Summary
Objective
Ischemic stroke is a major cause of morbidity and mortality in elderly men. Our main objective was to examine if testosterone (T) or dihydrotestosterone (DHT) were associated with incident ischemic stroke in elderly men.
Design
Cohort study
Participants
Elderly men in the Cardiovascular Health Study who had no history of stroke, heart disease, or prostate cancer as of 1994 and were followed until December 2010.
Measurements
Adjudicated ischemic stroke
Results
Among 1032 men (mean age 76, range 66-97), followed for a median of 10 years, 114 had an incident ischemic stroke. Total T and free T were not significantly associated with stroke risk while DHT had a nonlinear association with incident stroke (p=.006) in analyses adjusted for stroke risk factors. The lowest risk for stroke was at DHT levels of 50-75 ng/dL, with greater risk for stroke at DHT levels above 75 ng/dl or below 50ng/dl. Results were unchanged when SHBG was added to the model. Calculated free DHT had an inverse linear association with incident ischemic stroke with HR 0.77 (95% CI, 0.61, 0.98) per standard deviation in analyses adjusted for stroke risk factors.
Conclusions
DHT had a nonlinear association with stroke risk in which there was an optimal DHT level associated with the lowest stroke risk. Further studies are needed to confirm these results and to clarify if there is an optimal androgen range associated with the least risk for adverse outcomes in elderly men.
Keywords: androgens, testosterone, dihydrotestosterone, stroke, cohort
Introduction
Serum testosterone (T) levels progressively decline in men, starting in middle age, and by age 60 low T levels occur in 20%-40% of men.1-3 The exact threshold for low T In older men remains controversial and depends on the assay used. The Endocrine Sociey has recommended a threshold in older men of low total T 280-300 ng/dL,4 which is a level that is considered to be low in younger men. Although low T levels are common in older men, androgen deficiency (i.e., low T plus signs and symptoms of low T) is much less common and occurs in only 2-6% of men ages 30-79 years old.1, 5 Low T may be associated with an increased risk for stroke as it is associated with risk factors for stroke such as increased body mass index (BMI), diabetes, dyslipidemia, atherosclerosis, arterial stiffness, and atrial fibrillation.6-11 Dihydrotestosterone (DHT) may also be associated with an increased risk for stroke, as it is an active metabolite of T and is a much more potent androgen than T. DHT is associated with risk factors for stroke such as increased BMI, diabetes, dyslipidemia, prevalent cardiovascular disease, and ischemic heart disease mortality.12, 13 Sex hormone binding globulin (SHBG) is a carrier protein that tightly binds and transports T and DHT. SHBG may also be associated with an increased risk for stroke because low SHBG is associated with risk factors for stroke such as metabolic syndrome, diabetes, lipids, and cardiovascular outcomes.14-19 However, although T, DHT, and SHBG are associated with risk factors for stroke, it is possible that low levels of these hormones are not causally linked to stroke but are merely markers of poor health.
The purpose of this study was to examine if serum T, DHT, and SHBG were independently associated with incident ischemic stroke. Few studies have examined the association between T and SHBG and incident stroke and to our knowledge, no prior studies have evaluated the association between DHT and incident stroke. This is a clinically relevant question as stroke is the 4th leading cause of death in the United States and is responsible for significant morbidity and mortality, particularly in older men.20 Given the significant death and disability associated with stroke, the identification of potential novel risk factors for stroke could have a significant impact on public health. Based on available studies, we hypothesized that lower levels of T, DHT, and SHBG would be associated with an increased incidence of ischemic stroke in elderly men.
Methods
Study population
The Cardiovascular Health Study (CHS) is a longitudinal study that was initiated in 1989 to identify risk factors for cardiovascular disease in older adults.21 Eligible participants were 65 years or older, non-institutionalized, expected to stay in the area for 3 years, and able to give informed consent. Those excluded were hospice patients, wheelchair-bound, or receiving current treatment for cancer. Each study center's institutional review board approved the study and each participant provided written informed consent before participating in the study. Clinic examinations were performed annually from 1989 to 1999, and again in 2005. Men were included in the present study if they had no detectable heart disease, stroke, or prostate cancer as of the 1994 examination and had frozen sera available from that visit. Prevalent heart disease was defined as a history of myocardial infarction, coronary artery bypass graft (CABG), percutaneous coronary intervention (PCI), heart failure, or stroke
Assessment of the Primary Outcome
The primary outcome was incident fatal and non-fatal ischemic stroke as adjudicated by the CHS Events Committee, which included neurologists from each site.22 The information used to adjudicate stroke included Medicare data, hospital records, imaging studies, autopsy results, death certificates and interviews with attending physicians, subjects, and families. Based on this information, standardized algorithms were used to adjudicate stroke outcomes through December 2010. In situations in which there was insufficient evidence to classify a stroke as either hemorrhagic or ischemic, the stroke was classified as a stroke of unknown type. We limited the outcome to ischemic stroke because the risk factors for ischemic stroke (e.g., diabetes, dyslipidemia, atherosclerosis, atrial fibrillation) are more strongly associated with low T and DHT than risk factors for hemorrhagic stroke (thrombocytopenia, bleeding dyscrasias, and anticoagulant treatment).
