Abstract
Immune responses against lung-associated self-antigens (self-Ags) are hypothesized to play a role in the development of chronic lung graft rejection. We determined whether immune responses to lung self-Ags, K-alpha-1-tubulin (Kα1T) and Collagen V (Col-V) in the absence of alloimmunity, could promote airway inflammation and fibrosis. Following syngeneic murine orthotopic lung transplantation (LTx) we administered antibodies (Abs) to either Kα1T or Col-V or in combination to both of these self-Ags. As compared to recipients of isotype control Abs Kα1T Abs and/or Col-V Abs-treated recipients had marked lung graft cellular infiltration and bronchiolar fibrosis, This inflammation was also associated the accumulation of Kα1T and Col-V specific IFN-γ+ and IL-17+ T cells. Notably, the administration of Abs to Kα1T led to cellular and humoral immune responses to Col-V prior to development of fibrosis, and vice versa, indicating that epitope spreading can occur rapidly in an alloantigen independent manner. Collectively, these data support a model of chronic lung transplant rejection where the progressive loss of self-tolerance through epitope spreading promotes airway fibrosis. Strategies that target autoreactive Abs may be useful to inhibit chronic rejection of lung grafts.
INTRODUCTION
Long term outcomes following lung transplantation (LTx) remain poor due to development of chronic rejection (1, 2), clinically diagnosed as bronchiolitis obliterans syndrome (BOS). BOS is a fibro-proliferative process characterized by progressive decline in lung function. Several immunological and non-immunological factors have been attributed to BOS (3–7). The link between alloimmunity and chronic rejection is well recognized. This relationship is best exemplified by the finding that acute rejection is a major risk factor for chronic rejection (8). We demonstrated that antibodies (Abs) against self-antigens (self-Ags) such as K-alpha-1tubulin (Kα1T) and Collagen V (Col-V) often precede development of rejection (9). We also reported that preemptive Ab depletion in patients with detectable donor specific antibodies (DSA) post-LTx in having normal lung function lowers the risk for chronic rejection (6). However, some patients still developed BOS, despite clearance of DSA. These patients had persistence of Abs to self-Ags. On the other hand, in patients where both DSA and Abs to self-Ags were cleared, there was freedom from BOS suggesting self-Ags may play a pivotal role in the development of chronic rejection.
A link between alloimmunity and immune responses to self-Ags and chronic rejection has been proposed (9, 10). Earlier studies demonstrated that Abs to Kα1T can bind to epithelial cells, activate pro-inflammatory and pro-fibrotic growth factor signaling (11). Oral tolerance to Col-V has been shown to prevent rejection in rat lung allografts (12). Hence, we postulated that immune responses to self-Ags alone may play a pathogenic role for development of chronic lung rejection.
To define the effects of immune responses to self-Ags in the absence of alloimmune responses, we performed syngeneic mouse LTx (13). Syngeneic grafts have no evidence of inflammation for greater than 45 days whereas allografts were rejected by day 7 (13). Our results indicate that administration of Abs to lung associated self-Ags can lead to both cellular and humoral immune responses to other self-Ags expressed in lungs leading to inflammation and fibrosis in the transplanted lung.
MATERIALS AND METHODS
Animals and LTx
Six to eight week old male C57Bl/6 (H-2kb) were obtained (Jackson Laboratories, Bar Harbor, ME). Orthotopic left LTx was performed using cuff technique (13). For sham experiments, mice were ventilated for 1 hour (duration of mouse LTx). All animal studies performed with sterile precautions and approved by the Animal Studies Committee at Washington University School of Medicine.
Antibodies to Kα1T and Col-V
Rabbit polyclonal IgG Abs to Kα1T and Col-V were produced against Kα1T and Col-V proteins. Analysis of the specificity of the Abs were done by ELISA with plates coated with purified proteins (Col-V, Col-I and Col-II) (optical densities for Col-V: 0.863, Col-I: 0.124 and Col-II: 0.109). Purified Abs were endotoxin free by limulus amebocyte lysate assay. Abs to Kα1T or Col-V or both (n=5 per group) were administered intraperitoneally following LTx and to sham surgery mice (200μg/dose) on days 0 and weekly thereafter. Rabbit IgG was used as control.
Histology
Mice were sacrificed on day 45 following LTx. Sections were stained with hematoxylin-eosin and trichrome and analyzed blindly. Images were obtained on a Nikon Eclipse microscope (Nikon), and morphometric analysis performed using Nikon Elements software (Nikon).
Enzyme Linked Immunosorbent Assay (ELISA) for auto-Abs
Development of Abs to self-Ags Kα1T, Col-V, Col-I and Col-II was determined by ELISA using 30 and 45 days post-transplant sera (14). Concentration of Abs was calculated from a standard curve of known concentration of Abs and expressed as concentration in μg/mL.
