Abstract
Background
Retrospective studies indicate associations between TSER (thymidylate synthase enhancer region) genotypes and clinical outcomes in patients receiving 5-FU based chemotherapy, but well-controlled prospective validation has been lacking.
Methods
In this phase II study (NCT00515216 registered through ClinicalTrials.gov, http://clinicaltrials.gov/show/NCT00515216), patients with “good risk” TSER genotypes (at least one TSER*2 allele) were treated with FOLFOX chemotherapy to determine whether prospective patient selection can improve overall response rates (ORR) in patients with gastric and gastroesophageal junction (GEJ) cancers, compared with historical outcomes in unselected patients (estimated 43%).
Results
The ORR in genotype-selected patients was 39.1% (9 partial responses out of 23 evaluable patients, 95% CI, 22.2 to 59.2), not achieving the primary objective of improving ORR. An encouraging disease control rate (DCR, consisting of partial responses and stable diseases) of 95.7% was noted and patients with homozygous TSER*2 genotype showed better tumor response.
Conclusions
In this first prospective, multi-institutional study in patients with gastric or GEJ cancers, selecting patients with at least one TSER*2 allele did not improve the ORR but led to an encouraging DCR. Further studies are needed to investigate the utility of selecting patients homozygous for the TSER*2 allele and additional genomic markers in improving clinical outcomes for patients with gastric and GEJ cancers.
Trial Registration
ClinicalTrials.gov NCT00515216
Introduction
Cancers of the gastric cardia, gastroesophageal junction (GEJ) and distal esophagus have been rapidly increasing in incidence in the past decades, especially in patients younger than 40 years of age [1]. Unfortunately, 80–90% of newly diagnosed patients present with regional or distant metastatic disease and even with optimal therapy, the median survival in these patients is less than 1 year and survival at 5 years is essentially zero [2], [3]. Recent advances in our capacity to detect and target specific molecular lesions in cancer cells and to obtain genetic information from tumor tissues and patients allow for selection of optimal therapies, improving the outcomes for these aggressive cancers. For example, targeting HER-2 expressing gastroesophageal tumors with trastuzumab led to an improvement in the survival of patients with advanced disease [4]. However, for the majority of patients selection of initial therapy remains largely empiric as most of the regimens have similar response rates and median survival [3], [5]. Thus, there is a clear need for approaches that can guide treatment selection for the most effective regimens.
5-fluorouracil (5-FU) is one of the most commonly prescribed chemotherapy agents. It has demonstrated preclinical synergy with oxaliplatin in a variety of tumor types and this combination has demonstrated clear efficacy in treating patients with gastric and GEJ cancers [6]–. Thymidylate synthase (TS, encoded by the TYMS gene) is the critical enzyme in DNA synthesis and repair and it is the primary target for 5-FU and other folate-based antimetabolites [9]. Overexpression of TS has been linked to clinical resistance to TS-targeted agents including 5-FU [10]. In turn, genetic polymorphisms involving the promoter region of the TYMS gene, specifically, the number of tandem repeat sequences in the TS enhancer region (TSER - the 28-nucleotide G/C-rich sequence in the 5′-untranslated region) have been shown to be important determinants of tumoral TS expression [11]–[15]. The two most common TSER alleles are the two tandem repeats (TSER*2, allelic frequency = 0.2∼0.4) and the three tandem repeats (TSER*3, allelic frequency = 0.6∼0.8) [15].
Retrospective studies in colorectal cancer have demonstrated that individuals with TSER*3/*3 genotype had a significantly lower response rate and poorer outcomes to 5-FU compared with those with at least one TSER*2 allele [11], [16]–[18]. Additional retrospective analyses of TS expression or TSER genotypes in relation to clinical outcomes in patients with gastric cancers replicated these findings [19], [20]. More recently, findings from the first prospective study evaluating the utility of TSER genotypes in directing neoadjuvant chemoradiation for patients with rectal carcinoma demonstrated that patients treated with 5-FU-based chemoradiotherapy according to their TSER genotypes had improved tumor downstaging [21]. Together, these studies provide ample evidence that TS expression status and/or TSER genotyping may be useful in selecting patients who are likely to respond to treatment with 5-FU or its analogues.
This Phase II study was designed to prospectively select patients with “good risk” TSER genotypes (i.e. TSER*2/*2 or *2/*3) and treat them with a standard 5-FU-based regimen (FOLFOX; 5-FU, leucovorin, oxaliplatin) in order to improve clinical outcomes in patients with gastric and GEJ cancers. The primary end point of this study was to determine whether TSER genotype-directed chemotherapy would result in an improved overall response rate (ORR, 60% or higher) compared to historical control response rates in non-genotype selected patients (estimated 43%). The secondary end points were to retrospectively assess whether other genetic variations, in particular, additional polymorphic loci in the TYMS gene and other genes involved in the disposition and response to the FOLFOX regimen would influence the response in the treated patients.
Materials and Methods
Ethics Statement
The trial was done in accordance with the Declaration of Helsinki and ICH Good Clinical Practice. The study protocol was approved by the Vanderbilt University and Washington University Institutional Review Boards and all participants provided their written informed consent to participate in this study. The study was registered through ClinicalTrials.gov (Identifier: NCT00515216). The protocol for this trial and supporting TREND checklist are available as supporting information; see Checklist S1 and Protocol S1. The trial started (first patient enrolled) in June 2008 and ended (last patient completed the clinical study) in October 2010.
Eligibility
Adult (≥18 years) patients who had histologically or cytologically confirmed adenocarcinoma of the stomach or GEJ and had received no prior therapy for metastatic disease were eligible. Subjects could have received prior neoadjuvant or adjuvant therapy as long as the disease-free interval was longer than 6 months. Eligible patients had Eastern Cooperative Oncology Group (ECOG) performance status ≤2, adequate organ and bone marrow function, and an ability to understand and willingness to sign written informed consent. Patients with known active brain metastases, HIV on anti-retroviral therapy or other uncontrolled intercurrent illnesses were excluded.