Hormone levels and assays
Blood samples were obtained from men participating in the 1994 CHS examination. Time of day for sample collection was not recorded, but based on the study protocol we believe that most were collected before noon. The samples were stored at -70° C at the CHS Central Laboratory in Burlington, Vermont. In 2010, frozen serum samples from eligible participants were shipped on dry ice to an endocrine research laboratory (author AMM's laboratory), which has over 20 years of experience in conducting androgen assays. All assays were conducted in duplicate and the average value was used in these analyses. Total T and DHT were measured simultaneously using a liquid chromatography-tandem mass spectrometry assay.23 The lower limit of detection for total T was 1.0 ng/dL, with an intra-assay coefficient of variation (CV) of 4.9% and an inter-assay CV of 5.1%. The CDC Hormone Standardization Program certified the assay. The lower limit of detection for DHT was 0.02 ng/mL with an intra-assay CV of 5.9% and an inter-assay CV of 6.2%. Sex hormone binding globulin (SHBG) was assayed using a commercial radioimmunoassay kit with a time-resolved fluoro-immunoassay (Delfia, Perkin Elmer, Norton, OH). The lower limit of detection for SHBG was 0.5 nmol/L, with an intra-assay CV of 1.4% and an inter-assay CV of 6.6% at 31 nmol/L.
We determined calculated free T by the Vermeulen method, which correlates well with measures done by equilibrium dialysis,24 and the Mazer method.25 The calculated free T values by the Vermeulen and Mazer methods were highly correlated with each other (r= 0.998, p<0. 00001). However, we used the Mazer formula in the analyses, rather than the Vermeulen formula, because it allowed calculation of both free T and free DHT.26 Finally we determined repeated hormone measures in a subset of participants (n=74) who had longitudinal sera samples available from 1989, 1994, and 1996 to evaluate the reliability of a single hormone measure over several years by estimating the intra-class correlations (ICC) for total T and DHT.
Assessment of covariates
Descriptions of data collection methods, including instruments and protocols have been reported previously.21 The covariates were measured at the 1994 visit, unless otherwise noted, and included measures to characterize the population and known risk factors for incident stroke reported in a prior CHS investigation.22 These covariates were age, race, educational level, smoking status, alcohol intake, systolic and diastolic blood pressure, atrial fibrillation, diabetes, creatinine >1.25 mg/dL, HDL, total cholesterol and lipid-lowering, anti-hypertensive, antiplatelet and anticoagulation agents. Demographic variables and alcohol consumption were based on self-report. Smoking status was characterized by pack-years of smoking. Blood pressure was measured using standardized protocols. Medication use was ascertained using a validated medication inventory. Lab values obtained in 1992-1993 were lipids, creatinine and fasting glucose. We defined diabetes as fasting glucose ≥ 126 mg/dL, non-fasting glucose ≥ 200 mg/dL, or use of diabetes medication. We defined hypertension as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mm Hg, or physician diagnosis of hypertension combined with use of anti-hypertensive medication.
Statistical Analysis
Demographic and stroke risk factors were compared in men who had an ischemic stroke and those who did not using Chi-Square tests for categorical variables and t-tests for continuous variables. We modeled each hormone continuously to examine the shapes of the hormone-associations with incident ischemic stroke using generalized additive model plots and penalized cubic regression splines.27 The lowest and highest 2.5% of hormone values were excluded in generating the plots, to minimize the influence of extreme values, but all values were used in the regression analyses. Based on the spline plots, the simplest functional form that adequately characterized each association was selected. Nonlinearity of associations was tested with the gain statistic.28 If quadratic associations were found, the overall p-value for the hormone-association was determined by a likelihood ratio test, comparing a model without the linear and quadratic terms to one with both terms. In addition, we also reported the p value for the quadratic term alone to indicate the strength of the quadratic association. To aid the reader in interpretation of the results, nonlinear hormone associations were quantified by hormone intervals that were approximately equal to the standard deviation of the hormone. The interval of lowest risk was selected as the reference and then risk was evaluated at levels above and below the reference interval.