Enzyme-linked immunospot (ELISpot) for cellular immune responses to Kα1T and Col-V
Cellular immune responses to self-Ags Kα1T, Col-I and Col-V and control Col-II were enumerated using ELISpot (14). All antigens were endotoxin free. Cells cultured in medium or with phytohemagglutinin were used as negative and positive controls respectively. Results were represented as mean spots per million cells (spm) ±SD.
Col-V polyclonal antisera preadsorption to Col-I
1mg of human Col-I (Cell Sciences #CRC194) was added to 1ml of Affigel 15 (Biorad # 153-6051) and incubated 4 hours at 4°C. The beads were washed thrice with PBS (1500rpm for 5 min). Four mg of anti-Col-V was added to the beads loaded with Col-I protein and incubated overnight at 4°C, centrifuged (1500rpm for 5 min) at 4°C and the supernatant containing Abs specific to Col-V was collected. 500ng of recombinant Col-V (Sigma #C3657) and Col-I were loaded into 4–20% acrylamide gel and immuno-blotted with pre-absorbed and post-absorbed anti-Col-V. Absorption with Col-I resulted in complete depletion of Col-I Abs.
Statistical analysis
Morphometric analysis of tissue sections was performed using NIS-Elements Software (Nikon, Melville, NY). Analysis was performed on 5 different areas of the lungs and at least 5 fields were analyzed for each and the data presented as the average. Data was analyzed in SPSS v.19 (IBM Inc., Chicago, IL) and GraphPad Prism 5.0 (LaJolla, CA) and represented at mean±SD with at least 5 mice in each cohort. ChiSquare tests or Student T-tests were used as appropriate. Significance was set at p<0.05.
RESULTS
Administration of Abs to lung associated self-Ags (Kα1T and Col-V) induces airway inflammation and fibrosis following syngeneic murine LTx
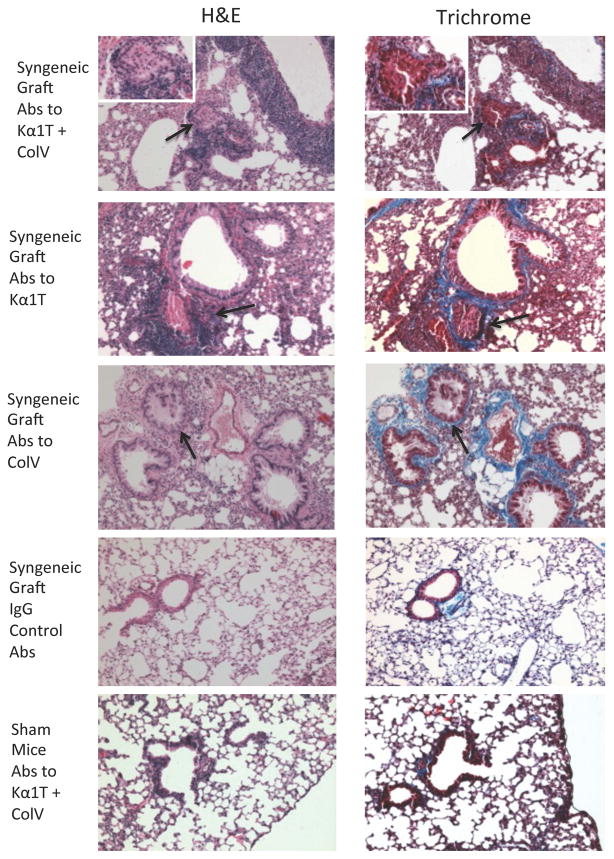
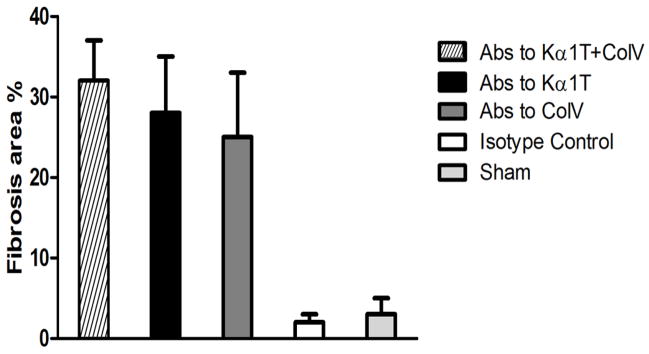
To determine whether administration of Abs to lung associated self-Ags in the absence of alloimmunity can lead to the airway fibrosis following murine LTx, Abs to either Col-V or Kα1T or both or control IgG were administered following syngeneic LTx. Syngeneic mouse LTx have demonstrated long-term graft survival (>45 days) with no evidence of rejection (13, 15). In contrast, histopathology of the transplanted lungs on day 45 demonstrated that 4 out of 5 mice that received Abs to both Kα1T and Col-V developed cellular infiltration around bronchioles and fibrosis (Table 1, Figures 1 & 2). Isotype control mice did not demonstrate any lesions (Figure 1) and had >45 days survival. Sham control also did not develop lesions (Figure 1). Lesions were limited to the transplanted left lungs and right lungs were histologically normal suggesting that LTx induced changes are necessary to expose self-Ags so that Abs can bind (16).
