Abstract
The alternate and optimized syntheses of the parent opioid fentanyl and its analogs are described. The routes presented exhibit high-yielding transformations leading to these powerful analgesics after optimization studies were carried out for each synthetic step. The general three-step strategy produced a panel of four fentanyls in excellent yields (73–78%) along with their more commonly encountered hydrochloride and citric acid salts. The following strategy offers the opportunity for the gram-scale, efficient production of this interesting class of opioid alkaloids.
Introduction
Very few synthetic drugs generate an immediate and powerful impact in the biomedical field shortly after their inception. This has been the case particularly within the areas of pre-surgical, surgical, and post-surgical anesthesiology where the need for fast acting, effective pain relievers is a key element in the overall patient care practice. Morphine (1) and Tramadol (2) (Fig. 1) are two opioid-based compounds that are widely recognized for being the gold standard prescriptions for patients with moderate to severe pain after surgery or with certain disease states (e.g. cancer) [1]–[6]. Due to their potency, these therapeutics, however, are also well known for their ability to foster chemical dependencies in patients and other users [7].
Figure 1. Various commonly administered opioids: (1) morphine, (2) Tramadol, (3) Demerol, and (4) fentanyl.
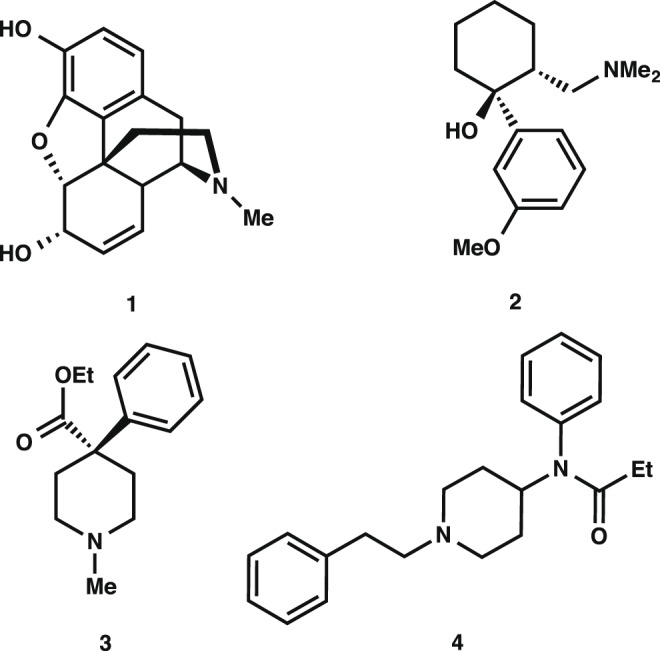
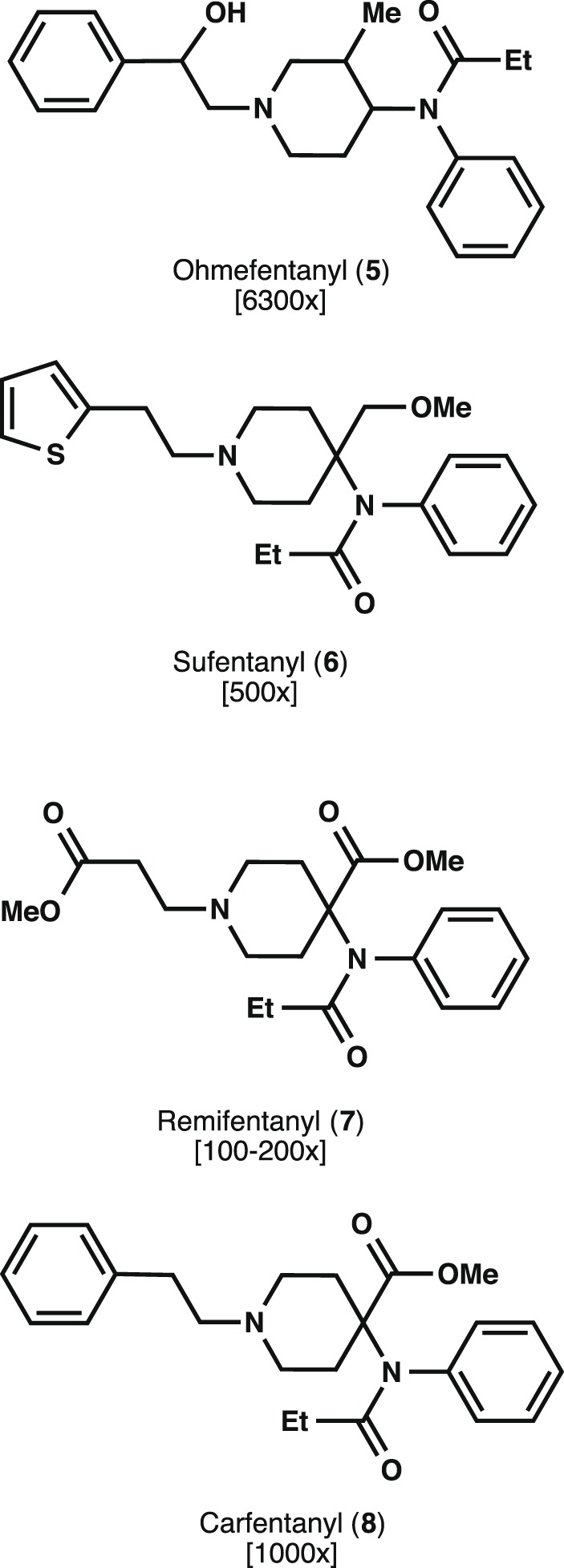
Though it is often difficult to surpass the established therapeutic records and efficiency profiles by the aforementioned drugs, occasionally new drug candidates are identified that accomplish this seemingly difficult feat. Such is the case for a class of synthetic alkaloids whose birth and swift entrance in the medical field of anesthesiology originated with the synthesis of fentanyl (4, Fig. 1) by Paul Janssen in 1960 [8]–[11]. Since its synthesis, inspired partly by the necessity to improve the potency and bioavailability of the structurally related opiate Demerol (3), fentanyl analogs with superior pharmacokinetic properties, onset time, and effective dosage have been successfully produced [12], [13]. Currently, a significant array of fentanyl analogs exists spanning a large range of physicochemical properties, which strictly determine their ultimate application. Some of these compounds, along with their potency relative to morphine, are given in Fig. 2 [12], [14].
Figure 2. Fentanyl analogs and their potencies relative to morphine.
With drugs of this kind, propensity of their users to become physiologically dependent has been reported, and indeed there exist issues involving the use of fentanyl and its analogs [15], [16]. For example, these compounds have been the epicenter of fatal incidents involving overdoses by users who self-administer quantities that are just minimally beyond the carefully prescribed doses for controlling pain in a clinical setting. Additionally, there has been documented military misuse of these compounds for their crowd controlling properties. As a particularly infamous case, the presumed use of gaseous/aerosolized fentanyl derivatives by Russian security forces to incapacitate terrorists during a Moscow theater hostage crisis in 2002 led to the death of 170 people, 127 of them hostages [17]–[21]. The powerful effects of these compounds at such low doses combined with the lack of medical training in cases of illicit use make these drugs extremely dangerous outside the clinical environment.