We used Cox proportional hazards regression models to estimate the relative risk of each hormone with incident ischemic stroke. Time at risk was calculated as the interval between the 1994 study examination when sera were obtained and date of incident ischemic stroke, death, or end of follow-up (December 2010). We evaluated the validity of the proportional hazards assumption using Schoenfeld residuals and found no meaningful violations. Men who died or had a hemorrhagic stroke or stroke of unknown type were censored at the time of death or stroke. Serial models were constructed to allow for different levels of adjustment. Model 1 adjusted for age. Model 2 adjusted for age, systolic blood pressure, anti-hypertensive medications, atrial fibrillation, diabetes, and the natural logarithm of pack-years smoked. Model 3 added lipid-lowering drugs, HDL, total cholesterol, high creatinine (>1.25 mg/dL), and replaced diabetes with the natural logarithm of fasting glucose and use of medications for diabetes. As an exploratory analysis, interactions of hormones with age, hypertension and diabetes were evaluated by forming cross product terms with each hormone, measured continuously. We also conducted a sensitivity analysis in which we excluded men who were treated with finasteride, which may lower DHT levels by approximately 70%29. All statistical analyses were conducted with STATA 12 and R.
Results
Characteristics of the study cohort
At the 1994 visit, 2057 men were alive and 1922 had some type of visit, for a response rate of 93.4%. The mean age of the cohort was 76.5 years (range 66-97) and African Americans comprised 14.8% of the sample. Despite the advanced age of the cohort, most men (84%) rated their health as good to excellent. Over a median follow-up of 10 years (maximum 16.5 years), 114 men had ischemic strokes. Men who had an ischemic stroke were more likely to have high systolic blood pressure, diabetes, and low calculated free DHT levels at the time of the T measure than men who did not experience an ischemic stroke. Table 1.
Table 1. Cohort Characteristics by Ischemic Stroke Status.
| Characteristic Mean (SD) | Total n=1032 | No stroken=918 | Stroke n=114 | p-value |
|---|---|---|---|---|
| Age, years | 76.5 (5.2) | 76.6 (5.2) | 76.3 (4.9) | .66 |
| Black race, n (%) | 153 (14.8) | 137 (14.9) | 16 (14.0) | .80 |
| High School Education, n (%) | 777 (75.3) | 686 (74.7) | 91 (79.8) | .23 |
| Good-excellent Health, n (%) | 867 (84.0) | 773 (84.2) | 94 (82.5) | .63 |
| Current smoking, n (%) | 105 (10.2) | 92 (10.0) | 13 (11.4) | .65 |
| Alcohol use, n (%) | 570 (55.2) | 502 (54.7) | 68 (59.7) | .32 |
| Systolic blood pressure | 132.2 (19.7) | 131.5 (19.7) | 138.2 (18.8) | .0006 |
| Diastolic blood pressure | 71.1 (10.8) | 71.0 (10.8) | 72.4 (11.0) | .19 |
| Hypertension, n (%) | 547 (53.0) | 478 (52.1) | 69 (60.5) | .09 |
| Atrial fibrillation, n (%) | 70 (6.8) | 61 (6.6) | 9 (7.9) | .62 |
| Diabetes, n (%) | 117 (11.3) | 96 (10.5) | 21 (18.4) | .01 |
| Lipid lowering medications, n (%) | 52 (5.0) | 47 (5.1) | 5 (4.4) | .74 |
| Anti-hypertensive medications, n (%) | 474 (45.9) | 423 (46.1) | 51 (44.7) | .79 |
| Warfarin, n (%) | 32 (3.1) | 28 (3.0) | 4 (3.5) | .79 |
| Creatinine>110.5 μmol/L, n (%) | 272 (27.7) | 244 (28.0) | 28 (25.5) | .58 |
| HDL cholesterol1, mmol/L | 1.24 (0.30) | 1.24 (0.30) | 1.23 (0.33) | .72 |
| Total cholesterol, 1 mmol/L | 4.91 (0.91) | 4.90 (0.91) | 4.97 (0.87) | .46 |
| Fasting Glucose, 1 mmol/L | 5.99 (1.69) | 5.94 (1.57) | 6.35 (2.44) | .08 |
| Total T, ng/dl | 389 (176) | 390 (173) | 384 (204) | .77 |
| Free T, ng/dl | 5.30 (2.2) | 5.31 (2.3) | 5.18 (1.9) | .50 |
| Total DHT, ng/dl | 45 (23) | 46 (23) | 42 (25) | .14 |
