energetics in general and available energy in particular in enabling vigorous, physically active lifestyles. The findings also partially contradict the perception that age-related declines in physical activity are primarily behaviorally and societally driven. In debilitated individuals there appears to be a critical level of energy required to maintain physical activity above levels normally associated with healthy aging and chronic disease prevention. Future research should target individuals who are either in or approaching the classification of having low fitness and are therefore susceptible to loss of functional independence and evaluate physiologically based approaches to increasing physical activity through enhancing energy availability.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging. Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
Footnotes
Disclosures: Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
References
- 1.Al-Hazzaa HM. Prevalence of physical inactivity in Saudi Arabia: a brief review. East Mediterr Health J. 2004;10(4-5):663–670. [PubMed] [Google Scholar]
- 2.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: What are the contributors? Annual Review of Public Health. 2005;26(1):421–443. doi: 10.1146/annurev.publhealth.26.021304.144437. [DOI] [PubMed] [Google Scholar]
- 3.Paffenbarger RS, Jr, Kampert JB, Lee IM, Hyde RT, Leung RW, Wing AL. Changes in physical activity and other lifeway patterns influencing longevity. Med Sci Sports Exerc. 1994;26(7):857–865. [PubMed] [Google Scholar]
- 4.Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333. doi: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50(5):889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 6.Hourigan SR, Nitz JC, Brauer SG, O'Neill S, Wong J, Richardson CA. Positive effects of exercise on falls and fracture risk in osteopenic women. Osteoporos Int. 2008;19(7):1077–1086. doi: 10.1007/s00198-007-0541-7. [DOI] [PubMed] [Google Scholar]
- 7.Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJ, Martin BW. Correlates of physical activity: why are some people physically active and others not? The Lancet. 380(9838):258–271. doi: 10.1016/S0140-6736(12)60735-1. [DOI] [PubMed] [Google Scholar]
- 8.Sallis JF, Hovell Melbourne F, Richard Hofstetter C, et al. A multivariate study of determinants of vigorous exercise in a community sample. Preventive Medicine. 1989;18(1):20–34. doi: 10.1016/0091-7435(89)90051-0. [DOI] [PubMed] [Google Scholar]
- 9.Simonsick EM, Guralnik JM, Fried LP. Who walks? Factors associated with walking behavior in disabled older women with and without self-reported walking difficulty. J Am Geriatr Soc. 1999;47(6):672–680. doi: 10.1111/j.1532-5415.1999.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 10.Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 11.Kirkwood TBL, Finch CE. Ageing: the old worm turns more slowly. Nature. 2002;419(6909):794–795. doi: 10.1038/419794a. [DOI] [PubMed] [Google Scholar]
- 12.Marden JH, Rogina B, Montooth KL, Helfand SL. Conditional tradeoffs between aging and organismal performance of Indy long-lived mutant flies. PNAS. 2003;100(6):3369–3373. doi: 10.1073/pnas.0634985100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Némoz-Bertholet F, Aujard F. Physical activity and balance performance as a function of age in a prosimian primate (Microcebus murinus) Experimental Gerontology. 2003;38(4):407–414. doi: 10.1016/S0531-5565(02)00244-9. [DOI] [PubMed] [Google Scholar]
- 14.Siwak CT, Tapp PD, Zicker SC, et al. Locomotor activity rhythms in dogs vary with age and cognitive status. Behav Neurosci. 2003;117(4):813–824. doi: 10.1037/0735-7044.117.4.813. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka H, Desouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. J Appl Physiol. 1997;83(6):1947–1953. doi: 10.1152/jappl.1997.83.6.1947. [DOI] [PubMed] [Google Scholar]
- 16.Schrack JA, Simonsick EM, Chaves PHM, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60(10):1811–1816. doi: 10.1111/j.1532-5415.2012.04153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: An emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(Suppl 2):S329–S336. doi: 10.1111/j.1532-5415.2010.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21(4):575–580. doi: 10.1093/geronj/21.4.575. [DOI] [PubMed] [Google Scholar]
