Abstract
Diabetes, more frequently type 1 but increasingly also type 2, commonly occurs in childhood. While more advanced diabetic kidney disease (DKD), e.g. loss of glomerular filtration rate (GFR), does not occur until adulthood, kidney biopsies show DKD structural changes as early as 1.5–5 years after the onset of type 1 diabetes. Earliest clinical sign of DKD, e.g. increased urine albumin excretion, commonly appears during childhood and adolescence and presents an important opportunity to detect and intervene on early DKD, perhaps more successfully than later in the disease course. Longitudinal studies of type 1 diabetes have enriched our understanding of the DKD natural history and modifiable risk factors for DKD progression. These studies have also shown that presence of DKD marks a subset of people with diabetes who are at the highest risk of early mortality, supporting an enhanced focus on DKD detection, prevention, and treatment. Early studies suggest that youth-onset type 2 diabetes is associated with a higher prevalence of comorbidities and risk factors and follows a more aggressive natural history. A deeper understanding of the natural history, risk factors, underlying mechanisms and therapeutic options for DKD in young-onset type 2 diabetes awaits further studies.
Keywords: diabetic kidney disease, type 1 diabetes, type 2 diabetes, microalbuminuria, macroalbuminuria, glomerular filtration rate, end-stage renal disease
Introduction
Diabetes is one of the most common chronic diseases affecting children and adolescents. There are currently more than 190,000 people younger than 20 years of age with diabetes in the US[1] and this number is projected to increase two-folds or more by 2050.[2] Historically, type 1 diabetes has been the predominant form of disease in children and adolescents. However, over the past two decades, the rise in childhood obesity has led to an increasing incidence of type 2 diabetes among children and adolescents, which now parallels and at times exceeds that of type 1 diabetes among minority youth, particularly after the age of 15.[1]
It has long been known that diabetes is associated with a significant increase in mortality, largely as a result of its long term complications. More recently, this excess mortality has been found to be concentrated in the subset of people with diabetes who develop kidney disease, both in type 1 [3, 4] and type 2 [5] diabetes. These observations highlight the importance of diabetic kidney disease (DKD) at least as a marker of a population at highest risk of mortality and perhaps as a risk factor directly contributing to excess mortality.
While more severe stages of DKD take decades to develop and are thus rarely observed in childhood, kidney biopsies as early as 1.5–5 years after diabetes onset show structural changes characteristic of DKD in both adults and children.[6–8] This suggests that the DKD course begins soon after diabetes onset and that this early interval may provide a critical time-frame for detection and intervention in the disease course, warranting intensive monitoring and modification of risk factors in children and adolescents. Perhaps more so than in adults, our current tools for early diagnosis of DKD in children and adolescents are few and flawed. Nonetheless, the heavy impact of childhood diabetes on morbidity and mortality later in life mandates our full use of all available tools and resources with a nuanced understanding of their strengths and limitations as well as a renewed effort towards development of new diagnostic tools and therapies.
This review will discuss the natural history and risk factors for development of DKD, the structural changes observed in DKD and the pathophysiological mechanisms causing those alterations. We will also discuss the differences in natural history and outcomes between type 1 and type 2 diabetes.
Natural history
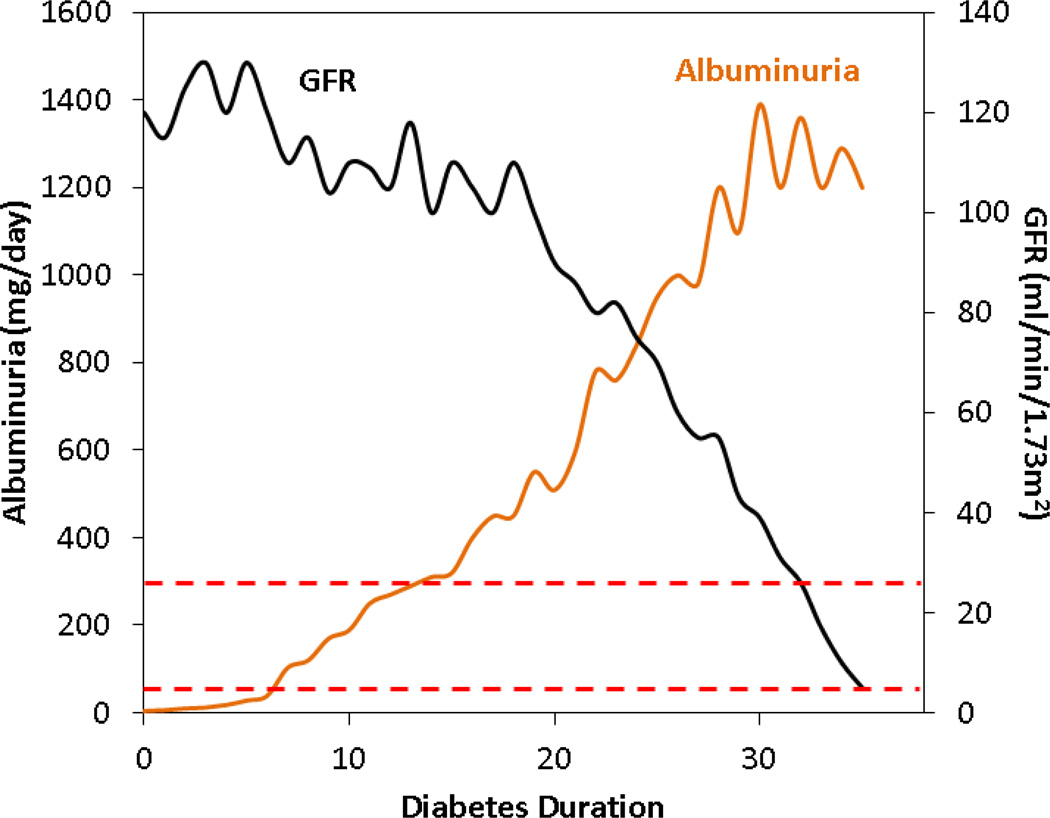
DKD natural history was classically described as an initial and progressive rise in urine albumin excretion, followed by progressive GFR loss and eventual development of end-stage renal disease (ESRD) over several decades (Figure 1). Microalbuminuria, defined as a urine albumin excretion of 30–299 mg/day (or an albumin to creatinine ratio of 30–299 mg/g creatinine in random samples) in at least 2 of 3 measurements, is the earliest sign of DKD and occurs in 26% of children and adolescents after 10 years and in 51% after 19 years of diabetes.[9] In the classic DKD presentation, once microalbuminuria develops, urine albumin excretion continues to rise, particularly in presence of uncontrolled risk factors. Macroalbuminuria, defined as a urine albumin excretion ≥300 mg/day (or albumin to creatinine ratio ≥300 mg/g creatinine), heralds the onset of overt DKD and is thought to inexorably lead to impaired GFR (defined as an estimated GFR <60ml/min/1.73m2) and eventually ESRD (Figure 1).