Fentanyl (prescribed more commonly by its trade name Sublimaze) is approximately 50–100 times more potent than morphine, a quality that has righteously cemented this drug and its congeners in the medical field as the primary choice for a fast acting anesthetic during perioperative procedures. Their modus operandi is believed to involve the binding to the transmembrane µ-opioid receptors on cell surfaces resulting in a cascade of intracellular signals that eventually results in their biological effect [22], [23]. Even though to date a detailed description of this receptor binding event remains undiscovered, a suitable model can be proposed based on the known binding of similar opioids to various nociceptive/opioid receptors for which few crystallographic structures have been solved [24]–[27].
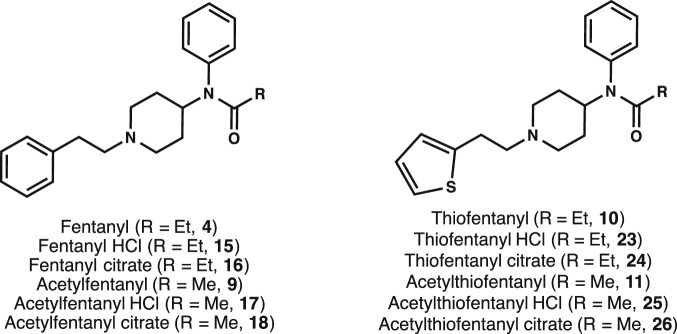
Due to the importance of this class of opioids in the biomedical field as well as their history of illicit use, it is not surprising that several synthetic routes have been devised for their construction since Janssen’s original disclosure [28]–[31]. However, most of these routes focus on specific transformations along the original sequence to eventually provide fentanyl (4) in moderate yields. The need to understand fentanyl receptor activation and to develop potential countermeasures for illicit use coupled to the lack of established procedures for procuring high quality materials in gram quantities prompted us to revisit and optimize the synthetic route for parent fentanyl 4 along with additional analogs. The routes described herein were optimized to obtain fentanyls in high yields using an efficient, three-step synthetic strategy. The four analogs that our efforts focused on are: fentanyl (4), acetylfentanyl (9), thiofentanyl (10) and acetylthiofentanyl (11). The opioids were synthesized as the free bases as well as their more clinically relevant hydrochloride and citric acid salts (Fig. 3).
Figure 3. Fentanyl analogs synthesized in this work.
Materials and Methods
Solvents used during the syntheses were removed by using a Büchi rotary evaporator R-200 equipped with a Büchi heating bath B-490 and coupled to a KNF Laboport Neuberger UN820 vacuum pump. Analytical thin layer chromatography (TLC) was conducted on Agela Technologies silica gel glass plates coupled with detection ceric ammonium molybdate (CAM), exposure to iodine vapor and/or UV light (λ = 254 nm). 1H NMR (600 MHz) and 13C NMR (150 MHz) were recorded in CDCl3 and D2O. Spectra were obtained using a Bruker Avance III 600 MHz instrument equipped with a Bruker QNP 5 mm cryoprobe (Bruker Biospin, Billerica, MA) at 30.0±0.1°C. NMR data is reported as follows: chemical shift (δ) (parts per million, ppm); multiplicity: s (singlet), d (doublet), t (triplet), q (quartet) and br (broad); coupling constants (J) are given in Hertz (Hz). 1H NMR chemical shifts are calibrated with respect to residual chloroform in CDCl3 centered at 7.26 ppm, whereas for 13C NMR, the center peak for CDCl3, centered at 77.0 ppm, was used for the calibration. All NMR spectra can be found in Information S1. HRMS analyses were obtained at the Forensic Science Center at the Lawrence Livermore National Laboratory using either Chemical Ionization (CI) or Electrospray Ionization (ESI). Elemental analyses were conducted at Galbraith Laboratories (Knoxville, TN).
Results and Discussion
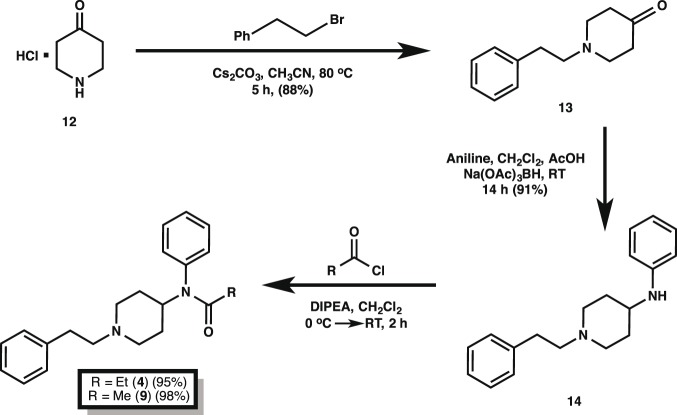
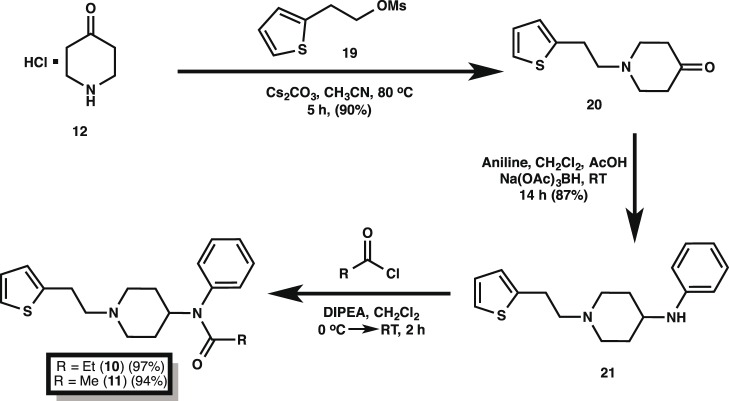
Our final, optimized synthetic path to fentanyl (4) is outlined in Fig. 4 and it begins with the alkylation of commercially available 4-piperidone monohydrate hydrochloride 12 with 2-(bromoethyl)benzene in the presence of cesium carbonate to furnish alkylated piperidone 13 in 88% yield. Reductive amination with aniline of 13 mediated by sodium triacetoxyborohydride in the presence of acetic acid yielded the 4-piperidineamine precursor 14 in excellent yield (91%). Lastly, piperidineamine 14 was acylated using propionyl chloride in the presence of Hunig’s base to provide fentanyl (4) in 95% yield. Likewise, piperidineamine 14 was treated with acetic anhydride in the presence of Hunig’s base to provide acetylfentanyl (9) in 98% yield. Conversion of 4 and 9 into their hydrochloride and citrate salts proceeded smoothly in nearly quantitative yields (Fig. 3). The synthesis of the thiofentanyl analogs was accomplished in a similar fashion as shown in Fig. 5. Thus, 4-piperidone monohydrate hydrochloride 12 was alkylated with 2-(thiophen-2-yl)ethyl methanesulfonate (19) [32] in the presence of cesium carbonate to give N-[2-(2-thienyl)ethyl]-4-piperidinone (20) in 90% yield. Reductive amination with aniline of 20 with sodium triacetoxyborohydride and acetic acid yielded the 4-piperidineamine precursor 21 in 87% yield. Lastly, piperidineamine 21 was acylated using propionyl chloride to provide thiofentanyl (10) in 97% yield. Likewise, piperidineamine 21 was treated with acetic anhydride in the presence of Hunig’s base to provide acetylthiofentanyl (11) in 94% yield. As before, conversion of 10 and 11 to their respective hydrochloride and citric acid salts was accomplished smoothly in nearly quantitative yields (Fig. 3).