| Free DHT, ng/dL | 0.26 (0.13) | 0.26 (0.14) | 0.24 (0.12) | .032 |
| SHBG, nmol/L | 64.9(29.9) | 64.9 (28.9) | 64.9 (37.6) | .99 |
From the 1992-93 exam; missing on 4-5% of participants; DHT- dihydrotestosterone, SHBG-sex hormone binding globulin; T testosterone
Testosterone and incident ischemic stroke
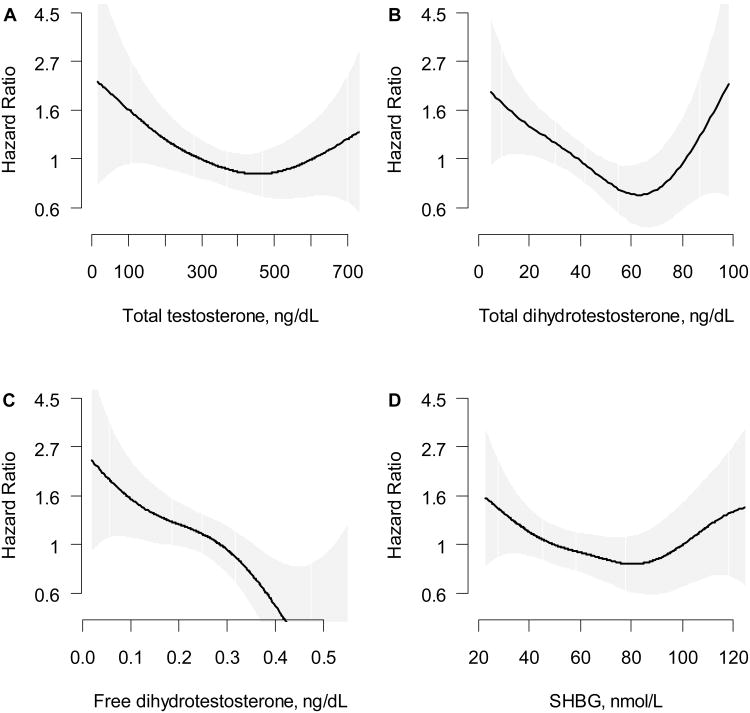
In age-adjusted models, total T had a nonlinear association that was marginally associated with incident stroke, with p=.074 for both the linear and quadratic terms and p=.009 for the quadratic term. The results were attenuated when either SHBG or DHT was added to the model (p=.16 and .25, respectively, for both the linear and quadratic terms.) The lowest risk for stroke occurred at total T levels of 400-600 ng/dl, which was set as the reference and then risk was examined at levels above and below this (Table 2 and Figure 1). In the fully adjusted model, total T had a marginal association with stroke (p=.055 for the combined linear and quadratic terms and p<. 03 for the quadratic term) and had no significant interactions with age, hypertension, or diabetes (p>.25 for all). Calculated free T was not associated with ischemic stroke in either linear or non-linear models.
Table 2. Total Testosterone and Incident Ischemic Stroke.
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| Total T, ng/dL | N | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| <200 | 107 | 1.87 (1.04, 3.35) | 1.38 (0.75, 2.54) | 1.46 (0.77, 2.75) |
| 200-400 | 487 | 1.01 (0.65, 1.57) | 0.91 (0.58, 1.42) | 0.90 (0.56, 1.45) |
| 400-600 | 331 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 600-800 | 85 | 1.50 (0.78, 2.91) | 1.54 (0.80, 2.98) | 1.73 (0.88, 3.39) |
| >800 | 22 | 1.55 (0.47, 5.04) | 1.54 (0.47, 5.07) | 1.69 (0.51, 5.60) |
| p-value* | .074 | .157 | .055 |
Based on a Likelihood ratio test of linear and quadratic terms.
Model 1: Adjusted for age
Model 2: Adjusted for age, ever smoked, pack-years (ln), systolic blood pressure, anti-hypertensive medications, diabetes, and atrial fibrillation.
Model 3: Adjusted for age, ever smoked, pack-years (ln), systolic blood pressure, anti-hypertensive medications, atrial fibrillation, total and HDL cholesterol, lipid-lowering medications, high creatinine, fasting glucose (ln) and use of insulin or oral hypoglycemic medications.
Figure 1.
The association of ischemic stroke risk with
A. Total testosterone (ng/dL) B. Total dihydrotestosterone (ng/dL) C. Free dihydrotestosterone (ng/dL) and D. SHBG (nmol/L). Models were fit using a penalized cubic spline and were adjusted for age. The lowest and highest 2.5% of hormone values were excluded to minimize the influence of extreme values but all values were used in the regression analyses.
Dihydrotestosterone (DHT) and incident ischemic stroke
In age-adjusted models, DHT had a significant nonlinear association with stroke (p=.0014 for both the linear and quadratic terms and p=.001 for the quadratic term). The lowest risk for stroke occurred at higher DHT levels of 50-75 ng/dL, which were above the mean DHT (45 ng/dl). This interval of lowest risk was set as the referent and risk was examined at levels above and below this (Table 3 and Figure 1). In the fully adjusted model, DHT was associated with stroke risk in a nonlinear manner (p=.006 for the combined linear and quadratic terms). When SHBG and total T were added to the model, DHT remained significant (p=.015). DHT had no significant interactions with age, hypertension, or diabetes (p>.25 for all). Calculated free DHT had an inverse linear association with incident stroke risk with a HR of 0.74 (0.58, 0.94) with a 26% decreased risk per standard deviation (0.135 ng/dl) increase in calculated free DHT in the fully adjusted model.