Table 1.
Prevalence of airway inflammation and fibrosis in mice at day 45 following administration of either Abs to Kα1T or Col-V or both or isotype control or sham surgery mice
| Mouse group | Airway inflammation | Fibrosis |
|---|---|---|
| Abs to Col-V+Kα1T | 4/5 (80%) | 4/5 (80%) |
| Abs to Kα1T | 4/5 (80%) | 4/5 (80%) |
| Abs to Col-V | 4/5 (80%) | 4/5 (80%) |
| Isotype control Abs | 1/5 (20%) | 1/5 (0%) |
| Sham mice | 1/5 (20%) | 0/5 (0%) |
Figure 1.
Representative histology on day 45 from mice that underwent syngeneic LTx (Bl/6 to Bl/6) and were administered Abs to Kα1T and Col-V, or Kα1T alone, or Col-V alone or isotype control Abs, or sham surgery (ventilation alone) mice that were administered Abs to Kα1T and Col-V. Figures on left represent H&E stains and right represent Trichrome stains. Images at 10x magnification and insets at 40x. Five mice in each group.
Figure 2.
Morphometric analysis of lung tissue slides with trichrome stains to quantify extent of fibrosis. Histological images were captured on a Nikon Eclipse microscope and analyzed by Nikon Elements software. Images from transplanted lung tissue in at least 3 different areas for each mouse in each group (total of 5 mice per group) were analyzed to quantify peribronchial fibrotic (blue areas around bronchioles in trichrome stains) per high-power field and represented as a percentage area of total.
Pathology is limited to transplanted lungs and does not affect other organs
To confirm that the administration of Abs to self-Ags following LTx affects the transplanted lung alone, and does not have a systemic response, we analyzed heart, liver and kidney tissues from all mice. None of the other organs, including native right lungs, demonstrated any signs of pathology (data not shown). This demonstrates that Abs to self-Ags induce specific pro-inflammatory and pro-fibrotic response in the transplanted organ alone.
Increase in self-reactive CD4+ T cells and Abs in LTx treated with Abs to self-Ags
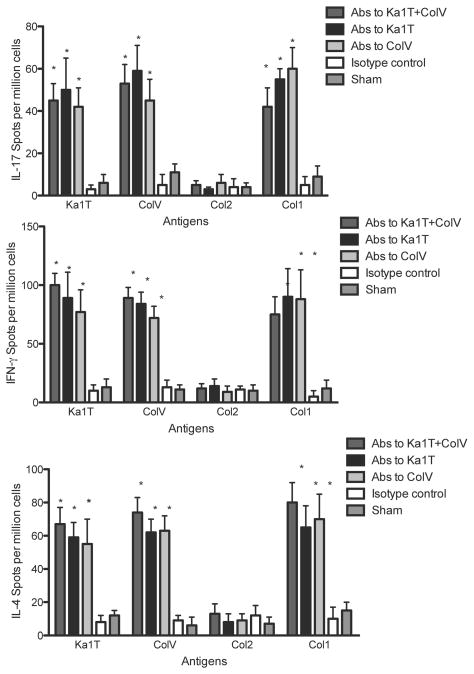
To determine the development of cellular responses against self-Ags, splenocytes from syngeneic LTx mice or sham control following Ab administration were isolated on day 45 and stimulated with Kα1T, Col-V, Col-I or Col-II. IFN-γ, IL-4, IL-17 secreting cells were enumerated using ELISpot. As shown in Figure 3, administration of Abs to self-Ags Kα1T and Col-V, either alone or in combination, resulted in a significant increase in IL-17 secreting cells to Kα1T and Col-V compared to control and sham mice (in spm±SD for Col-V+Kα1T, isotype control and sham Kα1T and Col-V: Col-V - 53±9 vs. 2±1 vs. 1±2, p=0.012; Kα1T - 67±10 vs. vs. 8±4 vs. 3±2, p=0.029). There was also a significant increase in self-Ag specific IFN-γ secreting cells (p=0.011) and IL-4 secreting cells (p=0.002) (Figure 3) in Abs administered LTx.