Figure 1.
The classic course of diabetic kidney disease (DKD) natural history. Classic DKD is thought to begin with rise of urine albumin excretion, initially to microalbuminuria (lower dashed red line), then proceed to macroalbuminuria (upper dashed line), with glomerular filtration rate (GFR) dropping only after macroalbuminuria.
More recent work has refined our understanding of the DKD natural history under current standards of care. First, longitudinal studies of type 1 diabetes have shown that while microalbuminuria does raise the odds of future DKD development, more adults[10–12] and children[13, 14] with microalbuminuria regress to normoalbuminuria (~30–40%) than progress to more advanced stages of DKD. In addition, microalbuminuria is not associated with an increased rate of GFR loss,[15] altogether suggesting that rather than being a committed marker of specific and irreversible DKD, microalbuminuria may represent reversible endothelial injury. Microalbuminuria regression to normoalbuminuria is more common with improved glycemic and blood pressure control, but is independent of renin angiotensin system (RAS) inhibition both in adults[12] and children.[13, 14] As such, the current KDOQI guidelines recommend treatment with RAS inhibitors only if microalbuminuria co-exists with other DKD risk factors (e.g. hypertension, diabetic retinopathy, dyslipidemia, etc).[16]
Secondly, while the classic presentation of macroalbuminuria preceding the impairment of GFR is observed in a majority of cases in type 1 diabetes, in a sizable minority GFR loss occurrs either after microalbuminuria (16%) or even in absence of any albuminuria (24%).[15] Thirdly, while macroalbuminuria is associated with a 50-fold greater risk of progression to impaired GFR,[17] 60% of people with macroalbuminuria maintained an estimated GFR >60 ml/min/1.73m2, even after 15 years of follow-up (Afkarian et al, submitted). These observations suggested that macroalbuminuria was a sign of substantial renal injury but that it did not set an inexorable course to impaired GFR or ESRD in all cases. In addition, significant renal injury could occur in absence of macro-or even microalbuminuria.
Interpretation of albuminuria in children and adolescents requires two additional considerations. First, the vast majority (75–95%) of proteinuria in children and adolescents is due to benign causes such as transient and orthostatic proteinuria.[18–22] Transient proteinuria may be associated with exercise, fever, cold, stress, dehydration or seizure and is reported to occur in as many as 50–74% of cases of observed proteinuria in children and adolescents.[18, 20] Orthostatic proteinuria, another etiology of benign childhood proteinuria, is reported in 6–20% [23] of healthy children and adolescents and leads to no adverse outcomes after up to 50 years of follow-up.[24, 25] Luckily, transient and orthostatic proteinuria can be readily identified by repeat measurement of urine protein excretion in absence of the conditions causing transient proteinuria and in a first-void urine sample to exclude orthostatic proteinuria.[26]
The second challenge in accurate interpretation of albuminuria in children is the likely misclassification of albuminuria status by application of a single threshold of 30 mg/day (or 30 mg/g of creatinine) in children of different age, size, gender and race. In children and adolescents, excretion of creatinine [27, 28] and albumin [27, 29] increases with age. Creatinine excretion is higher in boys [27, 30] and albumin excretion is higher in African Americans compared to Caucasians.[31] Using the single threshold of 30 mg/g in spot urine samples is likely to overestimate albumin excretion in younger, smaller and female children whose daily creatinine excretion is more likely to be much less than 1 gram.
Structural changes and their correlation with functional parameters
In biopsies performed in both adults and children with type 1 diabetes, the first observed alteration is the thickening of the glomerular [6] and tubular [8] basement membrane (GBM, TBM) at 1.5–2.5 years after diabetes onset, followed by mesangial matrix expansion with an increase in fractional volume of the mesangial matrix typically observed 5–7 years after diabetes onset.[7] Interstitial expansion is also observed, initially due to an increase in the cellular component and later on due to accumulation of fibrillar collagen in advanced DKD.[32] These various lesions progress at different rates within and between different patients with type 1 diabetes. However, by the time advanced DKD with renal insufficiency sets in, all patients display marked mesangial expansion and GBM thickening as well as interstitial expansion and fibrosis, tubular atrophy [33] and glomerulosclerosis.[34] Similar lesions are observed in children and adults with type 1 diabetes and normoalbuminuric DKD.[35] Diabetic retinopathy (DR) is nearly universal at the time of macroalbuminuria[36] and its severity is proportional to the degree of structural abnormalities in early DKD in type 1 diabetes.[37]
Renal structural lesions are much more heterogeneous in type 2 diabetes.[38, 39] Adults with type 2 diabetes and micro- or macroalbuminuria but GFR >60 ml/min/1.73m2 display typical DKD lesions in only 30% and 50% of cases, respectively, while the remainder show either atypical pathology (little or no diabetic glomerulopathy, with chronic tubulointerstitial fibrosis, arteriolar hyalinosis and glomerular sclerosis) or no significant pathology.[39] Interestingly, some degree of DR is present in all cases with typical DKD pathology while none of the cases with atypical pathology or non-significant injury have proliferative DR.[39] On the other hand, in type 2 diabetic patients with micro- or macroalbuminuria and impaired GFR (<60 ml/min/1.73m2), typical glomerular lesions are reported in 83% and 100%, respectively. However, less than 40% of those with impaired GFR and normoalbuminuria show typical DKD glomerular lesions, while a majority show either severe renovascular and/or tubulointerestitial changes or no identified cause for kidney disease.[40]
In type 1 diabetes, mesangial expansion is significantly and inversely correlated with GFR, presumably due to consequent reduction in glomerular capillary filtration surface area,[7] a feature which is strongly correlated with GFR loss.[41] Mesangial expansion is also strongly correlated with albuminuria [7, 42] and hypertension.[43] GBM thickening closely correlates with albuminuria, but less so with GFR or hypertension.[44] Interstitial expansion and global sclerosis are correlated with albuminuria and hypertension and inversely correlated with GFR.[7] These associations are similar, though less precise, in type 2 diabetes, with albuminuria correlating closely with both GBM width and mesangial expansion, while risk of progressive GFR loss in type 2 patients with albuminuria is significantly correlated with the extent of mesangial matrix expansion.[45] Notably, unlike the structural studies in type 1 diabetes, all of those in type 2 diabetes were conducted in adults. In the light of rising incidence and poor prognosis of type 2 diabetes in children and adolescents, as discussed below, similar structural studies in this population have the potential to add much needed depth to our understanding of the disease process.