Table 3. Total DHT and Incident Ischemic Stroke.
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| DHT, ng/dL | N | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| <25 | 168 | 2.38 (1.32, 4.29) | 2.05 (1.12, 3.75) | 2.20 (1.18, 4.12) |
| 25-50 | 487 | 1.63 (0.98, 2.72) | 1.63 (0.98, 2.72) | 1.67 (0.99, 2.83) |
| 50-75 | 279 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 75-100 | 71 | 2.13 (1.00, 4.56) | 2.45 (1.13, 5.27) | 2.45 (1.09, 5.50) |
| >100 | 27 | 1.47 (0.44, 4.98) | 1.60 (0.47, 5.42) | 1.89 (0.55, 6.53) |
| p-value* | .004 | .018 | .006 |
Based on a Likelihood ratio test of linear and quadratic terms.
Model 1: Adjusted for age
Model 2: Adjusted for age, ever smoked, pack-years (ln), systolic blood pressure, anti-hypertensive medications, diabetes, and atrial fibrillation.
Model 3: Adjusted for age, ever smoked, pack-years (ln), systolic blood pressure, anti-hypertensive medications, atrial fibrillation, total and HDL cholesterol, lipid-lowering medications, high creatinine, fasting glucose (ln) and use of insulin or oral hypoglycemic medications.
SHBG and incident ischemic stroke
In age-adjusted models, SHBG had a nonlinear relationship with stroke risk (p=.053 for both the linear and quadratic terms and p=.007 for the quadratic term). The lowest risk for stroke occurred at SHBG of 60-90 nmol/L (Table 4 and Figure 1). However, when total T or DHT were added to the model, the risk estimates were attenuated and SHBG was not associated with stroke risk. There were no significant interactions of SHBG with age, hypertension or diabetes.
Table 4. SHBG and Incident Ischemic Stroke.
| Model 1 | Model 2 | Model 3 | ||
|---|---|---|---|---|
| SHBG nmol/L | N | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| <30 | 72 | 1.93 (0.99, 3.78) | 1.40 (0.69, 2.83) | 1.41 (0.68, 2.92) |
| 30-60 | 462 | 1.12 (0.72, 1.75) | 1.07 (0.68, 1.68) | 1.06 (0.66, 1.70) |
| 60-90 | 338 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 90-120 | 114 | 1.38 (0.73, 2.59) | 1.37 (0.73, 2.58) | 1.52 (0.80, 2.89) |
| >120 | 46 | 1.57 (0.65, 3.80) | 1.63 (0.67, 3.93) | 1.71 (0.70, 4.16) |
| p-value* | .053 | .079 | .044 |
Based on a Likelihood ratio test of linear and quadratic terms.
Model 1: Adjusted for age
Model 2: Adjusted for age, ever smoked, pack-years (ln), systolic blood pressure, anti-hypertensive medications, diabetes, and atrial fibrillation.
Model 3: Adjusted for age, ever smoked, pack-years (ln), systolic blood pressure, anti-hypertensive medications, atrial fibrillation, total and HDL cholesterol, lipid-lowering medications, high creatinine, fasting glucose (ln) and use of insulin or oral hypoglycemic medications.
Sensitivity Analyses and Reliability of Hormone measures
In an age-adjusted, sensitivity analysis, men who were treated with finasteride (n=33) were excluded and there was no significant change in the results. (Results not shown.) The intra-class correlation coefficients (ICC) for 3 repeated hormone measures over a 7-year period were 0.82 for total T and 0.79 for DHT.
Discussion
To our knowledge, this is the first study to examine the association of T, DHT, and SHBG with incident ischemic stroke in elderly men without a history of heart disease or stroke. We had hypothesized that lower androgen and SHBG levels would be associated with increased risk for stroke. This hypothesis was confirmed for calculated free DHT, which had an inverse linear association with stroke risk. However for DHT, there was a non-linear association with stroke that persisted after adjusting for demographics, stroke risk factors, SHBG and total T. The lowest stroke-risk occurred at an intermediate DHT level of 50-75 ng/dl, which was higher than the mean DHT level. This suggests that above average DHT levels are associated with decreased risk, while either high DHT or low-intermediate DHT levels are associated with increased stroke-risk. Of note, few men had clearly high DHT levels >75 ng/dl, so the estimates of increased risk at clearly high DHT levels are less precise. Total T also had a nonlinear association with stroke, which was of borderline significance (p=.055). However, the association of total T with stroke was no longer significant after DHT was added to the model. It is unclear why DHT was more strongly associated with incident stroke than total T, but it may be because DHT is a much more potent androgen than total T. Further studies are needed to clarify this.