Figure 3.
Cellular immune responses against self-Ags. IL-17, IFN-γ, IL-10 and IL-4 ELISpot responses against Col-V, Kα1T, Col-II or Col-I in mice that underwent syngeneic LTx (Bl/6 to Bl/6) and were administered Abs to Kα1T and Col-V, or Kα1T alone, or Col-V alone or isotype control Abs, or sham surgery (ventilation alone) mice that were administered Abs to Kα1T and Col-V. Represented as mean spots per million cells of an average of three wells per mice with at least 5 mice in each group. *represents p<0.05 compared to isotype control and sham surgery mice.
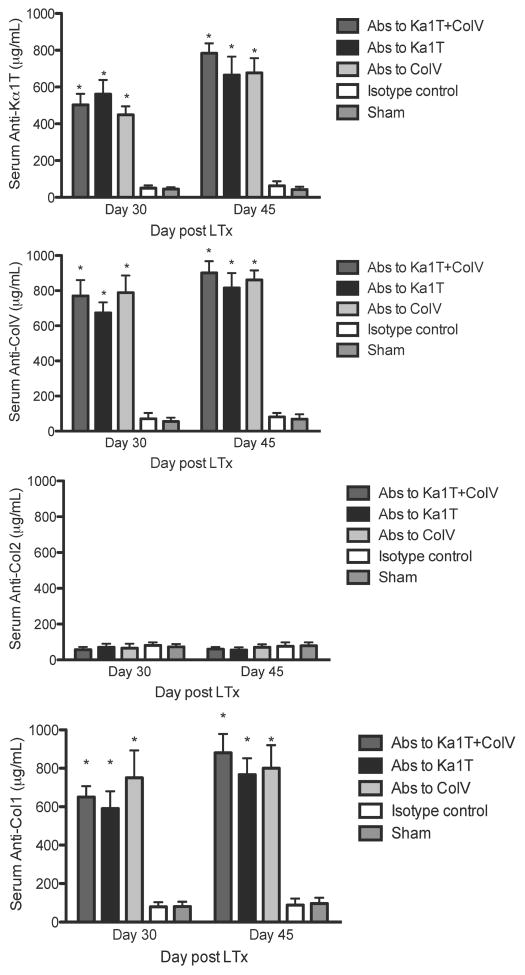
Another important finding is that administration of Abs to one of the self-Ags lead to immune responses to other tissue restricted self-Ags. As shown in Figure 3. Abs to self-Ags induced development of cellular responses (IL-17, IFN-γ and IL-4 secreting cells) not only to the self-Ags to which the Ab was specific but also to other self-Ag Col-I. In addition to cellular immune responses, the mice also developed humoral responses to other self-Ags (Figure 4).
Figure 4.
Humoral immune responses against self-Ags. ELISA to determine serum Abs (on day 30 and day 45) against Col-V, Kα1T, Col-II or Col-I in mice that underwent syngeneic LTx (Bl/6 to Bl/6) and were administered Abs to Kα1T and Col-V, or Kα1T alone, or Col-V alone or isotype control Abs, or sham surgery (ventilation alone) mice that were administered Abs to Kα1T and Col-V. Represented in μg/mL with at least 5 mice in each group. *represents p<0.05 compared to isotype control and sham surgery mice.
To eliminate the possibility the Col-I Ab reactivity within lung grafts was due to cross-reactivity to Col-I in our Col-V antiserum we preabsorbed these polyclonal Abs to Col-I proteins (Figure S1). Col-V antiserum preabsorbed to Col-I was then administered to three syngenic LTx and 30 days later lung grafts were analyzed for lesion development. Similar to recipients that received unabsorbed antiserum (Figure 4 versus Figure S2) recipients or preabsorbed serum also generated lesions. In addition, Abs (Figure S3) as well as IL-17 and IFN-γ-mediated T cell responses (data not shown) could be detected against Col-V, Kα1T and Col-I. However, there was no difference in Col-II specific IL-17, IFN-γ and IL-4 secreting cells. This demonstrates that Abs to self-Ags following LTx can induce both cellular and humoral immune responses to those self-Ags and also to other lung associated self-Ags in a tissue restricted manner.
One concern is that administration of rabbit Abs could result in the development of Abs against the rabbit proteins that could influence the outcome. However, ELISA performed using the serum from the LTx mice against plates coated with rabbit immunoglobulin (Sigma, St Louis, MO) demonstrated no detectable Abs against the rabbit immunoglobulins (OD 0.084).