Pathophysiology
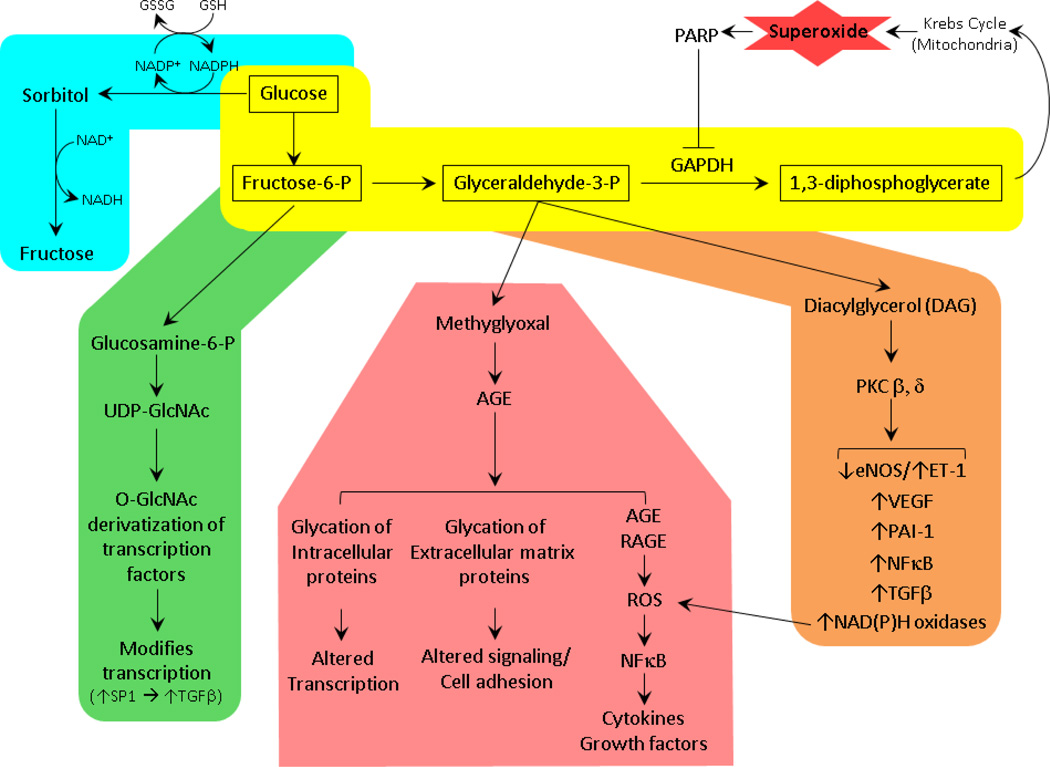
Animal models have shown dysregulation of many pathways in DKD and these findings have largely been corroborated in human studies. The Diabetes Control and Complications Trial (DCCT) demonstrated that hyperglycemia is the primary trigger for dysregulation of all these pathways. Hyperglycemia leads to end-organ damage in several tissues, perhaps particularly those which are not able to down-regulate glucose entry in hyperglycemia.[46] In 2001, Brownlee presented a unifying hypothesis [47] which aimed to connect all the implicated pathogenic pathways to elevated intracellular glucose concentration (Figure 2). Drawing on several lines of evidence, including hyperglycemia-induced increase in intracellular superoxide, the Brownlee hypothesis posited that elevated intracellular superoxide inhibits a key glycolytic enzyme Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) thus blocking metabolism and disposal of excess intracellular glucose. The resulting accumulation of intracellular glucose and its glycolytic intermediates, Fructose-6-Phosphate and Glyceraldehyde-3-phosphate, then feed into and induce four primary pathways: the polyol, hexosamine and Protein Kinase C pathways and non-enzymatic generation of Advanced Glycation End-products (AGE). These pathways in turn activate many other pathogenic signaling cascades, most notably the TGFβ and RAS pathways, leading to distal effects such as excessive production and accumulation of extracellular matrix components which give rise to the DKD structural and functional alterations discussed in previous sections, e.g. expansion of the mesangial matrix and thickening of GBM.
Figure 2.
Schematic of the unifying theory connecting hyperglycemia to distal pathophysiologic mechanisms via elevated intracellular superoxide. Hyperglycemia increases mitochondrial superoxide generation which activates PARP. PARP inhibits GAPDH by ADP-ribosylating it. Inhibition of GAPDH leads to buildup of preceding glycolytic intermediates which feed into and activate the four primary (diabetic kidney disease) DKD pathophysiological pathways, the polyol (blue), the Hexosamine (green), the AGE (pink) and PKC (orange). These pathways in turn trigger several distal cascades, upregulating expression of effector proteins such as components of the renin-angiotensin system and TGF-β
While hyperglycemia is widely believed to be the inciting pathogenic stimulus, recently controversy has surrounded the role of intracellular superoxide in triggering the subsequent pathophysiologic mechanisms. The hyperglycemia-induced increase in intracellular superoxide is at the core of the Brownlee hypothesis. However, recent observations of reduced superoxide levels in kidneys of DKD animal models [48] have led to an alternative hypothesis. This hypothesis suggests that hyperglycemia reduces superoxide generation which in turn decreases activity of 5’ AMP-activated protein Kinase (AMPK), a major energy-sensing enzyme, and PGC1, the master regulator of mitochondrial biogenesis. The resulting reduction in mitochondrial number and function promotes DKD progression. Pharmaceutical activation of AMPK and PGC1α were shown to increase kidney superoxide content and diminish activity of the distal pathogenic mechanisms, manifested in reduction of albuminuria and lower expression of fibronectin, collagen and TGF-β in the kidneys in several animal models of DKD.[48] Resolution of the controversy surrounding the effect of hyperglycemia on intracellular superoxide and its role in DKD pathogenesis awaits further studies.