The pathophysiology underlying the potential association between androgens and stroke is complex as androgens have differing effects related to age and actions at different receptors (androgen, estrogen, membrane bound and cytosolic receptors). In ischemic stroke models in castrated male animals, T and DHT are associated with both adverse30-33,34, 35,36 and protective effects.34, 37 The protective effects of androgens may be mediated via vasodilation, antiplatelet effects, and activation of the ERK/MAPK pathway,38,34, 35 while adverse effects may be mediated via vasoconstriction or inflammation.34, 39 In an animal model, androgen effects also varied by androgen levels with protective effects at physiologic T levels, but increased neuronal damage at both low and supra-physiologic T levels.35
In addition to the current study, another study recently reported that there appeared to be an optimal androgen range in elderly men. Specifically, in a study of elderly men, (mean age of 77), the third quartile of total T, free T and DHT was associated with the lowest risk for all-cause mortality and levels either above or below that were associated with increased risk. 40 It is interesting to note that the DHT interval associated with the lowest risk in that study, overlapped with the DHT interval associated with the lowest risk in the current study. The possibility of an optimal range for androgens may explain conflicting results in which both low and high levels of androgens were associated with risk. Specifically, low levels of T and DHT are associated with stroke and adverse CV events12, 41-43 while high levels of free T in a T-treatment trial were associated with cardiovascular events.44 If additional studies identify an optimal range for serum androgen levels, this would suggest that androgen levels be monitored in men who are treated with T, to avoid supra-physiologic or sub-physiologic T-levels. Monitoring of androgen levels following T treatment is currently recommended in the Endocrine Society Guidelines. 4 However, it is unclear if monitoring T levels occurs frequently in routine outpatient care as a recent study found that 25% of treated men did not have baseline T levels prior to T treatment. 45
Two other studies examined the association between T and incident stroke in elderly men without a history of stroke. In a study of Japanese-American men (ages 71-93), total T was not associated with incident adjudicated stroke,46 which is consistent with our study results and DHT was not examined as a risk factor for stroke. In another study of Australian men (ages 70 and older) low total and free T were both associated with incident stroke and transient ischemic attack (TIA) while SHBG was not and DHT was not examined as a risk factor for stroke.47 The non-significant association with SHBG is consistent with our study, while the significant association with total and free T differs from the current study. Our results may differ from that study47 due to differences in cohort definition and outcome ascertainment. Specifically, the current study excluded men with stroke and heart disease, while the other study47 excluded men with stroke and TIA, but included men with heart disease. In addition, the current study had an adjudicated outcome limited to ischemic stroke, while the other study47 used hospital discharge diagnoses of all stroke types and TIAs, which may be more subject to diagnostic error.
A limitation of this study is that there was only a single androgen measure, which may not be representative of the average serum androgen level.48 However, the high correlation of hormones in a subset of the cohort with repeated androgen levels suggests that a single serum measure was likely representative of the hormonal milieu of the individual. Another limitation is that although most of the blood samples were obtained in the morning there was not a standardized time to collect blood samples. This could influence the measured T levels, because serum T has a circadian rhythm with the highest T levels occurring in the morning. However, the effect of non-standardized blood collection times on study results may be minimal, since the circadian fluctuation in T levels is blunted in older men and DHT levels do not exhibit significant circadian variation. 48,49
Despite these weaknesses, the present study has several strengths. It is one of the first studies of incident stroke in older men to use highly sensitive and specific mass spectrometry to assay for total T and DHT. Prior studies that used immunoassay methods were unable to reliably and accurately assay for DHT because immunoassays do not have sufficient sensitivity or specificity to measure the low levels of serum DHT in men. Another strength is that the cohort was obtained from the CHS study, which was specifically designed to ascertain cardiovascular disease, so rigorous protocols were used to evaluate prevalent and incident cardiovascular disease. Few of the prior cohort studies of androgens and cardiovascular disease had a cohort with such well-characterized baseline cardiovascular disease and well-adjudicated clinical outcomes. Finally, a novel aspect of this study is the finding of a nonlinear relationship with DHT and incident ischemic stroke. If nonlinear relationships are replicated in other studies, it may help to explain some of the conflicting results of prior studies, as categorical or linear models would fail to detect a non-linear association.