Study Design and Treatment
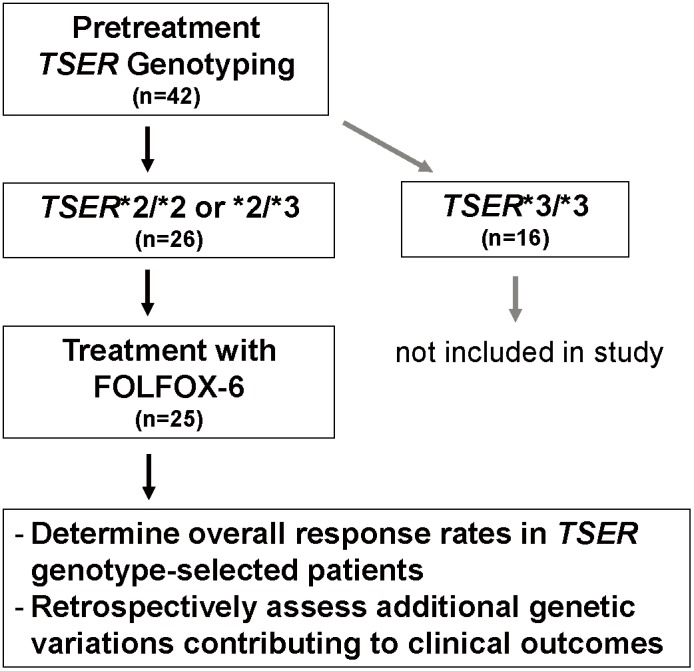
This was an open-label, non-randomized, investigator-initiated multi-center study involving prospective genotyping (study flow chart shown in Figure 1). Potentially eligible patients underwent a blood draw for TSER genotyping. Patients with TSER*3/*3 genotype were not included in study treatment. Patients with TSER*2/*2 or TSER*2/*3 genotypes received the modified FOLFOX-6 treatment consisting of oxaliplatin 85 mg/m2 and leucovorin 400 mg/m2 given over 2 hours along with 5-FU 400 mg/m2 given as an intravenous push over 5 minutes, followed by 5-FU 2,400 mg/m2 given as an intravenous infusion of 46 hours. This treatment was repeated every 2 weeks in the absence of unacceptable adverse events or disease progression.
Figure 1. A schematic diagram of the current phase II study design.
Assessment of Efficacy and Toxicity
The patients were reevaluated for response every 8 weeks. In addition to a baseline scan, confirmatory scans were performed not less than 4 weeks following initial documentation of objective response. Tumor response and progression were evaluated using Response Evaluation Criteria in Solid Tumors (RECIST). Partial response (PR) was defined as at least 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum LD. Progressive disease (PD) was defined as at least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions. Stable disease (SD) was defined as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 3.0. Dose modifications were made depending on the type and severity of toxicities observed.
Retrospective Genotyping Analyses
Retrospective genotyping analyses by restriction fragment length polymorphism (RFLP) were performed for two additional loci in the TYMS gene (G>C SNP within the second 28-bp tandem repeat of the 3R allele, rs34743033; 1494delTTAAAG in the 3′-UTR, rs34489327) and five loci in additional genes reported to be associated with response and toxicity to the FOLFOX regimen (ERCC1, c.354C>T, rs11615; ERCC2, c.2251A>C, rs13181; GSTP1, c.313A>G, rs1695; XRCC1, c.1196G>A, rs25487; and MDR1, c.3435C>T, rs1045642). Briefly, regions encompassing the respective polymorphisms were PCR amplified (Platinum PCR Supermix, Invitrogen) and subjected to restriction enzyme digest to yield the diagnostic fragmentation patterns (detailed conditions provided in Table S1 in File S1) [13], [22]–[30]. The digested and undigested products from each patient sample were visualized on 3% agarose gel along with positive controls whose genotypes were verified by direct sequencing.
Statistical Analyses
The primary objective of this study was to test whether selection of patients according to TSER genotypes would improve treatment response rates compared to the response rates previously reported in an unselected population. Sample size estimation was based on the assumed response rate of 43% with this regimen in an unselected population and improving the response rate to a minimum of 60% based on the retrospective data [19], [20]. An Optimum MinMax two-stage accrual design was employed [31]. In the initial step, 45 eligible patients were to be entered into the study, with a final accrual goal of 75 if ≥20 responses were observed in the first group. This design provides 90% statistical power to detect a difference of 17% with a two-sided significance level of <0.05. Unfortunately, the current study had to be terminated earlier than the initial stopping point due to insufficient funding. For 23 patients evaluable for tumor responses, univariate associations between genotypes and tumor response (PR and SD) were evaluated using Fisher’s exact test. Overall survival (OS) and progression free survival (PFS) were analyzed using Kaplan-Meier models and associations between genotypes and patient survival were assessed using log-rank tests. No sub-analyses were performed for potential associations between genotypes and toxicities since they were not pre-specified in our analysis plans. All statistical analyses were performed using R package (version 3.0.2).
Results
Patient characteristics
Patient baseline characteristics are listed in Table 1. Between June 2008 and October 2010, 42 patients with gastric and GEJ cancers were screened for their TSER genotypes; 26 patients (63.4%) had good risk genotypes (TSER*2/*2 or *2/*3) and enrolled onto the trial. One patient with a good risk genotype withdrew consent and was not treated on study. The majority of patients had gastric cancer (73%) and good performance status (69% ECOG PS of 1 or better). The median age of the participants was 56 years and a majority (62%) was male.