Another question worthy of further study is scarcity of novel diagnostic and therapeutic tools in the face of acknowledged advances in our understanding of the underlying DKD pathophysiology based on experimental animal models. Since the discovery of the beneficial effect of intensive diabetes treatment and RAS inhibition on DKD, no new therapies have proven successful. Most recently, dual RAS inhibition and activation of Nrf antioxidant pathway failed to impact DKD progression, joining a cadre of prior failed trials using inhibitors of advanced glycation end-products, protein kinase C and aldose reductase. Given the strong association of mortality with DKD, understanding, and remedying, the causes for this delay in translation from pathophysiology to novel diagnostic and therapeutic options are of significant public health impact.
Risk factors
The seminal DCCT/EDIC study included 195 participants who were 13–17 years old at enrollment, among whom intensive diabetes treatment was associated with 54% reduction in microalbuminburia and this benefit persisted during the EDIC study.[49] This established hyperglycemia, as measured by hemoglobin A1c, as the predominant risk factor for DKD. In addition female gender, diabetes duration, hypertension [50, 51], high normal urine albumin excretion [52] elevated LDL cholesterol, triglycerides,[53] high body mass index [54] and smoking [55] have been reported as risk factors for development of microalbuminuria in children and adolescents. Role of pre-pubertal diabetes duration in development of microalbuminuria has been the subject of some debate, but overall the total disease duration, and not the duration before or after puberty, appears to determine cumulative microalbuminuria risk [50, 56] and DKD structural alterations in the kidney.[57] Hemoglobin A1c,[9] male sex,[58] smoking,[59] baseline albumin excretion,[9] and serum uric acid[60] have been reported as risk factors for progression to macroalbuminuria in children and adolescents. Given the significant heritability observed for DKD,[61] family history of kidney disease is also a significant risk factor for DKD development.
Insulin resistance and type 2 diabetes in children and adolescents
In 2009, type 2 diabetes contributed to 23% of cases of diabetes among US children and adolescents, but was disproportionately more common among ethnic minorities. For example, among 15–19 year olds, type 2 diabetes caused 5.5% of diabetes among the non-Hispanic White, but 34–38% of diabetes in African-Americans, Hispanics and Asian Pacific Islanders and 80% of diabetes among American Indians.[1] Among youth diagnosed with type 1 diabetes, an additional 23% were insulin resistant with higher prevalence of obesity, again more commonly among ethnic minorities.[62]
Despite its lower overall prevalence compared to type 1 diabetes, the rise of type 2 diabetes in youth presents a grave public health problem for four reasons: youth with type 2 diabetes have a greater prevalence of risk factors for diabetic complications and mortality, show evidence of poor control of these risk factors, display more rapid progression of complications, higher rates of mortality and, most alarmingly, unabated progression of complications despite attempts to control risk factors.
First, compared with type 1 diabetes, young-onset type 2 diabetes is more frequently associated with several cardiovascular and mortality risk factors, even at diabetes onset. Hypertension is present in 24% of youth with type 2 vs. 6% of those with type 1 diabetes.[63] Dyslipidemia is also more common among youth with type 2 diabetes: an LDL >130 is present in 24% of children and adolescents with type 2 vs. 19% of type 1 diabetes; an HDL<40 is present in 44% vs. 12% of youth with type 2 and type 1 diabetes, respectively.[64] Children and adolescents with type 2 diabetes were also much more commonly obese (79%) than those with type 1 diabetes (13%).[65] Even more ominous is the considerable prevalence of early markers of cardiovascular disease at, or soon after, diabetes onset in youth with type 2 diabetes: microalbuminuria was observed in 22% vs. 9% of children and adolescent with type 2 vs type 1 diabetes.[66] Left ventricular hypertrophy was found in 22% of youth with type 2 diabetes up to 3 years after diagnosis.[67]
Secondly, risk factors appear to be less well controlled in children and adolescents with type 2 diabetes, compared to children with type 1 diabetes or adults. Poor glycemic control, defined as an HbA1c ≥9.5%, was reported in 27% of youth with type 2 diabetes, compared to 17% of those with type 1 diabetes and more commonly observed among ethnic minorities.[68] Only 32% of youth with type 2 diabetes, who had hypertension, were aware of their diagnosis and only 6.6% received treatment.[63] Among youth with dyslipidemia, an even smaller fraction (1%) received treatment.[64]
Thirdly, youth with type 2 diabetes see earlier and more rapid progression of DKD, not only compared to youth with type 1 diabetes [69–71] but also adults with type 2 diabetes.[72] In countries where incidence of DKD among youth with type 1 diabetes has declined in the past two decades, DKD due to type 2 diabetes has not.[69] This more rapid progression of DKD in type 2 diabetes is at least partly due to coexistence of other risk factors, including insulin resistance.[73] Consistent with higher burden of comorbidities and complications, young-onset type 2 diabetes was found to be associated with greater risk of mortality at a younger age and with significantly shorter disease duration than type 1 diabetes, despite comparable glycemic control.[74] Consequently, the US youth with type 2 diabetes have a 15 year shorter remaining life expectancy and markedly reduced quality of life due to development of severe and morbid diabetic complication by their 40’s.[75]
Perhaps most concerning were the recent reports from the Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) study, suggesting that young-onset type 2 diabetes and its complications may be much more aggressive and treatment-resistant than its adult counterpart. TODAY was a multicenter trial of 699 adolescents 10–17 years old, with less than 2 years of type 2 diabetes, BMI≥85th percentile, HbA1c ≤8% on metformin, controlled blood pressure and creatinine clearance >70ml/min, who were randomized to metformin alone, metformin and rosiglitazone or metformin and intensive lifestyle management.[76] Despite good compliance with medications, after a median of 1 year, 46% of participants failed glycemic control and had to start insulin. This was associated by a much more rapid loss of -cell function in this cohort than seen in adults.[77] After 4 years of follow-up, prevalence of microalbuminuria and hypertension rose by 3-fold. Hypertension was associated with male gender and increase in BMI and appeared resistant to treatment, requiring multiple medications in more than one-third of participants initially treated with one agent.[78] Dyslipidemia, defined as an LDL ≥130 mg/dL or use of LDL-lowering therapy, increased from 4.5 to 10.7% over 3 years of follow-up while elevated inflammatory markers (hsCRP, PAI-1 and homocysteine) continued to increase over time and were not affected by lipid-lowering therapy.[79]
Key summary points.