Cellular immune responses to self-Ags precede the development of humoral immune responses
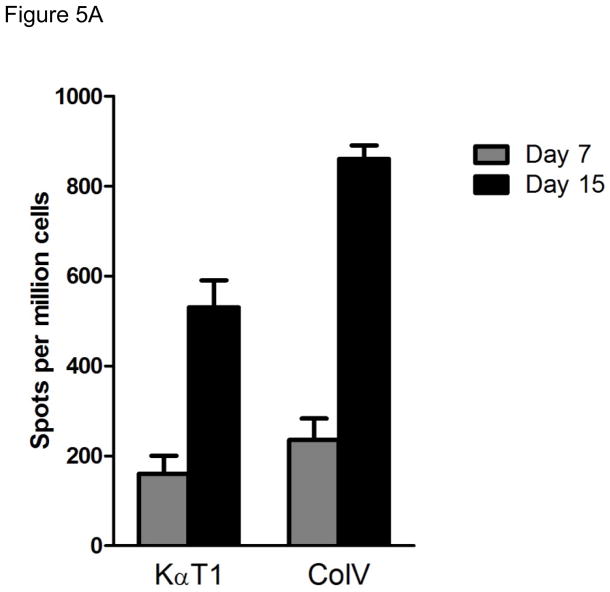
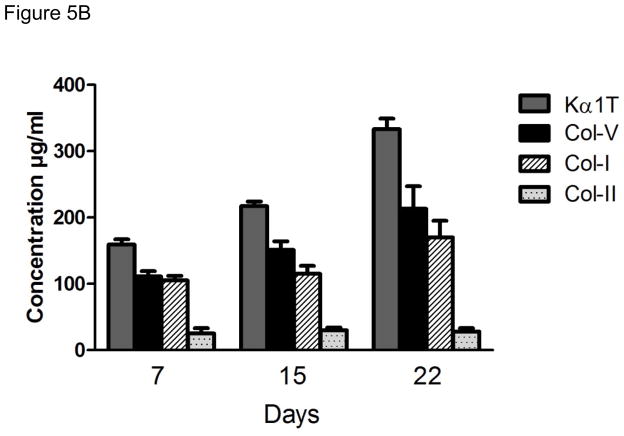
In order to determine the kinetics of cellular and humoral immune responses to self-Ags in the syngeneic murine LTx following administration of Abs to self-Ags we analyzed the cellular immune responses against Kα1T and Col-V on day 7 and 15 post-transplantation by ELISpot (IFN-γ and IL-4). Ab responses to Kα1T and Col-V were analyzed on day 7, 15 and 21 by ELISA. As shown in Figure 5A there was significant increases in the frequency of CD4 T cells secreting IFN-γ and IL-4 reactive to Kα1T (IFN-γ: 74±46, IL-4: 24±9) and Col-V (IFN-γ: 384±303, IL-4: 26±20) at day 15. However, Abs to Kα1T and Col-V in the sera of the mice were detectable only on day 21 post-LTx (Figure 5B). These results demonstrate that cellular immune responses to self-Ags precede humoral immunity.
Figure 5.
Kinetics of cellular and humoral immune responses against self-Ags. (A) IFN-γ, and IL-4 ELISpot responses against Col-V, and Kα1T, in mice that underwent syngeneic LTx (Bl/6 to Bl/6) and were administered Abs to Kα1T and Col-V were analyzed on day 7 and day 15. Represented as mean spots per million cells of an average of three wells per mice with at least 5 mice in each group. (B) ELISA was used to determine serum Abs (on day 7,15, 22) against Col-V, Kα1T, Col-II or Col-I in mice that underwent syngeneic LTx (Bl/6 to Bl/6) and were administered Abs to Kα1T and Col-V. Represented in μg/mL with at least 5 mice in each group.
Determination of reactivity to individual chains of Collagen V
In order to determine the reactivity of the sera to the individual Col-V chains, 1μg of the purified α1 and α2 chain of the Col-V (kindly provided by Dr. William Burlingham, University of Wisconsin) was electrophoresed on 4–12% Bis-Tris SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were blocked with 5% skim milk powder and probed with 1:250 diluted serum collected at 0, 7, 14, 21 and 28 days post-LTx following administration of Abs against Kα1T and Col-V and developed with HRP conjugated anti-mouse Ab. Western blot (Figure S4) demonstrated that mice administered with polyclonal Abs against self-Ags developed Abs against the α1 chain of the Col-V. There was no reactivity against the α2 chain of Col-V. These results demonstrate that Abs developed against the self-Ags in the mouse model of LTx is against the α1 chain of Col-V which is similar to that has been seen following human LTx.