Table 1. Baseline clinical and demographic characteristics of enrolled patients (n = 26).
| Characteristic | Patients (n = 26) | |
| No | % | |
| TSER genotypes | ||
| TSER*2/*2 | 5 | 19 |
| TSER*2/*3 | 21 | 81 |
| Gender | ||
| Male | 16 | 62 |
| Female | 10 | 38 |
| Age (years) | ||
| median | 56 | |
| range | 26–81 | |
| Ethnicity | ||
| Caucasians | 23 | 88 |
| African Americans | 2 | 8 |
| Not reported | 1 | 4 |
| ECOG performance status | ||
| 0 | 5 | 19 |
| 1 | 13 | 50 |
| 2 | 8 | 31 |
| Primary tumor type | ||
| Gastric | 19 | 73 |
| Gastroesophageal junction (GEJ) | 7 | 27 |
| Prior neoadjuvant or adjuvant therapy (>6 months) | 2 | 8 |
Treatment and toxicities
Twenty five patients received at least one dose of study treatment with the modified FOLFOX-6 regimen. A total of 128 cycles were administered, with a median of 5.5 cycles per patient (range, 0.5–15 cycles). The occurrence and the incidence of the main toxicities are reported in Table 2. The toxicities experienced by the study participants were within the expected range for patients receiving treatment with FOLFOX. The most common toxicities were hematologic with grade 3 and 4 neutropenia, leukopenia and anemia recorded in 8 out of 25 (32%), 4 out of 25 (16%), and one out of 25 (4%) patients, respectively. Only two out of 25 patients experienced grade 3 gastrointestinal toxicity and no participants had grade 4 gastrointestinal toxicity. Neurotoxicity was common and was observed in 44% (grade 1 in 20%, grade 2 in 16% and grade 3 in 8%) of the patients. Fatigue was also common and was observed in 52% (grade 1 in 40%, grade 2 in 4% and grade 3 in 8%) of the patients. No treatment-related deaths were reported.
Table 2. Toxicities observed in the treated patients (n = 25).
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||
| No | % | No | % | No | % | No | % | |
| Leukopenia (total WBC) | 4 | 16 | 5 | 20 | 4 | 16 | 0 | 0 |
| Neutropenia | 3 | 12 | 2 | 8 | 4 | 16 | 4 | 16 |
| Lymphopenia | 3 | 12 | 4 | 16 | 1 | 4 | 0 | 0 |
| Anemia | 13 | 52 | 6 | 24 | 1 | 4 | 0 | 0 |
| Thrombocytopenia | 9 | 36 | 3 | 12 | 0 | 0 | 0 | 0 |
| Nausea | 12 | 48 | 2 | 8 | 1 | 4 | 0 | 0 |
| Vomiting | 3 | 12 | 3 | 12 | 1 | 4 | 0 | 0 |
| Diarrhea | 8 | 32 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mucositis/stomatitis | 3 | 12 | 1 | 4 | 0 | 0 | 0 | 0 |