In 2009, diabetes affected more than 190,000 children and adolescents in the US, where its prevalence is projected to increase two-folds or more by 2050.
Majority (75–95%) of proteinuria in children and adolescents is due to benign causes (orthostatic and transient proteinuria), which have to be ruled out before further evaluation for micro- or macroalbuminuria.
Using random albumin or protein to creatinine ratio may overestimate albuminuria in younger, smaller and female children, where timed urine albumin excretion may be more appropriate.
Microalbuminuria, defined as a urine albumin excretion of 30–299 mg/day (or albumin to creatinine ratio 30–299 mg/g), is the earliest available sign of predisposition to DKD. Microalbuminuria can regress, remain unchanged or progress to macroalbuminuria.
Risk factors for microalbuminuria are high A1c, blood pressure, LDL, triglycerides, high normal urine albumin excretion, obesity and smoking. Microalbuminuria is more likely to regress with improved control of risk factors (A1c, blood pressure, smoking cessation).
Macroalbuminuria, a urine albumin excretion ≥300 mg/day or albumin to creatinine ratio ≥300 mg/g, signals more severe kidney injury, but is also more likely to progress slowly with control of risk factors (A1c, blood pressure, smoking).
Significant DKD can be present even in absence of elevated urine albumin excretion, emphasizing the need for optimal risk factor control even in absence of albuminuria.
Earliest DKD structural changes in the kidneys appear as early as 1.5–5 years after type 1 diabetes onset.
Historically, type 1 diabetes was the predominant form of diabetes in children and adolescents. With the rise of childhood obesity, prevalence of type 2 diabetes has increased to 23% of cases of diabetes in the US children and adolescents, and 34–80% of diabetes among US ethnic minorities.
Young onset type 2 diabetes is associated with more co-morbidities and risk factors, faster progression of DKD and other complications and higher mortality than type 1 diabetes and may be more aggressive and treatment resistant than type 2 diabetes in adults.
Acknowledgements
Supported by grant K23DK089017 from the NIDDK and a Norman S. Coplon Extramural Grant from Satellite Healthcare.
Glossary
- AGE
Advanced Glycation End-products
- eNOS
endothelial Nitric Oxide Synthase
- ET-1
Endothelin 1
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GSH
reduced form of Glutathione
- GSSG
oxidized glutathione
- PAI
Plasminogen Activator Inhibitor
- PARP
Poly (ADP ribose) polymerase
- PKC
Protein Kinase C
- RAGE
Receptor for AGE
- ROS
Reactive Oxygen Species
- VEGF
Vascular Endothelial Growth Factor.
Multiple choice questions
- A 15 year old boy with 10 years of type 1 diabetes has a urine albumin excretion of 280 mg/day based on a timed mid-day urine sample. What is the next best step?
- Start an angiotensin converting enzyme inhibiting agent
- Obtain a repeat urine sample to confirm microalbuminuria (random albumin/creatinine ratio is adequate)
- Obtain a repeat first-void urine sample to exclude orthostatic proteinuria
- Determine if patient had fever, dehydration, seizure, intense exercise or any other causes of transient proteinuria prior to collection of the urine sample
- c and d
- A 7-year old girl with 5 years of type 1 diabetes and normal BP presents with a urine albumin/creatinine ratio of 150 mg/g in a first-void urine sample and you are assured by the parents that she has had no recent fever, seizure, dehydration or any potential causes for transient proteinuria. What is the next best step?
- Start an angiotensin converting enzyme inhibiting agent
- Repeat her urine albumin excretion in a timed urine collection to estimate her daily creatinine excretion.
- Optimize risk factors (A1c, blood pressure); she is too young to receive angiotensin converting enzyme inhibiting agents
- Repeat her urine albumin excretion in another random sample
- There is no need to follow her albumin excretion. DKD does not develop this soon after diabetes onset.
- A 12 year old Samoan girl with 7 years of type 2 diabetes presents with BP 150/95, A1c 10, BMI 32, LDL 190 and a urine albumin excretion of 200 mg/day. What is the next best management step to reduce her risk of progression to advanced DKD and other diabetic complications?
- controlling A1c
- Controlling blood pressure
- Encouraging weight loss
- Treating macroalbuminuria (RAS inhibition)
- Treating dyslipidemia
- Workup of secondary hypertension
- All of the above
- A 11 year old boy presents with 6 years of type 1 diabetes, poor glycemic control (A1c 11), poorly controlled hypertension (BP 165/94), 2 gram/day proteinuria, hematuria and GFR ~50. What is the best management?
- Control risk factors (A1c, BP). This is most likely rapidly progressing DKD.
- Initiate workup for other causes of kidney disease
- Initiate workup for secondary hypertension
- Obtain a repeat first-void urine sample to exclude orthostatic proteinuria
- b and c
- A 16 year old Hispanic girl with recently diagnosed type 2 diabetes presents with poorly controlled diabetes (A1c 9.8), hypertension (BP 163/98), dyslipidemia (LDL 189), obesity (BMI 38) and an elevated urine albumin excretion (130 mg/g). Which of the following statements is incorrect?
- Her albuminuria must be benign or due to other causes of kidney disease because it takes at least 1 year to even see the first structural changes of diabetes and several more years to develop albuminuria.
- Given her young age, her hypertension requires workup to exclude causes of secondary hypertension
- She is at high risk for development and rapid progression of overt DKD despite her short diabetes duration because of many coexisting risk factors, including insulin resistance, hypertension, poor glycemic control and dyslipidemia.
- She is at high risk of glycemic failure on oral agents and requiring transition to insulin.
- Her presentation is consistent with early DKD as young onset type 2 diabetes is often associated with micraoalbuminuria and other risk factors at diagnosis.
- In this young person with high risk of early mortality, weight loss is one of the most significant risk factors to modify.