In conclusion, in this well-characterized cohort of elderly men, DHT had a non-linear association with incident ischemic stroke with the lowest risk for stroke occurring at a serum DHT level that was above the mean DHT. These results suggest that there may be an optimal DHT range that is associated with the least risk for incident ischemic stroke. In contrast, calculated free DHT had an inverse linear association with incident ischemic stroke. However, although DHT and calculated free DHT were associated with incident stroke, these results do not imply a causal association as due to the observational study design it is possible that some unmeasured factor is contributing to the observed association. Further studies are needed to confirm these results and to clarify if there is an optimal androgen range associated with the least risk for adverse outcomes in elderly men.
Supplementary Material
Acknowledgments
Funding Sources: This project was supported by the VA Research Service, the VA Epidemiology Research and Information Center (ERIC), and the VA Geriatric Research and Information Center (GRECC) and by 1R01HL091952. This research was supported by contracts HHSN268201200036C, N01-HC-85239, N01 HC-55222, N01-HC-85079, N01-HC-85080, N01-HC-85081, N01-HC-85082, N01-HC-85083, N01-HC-85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG-023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Footnotes
Disclosures. Dr. Matsumoto has had research support from AbbVie and GSK; on Advisory Boards for Lilly, Endo Pharmaceuticals and Forendo: and is on the Editorial Board for UpToDate.
No other authors have disclosures to make.
References
- 1.Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 2.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26:833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 5.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 6.Fukui M, Ose H, Kitagawa Y, Yamazaki M, Hasegawa G, Yoshikawa T, Nakamura N. Relationship between low serum endogenous androgen concentrations and arterial stiffness in men with type 2 diabetes mellitus. Metabolism. 2007;56:1167–1173. doi: 10.1016/j.metabol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Corona G, Mannucci E, Lotti F, Fisher AD, Bandini E, Balercia G, Forti G, Maggi M. Pulse pressure, an index of arterial stiffness, is associated with androgen deficiency and impaired penile blood flow in men with ED. J Sex Med. 2009;6:285–293. doi: 10.1111/j.1743-6109.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 8.Makinen J, Jarvisalo MJ, Pollanen P, Perheentupa A, Irjala K, Koskenvuo M, Huhtaniemi I, Raitakari OT. Increased carotid atherosclerosis in andropausal middle-aged men. J Am Coll Cardiol. 2005;45:1603–1608. doi: 10.1016/j.jacc.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 9.Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, Karlsson MK, Ljunggren O, Vandenput L, Mellstrom D, Tivesten A. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011;58:1674–1681. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai J, Zhou D, Xia S, Shang Y, Want L, Zheng L, Zhu J. Reduced testosterone levels in males with lone atrial fibrillation. Clin Cardiol. 2009;32:43–46. doi: 10.1002/clc.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Norman PE, Flicker L. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. J Clin Endocrinol Metab. 2012;97:4030–4039. doi: 10.1210/jc.2012-2265. [DOI] [PubMed] [Google Scholar]
- 13.Araujo AB, Kupelian V, Page ST, Handelsman DJ, Bremner WJ, McKinlay JB. Sex steroids and all-cause and cause-specific mortality in men. Arch Intern Med. 2007;167:1252–1260. doi: 10.1001/archinte.167.12.1252. [DOI] [PubMed] [Google Scholar]
- 14.Bhasin S, Jasjua GK, Pencina M, D'Agostino R, Sr, Coviello AD, Vasan RS, Travison TG. Sex hormone-binding globulin, but not testosterone, is associated prospectively and independently with incident metabolic syndrome in men: the framingham heart study. Diabetes Care. 2011;34:2464–2470. doi: 10.2337/dc11-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brand JS, Wareham NJ, Dowsett M, Folkerd E, van der Schouw YT, Luben RN, Khaw KT. Associations of endogenous testosterone and SHBG with glycated haemoglobin in middle-aged and older men. Clin Endocrinol (Oxf) 2011;74:572–578. doi: 10.1111/j.1365-2265.2010.03951.x. [DOI] [PubMed] [Google Scholar]
- 16.Chubb SA, Hyde Z, Almeida OP, Flicker L, Norman PE, Jamrozik K, Hankey GJ, Yeap BB. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. Eur J Endocrinol. 2008;158:785–792. doi: 10.1530/EJE-07-0893. [DOI] [PubMed] [Google Scholar]
- 17.Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91:843–850. doi: 10.1210/jc.2005-1326. [DOI] [PubMed] [Google Scholar]
- 18.Tsai EC, Matsumoto AM, Fujimoto WY, Boyko EJ. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27:861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 19.Kalme T, Seppala M, Qiao Q, Koistinen R, Nissinen A, Harrela M, Loukovaara M, Leinonen P, Tuomilehto J. Sex hormone-binding globulin and insulin-like growth factor-binding protein-1 as indicators of metabolic syndrome, cardiovascular risk, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90:1550–1556. doi: 10.1210/jc.2004-0762. [DOI] [PubMed] [Google Scholar]