| Taste alteration (dysgeusia) | 8 | 32 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vision-blurred vision | 3 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| Allergic reaction/hypersensitivity | 0 | 0 | 1 | 4 | 0 | 0 | 0 | 0 |
| Rash: hand-foot skin reaction | 2 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| AST, SGOT | 8 | 32 | 2 | 8 | 1 | 4 | 0 | 0 |
| ALT, SGPT | 10 | 40 | 2 | 8 | 0 | 0 | 0 | 0 |
| Neuropathy: sensory | 5 | 20 | 4 | 16 | 2 | 8 | 0 | 0 |
| Fatigue (asthenia, lethargy, malaise) | 10 | 40 | 1 | 4 | 2 | 8 | 0 | 0 |
Response to FOLFOX regimen, recurrence and survival and TSER genotypes
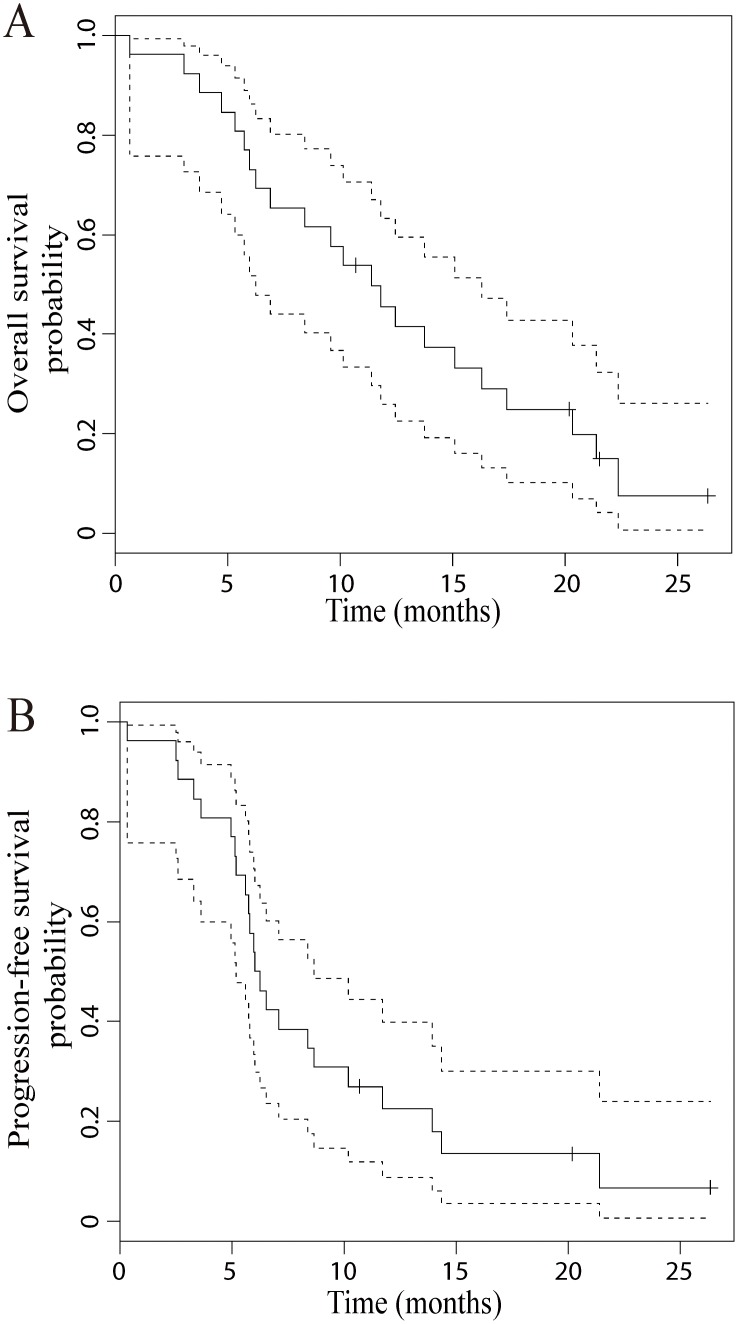
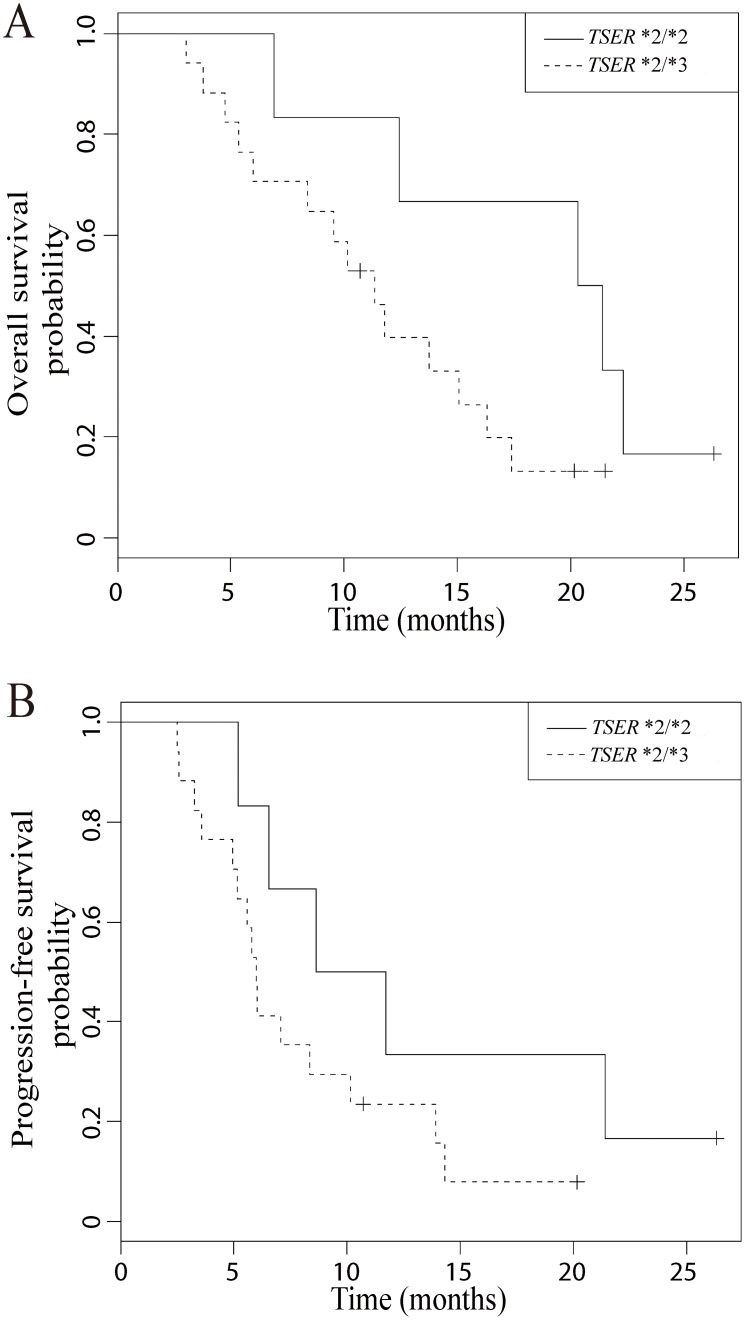
Response to the modified FOLFOX-6 regimen in the 23 evaluable patients is shown in Table 3. Nine patients experienced PR and no patient experienced complete response (CR), making the overall response rate (CR+PR) to be 39.1% (95% confidence interval (CI, Wilson), 22.2 to 59.2). When tumor responses are compared between the two TSER genotype groups, patients with the TSER*2/*2 genotype experienced a greater frequency of PR and a less frequency of SD compared to those with the TSER*2/*3 genotype (p = 0.02, Fisher’s exact test, Table 3). On the other hand, only one out of 23 patients experienced PD, for an observed disease control rate (CR+PR+SD) of 95.7% (95% CI, 79.0 to 99.2). OS and PFS plots for the enrolled patients are shown in Figure 2. Median values for OS and PFS were 11.4 months (95% CI, 6.3 to 16.3) and 6.2 months (95% CI, 5.2 to 8.6), respectively. For both OS and PFS, patients with the TSER*2/*2 genotype had a trend towards an improvement in median OS and PFS in comparison to those patients with the TSER*2/*3 genotype (Figure 3, OS, 20.9 vs 11.4 months; PFS, 10.2 vs 6.0 months).
Table 3. Tumor responses to the FOLFOX regimen in patients of differing TSER genotypes.
| Tumor response | TSER genotypes | P-valuea | |||
| TSER*2/*2 | TSER*2/*3 | ||||
| (n = 6) | (n = 17) | ||||
| No | % | No | % | ||
| PD (n = 1) | 0 | 0 | 1 | 5.9 | 0.02 |
| PR (n = 9) | 5 | 83.3 | 4 | 23.5 | |
| SD (n = 13) | 1 | 16.7 | 12 | 70.6 | |
PD, progressive disease; PR, partial response; SD, stable disease.