Answers:
1. e
2. b
3. g
4. e
5. a
References
- 1.Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM, Liese AD, Linder B, Mayer-Davis EJ, Pihoker C, Saydah SH, Standiford DA, Hamman RF for the SfDiYSG. Prevalence of Diabetes Mellitus in U.S. Youth in 2009: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2014;37:402–408. doi: 10.2337/dc13-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, Rodriguez BL, Standiford D. Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35:2515–2520. doi: 10.2337/dc12-0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groop PH, Thomas MC, Moran JL, Waden J, Thorn LM, Makinen VP, Rosengard-Barlund M, Saraheimo M, Hietala K, Heikkila O, Forsblom C, FinnDiane Study G. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58:1651–1658. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease: 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53:2312–2319. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, de Boer IH. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterby R. Morphometric studies of the peripheral glomerular basement membrane in early juvenile diabetes. I. Development of initial basement membrane thickening. Diabetologia. 1972;8:84–92. doi: 10.1007/BF01235631. [DOI] [PubMed] [Google Scholar]
- 7.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brito PL, Fioretto P, Drummond K, Kim Y, Steffes MW, Basgen JM, Sisson-Ross S, Mauer M. Proximal tubular basement membrane width in insulin-dependent diabetes mellitus. Kidney Int. 1998;53:754–761. doi: 10.1046/j.1523-1755.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 9.Amin R, Widmer B, Prevost AT, Schwarze P, Cooper J, Edge J, Marcovecchio L, Neil A, Dalton RN, Dunger DB. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ (Clinical research ed) 2008;336:697–701. doi: 10.1136/bmj.39478.378241.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 11.Rossing P, Hougaard P, Parving HH. Progression of microalbuminuria in type 1 diabetes: ten-year prospective observational study. Kidney Int. 2005;68:1446–1450. doi: 10.1111/j.1523-1755.2005.00556.x. [DOI] [PubMed] [Google Scholar]
- 12.de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD, Diabetes C, Complications Trial/Epidemiology of Diabetes I, Complications Study Research G. White NH, Danis RP, Davis MD, Hainsworth D, Hubbard LD, Nathan DM. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171:412–420. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudberg S, Dahlquist G. Determinants of progression of microalbuminuria in adolescents with IDDM. Diabetes Care. 1996;19:369–371. doi: 10.2337/diacare.19.4.369. [DOI] [PubMed] [Google Scholar]
- 14.Salardi S, Balsamo C, Zucchini S, Maltoni G, Scipione M, Rollo A, Gualandi S, Cicognani A. High rate of regression from micro-macroalbuminuria to normoalbuminuria in children and adolescents with type 1 diabetes treated or not with enalapril: the influence of HDL cholesterol. Diabetes Care. 2011;34:424–429. doi: 10.2337/dc10-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molitch ME, Steffes M, Sun W, Rutledge B, Cleary P, de Boer IH, Zinman B, Lachin J Epidemiology of Diabetes I, Complications Study G. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33:1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Kidney F. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. AmJ Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 17.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B. Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. New Engl J Med. 2011;365:2366–2376. doi: 10.1056/NEJMoa1111732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodge WF, West EF, Smith EH, Bruce H., 3rd Proteinuria and hematuria in schoolchildren: epidemiology and early natural history. J Pediar. 1976;88:327–347. doi: 10.1016/s0022-3476(76)81012-8. [DOI] [PubMed] [Google Scholar]
- 19.Vehaskari VM, Rapola J. Isolated proteinuria: analysis of a school-age population. J Pediatr. 1982;101:661–668. doi: 10.1016/s0022-3476(82)80287-4. [DOI] [PubMed] [Google Scholar]
- 20.Park YH, Choi JY, Chung HS, Koo JW, Kim SY, Namgoong MK, Park YS, Yoo KH, Lee KY, Lee DY, Lee SJ, Lee JE, Chung WY, Hah TS, Cheong HI, Choi Y, Lee KS. Hematuria and proteinuria in a mass school urine screening test. Pediatr Nephrol. 2005;20:1126–1130. doi: 10.1007/s00467-005-1915-8. [DOI] [PubMed] [Google Scholar]
- 21.Chandar J, Gomez-Marin O, del Pozo R, Sanders L, Montane B, Abitbol C, Strauss J, Zilleruelo G. Role of routine urinalysis in asymptomatic pediatric patients. Clin Pediatr. 2005;44:43–48. doi: 10.1177/000992280504400105. [DOI] [PubMed] [Google Scholar]
- 22.Sebestyen JF, Alon US. The teenager with asymptomatic proteinuria: think orthostatic first. Clin Pediatr. 2011;50:179–182. doi: 10.1177/0009922810380904. [DOI] [PubMed] [Google Scholar]
- 23.Brandt JR, Jacobs A, Raissy HH, Kelly FM, Staples AO, Kaufman E, Wong CS. Orthostatic proteinuria and the spectrum of diurnal variability of urinary protein excretion in healthy children. Pediatr Nephrol. 2010;25:1131–1137. doi: 10.1007/s00467-010-1451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rytand DA, Spreiter S. Prognosis in postural (orthostatic) proteinuria: forty to fifty-year follow-up of six patients after diagnosis by Thomas Addis. New Engl J Med. 1981;305:618–621. doi: 10.1056/NEJM198109103051105. [DOI] [PubMed] [Google Scholar]
- 25.Springberg PD, Garrett LE, Jr, Thompson AL, Jr, Collins NF, Lordon RE, Robinson RR. Fixed and reproducible orthostatic proteinuria: results of a 20-year follow-up study. Ann Intern Med. 1982;97:516–519. doi: 10.7326/0003-4819-97-4-516. [DOI] [PubMed] [Google Scholar]
- 26.Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, Ingelfinger J. Evaluation and management of proteinuria and nephrotic syndrome in children: recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE) Pediatrics. 2000;105:1242–1249. doi: 10.1542/peds.105.6.1242. [DOI] [PubMed] [Google Scholar]
- 27.Skinner AM, Addison GM, Price DA. Changes in the urinary excretion of creatinine, albumin and N-acetyl-beta-D-glucosaminidase with increasing age and maturity in healthy schoolchildren. Eur J Pediatr. 1996;155:596–602. doi: 10.1007/BF01957912. [DOI] [PubMed] [Google Scholar]