Critical role of T cells in the induction of immune response to self-Ags and development of fibroproliferation
In order to determine the role of T cells in the development of immune responses to self-Ags and development of fibroproliferation, we performed syngeneic LTx in B6.129P2.Tcrbtm1MomTcrdtm1Mom/J mice (Jackson Laboratory, Bar Harbor, MA) and administered Abs to Kα1T and Col-V. Histopathology of the lungs on day 45 demonstrated no evidence of rejection including lack of cellular infiltration and lack of fibroproliferation (Figure 6). Analysis of the serum by ELISA was also negative against the self-Ags (Kα1T and Col-V). Further, T cell responses were undetectable by ELISpot against Kα1T and Col-V. These results demonstrate that T cell responses play a crucial role in the induction of immune response against self-Ags and development of fibroproliferation.
Figure 6.
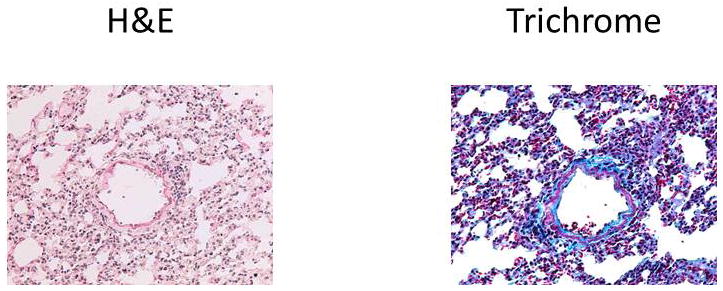
Representative histology on day 45 from mice that underwent syngeneic LTx (Bl/6 to B6.192P2.Tcrbtm1Mom Tcrdtm1Mom/J) and were administered Abs to Kα1T and Col-V. Figures on left represent H&E stains and right represent Trichrome stains. Images at 10x magnification. Five mice in each group.
DISCUSSION
Lung transplantation is lifesaving in patients with end stage pulmonary diseases. However, long term outcomes continue to be affected by chronic rejection (BOS) (1). Both immune and non-immune risk factors have been linked to development of chronic rejection following human LTx. Chronic rejection is characterized by immune mediated tissue inflammatory responses that lead to cellular infiltration, damage to parenchyma leading to fibroproliferative changes around the terminal vessels and bronchioles. There is growing evidence that besides alloimmune responses (5, 17, 18) immune responses developed against lung associated self-Ags – Kα1T and Col-V (6, 9, 10, 19) may play an important role in the immunopathogenesis of BOS following human LTx. In this study, we demonstrate that immune responses to lung tissue restricted self-Ags alone in the absence of alloimmune responses can induce inflammation and fibroproliferative changes. Administration of Abs to Kα1T, Col-V alone or in combination resulted in cellular infiltration, inflammation and fibrosis around the terminal airways. Further, there was induction of self-Ag specific IL-17 and IFN-γ (Figure 3) responses following syngeneic LTx. In our earlier studies we observed that administration of anti-keratin Abs into the native lung does not induce OAD lesions (14).
An important finding is that administration of Abs specific to one of the lung associated self-Ag lead to “spreading” of immune responses to other self-Ags, i.e. mice administered Abs against Kα1T developed both cellular and humoral immune response not only to Kα1T but also to Col-V and Col-I. This was, however, a tissue-restricted response as seen by an absence of immune responses to Col-II, which is not expressed much in the lung tissues as well as the lack of inflammation in other organs. In order to rule out that this is not due to cross reactivity to Col-I in the polyclonal Abs to Col-V administered, we performed absorption of the sera with Col-I. The resulting polyclonal containing only Col-V specificity when administered to murine LTx demonstrated OAD with fibrosis on day 30 and further developed Abs to Kα1T as well as Col-I. This strongly suggests that the induction of immune responses to Col-I is not due to cross reactivity but due to spreading of the immune responses. Further, the development of Ab responses to Col-V was restricted to α1 chain of Col-V as seen in human LTx.
In a previous study on LTx patients – presence of pre-transplant Abs to Kα1T or, Col-V increased the risk of PGD and BOS. Thus, when patients develop de novo Abs against one of these Ags, the inflammatory response may facilitate the induction of IL-17 and IFN-γ response to other self-Ags that may further perpetuate each other leading to chronic rejection. Studies have also demonstrated that immune responses to cardiac myosin play a crucial role in chronic rejection of heart allografts (20, 21) that is mediated through the production of auto-Abs (22). Further, immunization with cardiac myosin can lead to induction of chronic rejection in a syngeneic heart transplant model (23) and modulation of immune responses against cardiac myosin can prolong the heart graft survival (22). Although, in this study, we determined immune responses against only Kα1T, Col-V and Col-I, it is possible that there may be development of autoimmune response against other unknown lung self-Ags.