Calculated using Fisher’s exact test for the association between response (PR and SD) and TSER genotype.
Figure 2. Kaplan-Meier curves showing overall survival (A) and progression free survival (B) with 95% confidence intervals (CI) in the patients enrolled.
Figure 3. Kaplan-Meier curves showing overall survival (A) and progression free survival (B) according to TSER genotypes.
Potential associations between tumor response and retrospectively analyzed genetic variations
For 21 patients, the retrospective analyses for additional genotypes reported to be associated with treatment responses were completed (the genotypic and allelic frequencies of the interrogated variations are summarized in Table S2 in File S1). Of note, the frequency of TSER*2 allele is higher in our current study than those reported in the literature since the patients with the TSER*3/*3 genotype were excluded in this study. When the two additional loci in the TYMS genes (rs34743033 and rs34489327) were assessed for their potential association with tumor response (PR vs SD), the results did not indicate any statistically significant association (Table 4). Among the five additional genotypes analyzed, two genetic variations in the XRCC1 (c.1196G>A, rs25487) and MDR1 (c.3435C>T, rs1045642) genes displayed a trend supportive of their potential association with tumor response (p = 0.050 and 0.056, respectively, Fisher’s exact test, Table 4). However, no apparent association was observed when OS and PFS times were compared among the groups with differing genotypes at the five loci (Table S3 in File S1).
Table 4. Univariate association between additional retrospectively analyzed genotypes and tumor response (PR: partial response and SD: stable disease).
| Gene | Genotype | Tumor response | P-valuea | |||
| PR (N = 9) | SD (N = 11) | |||||
| No. | % | No. | % | |||
| TYMS | 5′-UTR TSER + G>C (rs34743033) | |||||
| TSER*2/*2 | 5 | 56 | 1 | 9 | ||
| TSER*2/*3(G) | 2 | 22 | 4 | 36 | ||
| TSER*2/*3(C) | 2 | 22 | 6 | 55 | 0.137 | |
| TYMS | 3′-UTR 1494delTTAAAG(6 bp) (rs34489327) | |||||
| +6 bp/+6 bp | 5 | 56 | 6 | 55 | ||
| +6 bp/−6 bp | 4 | 44 | 5 | 45 | 1 | |
| ERCC1 | c.354C>T (rs11615) | |||||
| C/C | 2 | 22 | 1 | 9 | ||
| C/T | 4 | 45 | 8 | 73 | ||
| T/T | 3 | 33 | 2 | 18 | 0.552 | |
| ERCC2 | c.2251A>C (rs13181) | |||||
| A/A | 5 | 56 | 3 | 27 | ||
| A/C | 3 | 33 | 6 | 55 | ||
| C/C | 1 | 11 | 2 | 18 | 0.485 | |
| GSTP1 | c.313A>G (rs1695) | |||||
| A/A | 4 | 45 | 8 | 73 | ||
| A/G | 3 | 33 | 2 | 18 | ||
| G/G | 2 | 22 | 1 | 9 | 0.552 | |
| XRCC1 | c.1196G>A (rs25487) | |||||
| A/A | 1 | 11 | 6 | 55 | ||
| G/A | 6 | 67 | 5 | 45 | ||
| G/G | 2 | 22 | 0 | - | 0.050 | |
| MDR1 | c.3435C>T (rs1045642) | |||||
| C/C | 0 | - | 1 | 9 | ||
| C/T | 9 | 100 | 6 | 55 | ||
| T/T | 0 | - | 4 | 36 | 0.056 | |
Calculated using Fisher’s exact test.
Discussion
This clinical study is unique in that we for the first time applied the strategy of prospectively assessing TSER genotypes to improve clinical outcomes in gastric and GEJ cancers. Our initial hypothesis was that selection of patients with good risk TSER genotypes would improve the response rate for FOLFOX to 60%, a 17% increase over the historic control response rate of 43% observed in non-genotype selected patients. The observed response rate in this study was 39.1% (9 PR out of 23 patients evaluable for tumor response, 95% CI 22.2 to 59.2), not achieving the primary study endpoint. The median OS and PFS times of 11.3 and 6.2 months in patients with good risk TSER genotypes were also comparable to those reported in non-genotype selected populations (Figure 2). Although the observed response rate did not support the utility of TSER genotyping as a treatment selection guide, it should also be noted that the enrolled patients experienced a very promising disease control rate of 95.7% (9 PR and 13 SD out of 23 patients), higher than those reported in the literature [32]–[36]. The high disease control rate in the current study may provide the first prospectively obtained evidence for the utility of TSER genotyping in improving clinical outcomes in patients with gastric and GEJ cancers.
The apparent lack of treatment outcome improvement in patients with good risk TSER genotypes may be related to the small number of patients enrolled in the current study, therefore necessitating further validation of this approach in a larger clinical trial. It should be however noted that we observed a very encouraging response rate of 83.3% in patients with the homozygous TSER*2/*2 genotype (5 out of 6 patients experienced PR). This observation was from a very small number of patients, but it suggests that the improvement in clinical outcomes in patients with gastric and GEJ cancers may require selection of patients with two TSER*2 alleles. Alternatively, the current findings may be related to the presence of multiple molecular/genetic factors contributing to chemotherapy response. Strategies involving more than one host and tumoral markers will likely be more successful in guiding therapeutic decision making. Currently we cannot rule out the possibility that TSER genotypes may have prognostic value, independent of therapy, in patients with gastric and GEJ cancers as previously reported in patients with colorectal cancer [37], [38]. Consideration of the above-mentioned aspects will be important in the design of future clinical trials which can evaluate multifactorial treatment selection approaches in a larger number of patients.