- 28.Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75:561–569. doi: 10.1093/ajcn/75.3.561. [DOI] [PubMed] [Google Scholar]
- 29.Davies AG, Postlethwaite RJ, Price DA, Burn JL, Houlton CA, Fielding BA. Urinary albumin excretion in school children. Arch Dis Child. 1984;59:625–630. doi: 10.1136/adc.59.7.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, Kusek JW, Byrd-Holt D, Narayan KM, Herman WH, Jones CP, Salive M, Agodoa LY. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 31.Hanevold CD, Pollock JS, Harshfield GA. Racial differences in microalbumin excretion in healthy adolescents. Hypertension. 2008;51:334–338. doi: 10.1161/HYPERTENSIONAHA.107.098095. [DOI] [PubMed] [Google Scholar]
- 32.Katz A, Caramori ML, Sisson-Ross S, Groppoli T, Basgen JM, Mauer M. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int. 2002;61:2058–2066. doi: 10.1046/j.1523-1755.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 33.Lane PH, Steffes MW, Fioretto P, Mauer SM. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int. 1993;43:661–667. doi: 10.1038/ki.1993.95. [DOI] [PubMed] [Google Scholar]
- 34.Osterby R, Gundersen HJ, Nyberg G, Aurell M. Advanced diabetic glomerulopathy. Quantitative structural characterization of nonoccluded glomeruli. Diabetes. 1987;36:612–619. doi: 10.2337/diab.36.5.612. [DOI] [PubMed] [Google Scholar]
- 35.Caramori ML, Fioretto P, Mauer M. Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: an indicator of more advanced glomerular lesions. Diabetes. 2003;52:1036–1040. doi: 10.2337/diabetes.52.4.1036. [DOI] [PubMed] [Google Scholar]
- 36.Parving HH, Hommel E, Mathiesen E, Skott P, Edsberg B, Bahnsen M, Lauritzen M, Hougaard P, Lauritzen E. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. BMJ. 1988;296:156–160. doi: 10.1136/bmj.296.6616.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein R, Zinman B, Gardiner R, Suissa S, Donnelly SM, Sinaiko AR, Kramer MS, Goodyer P, Moss SE, Strand T, Mauer M Renin-Angiotensin System S. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the Renin-Angiotensin System Study. Diabetes. 2005;54:527–533. doi: 10.2337/diabetes.54.2.527. [DOI] [PubMed] [Google Scholar]
- 38.Osterby R, Gall MA, Schmitz A, Nielsen FS, Nyberg G, Parving HH. Glomerular structure and function in proteinuric type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:1064–1070. doi: 10.1007/BF02374500. [DOI] [PubMed] [Google Scholar]
- 39.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, Sambataro M, Abaterusso C, Baggio B, Crepaldi G, Nosadini R. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia. 1996;39:1569–1576. doi: 10.1007/s001250050616. [DOI] [PubMed] [Google Scholar]
- 40.Ekinci EI, Jerums G, Skene A, Crammer P, Power D, Cheong KY, Panagiotopoulos S, McNeil K, Baker ST, Fioretto P, Macisaac RJ. Renal structure in normoalbuminuric and albuminuric patients with type 2 diabetes and impaired renal function. Diabetes Care. 2013;36:3620–3626. doi: 10.2337/dc12-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellis EN, Steffes MW, Goetz FC, Sutherland DE, Mauer SM. Glomerular filtration surface in type I diabetes mellitus. Kidney Int. 1986;29:889–894. doi: 10.1038/ki.1986.82. [DOI] [PubMed] [Google Scholar]
- 42.Chavers BM, Bilous RW, Ellis EN, Steffes MW, Mauer SM. Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. New Engl J Med. 1989;320:966–970. doi: 10.1056/NEJM198904133201503. [DOI] [PubMed] [Google Scholar]
- 43.Mauer SM, Sutherland DE, Steffes MW. Relationship of systemic blood pressure to nephropathology in insulin-dependent diabetes mellitus. Kidney Int. 1992;41:736–740. doi: 10.1038/ki.1992.115. [DOI] [PubMed] [Google Scholar]
- 44.Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27:195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nosadini R, Velussi M, Brocco E, Bruseghin M, Abaterusso C, Saller A, Dalla Vestra M, Carraro A, Bortoloso E, Sambataro M, Barzon I, Frigato F, Muollo B, Chiesura-Corona M, Pacini G, Baggio B, Piarulli F, Sfriso A, Fioretto P. Course of renal function in type 2 diabetic patients with abnormalities of albumin excretion rate. Diabetes. 2000;49:476–484. doi: 10.2337/diabetes.49.3.476. [DOI] [PubMed] [Google Scholar]
- 46.Kaiser N, Sasson S, Feener EP, Boukobza-Vardi N, Higashi S, Moller DE, Davidheiser S, Przybylski RJ, King GL. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42:80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 47.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 48.Dugan LL, You YH, Ali SS, Diamond-Stanic M, Miyamoto S, Decleves AE, Andreyev A, Quach T, Ly S, Shekhtman G, Nguyen W, Chepetan A, Le TP, Wang L, Xu M, Paik KP, Fogo A, Viollet B, Murphy A, Brosius F, Naviaux RK, Sharma K. AMPK dysregulation promotes diabetes-related reduction of superoxide and mitochondrial function. J Clin Invest. 2013;123:4888–4899. doi: 10.1172/JCI66218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3. [DOI] [PubMed] [Google Scholar]
- 50.Holl RW, Grabert M, Thon A, Heinze E. Urinary excretion of albumin in adolescents with type 1 diabetes: persistent versus intermittent microalbuminuria and relationship to duration of diabetes, sex, and metabolic control. Diabetes Care. 1999;22:1555–1560. doi: 10.2337/diacare.22.9.1555. [DOI] [PubMed] [Google Scholar]
- 51.Daniels M, DuBose SN, Maahs DM, Beck RW, Fox LA, Gubitosi-Klug R, Laffel LM, Miller KM, Speer H, Tamborlane WV, Tansey MJ Network TDEC. Factors associated with microalbuminuria in 7,549 children and adolescents with type 1 diabetes in the T1D Exchange clinic registry. Diabetes Care. 2013;36:2639–2645. doi: 10.2337/dc12-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olsen BS, Sjolie A, Hougaard P, Johannesen J, Borch-Johnsen K, Marinelli K, Thorsteinsson B, Pramming S, Mortensen HB. A 6-year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy.Danish Study Group of Diabetes in Childhood. J Diabetes Complications. 2000;14:295–300. doi: 10.1016/s1056-8727(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 53.Jenkins AJ, Lyons TJ, Zheng D, Otvos JD, Lackland DT, McGee D, Garvey WT, Klein RL Group DER. Lipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathy. Kidney Int. 2003;64:817–828. doi: 10.1046/j.1523-1755.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- 54.Stone ML, Craig ME, Chan AK, Lee JW, Verge CF, Donaghue KC. Natural history and risk factors for microalbuminuria in adolescents with type 1 diabetes: a longitudinal study. Diabetes Care. 2006;29:2072–2077. doi: 10.2337/dc06-0239. [DOI] [PubMed] [Google Scholar]