The mechanisms by which Abs against self-Ags are induced, or how they act once formed are not well understood. Absence of any immunological or pathological changes in sham animals (ventilation alone) suggests that early post-LTx inflammatory response may be necessary. Ischemia reperfusion injury during the transplant may lead to exposure of cryptic self-Ags to the immune system. There is evidence that following rat LTx Col-V is released into bronchoalveolar lavage fluid (16, 24). It is important to note that such a phenomenon is not only limited to allografts but also in syngeneic grafts (16). Hence, we hypothesize that Abs administered to syngeneic LTx have the capacity to bind the exposed self-Ags resulting in pro-inflammatory and pro-fibrotic responses. The critical role for autoimmune responses in the peri-transplant period influencing such changes is supported by studies in human LTx where pre-existing autoimmunity prior to transplant increased the risk of PGD, alloimmunity and BOS (10).
Th17 cells specific to self-Ags play an important role in development of self-Ags specific immune responses. Although we specifically didn’t block Th17, previous studies from our laboratory, and others, have shown that blocking of Th17 can prevent OAD (14, 25). In addition, the balancing role of IFN-γ and IL-17 responses in development of self-Ag induced airway fibroproliferative responses is an area of future investigation.
It is still not clear how the immune response spread to other tissue-restricted antigens. We propose that once Abs are bound to one of the self-Ags, it leads to opsonization of the membrane by Ag presenting cells leading to further activation, phenotypic switch leading to immune response to all the antigens present on the phagocytized membrane leading to spreading of immune responses to other self-Ags, and likely to alloantigen’s in allografts. These mechanistic studies are currently being investigated.
In conclusion, this study demonstrates that immune responses to lung associated self-Ags in the absence of alloimmunity can lead to airway inflammation and fibrosis in murine syngeneic transplants. This supports the hypothesis that de novo development of Abs to lung associated self-Ags following LTx can induce cellular immune responses which predisposes to development of chronic rejection. Strategies to detect and deplete such Abs during post-transplant may represent a novel approach to prevent BOS following human LTx.
Supplementary Material
Acknowledgments
This work was supported by NIH HL092514 and the BJC Foundation (TM). NIH HL094601 (AG). The authors would like to thank Ms. Billie Glasscock for assistance in preparation of this manuscript.
Abbreviations
- Abs
antibodies
- BOS
bronchiolitis obliterans syndrome
- Col-V
Collagen V
- DSA
donor specific antibodies
- Kα1T
K-alpha-1-Tubulin
- LTx
lung transplant
- self-Ags
self-antigens
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 2011 Oct;30(10):1104–22. doi: 10.1016/j.healun.2011.08.004. Epub 2011/10/04. eng. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010 Oct;29(10):1104–18. doi: 10.1016/j.healun.2010.08.004. Epub 2010/09/28. eng. [DOI] [PubMed] [Google Scholar]
- 3.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007 Mar 1;175(5):507–13. doi: 10.1164/rccm.200608-1079OC. Epub 2006/12/13. eng. [DOI] [PubMed] [Google Scholar]
- 4.Davis RD, Jr, Lau CL, Eubanks S, Messier RH, Hadjiliadis D, Steele MP, et al. Improved lung allograft function after fundoplication in patients with gastroesophageal reflux disease undergoing lung transplantation. J Thorac Cardiovasc Surg. 2003 Mar;125(3):533–42. doi: 10.1067/mtc.2003.166. Epub 2003/03/27. eng. [DOI] [PubMed] [Google Scholar]
- 5.Girnita AL, Duquesnoy R, Yousem SA, Iacono AT, Corcoran TE, Buzoianu M, et al. HLA-specific antibodies are risk factors for lymphocytic bronchiolitis and chronic lung allograft dysfunction. Am J Transplant. 2005 Jan;5(1):131–8. doi: 10.1111/j.1600-6143.2004.00650.x. Epub 2005/01/08. eng. [DOI] [PubMed] [Google Scholar]
- 6.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to K-alpha 1 Tubulin and Collagen V Are Associated With Chronic Rejection After Lung Transplantation. Am J Transplant. 2012 Aug;12(8):2164–71. doi: 10.1111/j.1600-6143.2012.04079.x. Epub 2012/05/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. Am J Respir Crit Care Med. 2004 Jul 15;170(2):181–7. doi: 10.1164/rccm.200310-1359OC. Epub 2004/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 8.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002 Feb;21(2):271–81. doi: 10.1016/s1053-2498(01)00360-6. Epub 2002/02/09. eng. [DOI] [PubMed] [Google Scholar]