The findings from another prospective study with selection of patients on the basis of TSER genotypes have been recently reported in rectal cancer patients receiving neoadjuvant chemoradiation (n = 135) [21]. In this particular study, patients with good risk TSER genotypes (at least one TSER*2 allele) were treated with standard 5-FU-based chemoradiation. Patients with good risk genotypes had higher rates of downstaging and pathologic complete response than reported in unselected populations. Patients with poor risk TSER genotypes (harboring no TSER*2 allele) were treated with irinotecan in addition to 5-FU-based chemoradiation. Patients in the poor risk groups receiving additional therapies also showed higher rates of downstaging and pathologic complete response than those for unselected population. This study [21] however did not address whether patients in the poor risk group would have had poorer clinical outcomes if they had not received additional irinotecan therapy. Nevertheless, these findings certainly support the utility of this single genotype-based strategy in improving the clinical outcomes of rectal cancer patients.
No improvement in the overall response rate from this study echo the inconsistent results observed in previous studies utilizing TSER genotyping in treating patients with gastric and esophageal cancers [6], . Less than expected response rates in our current study as well as other previously reported retrospective studies may be explained by the potential effect of oxaliplatin on tumoral TS expression. The clinical synergy of combining 5-FU with oxaliplatin is well documented and is key to the success and frequent use of this combination regimen [42]–[44]. One proposed explanation for this synergy is oxaliplatin-induced TS downregulation by as yet unexplained mechanisms [45], [46]. If oxaliplatin were to cause tumoral TS downregulation or any potential molecular changes influencing tumor response, any benefits from pretreatment TSER genotyping may have been obscured in our current study. Interestingly, some in vitro studies also indicate that irinotecan may also decrease TS activities and protein levels where irinotecan could overcome 5-FU resistance in tumors [47], [48]. These observations could help to explain the favorable response results in the “poor risk” patients who received irinotecan in the Tan et al. study [21]. However, our current study design of a single arm (patients with the favorable TSER genotypes only) does not allow us to gather treatment outcome for patients with the unfavorable TSER genotypes. Our initial study design was indeed two-armed (favorable vs. unfavorable TSER genotypes). But, the reviewers in the NIH study section recommended that we revise our study to be single armed for patients with favorable TSER genotypes given the patient numbers and other resources available. In future clinical trials, it would be certainly interesting to prospectively compare treatment outcomes of patients with favorable and unfavorable TSER genotypes.
Retrospective analyses for two additional TYMS genotypes showed no significant association with tumor response (Table 4). For additional non-TYMS genes analyzed, XRCC1 (c.1196G>A, rs25487) and MDR1 (c.3435C>T, rs1045642) showed a potential association to tumor response (Table 4). We performed these analyses based on the previous findings on the potential association between these genotypes and clinical outcomes in colorectal cancer patients treated with FOLFOX [24], [29], [40]. However, we did not observe any strong association between the tested genotypes and tumor response, except a trend reflective of potential associations for XRCC1 and MDR1. Similar negative results have been reported from retrospective analyses of TYMS and non-TYMS genotypes in relation to the histopathological tumor responses in patients with gastric and esophageal cancers [39], [41], [49]. Interestingly, the polymorphisms in XRCC1 and MDR1 have been associated with clinical outcomes in gastric and colorectal cancer [24], [50]. Further investigation in larger clinical trials is warranted to determine whether our current findings are indicative of varying impact of the tested genotypes in different tumor types.
In summary, our strategy to improve response rates in patients with gastric and GEJ cancers by pretreatment TSER genotyping was unsuccessful, but even in the setting of some limitations of our current study the high disease control rate was encouraging. The future applicability of a single polymorphism strategy to guide therapy selection, while feasible and attractive, does not appear to yield results of sufficient impact to be generally applicable. Our approach may aid in selecting patients who require chemotherapy intensification to achieve favorable results by adding oxaliplatin or irinotecan and avoid chemotherapy toxicities in those who may have a favorable outcome with 5-FU alone. The contemporary confluence of our capacity to molecularly target cancers and the advancement of our ability to characterize patients and their cancers using molecular genetics will allow for multiple variables to be considered and applied towards improvements in cancer care. TYMS polymorphism status does not appear to be a singularly important treatment selection factor, but it may be a key contributor to favorable outcomes in a multivariable genomically based therapeutic approach.
Supporting Information
Supporting Tables.
(DOCX)
TREND Checklist.
(PDF)
Trial Protocol.