Figure 4. Synthesis of fentanyl and acetylthiofentanyl.
Yields reflect the isolated materials by column chromatography after each step and using the optimized conditions (cf. Table 1). Citrate and hydrochloride salts for each analog were obtained in nearly quantitative yields by treating the free bases at the end of these routes with the corresponding acids.
Figure 5. Synthesis of thiofentanyl and acetylthiofentanyl.
Yields reflect the isolated materials by column chromatography after each step and using the optimized conditions (cf. Table 1). Citrate and hydrochloride salts for each analog were obtained in nearly quantitative yields by treating the free bases at the end of these routes with the corresponding acids.
Due to the low-yielding characteristics of our initial attempts, we decided to explore optimization studies for the synthesis of fentanyl (4) and then apply these to the syntheses of the analogs. Several conditions for each one of the steps composing the overall sequence were considered and evaluated (Table 1). We deduced that optimal conditions discovered for the synthesis of 4 could be directly translated to the syntheses of fentanyls 9–11 as they all share a common synthetic pathway. Thus, it was found that the use of acetonitrile instead of dimethylformamide increased the yields of the first alkylation step from 72 to 88% (Table 1, entries 1 and 2). This was also observed during the synthesis of the thiofentanyl precursor (20) that made use of the mesylate (19) as the alkylating species where the yield markedly increased from 62 to 83% (Table 1, entries 3 and 4). For the reductive amination (RA) step, the need for an equimolar amount of acetic acid was noted as this resulted in the efficient conversion of ketone 13 into the piperidineamine precursor 14 in the presence of sodium triacetoxyborohydride (Table 1, entry 5) [33], [34]. Carrying out the reductive amination under the same conditions but switching the hydride source to either sodium cyanoborohydride or sodium borohydride resulted in significant loss of yield at room temperature (Table 1, entries 6 and 7). However, use of the latter hydride reagents under refluxing conditions (80°C) increased their yields significantly (Table 1, entries 8 and 9). Lastly, for the acylation step of the sequence, the use of either propanoyl chloride or propanoic anhydride resulted in nearly identical yields (95% vs. 94%) regardless of the solvent to carry out the transformation (pyridine or dichloromethane) (Table 1, entries 10–12).
Table 1. Optimization steps for the synthesis of fentanyl (4); aisolated yield; balkylation in the synthesis of thiofentanyl derivatives; creductive amination.
| Entry | Synthetic Step | Reagents/Conditions | T (°C) | Yielda (%) |
| 1 | Alkylation | PhCH2CH2Br, Cs2CO3, DMF | 80 | 72 |
| 2 | PhCH2CH2Br, Cs2CO3, CH3CN | 80 | 88 | |
| 3 | R-OMs (19), Cs2CO3, DMFb | 80 | 62 | |
| 4 | R-OMs (19), Cs2CO3, CH3CNb | 80 | 83 | |
| 5 | RAc | Na(OAc)3BH, CH2Cl2, AcOH | 25 | 91 |
| 6 | NaCNBH3, CH2Cl2, AcOH | 25 | 64 | |
| 7 | NaBH4, CH2Cl2, AcOH | 25 | 52 | |
| 8 | NaCNBH3, CH2Cl2, AcOH | 80 | 86 | |
| 9 | NaBH4, CH2Cl2, AcOH | 80 | 84 | |
| 10 | Acylation | Propanoyl anhydride, pyridine | 25 | 94 |
| 11 | Propanoyl chloride, pyridine | 25 | 95 | |
| 12 | Propanoyl chloride, DIPEA, CH2Cl2 | 25 | 95 |
Synthesis
N-phenylethylpiperidin-4-one (13)
4-piperidone monohydrate hydrochloride (12) 22.0 g, 143.2 mmol) was dissolved in acetonitrile (400 mL) in a 1 L round-bottom flask equipped with a large stir bar and a condenser. The colorless solution was treated sequentially with cesium carbonate (Cs2CO3, 102.6 g, 315 mmol, 2.2 equiv.) and (2-bromoethyl)benzene (17.8 mL, 24.1 g, 130.2 mmol) at ambient temperature. The resulting suspension was vigorously stirred and refluxed at 80°C for 5 h. After 5 hours, the mixture was cooled to ambient temperature, transferred to a separatory funnel and partitioned (CH2Cl2//H2O). The organic phase was washed with brine (NaCl/H2O, 3×100 mL), satd. NaHCO3 (2×100 mL), dried over Na2SO4 and concentrated in vacuo to give a yellow oil. The oily mixture was purified by flash column chromatography (1∶1 → 7∶3 EtOAc/hexanes) to give 13 as a light yellow oil (23.3 g, 88%). Rf = 0.25 (1∶1 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.31−7.28 (m, 2H), 7.22−7.19 (m, 3H), 2.85−2.83 (m, 2H), 2.82 (t, J = 6.0, 4H), 2.74−2.71 (m, 2H), 2.47 (t, J = 6.0, 4H); 13C NMR (150 MHz, CDCl3) δ 209.0, 140.0, 128.7, 128.4, 126.2, 59.3, 53.1, 41.2, 34.1; HRMS (CI) m/z calcd for C13H17NO [M+]: 203.1310; found 203.1309; Anal. Calcd for C13H17NO: C, 76.81; H, 8.43; N, 6.89. Found: C, 76.49; H, 8.71; N, 6.98.
N-[1-(2-phenylethyl)-4-piperidinyl]aniline (14)
Aniline (8.1 mL, 8.24 g, 88.5 mmol) was taken up in methylene chloride (240 mL) in a 500 mL round-bottom flask equipped with a stir bar. The light brown solution was placed on an ice bath and treated dropwise with acetic acid (5.0 mL, 88.5 mmol). To the mixture, N-phenylethylpiperidin-4-one (13) (18.0 g, 88.5 mmol) was added as a solution in methylene chloride (60 mL), followed by the careful, slow addition of sodium triacetoxyborohydride (28.1 g, 132.8 mmol, 1.5 equiv.) in small portions. The reaction mixture was stirred at ambient temperature for 14 h. After this time, methanol (100 mL) was added to the mixture and all contents transferred to a separatory funnel. The mixture was partitioned (CH2Cl2//saturated NaHCO3). Once neutralized, the organic phase was washed with brine (NaCl/H2O, 3×100 mL), dried over Na2SO4 and concentrated in vacuo to give a light brown oil. The oily mixture was purified by flash column chromatography (1∶1 → 9∶1 EtOAc/hexanes) to give 14 as a light yellow oil (22.6 g, 91%). Rf = 0.22 (7∶3 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.28−7.27 (m, 2H), 7.21−7.18 (m, 3H), 7.16−7.15 (m, 2H), 6.68 (tt, J = 7.2, 1.2, 1H), 6.61−6.59 (m, 2H), 3.50 (br, 1H), 3.33−3.30 (m, 1H), 2.96−2.94 (m, 2H), 2.83−2.80 (m, 2H), 2.63−2.60 (m, 2H), 2.21 (t, J = 12.0, 2H), 2.10−2.07 (m, 2H), 1.53−1.47 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 147.1, 140.4, 129.3, 128.7, 128.4, 126.0, 117.2, 113.3, 60.7, 52.5, 50.2, 33.9, 32.6; HRMS (CI) m/z calcd for C19H24N2 [M+]: 280.1939; found 280.1937; Anal. Calcd for C19H24N2: C, 81.38; H, 8.63; N, 9.99. Found: C, 81.22; H, 8.68; N, 10.08.