- 9.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011 Jun;30(6):624–31. doi: 10.1016/j.healun.2011.01.708. Epub 2011/03/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010 Oct;90(4):1094–101. doi: 10.1016/j.athoracsur.2010.06.009. Epub 2010/09/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiriveedhi V, Gelman AE, Mohanakumar T. HIF-1alpha signaling by airway epithelial cell K-alpha1-tubulin: role in fibrosis and chronic rejection of human lung allografts. Cell Immunol. 2012;273(1):59–66. doi: 10.1016/j.cellimm.2011.11.006. Epub 2011/12/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Y, Sekine Y, Yoshida S, Yasufuku K, Petrache I, Benson HL, et al. Type V collagen-induced oral tolerance plus low-dose cyclosporine prevents rejection of MHC class I and II incompatible lung allografts. J Immunol. 2009 Jul 1;183(1):237–45. doi: 10.4049/jimmunol.0804028. Epub 2009/06/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krupnick AS, Lin X, Li W, Okazaki M, Lai J, Sugimoto S, et al. Orthotopic mouse lung transplantation as experimental methodology to study transplant and tumor biology. Nat Protoc. 2009;4(1):86–93. doi: 10.1038/nprot.2008.218. Epub 2009/01/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009 Jan 1;182(1):309–18. doi: 10.4049/jimmunol.182.1.309. Epub 2008/12/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Vleeschauwer S, Jungraithmayr W, Wauters S, Willems S, Rinaldi M, Vaneylen A, et al. Chronic rejection pathology after orthotopic lung transplantation in mice: the development of a murine BOS model and its drawbacks. PLoS One. 2012;7(1):e29802. doi: 10.1371/journal.pone.0029802. Epub 2012/01/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006 Apr;6(4):724–35. doi: 10.1111/j.1600-6143.2006.01236.x. Epub 2006/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 17.Palmer SM, Davis RD, Hadjiliadis D, Hertz MI, Howell DN, Ward FE, et al. Development of an antibody specific to major histocompatibility antigens detectable by flow cytometry after lung transplant is associated with bronchiolitis obliterans syndrome. Transplantation. 2002 Sep 27;74(6):799–804. doi: 10.1097/00007890-200209270-00011. Epub 2002/10/05. eng. [DOI] [PubMed] [Google Scholar]
- 18.Angaswamy N, Saini D, Ramachandran S, Nath DS, Phelan D, Hachem R, et al. Development of antibodies to human leukocyte antigen precedes development of antibodies to major histocompatibility class I-related chain A and are significantly associated with development of chronic rejection after human lung transplantation. Hum Immunol. 2010 Jun;71(6):560–5. doi: 10.1016/j.humimm.2010.02.021. Epub 2010/03/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007 Nov;117(11):3498–506. doi: 10.1172/JCI28031. Epub 2007/10/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolls HK, Kishimoto K, Dong VM, Illigens BM, Sho M, Sayegh MH, et al. T-cell response to cardiac myosin persists in the absence of an alloimmune response in recipients with chronic cardiac allograft rejection. Transplantation. 2002 Oct 15;74(7):1053–7. doi: 10.1097/00007890-200210150-00028. [DOI] [PubMed] [Google Scholar]
- 21.Rolls HK, Kishimoto K, Illigens BM, Dong V, Sayegh MH, Benichou G, et al. Detection of cardiac myosin-specific autoimmunity in a model of chronic heart allograft rejection. Transplantation proceedings. 2001 Nov-Dec;33(7–8):3821–2. doi: 10.1016/s0041-1345(01)02617-3. [DOI] [PubMed] [Google Scholar]
- 22.Fedoseyeva EV, Kishimoto K, Rolls HK, Illigens BM, Dong VM, Valujskikh A, et al. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. Journal of immunology. 2002 Aug 1;169(3):1168–74. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 23.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. Journal of immunology. 1999 Jun 1;162(11):6836–42. [PubMed] [Google Scholar]
- 24.Haque MA, Mizobuchi T, Yasufuku K, Fujisawa T, Brutkiewicz RR, Zheng Y, et al. Evidence for immune responses to a self-antigen in lung transplantation: role of type V collagen-specific T cells in the pathogenesis of lung allograft rejection. J Immunol. 2002 Aug 1;169(3):1542–9. doi: 10.4049/jimmunol.169.3.1542. Epub 2002/07/23. eng. [DOI] [PubMed] [Google Scholar]
- 25.Fan L, Benson HL, Vittal R, Mickler EA, Presson R, Fisher AJ, et al. Neutralizing IL-17 prevents obliterative bronchiolitis in murine orthotopic lung transplantation. Am J Transplant. 2011 May;11(5):911–22. doi: 10.1111/j.1600-6143.2011.03482.x. Epub 2011/04/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.