- 19.Brach JS, Simonsick EM, Kritchevsky S, et al. The association between physical function and lifestyle activity and exercise in the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2004;52(4):502–509. doi: 10.1111/j.1532-5415.2004.52154.x. [DOI] [PubMed] [Google Scholar]
- 20.Rundle A, Hagins M, Orjuela M, Mooney L, Kim M, Perera F. Traditional physical activity indexes derived from the Harvard alumni activity survey have low construct validity in a lower income, urban population. J Urban Health. 2007;84(5):722–732. doi: 10.1007/s11524-007-9212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS., Jr Accuracy of the college alumnus physical activity questionnaire. J Clin Epidemiol. 1993;46(12):1403–1411. doi: 10.1016/0895-4356(93)90140-v. [DOI] [PubMed] [Google Scholar]
- 22.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 23.Paffenbarger RS, Jr, Hyde RT, Hsieh CC, Wing AL. Physical activity, other life-style patterns, cardiovascular disease and longevity. Acta Med Scand Suppl. 1986;711:85–91. doi: 10.1111/j.0954-6820.1986.tb08936.x. [DOI] [PubMed] [Google Scholar]
- 24.Manson JE, Hu FB, Rich-Edwards JW, et al. A Prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. New England Journal of Medicine. 1999;341(9):650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 25.Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54(1):127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 26.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC long distance corridor walk. J Am Geriatr Soc. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 27.Parvataneni K, Ploeg L, Olney SJ, Brouwer B. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clinical Biomechanics. 2009;24(1):95–100. doi: 10.1016/j.clinbiomech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Ekelund U, Brage S, Besson H, Sharp S, Wareham NJ. Time spent being sedentary and weight gain in healthy adults: reverse or bidirectional causality? Am J Clin Nutr. 2008;88(3):612–617. doi: 10.1093/ajcn/88.3.612. [DOI] [PubMed] [Google Scholar]
- 29.Metcalf BS, Hosking J, Jeffery AN, Voss LD, Henley W, Wilkin TJ. Fatness leads to inactivity, but inactivity does not lead to fatness: a longitudinal study in children (EarlyBird 45) Arch Dis Child. 2011;96(10):942–947. doi: 10.1136/adc.2009.175927. [DOI] [PubMed] [Google Scholar]
- 30.Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53(3):M214–221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 31.Schrack JA, Simonsick EM, Ferrucci L. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am J Phys Med Rehabil. 2013;92(1):28–35. doi: 10.1097/PHM.0b013e3182644165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cress ME, Meyer M. Maximal voluntary and functional performance needed for independence in adults aged 65 to 97 years. Phys Ther. 2003;83(1):37–48. [PubMed] [Google Scholar]
- 33.Morey MC, Pieper CF, Cornoni-Huntley J. Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults? Med Sci Sports Exerc. 1998;30(8):1223–1229. doi: 10.1097/00005768-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Posner JD, McCully KK, Landsberg LA, et al. Physical determinants of independence in mature women. Archives of Physical Medicine and Rehabilitation. 1995;76(4):373–380. doi: 10.1016/S0003-9993(95)80664-4. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 36.Morris JN, Clayton DG, Everitt MG, Semmence AM, Burgess EH. Exercise in leisure time: coronary attack and death rates. Br Heart J. 1990;63(6):325–334. doi: 10.1136/hrt.63.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris JN, Kagan A, Pattison DC, Gardner MJ. Incidence and prediction of ischaemic heart-disease in London busmen. Lancet. 1966;2(7463):553–559. doi: 10.1016/s0140-6736(66)93034-0. [DOI] [PubMed] [Google Scholar]
- 38.Fiatarone MA, O'Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 39.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 40.Gibala MJ, Little JP, MacDonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. The Journal of Physiology. 2012;590(5):1077–1084. doi: 10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]