Fentanyl (4)
N-[1-(2-phenylethyl)-4-piperidinyl]aniline (14) (1.35 g, 4.8 mmol) was dissolved in methylene chloride (40 mL) in a 100 mL round bottom flask equipped with a small stir bar and was treated with diisopropylethylamine (DIPEA, 1.68 mL, 1.24 g, 9.6 mmol, 2.0 equiv.). The solution was cooled with an ice bath and treated dropwise with propionyl chloride (0.83 mL, 0.88 g, 9.6 mmol, 2.0 equiv.). The resulting mixture was stirred for 2 h at ambient temperature. The mixture was transferred to a separatory funnel and partitioned (CH2Cl2//H2O). The organic phase was washed with brine (NaCl/H2O, 1×50 mL), satd. NaHCO3 (1×50 mL), dried over anhydrous Na2SO4 and evaporated in vacuo at 40°C to give a yellow oil that was purified by flash column chromatography (3∶7 → 7∶3 EtOAc/hexanes) to furnish fentanyl (4) as a light yellow oil (1.53 g, 95%). Rf = 0.28 (1∶1 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.48−7.37 (m, 3H), 7.33−7.27 (m, 2H), 7.25−7.17 (m, 3H), 7.13−7.05 (m, 2H), 4.88−4.71 (br, 1H), 3.83−3.47 (br, 2H), 3.20−3.09 (br, 2H), 3.09−2.99 (br, 2H), 2.82−2.70 (br, 2H), 2.13−1.99 (br, 4H), 1.94 (q, J = 7.4, 2H), 1.01 (t, J = 7.4, 3H); 13C NMR (150 MHz, CDCl3) δ 174.0, 138.1, 137.0, 129.9, 129.8, 129.0, 128.9, 128.7, 127.0, 59.1, 52.6, 50.7, 31.3, 28.4, 28.0, 9.5; HRMS (CI) m/z calcd for C22H28N2O [M+]: 336.2202; found 336.2201; Anal. Calcd for C22H28N2O: C, 78.53; H, 8.39; N, 8.33. Found: C, 78.42; H, 8.05; N, 8.62.
Fentanyl hydrochloride (15)
Fentanyl (4) (138 mg, 0.41 mmol) was dissolved in diethyl ether (4 mL) in a 20 mL scintillation vial and treated at ambient temperature with 2.0 M HCl/Et2O solution (205 µL, 0.41 mmol) using a pipette. Upon addition of the acid, the colorless solution became a white suspension. The resulting suspension was stirred at ambient temperature for 2 hours and then filtered using suction filtration. The white solid was washed with diethyl ether (3×10 mL) and placed under vacuum overnight. Fentanyl hydrochloride (15) was obtained as a white solid (148 mg, 97%). 1H NMR (600 MHz, D2O) δ 7.53−7.50 (m, 3H), 7.36 (m, 2H), 7.32−7.24 (m, 5H), 4.77−4.61 (m, 1H) (overlaps with HOD), 3.66−3.61 (m, 2H), 3.32 (d, J = 8.4, 2H), 3.15 (d, J = 12.6, 2H), 3.00 (d, J = 8.4, 2H), 2.12−2.10 (m, 2H), 2.00 (q, J = 7.2, 2H), 1.64−1.58 (m, 2H), 0.92 (t, J = 7.2, 3H); 13C NMR (150 MHz, D2O) δ 177.5, 137.2, 136.3, 129.8, 129.3, 129.1, 128.8, 127.4, 57.6, 52.1, 50.0, 29.8, 28.2, 27.5, 9.0; TOF-MS (ESI) m/z calcd for C22H29N2O [M+H+]: 337.2274; found 337.2272; Anal. Calcd for C22H29ClN2O: C, 70.85; H, 7.84; N, 7.51. Found: C, 70.51; H, 8.03; N, 7.53.
Fentanyl citrate (16)
Fentanyl (4) (148 mg, 0.44 mmol) was dissolved in MeOH (4 mL) in a 20 mL scintillation vial and treated with citric acid (85 mg, 0.44 mmol). The clear solution was stirred at ambient temperature for 2 hours. The methanol was removed in vacuo at 50°C to obtain a glassy solid that upon scrapping from the surface of the vial yielded a white solid that was placed under vacuum overnight. Fentanyl citrate (16) was obtained as a white solid (221 mg, 95%). 1H NMR (600 MHz, D2O) δ 7.51−7.46 (m, 3H), 7.35−7.32 (m, 2H), 7.28−7.27 (m, 1H), 7.25−7.23 (m, 2H), 7.22−7.20 (m, 1H), 4.77−4.61 (m, 1H) (overlaps with HOD), 3.60−3.57 (m, 2H), 3.30−3.27 (m, 2H), 3.11 (td, J = 12.6, 3.0, 2H), 2.98−2.96 (m, 2H), 2.94 (d, J = 15.6, 2H), 2.78 (d, J = 15.6, 2H), 2.09−2.08 (m, 2H), 1.97 (q, J = 7.2, 2H), 1.57 (qd, J = 13.2, 3.6, 2H), 0.89 (t, J = 7.2, 3H); 13C NMR (150 MHz, D2O) δ 177.5, 177.0, 173.6, 137.2, 136.3, 129.8, 129.3, 129.1, 128.8, 127.4, 73.4, 57.6, 52.1, 50.0, 43.3, 29.9, 28.2, 27.5, 9.0; HRMS (CI) m/z calcd for C22H29N2O [M+H+]: 337.2274; found 337.2284; Anal. Calcd for C28H36N2O8: C, 63.62; H, 6.86; N, 5.30. Found: C, 63.88; H, 7.02; N, 5.66.
Acetylfentanyl (9)
N-[1-(2-phenylethyl)-4-piperidinyl]aniline (14) (1.50 g, 5.34 mmol) was dissolved in methylene chloride (50 mL) in a 100 mL round bottom flask with a small stir bar and was treated with diisopropylethylamine (DIPEA, 1.86 mL, 1.38 g, 10.7 mmol, 2.0 equiv.). The solution was cooled with an ice bath and treated dropwise with acetic anhydride (1.0 mL, 1.1 g, 10.7 mmol, 2.0 equiv.). The resulting mixture was stirred for 2 h at ambient temperature. The mixture was transferred to a separatory funnel and partitioned (CH2Cl2//H2O). The organic phase was washed with brine (NaCl/H2O, 1×50 mL), satd. NaHCO3 (1×50 mL), dried over anhydrous Na2SO4 and evaporated in vacuo at 40°C to give a yellow oil that was purified by flash column chromatography (3∶7 → 7∶3 EtOAc/hexanes) to furnish acetylfentanyl (9) as a light yellow oil (1.68 g, 98%). Rf = 0.28 (1∶1 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.45−7.36 (m, 3H), 7.30−7.25 (m, 2H), 7.20−7.16 (m, 3H), 7.11−7.09 (m, 2H), 4.69 (tt, J = 12.0, 4.2, 1H), 3.42−3.36 (br, 1H), 3.04−3.00 (m, 2H), 2.80−2.73 (m, 2H), 2.60−2.55 (m, 2H), 2.20 (td, J = 12.0, 2.4, 2H), 1.86−1.82 (m, 2H), 1.77 (s, 3H), 1.47 (qd, J = 12.6, 4.2, 2H); 13C NMR (150 MHz, CDCl3) δ 170.3, 140.2, 139.3, 130.3, 129.4, 128.6, 128.4, 128.4, 126.1, 60.4, 53.0, 52.1, 33.7, 30.4, 23.5; HRMS (CI) m/z calcd for C21H26N2O [M+]: 322.2045; found 322.2043; Anal. Calcd for C21H26N2O: C, 78.22; H, 8.13; N, 8.69. Found: C, 78.26; H, 8.11; N, 8.75.