(PDF)
Acknowledgments
The Vanderbilt University CHGR/CRC DNA Resources Core provided technical assistance for this work.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported in part by the grants from the National Cancer Institute, NIH (R21 CA123881, K23 CA098011), the National Center for Research resources, NIH (5 M01 RR-000095). Additional funding was provided by BJC Foundation Cancer Frontier Fund and Sanofi-Aventis. The authors confirm that the funding agencies, with the exception of the NIH, had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Brown LM, Devesa SS (2002) Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 11: 235–256. [DOI] [PubMed] [Google Scholar]
- 2. Polednak AP (2003) Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer 105: 98–100. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, et al. (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358: 36–46. [DOI] [PubMed] [Google Scholar]
- 4. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376: 687–697. [DOI] [PubMed] [Google Scholar]
- 5. Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, et al. (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24: 4991–4997. [DOI] [PubMed] [Google Scholar]
- 6. Keam B, Im SA, Han SW, Ham HS, Kim MA, et al. (2008) Modified FOLFOX-6 chemotherapy in advanced gastric cancer: Results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer 8: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oh SY, Kwon HC, Seo BG, Kim SH, Kim JS, et al. (2007) A phase II study of oxaliplatin with low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFOX-4) as first line therapy for patients with advanced gastric cancer. Acta Oncol 46: 336–341. [DOI] [PubMed] [Google Scholar]
- 8. De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, et al. (2005) A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 92: 1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu E, Moto AC, Fogarasi MC (2001) Cancer: Principles & Practice of Oncology; De Vita JVT, Hellman S, Rosenberg SA, editors. Philadelphia: Lippincott Williams & Wilkins. 388–415 p. [Google Scholar]
- 10. Marsh S, McLeod HL (2001) Thymidylate synthase pharmacogenetics in colorectal cancer. [see comment]. Clin Colorectal Cancer 1: 175–178 discussion 179–181. [DOI] [PubMed] [Google Scholar]
- 11. Horie N, Aiba H, Oguro K, Hojo H, Takeishi K (1995) Functional analysis and DNA polymorphism of the tandemly repeated sequences in the 5′-terminal regulatory region of the human gene for thymidylate synthase. Cell Struct Funct 20: 191–197. [DOI] [PubMed] [Google Scholar]
- 12. Kawakami K, Omura K, Kanehira E, Watanabe Y (1999) Polymorphic tandem repeats in the thymidylate synthase gene is associated with its protein expression in human gastrointestinal cancers. Anticancer Res 19: 3249–3252. [PubMed] [Google Scholar]
- 13. Ulrich CM, Bigler J, Velicer CM, Greene EA, Farin FM, et al. (2000) Searching expressed sequence tag databases: discovery and confirmation of a common polymorphism in the thymidylate synthase gene. Cancer Epidemiol Biomarkers Prev 9: 1381–1385. [PubMed] [Google Scholar]
- 14. Kawakami K, Salonga D, Park JM, Danenberg KD, Uetake H, et al. (2001) Different lengths of a polymorphic repeat sequence in the thymidylate synthase gene affect translational efficiency but not its gene expression. Clin Cancer Res 7: 4096–4101. [PubMed] [Google Scholar]
- 15. Marsh S, Collie-Duguid ES, Li T, Liu X, McLeod HL (1999) Ethnic variation in the thymidylate synthase enhancer region polymorphism among Caucasian and Asian populations. Genomics 58: 310–312. [DOI] [PubMed] [Google Scholar]
- 16. Iacopetta B, Grieu F, Joseph D, Elsaleh H (2001) A polymorphism in the enhancer region of the thymidylate synthase promoter influences the survival of colorectal cancer patients treated with 5-fluorouracil. Br J Cancer 85: 827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pullarkat ST, Stoehlmacher J, Ghaderi V, Xiong YP, Ingles SA, et al. (2001) Thymidylate synthase gene polymorphism determines response and toxicity of 5-FU chemotherapy. Pharmacogenomics J 1: 65–70. [DOI] [PubMed] [Google Scholar]
- 18. Villafranca E, Okruzhnov Y, Dominguez MA, Garcia-Foncillas J, Azinovic I, et al. (2001) Polymorphisms of the repeated sequences in the enhancer region of the thymidylate synthase gene promoter may predict downstaging after preoperative chemoradiation in rectal cancer. J Clin Oncol 19: 1779–1786. [DOI] [PubMed] [Google Scholar]
- 19. Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, et al. (1998) ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 16: 309–316. [DOI] [PubMed] [Google Scholar]
- 20. Yeh KH, Shun CT, Chen CL, Lin JT, Lee WJ, et al. (1998) High expression of thymidylate synthase is associated with the drug resistance of gastric carcinoma to high dose 5-fluorouracil-based systemic chemotherapy. Cancer 82: 1626–1631. [DOI] [PubMed] [Google Scholar]
- 21. Tan BR, Thomas F, Myerson RJ, Zehnbauer B, Trinkaus K, et al. (2011) Thymidylate synthase genotype-directed neoadjuvant chemoradiation for patients with rectal adenocarcinoma. J Clin Oncol 29: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henriquez-Hernandez LA, Murias-Rosales A, Hernandez Gonzalez A, Cabrera De Leon A, Diaz-Chico BN, et al. (2009) Gene polymorphisms in TYMS, MTHFR, p53 and MDR1 as risk factors for breast cancer: a case-control study. Oncol Rep 22: 1425–1433. [DOI] [PubMed] [Google Scholar]
- 23. Lincz LF, Scorgie FE, Garg MB, Ackland SP (2007) Identification of a novel single nucleotide polymorphism in the first tandem repeat sequence of the thymidylate synthase 2R allele. Int J Cancer 120: 1930–1934. [DOI] [PubMed] [Google Scholar]
- 24. Huang MY, Huang ML, Chen MJ, Lu CY, Chen CF, et al. (2011) Multiple genetic polymorphisms in the prediction of clinical outcome of metastatic colorectal cancer patients treated with first-line FOLFOX-4 chemotherapy. Pharmacogenet Genomics 21: 18–25. [DOI] [PubMed] [Google Scholar]