- 55.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care. 2002;25:859–864. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 56.Schultz CJ, Konopelska-Bahu T, Dalton RN, Carroll TA, Stratton I, Gale EA, Neil A, Dunger DB. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes Vare. 1999;22:495–502. doi: 10.2337/diacare.22.3.495. [DOI] [PubMed] [Google Scholar]
- 57.Drummond KN, Kramer MS, Suissa S, Levy-Marchal C, Dell'Aniello S, Sinaiko A, Mauer M International Diabetic Nephropathy Study G. Effects of duration and age at onset of type 1 diabetes on preclinical manifestations of nephropathy. Diabetes. 2003;52:1818–1824. doi: 10.2337/diabetes.52.7.1818. [DOI] [PubMed] [Google Scholar]
- 58.Raile K, Galler A, Hofer S, Herbst A, Dunstheimer D, Busch P, Holl RW. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care. 2007;30:2523–2528. doi: 10.2337/dc07-0282. [DOI] [PubMed] [Google Scholar]
- 59.Couper JJ, Staples AJ, Cocciolone R, Nairn J, Badcock N, Henning P. Relationship of smoking and albuminuria in children with insulin-dependent diabetes. Diabetes Med. 1994;11:666–669. doi: 10.1111/j.1464-5491.1994.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 60.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes. 2009;58:1668–1671. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krolewski AS, Canessa M, Warram JH, Laffel LM, Christlieb AR, Knowler WC, Rand LI. Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. New Engl J Med. 1988;318:140–145. doi: 10.1056/NEJM198801213180303. [DOI] [PubMed] [Google Scholar]
- 62.Dabelea D, Pihoker C, Talton JW, D'Agostino RB, Jr, Fujimoto W, Klingensmith GJ, Lawrence JM, Linder B, Marcovina SM, Mayer-Davis EJ, Imperatore G, Dolan LM Study SfDiY. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34:1628–1633. doi: 10.2337/dc10-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez BL, Dabelea D, Liese AD, Fujimoto W, Waitzfelder B, Liu L, Bell R, Talton J, Snively BM, Kershnar A, Urbina E, Daniels S, Imperatore G, Group SS. Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the SEARCH for diabetes in youth study. J Pediatr. 2010;157:245–251. e241. doi: 10.1016/j.jpeds.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 64.Kershnar AK, Daniels SR, Imperatore G, Palla SL, Petitti DB, Pettitt DJ, Marcovina S, Dolan LM, Hamman RF, Liese AD, Pihoker C, Rodriguez BL. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2006;149:314–319. doi: 10.1016/j.jpeds.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 65.Liu LL, Lawrence JM, Davis C, Liese AD, Pettitt DJ, Pihoker C, Dabelea D, Hamman R, Waitzfelder B, Kahn HS Group SfDiYS. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2010;11:4–11. doi: 10.1111/j.1399-5448.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 66.Maahs DM, Snively BM, Bell RA, Dolan L, Hirsch I, Imperatore G, Linder B, Marcovina SM, Mayer-Davis EJ, Pettitt DJ, Rodriguez BL, Dabelea D. Higher prevalence of elevated albumin excretion in youth with type 2 than type 1 diabetes: the SEARCH for Diabetes in Youth study. Diabetes Care. 2007;30:2593–2598. doi: 10.2337/dc07-0450. [DOI] [PubMed] [Google Scholar]
- 67.Ettinger LM, Freeman K, DiMartino-Nardi JR, Flynn JT. Microalbuminuria and abnormal ambulatory blood pressure in adolescents with type 2 diabetes mellitus. J Pediatr. 2005;147:67–73. doi: 10.1016/j.jpeds.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Petitti DB, Klingensmith GJ, Bell RA, Andrews JS, Dabelea D, Imperatore G, Marcovina S, Pihoker C, Standiford D, Waitzfelder B, Mayer-Davis E Group SfDiYS. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155:668–672. e661–663. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yokoyama H, Okudaira M, Otani T, Sato A, Miura J, Takaike H, Yamada H, Muto K, Uchigata Y, Ohashi Y, Iwamoto Y. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58:302–311. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 70.Scott A, Toomath R, Bouchier D, Bruce R, Crook N, Carroll D, Cutfield R, Dixon P, Doran J, Dunn P, Hotu C, Khant M, Lonsdale M, Lunt H, Wiltshire E, Wu D. First national audit of the outcomes of care in young people with diabetes in New Zealand: high prevalence of nephropathy in Maori and Pacific Islanders. The N Z Med J. 2006;119:U2015. [PubMed] [Google Scholar]
- 71.Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35:1265–1271. doi: 10.2337/dc11-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. J Am Med Assoc. 2006;296:421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 73.Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, Maahs DM. Early diabetic nephropathy: a complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care. 2013;36:3678–3683. doi: 10.2337/dc13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Constantino MI, Molyneaux L, Limacher-Gisler F, Al-Saeed A, Luo C, Wu T, Twigg SM, Yue DK, Wong J. Long-Term Complications and Mortality in Young-Onset Diabetes: Type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36:3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rhodes ET, Prosser LA, Hoerger TJ, Lieu T, Ludwig DS, Laffel LM. Estimated morbidity and mortality in adolescents and young adults diagnosed with Type 2 diabetes mellitus. Diabetes Med. 2012;29:453–463. doi: 10.1111/j.1464-5491.2011.03542.x. [DOI] [PubMed] [Google Scholar]
- 76.Group TS, Zeitler P, Epstein L, Grey M, Hirst K, Kaufman F, Tamborlane W, Wilfley D. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Group TS. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and beta-cell function in TODAY. Diabetes Care. 2013;36:1749–1757. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.TODAY Study Group: Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1735–1741. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.TODAY Study Group: Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36:1758–1764. doi: 10.2337/dc12-2388. [DOI] [PMC free article] [PubMed] [Google Scholar]