Acetylfentanyl hydrochloride (17)
Acetylfentanyl (9) (156 mg, 0.48 mmol) was dissolved in diethyl ether (4 mL) in a 20 mL scintillation vial and treated at ambient temperature with 2.0 M HCl/Et2O solution (242 µL, 0.48 mmol) using a pipette. Upon addition of the acid, the colorless solution became a white suspension. The resulting suspension was stirred at ambient temperature for 2 hours and then filtered using suction filtration. The white solid was washed with diethyl ether (3×10 mL) and placed under vacuum overnight. Acetylfentanyl hydrochloride (17) was obtained as a white solid (165 mg, 96%). 1H NMR (600 MHz, D2O) δ 7.65−7.44 (m, 3H), 7.33−7.30 (m, 2H), 7.27−7.24 (m, 1H), 7.23−7.20 (m, 4H), 4.77−4.61 (m, 1H) (overlaps with HOD), 3.60−3.54 (m, 2H), 3.30−3.24 (m, 2H), 3.13−3.10 (m, 2H), 3.07−2.90 (m, 2H), 2.09−2.04 (m, 2H), 1.72 (s, 3H), 1.60−1.53 (m, 2H); 13C NMR (150 MHz, D2O) δ 174.1, 137.6, 136.2, 129.8, 129.7, 129.3, 129.1, 128.8, 127.4, 57.6, 52.1, 50.0, 29.9, 27.4, 22.5; TOF-MS (ESI) m/z calcd for C21H27N2O [M+H+]: 323.2118; found 323.2124; Anal. Calcd for C21H27ClN2O: C, 70.28; H, 7.58; N, 7.81. Found: C, 69.97; H, 7.72; N, 7.91.
Acetylfentanyl citrate (18)
Acetylfentanyl (9) (150 mg, 0.46 mmol) was dissolved in MeOH (4 mL) in a 20 mL scintillation vial and treated with citric acid (90 mg, 0.46 mmol). The clear solution was stirred at ambient temperature for 2 hours. The methanol was removed in vacuo at 50°C to obtain a glassy solid that upon scrapping from the surface of the vial yielded a white solid that was placed under vacuum overnight. Fentanyl citrate (18) was obtained as a white solid (225 mg, 95%). 1H NMR (600 MHz, D2O) δ 7.53−7.47 (m, 3H), 7.36−7.33 (m, 2H), 7.30−7.26 (m, 1H), 7.26−7.22 (m, 4H), 4.77−4.61 (m, 1H) (overlaps with HOD), 3.62−3.58 (m, 2H), 3.31−3.28 (m, 2H), 3.11 (td, J = 13.2, 2.4, 2H), 2.99−2.96 (m, 2H), 2.87 (d, J = 15.6, 2H), 2.73 (d, J = 15.6, 2H), 2.12−2.10 (m, 2H), 1.74 (s, 3H), 1.58 (qd, J = 14.4, 3.0, 2H); 13C NMR (150 MHz, D2O) δ 178.3, 174.5, 174.1, 137.6, 136.3, 129.8, 129.7, 129.3, 129.1, 128.8, 127.4, 73.7, 57.6, 52.1, 49.9, 48.9, 43.6, 29.8, 27.4, 22.5; TOF-MS (ESI) m/z calcd for C21H27N2O [M+H+]: 323.2118; found 323.2127; Anal. Calcd for C27H34N2O8: C, 63.02; H, 6.66; N, 5.44. Found: C, 63.38; H, 6.69; N, 5.78.
2-(Thiophen-2-yl)ethyl methanesulfonate (19) [32]
2-Thiophene ethanol (5.0 g, 39 mmol) was taken up in methylene chloride (50 mL) in a 250 mL round bottom flask and treated with triethylamine (TEA, 6.5 mL, 46.8 mmol). The resulting dark brown solution was cooled with an ice bath and treated with mesyl chloride (MsCl, 3.7 mL, 46.8 mmol, 1.2 equiv.). The ice bath was removed and the resulting brown solution was allowed to warm to ambient temperature where it was stirred overnight. The brown solution was transferred to a separatory funnel and partitioned (CH2Cl2//H2O). The organic phase was washed with brine (NaCl/H2O, 3×50 mL), satd. NaHCO3 (1×50 mL), dried over anhydrous Na2SO4 and evaporated in vacuo at 50°C to give a brown oil that was purified by flash column chromatography (hexanes → 3∶7 EtOAc/hexanes) to furnish thiophene mesylate 19 as a light brown oil (6.76 g, 84%). Rf = 0.54 (3∶7 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.91 (dd, J = 4.8, 0.6, 1H), 6.95 (dd, J = 5.4, 3.6, 1H), 6.91−6.90 (m, 1H), 4.42 (t, J = 6.6, 2H), 3.27 (t, J = 6.6, 2H), 2.92 (s, 3H); 13C NMR (150 MHz, CDCl3) δ 138.1, 127.1, 126.2, 124.5, 69.7, 37.4, 29.8; HRMS (CI) m/z calcd for C7H10O3S2 [M+]: 206.0071; found 206.0070.
N-[2-(2-thienyl)ethyl]-4-piperidinone (20)
4-piperidone monohydrate hydrochloride (12) (2.22 g, 14.5 mmol) was dissolved in acetonitrile (80 mL) in a 250 mL round-bottom flask equipped with a stir bar and a condenser. The colorless solution was treated sequentially with cesium carbonate (Cs2CO3, 10.4 g, 31.9 mmol, 2.2 equiv.) and 2-(thiophen-2-yl)ethyl methanesulfonate (19) (3.0 g, 14.5 mmol) at ambient temperature. The resulting suspension was vigorously stirred and refluxed at 80°C for 5 h. After 5 hours, the mixture was cooled to ambient temperature, transferred to a separatory funnel and partitioned (CH2Cl2//H2O). The organic phase was washed with brine (NaCl/H2O, 3×50 mL), saturated NaHCO3 (2×50 mL), dried over Na2SO4 and concentrated in vacuo to give a yellow oil. The oily mixture was purified by flash column chromatography (1∶1 → 7∶3 EtOAc/hexanes) to give 20 as a light yellow oil (2.7 g, 90%). Rf = 0.25 (1∶1 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.14 (dd, J = 5.4, 1.2, 1H), 6.92 (dd, J = 5.4, 3.6, 1H), 6.84 (dq, J = 3.6, 1.2, 1H), 3.04 (t, J = 7.2, 2H), 2.82 (t, J = 6.0, 4H), 2.77 (t, J = 7.2, 2H), 2.48 (t, J = 6.0, 4H); 13C NMR (150 MHz, CDCl3) δ 209.0, 142.4, 126.6, 124.7, 123.7, 60.4, 53.0, 41.3, 28.3; HRMS (CI) m/z calcd for C11H15NOS [M+]: 209.0874; found 209.0872; Anal. Calcd for C11H15NOS: C, 63.12; H, 7.22; N, 6.69; Found: C, 63.01; H, 7.16; N, 6.77.