- 25. Huang MY, Fang WY, Lee SC, Cheng TL, Wang JY, et al. (2008) ERCC2 2251A>C genetic polymorphism was highly correlated with early relapse in high-risk stage II and stage III colorectal cancer patients: a preliminary study. BMC Cancer 8: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang XH, Zhang X, Zhang L, Chen Q, Yang Z, et al. (2012) XRCC1 Arg399Gln was associated with repair capacity for DNA damage induced by occupational chromium exposure. BMC Res Notes 5: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lunn RM, Langlois RG, Hsieh LL, Thompson CL, Bell DA (1999) XRCC1 polymorphisms: effects on aflatoxin B1-DNA adducts and glycophorin A variant frequency. Cancer Res 59: 2557–2561. [PubMed] [Google Scholar]
- 28. Zhang Z, Wan J, Jin X, Jin T, Shen H, et al. (2005) Genetic polymorphisms in XRCC1, APE1, ADPRT, XRCC2, and XRCC3 and risk of chronic benzene poisoning in a Chinese occupational population. Cancer Epidemiol Biomarkers Prev 14: 2614–2619. [DOI] [PubMed] [Google Scholar]
- 29. Mandola MV, Stoehlmacher J, Muller-Weeks S, Cesarone G, Yu MC, et al. (2003) A novel single nucleotide polymorphism within the 5′ tandem repeat polymorphism of the thymidylate synthase gene abolishes USF-1 binding and alters transcriptional activity. Cancer Res 63: 2898–2904. [PubMed] [Google Scholar]
- 30. Kawakami K, Watanabe G (2003) Identification and functional analysis of single nucleotide polymorphism in the tandem repeat sequence of thymidylate synthase gene. Cancer Res 63: 6004–6007. [PubMed] [Google Scholar]
- 31. Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 32. Ocvirk J, Rebersek M, Skof E, Hlebanja Z, Boc M (2012) Randomized prospective phase II study to compare the combination chemotherapy regimen epirubicin, cisplatin, and 5-fluorouracil with epirubicin, cisplatin, and capecitabine in patients with advanced or metastatic gastric cancer. Am J Clin Oncol 35: 237–241. [DOI] [PubMed] [Google Scholar]
- 33. Moon YW, Rha SY, Jeung HC, Kim C, Hong MH, et al. (2010) Outcomes of multiple salvage chemotherapy for advanced gastric cancer: implications for clinical practice and trial design. Cancer Chemother Pharmacol 66: 797–805. [DOI] [PubMed] [Google Scholar]
- 34. Moehler M, Mueller A, Trarbach T, Lordick F, Seufferlein T, et al. (2011) Cetuximab with irinotecan, folinic acid and 5-fluorouracil as first-line treatment in advanced gastroesophageal cancer: a prospective multi-center biomarker-oriented phase II study. Ann Oncol 22: 1358–1366. [DOI] [PubMed] [Google Scholar]
- 35. Koizumi W, Takiuchi H, Yamada Y, Boku N, Fuse N, et al. (2010) Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol 21: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 36. Amarantidis K, Xenidis N, Chelis L, Chamalidou E, Dimopoulos P, et al. (2011) Docetaxel plus oxaliplatin in combination with capecitabine as first-line treatment for advanced gastric cancer. Oncology 80: 359–365. [DOI] [PubMed] [Google Scholar]
- 37. Edler D, Hallstrom M, Johnston PG, Magnusson I, Ragnhammar P, et al. (2000) Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin Cancer Res 6: 1378–1384. [PubMed] [Google Scholar]
- 38. Popat S, Matakidou A, Houlston RS (2004) Thymidylate synthase expression and prognosis in colorectal cancer: a systematic review and meta-analysis. J Clin Oncol 22: 529–536. [DOI] [PubMed] [Google Scholar]
- 39. Ott K, Vogelsang H, Marton N, Becker K, Lordick F, et al. (2006) The thymidylate synthase tandem repeat promoter polymorphism: A predictor for tumor-related survival in neoadjuvant treated locally advanced gastric cancer. Int J Cancer 119: 2885–2894. [DOI] [PubMed] [Google Scholar]
- 40. Kawakami K, Graziano F, Watanabe G, Ruzzo A, Santini D, et al. (2005) Prognostic role of thymidylate synthase polymorphisms in gastric cancer patients treated with surgery and adjuvant chemotherapy. Clin Cancer Res 11: 3778–3783. [DOI] [PubMed] [Google Scholar]
- 41. Okuno T, Tamura T, Yamamori M, Chayahara N, Yamada T, et al. (2007) Favorable genetic polymorphisms predictive of clinical outcome of chemoradiotherapy for stage II/III esophageal squamous cell carcinoma in Japanese. Am J Clin Oncol 30: 252–257. [DOI] [PubMed] [Google Scholar]
- 42. Lordick F, Lorenzen S, Stollfuss J, Vehling-Kaiser U, Kullmann F, et al. (2005) Phase II study of weekly oxaliplatin plus infusional fluorouracil and folinic acid (FUFOX regimen) as first-line treatment in metastatic gastric cancer. Br J Cancer 93: 190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, et al. (2004) Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer 91: 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, et al. (2003) Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol 21: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 45. Yeh KH, Cheng AL, Wan JP, Lin CS, Liu CC (2004) Down-regulation of thymidylate synthase expression and its steady-state mRNA by oxaliplatin in colon cancer cells. Anticancer Drugs 15: 371–376. [DOI] [PubMed] [Google Scholar]
- 46. Chen CC, Chen LT, Tsou TC, Pan WY, Kuo CC, et al. (2007) Combined modalities of resistance in an oxaliplatin-resistant human gastric cancer cell line with enhanced sensitivity to 5-fluorouracil. Br J Cancer 97: 334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fukushima M, Sakamoto K, Ohshimo H, Nakagawa F, Taguchi T (2010) Irinotecan overcomes the resistance to 5-fluorouracil in human colon cancer xenografts by down-regulation of intratumoral thymidylate synthase. Oncol Rep 24: 835–842. [DOI] [PubMed] [Google Scholar]
- 48. Torigoe S, Ogata Y, Matono K, Shirouzu K (2009) Molecular mechanisms of sequence-dependent antitumor effects of SN-38 and 5-fluorouracil combination therapy against colon cancer cells. Anticancer Res 29: 2083–2089. [PubMed] [Google Scholar]
- 49. Toriumi F, Kubota T, Saikawa Y, Yoshida M, Otani Y, et al. (2004) Thymidylate synthetase (TS) genotype and TS/dihydropyrimidine dehydrogenase mRNA level as an indicator in determining chemosensitivity to 5-fluorouracil in advanced gastric carcinoma. Anticancer Res 24: 2455–2463. [PubMed] [Google Scholar]
- 50. Li Y, Yan PW, Huang XE, Li CG (2011) MDR1 gene C3435 T polymorphism is associated with clinical outcomes in gastric cancer patients treated with postoperative adjuvant chemotherapy. Asian Pac J Cancer Prev 12: 2405–2409. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Tables.
(DOCX)
TREND Checklist.
(PDF)
Trial Protocol.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.