- 20.Vagnerova K, Koerner IP, Hurn PD. Gender and the injured brain. Anesth Analg. 2008;107:201–214. doi: 10.1213/ane.0b013e31817326a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 22.Longstreth WT, Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, Furberg CD. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology. 2001;56:368–375. doi: 10.1212/wnl.56.3.368. [DOI] [PubMed] [Google Scholar]
- 23.Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:3200–3206. doi: 10.1002/rcm.3205. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 25.Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74:512–519. doi: 10.1016/j.steroids.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Evans RW. Neurologic aspects of hyperventilation syndrome. Semin Neurol. 1995;15:115–125. doi: 10.1055/s-2008-1041015. [DOI] [PubMed] [Google Scholar]
- 27.Hastie T, Hibishani R. Generalized additve models. Chapman & Hall/CRC; Boca Raton: 1990. [Google Scholar]
- 28.Hastie TJ, Tibsharani RJ. Generalized additive models. Chapman Hall/CRC; Boca Raton: 1990. [Google Scholar]
- 29.Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. World J Urol. 2002;19:413–425. doi: 10.1007/s00345-002-0248-5. [DOI] [PubMed] [Google Scholar]
- 30.Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia-reperfusion injury in an animal model. J Appl Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- 31.Vagnerova K, Liu K, Ardeshiri A, Cheng J, Murphy SJ, Hurn PD, Herson PS. Poly (ADP-ribose) polymerase-1 initiated neuronal cell death pathway--do androgens matter? Neuroscience. 2010;166:476–481. doi: 10.1016/j.neuroscience.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. 1Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 33.Cheng J, Alkayed NJ, Hurn PD. 1Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–1562. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Littleton-Kearney M, Hurn PD. Testosterone as a modulator of vascular behavior. Biol Res Nurs. 2004;5:276–285. doi: 10.1177/1099800403262927. [DOI] [PubMed] [Google Scholar]
- 35.Nakano T, Hurn PD, Herson PS, Traystman RJ. *Testosterone exacerbates neuronal damage following cardiac arrest and cardiopulmonary resuscitation in mouse. Brain Res. 2010;1357:124–130. doi: 10.1016/j.brainres.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. *1Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng J, Uchida M, Zhang W, Grafe MR, Herson PS, Hurn PD. Role of salt-induced kinase 1 in androgen neuroprotection against cerebral ischemia. J Cereb Blood Flow Metab. 2010;31:339–350. doi: 10.1038/jcbfm.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gatson JW, Singh M. DHT has neuroprotective effect by activation of ERK/MPK pathway. Endocrinology. 148:2458–2464. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- 39.Cheng J, Alkayed NJ, Hurn PD. High-dose DHT exacerbates cerebral ischemic damage, potentially by enhanced inflammation, dysregulation of BBB and imbalance, effects on extracellular matrix, apoptosis and ionic balance (Cheng J, J b 27:1553-1562, 2007) J Cerebr Blood Flow Metab. 2007;27:1553–1562. [Google Scholar]
- 40.Yeap BB, Alfonso H, Chubb SA, Handelsman DJ, Hankey GJ, Almeida OP, Golledge J, Norman PE, Flicker L. In older men an optimal plasma testosterone is associated with reduced all-cause mortality and higher dihydrotestosterone with reduced ischemic heart disease mortality, while estradiol levels do not predict mortality. J Clin Endocrinol Metab. 2014;99:E9–18. doi: 10.1210/jc.2013-3272. [DOI] [PubMed] [Google Scholar]
- 41.Haring R, John U, Volzke H, Nauck M, Dorr M, Felix SB, Wallaschofski H. Low testosterone concentrations in men contribute to the gender gap in cardiovascular morbidity and mortality. Gend Med. 2012;9:557–568. doi: 10.1016/j.genm.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Hyde Z, Norman PE, Flicker L, Hankey GJ, Almeida OP, McCaul KA, Chubb SA, Yeap BB. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study. J Clin Endocrinol Metab. 2012;97:179–189. doi: 10.1210/jc.2011-1617. [DOI] [PubMed] [Google Scholar]
- 43.Keating NL, O'Malley J, Smith MR. Diabetes and cardiovasuclar disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 44.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med. 2013;173:1465–1466. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abbott RD, Launer LJ, Rodriguez BL, Ross GW, Wilson PW, Masaki KH, Strozyk D, Curb JD, Yano K, Popper JS, Petrovitch H. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–568. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- 47.Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94:2353–2359. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 48.Brambilla DJ, O'Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007;67:853–862. doi: 10.1111/j.1365-2265.2007.02976.x. [DOI] [PubMed] [Google Scholar]
- 49.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.