N-phenyl-1-(2-(thiophen-2-yl)ethyl)piperidin-4-amine (21)
Aniline (0.53 mL, 5.7 mmol) was taken up in methylene chloride (50 mL) in a 250 mL round-bottom flask equipped with a stir bar. The light brown solution was placed on an ice bath and treated dropwise with acetic acid (0.32 mL, 5.7 mmol). To the mixture, N-[2-(2-thienyl)ethyl]-4-piperidinone (20) (1.2 g, 5.7 mmol) was added as a solution in methylene chloride (30 mL), followed by the careful, slow addition of sodium triacetoxyborohydride (1.8 g, 8.6 mmol, 1.5 equiv.) in small portions. The reaction mixture was stirred at ambient temperature for 14 h. After this time, methanol (40 mL) was added to the mixture and all contents transferred to a separatory funnel. The mixture was partitioned (CH2Cl2//saturated NaHCO3). Once neutralized, the organic phase was washed with brine (NaCl/H2O, 3×50 mL), dried over Na2SO4 and concentrated in vacuo to give a light brown oil. The oily mixture was purified by flash column chromatography (1∶1 → 9∶1 EtOAc/hexanes) to give 21 as a light yellow oil (1.42 g, 87%). Rf = 0.33 (7∶3 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.18−7.15 (m, 2H), 7.13 (dd, J = 5.4, 1.2, 1H), 6.92 (dd, J = 5.4, 3.6, 1H), 6.84−6.82 (m, 1H), 6.69 (tt, J = 7.2, 0.6, 1H), 6.62−6.58 (m, 1H), 3.52 (br, 1H), 3.34−3.31 (m, 1H), 2.13 (t, J = 6.6, 2H), 2.95−2.93 (m, 2H), 2.68 (t, J = 6.6, 2H), 2.22 (td, J = 13.2, 2.4, 2H), 2.10−2.07 (m, 2H), 1.54−1.51 (m, 2H); 13C NMR (150 MHz, CDCl3) δ 147.1, 142.9, 129.3, 126.6, 124.6, 123.5, 117.2, 113.3, 60.0, 52.4, 49.9, 32.6, 28.0; HRMS (CI) m/z calcd for C17H22N2S [M+]: 286.1504; found 286.1503; Anal. Calcd for C17H22N2S: C, 71.29; H, 7.74; N, 9.78; Found: C, 71.16; H, 7.75; N, 9.66.
Thiofentanyl (10)
N-phenyl-1-(2-(thiophen-2-yl)ethyl)piperidin-4-amine (21) (2.21 g, 7.74 mmol) was dissolved in methylene chloride (50 mL) in a 100 mL round bottom flask equipped with a stir bar and was treated with diisopropylethylamine (DIPEA, 2.0 mL, 1.5 g, 11.6 mmol, 1.5 equiv.). The solution was cooled with an ice bath and treated dropwise with propionyl chloride (0.99 mL, 11.6 mmol). The resulting mixture was stirred for 2 h at ambient temperature. The mixture was transferred to a separatory funnel and partitioned (CH2Cl2//H2O). The organic phase was washed with brine (NaCl/H2O, 1×50 mL), satd. NaHCO3 (1×50 mL), dried over anhydrous Na2SO4 and evaporated in vacuo at 40°C to give a yellow oil that was purified by flash column chromatography (3∶7 → 7∶3 EtOAc/hexanes) to furnish thiofentanyl (10) as a light yellow oil (2.57 g, 97%). Rf = 0.28 (1∶1 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.38−7.33 (m, 3H), 7.09−7.06 (m, 2H), 6.88 (dd, J = 4.8, 3.0, 1H), 6.77−6.75 (m, 1H), 4.67 (tt, J = 12.0, 4.2, 1H), 2.97−2.92 (m, 4H), 2.61−2.58 (m, 2H), 2.17 (td, J = 12.0, 1.8, 2H), 1.91 (q, J = 7.8, 2H), 1.81−1.78 (br s, 1H), 1.40 (qd, J = 11.4, 3.6, 2H), 1.00 (t, J = 7.8, 3H); 13C NMR (150 MHz, CDCl3) δ 173.5, 142.6, 138.8, 130.4, 129.3, 128.3, 126.6, 124.5, 123.4, 60.0, 53.0, 52.1, 30.5, 28.5, 27.8, 9.6; HRMS (CI) m/z calcd for C20H26N2OS [M+]: 342.1766; found 342.1765; Anal. Calcd for C20H26N2OS: C, 70.14; H, 7.65; N, 8.18; Found: C, 70.11; H, 7.63; N, 8.10.
Thiofentanyl hydrochloride (23)
Thiofentanyl (10) (300 mg, 0.87 mmol) was dissolved in diethyl ether (6 mL) in a 20 mL scintillation vial and treated at ambient temperature with 2.0 M HCl/Et2O solution (435 µL, 0.87 mmol) using a pipette. Upon addition of the acid, the colorless solution became a white suspension. The resulting suspension was stirred at ambient temperature for 2 hours and then filtered using suction filtration. The white solid was washed with diethyl ether (3×10 mL) and placed under vacuum overnight. Thiofentanyl hydrochloride (23) was obtained as a white solid (319 mg, 97%). 1H NMR (600 MHz, D2O) δ 7.52−7.47 (m, 3H), 7.30 (dd, J = 5.4, 1.2, 1H), 7.25−7.22 (m, 2H), 6.98 (dd, J = 4.8, 3.0, 1H), 6.94−6.93 (m, 1H), 3.59−3.57 (m, 2H), 3.35 (t, J = 7.2, 2H), 3.23 (t, J = 7.2, 2H), 3.17−3.15 (m, 2H), 2.09−2.08 (m, 2H), 1.98 (q, J = 7.8, 2H), 1.62−1.58 (m, 2H), 0.90 (t, J = 7.8, 3H); 13C NMR (150 MHz, D2O) δ 177.5, 138.1, 137.2, 129.8, 129.3, 127.6, 126.5, 125.3, 57.6, 50.0, 28.2, 27.4, 24.2, 9.0; TOF-MS (ESI) m/z calcd for C20H26N2OS [M+H+]: 343.1839; found 343.1812; Anal. Calcd for C20H27ClN2OS: C, 63.39; H, 7.18; N, 7.39; Found: C, 63.14; H, 7.25; N, 7.42.
Thiofentanyl citrate (24)
Thiofentanyl (10) (360 mg, 1.05 mmol) was dissolved in MeOH (5 mL) in a 20 mL scintillation vial and treated with citric acid (201 mg, 1.05 mmol). The clear solution was stirred at ambient temperature for 2 hours. The methanol was removed in vacuo at 50°C to obtain a glassy solid that upon scrapping from the surface of the vial yielded a white solid that was placed under vacuum overnight. Thiofentanyl citrate (24) was obtained as a white solid (538 mg, 96%). 1H NMR (600 MHz, D2O) δ 7.51−7.49 (m, 3H), 7.30 (dd, J = 4.8, 1.2, 1H), 7.23 (m, 2H), 6.98 (dd, J = 4.8, 3.6, 1H), 6.94−6.93 (m, 1H), 3.60−3.58 (m, 2H), 3.35 (t, J = 7.8, 2H), 3.24 (t, J = 7.8, 2H), 3.15−3.11 (m, 2H), 2.84 (d, J = 15.6, 2H), 2.72 (d, J = 15.6, 2H), 2.10−2.08 (m, 2H), 1.98 (q, J = 7.2, 2H), 1.59 (qd, J = 13.8, 3.6, 2H), 0.90 (t, J = 7.2, 3H); 13C NMR (150 MHz, D2O) δ 178.6, 177.5, 174.7, 138.1, 137.2, 129.8, 129.8, 129.3, 127.6, 126.5, 125.3, 73.8, 57.6, 52.1, 49.9, 28.2, 27.4, 24.1, 9.0; TOF-MS (ESI) m/z calcd for C20H26N2OS [M+H+]: 343.1839; found 343.1842; Anal. Calcd for C20H27ClN2OS: C, 58.41; H, 6.41; N, 5.24; Found: C, 58.70; H, 6.55; N, 5.41.
Acetylthiofentanyl (11)
N-phenyl-1-(2-(thiophen-2-yl)ethyl)piperidin-4-amine (21) (1.2 g, 4.2 mmol) was dissolved in methylene chloride (50 mL) in a 100 mL round bottom flask equipped with a stir bar and was treated with diisopropylethylamine (DIPEA, 0.98 mL, 0.72 g, 8.4 mmol). The solution was cooled with an ice bath and treated dropwise with acetic anhydride (0.52 mL, 0.56 g, 8.4 mmol). The resulting mixture was stirred for 2 h at ambient temperature. The mixture was transferred to a separatory funnel and partitioned (CH2Cl2//H2O). The organic phase was washed with brine (NaCl/H2O, 1×10 mL), satd. NaHCO3 (1×10 mL), dried over anhydrous Na2SO4 and evaporated in vacuo at 40°C to give a yellow oil that was purified by flash column chromatography (3∶7 → 7∶3 EtOAc/hexanes) to furnish acetylthiofentanyl (11) as a light yellow oil (1.3 g, 94%). Rf = 0.28 (1∶1 EtOAc/hexanes); 1H NMR (600 MHz, CDCl3) δ 7.40−7.34 (m, 3H), 7.09−7.07 (m, 3H), 6.88 (d, J = 5.4, 3.6, 1H), 6.77−6.75 (m, 1H), 4.66 (tt, J = 12.0, 4.2, 1H), 2.99−2.93 (m, 4H), 2.62−2.58 (m, 2H), 2.19 (td, J = 12.6, 2.4, 2H), 1.81−1.79 (m, 2H), 1.74 (s, 3H), 1.43 (qd, J = 12.0, 3.6, 2H); 13C NMR (150 MHz, CDCl3) δ 170.3, 142.5, 139.4, 130.2, 129.4, 128.4, 126.7, 124.6, 123.4, 59.9, 52.9, 52.1, 30.4, 27.7, 23.5; HRMS (CI) m/z calcd for C19H24N2OS [M+]: 328.1609; found 328.1613; Anal. Calcd for C19H24N2OS: C, 69.48; H, 7.36; N, 8.53; Found: C, 69.44; H, 7.28; N, 8.46.
Acetylthiofentanyl hydrochloride (25)
Acetylthiofentanyl (11) (260 mg, 0.79 mmol) was dissolved in diethyl ether (3 mL) in a 20 mL scintillation vial and treated at ambient temperature with 2.0 M HCl/Et2O solution (396 µL, 0.79 mmol) using a pipette. Upon addition of the acid, the colorless solution became a white suspension. The resulting suspension was stirred at ambient temperature for 2 hours and then filtered using suction filtration. The white solid was washed with diethyl ether (2×5 mL) and placed under vacuum overnight. Acetylthiofentanyl hydrochloride (25) was obtained as a white solid (273 mg, 95%). 1H NMR (600 MHz, D2O) δ 7.50−7.45 (m, 3H), 7.27 (dd, J = 5.4, 2.1, 1H), 7.22−7.21 (m, 2H), 6.95 (dd, J = 4.8, 3.6, 1H), 6.92−6.90 (m, 1H), 3.57−3.56 (m, 2H), 3.33 (t, J = 7.2, 2H), 3.21 (t, J = 7.2, 2H), 3.13−3.10 (m, 2H), 2.08−2.06 (m, 2H), 1.72 (s, 3H), 1.61−1.56 (m, 2H); 13C NMR (150 MHz, D2O) δ 174.1, 138.0, 137.6, 129.8, 129.6, 129.3, 127.5, 126.4, 125.3, 57.5, 52.1, 49.9, 27.3, 24.1, 22.4; TOF-MS (ESI) m/z calcd for C19H25N2OS [M+H+]: 329.1682; found 329.1666; Anal. Calcd for C19H25ClN2OS: C, 62.53; H, 6.91; N, 7.68; Found: C, 62.25; H, 7.00; N, 7.63.
Acetylthiofentanyl citrate (26)
Acetylthiofentanyl (11) (282 mg, 0.86 mmol) was dissolved in MeOH (3 mL) in a 20 mL scintillation vial and treated with citric acid (165 mg, 0.86 mmol). The clear solution was stirred at ambient temperature for 2 hours. The methanol was removed in vacuo at 50°C to obtain a white solid that was placed under vacuum overnight. Acetylthiofentanyl citrate (26) was obtained as a white solid (438 mg, 98%). 1H NMR (600 MHz, D2O) δ 7.49−7.44 (m, 3H), 7.26 (dd, J = 4.8, 1.2, 1H), 7.20−7.19 (m, 2H), 6.94 (dd, J = 4.8, 3.0, 1H), 6.91−6.90 (m, 1H), 3.56−3.54 (m, 2H), 3.31 (t, J = 7.8, 2H), 3.20 (t, J = 7.2, 2H), 3.11−3.07 (m, 2H), 2.80 (d, J = 15.6, 2H), 2.68 (d, J = 15.6, 2H), 2.07−2.04 (m, 2H), 1.70 (s, 3H), 1.58−1.52 (m, 2H); 13C NMR (150 MHz, D2O) δ 178.3, 174.4, 174.1, 138.1, 137.5, 129.8, 129.6, 129.3, 127.5, 126.4, 125.2, 73.7, 57.5, 52.0, 49.3, 43.5, 27.3, 24.1, 22.4; TOF-MS (ESI) m/z calcd for C19H25N2OS [M+H+]: 329.1682; found 329.1688; Anal. Calcd for C25H32N2O8S: C, 57.68; H, 6.20; N, 5.38; Found: C, 57.83; H, 6.52; N, 5.53.
Conclusion
The efficient syntheses of fentanyl and three other analogs (along with their hydrochloride and citric acid salts) have been accomplished. The three-step synthetic route was subject to optimization studies furnishing a process that generates the target fentanyls in high yields (73−78%). Thus, the syntheses described herein provide an efficient protocol for the construction of these interesting opioids for in depth biochemical as well as crystallographic studies.
Supporting Information
Proton (1H) and Carbon (13C) NMR spectra for the fentanyl panel (free bases and salts) and their synthetic intermediates. A more specific table of contents can be located in the document.
(PDF)
Acknowledgments
Disclaimer: This document (LLNL-JRNL-656516) was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor Lawrence Livermore National Security, LLC, nor any of their employees makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or Lawrence Livermore National Security, LLC. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or Lawrence Livermore National Security, LLC, and shall not be used for advertising or product endorsement purposes.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors (CAV, BPM, and RNL) received funding from Lawrence Livermore National Laboratory (llnl.gov) under the Laboratory Directed Research and Development (LDRD) program, project number 14-ERD-048. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Friedrichsdorf SJ, Postier A (2014) Management of breakthrough pain in children with cancer. J. Pain Res. 7: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee SK, Dawson J, Lee JA, Osman G, Levitin MO, et al. (2014) Management of cancer pain: 1. Wider implications of orthodox analgesics. Int. J. Gen. Med. 7: 49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grass JA (1992) Fentanyl: clinical use as postoperative analgesic: epidural/intrathecal route. J. Pain Symptom Manag. 7: 419–430. [DOI] [PubMed] [Google Scholar]
- 4. Grass JA (1992) Sufentanil: clinical use as postoperative analgesic: epidural/intrathecal route. J. Pain Symptom Manag. 7: 271–286. [DOI] [PubMed] [Google Scholar]
- 5. Grass JA (2000) The role of epidural anesthesia and analgesia in postoperative outcome. Anesthesiol. Clin. North America. 18(2): 407–428. [DOI] [PubMed] [Google Scholar]
- 6. Lehmann KA (2005) Recent developments in patient-controlled analgesia. J. Pain Symptom Manag. 29: S72–S89. [DOI] [PubMed] [Google Scholar]
- 7. Mercadante S (2011) New opioids. Curr. Pain Headache R. 15: 244–249. [DOI] [PubMed] [Google Scholar]
- 8. Janssen PAJ, Eddy NB (1960) Compounds related to pethidine-IV. New general chemical methods of increasing the analgesic activity of pethidine. J. Med. Chem. 2: 31–45. [DOI] [PubMed] [Google Scholar]
- 9. Janssen PAJ (1961) Pirinitramide (R 3365), a potent analgesic with unusual chemical structure. J. Pharm. Pharmacol. 13: 513–530. [DOI] [PubMed] [Google Scholar]
- 10. Stanley TH (2005) Fentanyl. J. Pain Symptom Manag. 29: S67–S71. [DOI] [PubMed] [Google Scholar]
- 11. Stanley TH (1992) The history and development of the fentanyl series. J. Pain Symptom Manag. 7: S3–S7. [DOI] [PubMed] [Google Scholar]
- 12. Maciejewski D (2012) Sufentanil in anaesthesiology and intensive therapy. Anasthesiol. Intensive Ther. 44: 35–41. [PubMed] [Google Scholar]
- 13. Vardanyan RS, Hruby VJ (2014) Fentanyl-related compounds and derivatives: current status and future prospects for pharmaceutical applications. Future Med. Chem. 6: 385–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon DB (2006) Oral transmucosal fentanyl citrate for cancer breakthrough pain: a review. Oncol. Nurs. Forum 33: S6–S13. [DOI] [PubMed] [Google Scholar]
- 15. Kronstrand R, Druid H, Holmgren P, Rajs J (1997) A cluster of fentanyl-related deaths among drug addicts in Sweden. Forensic Sci. Int. 88: 185–195. [DOI] [PubMed] [Google Scholar]
- 16.Between March–May of 2013, acetylfentanyl, an analog six times more potent than morphine was linked to 14 overdose deaths in Rhode Island, while on February of this year, this designer opioid drug was the cause of 3 overdose deaths in North Carolina. For the Rhode Island case please see: Centers for Disease Control and Prevention (2013) MMWR Morb. Mortal Wkly. Rep. 62: 703–704. [Google Scholar]
- 17. Chauhan S, Chauhan S, D’Cruz R, Faruqi S, Singh KK, et al. (2008) Chemical warfare agents. Environ. Tox. Pharm. 26: 113–122. [DOI] [PubMed] [Google Scholar]
- 18. Wax PM, Becker CE, Curry SC (2003) Unexpected “gas” casualties in Moscow: a medical toxicology perspective. Ann. Emerg. Med. 41: 700–705. [DOI] [PubMed] [Google Scholar]
- 19. Rieder J, Keller C, Hoffmann G, Lirk P (2003) Moscow theatre siege and anaesthetic drugs. Lancet 361: 1131. [DOI] [PubMed] [Google Scholar]
- 20. Coupland RM (2003) Incapacitating chemical weapons: a year after the Moscow theatre siege. Lancet 362: 1346. [DOI] [PubMed] [Google Scholar]
- 21. George AV, Lu JJ, Pisano MV, Metz J, Erickson TB (2010) Carfentanil–an ultra potent opioid. Am. J. Emerg. Med. 28: 530–532. [DOI] [PubMed] [Google Scholar]
- 22. Sirohi S, Dighe SV, Walker EA, Yoburn BC (2008) The analgesic efficacy of fentanyl: relationship to tolerance and mu-opioid receptor regulation. Pharmacol. Biochem. Behav. 91: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duttaroy A, Yoburn BC (1995) The effect of intrinsic efficacy on opioid tolerance. Anesthesiology 82: 1226–1236. [DOI] [PubMed] [Google Scholar]
- 24. Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, et al. (2012) Crystal structure of the µ-opioid receptor bound to a morphinan antagonist. Nature 485: 321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu H, Wacker D, Mileni M, Katritch V, Han GW, et al. (2012) Structure of the human κ-opioid receptor in complex with JDTic. Nature 485: 327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson AA, Liu W, Chun E, Katritch V, Wu H, et al. (2012) Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, et al. (2012) Structure of the δ-opioid receptor bound to naltrindole. Nature 485: 400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gupta PK, Kumaran G, Ambuja P, Malhotra RC (2005) A convenient one pot synthesis of fentanyl. J. Chem. Res. 7: 452–453. [Google Scholar]
- 29. Watson AJA, Maxwell AC, Williams JMJ (2011) Borrowing hydrogen methodology for amine synthesis under solvent-free microwave conditions. J. Org. Chem. 76: 2328–2331. [DOI] [PubMed] [Google Scholar]
- 30. Ghaffarzadeh M, Joghan SS, Faraji F (2012) A new method for the synthesis of amides from imines. Tetrahedron Lett. 53: 203–206. [Google Scholar]
- 31.Gant TG, Sarshar S (2010) US Patent 20100016365.
- 32. Albanese D, Ghidoli C, Zenoni M (2008) Concise Synthesis of Vinylheterocycles through β-Elimination under Solventless Phase Transfer Catalysis Conditions. Org. Process Res. Dev. 12 736–739. [Google Scholar]
- 33. Burova SA, Davis R, Fitzgerald RN, Johnson BS, Matsuoka RT (2007) General Synthesis of 1-Substituted 2-Methylbenzimidazoles from Ketones and 2-Aminoacetanilide. Synth. Commun. 37: 3029–3039. [Google Scholar]
- 34. Balannik V, Wang J, Ohigashi Y, Jing X, Magavern E, et al. (2009) Design and Pharmacological Characterization of Inhibitors of Amantadine-Resistant Mutants of the M2 Ion Channel of Influenza A Virus. Biochemistry 48: 11872–11882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proton (1H) and Carbon (13C) NMR spectra for the fentanyl panel (free bases and salts) and their synthetic intermediates. A more specific table of contents can be located in the document.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.